Abstract
Vacuolar proton-translocating ATPase (V-ATPase) is located in fungal vacuolar membranes. It is involved in multiple cellular processes, including the maintenance of intracellular ion homeostasis by maintaining acidic pH within the cell. The importance of V-ATPase in virulence has been demonstrated in several pathogenic fungi, including Candida albicans. However, it remains to be determined in the clinically important fungal pathogen Candida glabrata. Increasing multidrug resistance of C. glabrata is becoming a critical issue in the clinical setting. In the current study, we demonstrated that the plecomacrolide V-ATPase inhibitor bafilomycin B1 exerts a synergistic effect with azole antifungal agents, including fluconazole and voriconazole, against a C. glabrata wild-type strain. Furthermore, the deletion of the VPH2 gene encoding an assembly factor of V-ATPase was sufficient to interfere with V-ATPase function in C. glabrata, resulting in impaired pH homeostasis in the vacuole and increased sensitivity to a variety of environmental stresses, such as alkaline conditions (pH 7.4), ion stress (Na+, Ca2+, Mn2+, and Zn2+ stress), exposure to the calcineurin inhibitor FK506 and antifungal agents (azoles and amphotericin B), and iron limitation. In addition, virulence of C. glabrata Δvph2 mutant in a mouse model of disseminated candidiasis was reduced in comparison with that of the wild-type and VPH2-reconstituted strains. These findings support the notion that V-ATPase is a potential attractive target for the development of effective antifungal strategies.
Introduction
Invasive candidiasis is one of the most frequent fungal infections among a wide spectrum of immunocompromised patients, with the in-hospital mortality rates reported to be as high as 20–40% even among patients who receive antifungal therapy [1]. The therapeutic options currently available to treat invasive candidiasis are limited to only four classes of antifungal agents: azoles, echinocandins, polyenes, and fluoropyrimidines. Further, the incidence rates of candidemia caused by non-albicans Candida species are increasing and antifungal resistance of these species has emerged as a serious problem in clinical practice [1–3]. The rise of multidrug resistance with unfavorable therapeutic outcome among Candida glabrata infections became a critical healthcare issue in the last decade [4–6]. Therefore, the development of novel antifungal strategies is urgently needed.
Recent studies highlight vacuolar proton-translocating ATPase (V-ATPase) as an attractive target for drug discovery (reviewed in [7]). V-ATPase is an ATP-driven proton pump present in the endomembranes of all eukaryotic organisms [8, 9]. In particular, this proton pump is present in fungal vacuolar membranes, where it plays an important role in the maintenance of intracellular ion homeostasis by maintaining acidic pH within cell [10–12]. The V-ATPase is composed of 14 subunits that form two domains, a membrane-integral V0 domain and a cytoplasmic V1 domain; and assembly factors, including Vph2 (Vma12), Vma21, Vma22, and Pkr1, are required for the assembly of a functional yeast V-ATPase [9, 13–15]. In Saccharomyces cerevisiae, V-ATPase synthesis and assembly are lost upon deletion of VPH2, leading to changes in ion sensitivity, including calcium sensitivity [16]. Previously, we demonstrated that V-ATPase also plays an important role in endogenous and exogenous oxidative stress response by regulating the expression and activity levels of the superoxide dismutase Sod2 and catalase Cta1, respectively, in C. glabrata [17].
Previous studies with mutant strains of Histoplasma capsulatum, Cryptococcus neoformans, and C. albicans lacking specific subunits of V-ATPase demonstrated that loss of V-ATPase function leads to vacuolar alkalinization and attenuation of in vivo virulence [18–21]. However, the link between V-ATPase function and virulence in C. glabrata has not been reported. In the current study, we investigated the effects of V-ATPase defect in C. glabrata on responses to various environmental stresses, antifungal resistance, and virulence.
Materials and methods
Strains, culture conditions, and compounds
C. glabrata strain CBS138 [22] was used as a wild-type control. C. glabrata Δvph2 deletion mutant lacking the entire VPH2 open reading frame (NCBI accession no.: XP_448720, Candida genome database ID: CAGL0K11594g) and a VPH2-reconstituted strain, in which an intact VPH2 was reintroduced at the native locus in the genome of the Δvph2 mutant, were constructed previously [17]. C. glabrata cells were propagated in yeast peptone dextrose (YPD) medium [1% (wt/vol) yeast extract, 2% (wt/vol) peptone, and 2% (wt/vol) glucose] or synthetic complete medium (SC) [0.67% (wt/vol) yeast nitrogen base with amino acids and 2% (wt/vol) glucose] at 30°C, unless otherwise specified. Media were solidified by the addition of 1.5% (wt/vol) agar. Fluconazole, voriconazole, amphotericin B, and FK506 were purchased from Sigma-Aldrich (St. Louis, MO). Bafilomycin B1 was purchased from Santa Cruz Biotechnology (Dallas, TX). Desferrioxamine (DFO) was purchased from EMD Chemicals (San Diego, CA) and bathophenanthroline disulfonate (BPS) was from MP Biomedicals (Solon, OH). Voriconazole, bafilomycin B1, and FK506 were dissolved in dimethyl sulfoxide and other compounds were dissolved in distilled water. Cell growth was not affected by exposure to the quantities of dimethyl sulfoxide used in the current study.
Drug susceptibility assays
Susceptibility to fluconazole, voriconazole, and the V-ATPase inhibitor bafilomycin B1, alone or in combination, was examined by using broth microdilution test, essentially according to the Clinical and Laboratory Standards Institute (CLSI) M27-S4 protocol [23] and the previous report [24] with minor modifications. Briefly, C. glabrata cells were incubated in SC at 35°C for 48 h. The minimum drug concentration that inhibited cell growth by more than 80% relative to drug-free control was defined as the minimum inhibitory concentration (MIC). Fractional inhibitory concentration (FIC) was calculated by using the following formula: FIC for drug A = (MIC of drug A in combination with drug B)/(MIC of drug A alone). The sum of FIC for drug A and FIC for drug B was defined as the FIC index (FICI). Drug interaction was classified as synergistic if FICI was ≤0.5 [25].
Spot dilution test was performed as described previously [26]. Briefly, the density of logarithmic-phase cultures in SC was adjusted to the concentration of 2 × 107 cells/ml. Serial 10-fold dilutions in SC were then prepared, and 5 μl of each dilution was spotted onto SC plates containing the test compound at the desired concentrations. Plates were incubated at 30°C for 48 h and photographed.
All sensitivity tests were performed on at least three separate occasions to ensure reproducibility.
Staining of fungal cells
Vacuolar staining with the styryl dye N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl)pyridinium dibromide (FM4-64; Thermo Fisher Scientific, Molecular Probes, Eugene, OR) and the pH-sensitive fluorophore 2N,7N-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM; Thermo Fisher Scientific, Molecular Probes) was performed as described previously [27, 28] with few modifications. Briefly, logarithmic-phase cells of C. glabrata were washed and resuspended in SC broth (pH 5.0). FM4-64 was added to cell suspensions (final concentration: 5 μM) and the mixtures were incubated at 30°C for 15 min to stain vacuole membranes. Cells were washed in SC with agitation for 90 min and resuspended in SC. BCECF-AM was added to cell suspensions (final concentration: 18 μM) and incubated at 30°C for 60 min. Cells were washed twice in SC, and microscopic examination was performed immediately after washing. Images were acquired using a Carl Zeiss LSM780 confocal laser-scanning microscope and processed using ZEN 2011 software (Carl Zeiss, Jena, Germany). The excitation and emission parameters were as follows: 560 and 605 nm, respectively, for FM4-64; and 470 and 535 nm, respectively, for BCECF-AM.
Virulence assay
Specific pathogen-free 8-week-old female BALB/c mice, weighing approximately 20 g, were purchased from Charles River Laboratories Japan (Yokohama, Japan). All mice had free access to food and water and were housed in a light–and temperature–controlled room at the Biomedical Research Center, Life Science Support Center, Nagasaki University. The health status of all mice was monitored at least daily throughout the experiments. All animal experiments were performed in full compliance with the Guide for the Care and Use of Laboratory Animals [29] and all institutional regulations and guidelines for animal experimentation, after pertinent review and approval by the Institutional Animal Care and Use Committee of Nagasaki University (protocol number 1407281164).
Logarithmic-phase cells of C. glabrata wild-type, Δvph2, and VPH2-reconstituted strains were harvested, washed, and resuspended in sterile saline, and cell density was adjusted to 4 × 108 cells/ml. The actual colony forming units (CFUs) used were confirmed by plating serial dilutions of the cell suspensions on YPD plates and incubating at 30°C overnight. Mice (n = 7 for wild-type, n = 9 for Δvph2, and n = 8 for Δvph2 + VPH2, per experiment) were inoculated with 0.2 ml of each cell suspension via the lateral tail vein. Mice were euthanized by carbon dioxide-induced asphyxia 7 d after the injection, and the spleen, liver, and both kidneys were excised. The organs were homogenized in sterile saline using a Shake Master NEO (Bio Medical Science, Tokyo, Japan). The homogenates were appropriately diluted in sterile saline and plated on YPD agar. Colonies were counted after 48 h of incubation at 30°C and CFUs per organ were calculated. A P-value of <0.05 (Kruskal-Wallis test with Dunn’s post-test) was considered to represent statistical significance.
Results
Synergistic effects of azoles and the V-ATPase inhibitor bafilomycin B1 against C. glabrata
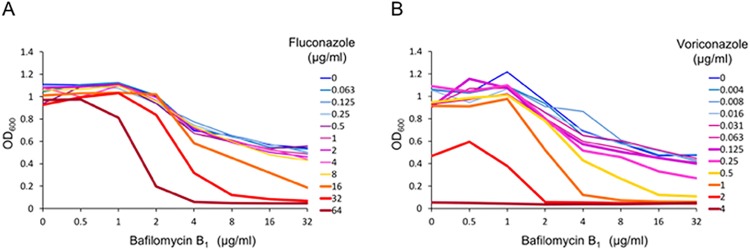
Azole antifungals, including fluconazole and voriconazole, inhibit the biosynthesis of ergosterol, the major component of fungal cell membrane, by targeting lanosterol 14α-demethylase encoded by ERG11 [30]. V-ATPase is pharmacologically inhibited by the plecomacrolide bafilomycin B1, which binds to the V0 subunit of V-ATPase, and simultaneously interferes with ATP hydrolysis and proton transport [31, 32]. To examine the effect of bafilomycin B1 on azole susceptibility of C. glabrata wild-type strain, we performed a checkerboard assay using serial 2-fold dilutions of the drugs. In the assay, MICs of fluconazole, voriconazole, and bafilomycin B1 were determined to be >64, 4, and >32 μg/ml, respectively (Fig 1). FIC indices of the combination of fluconazole and bafilomycin B1, and the combination of voriconazole and bafilomycin B1 were 0.375 and 0.313, respectively, indicating synergistic effects of these azoles and bafilomycin B1 against C. glabrata.
Fig 1. Synergistic effects of azole antifungals and the V-ATPase inhibitor bafilomycin B1 against C. glabrata wild-type strain.
Checkerboard assay was performed using serial 2-fold dilutions of drugs. (A) Data for the combination of fluconazole and bafilomycin B1. (B) Data for the combination of voriconazole and bafilomycin B1. Plates were incubated at 35°C for 48 h and the optical density at 600 nm (OD600) was determined. The graphs are representative of three independent replicate experiments.
Deletion of the V-ATPase assembly factor gene VPH2 leads to impaired vacuole acidification in C. glabrata
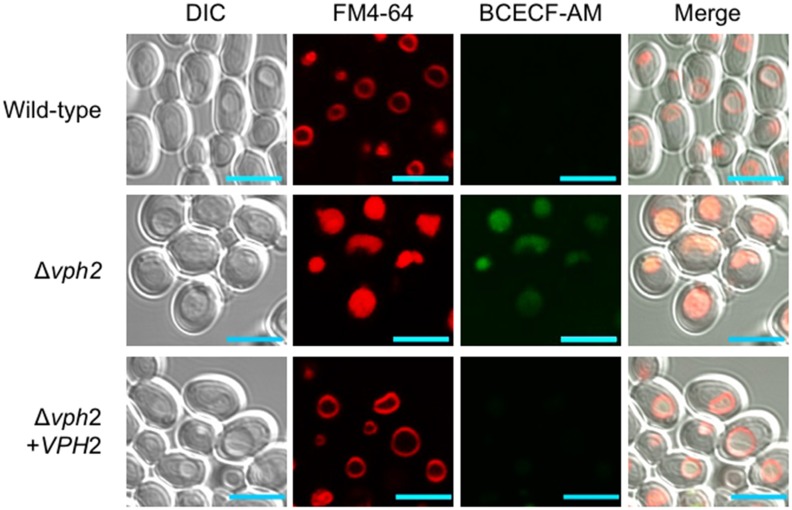
To investigate the role of V-ATPase in C. glabrata in detail, we analyzed the phenotype of the Δvph2 mutant, by comparing it with that of the wild-type and VPH2-reconstituted strains. First, C. glabrata cells were incubated with FM4-64, which selectively stains yeast vacuolar membranes and may be detected by red fluorescence [33, 34]. The wild-type and VPH2-reconstituted strains exhibited the typical ring-staining pattern of the vacuole membrane, while FM4-64 accumulated within the vacuole lumen in the Δvph2 mutant (Fig 2). The impaired trafficking of FM4-64 to the vacuolar membrane in the Δvph2 mutant was consistent with endocytosis defects demonstrated by C. albicans vma mutants [21] and C. albicans cells treated with fluconazole [28].
Fig 2. Vacuole staining.
Logarithmic-phase cells of C. glabrata were prepared in SC broth. Vacuolar membranes were first stained with FM4-64. After washing, the pH-sensitive fluorophore BCECF-AM was added to cell suspensions. Note the accumulation of FM4-64 and BCECF-AM within the vacuole lumen of the Δvph2 mutant. Scale bars, 5 μm. The images are representative of three independent replicate experiments.
The cells were then labeled with the pH-sensitive fluorescent dye BCECF-AM. The dye labeled the vacuoles of the Δvph2 mutant but not those of the wild-type and VPH2-reconstituted strains (Fig 2). This indicated impaired vacuolar acidification in the Δvph2 mutant, as would be expected after loss of proton pump capacity.
Loss of Vph2 results in increased fungal sensitivity to various environmental stresses
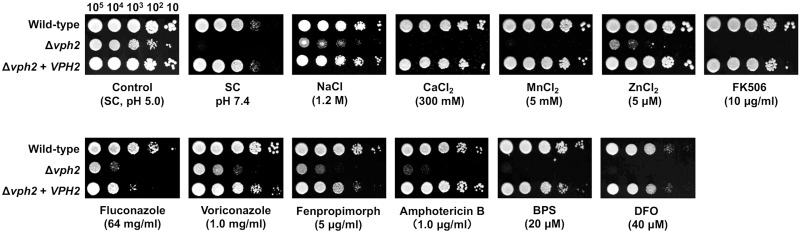
The phenotype of the Δvph2 mutant was further examined by spot dilution assays. In agreement with the notion of impaired vacuole acidification even under acidic conditions (Fig 2), the Δvph2 mutant exhibited a growth defect at pH 5.0 and was unable to grow at pH 7.4 (Fig 3). The Δvph2 mutant also displayed increased sensitivity to ion stress induced by excess of NaCl, CaCl2, MnCl2, and ZnCl2 in the growth medium.
Fig 3. Spot dilution assay.
Serial 10-fold dilutions of logarithmic-phase cells of C. glabrata were spotted onto SC plates containing the indicated compounds at the specified concentrations. Plates were incubated at 30°C for 48 h and photographed. The images are representative of three independent replicate experiments. BPS, bathophenanthroline disulfonate; and DFO, desferrioxamine.
The Ca2+/calmodulin-dependent protein phosphatase calcineurin plays a critical role in maintaining intracellular ion homeostasis and cell integrity. Further, simultaneous loss of certain subunits of V-ATPase and calcineurin is synthetically lethal in S. cerevisiae [35, 36]. In agreement with the findings for S. cerevisiae, the C. glabrata Δvph2 mutant was unable to grow in the presence of the calcineurin inhibitor FK506 (Fig 3). In addition to fluconazole and voriconazole, the Δvph2 mutant displayed increased susceptibility to fenpropimorph, which inhibits C-8 sterol isomerase (Erg2) and C-14 sterol reductase (Erg24) in the ergosterol biosynthesis pathway [37, 38], and amphotericin B, which directly targets ergosterol [39]. Finally, the Δvph2 mutant also exhibited growth defects under iron-limited conditions induced by the inclusion of the bacterial siderophore DFO or the Fe2+-chelator BPS in the growth medium.
Loss of Vph2 results in reduced fungal virulence in a murine model of disseminated candidiasis
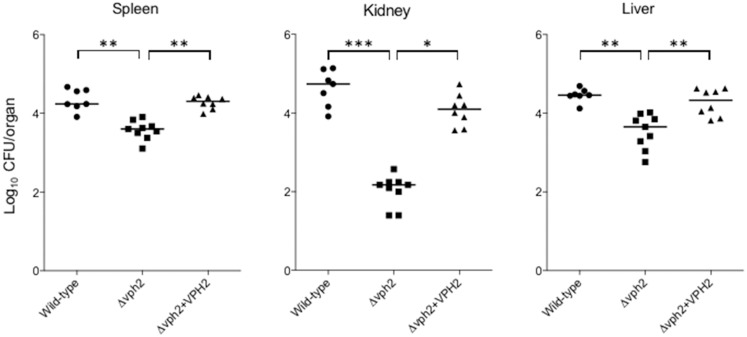
The effect of VHP2 deletion on the virulence of C. glabrata was examined using a mouse model of disseminated candidiasis. No mice died prior to euthanasia in the experiments. Fungal burdens in the examined organs of immunocompetent mice infected with the Δvph2 mutant were significantly lower than those in mice infected with the wild-type or VPH2-reconstituted strains (Fig 4). This suggested that V-ATPase plays an important role in the virulence in C. glabrata.
Fig 4. Fungal virulence in a mouse model of disseminated candidiasis.
Eight-week-old female BALB/c mice were intravenously inoculated with 8 × 107 cells of each C. glabrata strain (wild-type, n = 7; Δvph2, n = 9; and Δvph2 + VPH2, n = 8; per experiment). The mice were sacrificed 7 d after inoculation and CFUs per organ in specific organs were determined. The geometric mean is shown as a bar. Data representative of two independent experiments are shown. The C. glabrata strains used were: wild-type (CBS138), filled circles; Δvph2 mutant, squares; and VPH2-reconstituted strain, triangles. *P < 0.05, **P < 0.01, ***P < 0.001 (Kruskal-Wallis test with Dunn’s post-test).
Discussion
Overcoming the antifungal resistance of C. glabrata in the clinical setting is a pressing issue. In the current study, we demonstrated the synergistic effect of the V-ATPase inhibitor bafilomycin B1 and azole antifungals against a C. glabrata wild-type strain. Azole antifungals exert an antifungal effect partly by impairing vacuolar acidification, since ergosterol is required for V-ATPase to function efficiently [28]. The concurrent disruption of ergosterol and V-ATPase was induced by exposing the Δvph2 mutant to ergosterol inhibitors, leading to the severe growth impairment of the Δvph2 mutant (Fig 3). Some C. glabrata Δvph2 phenotypes were anticipated based on the published findings in S. cerevisiae and C. albicans. However, in the current study, we demonstrated for the first time that the loss of Vph2 in C. glabrata results in a V-ATPase defect, which leads to impaired vacuolar pH homeostasis and increased sensitivity to a variety of environmental stresses, as well as attenuated virulence in mice. The growth defect of the Δvph2 mutant could contribute to the enhanced susceptibility to diverse drugs tested and decreased virulence.
The Δvph2 mutant was unable to grow under iron-limiting conditions. Requirement of V-ATPase for iron homeostasis was also demonstrated in H. capsulatum [19]. Iron acquisition and iron homeostasis are important virulence factors in pathogenic fungi. For example, C. albicans must obtain hemoglobin iron to survive under the iron-limiting conditions in host tissues, and functional V-ATPase is required for iron acquisition in the microorganism [40].
Targeting a conserved protein that plays an essential role in human and fungal cells is challenging as it entails averting host toxicity. For instance, V-ATPase is present in the renal tubules and osteoclasts in mammals, including human [8]. However, although V-ATPase is highly conserved in eukaryotes, some major differences between mammalian and fungal V-ATPases exist, particularly with respect to the isoform composition of subunits and in the regulation of complex disassembly [41–45]. The different numbers and types of isoforms have been developed for most subunits of the mammalian V-ATPase [7]. The sequence conservation between S. cerevisiae and human V-ATPase subunits is 51–60% similarity and 31–41% identity, depending on the subunit and isoform [44]. C. glabrata VPH2 encodes a putative protein of 209 amino acids, with a molecular mass of 23.4 kDa. The deduced amino acid sequences of C. glabrata VPH2 share 61.8% similarity and 41.9% identity with those of S. cerevisiae VPH2 (NCBI Gene ID 853741, NCBI accession no. CAA81960), but only 32.6% similarity and 18.1% identity with those of a human homolog (TMEM199: NCBI Gene ID 147007, NCBI accession no. NP_689677) (S1 Fig). These different features could potentially be exploited to selectively target V-ATPase of pathogenic fungi.
In conclusion, in the current study, we provided evidence that disruption of C. glabrata V-ATPase function by deleting VPH2 impairs the fungal response to various environmental stresses and results in the attenuation of virulence of this clinically important fungal pathogen, supporting the notion that V-ATPase is an attractive antifungal target.
Supporting information
Identical and similar amino acids are shown as darkly shaded and lightly shaded regions, respectively. GenBank accession number: C. glabrata VPH2, XP_448720; S. cerevisiae VPH2, CAA81960; and TMEM199, NP_689677.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was partially supported by the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED) (https://www.amed.go.jp/en/program/list/01/06/002.html) (grant number JP18fk0108008 to T.M. and S.K.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445–1456. 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- 2.Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2017;7:2173 10.3389/fmicb.2016.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017;10:249–259. 10.2147/IDR.S124918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(Suppl 3):S445–S451. 10.1093/infdis/jix131 [DOI] [PubMed] [Google Scholar]
- 5.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. 10.1016/S1473-3099(17)30316-X [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol. 2012;50(4):1199–1203. 10.1128/JCM.06112-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayek SR, Lee SA, Parra KJ. Advances in targeting the vacuolar proton-translocating ATPase (V-ATPase) for anti-fungal therapy. Front Pharmacol. 2014;5:4 10.3389/fphar.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8(11):917–929. 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- 9.Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev. 2006;70(1):177–191. 10.1128/MMBR.70.1.177-191.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond S, Forgac M. The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J Biol Chem. 2008;283(52):36513–36521. 10.1074/jbc.M805232200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham LA, Stevens TH. Assembly of the yeast vacuolar proton-translocating ATPase. J Bioenerg Biomembr. 1999;31(1):39–47. [DOI] [PubMed] [Google Scholar]
- 12.Huss M, Vitavska O, Albertmelcher A, Bockelmann S, Nardmann C, Tabke K, et al. Vacuolar H(+)-ATPases: intra- and intermolecular interactions. Eur J Cell Biol. 2011;90(9):688–695. 10.1016/j.ejcb.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 13.Davis-Kaplan SR, Compton MA, Flannery AR, Ward DM, Kaplan J, Stevens TH, et al. PKR1 encodes an assembly factor for the yeast V-type ATPase. J Biol Chem. 2006;281(42):32025–32035. 10.1074/jbc.M606451200 [DOI] [PubMed] [Google Scholar]
- 14.Graham LA, Hill KJ, Stevens TH. Assembly of the yeast vacuolar H+-ATPase occurs in the endoplasmic reticulum and requires a Vma12p/Vma22p assembly complex. J Cell Biol. 1998;142(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane PM, Stevens TH. The long physiological reach of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr. 2007;39(5–6):415–421. 10.1007/s10863-007-9112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata R, Umemoto N, Ho MN, Ohya Y, Stevens TH, Anraku Y. VMA12 is essential for assembly of the vacuolar H(+)-ATPase subunits onto the vacuolar membrane in Saccharomyces cerevisiae. J Biol Chem. 1993;268(2):961–967. [PubMed] [Google Scholar]
- 17.Nishikawa H, Miyazaki T, Nakayama H, Minematsu A, Yamauchi S, Yamashita K, et al. Roles of vacuolar H+-ATPase in the oxidative stress response of Candida glabrata. FEMS Yeast Res. 2016;16(5). pii: fow054. 10.1093/femsyr/fow054 [DOI] [PubMed] [Google Scholar]
- 18.Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson PR. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol Microbiol. 2001;42(4):1121–1131. [DOI] [PubMed] [Google Scholar]
- 19.Hilty J, Smulian AG, Newman SL. The Histoplasma capsulatum vacuolar ATPase is required for iron homeostasis, intracellular replication in macrophages and virulence in a murine model of histoplasmosis. Mol Microbiol. 2008;70(1):127–139. 10.1111/j.1365-2958.2008.06395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patenaude C, Zhang Y, Cormack B, Köhler J, Rao R. Essential role for vacuolar acidification in Candida albicans virulence. J Biol Chem. 2013;288(36):26256–26264. 10.1074/jbc.M113.494815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poltermann S, Nguyen M, Günther J, Wendland J, Härtl A, Künkel W, et al. The putative vacuolar ATPase subunit Vma7p of Candida albicans is involved in vacuole acidification, hyphal development and virulence. Microbiology. 2005;151(Pt 5):1645–1655. 10.1099/mic.0.27505-0 [DOI] [PubMed] [Google Scholar]
- 22.Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, et al. Genome evolution in yeasts. Nature. 2004;430(6995):35–44. 10.1038/nature02579 [DOI] [PubMed] [Google Scholar]
- 23.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. CLSI document M27-S4. Wayne, PA.: Clinical and Laboratory Standards Institute; 2012.
- 24.Nagayoshi Y, Miyazaki T, Shimamura S, Nakayama H, Minematsu A, Yamauchi S, et al. Unexpected effects of azole transporter inhibitors on antifungal susceptibility in Candida glabrata and other pathogenic Candida species. PLoS One. 2017;12(7):e0180990 10.1371/journal.pone.0180990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. Combination antifungal therapy. Antimicrob Agents Chemother. 2004;48(3):693–715. 10.1128/AAC.48.3.693-715.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki T, Nakayama H, Nagayoshi Y, Kakeya H, Kohno S. Dissection of Ire1 functions reveals stress response mechanisms uniquely evolved in Candida glabrata. PLoS Pathog. 2013;9(1):e1003160 10.1371/journal.ppat.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RM, Allen C, Melman SD, Waller A, Young SM, Sklar LA, et al. Identification of inhibitors of vacuolar proton-translocating ATPase pumps in yeast by high-throughput screening flow cytometry. Anal Biochem. 2010;398(2):203–211. 10.1016/j.ab.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YQ, Gamarra S, Garcia-Effron G, Park S, Perlin DS, Rao R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010;6(6):e1000939 10.1371/journal.ppat.1000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 2011.
- 30.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11(6):272–279. [DOI] [PubMed] [Google Scholar]
- 31.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85(21):7972–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drose S, Bindseil KU, Bowman EJ, Siebers A, Zeeck A, Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry. 1993;32(15):3902–3906. [DOI] [PubMed] [Google Scholar]
- 33.Bairwa G, Rasheed M, Taigwal R, Sahoo R, Kaur R. GPI (glycosylphosphatidylinositol)-linked aspartyl proteases regulate vacuole homoeostasis in Candida glabrata. Biochem J. 2014;458(2):323–334. 10.1042/BJ20130757 [DOI] [PubMed] [Google Scholar]
- 34.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128(5):779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett-Engele P, Moilanen B, Cyert MS. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H(+)-ATPase. Mol Cell Biol. 1995;15(8):4103–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemenway CS, Dolinski K, Cardenas ME, Hiller MA, Jones EW, Heitman J. vph6 mutants of Saccharomyces cerevisiae require calcineurin for growth and are defective in vacuolar H(+)-ATPase assembly. Genetics. 1995;141(3):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia N, Arthington-Skaggs B, Lee W, Pierson CA, Lees ND, Eckstein J, et al. Candida albicans sterol C-14 reductase, encoded by the ERG24 gene, as a potential antifungal target site. Antimicrob Agents Chemother. 2002;46(4):947–957. 10.1128/AAC.46.4.947-957.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcireau C, Guilloton M, Karst F. In vivo effects of fenpropimorph on the yeast Saccharomyces cerevisiae and determination of the molecular basis of the antifungal property. Antimicrob Agents Chemother. 1990;34(6):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A. 2012;109(7):2234–2239. 10.1073/pnas.1117280109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman Z, Shemer R, Conibear E, Kornitzer D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol Microbiol. 2008;69(1):201–217. 10.1111/j.1365-2958.2008.06277.x [DOI] [PubMed] [Google Scholar]
- 41.Finnigan GC, Hanson-Smith V, Stevens TH, Thornton JW. Evolution of increased complexity in a molecular machine. Nature. 2012;481(7381):360–364. 10.1038/nature10724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jefferies KC, Cipriano DJ, Forgac M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys. 2008;476(1):33–42. 10.1016/j.abb.2008.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kane PM. Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr Protein Pept Sci. 2012;13(2):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahman S, Yamato I, Saijo S, Mizutani K, Ishizuka-Katsura Y, Ohsawa N, et al. Biochemical and biophysical properties of interactions between subunits of the peripheral stalk region of human V-ATPase. PLoS One. 2013;8(2):e55704 10.1371/journal.pone.0055704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry. 2010;49(23):4715–4723. 10.1021/bi100397s [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identical and similar amino acids are shown as darkly shaded and lightly shaded regions, respectively. GenBank accession number: C. glabrata VPH2, XP_448720; S. cerevisiae VPH2, CAA81960; and TMEM199, NP_689677.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.