Abstract
Background
We previously reported regenerative therapies for decompensated cirrhosis based on peripheral venous drip infusion using non-cultured whole bone marrow (BM) cells, or the less invasive cultured BM-derived mesenchymal stem cells (BMSCs). Here, we assessed the efficacy and safety of hepatic arterial infusion using cultured autologous BMSCs, comparing it with peripheral infusion, using our established canine liver fibrosis model.
Methods
Canine BM cells were harvested and cultured, and the resultant BMSCs were returned to carbon tetrachloride (CCl4)-induced liver cirrhosis model canines via either a peripheral vein (Vein group) or hepatic artery (Artery group). A variety of assays were performed before and 4, 8, and 12 weeks after BMSC infusion, and liver fibrosis and indocyanine green (ICG) half-life (t1/2) were compared to those in a control group that received CCl4 but not BMSCs. The safety of this approach was evaluated by contrast-enhanced computed tomography (CT) and serial blood examinations after infusion.
Results
Four weeks after infusing BMSCs, a significant improvement was observed in the Vein group (n = 8) compared to outcome in the Control group (n = 10), along with a decrease in ICG t1/2. In the Artery group (n = 4), ICG t1/2 was significantly shorter than that in the Vein group at 8 weeks (Δt1/2: −3.8 ± 1.7 min vs. +0.4 ± 2.4 min; p < 0.01) and 12 weeks (Δt1/2: −4.2 ± 1.7 min vs. +0.4 ± 2.7 min; p < 0.01) after BMSC administration. Post-infusion contrast-enhanced CT showed no liver infarction, and blood tests showed no elevations in either serum lactate dehydrogenase concentrations or hypercoagulability.
Conclusions
We confirmed the efficacy and safety of the hepatic arterial infusion of cultured autologous BMSCs using a canine model, thereby providing non-clinical proof-of-concept.
Introduction
Liver cirrhosis represents the advanced stage of liver fibrosis, presenting as regenerative nodules surrounded by fibrotic bands that develop in response to chronic liver injury [1–3]. Currently, the most effective therapy for advanced cirrhosis is liver transplantation. However, the procedure has several limitations including the lack of donors, surgical complications, immunological suppression, and high medical costs [4]. Therefore, novel alternative therapies for decompensated liver cirrhosis are urgently needed.
In recent years, several studies using animal models of liver diseases have demonstrated that bone marrow (BM) cell transplantation might accelerate liver regeneration and reduce hepatic fibrosis [5–8].
Accordingly, we developed autologous BM cell infusion (ABMi) therapy for liver cirrhosis and reported its efficacy and safety for liver cirrhosis [9,10]. However, since ABMi therapy requires BM aspiration under general anesthesia, some patients are excluded due to poor liver or cardiopulmonary functions. In an effort to expand the applicability of ABMi therapy, we developed a less invasive method for liver regeneration therapy using autologous BM-derived mesenchymal stem cells (BMSCs) cultured from small amounts of BM fluid aspirated under local anesthesia. However, before human clinical trials can be considered, the safety and efficacy of cultured autologous BMSC infusion must be confirmed in medium-to-large animals. To this end, we previously established a useful canine liver fibrosis model generated through the repeated administration of carbon tetrachloride (CCl4) through a catheter for 10 weeks. Using this model, we found that peripheral venous infusion using cultured autologous BMSCs could improve liver fibrosis without adverse effects, suggesting that this could represent a less invasive therapeutic option [11].
Here, to develop liver regeneration therapy with enhanced therapeutic effects, we evaluated the efficacy and safety of the hepatic arterial infusion of cultured autologous BMSCs, comparing it with peripheral infusion, using our established canine model of liver fibrosis.
Materials and methods
Animals and ethics
Twenty-two beagles (1–2 years of age; 10 males and 12 females) were used in this study. These animals included six canines in the control group and four in the peripheral venous infusion group, from which 0- and 4-week data were used from our previous study [11]. These canines were housed in our animal facility and treated in accordance with the university’s Animal Care Guidelines. In particular, they were housed in individual metal cages (100 cm in width and 120 cm in height) and given standard solid food (Oriental Yeast Co., Tokyo, Japan), according to dietary recommendations that were based on body weight, age, and activity level of each canine. They had free access to water at all times and were housed in a climate-controlled building at 22–23 °C, with a 16-h light/8-h dark cycle. They were taken for walks daily in the evening (15:00 to 17:00) and were inspected at least three times per day to monitor their health and well-being. Facility staff members including our group checked several health conditions as follows: appetite, thirst, defecation, urination, behavior and lethargy, etc. After surgery, we also checked these health conditions at least three times per day. This study was approved by the Institutional Animal Care and Use Committee of Yamaguchi University, “Ube” area. (Approval number: 21–033).
Catheter implantation
All catheter implantations were performed as previously described [11]. Briefly, this was performed under general anesthesia for all canines. The stomach was directly punctured with an 18-gauge Teflon IV catheter (6-French P-U catheter; Toray Medical Co. Ltd., Tokyo, Japan). An infusion port (P-U Celsite port; Toray) was then placed in a subcutaneous pocket created on the back. Carbon tetrachloride (CCl4, Wako, Osaka, Japan) was administered from the port, which essentially represented oral administration. Intramuscular buprenorphine at 10 μg/kg body weight (BW) and subcutaneous cefovecin (Convenia; Zoetis Japan, Tokyo, Japan) at 8 mg/kg BW were given at the end of the procedure for post-operative analgesia and to prevent infections, respectively. As stated, we checked several health conditions after surgery at least three times per day.
Aspiration of bone marrow fluid, culture, and infusion of BMSCs
After catheter implantation, a 16-gauge biopsy needle (Angiotech, Gainesville, FL) was used to puncture the proximal humerus of each canine and BM was aspirated using a 5-mL syringe filled with 1 mL of heparin. To culture BMSCs, the aspirated BM fluid was seeded in T-75 flasks (Life Technologies Corp., Grand Island, NY) and cultured with 10% fetal bovine serum (Life Technologies) and 100 μg/mL gentamicin (Life Technologies) in Dulbecco’s modified Eagle Medium (DMEM, Life Technologies) in a 5% CO2 incubator at 37 °C. After 2 days of incubation, non-adherent cells were removed during medium replacement. The culture medium was changed every 2 days for 2–3 weeks. Expanded BMSCs were detached using cell detachment solution and then collected.
Experimental model (induction of liver fibrosis)
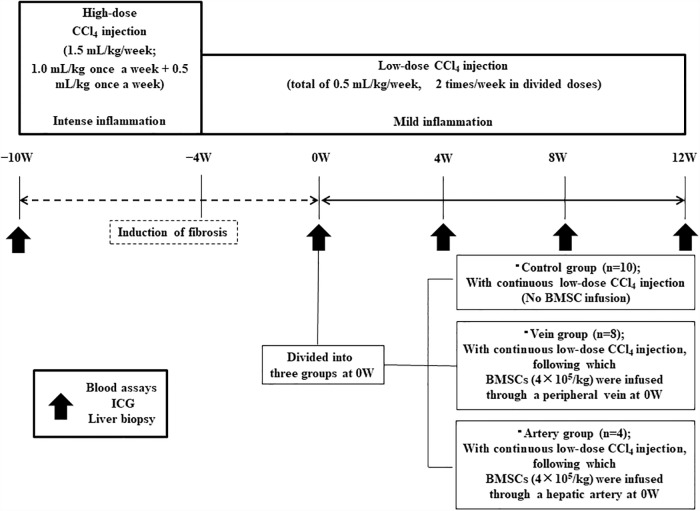
CCl4 was diluted 1:1 in corn oil before injecting it intra-gastrically via the catheter. Injections ware repeated for 10 weeks using the implanted catheter (high-dose period: 1.0 mL/kg BW once per week and 0.5 mL/kg BW once per week for 6 weeks; low-dose period: 0.25 mL/kg BW twice per week for 4 weeks) to induce liver fibrosis (Fig 1). After the infusion of BMSCs, low-dose CCl4 was further administrated for 12 weeks. CCl4 injections were performed on the first and fourth days of each week.
Fig 1. Experimental protocol for canine model of liver fibrosis and treatment.
The canines were administered repeated carbon tetrachloride (CCl4) injections through the implanted catheter for 10 weeks (high-dose period: 1.0 mL/kg body weight once per week and 0.5 mL/kg body weight once per week for 6 weeks; low-dose period: 0.25 mL/kg body weight twice per week for 4 weeks). CCl4 was injected on the first and fourth days of each week. At 0 weeks (0W; i.e. after 10 weeks of CCl4 injections), the canines were divided into three groups. In the control group, low-dose CCl4 injection was continued for a further 12 weeks without bone marrow-derived mesenchymal stem cell (BMSC) infusion. In the Vein group, cultured autologous canine BMSCs (4 × 105/kg) were infused through a peripheral vein, and low-dose CCl4 injections were continued for 12 more weeks. In the Artery group, cultured autologous BMSCs (4 × 105/kg) were infused through a hepatic artery using angiography, and low-dose CCl4 injections were continued for 12 more weeks. In all three groups, blood assays, indocyanine green (ICG) testing, and liver biopsy based on ultrasonography were performed at −10W, 0W, 4W, 8W, and 12W, as indicated by the arrows.
Experimental groups
After 10 weeks of CCl4 injections, the 22 canines were divided into three groups including the Control, Vein, and Artery groups. This point was defined as “0W”. Subsequently, the 10 animals in the Control group received low-dose CCl4 injection for a further 12 weeks. The eight canines in the Vein group were infused with cultured autologous BMSCs (4 × 105/kg) through a peripheral vein and low-dose CCl4 injection was continued for 12 more weeks. The four canines in the Artery group were infused with cultured autologous BMSCs (4 × 105/kg) through a hepatic artery using angiography, and low-dose CCl4 injection was continued for 12 more weeks.
Serial examinations including blood tests, ultrasonography-guided liver biopsies, and indocyanine green (ICG) tests were performed before and 10, 14, 18, and 22 weeks after commencing CCl4 injection (i.e. at −10W, 0W, 4W, 8W and 12W; Fig 1). Furthermore, abdominothoracic contrast-enhanced computed tomography (CE-CT) was performed 30 min after BMSC-infusion to evaluate the presence of liver infarction and portosystemic shunting (PSS).
Safety evaluations with an increased BMSC dose
BMSCs were increased to 1.2 × 106 cells/kg and infused through a hepatic artery into the canine model of liver fibrosis to test the effects of a higher dose (n = 1). The general condition and vital signs were monitored before and after BMSC infusion. Blood parameters were measured before BMSC infusion and at 1, 3, 5, and 7 days after infusion. Seven days after BMSC infusion, after collecting blood samples, we conducted a liver biopsy to evaluate the presence of liver infarction based on histological analysis.
Laboratory tests
The blood samples collected at the stipulated time points were used to assess serum albumin, alanine aminotransferase, aspartate aminotransferase, bilirubin, prothrombin time PT, anti-thrombin3 (AT3) activity, and fibrin degradation products.
ICG tests to assess liver functions
A 22-G indwelling venous cannula was inserted into the internal jugular vein and blood was collected. Following the collection of blood for the 0 time-point sample, 0.1 mg/kg of ICG (Diagnogreen Inj.; Daiichi Sankyo Co. Ltd., Tokyo, Japan) was administered via the peripheral vein and blood was collected 5, 10, 15, 20, and 30 min later from the maintained venous route. Blood samples were then centrifuged and sera were harvested from blood samples and analyzed for ICG content using an ICG Meter (Fuchu Giken, Inc., Tokyo, Japan) The difference in the ICG half-life (t1/2), or ΔICG (min), was calculated using the formula, ΔICG (min) = ICG t1/2 (min) at 4W, 8W, or 12W –ICG t1/2 (min) at 0W.
CE-CT imaging
CE-CT examinations were performed using an eight-detector-row CT system (ECLOS 8; Hitachi Medical Corp., Tokyo, Japan). All canines were first placed in ventral recumbency and all CT scans were performed during apnea under anesthesia. Canines were administered 2 mL/kg iopamidol with an iodine concentration of 370 mg/mL (Oiparomin 370; Fuji Pharmaceutical Co., Toyama, Japan) as intravenous contrast medium.
Liver biopsy
After the intramuscular injection of 20 μg/kg medetomidine (Dorbene; Kyoritsu Seiyaku Corp., Tokyo, Japan), the canines were placed in the left lateral decubitus position. The skin over the biopsy site was shaved and then disinfected with povidone-iodine. The local anesthetic (0.5% lidocaine; Pfizer, Tokyo, Japan) was injected into a small area of the skin and into tissues over part of the liver, and ultrasonography-guided liver biopsies were collected using a 16-gauge biopsy needle (Aragon Medical Devices, Plano, TX). After removing the biopsy needle, the puncture site was manually compressed and atipamezole hydrochloride was injected intramuscularly.
Some samples were fixed in 4% paraformaldehyde overnight and used for the evaluation of liver fibrosis by Sirius-red staining, whereas others were stored at −80 °C for real-time quantitative polymerase chain reaction (RT-PCR). These experiments including ultrasonography-guided liver biopsies were performed over time. We analyzed gene expression related to liver fibrosis by RT-PCR using ultrasonography-guided biopsied specimens; therefore, we have been taking care of these canines after this study, and we did not sacrifice them.
Sirius-red staining
Paraffin-embedded liver samples were sectioned (3-μm) and stained with Sirius-red staining. For this, paraffin sections were de-waxed, hydrated, and stained with Sirius-red solution (1.3% aqueous picric acid/0.1% Direct Red 80) for 15–20 min to detect liver fibrosis. Samples were then washed with 0.5% acetic acid for 1 min and dehydrated using 100% ethanol for 5 min. Samples were cleared in xylene for 10 min and then mounted in a resinous medium.
Histomorphometry
Histomorphometry was performed using an imaging system coupled to a fluorescence microscope (Biorevo BZ9000; Keyence, Osaka, Japan). The fibrotic area was calculated as the percent Sirius red-stained area relative to the total sample using a BZ Analyzer II (Keyence). Vessels stained with Sirius red were excluded from the calculation. We defined the percent fibrotic area as the fibrosis level. The difference in the fibrotic area, or Δfibrosis level (%), was calculated using the formula Δfibrosis level (%) = fibrosis level (%) at 4W, 8W, or 12W − fibrosis level (%) at 0W.
Angiography and intra-arterial BMSC injection
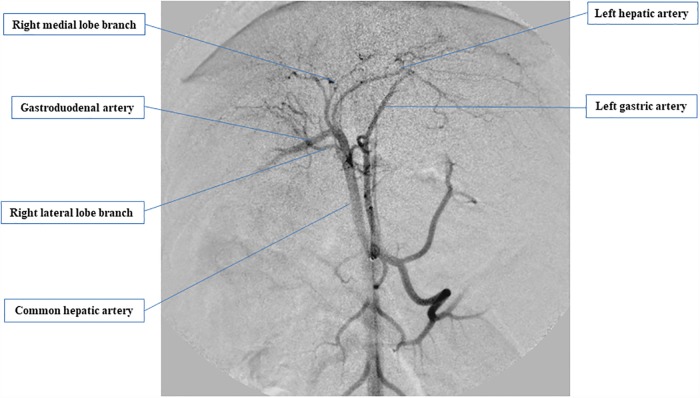
Anesthesia was induced via the slow intravenous administration of propofol (1% intravenous propofol, 7 mg/kg; Maruishi Pharmaceutical Co, Osaka, Japan) and maintained with isoflurane (isoflurane, 1.4–2.5%; Dainippon Sumitomo Pharma Co, Tokyo, Japan) and oxygen. All canines were administered an antibiotic (cefazolin sodium, 25 mg/kg intravenously) and analgesic (buprenorphine, 20 mg/kg intramuscularly) after induction. The right femoral artery was punctured with a 20-G needle and cannulated with a 4-French (Fr) introducer sheath (Radifocus introducer sheath; Terumo Co, Tokyo, Japan). A guidewire (SURF, diameter: 0.89 mm, angled, 80 cm; Piolax Medical Devices Inc., Yokohama, Japan) and catheter (PA catheter, 4 Fr, 40 cm; Terumo Clinical Supply Co, Gifu, Japan) were inserted into the aorta and celiac artery with fluoroscopic guidance (ARCADIS Varic; Siemens Healthcare Japan, Tokyo, Japan). A microguidewire (Radifocus guidewire, diameter: 0.41 mm, angled, 100 cm; Terumo Clinical Supply Co.) was advanced into the common hepatic artery through the catheter. Then, a 2.1-Fr microcatheter (Sniper 2, 80 cm; Terumo Clinical Supply Co, Gifu, Japan) was placed in the common hepatic artery prior to injection of the contrast agent (Optiray 350; Covidien Co, Tokyo, Japan) under digital subtraction angiography (Fig 2).
Fig 2. Ventral-dorsal digital subtraction images of a canine.
Hepatic arteriogram was performed after the injection of contrast medium through a 4-French catheter placed in the common hepatic artery. We inserted a catheter in the left hepatic artery, right medial lobe branch, and right lateral lobe branch and injected bone marrow-derived mesenchymal stem cells (BMSCs) via each of these arteries at doses of 2 × 105 cell/kg, 1 × 105 cell/kg, and 1 × 105 cell/kg, respectively.
Next, we inserted a catheter into the left hepatic artery, right medial lobe branch, and right lateral lobe branch and administered BMSCs via each of these arteries at doses of 2 × 105 cells/kg, 1 × 105 cells/kg, and 1 × 105 cells/kg, respectively. After removing the sheath, the puncture site was manually compressed. Cefazolin sodium (25 mg/kg, intravenously) and buprenorphine (20 mg/kg, intramuscularly) were administered after treatment.
RT-PCR analysis
Total RNA extraction was performed on liver biopsy specimens using an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). For cDNA synthesis, Taqman reverse transcription reagents were used as described in the manufacturer’s manual (Roche Diagnostics, Indianapolis, IN). Variations in gene expression were analyzed using a Step One Plus real-time PCR system (Life Technologies) with SYBR green. Relative quantification of gene expression was performed using ribosomal protein 18 as an internal control. The primers used were as follows: canine collagen, type1, alpha2 (COL1A2) primers: sense (5′-CCCAGCCAAGAACTGGTACAGAA-3′), antisense (5′-CGCATGAAGGCGAGTTGAGTAG-3′); canine collagen, type3, alpha1 (COL3) primers: sense (5′-CATCTCGGCACAGCAGCAA-3′), antisense (5′-CAGATCCTGAGTCACAGACGCATA-3′); canine tissue inhibitor of metalloproteinase 1 (TIMP-1) primers: sense (5′-TTCACCAAGACCTATGCTGCTGCTG-3′), antisense (5′-AGTTGCATATCCCTGGCTCTC-3′).
Statistical analysis
Data were analyzed using a Student’s t-test and paired t-test. Values of p < 0.05 were considered statistically significant. Data are presented as the mean ± standard deviation.
Results
Assessment of fibrotic area
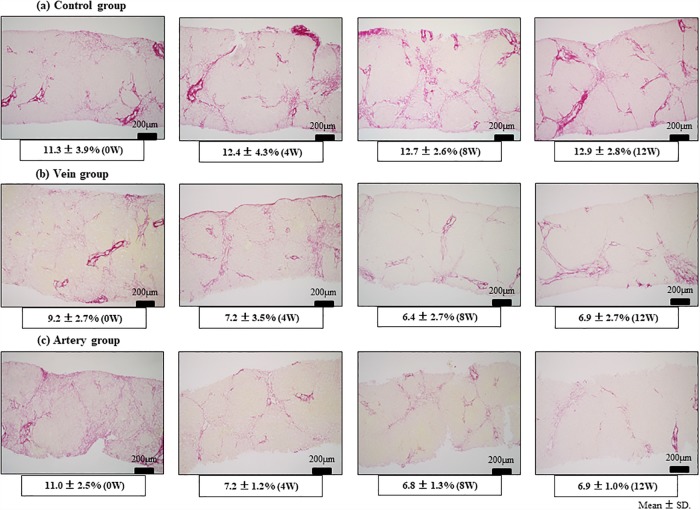
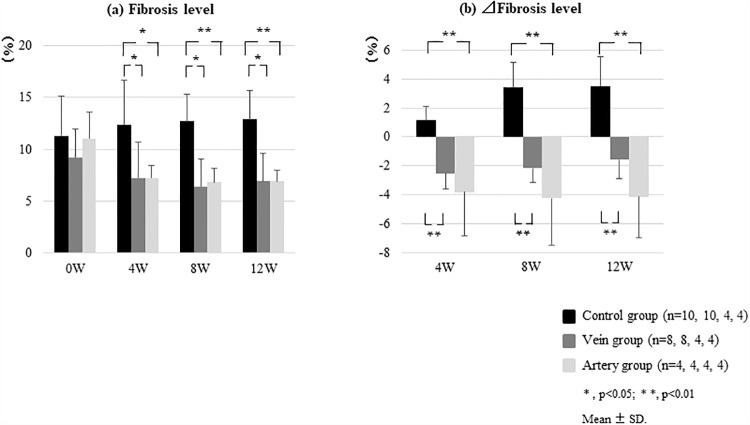
First, adverse events associated with catheter implantations were not observed, and US-guided liver biopsy was performed over time. Bridging fibrosis was confirmed at 0W based on Sirius red staining, which demonstrated pseudo-lobules in some samples. The fibrosis level in the Control group increased from 11.3 ± 3.9% (0W) to 12.4 ± 4.3% (4W), 12.7 ± 2.6% (8W), and 12.9 ± 2.8% (12W), whereas that in the Vein group decreased from 9.2 ± 2.9% (0W) to 7.2± 3.5% (4W), 6.4 ± 2.7% (8W), and 6.9 ± 2.7% (12W) (Fig 3a and 3b and S1 Table). In the Artery group, the fibrosis level also significantly decreased from 11.0 ± 2.5% (0W) to 7.2 ± 1.2% (4W; p < 0.05), 6.8 ± 1.3% (8W; p < 0.05), and 6.9 ± 1.0% (12W; p < 0.05; Figs 3C and 4A). In terms of the Δfibrosis area 4 weeks after BMSC infusion, there was a significant improvement in fibrosis in both the Vein (Δfibrosis area: −2.5 ± 1.1%) and Artery groups (−3.8 ± 3.0%) relative to that in the Control group (+0.9 ± 1.0%). However, the Artery group showed a greater effect, and this effect was maintained even at 8 (−4.3 ± 3.2%) and 12 (−4.2 ± 2.8%) weeks (Fig 4b).
Fig 3. Liver fibrosis in different treatment groups as assessed by Sirius-red staining.
The canines were administered repeated carbon tetrachloride (CCl4) injections to induce liver fibrosis and treated with bone marrow-derived mesenchymal stem cells (BMSCs) via the peripheral vein (Vein group) or hepatic artery (Artery group). (a) In the Control group, the fibrosis level increased over time from 11.3 ± 3.9% (0W) to 12.4 ± 4.3% (4W), 12.7 ± 2.6% (8W), and 12.9 ± 2.8% (12W). (b) In the Vein group, the fibrosis level decreased from 9.2 ± 2.7% (0W) to 7.2± 3.5% (4W), 6.4 ± 2.7% (8W), and 6.9 ± 2.7% (12W). (c) In the Artery group, the fibrosis level decreased significantly from 11.0 ± 2.5% (0W) to 7.2 ± 1.2% (4W; p < 0.05), 6.8 ± 1.3% (8W; p < 0.05), and 6.9 ± 1.0% (12W; p < 0.05). 4W, 8W, and 12W denote weeks post-BMSC administration.
Fig 4. Quantification of liver fibrosis assessed by Sirius red staining in different treatment groups.
The canines were administered repeated carbon tetrachloride (CCl4) injections to induce liver fibrosis and treated with bone marrow-derived mesenchymal stem cells (BMSCs) via the peripheral vein (Vein group) or hepatic artery (Artery group). (a) Significant improvements in fibrosis level were found in both the Vein and Artery groups with respect to that in the Control group at 4, 8, and 12 weeks post-BMSC administration. (b) With respect to the change in (Δ) fibrotic area, a significant improvement in fibrosis was found at 4 weeks post-BMSC administration in the Vein (Δfibrotic area: −2.5 ± 1.1%) and Artery groups (−3.8 ± 3.0%) relative to that in the Control group (+0.9 ± 1.0%). A greater effect was found in the Artery group, and this effect was maintained even after 8 (−4.3 ± 3.2%) and 12 (−4.2 ± 2.8%) weeks.
ICG test results
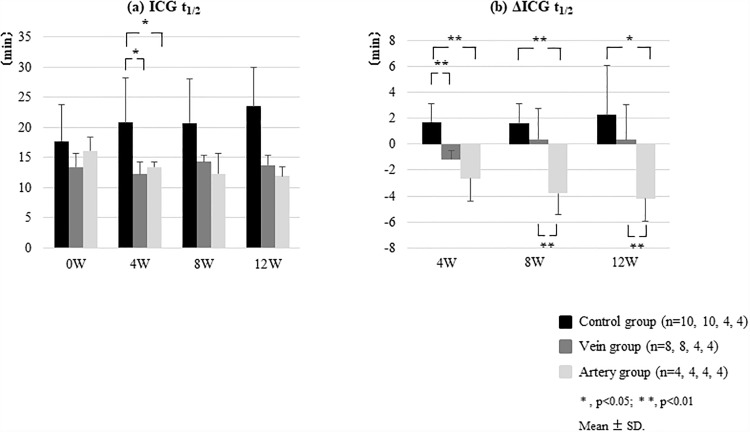
The ICG t1/2 increased over time in the Control group from 17.7 ± 3.4 min (0W) to 20.8 ± 6.3 min (4W), 20.7 ± 4.1 min (8W), and 23.5 ± 5.0 min (12W). In the Vein group, the ICG t1/2 slightly decreased at 4 weeks, from 13.4 ± 2.3 min (0W) to 12.2 ± 2.0 min (4W); however, from 8 weeks on, it increased to 14.3 ± 1.1 min (8W) and 13.7 ± 1.7 min (12W). In the Artery group, the ICG t1/2 decreased from 16.1 ± 2.3 min (0W) to 13.4 ± 0.8 min (4W), and this value was subsequently maintained (12.3 ± 3.3 min (8W) and 12.0 ± 1.5 min (12W; Fig 5a and S2 Table).
Fig 5. Therapeutic effects of transfused BMSCs in a CCl4-induced canine liver fibrosis model.
After the induction of liver fibrosis, canines were treated with BMSCs via the peripheral vein (Vein group) or hepatic artery (Artery group). (a) Indocyanine green (ICG) half-life (t1/2) in the Vein group was slightly decreased at 4 weeks post-BMSC administration, from 13.4 ± 2.3 min (0W) to 12.2 ± 2.0 min (4W), but increased from 8 weeks onward to 14.3 ± 1.1 min (8W) and 13.7 ± 1.7 min (12W). In the Artery group, the ICG t1/2 decreased from 16.1 ± 2.3 min (0W) to 13.4 ± 0.8 min (4W), and this value was subsequently maintained (12.3 ± 3.3 min (8W), 12.0 ± 1.5 min (12W)). (b) The ΔICG t1/2 value was significantly decreased in the Vein group compared to that in the Control group at 4 weeks post-BMSC administration (ΔICG t1/2 (min): −1.2 ± 0.7 vs. 1.7 ± 1.4; p < 0.01). Moreover, the ICG t1/2 in the Artery group (n = 4) was significantly decreased at 8 weeks (Δt1/2: −4.2 ± 1.7 min vs. +0.4 ± 2.7 min; p < 0.01) and 12 weeks after BMSC administration (Δt1/2: −4.2 ± 1.7 min vs. +0.4 ± 2.7 min; p < 0.05) even when compared to that in the Vein group. All error bars represent the standard deviation of the mean.
The ΔICG t1/2 values in the Vein group (n = 8) were significantly lower than those in the Control group (n = 10) at 4 weeks following BMSC administration (ΔICG t1/2 (min): −1.2 ± 0.7 vs. 1.7 ± 1.4; p < 0.01); however, no obvious significant differences were found at 8 or 12 weeks post-BMSC administration. In the Artery group (n = 4), ICG t1/2 values were significantly lower at 8 weeks (Δt1/2: −4.2 ± 1.7 min vs. +0.4 ± 2.7 min; p < 0.01) and 12 weeks after BMSC administration (Δt1/2: −4.2 ± 1.7 min vs. +0.4 ± 2.7 min; p < 0.05) even when compared to those in the Vein group (Fig 5b).
Biochemical results
In the Control group, albumin and AT3 gradually tended to become exacerbated. In the Vein group, although improvement was observed at 4 weeks, subsequent gradual exacerbation was observed. However, in the Artery group, this improvement at 4 and 8 weeks was maintained at 12 weeks (Table 1 and S3 Table).
Table 1. Clinical laboratory test results.
| Control group | Vein group | Artery group | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0W (n = 10) |
4W (n = 10) |
8W (n = 4) |
12W (n = 4) |
0W (n = 8) |
4W (n = 8) |
8W (n = 4) |
12W (n = 4) |
0W (n = 4) |
4W (n = 4) |
8W (n = 4) |
12W (n = 4) |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Alb (g/dL) | 2.5±0.3 | 2.3±0.3 | 2.4±0.2 | 2.4±0.3 | 2.7±0.2 | 2.8±0.2 | 2.7±0.2 | 2.5±0.1 | 2.5±0.1 | 2.6±0.1 | 2.5±0.1 | 2.6±0.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| AST (U/L) | 60.2±31.5 | 85.1±39.9 | 81.7±83.8 | 70.3±39.9 | 59.5±36.3 | 50.1±21.9 | 60.1±31.5 | 42.3±14.5 | 83.0±53.8 | 54.0±20.6 | 51.5±28.3 | 35.0±5.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| ALT (U/L) | 662.1±663.4 | 850.2±498.8 | 619.0±717.2 | 506.8±232.3 | 374.8±520.1 | 327.6±382.4 | 472.8±572.5 | 232.5±274.9 | 689.5±728.8 | 649.8±583.1 | 506.8±615.5 | 221.5±144.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| LDH (U/L) | 163.3±100.2 | 169.4±66.1 | 96.8±23.4 | 89.5±24.5 | 146.5±21.1 | 153.8±73.6 | 168.5±75.8 | 174.8±79.9 | 146.0±41.8 | 117.8±26.3 | 130.0±46.2 | 118.8±45.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| PT (sec) | 9.5±1.8 | 8.9±1.2 | 9.3±2.4 | 9.7±2.5 | 7.4±0.7 | 7.3±0.9 | 7.1±0.8 | 6.7±0.5 | 9.7±3.1 | 8.3±1.0 | 8.3±1.0 | 8.0±1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| AT3 (%) | 102.3±21.0 | 97.9±14.8 | 97.3±25.8 | 94.8±30.4 | 112.1±11.7 | 116.5±11.4 | 113.3±17.7 | 108.0±9.9 | 99.0±15.3 | 106.5±12.9 | 108.3±9.1 | 113.8±7.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||
Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT, prothrombin time; AT3, antithrombin 3. Data are expressed as mean ± standard deviation.
Expression of liver fibrosis-related genes
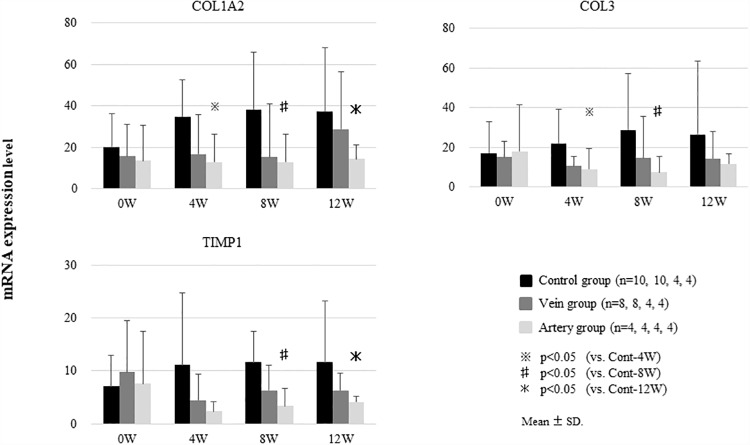
The expression of COL1A2, COL3, and TIMP-1 mRNA, which encode proteins involved in hepatic fibrosis, was analyzed. Expression levels of all genes tended to decrease in the Vein group compared to those in the Control group, although the differences were not significant. However, in the Artery group, the levels of all genes were significantly decreased compared to those in the Control group at 4, 8, and 12 weeks (Fig 6).
Fig 6. Expression of liver fibrosis-related genes after the induction of liver fibrosis and BMSC treatment.
After the induction of liver fibrosis, canines were treated with BMSCs via the peripheral vein (Vein group) or hepatic artery (Artery group). The expression of COL1A2, COL3, and TIMP-1 mRNA tended to decrease in the Vein group compared to that the Control group, although the differences were not significant. In the Artery group, the expression levels of COL1A2, COL3, and TIMP-1 were significantly lower than those in the Control group at 4, 8, and 12 weeks. Data show the mean ± standard deviation.
Safety evaluations
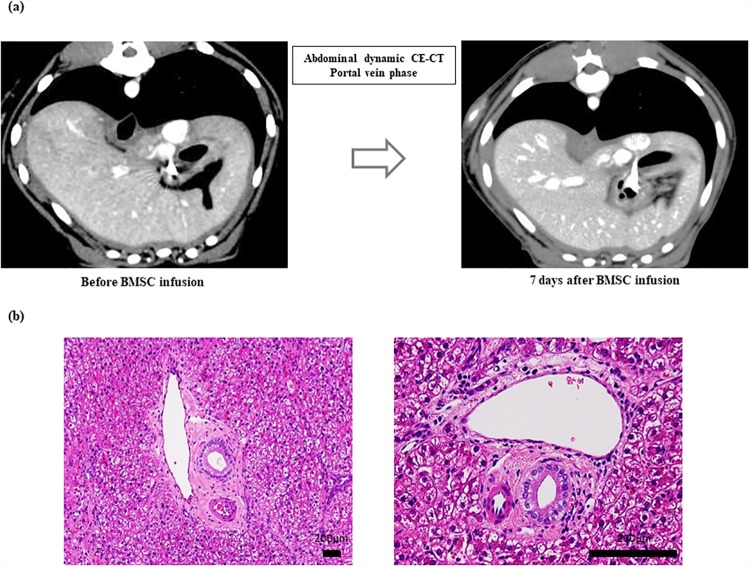
Post-infusion contrast-enhanced CT showed no sign of liver infarction and the absence of major PSS (Fig 7a). Further, we did not observe oxygen desaturation, a remarkable change in pulse rate, or a decline in general condition after BMSC infusion in any of the canines including the canine used to test an increased dose. Moreover, in the canine infused with a higher number of BMSCs (1.2 × 106 cells/kg), blood tests showed no elevation in either serum lactate dehydrogenase concentrations or hypercoagulability (Table 2). Moreover, arterial embolization and necrotic biliary areas were not observed based on hematoxylin and eosin staining of the liver tissue (Fig 7b).
Fig 7. Contrast-enhanced computed tomography (CE-CT) and photomicrographs of liver tissue samples after BMSC application.
(a) After the infusion of CE-CT, no evidence of liver infarction and the absence of major portosystemic shunts (PSS) were observed. (b) Arterial embolization and biliary necrotic images were not observed in the liver tissues following hematoxylin and eosin staining.
Table 2. Clinical laboratory test results in the safety evaluation.
| Before | Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|---|
| Alb (g/dL) | 2.6±0.2 | 2.9±0.3 | 3.1±0.2 | 3.1±0.3 | 2.8±0.2 |
| AST (U/L) | 37.2±8.5 | 47.1±9.3 | 33.5±5.9 | 36.1±12.4 | 33.8±9.3 |
| ALT (U/L) | 1302.1±532.3 | 1166.5±368.9 | 799.1±132.4 | 575.8±122.9 | 387.3±92.3 |
| LDH (U/L) | 41.6±14.5 | 203.1±86.1 | 99.2±22.8 | 64.0±19.2 | 70.7±27.9 |
| PT (sec) | 7.5±1.2 | 7.0±1.1 | 6.8±0.9 | 6.6±1.1 | 6.8±0.8 |
| FDP (μg/mL) | 1.0±0.1 | 1.4±0.1 | 2.4±0.2 | 2.4±0.2 | 1.4±0.1 |
| D-dimer (μg/mL) | 0.4±0.1 | 0.5±0.1 | 1.2±0.1 | 1.1±0.1 | 0.5±0.1 |
| AT3 (%) | 99.2±12.5 | 126.3±21.8 | 150.2±26.1 | 149.9±19.2 | 132.7±24.5 |
Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT, prothrombin time; FDP, fibrin degradation products; AT3, antithrombin 3. Data are expressed as mean ± standard deviation.
Discussion
The present study showed the efficacy and safety of hepatic artery infusion using cultured autologous BMSCs based on a canine liver fibrosis model.
In a previous study, we placed a port and a catheter in beagles, and using this system, administered CCl4 over a 10-week period to induce liver fibrosis. We then administered cultured autologous BMSCs via peripheral veins and confirmed the safety and efficacy of this approach for the treatment of fibrosis [11].
In an attempt to increase the efficacy of this treatment, we considered the selective administration of BMSCs into the liver via the hepatic artery to facilitate more effective liver regeneration. Hence, in this study, we administered cultured autologous BMSCs into the hepatic artery, and compared treatment efficacy and safety between the two administration routes.
Using canines, we established a technique based on abdominal angiography for the administration of BMSCs via the hepatic artery, which is used routinely in medical practice. Canine abdominal blood vessels differ from those of humans in that after an artery branches from the common hepatic artery toward the outer right liver lobe, the gastroduodenal artery branches into the artery leading to the inner right lobe and the artery leading to the left lobe. In a joint study carried out at the Veterinary Surgery Laboratory of Yamaguchi University Joint Faculty of Veterinary Medicine, the abdominal vascular course of 30 beagles was studied using 3-D CT, and in 80% of these animals, the blood vessel to the outer right lobe branched before the gastroduodenal artery [12]. Based on this vascular anatomy, which is different from that of humans, we introduced a catheter into the left hepatic artery, the right medial lobe branch, and the right lateral lobe branch, and administered BMSCs at 2 × 105 cells/kg 1 × 105 cells/kg, and 1 × 105 cells/kg, respectively.
We also assessed the ICG t1/2 to evaluate liver function. Boothe et al. proved the diagnostic benefits of using ICG disposition kinetics as a method to evaluate hepatic function in canines with progressive liver disease [13]. However, in cases with PSS, the results of ICG testing might exaggerate the diminished functional reserve [14, 15]. Since we confirmed the absence of PSS in our experimental model by CE-CT (Fig 7A), the prolonged ICG t1/2 observed in our canine model is a true reflection of liver function.
At 4 weeks after the administration of BMSCs, the ICG t1/2 increased in the Control group but decreased in the Vein and Artery groups. Moreover, whereas the ICG t1/2 increased in the Vein group at 8 weeks post-BMSC administration, the effects of treatment were maintained in the Artery group at 8 and 12 weeks post-administration. Consequently, the effects of BMSCs injected via a peripheral vein in this canine liver fibrosis model were suggested to last for approximately 4 weeks. In contrast, with the administration of BMSCs via the hepatic artery, it is anticipated that these effects can be maintained for at least 12 weeks after administration.
Boothe et al. induced hepatic disease in canines with dimethylnitrosamine, and reported that Alb, T-bil, and prothrombin time were only slightly different between mild and severe hepatic disease groups, and that AT3 activity correlated with hepatic disease severity, similar to that observed in ICG experiments [13]. In our study, AT3 decreased in the Vein group 8 weeks after BMSC administration, similar to results obtained for the ICG experiment, although the decrease in the Artery group was maintained at 8 and 12 weeks post-administration.
Whereas the expression of mRNA involved in hepatic fibrosis tended to be lower in the Vein group than the Control group, it was significantly lower at 4, 8, and 12 weeks in the Artery group, compared to that in the Control group. Kanemoto et al. reported that the expression of these genes correlates well with the histologic degree of fibrosis in canines [16]. Hence, assuming that the reduced expression of these genes in the Artery group is consistent with the degree of fibrosis (as suggested by the results of Kanemoto et al.), it appears that a stronger anti-fibrotic effect occurred in the Artery group than in the Vein group.
The mechanisms underlying BMSC-mediated improvements in fibrosis remain to be clarified. Several studies have suggested that mesenchymal stem cells (MSCs) exert their effects on different aspects of fibrosis to facilitate this improvement. The first of these is the effect of MSCs on reducing liver cell damage. MSCs are believed to prevent liver cell damage by suppressing oxidative stress induced at the time of injury and by downregulating inflammatory cytokines such as TNF-α and IFN-γ [17, 18]. Therefore, they are thought to have an anti-inflammatory effect. At the same time, some reports have suggested that MSCs have immunosuppressive and immunoregulatory effects, and that they inhibit T cell proliferation during liver inflammation and promote regulatory T cell proliferation [19, 20]. It has also been reported that MSCs tend to suppress inflammation through their effects on macrophages and dendritic cells, which indirectly inhibits the action of stellate cells and is involved in the inhibition of fibrosis [21]. In addition, there are many aspects related to the direct effects of MSCs that are unclear, although numerous reports have reported improved fibrosis following the administration of MSCs. Thus, based on these effects on the abatement of liver disease and the suppression of inflammation, it appears likely that MSCs exert a secondary effect by indirectly inducing endogenous mechanisms to improve fibrosis. Further clarification of such mechanisms will be an area for future work.
The optimal route of MSC delivery to the liver is an important concern. Most studies have used an intravenous route, although a large proportion of MSCs that are injected via this route become trapped in the lungs upon first passage [22–25]. Moreover, several studies have revealed that intra-arterial injection can significantly decrease the number of MSCs that are trapped within the lungs, thus increasing their uptake by other organs, especially the liver [26–28]. We, therefore hypothesized that the administration of MSCs via the hepatic artery, rather than via a peripheral vein, would prevent MSCs from becoming trapped in the lungs, ensuring greater accumulation in the liver, and leading to improved liver function and the suppression of liver fibrosis. However, even in our canine liver fibrosis model, the engraftment of administered cells needs to be analyzed and studied in the future. Suk et al. also reported that BMSC administration into the hepatic artery could augment improvements in fibrosis and Child-Pugh scores, an index of liver function, in the group administered cells via both routes compared to those in the single administration group [29].
BMSCs can be cryopreserved once they have been cultured, allowing for frequent administration while reducing burden to the patient; this will likely lead to a more lasting therapeutic effect. Since multiple administrations of cryopreserved autologous BMSCs will likely bring about greater therapeutic efficacy for hepatic fibrosis, it will be important to perform comparative studies to test this in the future.
In conclusion, we confirmed the efficacy and safety of the hepatic artery infusion of cultured autologous BMSCs for the treatment of liver fibrosis using a canine model, therefore providing non-clinical proof-of-concept for this approach.
Supporting information
(Upper table) Fibrosis level (%) is the mean of the levels of Sirius red stained area in liver biopsied section. (Lower table) ΔFibrosis level (%) was calculated using the formula; Δfibrosis level (%) = fibrosis level (%) at 4W, 8W, or 12W − fibrosis level (%) at 0W. Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; SD, the standard deviation of the mean.
(XLSX)
(Upper table) the mean and SD of ICG t1/2 (min). (Lower table) the mean and SD of ΔICG t1/2 (min). ΔICG t1/2 (min) was calculated using the formula; ΔICG t1/2 (min) = ICG t1/2 (min) at 4W, 8W, or 12W –ICG t1/2 (min) at 0W. Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; SD, the standard deviation of the mean.
(XLSX)
Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT, prothrombin time; AT3, antithrombin 3. Data are expressed as mean ± standard deviation (SD). Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; C-1~C-10, each canine in Control group; V-1~V-8, each canine in Vein group, A-1~A-4, each canine in Artery group.
(XLSX)
Acknowledgments
We thank Ms. Mariko Yamada, Ms. Kumie Ota, Ms. Risa Mochizuki, Mr. Yasuhisa Oishi, Ms. Ikuyo Yoshida, Mr. Koushiro Ozono, Mr. Takafumi Kanematsu, Mr. Katsuhiro Kesamaru, Mr. Hironori Morisaki, Ms. Miku Yamashita, Mr. Yutaro Kitaura, Mr. Ko Nakasumi, Mr. Kazuaki Igari, Mr. Shotaro Eto, Mr. Kyosuke Hidari, Mr. Masahiro Imagawa, Mr. Tsunehiro Isozaki, Mr. Kentaro Itoh, Mr. Haruhi Sakai, Ms. Mai Sakai, Mr. Hiroya Inoue, Mr. Fumiya Yamane, Mr. Masato Hiyama, and Ms. Saori Shinzato for their excellent technical assistance.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
The development of this model was supported by the Japanese Highway Program for the Realization of Regenerative Medicine by the Japan Science and Technology Agency (JST) and the Japan Agency for Medical Research and Development (AMED). The Project of Promotion for The Research and Development (R&D) Center on Regenerative Medicine in Yamaguchi Prefecture. Japan Society for the Promotion of Science JSPS KAKENHI Grant Numbers JP17H04162.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141: 1572–1585. 10.1053/j.gastro.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18: 1–21. 10.3350/kjhep.2012.18.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim G, Cho YZ, Baik SK. Assessment for risk of bias in systematic reviews and meta-analyses in the field of hepatology. Gut Liver. 2015;9: 701–706. 10.5009/gnl14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol. 2011;3: 215–218 10.4254/wjh.v3.i8.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho KA, Ju SY, Cho SJ, Jung YJ, Woo SY, Seoh JY, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33: 772–777. 10.1016/j.cellbi.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 6.Hardjo M, Miyazaki M, Sakaguchi M, Masaka T, Ibrahim S, Kataoka K, et al. Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant. 2009;18: 89–99. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, Takami T, Terai S, Sakaida I. Autologous bone marrow cell infusions suppress tumor initiation in hepatocarcinogenic mice with liver cirrhosis. J Gastroenterol Hepatol. 2012;27(Suppl): 104–111. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011;4: 391–417. 10.1177/1756283X11413002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24: 2292–2298. 10.1634/stemcells.2005-0542 [DOI] [PubMed] [Google Scholar]
- 10.Terai S, Sakaida I. Autologous bone marrow cell infusion therapy for liver cirrhosis patients. J Hepatobiliary Pancreat Sci. 2011; 18:23–25. 10.1007/s00534-010-0305-1 [DOI] [PubMed] [Google Scholar]
- 11.Matsuda T, Takami T, Sasaki R, Nishimura T, Aibe Y, Paredes BD, et al. A canine liver fibrosis model to develop a therapy for liver cirrhosis using cultured bone marrow-derived cells. Hepatol Commun. 2017;1: 691–703. 10.1002/hep4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oishi Y, Tani K, Nakazawa H, Itamoto K, Haraguchi T, Taura Y. Anatomical evaluation of hepatic vascular system in healthy beagles using X-ray contrast computed tomography. J Vet Med Sci. 2015;77: 925–929. 10.1292/jvms.14-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boothe DM, Brown SA, Jenkins WL, Green RA, Cullen JM, Corrier DE. Indocyanine green disposition in healthy dogs and dogs with mild, moderate, or severe dimethylnitrosamine-induced hepatic disease. Am J Vet Res. 1992;53: 382–388. [PubMed] [Google Scholar]
- 14.Nanashima A, Yamaguchi H, Shibasaki S, Morino S, Ide N, Takeshita H, et al. Relationship between indocyanine green test and technetium-99m galactosyl serum albumin scintigraphy in patients scheduled for hepatectomy: Clinical evaluation and patient outcome. Hepatol Res. 2004;28: 184–190. 10.1016/j.hepres.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21: 434–439. [PubMed] [Google Scholar]
- 16.Kanemoto H, Ohno K, Sakai M, Takahashi M, Fujino Y, Tsujimoto H. Expression of fibrosis-related genes in canine chronic hepatitis. Vet Pathol. 2011;48: 839–845. 10.1177/0300985810388523 [DOI] [PubMed] [Google Scholar]
- 17.Quintanilha LF, Takami T, Hirose Y, Fujisawa K, Murata Y, Yamamoto N, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res. 2014;44: E206–217. 10.1111/hepr.12204 [DOI] [PubMed] [Google Scholar]
- 18.Nagaishi K, Ataka K, Echizen E, Arimura Y, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic hepatocyte damage in mice by inhibiting infiltration of bone marrow-derived cells. Hepatology. 2014;59: 1816–1829 10.1002/hep.26975 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc. 2009;41: 4352–4356 10.1016/j.transproceed.2009.08.072 [DOI] [PubMed] [Google Scholar]
- 20.Huang B, Cheng X, Wang H, Huang W, la Ga Hu Z, Wang D, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14: 45 10.1186/s12967-016-0792-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ, et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7: e2524 10.1038/cddis.2016.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3: 297 10.3389/fimmu.2012.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18: 683–692. 10.1089/scd.2008.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assis AC, Carvalho JL, Jacoby BA, Ferreira RL, Castanheira P, Diniz SO, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19: 219–230. 10.3727/096368909X479677 [DOI] [PubMed] [Google Scholar]
- 25.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108: 863–868. 10.1161/01.CIR.0000084828.50310.6A [DOI] [PubMed] [Google Scholar]
- 26.Makela T, Takalo R, Arvola O, Haapanen H, Yannopoulous F, Blanco R, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17: 392–402. [DOI] [PubMed] [Google Scholar]
- 27.Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38: 961–967. 10.1016/j.nucmedbio.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 28.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39: 1569–1574 10.1161/STROKEAHA.107.502047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64: 2185–2197 10.1002/hep.28693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Upper table) Fibrosis level (%) is the mean of the levels of Sirius red stained area in liver biopsied section. (Lower table) ΔFibrosis level (%) was calculated using the formula; Δfibrosis level (%) = fibrosis level (%) at 4W, 8W, or 12W − fibrosis level (%) at 0W. Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; SD, the standard deviation of the mean.
(XLSX)
(Upper table) the mean and SD of ICG t1/2 (min). (Lower table) the mean and SD of ΔICG t1/2 (min). ΔICG t1/2 (min) was calculated using the formula; ΔICG t1/2 (min) = ICG t1/2 (min) at 4W, 8W, or 12W –ICG t1/2 (min) at 0W. Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; SD, the standard deviation of the mean.
(XLSX)
Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT, prothrombin time; AT3, antithrombin 3. Data are expressed as mean ± standard deviation (SD). Control group (n = 10, 10, 4, 4), Vein group (n = 8, 8, 4, 4), Artery group (n = 4, 4, 4, 4). W, week; C-1~C-10, each canine in Control group; V-1~V-8, each canine in Vein group, A-1~A-4, each canine in Artery group.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.