Abstract
Importance:
The fecal immunochemical test (FIT) is commonly used for colorectal cancer (CRC) screening and positive tests require follow-up colonoscopy. However, follow-up intervals vary markedly, which may result in neoplastic progression.
Objective:
Evaluate whether time to colonoscopy after a positive FIT is associated with an increased risk of CRC outcomes at the follow-up colonoscopy.
Design:
Retrospective cohort study from January 1, 2010 through December 31, 2014.
Setting:
Kaiser Permanente Northern and Southern California, two large community-based integrated healthcare delivery organizations.
Participants:
70,124 CRC screening-eligible FIT-positive patients, ages 50–75 years, who had a follow-up colonoscopy.
Exposure:
Time (days) to colonoscopy after a positive FIT.
Main Outcomes and Measures:
Risk of any CRC and advanced-stage disease. Odds ratios (OR) and 95% confidence intervals (CI) were adjusted for patient demographics and baseline risk factors.
Results:
Among 70,124 FIT-positive patients (median [interquartile range] age, 61 [55, 67] years; 36,976 [52.7%] male), follow-up colonoscopies were completed in 86% of patients (median [interquartile range] time: 37 [23, 62] days). There were 2,191 cases of any CRC and 601 cases of advanced-stage disease diagnosed. Compared to colonoscopy follow-up within 8–30 days, there was no significant increase in risk for follow-up at 2, 3, or 4–6 months for any CRC (30, 28, 31, and 31 cases/1,000 patients, respectively) or advanced-stage disease (8, 7, 7, and 9 cases/1,000 patients, respectively). A non-significant increase in risk was seen starting at 7–9 months (any CRC: OR, 1.30; 95% CI, 0.99–1.72; 43 cases/1,000 patients and advanced-stage disease: OR, 1.32; 95% CI, 0.80–2.18; 13 cases/1,000 patients). Risks were significantly higher for examinations at 10–12 months (any CRC: OR, 1.48; 95%CI, 1.05–2.08; 49 cases/1,000 patients and advanced-stage disease: OR, 1.97; 95% CI, 1.14–3.42; 19 cases/1,000 patients) and >12 months (any CRC: OR, 2.25; 95% CI, 1.89–2.68; 76 cases/1,000 patients and advanced-stage disease: OR, 3.22; 95% CI, 2.44–4.25; 31 cases/1,000 patients).
Conclusions and Relevance:
Among patients with a positive FIT, compared to time to follow-up at 8–30 days, follow-up colonoscopy at >6 months was increasingly associated with a higher risk of any CRC and advanced-stage disease.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 Screening reduces mortality through removal of precancerous polyps and treatment of early-stage cancers.2 The US Preventive Services Task Force endorses multiple screening approaches for early detection of CRC, including fecal immunochemical test (FIT) screening.2 FIT screening is commonly used worldwide3,4 and because of its sensitivity, effectiveness, low cost, and ability to be distributed by mail, it is an increasingly common method for meeting CRC screening goals in the United States.
A positive FIT needs to be followed by a complete colon examination, typically with colonoscopy;5 however, recommendations for how quickly to complete follow-up differ and lack a strong evidence base.6–8 In practice there is marked variation in time to follow-up after a positive stool test, which may result in neoplastic progression. Few studies have evaluated CRC outcomes associated with variation in time to follow-up. Two studies of military veterans reported no association between longer intervals from positive test to colonoscopy and either cancer stage or survival, but small sample sizes limited power.9,10 Given CRC screening theoretically impacts every adult who reaches screening age, and the increasing adoption of FIT screening worldwide, there is a critical need to provide evidence-based follow-up recommendations. The present study tested the hypothesis that longer time to colonoscopy after a positive FIT increases the risk of any CRC and advanced-stage disease.
METHODS
Study Population and Oversight
This was a retrospective cohort study of Kaiser Permanente Northern California (KPNC) and Southern California (KPSC) health plan members. These integrated health care delivery organizations serve approximately 7.5 million members throughout California, and the diverse membership is similar to the region’s census demographics.11–13 The study was approved by the local institutional review boards and a waiver was granted for obtaining written informed consent from study participants.
Organized CRC Screening Programs
The health plans initiated organized FIT outreach in 2006.14 Each year screening-eligible health plan members ages 50–75 years and not up-to-date with screening by other methods are mailed a FIT kit (OC FIT-CHEK, Polymedco Inc.). Patients mail completed kits to regional laboratories where they are analyzed using OC-Sensor Diana (Polymedco Inc., positive result ≥100 ng/mL [20 μg/g]). FIT kits are also distributed in-person to patients not up-to-date at office visits or when receiving a flu shot. FIT-positive patients are referred by their physician or contacted by the gastroenterology department for colonoscopy scheduling.
Study Eligibility Criteria
Members were eligible for the study if they were 50–75 years of age and completed FIT screening between January 1, 2010 and October 31, 2012 for KPSC members and January 1, 2010 and July 31, 2013 for KPNC members. Among those with a positive FIT, patients were excluded if they had: a prior history of CRC; <1 year of membership after FIT screening and no record of a colonoscopy during that period; a >3 month gap in membership after screening; <1 year of membership prior to screening (to record prior out-of-system endoscopy procedures and diagnoses); a colonoscopy within 10 years or sigmoidoscopy within 5 years before FIT screening; or a colonoscopy or CRC diagnosis 1–7 days after their positive FIT (because these FIT may represent diagnostic rather than screening tests).
Follow-up Time Intervals and Cancer Outcomes
The exposure was the time elapsed between a positive FIT and subsequent colonoscopy. Time was examined as a continuous variable and in 7 intervals; the reference group was 8–30 days and comparison categories were 2 months (31–60 days), 3 months (61–90 days), 4–6 months (91–180 days), 7–9 months (181–272 days), 10–12 months (273–365 days), and >12 months (366–1751 days). The intervals were chosen to evaluate published follow-up recommendations (i.e., ≤31 days [European recommendation] and ≤60 days [Canadian and Veterans Health Administration recommendation]); to provide calendar month intervals as practical cut-offs (i.e., 1, 2, 3, 4–6, 7–9, 10–12, and >12 months); and to balance sample sizes based on outcome distributions.
The primary outcomes were any colorectal adenocarcinoma diagnosed at or within 6 months after the follow-up colonoscopy, cancer by stage, advanced-stage disease, and adenomas with advanced histology (i.e., tubulovillous and villous adenomas) (96% of diagnoses were within ≤1 month after the colonoscopy). Diagnoses within 6 months were included to account for colonoscopies repeated due to poor bowel preparation, incomplete examination or excision, patient intolerance, etc. Adenoma size was not available electronically.
Data Sources
FIT results and dates were obtained from laboratory databases. Colonoscopy procedures were identified using Current Procedural Terminology codes (44388–44394, 44397, 45355, 45378–45392), International Classification of Disease procedure codes (45.21–45.23, 45.25, 45.42, 45.43, 98.04), and Healthcare Common Procedure Coding System codes (G0105, G0121). Colorectal adenocarcinoma diagnoses and cancer stage were obtained from Kaiser Permanente cancer registries, which report to the Surveillance, Epidemiology and End-Results (SEER) program and capture >99% of cancers diagnosed among members, compared with manual review. Advanced-stage cancers were defined as stage III (regional lymph node involvement) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system or, for those without such staging, as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to the regional lymph nodes), or code 7 (distant metastasis) according to the SEER Program Coding and Staging Manual 2013.15 Adenomas with advanced histology were identified using Systematized Nomenclature of Medicine codes in pathology databases linked to the date of the colonoscopy exam. Validation studies confirmed high levels (>95%) of sensitivity and accuracy for capture and classification of colonoscopy exams, adenoma diagnoses, histology, and cancers.16
Statistical Analyses
P-values for differences in baseline characteristics were derived from chi-squared tests. Crude rates and 95% CIs were calculated as cases/1,000 patients who completed a colonoscopy. Risk analyses utilized multivariable logistic regression models; odds ratios (OR) and 95% confidence intervals (CI) were adjusted for sex; age at FIT screening (50–54, 55–59, 60–64, 65–69, 70–75 years); self-reported race/ethnicity (non-Hispanic white, Hispanic, black, Asian/Pacific Islander, and other/unknown); body mass index (<25.0, 25.0–29.9, ≥30 kg/m2, unknown); region (KPNC, KPSC); FIT screening year; completion of previous FIT screening (ever and in the prior year); and in the year before FIT screening, receipt of the flu or pneumonia vaccine, presence of gastrointestinal symptoms (bleeding or blood in stool, unexplained weight loss, abdominal pain, diarrhea, diverticulitis, inflammatory bowel disease, or Lynch syndrome), diagnosis of iron-deficiency anemia or diabetes, current smoker, number of primary care visits (0, 1, 2–3, ≥4), and number of days hospitalized (0–1, 2–3, ≥4). Hypothesis testing was two-sided with α = .05. Sensitivity analyses included redefining the reference group to include patients whose exams were performed 1–30 days (to include the earliest exams, though these have greater risk of being symptom-driven), 8–60 days and 8–90 days after a positive FIT; excluding follow-up colonoscopies >24 months after a positive FIT; including members who had <1 year of membership prior to FIT screening, or who had a colonoscopy within 10 years or sigmoidoscopy within 5 years prior to FIT screening; and adding an exposure category of 1–7 days. To test for effect modification, interaction terms were added to the main model for each covariate and time was included as a continuous variable; likelihood ratio tests generated a p-value for each time-by-covariate interaction. Stratified models are presented when interaction p<0.10; OR point estimates represent the overall risk estimate for each additional 30-day delay in follow-up compared to follow-up at 8–30 days. Analyses were performed with SAS version 9.3 (SAS Institute) and Stata version 10.1 (StataCorp).
RESULTS
Of 1,258,039 members aged 50–75 years who completed FIT screening, 106,520 (8.5%) were FIT positive (Figure 1). Of these, 51 were excluded for history of CRC; 2,873 for <1 year of membership after FIT screening and no record of a colonoscopy during that period; 17 for a membership gap >3 months after screening; 9,771 for <1 year of membership prior to FIT screening; 10,873 for a colonoscopy <10 years or sigmoidoscopy <5 years before FIT screening; and 1,417 for colonoscopy or CRC diagnosis 1–7 days after their positive FIT. Of the remaining 81,518 FIT-positive individuals, 70,124 (86.0% and 65.8% of 106,520) received a follow-up colonoscopy by the end of the study period.
Figure 1. Patient flow diagram.
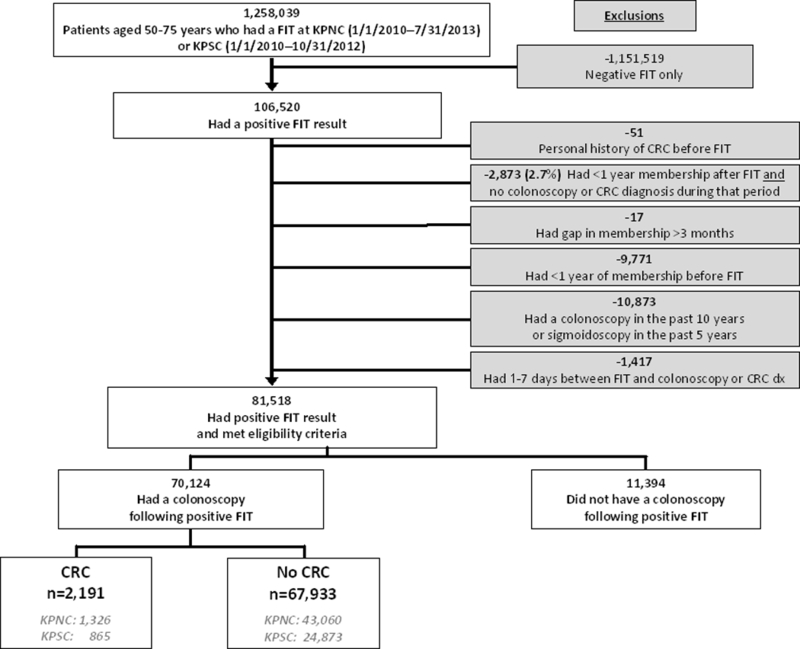
Abbreviations: CRC, colorectal cancer; dx, diagnosis; FIT, fecal immunochemical test; KPNC, Kaiser Permanente Northern California; KPSC, Kaiser Permanente Southern California; n, number.
Characteristics of the Cohort
Of the 81,518 study-eligible FIT-positive patients, 33.3% received a colonoscopy within 30 days, 63.6% within 2 months, 74.2% within 3 months, 80.6% within 6 months, and 83.2% within 12 months; completion rates were similar in the total group of 106,520 FIT-positive patients (eFigure 1). Among the 70,124 patients who received a follow-up colonoscopy (Table 1), the median [IQR] age was 61 [55, 67] years, 52.7% were male, 56.1% were non-Hispanic white, and 42.2% had a body mass index of 30.0 kg/m2 or greater. The median [IQR] time to colonoscopy was 37 [23, 62] days. Baseline covariates across time-to-colonoscopy exposure groups were typically within a few percentage points (Table 1), although even small differences were significant given the large sample size.
Table 1.
Characteristics and CRC outcomes in patients who received a colonoscopy after a positive FIT.
| Time to colonoscopya | 8–30 days | 2 months | 3 months | 4–6 months | 7–9 months | 10–12 months | >12 months | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristicsb | N | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) |
| Total | 27,176 | 24,644 | 8,666 | 5,251 | 1,335 | 748 | 2,304 | 70,124 | ||||||||
| Region | ||||||||||||||||
| KPNC | 14,473 | (53.3) | 17,102 | (69.4) | 6,233 | (71.9) | 3,781 | (72.0) | 978 | (73.3) | 501 | (67.0) | 1,318 | (57.2) | 44,386 | (63.3) |
| KPSC | 12,703 | (46.7) | 7,542 | (30.6) | 2,433 | (28.1) | 1,470 | (28.0) | 357 | (26.7) | 247 | (33.0) | 986 | (42.8) | 25,738 | (36.7) |
| Sex | ||||||||||||||||
| Female | 12,772 | (47.0) | 11,678 | (47.4) | 4,111 | (47.4) | 2,527 | (48.1) | 631 | (47.3) | 353 | (47.2) | 1,076 | (46.7) | 33,148 | (47.3) |
| Male | 14,404 | (53.0) | 12,966 | (52.6) | 4,555 | (52.6) | 2,724 | (51.9) | 704 | (52.7) | 395 | (52.8) | 1,228 | (53.3) | 36,976 | (52.7) |
| Age (years) | ||||||||||||||||
| 50–54 | 6,755 | (24.9) | 5,787 | (23.5) | 1,975 | (22.8) | 1,273 | (24.2) | 319 | (23.9) | 166 | (22.2) | 526 | (22.8) | 16,801 | (24.0) |
| 55–59 | 5,655 | (20.8) | 5,132 | (20.8) | 1,784 | (20.6) | 1,098 | (20.9) | 289 | (21.6) | 145 | (19.4) | 478 | (20.7) | 14,581 | (20.8) |
| 60–64 | 5,685 | (20.9) | 5,167 | (21.0) | 1,899 | (21.9) | 1,161 | (22.1) | 272 | (20.4) | 182 | (24.3) | 487 | (21.1) | 14,853 | (21.2) |
| 65–69 | 4,818 | (17.7) | 4,501 | (18.3) | 1,556 | (18.0) | 844 | (16.1) | 226 | (16.9) | 124 | (16.6) | 414 | (18.0) | 12,483 | (17.8) |
| 70–75 | 4,263 | (15.7) | 4,057 | (16.5) | 1,452 | (16.8) | 875 | (16.7) | 229 | (17.2) | 131 | (17.5) | 399 | (17.3) | 11,406 | (16.3) |
| Median (IQR) | 61 | (55,67) | 61 | (55,67) | 61 | (55,67) | 60 | (55,67) | 61 | (55,67) | 61 | (55,67) | 61 | (55,67) | 61 | (55,67) |
| Race/Ethnicity | ||||||||||||||||
| Non-Hispanic White | 15,178 | (55.9) | 14,123 | (57.3) | 4,929 | (56.9) | 2,828 | (53.9) | 708 | (53.0) | 387 | (51.7) | 1,197 | (52.0) | 39,350 | (56.1) |
| Hispanic | 5,103 | (18.8) | 3,923 | (15.9) | 1,357 | (15.7) | 837 | (15.9) | 234 | (17.5) | 138 | (18.4) | 435 | (18.9) | 12,027 | (17.2) |
| Black | 2,178 | ( 8.0) | 1,938 | (7.9) | 677 | (7.8) | 499 | (9.5) | 122 | (9.1) | 64 | (8.6) | 255 | (11.1) | 5,733 | (8.2) |
| Asian/Pacific Islander | 3,745 | (13.8) | 3,805 | (15.4) | 1,407 | (16.2) | 905 | (17.2) | 224 | (16.8) | 136 | (18.2) | 340 | (14.8) | 10,562 | (15.1) |
| Other | 972 | ( 3.6) | 855 | (3.5) | 296 | (3.4) | 182 | (3.5) | 47 | (3.5) | 23 | (3.1) | 77 | (3.3) | 2,452 | (3.5) |
| Year FIT screened | ||||||||||||||||
| 2010 | 6,246 | (23.0) | 6,210 | (25.2) | 2,726 | (31.5) | 1,747 | (33.3) | 518 | (38.8) | 292 | (39.0) | 956 | (41.5) | 18,695 | (26.7) |
| 2011 | 7,827 | (28.8) | 7,122 | (28.9) | 3,200 | (36.9) | 2,053 | (39.1) | 460 | (34.5) | 246 | (32.9) | 791 | (34.3) | 21,699 | (30.9) |
| 2012 | 9,018 | (33.2) | 7,792 | (31.6) | 2,010 | (23.2) | 1,076 | (20.5) | 250 | (18.7) | 157 | (21.0) | 463 | (20.1) | 20,766 | (29.6) |
| 2013 | 4,085 | (15.0) | 3,520 | (14.3) | 730 | (8.4) | 375 | (7.1) | 107 | (8.0) | 53 | (7.1) | 94 | (4.1) | 8,964 | (12.8) |
| Body mass index | ||||||||||||||||
| <25.0 kg/m2 | 6,007 | (22.1) | 5,514 | (22.4) | 2,020 | (23.3) | 1,150 | (21.9) | 313 | (23.4) | 158 | (21.1) | 504 | (21.9) | 15,666 | (22.3) |
| 25.0–29.9 kg/m2 | 9,754 | (35.9) | 8,590 | (34.9) | 2,951 | (34.1) | 1,795 | (34.2) | 453 | (33.9) | 253 | (33.8) | 817 | (35.5) | 24,613 | (35.1) |
| ≥30 kg/m2 | 11,349 | (41.8) | 10,448 | (42.4) | 3,649 | (42.1) | 2,283 | (43.5) | 564 | (42.2) | 337 | (45.1) | 971 | (42.1) | 29,601 | (42.2) |
| Unknown | 66 | ( 0.2) | 92 | ( 0.4) | 46 | ( 0.5) | 23 | ( 0.4) | 5 | ( 0.4) | 12 | ( 0.5) | 244 | ( 0.3) | ||
| Distribution of FIT to patient | ||||||||||||||||
| In-person | 7,472 | (27.5) | 5,768 | (23.4) | 1,991 | (23.0) | 1,300 | (24.8) | 385 | (28.8) | 227 | (30.3) | 773 | (33.6) | 17,916 | (25.5) |
| FIT mailed | 19,704 | (72.5) | 18,876 | (76.6) | 6,675 | (77.0) | 3,951 | (75.2) | 950 | (71.2) | 521 | (69.7) | 1,531 | (66.4) | 52,208 | (74.5) |
| Previously FIT screened | ||||||||||||||||
| Yes | 20,651 | (76.0) | 18,489 | (75.0) | 6,231 | (71.9) | 3,738 | (71.2) | 907 | (67.9) | 508 | (67.9) | 1,536 | (66.7) | 52,060 | (74.2) |
| FIT screened in prior year | ||||||||||||||||
| Yes | 7,400 | (27.2) | 7,249 | (29.4) | 2,454 | (28.3) | 1,423 | (27.1) | 317 | (23.7) | 162 | (21.7) | 508 | (22.0) | 19,513 | (27.8) |
| Vaccinated in prior yearc | ||||||||||||||||
| Yes | 15,170 | (55.8) | 13,478 | (54.7) | 4,635 | (53.5) | 2,738 | (52.1) | 657 | (49.2) | 363 | (48.5) | 1,099 | (47.7) | 38,140 | (54.4) |
| GI symptoms in prior yeard | ||||||||||||||||
| Yes | 3,190 | (11.7) | 2,654 | (10.8) | 915 | (10.6) | 602 | (11.5) | 194 | (14.5) | 100 | (13.4) | 313 | (13.6) | 7,968 | (11.4) |
| Anemia in prior yeare | ||||||||||||||||
| Yes | 912 | ( 3.4) | 823 | ( 3.3) | 325 | ( 3.8) | 238 | ( 4.5) | 88 | ( 6.6) | 42 | ( 5.6) | 134 | ( 5.8) | 2,562 | ( 3.7) |
| Diabetes in prior year | ||||||||||||||||
| Yes | 5,831 | (21.5) | 5,797 | (23.5) | 2,145 | (24.8) | 1,300 | (24.8) | 338 | (25.3) | 183 | (24.5) | 642 | (27.9) | 16,236 | (23.2) |
| Smoking in prior year | ||||||||||||||||
| Yes | 4,147 | (15.3) | 3,724 | (15.1) | 1,274 | (14.7) | 842 | (16.0) | 220 | (16.5) | 140 | (18.7) | 466 | (20.2) | 10,813 | (15.4) |
| Primary care visits in prior year | ||||||||||||||||
| 0 | 2,945 | (10.8) | 2,967 | (12.0) | 1,064 | (12.3) | 641 | (12.2) | 164 | (12.3) | 103 | (13.8) | 307 | (13.3) | 8,191 | (11.7) |
| 1 | 5,401 | (19.9) | 4,963 | (20.1) | 1,706 | (19.7) | 996 | (19.0) | 230 | (17.2) | 144 | (19.3) | 441 | (19.1) | 13,881 | (19.8) |
| 2–3 | 9,339 | (34.4) | 8,234 | (33.4) | 2,882 | (33.3) | 1,653 | (31.5) | 448 | (33.6) | 214 | (28.6) | 724 | (31.4) | 23,494 | (33.5) |
| ≥4 | 9,491 | (34.9) | 8,480 | (34.4) | 3,014 | (34.8) | 1,961 | (37.3) | 493 | (36.9) | 287 | (38.4) | 832 | (36.1) | 24,558 | (35.0) |
| Inpatient days in prior year | ||||||||||||||||
| 0–1 | 25,255 | (92.9) | 22,870 | (92.8) | 7,997 | (92.3) | 4,696 | (89.4) | 1,171 | (87.7) | 654 | (87.4) | 2,042 | (88.6) | 64,685 | (92.2) |
| 2–3 | 867 | ( 3.2) | 720 | ( 2.9) | 242 | ( 2.8) | 186 | ( 3.5) | 52 | ( 3.9) | 44 | ( 5.9) | 89 | ( 3.9) | 2,200 | ( 3.1) |
| ≥4 | 1,054 | ( 3.9) | 1,054 | ( 4.3) | 427 | ( 4.9) | 369 | ( 7.0) | 112 | ( 8.4) | 50 | ( 6.7) | 173 | ( 7.5) | 3,239 | ( 4.6) |
| CRC-Related Outcomesb | ||||||||||||||||
| Advanced adenomaf | ||||||||||||||||
| Yes | 2,135 | ( 8.1) | 2,168 | ( 9.0) | 779 | ( 9.3) | 429 | ( 8.4) | 114 | ( 8.9) | 75 | (10.5) | 247 | (11.6) | 5,947 | ( 8.8) |
| Any CRC | ||||||||||||||||
| Yes | 807 | ( 3.0) | 685 | ( 2.8) | 265 | ( 3.1) | 165 | ( 3.1) | 58 | ( 4.3) | 37 | ( 4.9) | 174 | ( 7.6) | 2,191 | ( 3.1) |
| Advanced-stage CRCg | ||||||||||||||||
| Yes | 219 | ( 0.8) | 173 | ( 0.7) | 60 | ( 0.7) | 46 | ( 0.9) | 17 | ( 1.3) | 14 | ( 1.9) | 72 | ( 3.1) | 601 | ( 0.9) |
| Unknown | 3 | ( 0.0) | 2 | ( 0.0) | 2 | ( 0.0) | 2 | ( 0.0) | 0 | 1 | ( 0.1) | 4 | ( 0.2) | 14 | ( 0.0) | |
| CRC Stage | ||||||||||||||||
| 0 | 129 | ( 0.5) | 113 | ( 0.5) | 39 | ( 0.5) | 32 | ( 0.6) | 7 | ( 0.5) | 6 | ( 0.8) | 17 | ( 0.7) | 343 | ( 0.5) |
| I | 314 | ( 1.2) | 275 | ( 1.1) | 122 | ( 1.4) | 48 | ( 0.9) | 19 | ( 1.4) | 5 | ( 0.7) | 40 | ( 1.7) | 823 | ( 1.2) |
| II | 142 | ( 0.5) | 122 | ( 0.5) | 42 | ( 0.5) | 37 | ( 0.7) | 15 | ( 1.1) | 11 | ( 1.5) | 41 | ( 1.8) | 410 | ( 0.6) |
| III | 169 | ( 0.6) | 133 | ( 0.5) | 56 | ( 0.6) | 32 | ( 0.6) | 12 | ( 0.9) | 9 | ( 1.2) | 49 | ( 2.1) | 460 | ( 0.7) |
| IV | 50 | ( 0.2) | 40 | ( 0.2) | 4 | ( 0.0) | 14 | ( 0.3) | 5 | ( 0.4) | 5 | ( 0.7) | 23 | ( 1.0) | 141 | ( 0.2) |
| Unknown | 3 | ( 0.0) | 2 | ( 0.0) | 2 | ( 0.0) | 2 | ( 0.0) | 0 | 1 | ( 0.1) | 4 | ( 0.2) | 14 | ( 0.0) | |
| No CRC | 26,369 | (97.0) | 23,959 | (97.2) | 8,401 | (96.9) | 5,086 | (96.9) | 1,277 | (95.7) | 711 | (95.1) | 2,130 | (92.4) | 67,933 | (96.9) |
Abbreviations: CRC, colorectal cancer; FIT, fecal immunochemical test; GI, gastrointestinal; IQR, interquartile range (25th, 75th percentiles); n, number; SD, standard deviation; %, column percent.
Time to colonoscopy intervals [months (days)]: 2 months (31–60 days), 3 months (61–90 days), 4–6 months (91–180 days), 7–9 months (181–272 days), 10–12 months (273–365 days), and >12 months (366–1571 days).
p<0.001 for differences in proportions across time intervals for all variables except sex (p=0.82).
Vaccinated in prior year refers to receipt of the flu or pneumonia vaccine in the year before FIT screening.
GI symptoms include bleeding or blood in stool, unexplained weight loss, abdominal pain, diarrhea, or diverticulitis diagnosed in the year before FIT, and to inflammatory bowel disease, or Lynch syndrome diagnosis any time before FIT.
Anemia refers to iron-deficiency anemia.
Advanced adenoma refers to adenomas with advanced histology (i.e., tubullovillous and villous adenomas).
Advanced-stage cancers were defined as stage III (regional lymph node involvement) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system or, for those without such staging, as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to the regional lymph nodes), or code 7 (distant metastasis) according to the SEER Program Coding and Staging Manual 2013.
Time to Colonoscopy and Risk of CRC Outcomes
Longer time between positive FIT and colonoscopy follow-up increased the risk of CRC outcomes. Compared to follow-up at 8–30 days, for each additional 30-day delay the OR for any CRC was 1.03 (95% CI: 1.03–1.04; 2,191 cases/70,124 total=31 cases/1,000 patients) and for advanced-stage disease was 1.05 (95% CI: 1.04–1.06; 601 cases/70,110 total=9 cases/1,000 patients); however, the relationship was not linear (Figures 2 and 3). Compared to patients who received follow-up within 8–30 days, there was no significant increase in risk of CRC outcomes for examinations within 2, 3 or 4–6 months (Figures 2 and 3). A non-significant increase in risk of any CRC and advanced-stage disease was seen starting at 7–9 months, and risks were significantly higher at 10–12 months and >12 months. Specifically, starting with follow-up at 7–9 months, there was a higher risk of stage II CRC (OR, 1.88; 95% CI, 1.09–3.23; 15 cases/1,292 total=12 cases/1,000 patients). At 10–12 months, the risk was higher for any CRC (OR, 1.48; 95% CI, 1.05–2.08; 37 cases/748 total=49 cases/1,000 patients), advanced-stage disease (OR, 1.97; 95% CI, 1.14–3.42; 14 cases/747 total=19 cases/1,000 patients), stage II CRC (OR, 2.39; 95% CI, 1.28–4.46; 11 cases/722 total=15 cases/1,000), and stage IV CRC (OR, 2.71; 95% CI, 1.06–6.89; 5 cases/716 total=7 cases/1,000 patients). At >12 months, the risk was higher for advanced adenomas (OR, 1.32; 95% CI, 1.15–1.52; 247 cases/2,130 total=116 cases/1,000 patients), any CRC (OR, 2.25; 95% CI, 1.89–2.68; 174 cases/2,304 total=76 cases/1,000 patients), advanced-stage disease (OR, 3.22; 95% CI, 2.44–4.25; 72 cases/2,300 total=31 cases/1,000 patients), stage II CRC (OR, 2.94; 95% CI, 2.05–4.20; 41 cases/2,171 total=19 cases/1,000 patients), stage III CRC (OR, 3.07; 95% CI, 2.21–4.27; 49 cases/2,179 total=22 cases/1,000 patients), and stage IV CRC (OR, 3.86; 95% CI, 2.32–6.44; 23 cases/2,153 total=11 cases/1,000 patients). Compared to no adjustment, accounting for common baseline factors (e.g., age, sex, race/ethnicity, comorbidity, and prior FIT screening) moderately reduced the associations (eTable 1), but did not change their direction; adjustment for additional factors related to health and health care utilization slightly strengthened the associations.
Figure 2. Time to colonoscopy after a positive FIT and adjusted riska of CRC outcomes (panel A and B).

Abbreviations: Adv-stage, advanced-stage; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test. aAdjusted for sex; age; race/ethnicity; body mass index; region; FIT screening year; completion of previous FIT screening (ever and in the prior year); and in the year prior to FIT screening, receipt of the flu or pneumonia vaccine, presence of gastrointestinal symptoms (bleeding or blood in stool, unexplained weight loss, abdominal pain, diarrhea, diverticulitis, inflammatory bowel disease, or Lynch syndrome), diagnosis of iron-deficiency anemia or diabetes, current smoker, number of primary care visits, and number of days hospitalized. Models for any CRC include the entire population. Models for advanced adenoma exclude 2,191 patients diagnosed with CRC. Models for advanced-stage CRC exclude 14 patients with CRC of unknown stage. Models for stage-specific CRC exclude patients with CRC of any stage other than the specified stage. The adjusted advanced-stage CRC model dropped 244 patients with unknown BMI because no patient with unknown BMI had this outcome. The adjusted models for CRC stages 0, III, and IV dropped 242 patients with unknown BMI because no patient with unknown BMI had these outcomes. The adjusted CRC stage IV model dropped 2435 patients with unknown race/ethnicity because no patient with unknown race/ethnicity had this outcome.
Figure 3. Time to colonoscopy after a positive FIT and crude CRC rates (panel A and B).

Abbreviations: Adv-stage, advanced-stage; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test.
In sensitivity analyses (Table 2), the pattern of increased OR estimates for any CRC and/or advanced-stage disease with examinations 10–12 months and >12 months post FIT persisted with different reference group definitions and when individuals were excluded if colonoscopy was performed >24 months after a positive FIT (thereby excluding people unlikely to have a cancer, given they had not developed signs or symptoms after extended follow-up). When 20,644 originally-excluded patients who had either <1 year of membership prior to FIT screening or were up-to-date with screening by prior endoscopy were included, risk was higher only for follow-up at >12 months. With 8–60 days and 8–90 days as the reference group, the risk of any CRC was also higher in the 7–9 months exposure group. As expected, the 1–7 days exposure group had a higher risk of adverse outcomes, given extremely rapid follow-up (within a week) likely represents a high-risk group.
Table 2.
Time to colonoscopy after a positive FIT: sensitivity analyses
| Time to colonoscopya | Any CRC |
Advanced-stage CRCb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Totalc | Rated | (95% CI) | Adj ORe |
(95% CI) | Cases/Totalc | Rated | (95% CI) | Adj ORe |
(95% CI) | |
| Reference group: 1–30 days | ||||||||||
| 1–30 days | 871 / 28,567 | 30 | ( 28, 32) | 1.00 | (reference) | 248 / 28,564 | 9 | ( 8, 10) | 1.00 | (reference) |
| 2 months | 685 / 24,644 | 28 | ( 26, 30) | 0.90 | (0.81, 0.99) | 173 / 24,642 | 7 | ( 6, 8) | 0.79 | (0.65, 0.97) |
| 3 months | 265 / 8,666 | 31 | ( 27, 34) | 0.93 | (0.80, 1.07) | 60 / 8,664 | 7 | ( 5, 9) | 0.73 | (0.54, 0.97) |
| 4–6 months | 165 / 5,251 | 31 | ( 27, 36) | 0.95 | (0.80, 1.13) | 46 / 5,249 | 9 | ( 6, 11) | 0.91 | (0.66, 1.25) |
| 7–9 months | 58 / 1,335 | 43 | ( 32, 54) | 1.27 | (0.96, 1.67) | 17 / 1,335 | 13 | ( 7, 19) | 1.22 | (0.74, 2.01) |
| 10–12 months | 37 / 748 | 49 | ( 34, 65) | 1.44 | (1.02, 2.02) | 14 / 747 | 19 | ( 9, 28) | 1.83 | (1.06, 3.17) |
| >12 months | 174 / 2,304 | 76 | ( 65, 86) | 2.19 | (1.84, 2.60) | 72 / 2,300 | 31 | (24, 38) | 2.99 | (2.27, 3.93) |
| |
|
|||||||||
| Reference group: 8–60 days | ||||||||||
| |
|
|||||||||
| 8–60 days | 1,492 / 51,820 | 29 | ( 27, 30) | 1.00 | (reference) | 392 / 51,815 | 8 | ( 7, 8) | 1.00 | (reference) |
| 3 months | 265 / 8,666 | 31 | ( 27, 34) | 0.99 | (0.87, 1.14) | 60 / 8,664 | 7 | ( 5, 9) | 0.84 | (0.64, 1.11) |
| 4–6 months | 165 / 5,251 | 31 | ( 27, 36) | 1.02 | (0.86, 1.20) | 46 / 5,249 | 9 | ( 6, 11) | 1.06 | (0.78, 1.44) |
| 7–9 months | 58 / 1,335 | 43 | ( 32, 54) | 1.36 | (1.04, 1.79) | 17 / 1,335 | 13 | ( 7, 19) | 1.43 | (0.87, 2.33) |
| 10–12 months | 37 / 748 | 49 | ( 34, 65) | 1.54 | (1.10, 2.16) | 14 / 747 | 19 | ( 9, 28) | 2.13 | (1.24, 3.67) |
| >12 months | 174 / 2,304 | 76 | ( 65, 86) | 2.34 | (1.98, 2.77) | 72 / 2,300 | 31 | (24, 38) | 3.48 | (2.68, 4.52) |
| |
|
|||||||||
| Reference group: 8–90 days | ||||||||||
| |
|
|||||||||
| 8–90 days | 1,757 / 60,486 | 29 | ( 28, 30) | 1.00 | (reference) | 452 / 60,479 | 7 | ( 7, 8) | 1.00 | (reference) |
| 4–6 months | 165 / 5,251 | 31 | ( 27, 36) | 1.02 | (0.87, 1.20) | 46 / 5,249 | 9 | ( 6, 11) | 1.09 | (0.80, 1.48) |
| 7–9 months | 58 / 1,335 | 43 | ( 32, 54) | 1.36 | (1.04, 1.79) | 17 / 1,335 | 13 | ( 7, 19) | 1.47 | (0.90, 2.40) |
| 10–12 months | 37 / 748 | 49 | ( 34, 65) | 1.54 | (1.10, 2.16) | 14 / 747 | 19 | ( 9, 28) | 2.19 | (1.28, 3.77) |
| >12 months | 174 / 2,304 | 76 | ( 65, 86) | 2.35 | (1.99, 2.77) | 72 / 2,300 | 31 | (24, 38) | 3.58 | (2.76, 4.63) |
| |
|
|||||||||
| Excludes colonoscopies >24 months after FIT | ||||||||||
| |
|
|||||||||
| 8–30 days | 807 / 27,176 | 30 | ( 28, 32) | 1.00 | (reference) | 219 / 27,173 | 8 | ( 7, 9) | 1.00 | (reference) |
| 2 months | 685 / 24,644 | 28 | ( 26, 30) | 0.92 | (0.82, 1.02) | 173 / 24,642 | 7 | ( 6, 8) | 0.85 | (0.69, 1.04) |
| 3 months | 265 / 8,666 | 31 | ( 27, 34) | 0.95 | (0.82, 1.10) | 60 / 8,664 | 7 | ( 5, 9) | 0.78 | (0.58, 1.04) |
| 4–6 months | 165 / 5,251 | 31 | ( 27, 36) | 0.98 | (0.82, 1.16) | 46 / 5,249 | 9 | ( 6, 11) | 0.98 | (0.70, 1.35) |
| 7–9 months | 58 / 1,335 | 43 | ( 32, 54) | 1.30 | (0.99, 1.71) | 17 / 1,335 | 13 | ( 7, 19) | 1.31 | (0.79, 2.16) |
| 10–12 months | 37 / 748 | 49 | ( 34, 65) | 1.47 | (1.04, 2.07) | 14 / 747 | 19 | ( 9, 28) | 1.97 | (1.14, 3.42) |
| >12 months | 105 / 1,521 | 69 | ( 56, 82) | 2.13 | (1.72, 2.64) | 42 / 1,520 | 28 | (19, 36) | 2.98 | (2.12, 4.18) |
| |
|
|||||||||
| Includes <1 year membership before FIT, or prior endoscopy | ||||||||||
| |
|
|||||||||
| 8–30 days | 999 / 33,924 | 29 | ( 28, 31) | 1.00 | (reference) | 283 / 33,920 | 8 | ( 7, 9) | 1.00 | (reference) |
| 2 months | 837 / 30,124 | 28 | ( 26, 30) | 0.92 | (0.84, 1.01) | 210 / 30,121 | 7 | ( 6, 8) | 0.81 | (0.67, 0.97) |
| 3 months | 313 / 10,604 | 30 | ( 26, 33) | 0.94 | (0.82, 1.07) | 72 / 10,600 | 7 | ( 5, 8) | 0.74 | (0.57, 0.97) |
| 4–6 months | 199 / 6,539 | 30 | ( 26, 35) | 0.95 | (0.81, 1.11) | 55 / 6,537 | 8 | ( 6, 11) | 0.89 | (0.66, 1.20) |
| 7–9 months | 72 / 1,700 | 42 | ( 33, 52) | 1.29 | (1.00, 1.65) | 21 / 1,700 | 12 | ( 7, 18) | 1.23 | (0.79, 1.94) |
| 10–12 months | 42 / 963 | 44 | ( 31, 57) | 1.29 | (0.94, 1.77) | 14 / 962 | 15 | ( 7, 22) | 1.42 | (0.82, 2.44) |
| >12 months | 205 / 3,072 | 67 | ( 58, 76) | 2.03 | (1.73, 2.38) | 82 / 3,066 | 27 | (21, 32) | 2.67 | (2.07, 3.44) |
| |
|
|||||||||
| Includes 1–7 days exposure category | ||||||||||
| |
|
|||||||||
| 1–7 days | 64 / 1,391 | 46 | ( 35, 57) | 1.45 | (1.11, 1.89) | 29 / 1,391 | 21 | (13, 28) | 2.38 | (1.60, 3.55) |
| 8–30 days | 807 / 27,176 | 30 | ( 28, 32) | 1.00 | (reference) | 219 / 27,173 | 8 | ( 7, 9) | 1.00 | (reference) |
| 2 months | 685 / 24,644 | 28 | ( 26, 30) | 0.91 | (0.82, 1.02) | 173 / 24,642 | 7 | ( 6, 8) | 0.84 | (0.69, 1.03) |
| 3 months | 265 / 8,666 | 31 | ( 27, 34) | 0.95 | (0.82, 1.09) | 60 / 8,664 | 7 | ( 5, 9) | 0.77 | (0.58, 1.03) |
| 4–6 months | 165 / 5,251 | 31 | ( 27, 36) | 0.97 | (0.82, 1.16) | 46 / 5,249 | 9 | ( 6, 11) | 0.97 | (0.70, 1.34) |
| 7–9 months | 58 / 1,335 | 43 | ( 32, 54) | 1.30 | (0.98, 1.71) | 17 / 1,335 | 13 | ( 7, 19) | 1.30 | (0.79, 2.15) |
| 10–12 months | 37 / 748 | 49 | ( 34, 65) | 1.47 | (1.04, 2.07) | 14 / 747 | 19 | ( 9, 28) | 1.95 | (1.12, 3.39) |
| >12 months | 174 / 2,304 | 76 | ( 65, 86) | 2.24 | (1.88, 2.67) | 72 / 2,300 | 31 | (24, 38) | 3.20 | (2.43, 4.22) |
Abbreviations: Adj, adjusted; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test; GI, gastrointestinal; n, number; OR, odds ratio.
Time to colonoscopy intervals [months (days)]: 2 months (31–60 days), 3 months (61–90 days), 4–6 months (91–180 days), 7–9 months (181–272 days), 10–12 months (273–365 days), and >12 months (366–1571 days).
Advanced-stage cancers were defined as stage III (regional lymph node involvement) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system or, for those without such staging, as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to the regional lymph nodes), or code 7 (distant metastasis) according to the SEER Program Coding and Staging Manual 2013.
Cases/Total refers to the number of cases per the total number of patients who had a colonoscopy after a positive FIT.
Rates (and 95% CIs) are per 1,000 patients who had a colonoscopy after a positive FIT.
Adjusted for sex; age; race/ethnicity; body mass index (BMI); region; FIT screening year; completion of previous FIT screening (ever and in the prior year); and in the year prior to FIT screening, receipt of the flu/pneumonia vaccine, presence of gastrointestinal symptoms (bleeding or blood in stool, unexplained weight loss, abdominal pain, diarrhea, diverticulitis, inflammatory bowel disease, or Lynch syndrome), diagnosis of iron-deficiency anemia or diabetes, current smoker, number of primary care visits, and number of days hospitalized. Models for any CRC include the entire population. Models for advanced adenoma exclude 2,191 patients diagnosed with CRC. Models for advanced-stage CRC exclude 14 patients with CRC of unknown stage. Models for stage-specific CRC exclude patients with CRC of any stage other than the specified stage. The adjusted advanced-stage CRC model dropped 244 patients with unknown BMI because no patient with unknown BMI had this outcome. The adjusted models for CRC stages 0, III, and IV dropped 242 patients with unknown BMI because no patient with unknown BMI had these outcomes. The adjusted CRC stage IV model dropped 2435 patients with unknown race/ethnicity because no patient with unknown race/ethnicity had this outcome.
The associations between time to colonoscopy and risk of any CRC and advanced-stage disease differed somewhat across strata of age, prior FIT screening, and no preventive vaccinations in the year before FIT screening (eTable 2); region was also an effect modifier for advanced-stage disease. However, the differences were small, with the exception of age, and significant associations persisted across all strata. For example, similar increases in risk for advanced-stage disease were found for patients with and without prior FIT screening (OR 1.05; 95% CI 1.04–1.07 vs. OR=1.04; 95% CI 1.02–1.06, respectively). Also, stronger associations for both any CRC and advanced-stage disease were found among older patients than younger patients, though significant associations were found for both groups.
DISCUSSION
In a large community-based setting, compared to colonoscopies performed 8–30 days after a positive FIT, there was no increase in risk of CRC outcomes for colonoscopies completed within 6 months after a positive FIT. There was a higher risk of stage II CRC at 7–9 months; of any CRC, advanced-stage disease, and stage II and IV CRC at 10–12 months; and of advanced adenomas, any CRC, advanced-stage disease, and stage II-IV CRCs at >12 months.
Time intervals between a positive fecal test and colonoscopy follow-up vary widely in practice.17–33 In studies among veterans and within a public health care system, for example, the average and median times to colonoscopy were 103 days and 174 days, respectively.25,33 Longer intervals could increase the chance of neoplastic progression, while short intervals may substantially increase patient and clinician burdens without benefiting cancer outcomes. In the current study, nearly 75% of FIT-positive patients received a colonoscopy within 90 days. This required rapid communication of positive results to patients and physicians, sufficient colonoscopy access, rapid scheduling, and tracking exam completion.14 However, even with one of the most rapid follow-up rates reported to date,33 only one-third of FIT-positive patients received a follow-up colonoscopy within 30 days.
Guidelines for colonoscopy follow-up vary and lack supporting data. In 2006, a Canadian consensus group recommended colonoscopy follow-up within 2 months of a positive fecal test, although no rationale was provided.7 In 2007, the Veterans Health Administration issued a directive that a colonoscopy be performed within 60 days of a positive screening test;6 however, a subsequent report found insufficient evidence to support the recommendation.8 Similarly, in 2012, European guidelines recommended colonoscopy within 31 days after referral for a positive fecal screening test, despite a lack of evidence for effectiveness. Given the lack of supporting evidence for recommendations, and the substantial difficulties for patients and clinicians to rapidly schedule and complete sedated examinations which require time off from work, a person to accompany the patient home, and skilled personnel,34 current United States consensus guidelines offer no recommendation regarding the time interval between a positive FIT and follow-up colonoscopy.2,5
Prior studies have mainly explored risk factors for different times to follow-up colonoscopy17–24,26,28–31 and methods for improving follow-up,25,32,35–37 rather than the actual consequences of different times to follow-up on cancer outcomes. An analysis of 100 veterans referred for colonoscopy after a positive fecal test reported no association between follow-up time and CRC stage.10 A study of 231 veterans, which, due to sample size limitations primarily evaluated trends rather than specific time intervals, reported that each additional 30-day wait for colonoscopy after a positive fecal test was associated with an increased risk of any adenoma (OR, 1.10; 95% CI: 1.02–1.19), but did not achieve statistical significance for advanced neoplasia (advanced adenomas or intramucosal carcinoma) or invasive cancers.9 Both studies included single sites with predominantly male populations. A Canadian study of 246 CRC patients reported no association between wait-time and node positivity or presence of distant metastases at diagnosis.38 A modeling study reported that, compared to colonoscopy within 2 weeks of a positive fecal test, waiting 12 months might reduce the total years-of-life gained from screening by an estimated 9%.39 While the modeling study reported a steady increase in risk between the duration of the delay and screening benefits lost, the current study only found evidence for a higher risk of adverse CRC outcomes for colonoscopies performed >6 months after a positive FIT. Therefore, although the time interval from colorectal polyp initiation to CRC is believed to span years, our study findings suggest that by the time a lesion is detectable by FIT, further lesion progression may occur as soon as 7–9 months after testing positive. Thus, completing colonoscopy follow-up within 3 months of a positive fecal test appears to be a prudent recommendation, to provide a margin of safety.
Study strengths include its large size and number of CRC outcomes; comprehensive capture of FIT and cancer results; a multi-medical center, community-based, diverse population; validated approaches for capturing pathology data and colonoscopy exams; histological confirmation of adenomas; validated SEER cancer registries; evaluation of a large number of possible confounding factors; and evaluation of assumptions through sensitivity analyses.
Limitations include the observational design and potential influence of unmeasured confounders, although the large number of patients allowed well-powered evaluations of a large number of possible confounding factors. Increases in risk over time were seen across all strata of potential confounders, including among patients with and without prior screening, comorbidities, and healthcare seeking behaviors. These findings support the biologic hypothesis that delays result in progression from polyps to cancer and from less advanced to more advanced cancers. Measures of colonoscopy quality were not available for all patients; however, a large-scale chart review in the study population demonstrated cecal intubation rates of 97.7% and adequate-to-excellent bowel preparations in 92.0% of exams.40 Finally, adenoma size was not available; thus, advanced adenomas were defined only by advanced histology.
CONCLUSIONS
Among patients with a positive FIT, compared to colonoscopy follow-up at 8–30 days, follow-up at 7–9 months was associated with an increased risk of stage II CRC; follow-up at 10–12 months was associated with a higher risk of any CRC, advanced-stage disease, and stage II and IV CRC; and follow-up at >12 months was associated with a higher risk of advanced adenomas, any CRC, advanced-stage disease, and stage II-IV CRC. Thus, in screening-eligible patients, a follow-up colonoscopy within 6 months after a positive FIT may minimize the risk of neoplastic progression; within 3 months may provide an additional margin of safety.
Supplementary Material
Key Points.
Question:
Is time to colonoscopy after a positive fecal immunochemical test (FIT) associated with an increased risk of colorectal cancer (CRC)?
Findings:
In this retrospective cohort study of 70,124 FIT-positive patients, there was no increase in risk of CRC if colonoscopy follow-up after a positive FIT occurred within 6 months. Follow-up after 6 months was increasingly associated with a higher risk of any CRC and advanced-stage disease.
Meaning:
After a positive FIT, a follow-up colonoscopy within 6 months may minimize the risk of neoplastic progression; within 3 months may provide an additional margin of safety.
Acknowledgement Section
Funding/Support: This study was conducted within the National Cancer Institute-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes (grant U54 CA163262).
Role of the Funder/Sponsor: The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Abbreviations:
- FIT
fecal immunochemical test
- CI
confidence interval
- CRC
colorectal cancer
- IQR
interquartile range
- KPNC
Kaiser Permanente Northern California
- KPSC
Kaiser Permanente Southern California
- n
sample size
- OR
odds ratio
- PROSPR
Population-based Research Optimizing Screening through Personalized Regimens
- SEER
Surveillance Epidemiology and End Results
Footnotes
Conflict of Interest Disclosures: No conflicts of interest were reported.
References
- 1.Colorectal Cancer Statistics. http://www.cdc.gov/cancer/colorectal/statistics/index.htm. Accessed 3/29/16.
- 2.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 3.Recommendations on screening for colorectal cancer in primary care. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2016;188(5):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: tests, strategies, and perspectives. Front Public Health. 2014;2:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. [DOI] [PubMed] [Google Scholar]
- 6.Administration VH. VHA Directive 2007–004: colorectal cancer screening. Washington, DC: Department of Veterans Affairs; 2007. [Google Scholar]
- 7.Paterson WG, Depew WT, Pare P, et al. Canadian consensus on medically acceptable wait times for digestive health care. Can J Gastroenterol. 2006;20(6):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson K CS, Humphrey L, Helfand M. Patients with positive screening fecal occult blood tests: evidence brief on the relationship between time delay to colonoscopy and colorectal cancer outcomes, VA-ESP Project #09–199; 2013.
- 9.Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Digestive diseases and sciences. 2009;54(11):2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wattacheril J, Kramer JR, Richardson P, et al. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther. 2008;28(9):1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? 2006. http://www.dor.kaiser.org/dor/mhsnet/public/kpnc_community.htm.
- 13.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33(1):101–110. [DOI] [PubMed] [Google Scholar]
- 15.SEER Program Coding and Staging Manual 2013. 2013; http://seer.cancer.gov/manuals/2013/SPCSM_2013_maindoc.pdf.
- 16.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. Journal of gastroenterology and hepatology. 2012;27(6):1070–1077. [DOI] [PubMed] [Google Scholar]
- 18.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Diseases of the colon and rectum. 2006;49(7):1002–1010. [DOI] [PubMed] [Google Scholar]
- 19.Morris S, Baio G, Kendall E, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107(5):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paszat L, Rabeneck L, Kiefer L, Mai V, Ritvo P, Sullivan T. Endoscopic follow-up of positive fecal occult blood testing in the Ontario FOBT Project. Can J Gastroenterol. 2007;21(6):379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields HM, Weiner MS, Henry DR, et al. Factors that influence the decision to do an adequate evaluation of a patient with a positive stool for occult blood. The American journal of gastroenterology. 2001;96(1):196–203. [DOI] [PubMed] [Google Scholar]
- 22.Steele RJ, Kostourou I, McClements P, et al. Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. J Med Screen. 2010;17(2):68–74. [DOI] [PubMed] [Google Scholar]
- 23.Ferrat E, Le Breton J, Veerabudun K, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer. 2013;109(6):1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partin MR, Burgess DJ, Burgess JF Jr., et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(2):422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell AA, Nugent S, Ordin DL, Noorbaloochi S, Partin MR. Evaluation of a VHA collaborative to improve follow-up after a positive colorectal cancer screening test. Medical care. 2011;49(10):897–903. [DOI] [PubMed] [Google Scholar]
- 26.Correia A, Rabeneck L, Baxter NN, et al. Lack of follow-up colonoscopy after positive FOBT in an organized colorectal cancer screening program is associated with modifiable health care practices. Prev Med. 2015;76:115–122. [DOI] [PubMed] [Google Scholar]
- 27.Rabeneck L, Tinmouth JM, Paszat LF, et al. Ontario’s ColonCancerCheck: results from canada’s first province-wide colorectal cancer screening program. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(3):508–515. [DOI] [PubMed] [Google Scholar]
- 28.Rao SK, Schilling TF, Sequist TD. Challenges in the management of positive fecal occult blood tests. Journal of general internal medicine. 2009;24(3):356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Archives of internal medicine. 2011;171(3):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(6):1232–1235. [DOI] [PubMed] [Google Scholar]
- 31.Garman KS, Jeffreys A, Coffman C, Fisher DA. Colorectal cancer screening, comorbidity, and follow-up in elderly patients. Am J Med Sci. 2006;332(4):159–163. [DOI] [PubMed] [Google Scholar]
- 32.Miglioretti DL, Rutter CM, Bradford SC, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Medical care. 2008;46(9 Suppl 1):S91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valori R, Rey JF, Atkin WS, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012;44 Suppl 3:SE88–105. [DOI] [PubMed] [Google Scholar]
- 35.Myers RE, Turner B, Weinberg D, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38(4):375–381. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Kadiyala H, Bhagwath G, et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. The American journal of gastroenterology. 2009;104(4):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taplin SH, Haggstrom D, Jacobs T, et al. Implementing colorectal cancer screening in community health centers: addressing cancer health disparities through a regional cancer collaborative. Medical care. 2008;46(9 Suppl 1):S74–83. [DOI] [PubMed] [Google Scholar]
- 38.Janssen RM, Takach O, Nap-Hill E, Enns RA. Time to Endoscopy in Patients with Colorectal Cancer: Analysis of Wait-Times. Can J Gastroenterol Hepatol. 2016;2016:8714587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meester RG, Zauber AG, Doubeni CA, et al. Consequences of Increasing Time to Colonoscopy Examination Following Positive Result From Fecal Colorectal Cancer Screening Test. Clin Gastroenterol Hepatol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointestinal Endoscopy. 2015;81(3):575–582 e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


