Abstract
The development of a sterilizing vaccine against malaria remains one of the highest priorities for global health research. While sporozoite vaccines targeting the pre-erythrocytic stage show great promise, it has not been possible to maintain efficacy long-term, likely due to an inability of these vaccines to maintain effector memory T cell responses in the liver. Vaccines based on human cytomegalovirus (HCMV) might overcome this limitation since vectors based on rhesus CMV (RhCMV), the homologous virus in rhesus macaques (RM), elicit and indefinitely maintain high frequency, non-exhausted effector memory T cells in extralymphoid tissues, including the liver. Moreover, RhCMV strain 68–1 elicits CD8+ T cells broadly recognizing unconventional epitopes exclusively restricted by MHC-II and MHC-E. To evaluate the potential of these unique immune responses to protect against malaria, we expressed four Plasmodium knowlesi (Pk) antigens (CSP, AMA1, SSP2/TRAP, MSP1c) in RhCMV 68–1 or in Rh189-deleted 68–1, which additionally elicits canonical MHC-Ia-restricted CD8+ T cells. Upon inoculation of RM with either of these Pk Ag expressing RhCMV vaccines, we obtained T cell responses to each of the four Pk antigens. Upon challenge with Pk sporozoites we observed a delayed appearance of blood stage parasites in vaccinated RM consistent with a 75–80% reduction of parasite release from the liver. Moreover, the Rh189-deleted RhCMV/Pk vectors elicited sterile protection in one RM. Once in the blood, parasite growth was not affected. In contrast to T cell responses induced by Pk infection, RhCMV vectors maintained sustained T cell responses to all four malaria antigens in the liver post-challenge. The delayed appearance of blood stage parasites is thus likely due to a T cell-mediated inhibition of liver stage parasite development. As such, this vaccine approach can be used to efficiently test new T cell antigens, improve current vaccines targeting the liver stage and complement vaccines targeting erythrocytic antigens.
Introduction
Malaria is a global burden and the development of a vaccine is one of the highest priorities for global health research [1, 2]. Malaria is caused by Plasmodium parasites with P. falciparum (Pf) showing the highest mortality whereas Pk occurs naturally in monkeys in Southeast Asia, with frequent infections of humans [3]. Plasmodium is transmitted by Anopheles mosquitoes, which inoculate sporozoites (Spz) that infect the liver where parasites undergo extensive replication resulting in the release into the bloodstream of thousands of merozoites that infect erythrocytes. Since the pre-erythrocytic (PE) phase involves relatively few parasites and lasts only a few days, PE immunity is limited even in individuals regularly exposed to infection [4]. Instead, infected individuals predominantly develop immunity to erythrocytic parasites. However, antigens expressed on merozoites or erythrocytes display antigenic variation [5]. Thus, naturally acquired immunity to malaria is only partial, highly strain-specific, and short-lived [6]. Thus, a successful vaccine needs to be qualitatively and/or quantitatively different from natural immunity [7].
Sterilizing immunity has been achieved by repeatedly inoculating volunteers with radiation-attenuated Spz (RAS), genetically attenuated Spz (GAP) or chemoprophylaxis with live Spz (CPS) [8, 9]. Indeed, live attenuated Spz are currently in clinical development [10, 11]. However, the large-scale production, shipment, cost and delivery of such a vaccine is challenging. Moreover, large numbers of Spz have to be given intravenously to provide protection and at regular intervals to maintain sufficient immunity over time. Nevertheless, Spz immunizations clearly establish that sterilizing immunity against malaria is possible. The goal for subunit-based vaccines is therefore to match the success of Spz immunization but also to maintain protection over time.
Protection by Spz is predominantly mediated by cellular immunity, particularly interferon (IFN)γ producing CD8+ T cells, characterized as effector memory T cells (TEM) [9, 10, 12–16]. Whereas central memory T cells (TCM) reside in lymphoid organs from where they expand upon antigen exposure, TEM predominantly reside in non-lymphoid tissues, including the liver, enabling them to respond immediately to incoming pathogens [17]. However, all presently used vaccine vectors ultimately elicit TCM and thus are unable to maintain lasting, liver-resident TEM [18]. Since initial pathogen recognition, expansion, effector differentiation and migration of TCM requires at least one week, the short duration of the liver stage likely enables the parasite to escape control by TCM-mediated recall responses [19, 20]. Indeed, the short duration of the liver stage is likely an adaptive trait of the parasite to evade cellular immunity during this vulnerable period. Furthermore, all vaccine strategies currently in development against malaria elicit CD8+ T cells recognizing a limited set of immunodominant, “canonical” MHC-I restricted epitopes within a given antigen resulting in rather focused immune responses that might limit their efficacy [21].
A possible approach to overcome the current limitations of malaria vaccines is the use of CMV as a vector platform. Both HCMV and RhCMV maintain life-long TEM that average 10% of the total circulating memory T cell population [22]. Importantly, HCMV-specific TEM do not show signs of exhaustion: i.e. they retain the ability to produce multiple cytokines [17, 23]. Upon insertion of antigens from Simian Immunodeficiency Virus (SIV), RhCMV elicited and indefinitely maintained high frequency TEM against SIV in RM, including the liver [24, 25]. Importantly, multiple recombinant RhCMV vectors expressing individual SIV antigens and given sequentially or simultaneously provided unprecedented protection against highly pathogenic SIVmac239, with ~50% of vaccinated RM completely controlling and then clearing the SIV infection, the first documented immune-mediated clearance of a lentivirus [26]. RhCMV-based vectors expressing six to nine different antigens derived from Mycobacerium tuberculosis (TB) further demonstrated the best known protection of RM against intrabronchial challenge to which RM are exquisitely sensitive [27]. Thus, RhCMV-based vectors are a versatile vaccine platform that has shown unprecedented protection against both viral and bacterial pathogens in RM.
Unexpectedly, RhCMV-vectors differed from all other vaccine vectors not only in their ability to induce and maintain TEM, but also in their unprecedented capacity to modulate CD8+ T cell priming [28, 29]. RhCMV strain 68–1 lacking the viral tropism factors UL128 and UL130 elicits CD8+ T cells that exclusively recognize peptides in the context of MHC-II or MHC-E. In contrast, UL128/130-intact recombinants exclusively elicit conventional, MHC-Ia-restricted CD8+ T cells. However, these MHC-I restricted CD8+ T cells recognize “non-canonical” epitopes that are sub-dominant in other vector systems since priming of canonical CD8+ T cells is inhibited by the MHC-I targeting viral protein Rh189 (the RhCMV homologue of HCMV US11) [29]. Thus, RhCMV-based vectors can be programmed to elicit CD8+ T cells to non-overlapping peptides with different restriction elements [30].
To evaluate the potential of CMV-based vectors for malaria vaccines in the RhCMV/RM model, we inserted Pk antigens into RhCMV 68–1 in the presence or absence of Rh189. The four antigen panel (PK4) comprises the Pk homologs of circumsporozoite protein (CSP), apical membrane protein-1 (AMA1), sporozoite surface protein-2 (SSP2/TRAP) and the 42kDa C-terminal fragment of the major merozoite surface protein-1 (MSP1c) [31]. The PK4 panel has been previously tested in RM using various vector platforms given as heterologous prime/boost regimen that demonstrated partial, albeit short-lived protection upon Spz challenge [31–34]. We were able to elicit CD4+ and CD8+ T cell responses to each of the four antigens expressed by RhCMV as well as some antibodies to Spz and blood stage parasites. Here we show that immunization with RhCMV/PK4 significantly delayed the appearance of blood stage parasites. We interpret this result as evidence for an inhibition of liver stage development by Pk-specific T cells. Unlike T cell responses elicited by Spz-infection, the T cell immunity elicited by RhCMV-vectors was maintained over time suggesting that CMV-vectored malaria vaccines can improve and complement current vaccine strategies.
Results
Construction of RhCMV/PK4 and ΔRh186-9/PK4
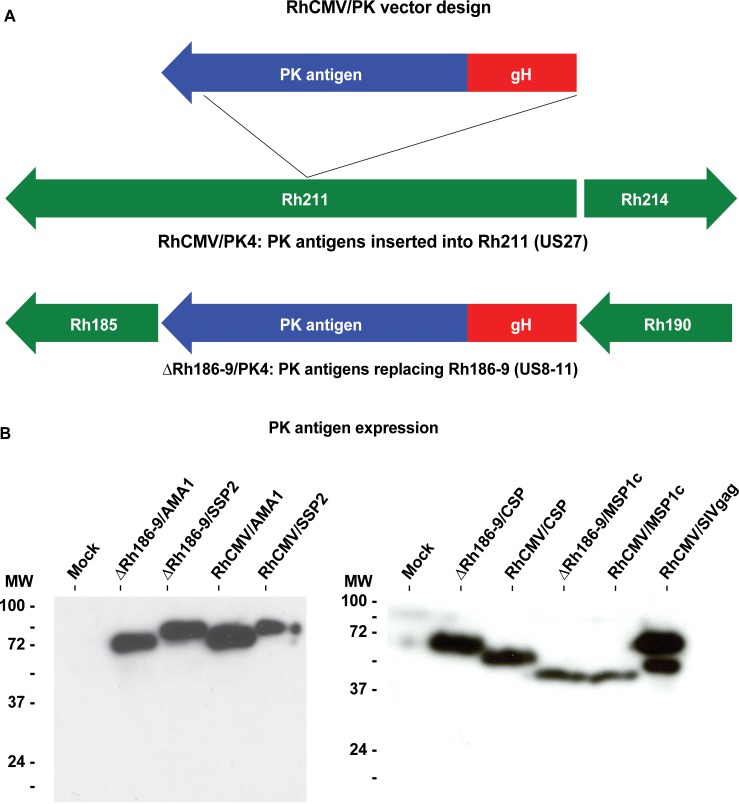
We inserted the four Pk antigens into 68–1 RhCMV and into 68–1 RhCMVΔRh186-189 lacking Rh189 responsible for inhibiting canonical, MHC-I restricted CD8+ T cell priming (deletion of Rh186-188 is irrelevant for T cell modulation [29]). Codon-optimized open reading frames (ORFs) for each of the four antigens were inserted downstream of the HCMV gH promoter [25] with each protein tagged with a FLAG-epitope. We selected the gH promoter since we were unable to recover some of the vectors that expressed the PK antigens under the more commonly used EF1α promoter, presumably since constitutive overexpression of PK antigens is not very well tolerated by infected cells. This is similar to the envelope protein of SIV which also needed to be expressed under the gH promoter to recover recombinant RhCMV [25]. Using bacterial artificial chromosome (BAC) recombineering, each expression cassette was inserted into Rh211 [35] of RhCMV 68–1 to generate the RhCMV/PK4 panel, or the cassette replaced Rh186-189 to generate the ΔRh186-9/PK4 panel (Fig 1A). The constructs were validated by next generation sequencing (S1 Fig). Upon recovery of virus, expression of the antigens was confirmed by immunoblot (Fig 1B). Due to an in-frame internal deletion within the repeat region of CSP in RhCMV/CSP (S2 Fig) the molecular weight of the resulting protein is lower compared to ΔRh186-9/CSP (Fig 1B). Since this truncation is not expected to reduce the number of potential T cell epitopes we did not repair the deletion.
Fig 1. RhCMV vectors expressing Pk antigens.
(A) Schematic of the Pk antigen expression cassettes inserted into the RhCMV genome. Expression cassettes containing the HCMV gH promoter and codon-optimized synthetic Pk genes encoding for the proteins AMA1, CSP, MSP1c (carboxy-terminal 42 kDa fragment), or SSP2 were inserted into the Rh211 gene (US27 in HCMV) to generate the RhCMV/PK4 panel. To generate the ΔRh186-9/PK4 panel, the expression cassettes were used to replace the gene region Rh186-189 encoding the RhCMV homologs of HCMV US8-11. To facilitate detection, the PK4 antigens were fused to the FLAG epitope sequence at their carboxy-terminus. (B) Immunoblots of Pk antigen expression by RhCMV vectors. Lysates of rhesus fibroblasts infected (MOI = 3) for 24 hours with RhCMV/PK4 or ΔRh186-9/PK4, or RhCMV/SIVgag included as control, were separated by SDS-PAGE and immunoblotted using anti-FLAG antibody. The molecular weight of control proteins is indicated. The left and right panels show the uncropped images of immunoblots of two different gels containing cell lysates that were from the same experiment. The exposure time for each blot was adjusted for optimal detection, with the left panel proteins being detectable after shorter exposure than the proteins shown on the right.
Immunogenicity of RhCMV/PK4 and ΔRh186-9/PK4
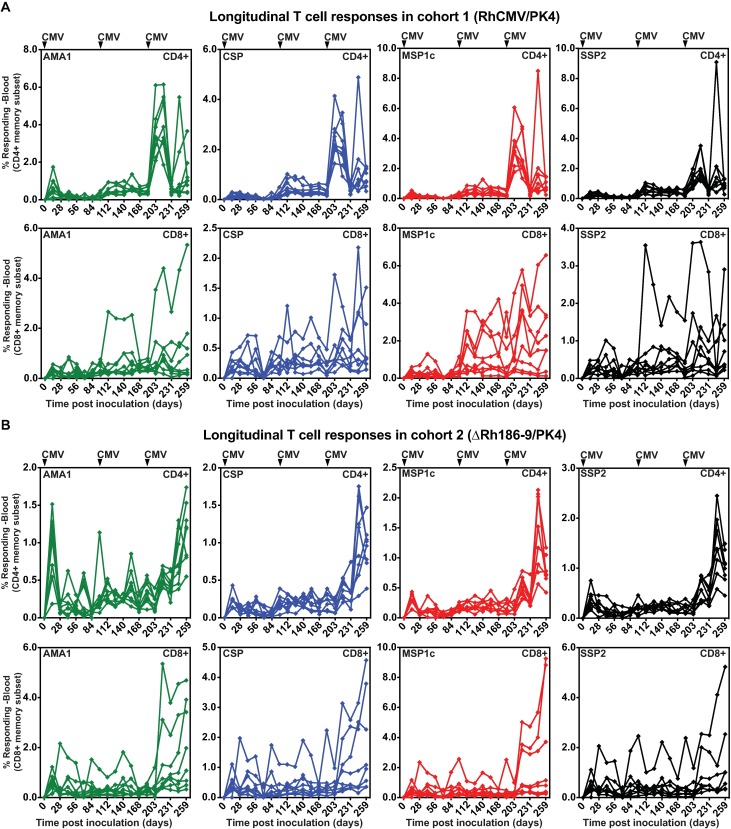
RhCMV-based vectors can be used repeatedly and in animals naturally infected with RhCMV due to CD8+ T cell evasion by Rh182 (US2), Rh183 (US3), Rh185 (US6) and Rh189 (US11). Viruses lacking Rh182-189 cannot infect RhCMV-seropositive RM [36] whereas RhCMV lacking Rh186-189, as used here, superinfect such RM [29]. We inoculated 5x106 plaque forming units (PFU) of each of the four RhCMV/PK4 or ΔRh186-9/PK4 vectors subcutaneously (SC) into RhCMV-seropositive male, juvenile RM of cohort 1 (n = 8) or cohort 2 (n = 8), respectively. T cell responses in peripheral blood mononuclear cells (PBMC) were measured by intracellular cytokine staining (ICS) using pools of 15mer peptides, overlapping by 4 amino-acids and spanning each of the PK4 antigens at biweekly intervals. All RM developed CD4+ and CD8+ T cells to all four Pk antigens (Fig 2).
Fig 2. T cell responses to Pk antigens.
Frequencies of Pk Ag-specific CD4+ and CD8+ T cells in the blood of animals inoculated with 5x106 PFU of each of the four RhCMV/PK4 recombinants (A) or ΔRh186-9/PK4 recombinants (B) at day 0 and with RhCMV/PK4 on days 98 and 189. The percentage of CD4+ or CD8+ T cells (corrected for memory T cells) responding to each of the four Pk antigens were measured by ICS using overlapping peptide pools at the indicated days. T cell responses are shown for each antigen in each individual animal over time.
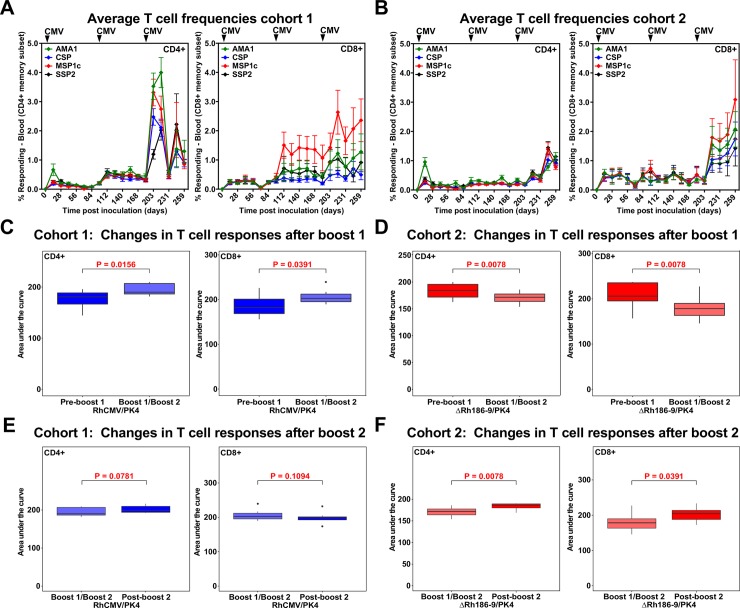
The immune response in both cohorts was boosted at day 98. We only used RhCMV/PK4 vectors for boosting in both cohorts because we observed in other studies that re-inoculation with Rh189-deleted vectors does not boost T cell responses (data not shown). However, while both CD4+ and CD8+ T cell responses were increased by boosting in cohort 1, average responses did not increase in cohort 2, they even decreased (Fig 3). Therefore, we boosted a second time on day 189 which resulted in a significant increase of both CD4+ and CD8+ T cell responses in cohort 2 whereas cohort 1 responses did not increase further (Fig 3). After the 2nd boost, CD8+ T cell responses were similar between the two cohorts (S3 Fig). However, both total and antigen-specific CD4+ T cell frequencies were significantly higher in cohort 1 compared to cohort 2 (S3 Fig). Thus, while all RM displayed robust T cell responses to all antigens after the second boost, it seems that deletion of Rh189 in the first inoculation resulted in reduced induction of CD4+ T cell responses possibly due to viral control by canonical CD8+ T cells. A schematic overview of the prime/boost regimen is shown in S4 Fig.
Fig 3. Impact of boosting on T cell responses elicited by RhCMV/PK4 and ΔRh186-9/PK4.
(A, B) Average frequencies of T cell responses to each of the antigens in cohort 1 (A) or cohort 2 (B) over time. (C-F) Impact of boosting on T cell responses. Statistical analysis of T cell response magnitudes, as determined by measuring the areas under the log10 curve (AUC) of T cell frequencies in each individual RM to all antigens determined by ICS. The boxplots show the median (horizontal line), interquartile range (shaded box), and range (whiskers and outlier points) of the total T cell responses to all antigens. Statistical significance was determined by Wilcoxon test. (C, D) Comparison of the AUC prior to the 1st boost and between boost 1 and 2 within cohort 1 (C) or cohort 2 (D). (E, F) Comparison of the AUC between boost 1 and 2 versus post-boost 2 within cohort 1 (E) or cohort 2 (D) over 91 days between boosts and for 84 days post boost 2.
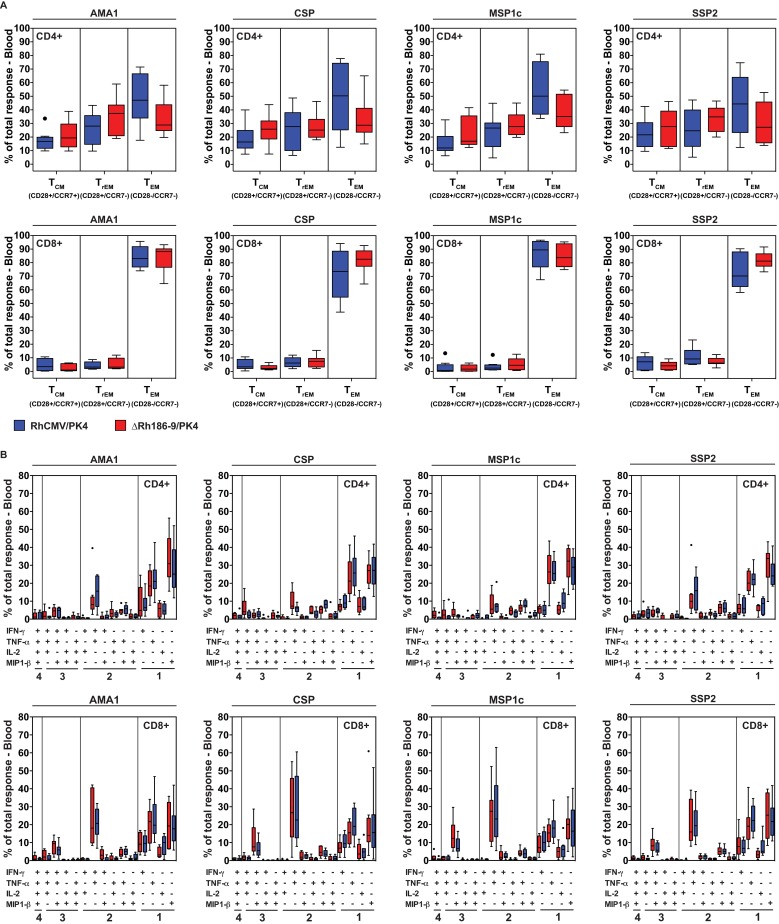
The T cell responses to both HCMV and RhCMV are characterized by their effector memory T cell differentiation and poly-functional phenotype, i.e. the ability to generate multiple cytokines in response to antigen. We therefore analyzed the memory phenotype and individual cytokine production of the T cells elicited to Pk antigens by RhCMV vectors after the 2nd boost. RhCMV vector-elicited PK4-specific CD8+ T cell responses were predominantly effector-differentiated, manifesting an almost exclusive TEM phenotype in both cohorts whereas CD4+ T cell responses displayed a mixed T cell phenotype including central memory, transitional memory and effector memory (Fig 4A). Importantly, a large percentage of malaria antigen-specific CD4+ and CD8+ T cells produced both TNF-α and IFN-γ (with or without MIP-1β), with the remainder generating individual cytokines including IL-2 (Fig 4B). These observations are consistent with previous reports for SIV and TB antigens [25, 27] and highlight the effector functionality of the RhCMV-elicited T cells.
Fig 4. Memory phenotype and cytokine production by Pk-antigen specific T cells elicited by RhCMV.
(A) Boxplots comparing the memory differentiation of the RhCMV/PK4 or ΔRh186-9/PK4-elicited CD4+ and CD8+ memory T cells in peripheral blood responding to each Pk antigen with TNF-α and/or IFN-γ production after the 2nd boost (day 259). Memory differentiation state was based on CD28 vs. CCR7 expression, delineating central memory (TCM), transitional effector memory (TTREM), and effector memory (TEM), as designated. (B) Boxplots comparing the frequency of vaccine-elicited CD4+ and CD8+ memory T cells in peripheral blood responding to each of the Pk antigens with production of TNF-α, IFN-γ, IL-2 or MIP-1β, alone and in all combinations.
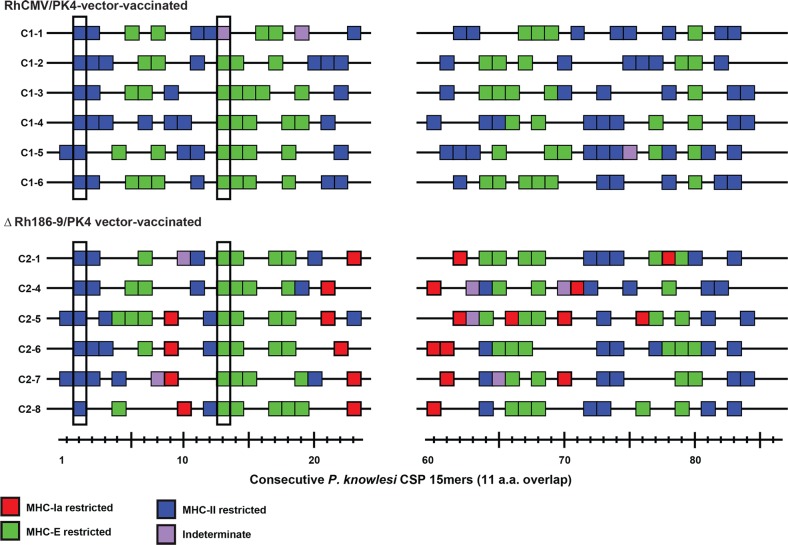
We previously demonstrated that strain 68–1 RhCMV vectors elicit unconventional CD8+ T cell responses that are restricted by MHC-II and MHC-E to SIV and TB antigens [27, 28]. We further showed that 68–1 RhCMV/SIV vectors deleted for Rh186-9 elicit additional CD8+ T cells that target “canonical” MHC-Ia restricted epitopes, i.e. epitopes known to be immunodominant in SIV-infected animals, or animals immunized with conventional vector systems [29]. Therefore, it is expected that 68-1-derived RhCMV/PK4 will elicit MHC-II and MHC-E-restricted CD8+ T cells whereas ΔRh186-9/PK4 will additionally elicit MHC-Ia restricted CD8+ T cells. To verify that this is indeed the case we measured the CSP-specific T cell responses of six monkeys in each cohort at the individual peptide level and then determined their MHC-restriction using blocking antibodies (W6/32 for MHC-I) or blocking peptides (invariant chain-derived CLIP for MHC-II and peptide VL9 for MHC-E). We used only the non-repeat regions for this epitope mapping since repeat regions contain the same T cell epitope multiple times. As shown in Fig 5, stimulation by CSP peptides of CD8+ T cells from RhCMV/PK4 immunized, cohort 1 animals was either blocked by CLIP or VL9 peptides indicative of MHC-II or MHC-E restriction, respectively. In contrast, stimulation by several CSP peptides recognized by cohort 2 CD8+ T cells was not blocked by either peptide, but was inhibited in the presence of pan-MHC-I antibody W6/32 consistent with classical MHC-I presentation. In addition, we identified two “supertopes”‘, i.e. peptides recognized in each RM, one for each MHC-II and MHC-E (Fig 5). As described previously, the ability to present the same peptide regardless of the MHC allotype of each animal is explained by the high conservation of MHC-E and by promiscuous binding to MHC-II [28]. Since these observations are consistent with our previous results obtained with SIV antigens we conclude that the CD8+ T cell response to all four PK antigens was restricted by MHC-II and MHC-E in cohort 1 and by MHC-I, MHC-II and MHC-E in cohort 2.
Fig 5. MHC restriction analysis of RhCMV/CSP and ΔRh186-9/CSP-elicited CD8+ T cell responses.
CSP-specific CD8+ T cells were epitope-mapped in six animals of each cohort 1 (upper panel) and 2 (lower panel) using flow cytometric ICS to detect recognition of each consecutive, overlapping 15mer peptide comprising the indicated amino-terminal and carboxy-terminal region of Pk CSP. Peptides resulting in specific CD8+ T cell responses are indicated by a box, with the color of the box designating MHC restriction as determined by blocking with the anti-pan-MHC-I mAb W6/32, the MHC-E blocking peptide VL9 and the MHC-II blocking peptide CLIP as previously described [28, 29]. Highlighted are peptides recognized by T cells in every animal.
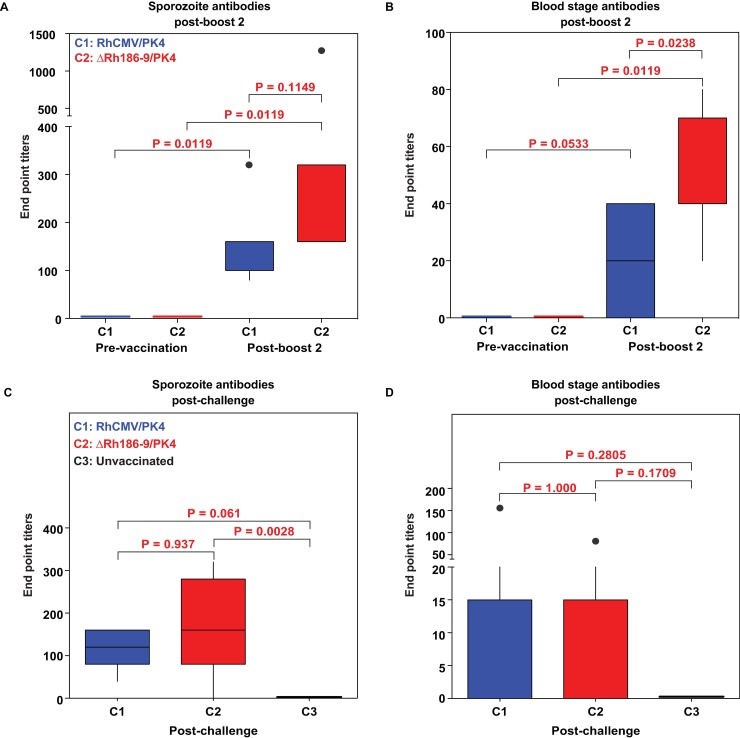
To examine whether the two boost regimen elicited antibody responses to Spz or infected erythrocytes, we used immunofluorescence assays (IFA) of whole parasites to measure antibody titers [37]. In sera collected after the 2nd boost (day 203) we observed modest, but significant, antibody titers to Spz in all immunized animals compared to pre-vaccine sera (Fig 6A). Interestingly, the Spz-staining pattern was typical for AMA1 and SSP2/TRAP that localize to intracellular compartments, but lacked typical CSP-staining (data not shown). Antibody responses to blood stage parasites were generally lower than those to Spz (Fig 6B) consistent with the fact that only two antigens are expressed in the blood stage (AMA1 and MSP1) whereas three are expressed in Spz (CSP, SSP2, AMA1). Interestingly, cohort 2 animals had higher titers to blood stage parasites than cohort 1 animals, 3 of which were antibody negative, suggesting that deletion of Rh189 has opposite effects on CD4+ T cells and antibody responses.
Fig 6. Antibody responses to P. knowlesi sporozoites and blood stage parasites.
(A, B) Endpoint titers of IgG antibodies in serum collected from cohort 1 (RhCMV/PK4) or cohort 2 (ΔRh186-9/PK4) prior to immunization on day 0 or on day 203 (post-2nd boost). Pk parasite stage-specific antibodies were measured by IFA to Pk Spz (A) and to Pk-infected RBC (B). (C, D) Endpoint titers to Spz (C) and blood stage parasites (D) determined by IFA at day 14 post-challenge for each animal in the indicated cohorts. Statistical significance for differences in Spz or blood stage antibody titers measured by IFA was determined using the Wilcoxon test. Unadjusted Wilcoxon test p-values comparing IFA results across groups are displayed as boxplots showing the median (horizontal line), interquartile range (box), and range (whiskers and outlier points).
Sporozoite challenge
Approximately one month prior to challenge, we assigned an age and sex-matched group of RhCMV-seropositive RM (cohort 3, n = 8) (S4 Fig). All cohorts were challenged on the same day with 100 Pk Spz freshly isolated from Anopheles dirus mosquitoes that had been fed on Pk-infected RM two weeks earlier[38]. Protection was assessed by daily blood examination starting 6 days after Spz challenge using Giemsa-stained thin blood smears made from ear-prick blood. RM with parasitemias exceeding 2% were treated with chloroquine and artesunate.
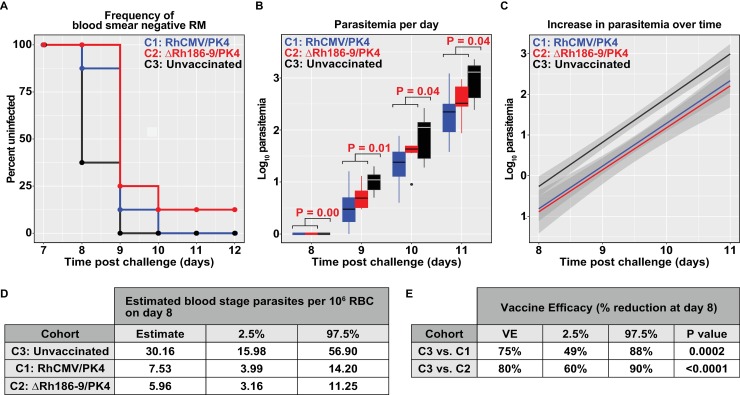
5/8 (62.5%) of control RM were positive for blood stage parasites on day 8 after challenge whereas 15/16 (93.75%) of vaccinated RM remained blood-stage free until day 9 with 3/16 (18.75%) being blood-stage free until day 10 (Fig 7A, S5 Fig). One of the RM in cohort 2 remained parasite-free throughout. 75% of control RM, but only 37.5% of cohort 1 and 2 animals, needed to be treated on day 11. On each of the days 8–11 the mean parasitemia was significantly lower in immunized RM compared to control animals (Fig 7B). There was no significant difference between the two vaccine groups although its noteworthy that the only sterilely protected RM was in cohort 2. Consistent with TEM being ineffective against blood-stage parasites and the lack of substantial antibodies to the blood stage, the increase in parasitemia over time (as indicated by the slope of the curve) was not reduced in vaccinated RM (Fig 7C). Using the parallel increases in parasitemia observed in control and vaccine groups we were able to estimate the average blood stage parasite burden on day 8, the first day parasite were detectable in blood. Control RM were predicted to have 30 parasites per 106 red blood cells (RBC), whereas RhCMV/PK4 and ΔRh186-9/PK4 immunized RM were predicted to have 7.5 and 6 parasites per 106 RBC, respectively (Fig 7D). This result corresponds to a 75 and 80% reduction in parasite load in the blood (Fig 7E). The most likely explanation for this significant reduction of blood stage parasites is that RhCMV-vector-induced immune responses reduced the liver stage parasite burden, presumably by partially eliminating infected hepatocytes or by inhibiting parasite development in the liver, thus resulting in reduced or delayed release of merozoites. However, once released into the blood stream, the RhCMV-based immune responses were unable to slow parasite growth.
Fig 7. Blood stage parasitemia upon Spz challenge.
Each cohort was challenged with 100 Spz at day 0 and blood stage parasitemia was monitored daily by Giemsa stained thin blood smears starting at day 6 post-challenge. (A) Percent of animals without detectable parasitemia at the indicated days post-challenge. The percentage of uninfected RM is shown for each cohort: C1 = animals immunized with the RhCMV/PK4 vector panel, C2 = animals immunized with the ΔRh186-9/PK4 panel, C3 = non-immunized animals. (B) Boxplots of log10 parasitemia per 20,000 RBC at the indicated days show the median (horizontal line), interquartile range (shaded box), and range (whiskers and outlier points) among RM with detectable parasitemia, by day. Statistical significance as determined by unadjusted Wilcoxon test of C1 and C2 versus group C3 is shown above each plot. (C) Linear model fit to the log10-transformed parasitemia values with shaded bands indicating pointwise 95% confidence interval. The parasitemia data to days 8–11 fit a linear regression model and the intercepts for each of the vaccine groups differ significantly (P<0.0001, ANOVA F test, see Methods) from the control group whereas the slopes did not differ (P = 0.9262). This corresponds to an impact on the number of parasites present on day 8. (D) Estimated mean day 8 parasitemia by group and 95% confidence interval are shown in units of 106 RBC. These are estimated from the coefficients of the group terms in a simple linear model relating parasitemia over days 8–11 post-challenge to day and group, as described in Methods. (E) Estimated percent reduction in mean day 8 parasitemia by treated group when compared to control RM, with 95% confidence intervals. These are estimated from the coefficients of the group terms in a simple linear model relating parasitemia over days 8–11 post-challenge to day and group, as described in Methods.
Post-challenge analysis
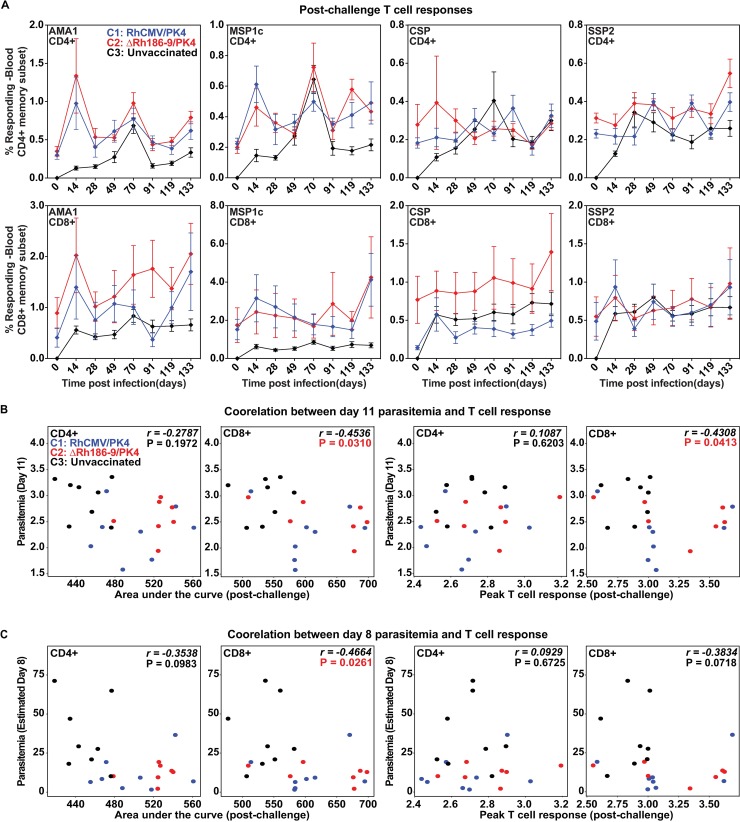
All RM in the control group developed de novo T cell responses to all four antigens beginning at day 14 post-challenge (Fig 8A). However, there was no difference in the AUC prior to challenge and post-challenge in each vaccine group and post-challenge T cell responses did not display a robust increase after challenge (Fig 8A). This observation is consistent with the fact that RhCMV-induced TEM do not mount an anamnestic response to antigen [17]. Moreover, epitopes recognized by CD8+ T cells induced by strain 68–1 are not expected to overlap with epitopes recognized by CD8+ T cells induced by Pk, given their unconventional MHC restriction (Fig 5). While there might be some overlap between T cells elicited by Spz challenge and canonical, MHC-I restricted CD8+ T cells elicited by Rh189-deleted vectors, only few epitopes would be expected to be shared which is unlikely to affect global T cell response measurements.
Fig 8. Immune responses in response to parasite challenge.
Animals in cohorts 1 and 2 were challenged with 100 PK Spz on day 273 after the first inoculation with RhCMV vectors. Animals in cohort 3 were challenged on the same day. AA) Average CD8+ and CD4+ T cell response frequencies (+/- SD) for each of the four antigens measured in the PBMC of the indicated cohorts by ICS at the indicated time points post-challenge. (B) Post-challenge CD8+ T cell levels correlate with reduced viremia. Scatterplots show association between post-challenge area under the ICS response measurement curve (AUC, left) or the Peak T cell response (right) versus observed Day 11 parasitemia (B) or estimated Day 8 parasitemia (C). Spearman rho (r) values and corresponding p-values shown on each panel indicate significant inverse correlations between CD8+ T cell responses and parasitemia outcome.
We also examined whether Pk-infection boosted antibody responses to Spz or infected erythrocytes using IFA to measure antibody levels at day 14 post-boost. However, we did not observe an increase in antibody responses elicited by RhCMV-vectors (Fig 6C and 6D) compared to pre-challenge levels (Fig 6A). In fact, the vaccine-induced antibody levels were lower after challenge. One possible explanation is that the antibodies elicited by the vaccine target different epitopes than those elicited by infection. However, Spz-challenge did not elicit a measurable de novo antibody response to whole parasites in any of the control RM (Fig 6C and 6D). Thus, the transient infection that occurred in all but one RM did not seem to be sufficiently immunogenic to elicit Pk-specific antibodies or boost vaccine-induced antibodies.
In the vaccinated RM that developed blood stage parasitemia there was heterogeneity with respect to levels of parasitemia or day of treatment (S5 Fig). Therefore, we examined whether pre-challenge T cell response magnitudes in cohorts 1 and 2 would correlate with the level of parasitemia measured on day 11 or the estimated parasitemia on day 8 (the sterile animal was not included in this analysis). However, neither AUC nor peak levels of CD8+ or CD4+ T cell frequencies post-2nd boost or across the entire vaccination period displayed a significant correlation with parasitemia upon Spz challenge. In contrast, there was a significant inverse correlation between the magnitude of the post-challenge CD8+ T cell responses, either AUC or peak of ICS results, and parasitemia on days 8 and 11 (Fig 8B). In contrast, CD4+ T cell responses did not show such a correlation. Since the observed correlation is largely driven by the lower T cell response frequencies and increased parasitemia in the control cohort compared to the vaccine cohorts, it may simply reflect the fact that the vaccinated animals (all of whom had pre-challenge Pk-specific CD8+ T cell responses) were partially protected. However, it is interesting that this correlation was only observed for CD8+ T cells, but not for CD4+ T cells although both T cell sub-populations were strongly induced by vaccination. This result is thus consistent with an important role of CD8+ T cells in delaying blood stage parasitemia.
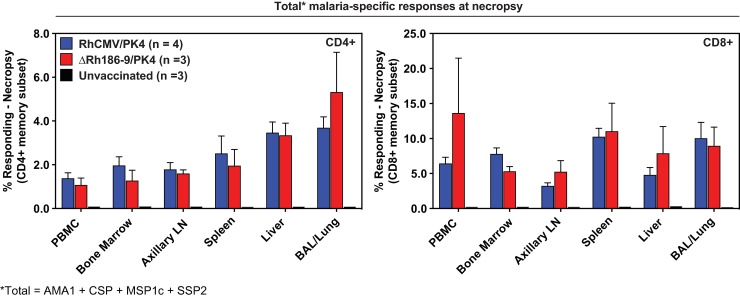
RhCMV-elicited T cell responses are generally maintained in extralymphoid tissues for the life of immunized animals. We therefore determined the tissue distribution of PK4-specific T cells in several RhCMV/PK4 and ΔRh186-9/PK4-immunized animals as well as control animals more than one year post-challenge (S4 Fig). Four animals from cohort 1 and three animals from cohorts 2 and 3 were necropsied and T cells were isolated from multiple tissues and analyzed by ICS for each individual antigen (Fig 9 and S6 Fig). As we have observed in the past for SIV antigens [24], average CD4+ and CD8+ T cell frequencies ranged from 0.5 to 5% of memory T cells in each tissue with significant T cell responses in the liver against each of the four antigens. Importantly, T cell responses to any of the antigens were undetectable in any tissue of the control animals (Fig 9), despite the fact that all control animals had PK4-specific T cells in the blood immediately after Spz challenge (Fig 8). Thus, T cell responses elicited by the transient Pk-infection occurring in control animals seemed short-lived. The robust T cell responses measured in the vaccinated cohorts more than one year after challenge are thus due to continued stimulation by RhCMV vectors and not due to the transient Pk-infection that occurred during challenge. These results further support the notion that CMV-vectored malaria vaccines are unique in their ability to maintain high frequency effector memory T cells in the liver.
Fig 9. Post-challenge analysis of combined PK4-specific CD4+ and CD8+ T cell responses in individual tissues.
Flow cytometric ICS results of peripheral blood and tissue CD94+ and CD8+ T cell responses to the peptide mixes comprising each of the four PK antigens in 4 animals of cohort 1 (RhCMV/PK4), three animals of cohort 2 (ΔRh186-9/PK4) and three animals of control cohort 3. The sum of average response frequencies (+SEM), corrected for memory T cells, is shown for the indicated tissues. T cell response frequencies in the control cohort were below the detection limit.
Discussion
Our results suggest a significant T cell-mediated control of PE, but not blood stage, parasites by RhCMV-vectored malaria vaccines consistent with the pattern of control expected for a TEM-dominated immune response. Although we detected antibody responses after 2 boosts, these were modest and particularly low towards blood stage parasites. Similarly, antibodies to SIV and TB antigens were absent or barely detectable in previous RhCMV-vector studies [25] [27].Since infected erythrocytes do not present antigen and cannot be targeted by T cells, it is not unexpected that RhCMV-mediated immunity was unable to control erythrocytic parasites. The fact that blood stage parasites replicated with similar kinetics in vaccinated and control animals suggests strongly that anti-blood stage antibodies did not impact parasitemia. In contrast, in several previous heterologous prime/boost studies using the PK4 panel, parasites were cleared from the blood in some immunized monkeys, even after reaching significant parasitemia possibly due to the fact that heterologous prime/boost vaccines elicit both T cells and antibodies [32–34]. However, protection by heterologous prime/boost PK4 vaccines was short-lived since protection was lost after 6 months [32]. Although we did not re-challenge the RM in our study, we would expect that the observed delay of blood stage parasitemia will be maintained for life since RhCMV-elicited TEM frequencies do not decrease over time [24]. Indeed, robust blood and tissue T cell frequencies were measured more than one year after challenge in vaccinated animals whereas the T cell response in control animals that were infected during challenge was no longer detectable. Thus, the mechanism of protection by Spz or heterologous prime/boost vaccination and RhCMV-vaccination is likely quite different, as reported in the SIV challenge models [24].
We calculated that between 75–80% fewer parasites were released into the bloodstream in vaccinated as compared to unimmunized animals and one of the immunized animals did not develop parasitemia. This relatively low frequency of sterile protection is similar to most previous Pk challenge studies in RM vaccinated with all or some of the PK4 antigens [31–34]. Only one study reported sterile, albeit short-lived, protection of more than one or two animals per cohort, but this experiment has not yet been repeated [32]. Partial protection was also reported for RAS immunization which protected 5 out of 9 animals upon Spz challenge [39] whereas CPS immunization of 8 RM resulted in sterile protection of 2 RM and delayed parasitemia in 2 RM upon challenge by mosquito bite [40]. In contrast, >90% sterile protection is routinely observed in humans vaccinated with RAS [41–45] or CPS [9, 13, 46, 47] and challenged with P. falciparum Spz. Thus, Pk challenge of RM seems to be more stringent than Pf challenge of humans. Conceivably, HCMV-based vectors expressing Pf antigens might provide better protection in human challenge studies than in the RhCMV/Pk model. Live-attenuated HCMV-based vaccines are currently in development for HIV and TB, and similar approaches could be used for Pf.
Protection by attenuated Spz is mediated by CD8+ T cells in RM [39] as well as in humans [9, 10]. It is also likely that the delay of blood stage parasitemia by the RhCMV-based malaria vaccines described here was mediated by CD8+ T cells since post-challenge CD8+ T cell responses, but not CD4+ T cells, correlated with decreased parasitemia. Moreover, the increased frequency of CD4+ T cells observed in cohort 1 compared to cohort 2 did not result in increased protection. In fact, the only sterile animal was observed in cohort 2. Thus, our results are consistent with a CD8+-mediated immune mechanism of protection.
We recently described that RhCMV strain 68-1-derived vectors elicit unconventional CD8+ T cell responses exclusively restricted by MHC-II and MHC-E instead of MHC-I [29, 48]. These unconventional CD8+ T cells are an intrinsic feature of the 68–1 strain resulting in MHC-II and MHC-E-restricted responses to heterologous antigens inserted into RhCMV, as previously shown for SIV and TB, as well as to RhCMV proteins [27–29]. We confirmed this unconventional MHC-restriction for malaria antigens by mapping the CSP-specific CD8+ T cells response to individual peptides and demonstrated their MHC-restriction profile with blocking reagents. Consistent with our previous observations, we observed that RhCMV/PK4 elicited CD8+ T cells were exclusively restricted by MHC-II and MHC-E. Thus, the specificities of the CD8+ T cells elicited by the vectors used in cohort 1 are unlike those elicited by Spz-immunization or by other vaccine strategies. The additional deletion of Rh189 in cohort 2 vectors further added MHC-I restricted CD8+ T cells consistent with our previous observation that 68–1ΔRh189 vectors elicit a combination of MHC-I, MHC-II and MHC-E responses to SIV antigens [29, 48]. However, the total T cell response frequency, particularly CD8+ T cells, elicited by ΔRh186-9/PK4 was rather low and required two boosts with RhCMV/PK4 which will only boost unconventional CD8+ T cells. Moreover, the similar delay in parasitemia observed in RM in cohort 1 and cohort 2 suggests that the unconventionally restricted CD8+ T cells are largely, if not completely, responsible for any CD8+ T cell-mediated efficacy. Nevertheless, it is noteworthy that the only sterilely protected RM was in cohort 2, suggesting the possibility that including MHC-I-restricted CD8+ T cells might enhance efficacy in some RM. However, it should be noted that deletion of Rh189 only provides a selected subset of MHC-I epitopes that were termed “canonical” since they are immunodominant in non-CMV contexts [29]. This is reflected by the fact that, on average, only about half the number of peptides was restricted by MHC-I compared to MHC-II or MHC-E in cohort 2 animals. In contrast, wildtype-like vectors in which the RhCMV homologs of UL128 and UL130 were “repaired” elicit much broader CD8+ T cell responses to “non-canonical” MHC-I epitopes that are subdominant in other vector systems [29]. It will thus be interesting to include such repaired vectors expressing Pk antigens in future studies. Since very little is known about the ability of hepatocytes to present malaria antigens in the context of different MHC molecules, immunization with different vector backbones will reveal protective CD8+ T cell sub-populations targeting either conventional or unconventional epitopes.
The four antigens selected in this study are the Pk homologs of Pf antigens that have been extensively studied in various clinical trials using a variety of vaccine platforms. CSP is the antigen in the most advanced malaria vaccine, RTS,S, which partially protects young children from disease [49, 50] and SSP2/TRAP has shown great promise in a number of human challenge studies [19, 48, 51, 52]. Similar to our findings, the blood stage antigens MSP1 and AMA1 expressed by viral vectors did not reduce blood stage parasite growth rates but delayed time to diagnosis suggesting T cell mediated control of the PE stage [48]. Unfortunately, protection by subunit vaccines has not yet achieved the level of protection obtained with attenuated Spz in human challenge studies [53] possibly because the parasite antigens targeted by protective CD8+ T cells elicited by Spz-vaccination are not known. However, various strategies are currently being employed to identify such antigens [54, 55] and these approaches are likely to yield novel vaccine candidates that could be tested in the RhCMV/PK model. A unique aspect of RhCMV-vectors is that multiple vectors can be combined to elicit immune responses to several antigens without interference. In previous work we co-inoculated five vectors expressing five different SIV antigens [24] or four vectors expressing nine different M. tuberculosis antigens [27]. In each case, we were able to elicit T cell responses to each of the antigens without observing any interference from the others. In contrast, the immune responses to MSP1 dominated over AMA1 and SSP2/TRAP in humans inoculated with pox- and adenoviral vectors [48]. Thus, the RhCMV/PK system might be an ideal platform to rapidly test large numbers of potential vaccine candidates for protection.
The tissue distribution of T cells is an inherent feature of RhCMV-vectored vaccines that is irrespective of the heterologous antigen used or the route of inoculation. As observed in previous studies with SIV antigens [24], CD8+ T cell responses to each of the four PK antigens were particularly elevated in the liver, a result that is consistent with liver-localized CD8+ T cells controlling parasites in the liver. The unique ability of RhCMV vectors to elicit and indefinitely maintain a robust and sustained T cell response to malaria antigens in the liver renders vectors based on HCMV attractive for further development of vaccines targeting the liver stage either by themselves or in combination with traditional vaccines or vaccine vectors. Since the vast majority of individuals living in malaria-endemic regions are naturally infected with HCMV, any malaria vaccine must already be immunogenic in the context of a HCMV-immune host environment. Therefore, HCMV-vectored malaria vaccines can be combined with any other malaria vaccine to either broaden the T cell responses and epitope targeting to a given antigen, or to include a potent T cell component to an antibody-inducing vaccine, or additional antigens into existing vaccines.
Conclusion
This is the first report demonstrating control of malaria by unconventional TEM-inducing RhCMV vectors. The delay in the appearance of blood stage parasites suggests that these T cells significantly controlled the liver stage of the parasite. As such, this study established proof of principle for this novel approach for malaria vaccine development. There are multiple possibilities to further improve protection including, but not limited to, programming different CD8+ T cells using genetically distinct HCMV vectors, screening multiple novel antigens, and combining HCMV vectors with other vaccine strategies.
Materials and methods
Construction of recombinant RhCMV
FLAG-epitope tagged, codon-optimized Pk genes were synthesized by GeneArt based on Pk strain H: AMA1 (XP_002259339), CSP (XP_002259002), the C-terminal 328 AA of MSP1 (XP_002258582) and SSP2/TRAP (XP_002259987). Using the RhCMV 68–1 BAC [56] we generated the four vector panel RhCMV/PK4 by inserting expression cassettes of ORFs under control of the HCMV gH promoter into Rh211 using homologous recombination [25]. The ΔRh186-9/PK4 panel was generated by replacing the genomic region NT196625-NT199855 with the gH/PK expression cassette in the 68–1 BAC [29]. All BACs were analyzed by restriction digest and by NGS on an Illumina MiSeq sequencer to confirm genomic integrity. BACs were electroporated into rhesus fibroblasts to reconstitute virus. Pk antigen expression was confirmed by immunoblot of infected cell lysates and vaccine stocks were generated by the OHSU Molecular Virology Support Core.
Ethics statement
Purpose-bred adult rhesus macaques (Macaca mulatta) of Indian origin were used either at the Walter Reed Army Institute of Research/Naval Medical Research Center (WRAIR/NMRC) or the Oregon National Primate Research Center (ONPRC). Both facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The experiments were conducted in compliance with the Animal Welfare Act in accordance with the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council and approved by the respective Institutional Animal Care and Use Committees (IACUC) that adhere to national guidelines established in the Animal Welfare Act (7 U.S.C. Sections 2131–2159) and the Guide for the Care and Use of Laboratory Animals (8th Edition) as mandated by the U.S. Public Health Service Policy. The animal study protocols were reviewed and approved either by the IACUC of the WRAIR/NMRC or ONPRC in compliance with all applicable federal regulations governing the protection of animals and research under NMRC protocol 14-IDD-24LS (Production of malaria parasites using rhesus monkeys) or ONPRC protocol IS00002413–0963 (An Effector Memory T Cell-Inducing Subunit Vaccine against Malaria). In this study the major risk to the animals was from the malaria infection. Harm from malaria infection was minimized by treating with anti-malarial drugs at a parasitemia level low enough to prevent serious illness.
Rhesus macaques
For challenge experiments, Pk Spz were generated at the WRAIR/NMRC in two purpose-bred, male RM of Indian origin from the WRAIR breeding colony at Covance, Texas. RM were splenectomized prior to infection with blood-stage parasites and feeding of mosquitoes. For vaccine studies, 24 purpose-bred, pedigreed, male RM were used at ONPRC. At assignment, these RM were positive for RhCMV but free of Macacine herpesvirus 1, D-type simian retrovirus, simian T-lymphotrophic virus type 1, simian immunodeficiency virus, and TB. The RM were housed in Animal Biosafety Level-2 rooms with insect control. Animals were fed commercial monkey chow (Purina) and fresh fruit and vegetables provided daily; water was provided at all times via automatic watering system. All non-human primates were provided with environmental enrichment in the form of manipulanda (such as toys, wood stick, or mirror, etc.). Additionally, the enrichment program uses toys and food inside and outside the cage to promote species-specific behavior, such as a foraging boards and visualization of conspecifics as well as Music and TV on a rotational basis. Additional environmental enrichment included cage perches, daily interactions with animal care staff, daily treats and fresh fruits and vegetables as a food enrichment.
RM were sedated with ketamine HCl or Telazol for subcutaneous vaccine administration or intravenous Spz administration. All animals with malaria infections were closely monitored and treated with anti-malarial drugs, and adjunctive therapy as needed.
Malaria parasites
Plasmodium knowlesi H strain parasites were derived from stocks at the NIH.
Mosquitoes
Anopheles dirus subspecies A were provided by the Laboratory of Malaria and Vector Research, NIAID, NIH.
Immunization
RM of cohorts 1 (n = 8) and 2 (n = 8) were immunized by subcutaneous administration of 5x106 PFU of each RhCMV/PK4 or ΔRh186-9/PK4 vector, respectively, on day 0 by inoculating each of the four vectors in a separate site (right arm, left arm, right leg, left leg). RhCMV/PK4 vectors were administered twice again to both cohorts on days 98 and 190.
T cell assays
PK-specific CD4+ and CD8+ T cell responses were measured in PBMC by ICS [24, 25, 36]. Briefly, PBMC were incubated with consecutive 15mer peptide mixes (11 amino acid overlap) comprising the Pk proteins and the co-stimulatory molecules CD28 and CD49d (BD Biosciences) for 1h, followed by addition of Brefeldin A (Sigma-Aldrich) for an additional 8hrs. Co-stimulation without peptides served as background control. As previously described [28, 29], the MHC restriction (MHC-Ia, MHC-E, MHC-II) of a peptide-specific response was determined by pre-incubating isolated mononuclear cells for 1 hr at room temperature (prior to adding peptides and incubating per the standard ICS assay) with the following blockers: 1) the pan anti-MHC-I mAb W6/32 (10mg/ml), 2) the MHC-II-blocking CLIP peptide (MHC-II-associated invariant chain, amino acids 89–100; 20μM), and 3) the MHC-E-blocking VL9 peptide (VMAPRTLLL; 20μM). Blocking reagents were not washed, but remained throughout the assay. Stimulated cells were fixed, permeabilized and stained [24, 25, 36] using combinations of the following fluorochrome-conjugated mAbs: SP34-2 (CD3; Pacific Blue, Alexa700), L200 (CD4; AmCyan, BV510), SK-1 (CD8; PerCP-Cy5.5), MAB11 (TNFα; FITC, PE), B27 (IFNγ; APC), FN50 (CD69; PE-TexasRed), B56 (Ki-67; FITC), and in polycytokine analyses, JES6-5H4 (IL2; PE Cy-7). Data was collected on an LSR-II (BD Biosciences). Analysis was performed using FlowJo software (Tree Star). Lymphocytes were gated for CD3+ and progressive gating on CD4+ and CD8+ T cell subsets. Antigen-responding cells in both CD4+ and CD8+ T cell populations were determined by their intracellular expression of CD69 and one or more cytokines. After subtracting background, the raw response frequencies were memory corrected[24, 25, 36] using combinations of the following mAbs to define the memory vs. naïve subsets: SP34-2 (CD3; Alexa700, PerCP-Cy5.5), L200 (CD4; AmCyan), SK-1 (CD8; APC, PerCP-cy-5.5), MAB11 (TNFα; FITC), B27 (IFNγ; APC), FN50 (CD69; PE), CD28.2 (CD28; PE-TexasRed), DX2 (CD95; PE), 15053 (CCR7; Pacific Blue), and B56 (Ki-67; FITC). For memory phenotype and polycytokine analysis of Pk antigen-specific T cells, all cells expressing CD69 plus one or more cytokines were first Boolean gated, and then this overall Ag-responding population was subdivided into the subsets of interest on the basis of surface phenotype or cytokine production pattern [24, 25, 36].
Prior to necropsy, RM were euthanized a with sodium pentobarbital overdose (>50 mg/kg) and exsanguinated via the distal aorta. Mononuclear cell preparations were obtained from blood, bone marrow, lymph nodes, spleen, liver, intestinal mucosa, colon and broncho-alveolar lavage (BAL) as previously described [25].
Immunofluorescence assay (IFA)
RM sera were tested by IFA for reactivity to Spz and asexual erythrocytic stages of Pk as previously described [37]. To determine IgG titers erial dilutions of the sera were incubated for one hour at 37°C, washed and developed with FITC-labeled goat anti-human IgG for 30 minutes at 37°C in the presence of 0.005% Evan’s blue. End-point IgG titers were determined under a fluorescence microscope as the last titer showing specific reactivity to the parasite stage and recorded as digital pictures.
Production of Pk-infected mosquitoes
One splenectomized RM was anesthetized with ketamine and acepromazine and infected by intravenous injection of cryopreserved red blood cells infected with Pk. Female mosquitoes were used 5–7 days after emerging from pupae. Pint cartons of 100 mosquitoes were starved for 8hrs prior to the feeding; cartons were placed against the shaved skin of the infected RM, anesthetized as above, under drapes for darkness. After feeding for 30 minutes, mosquitoes not engorged with blood were removed, and the remaining mosquitoes were maintained at 26°C and 85% humidity. Cotton pads soaked in sugar solution were changed daily.
Spz challenge
Anopheles dirus mosquitoes were used on day 17 after they had fed on a Pk-infected RM. Spz were dissected by the Ozaki method into M199 medium (Sigma) with 5% normal RM serum. For challenge at day 273 post-inoculation with RhCMV, 100 Pk Spz were injected intravenously in 1 ml RPMI1640 with 5% normal rhesus serum. Starting at day 6 after challenge, blood was obtained by skin prick for thin film slides. After Giemsa staining, blood was examined under ×1000 magnification until 20,000 RBC were examined. Infected animals were treated when parasitemias reached 2% by intramuscular injections of artesunate (5 mg/kg single dose) and chloroquine diphosphate (25 mg/kg for three consecutive days). Animals were monitored for parasitemia the day after treatment and on days 17 and 20 post-challenge to ensure effectiveness of treatment in clearing malaria infections. The sterile animal was not treated but monitored daily until day 23 post-challenge.
Statistical analysis
Statistical analysis was conducted using R[57]. Data transformations were conducted prior to analysis (log10 transformations were applied to parasitemia and ICS measurements). Statistical significance was evaluated using a 5% type I error threshold on unadjusted values, and a 5% type I family-wise error threshold on adjusted p-values using the Holm method for multiplicity adjustment to control false discoveries across T cell frequencies measured by ICS or endpoint antibody titers measured by IFA[58]. All p-values shown are unadjusted except where noted. Boxplots were created using standard Tukey rules: boxes show interquartile range, IQR, with line at median, and whiskers extend to most extreme datapoints within 1.5*IQR from 25th and 75th percentiles, and outlier values outside of whiskers are plotted as points. P-values are shown across boxes only if significant (p≤0.05).
Comparisons across groups were conducted using two-sided Wilcoxon tests, and comparisons across time points were conducted using paired two-sided Wilcoxon tests[59].
Standard linear regression analysis was applied to relate the log10 parasitemia on days 8 through 11 to the day (coded as days post challenge minus 8, so the intercept is estimated day 8 log10 parasitemia). The model fit was good, especially with a simple model relating log10 parasitemia on each day to a group-specific intercept and a slope (adjusted R2 = 0.795), and also with a more complex model that allows the slope to vary by treatment group (adjusted R2 = 0.79). We employed standard ANOVA F-tests [59] to evaluate the significance of the interaction term (reflecting varying slope by treatment group) and found that the evidence did not support inclusion of the interaction term (P = 0.93) when comparing these two models, but when comparing the simpler model to the trivial model that ignores treatment group (adjusted R2 = 0.75), the ANOVA F-test P-value is highly significant (P = 2.3e-5), supporting the inclusion of group-specific intercepts, but not group-specific slopes, in the final model. Estimated Day 8 parasitemia for each RM was determined from the model fit. Due to discretization, observed Day 8 parasitemia values are either 0 or 1, but estimated Day 8 values from the model provide sufficient variation for immune correlates evaluation.
Vaccine efficacy was evaluated as percent reduction in estimated Day 8 parasitemia compared to the control group, with confidence intervals for the difference and p-values given by the linear model fit described above (using the simple model relating log10 parasitemia to days post infection minus 8, with group-specific intercepts), using the T distribution and test as is standard for evaluating coefficients of regression models.
Correlations were evaluated using Spearman’s method [60] (rank-transformation followed by Pearson correlation), and tested using the cor.test (., method = “spearman”) method in R.
Supporting information
The bacterial artificial chromosomes (BACs) of recombinant RhCMV constructs were sequenced by NGS and analyzed. All sequencing reads passing quality control were aligned to the de novo assembled consensus sequence of the viral genome. The consensus sequence was aligned with the parental RhCMV 68–1 BAC (Genbank Accession JQ795930) and the ORF map of the consensus sequences are shown. The bar indicates the percentage of nucleotide identity between the test and the reference sequences with green being 100% identical. The BAC cassette (green ORFs) is flanked by loxP sites (red). The only sequence difference between the parental BAC and the individual constructs is at the site of Pk antigen insertions (blow up below the full genome). In the RhCMV/PK4 vectors the antigens disrupt ORF Rh211. In the ΔRh186-9/PK4 vectors the antigens replace the ORFs Rh186, Rh187, Rh188 and 189.
(PDF)
Nucleotide sequence alignment and in silico translation of the CSP insert ΔRh186-9/CSP (upper sequence) and in RhCMV/CSP (lower sequence). The sequence was generated from DNA of virus isolated from the supernatant of infected rhesus fibroblasts. The in-frame deletion in the CSP region of RhCMV/CSP resulted in an internal truncation of the repeat region.
(PDF)
(A) Comparison of T cell response magnitudes, as determined by measuring the areas under the log10 curve (AUC) of T cell frequencies for each individual RM determined by ICS, between cohort 1 (RhCMV/PK4) and Cohort 2 (ΔRh186-9/PK4) over the entire immunization period. The boxplots graph shows the average (within 95% CI) median (horizontal line), interquartile range (shaded box), and range (whiskers and outlier points) of the total T cell responses to all antigens, whereas the table shows the p-values for the comparisons of each of the antigens individually. Statistical significance was determined by Wilcoxon test and we applied the Holm p-value adjustment method for controlling the family-wise error rate over the four genes. (B) Comparison of the peak T cell response over the immunization phase either for all antigens (boxplot graph) or for each antigen individually (table). Statistical analysis was as in A). (C) Comparisons of T cell response magnitudes (AUC) determined for cohort 1 and cohort 2 after the 2nd boost. Statistical analysis was as in A). (D) Comparisons of peak T cell response magnitudes determined for cohort 1 and cohort 2 after the 2nd boost. Statistical analysis was as in A).
(PDF)
Schematic of the RM cohorts, immunization schedule, challenge time points, post-challenge analysis and necropsy. Stars indicate the days when sera were collected for analysis of the antibody response. T cell functional assays indicate the day of blood collection for T cell phenotype analysis. The week (wk) post-vaccination of the animals necropsied in each cohort is indicated.
(PDF)
Parasitemia was determined as described in the Materials and Methods. Animals were treated with anti-malarial drugs when parasites exceeded 2% parasitemia (>400 infected RBC) on the indicated days.
(PDF)
Flow cytometric ICS results of peripheral blood and tissue CD4+ and CD8+ T cell responses to the peptide mixes comprising each of the four PK antigens in 4 animals of cohort 1 (RhCMV/PK4), 3 animals of cohort 2 (ΔRh186-9/PK4) and 3 animals of control cohort 3. The average response frequencies (+SEM), corrected for memory T cells, is shown for the indicated tissues for each of the antigens.
(PDF)
Acknowledgments
We thank Eric McDonald for managing the LSR and the OHSU Molecular Virology Support Core for generating virus stocks. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. For military service members or employees of the U.S. Government this work was prepared as part of official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported/funded by work unit number A1256, by the Military Infectious Disease Research Program project number F0351_13_NM, Naval Medical Research Center grant N62645-13-D-4021, and by the National Institute of Health grants R21AI103498, RO1AI059457 and RO1AI123182. This project was also supported by the National Center for Research Resources and the Office of Research Infrastructure Programs of the NIH through grant P51OD011092 as well as NIH Nonhuman Primate Reagent Resource (R24 RR016001) and National Institute of Allergy and Infectious Diseases contract (HHSN 2722000900037C). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Malaria Report 2014. 2014; Available from: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en.
- 2.Plowe CV, Alonso P, Hoffman SL. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J Infect Dis. 2009. December 1;200(11):1646–9. 10.1086/646613 [DOI] [PubMed] [Google Scholar]
- 3.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013. April;26(2):165–84. 10.1128/CMR.00079-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offeddu V, Thathy V, Marsh K, Matuschewski K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol. 2012. May 15;42(6):535–48. 10.1016/j.ijpara.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 5.Gupta S. Parasite immune escape: new views into host-parasite interactions. Curr Opin Microbiol. 2005. August;8(4):428–33. 10.1016/j.mib.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Früh K, Doumbo O, Muller HM, Koita O, McBride J, Crisanti A, et al. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991. April;59(4):1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010. October 29;33(4):555–66. 10.1016/j.immuni.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Epstein JE, Richie TL. The whole parasite, pre-erythrocytic stage approach to malaria vaccine development: a review. Curr Opin Infect Dis. 2013. October;26(5):420–8. 10.1097/QCO.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 9.Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017. February 23;542(7642):445–9. 10.1038/nature21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8 T cell immunity. Science. 2011. October 28;334(6055):475–80. 10.1126/science.1211548 [DOI] [PubMed] [Google Scholar]
- 11.Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. 2017. January 12;2(1):e89154 10.1172/jci.insight.89154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, et al. Longevity and Composition of Cellular Immune Responses Following Experimental Plasmodium falciparum Malaria Infection in Humans. PLoS Pathog. 2011. December;7(12):e1002389 10.1371/journal.ppat.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011. May 21;377(9779):1770–6. 10.1016/S0140-6736(11)60360-7 [DOI] [PubMed] [Google Scholar]
- 14.Douradinha B, van Dijk M, van Gemert GJ, Khan SM, Janse CJ, Waters AP, et al. Immunization with genetically attenuated P52-deficient Plasmodium berghei sporozoites induces a long-lasting effector memory CD8+ T cell response in the liver. J Immune Based Ther Vaccines. 2011;9(1):6 10.1186/1476-8518-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt NW, Harty JT. Cutting edge: attrition of Plasmodium-specific memory CD8 T cells results in decreased protection that is rescued by booster immunization. J Immunol. 2011. April 1;186(7):3836–40. 10.4049/jimmunol.1003949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008. September 16;105(37):14017–22. 10.1073/pnas.0805452105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012. June 15;188(12):5811–7. 10.4049/jimmunol.1102695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Barra E, Hodgson SH, Ewer KJ, Bliss CM, Hennigan K, Collins A, et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS One. 2014;9(12):e115161 10.1371/journal.pone.0115161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis. 2015. April 1;211(7):1076–86. 10.1093/infdis/jiu579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One. 2013;8(2):e55571 10.1371/journal.pone.0055571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimani D, Jagne YJ, Cox M, Kimani E, Bliss CM, Gitau E, et al. Translating the immunogenicity of prime-boost immunization with ChAd63 and MVA ME-TRAP from malaria naive to malaria-endemic populations. Mol Ther. 2014. November;22(11):1992–2003. 10.1038/mt.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005. September 5;202(5):673–85. 10.1084/jem.20050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ. T cell exhaustion. Nat Immunol. 2011. June;12(6):492–9. [DOI] [PubMed] [Google Scholar]
- 24.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011. May 26;473(7348):523–7. 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009. March;15(3):293–9. 10.1038/nm.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen SG, Piatak M Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013. October 3;502(7469):100–4. 10.1038/nature12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. [Article]. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016. January 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013. May 24;340(6135):1237874 10.1126/science.1237874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Früh K, Picker L. CD8+ T cell programming by cytomegalovirus vectors: applications in prophylactic and therapeutic vaccination. Curr Opin Immunol. 2017. July 19;47:52–6. 10.1016/j.coi.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers WO, Baird JK, Kumar A, Tine JA, Weiss W, Aguiar JC, et al. Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques. Infect Immun. 2001. September;69(9):5565–72. 10.1128/IAI.69.9.5565-5572.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang G, Shi M, Conteh S, Richie N, Banania G, Geneshan H, et al. Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: comparison of heterologous prime boost strategies. PLoS One. 2009;4(8):e6559 10.1371/journal.pone.0006559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss WR, Kumar A, Jiang G, Williams J, Bostick A, Conteh S, et al. Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens. PLoS One. 2007;2(10):e1063 10.1371/journal.pone.0001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers WO, Weiss WR, Kumar A, Aguiar JC, Tine JA, Gwadz R, et al. Protection of rhesus macaques against lethal Plasmodium knowlesi malaria by a heterologous DNA priming and poxvirus boosting immunization regimen. Infect Immun. 2002. August;70(8):4329–35. 10.1128/IAI.70.8.4329-4335.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malouli D, Nakayasu ES, Viswanathan K, Camp DG 2nd, Chang WL, Barry PA, et al. Reevaluation of the Coding Potential and Proteomic Analysis of the BAC-Derived Rhesus Cytomegalovirus Strain 68–1. J Virol. 2012. September;86(17):8959–73. 10.1128/JVI.01132-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010. April 2;328(5974):102–6. 10.1126/science.1185350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguiar JC, Hedstrom RC, Rogers WO, Charoenvit Y, Sacci JB Jr., Lanar DE, et al. Enhancement of the immune response in rabbits to a malaria DNA vaccine by immunization with a needle-free jet device. Vaccine. 2001. October 12;20(1–2):275–80. [DOI] [PubMed] [Google Scholar]
- 38.Murphy JR, Weiss WR, Fryauff D, Dowler M, Savransky T, Stoyanov C, et al. Using infective mosquitoes to challenge monkeys with Plasmodium knowlesi in malaria vaccine studies. Malar J. 2014;13:215 10.1186/1475-2875-13-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One. 2012;7(2):e31247 10.1371/journal.pone.0031247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichyangkul S, Spring MD, Yongvanitchit K, Kum-Arb U, Limsalakpetch A, Im-Erbsin R, et al. Chemoprophylaxis with sporozoite immunization in P. knowlesi rhesus monkeys confers protection and elicits sporozoite-specific memory T cells in the liver. PLoS One. 2017;12(2):e0171826 10.1371/journal.pone.0171826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieckmann KH, Beaudoin RL, Cassells JS, Sell KW. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull World Health Organ. 1979;57 Suppl 1:261–5. [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002. April 15;185(8):1155–64. 10.1086/339409 [DOI] [PubMed] [Google Scholar]
- 43.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013. September 20;341(6152):1359–65. 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- 44.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016. June;22(6):614–23. 10.1038/nm.4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A. 2017. March 07;114(10):2711–6. 10.1073/pnas.1615324114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009. July 30;361(5):468–77. 10.1056/NEJMoa0805832 [DOI] [PubMed] [Google Scholar]
- 47.Bijker EM, Schats R, Obiero JM, Behet MC, van Gemert GJ, van de Vegte-Bolmer M, et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: a double-blind randomized controlled clinical trial. PLoS One. 2014;9(11):e112910 10.1371/journal.pone.0112910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. 2012. December;20(12):2355–68. 10.1038/mt.2012.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agnandji ST, Fernandes JF, Bache EB, Ramharter M. Clinical development of RTS,S/AS malaria vaccine: a systematic review of clinical Phase I-III trials. Future Microbiol. 2015;10(10):1553–78. 10.2217/fmb.15.90 [DOI] [PubMed] [Google Scholar]
- 50.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312 10.1186/1475-2875-8-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogwang C, Kimani D, Edwards NJ, Roberts R, Mwacharo J, Bowyer G, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Science Translational Medicine. 2015. May 6, 2015;7(286):286re5 10.1126/scitranslmed.aaa2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836 10.1038/ncomms3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill AV. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci. 2011. October 12;366(1579):2806–14. 10.1098/rstb.2011.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aguiar JC, Bolton J, Wanga J, Sacci JB, Iriko H, Mazeika JK, et al. Discovery of Novel Plasmodium falciparum Pre-Erythrocytic Antigens for Vaccine Development. PLoS One. 2015;10(8):e0136109 10.1371/journal.pone.0136109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies DH, Duffy P, Bodmer JL, Felgner PL, Doolan DL. Large screen approaches to identify novel malaria vaccine candidates. Vaccine. 2015. December 22;33(52):7496–505. 10.1016/j.vaccine.2015.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang WL, Barry PA. Cloning of the full-length rhesus cytomegalovirus genome as an infectious and self-excisable bacterial artificial chromosome for analysis of viral pathogenesis. J Virol. 2003. May;77(9):5073–83. 10.1128/JVI.77.9.5073-5083.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Team RC. R: A language and environment for statistical computing. Vienna, Austria2015; Available from: https://www.R-project.org/.
- 58.Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, et al. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol. 2011. August 15;187(4):1722–32. 10.4049/jimmunol.1100560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007. December 11;104(50):19960–5. 10.1073/pnas.0705905104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Best DJB, Roberts DE. Algorithm AS 89: The Upper Tail Probabilities of Spearman's rho. Applied Statistics. 1975;24:377–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bacterial artificial chromosomes (BACs) of recombinant RhCMV constructs were sequenced by NGS and analyzed. All sequencing reads passing quality control were aligned to the de novo assembled consensus sequence of the viral genome. The consensus sequence was aligned with the parental RhCMV 68–1 BAC (Genbank Accession JQ795930) and the ORF map of the consensus sequences are shown. The bar indicates the percentage of nucleotide identity between the test and the reference sequences with green being 100% identical. The BAC cassette (green ORFs) is flanked by loxP sites (red). The only sequence difference between the parental BAC and the individual constructs is at the site of Pk antigen insertions (blow up below the full genome). In the RhCMV/PK4 vectors the antigens disrupt ORF Rh211. In the ΔRh186-9/PK4 vectors the antigens replace the ORFs Rh186, Rh187, Rh188 and 189.
(PDF)
Nucleotide sequence alignment and in silico translation of the CSP insert ΔRh186-9/CSP (upper sequence) and in RhCMV/CSP (lower sequence). The sequence was generated from DNA of virus isolated from the supernatant of infected rhesus fibroblasts. The in-frame deletion in the CSP region of RhCMV/CSP resulted in an internal truncation of the repeat region.
(PDF)
(A) Comparison of T cell response magnitudes, as determined by measuring the areas under the log10 curve (AUC) of T cell frequencies for each individual RM determined by ICS, between cohort 1 (RhCMV/PK4) and Cohort 2 (ΔRh186-9/PK4) over the entire immunization period. The boxplots graph shows the average (within 95% CI) median (horizontal line), interquartile range (shaded box), and range (whiskers and outlier points) of the total T cell responses to all antigens, whereas the table shows the p-values for the comparisons of each of the antigens individually. Statistical significance was determined by Wilcoxon test and we applied the Holm p-value adjustment method for controlling the family-wise error rate over the four genes. (B) Comparison of the peak T cell response over the immunization phase either for all antigens (boxplot graph) or for each antigen individually (table). Statistical analysis was as in A). (C) Comparisons of T cell response magnitudes (AUC) determined for cohort 1 and cohort 2 after the 2nd boost. Statistical analysis was as in A). (D) Comparisons of peak T cell response magnitudes determined for cohort 1 and cohort 2 after the 2nd boost. Statistical analysis was as in A).
(PDF)
Schematic of the RM cohorts, immunization schedule, challenge time points, post-challenge analysis and necropsy. Stars indicate the days when sera were collected for analysis of the antibody response. T cell functional assays indicate the day of blood collection for T cell phenotype analysis. The week (wk) post-vaccination of the animals necropsied in each cohort is indicated.
(PDF)
Parasitemia was determined as described in the Materials and Methods. Animals were treated with anti-malarial drugs when parasites exceeded 2% parasitemia (>400 infected RBC) on the indicated days.
(PDF)
Flow cytometric ICS results of peripheral blood and tissue CD4+ and CD8+ T cell responses to the peptide mixes comprising each of the four PK antigens in 4 animals of cohort 1 (RhCMV/PK4), 3 animals of cohort 2 (ΔRh186-9/PK4) and 3 animals of control cohort 3. The average response frequencies (+SEM), corrected for memory T cells, is shown for the indicated tissues for each of the antigens.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.