1 Summary
Previous research has found that, paradoxically, while older adults have more difficulty comprehending speech in challenging circumstances than younger adults, their brain responses track the envelope of the acoustic signal more robustly. Here we investigate this puzzle by using magnetoencephalography (MEG) source localization to determine the anatomical origin of this difference. Our results indicate that this robust tracking in older adults does not arise merely from having the same responses as younger adults but with larger amplitudes; instead, they recruit additional regions, inferior to core auditory cortex, with a short latency of ~30 ms relative to the acoustic signal.
2 Introduction
While older adults have more difficulty comprehending speech, especially in challenging circumstances, their brain responses track the speech envelope more robustly than the brain responses of younger adults [1,2]. Several candidate explanations might account for this observation. For example, older adults exhibit amplified responses to even simple tone stimuli [3,4], suggesting that increased speech tracking could be due to amplified representations of any auditory features. This might arise from central compensatory gain mechanisms that restore the representation of sounds at the cortical level despite degraded auditory brainstem responses [5,6]. Animal models also suggest that aging may alter the balance between excitatory and inhibitory processes (decreasing inhibition) in the cortex, acting at several levels along the auditory pathway [7–12], leading to stronger cortical stimulus-driven responses. On the other hand, there is also evidence for a reorganization of task-dependent networks, in which older adults recruit additional higher order cortical regions to compensate for age-related deficits [13–15], such as a reduction in working memory capacity [16]. Increased auditory attention, possibly due to increased listening effort, might also explain an increased response in core auditory cortex [17]. Finally, aging may also compromise the efficient use of cognitive resources because of decreased cortical network connectivity [13], leading to redundant processing across areas.
Consequently, increased neural speech tracking might arise from several different underlying changes. These changes can be distinguished by whether they uniformly affect responses that are also involved in younger adults, or whether they involve increased recruitment of additional regions. Here we investigate increased speech representation by using source localization to determine the anatomical source of the difference. Our results indicate that robust tracking in older adults does not arise merely from having the same responses as younger adults with larger amplitudes, but instead from recruiting additional regions, ventral to core auditory cortex, for processing speech.
3 Methods
MEG data were collected from a sample of 17 younger (18-27 yr, 3 men) and 15 older adults (61-73 yr, 5 men) with clinically normal hearing, described in detail in [1]. Here we analyze data from participants listening to two one-minute long segments of an audiobook rendition of The Legend of Sleepy Hollow (https://librivox.org/the-legend-of-sleepy-hollow-by-washington-irving). Each segment was repeated 3 times for a total of 6 minutes of data per subject.
For details on the basic MEG data analysis see [18]. Raw data were filtered between 1-8 Hz [2], downsampled to 200 Hz and projected to virtual current dipoles on the cortical surface using distributed minimum norm estimates [19]. These localized brain responses were individually modeled as driven by the analytic envelope of the acoustic stimulus, using a linear filter model [20]. Boosting was used to estimate the optimal filter known as the Temporal Response Function (TRF) [21,22]. A TRF describes the effect of elementary features of the speech envelope on the brain response signal at different latencies. Response functions were generated from a basis of 50 ms Hamming windows, distributed at 5 ms intervals in the kernel window of 0–500 ms. Thus, each TRF was the sum of up to 100 scaled and shifted Hamming windows with scaling values determined by boosting.
Model fits were evaluated based on the Fisher z-transformed Pearson correlation between predicted and actual source localized responses. To determine the predictive power of the model with bias correction, we subtracted from the correct model fit the model fit obtained from using a misaligned version of the same stimulus (obtained by switching the first and second half of the acoustic stimulus). Also, since the goal of this study was to analyze brain responses associated with time-locked auditory processing, the analysis was restricted to the temporal lobes of both hemispheres.
To localize the source of the higher predictability of older adults’ brain responses, z-difference maps were smoothed with a Gaussian kernel (STD = 5 mm) and compared between the two groups by performing independent-samples t-tests at all virtual current dipoles, while controlling for multiple comparisons using threshold-free cluster enhancement (TFCE) [23]. To statistically assess hemispheric differences, results from the right hemisphere were mapped to the left hemisphere as described in [18], and left-right difference maps were then compared between groups.
The area of significant group differences was then used as the region of interest (ROI) in which to analyze estimated response functions, and, specifically, to determine the latency of the responses contributing to the effect. For subsequent analyses, absolute values of the response functions were used to avoid any influence of arbitrary differences in current direction due to cortical surface orientation. The mean absolute value over source dipoles within the ROI was extracted for each time point of the response function. The result was an estimate of the magnitude of the response’s contribution to predictions at each time point. This time course exhibited characteristic peaks that were used to determine the latency at which older adults’ responses in the ROI were stronger than younger adults’ responses. A one-tailed independent t-test was performed at each time point, again corrected using TFCE.
Finally, to confirm that the difference in responses in the peak thus identified was indeed specific to the anatomical region identified for enhanced speech representation, the average absolute response during the peak was extracted for each dipole. The spatial distribution of this peak response was then compared between groups, again corrected with TFCE.
4 Results
Brain responses of older adults were predicted significantly better than responses of younger adults in a region of the left temporal lobe clearly outside core auditory cortex (Figure 1A, p = .001). Though this effect was not significant in the right hemisphere, there was no significant difference between hemispheres (p = .169). Group averaged model predictions suggest that for both groups, predictions were best near core auditory cortex (Figure 1B). In older adults, however, the area in which the model made good predictions extended further. The fact that the spatial peak of the difference is located inferior to the peak of the group average indicates that this is a true difference in neural source locations, rather than an artifact due to an amplitude difference combined with spatial dispersion of MEG source estimates, which would instead manifest as a difference peak close to the average peak.
Figure 1:
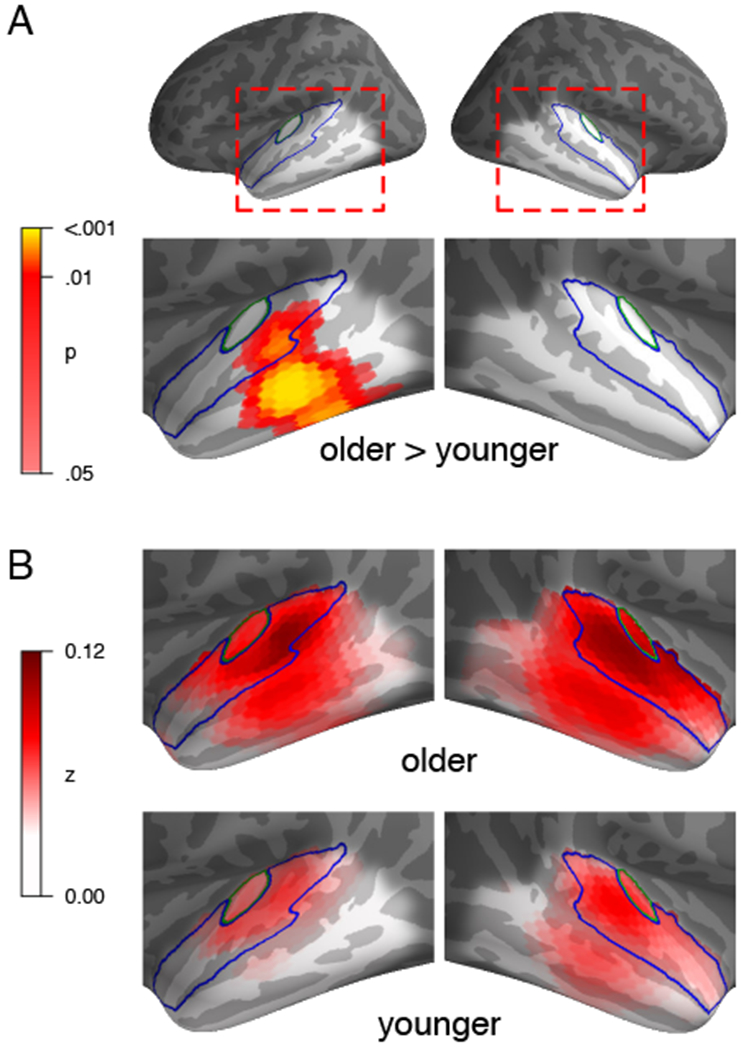
Model fit, expressed as Fisher z-transformed Pearson correlation between predicted and measured (source transformed) responses. A) Significantly better predicted brain responses in older adults than younger adults, in a region below left core auditory cortex (p ≤ .05 corrected within indicated bilateral temporal lobe). Outlines indicate Heschl’s gyrus (core auditory cortex, green) and superior temporal gyrus (blue). B) Average model prediction quality for each group separately.
The temporal dynamics of responses within this area were characterized using the average absolute TRF, which describes the extent to which elementary acoustic features affect brain responses at different delays. The TRF exhibited characteristic peaks around 30 and 100 ms [18,24], with an additional peak around 180 ms for older adults (Figure 2A). A significant difference between older and younger adults emerged in the earliest response peak (25 ms, p = .024, one-tailed). No other peak was significantly different, even when excluding the data encompassing the first peak up to 70 ms to implement a step-down procedure [25] (p = .229). The spatial distribution of the amplitude difference in the first peak, shown in Figure 2B, closely resembled the spatial extent of the model fit difference (Figure 1A). This confirms that the difference in response magnitude in the first peak was indeed specific to the region with improved model fit. Thus, increased speech tracking in older adults is at least partly due to an amplified early response in higher order auditory cortex.
Figure 2:
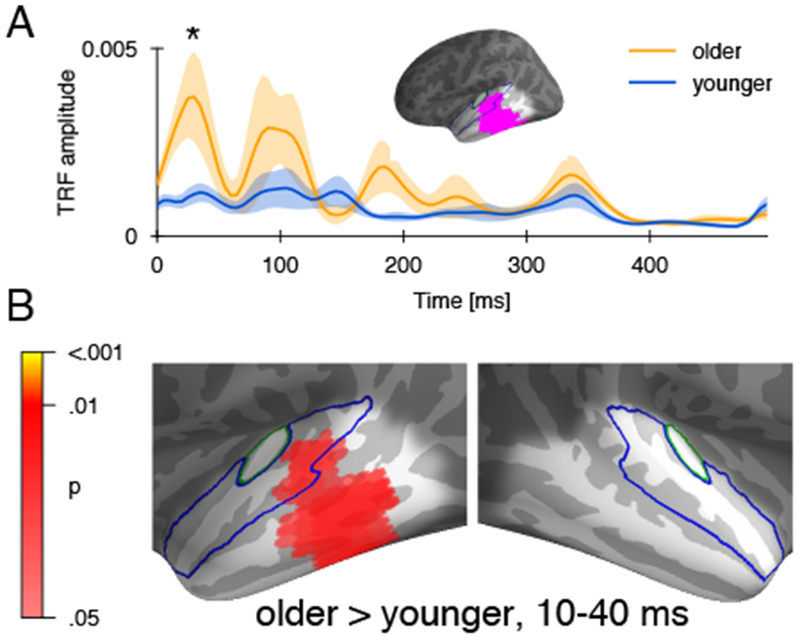
Response function in the region of difference. A) Mean absolute response function in the ROI based on significant difference in model fit (cf. Figure 1), with standard error. Older adults had significantly higher amplitude in the earliest response peak. B) Anatomical distribution of the difference in the absolute response strength during the window defined based on the first peak (10-40 ms). The anatomical distribution closely resembles the region of the difference in model fit.
5 Discussion
Compared to younger adults, older adults’ brain responses exhibit increased tracking of the acoustic envelope of speech in the 1-8 Hz range. Amplified envelope tracking may be due to increased neural activity, and not necessarily to enhanced encoding of acoustic features. Source localized MEG responses suggest that this effect is particularly pronounced in the left temporal lobe, lateral and inferior to core auditory cortex. This suggests that older adults, rather than exhibiting the same responses as younger adults with uniformly higher response amplitudes, disproportionately recruit higher order auditory cortex during speech perception.
Analysis of the response functions suggests that the largest contribution to this effect occurred in the earliest response peak with only about 30 ms latency. This peak is associated with processing of purely acoustic properties, as opposed to a later peak around 100 ms which is more sensitive to attended than unattended acoustic features [24]. This suggests that in older adults, the initial stage of cortical speech sound processing engaged a larger neural population in higher order auditory cortex.
This is consistent with several studies reporting that aging might alter the balance between inhibitory and excitatory neural mechanisms in the cortex [7,10–12]. The resulting increase in neural excitability and decrease in neural selectivity would lead to larger responses to auditory signals [26]. On the other hand, an early overrepresentation might also be an indication that older adults recruit greater neural resources at relatively low task loads [15]. This would be consistent with studies showing that older adults perform more poorly than younger adults during dual tasks (e.g. auditory and memory) [27–29], and with greater activation of speech motor cortex areas during speech discrimination [30]. However, the result that older adults’ increased responses originate outside of core auditory cortex cannot be explained by a simple effect of increased auditory attention [17].
Figure 2A suggests that older adults also exhibit a larger peak than younger adults around 180 ms. While this difference was not significant in this analysis, the difference does reach significance when the ROI is enlarged to include the whole brain (p = .017), consistent with improved stimulus reconstruction seen for older but not younger adults for time windows longer than 150 ms [1].
A recent large scale investigation suggests that age-related changes in simple tone-evoked magnetic fields are characterized by a cumulative delay, also associated with decreased grey matter volume in higher order auditory cortex [3] (the relation to increased amplitudes was not analyzed there). This region (delimited with the greater spatial confidence of MRI) lies within the region found here to exhibit increased speech tracking in older adults.
Together, these results suggest that altered auditory processing in older adults might be related to changes in early responses in higher order auditory areas. These may arise from a variety of phenomena, including degraded neural network communication, imbalance between inhibition and excitation, and inefficient use of cognitive resources.
Acknowledgments
This research was funded by NIH grant P01-AG055365
6 References
- 1.Presacco A et al. (2016) Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J. Neurophysiol 116, 2346–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Presacco A et al. (2016) Effect of informational content of noise on speech representation in the aging midbrain and cortex. J. Neurophysiol 116, 2356–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price D et al. (2017) Age-related delay in visual and auditory evoked responses is mediated by white- and grey-matter differences. Nat. Commun 8, 15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross B et al. (2010) Biological Markers of Auditory Gap Detection in Young, Middle-Aged, and Older Adults. PLoS ONE 5, e10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers AR et al. (2016) Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89, 867–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidelman GM et al. (2014) Age-related changes in the subcortical–cortical encoding and categorical perception of speech. Neurobiol. Aging 35, 2526–2540 [DOI] [PubMed] [Google Scholar]
- 7.Overton JA and Recanzone GH (2016) Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque. J. Neurophysiol 115, 2911–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspary DM et al. (1995) Central auditory aging: GABA changes in the inferior colliculus. Exp. Gerontol 30, 349–360 [DOI] [PubMed] [Google Scholar]
- 9.Caspary DM et al. (2005) Age-Related Changes in the Inhibitory Response Properties of Dorsal Cochlear Nucleus Output Neurons: Role of Inhibitory Inputs. J. Neurosci 25, 10952–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villers-Sidani E. de et al. (2010) Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc. Natl. Acad. Sci 107, 13900–13905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes LF et al. (2010) Processing of broadband stimuli across A1 layers in young and aged rats. Hear. Res 264, 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juarez-Salinas DL et al. (2010) Hierarchical and Serial Processing in the Spatial Auditory Cortical Pathway Is Degraded by Natural Aging. J. Neurosci 30, 14795–14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peelle JE et al. (2010) Neural Processing during Older Adults’ Comprehension of Spoken Sentences: Age Differences in Resource Allocation and Connectivity. Cereb. Cortex 20, 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingfield A and Grossman M (2006) Language and the Aging Brain: Patterns of Neural Compensation Revealed by Functional Brain Imaging. J. Neurophysiol 96, 2830–2839 [DOI] [PubMed] [Google Scholar]
- 15.Reuter-Lorenz PA and Cappell KA (2008) Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci 17, 177–182 [Google Scholar]
- 16.Schneider-Garces NJ et al. (2010) Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J. Cogn. Neurosci 22, 655–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woldorff MG et al. (1993) Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc. Natl. Acad. Sci 90, 8722–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodbeck C et al. (2018) Neural source dynamics of brain responses to continuous stimuli: Speech processing from acoustics to comprehension. NeuroImage 172, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramfort A et al. (2014) MNE software for processing MEG and EEG data. NeuroImage 86, 446–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalor EC et al. (2009) Resolving Precise Temporal Processing Properties of the Auditory System Using Continuous Stimuli. J. Neurophysiol 102, 349–359 [DOI] [PubMed] [Google Scholar]
- 21.David SV et al. (2007) Estimating sparse spectro-temporal receptive fields with natural stimuli. Netw. Comput. Neural Syst. 18, 191–212 [DOI] [PubMed] [Google Scholar]
- 22.Brodbeck C (2018) Eelbrain 0.27, Zenodo. [Google Scholar]
- 23.Smith SM and Nichols TE (2009) Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98 [DOI] [PubMed] [Google Scholar]
- 24.Ding N and Simon JZ (2012) Emergence of neural encoding of auditory objects while listening to competing speakers. Proc. Natl. Acad. Sci 109, 11854–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols TE and Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 15, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alain C et al. (2014) Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front. Syst. Neurosci 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson Gosselin P and Gagné J-P (2011) Older Adults Expend More Listening Effort Than Young Adults Recognizing Speech in Noise. J. Speech Lang. Hear. Res 54, 944–958 [DOI] [PubMed] [Google Scholar]
- 28.Tun PA et al. (2009) Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 24, 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward CM et al. (2016) Effects of Age, Acoustic Challenge, and Verbal Working Memory on Recall of Narrative Speech. Exp. Aging Res. 42, 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y et al. (2016) Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat. Commun 7, 12241. [DOI] [PMC free article] [PubMed] [Google Scholar]


