Abstract
Purpose:
Fungal keratitis can be difficult to medically treat. Topical antifungals are usually applied empirically as the initial option in treating fungal keratitis. Natamycin (NAT) and/or voriconazole (VRCZ) have been widely used in the treatment of fungal keratitis. However, Fusarium solani species complex (FSSC), which are the dominant species of fungal keratitis, are resistant to VRCZ. This study investigated in vitro efficacy of luliconazole (LLCZ), a new imidazole antifungal, against FSSC and other filamentous fungi.
Methods:
A total of 18 Fusarium isolates and 7 others were grown on potato dextrose agar at 30 and 37°C. For Fusarium, species identification and phylogenetic tree analysis were performed based on elongation factor-1α (EF-1α) DNA sequencing. The broth microdilution method was used for antifungal susceptibility testing of 11 antifungal drugs including LLCZ.
Results:
The 18 identified Fusarium isolates belonged to FSSC (n = 13), Fusarium oxysporum species complex (FOSC; n = 2), Fusarium chlamydosporum species complex (FCSC; n = 1), Fusarium incarnatum-equiseti species complex (FIESC; n = 1), and Fusarium fujikuroi species complex (FFSC; n = 1). We further divided 13 FSSC isolates into 3 clades, FSSC5 (n = 8), FSSC3 + 4 (n = 4), and FSSC9-a (n = 1), with 8 FSSC strains growing at 37°C. LLCZ showed lowest minimum inhibitory concentrations (MICs) against all tested filamentous fungi, with a MIC90 against the Fusarium species of 0.06 μg/mL, whereas MIC90 for NAT and VRCZ were 4 and 8 μg/mL, respectively.
Conclusions:
LLCZ has the strongest in vitro antifungal activity among all drugs used against broad-range filamentous fungi including FSSC. LLCZ may potentially be a new medical treatment option for fungal keratitis.
Key Words: luliconazole, Fusarium solani, fungal keratitis
Infectious keratitis is a major worldwide cause of corneal blindness that especially occurs in developing regions of the world.1 Causative organisms of infectious keratitis include bacteria, virus, fungi, Acanthamoeba, and other rare protozoa. Among these organisms, the treatment of fungal keratitis is the most challenging. Fungal keratitis requires surgical treatment including penetrating keratoplasty and evisceration to a greater degree than that observed for other causes of keratitis.2 The frequency of fungal keratitis in infectious keratitis varies according to geography, climate, occupation, and healthcare exposure.3 In tropical or agricultural countries, it has been reported that the proportion of fungal keratitis to total infectious keratitis is more than 60%.4,5 In many countries, the most predominantly reported fungal species is Fusarium, with proportions ranging from 25% to 73.3%.2,4–8 Thus, Fusarium keratitis is one of the most important types of infectious keratitis.
Recently, topical voriconazole (VRCZ) has been widely used in the treatment of fungal keratitis, and there are many reports on the successful treatment of fungal keratitis caused by Aspergillus fumigatus, Purpureocillium lilacinum, Alternaria species, and Beauveria bassiana.9–14 However, minimum inhibitory concentrations (MICs) of VRCZ against Fusarium species are higher than those of the other fungal species.15 It has been reported that polyenes such as amphotericin B and natamycin (NAT) are still most susceptible for the Fusarium species in vitro.16–20 Indeed, randomized clinical trials showed that NAT was more effective than VRCZ for Fusarium keratitis.21,22 However, disadvantages of using polyenes are that they are strongly toxic to human cells and their corneal penetration as drugs is poor.23 Therefore, there is a need for new antifungal ophthalmic solutions that can be used as a first-line drug for broad-range filamentous fungi.
Luliconazole (LLCZ) is a new imidazole antifungal agent with broad-spectrum antifungal activity that is structurally related to its predecessor, lanoconazole. LLCZ, lanoconazole and efinaconazole have been clinically used as topical drugs in the treatment of onychomycosis and dermatophytosis. These drugs are also known to be highly active against dermatophytes, with geometric mean MICs of LLCZ, lanoconazole, and efinaconazole reported to be 0.0005, 0.002, and 0.007 μg/mL, respectively.24 Posaconazole and ravuconazole are also new azoles that have yet to be topically applied in an ophthalmic setting. In our present study, we investigated the in vitro antifungal activity of LLCZ, lanoconazole, and other azoles against the Fusarium species isolated from fungal keratitis and other species of reference strains.
MATERIALS AND METHODS
Fungal Isolates, Colony Morphologies, and Growth Temperatures
A multicenter study of fungal keratitis in Japan performed by the Japanese Association for Ocular Infection collected 14 Fusarium isolates.8 Four culture collection strains of Fusarium species and other species (Aspergillus flavus, Alternaria alternata, B. bassiana, and Scedosporium apiospermum) were obtained from the Biological Resource Center, NITE (NBRC). We also examined P. lilacinum, which was isolated from fungal keratitis, and 2 Candida reference strains that were obtained from the American Type Culture Collection (ATCC) (Table 1).
TABLE 1.
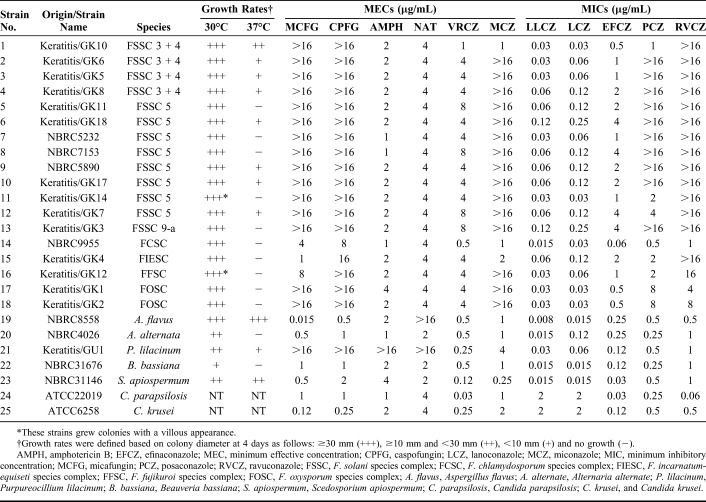
Results of Species Identifications, Growth Rates, Colony Morphologies, and Antifungal Susceptibilities of Isolates Included in the Study
The colony morphology on the surface and reverse side was observed on potato dextrose agar plates after a 4-day incubation at 30 and 37°C. Colony diameters on potato dextrose agar plates were measured.
Species Identification and Phylogenetic Tree Analysis
Genomic DNA of Fusarium isolates was extracted using a ZR Fungal/Bacterial DNA Kit in accordance with the supplier's instruction. DNA sequencing of the PCR product amplifying the elongation factor-1α (EF-1α) gene was performed with primer pairs, using a previously described method.25 Species identification was determined by comparing similar sequences with the Fusarium MLST database (http://www.cbs.knaw.nl/fusarium/).
The phylogenetic tree based on EF-1α sequences was constructed using the Molecular Evolutionary Genetics Analysis (MEGA) software version 7.0.26 (http://megasoftware.net) by the Neighbor-Joining method.26
Antifungal Agent and Antifungal Susceptibility Testing
Susceptibilities of 11 antifungal drugs (micafungin, caspofungin, amphotericin B, NAT, VRCZ, miconazole, LLCZ, lanoconazole, efinaconazole, posaconazole, and ravuconazole) in 3 major classes (echinocandins, polyenes, and azoles) were tested by the broth microdilution method that is based on the CLSI M38-A2 standard.27 MIC endpoints for newer azoles, which included LLCZ, lanoconazole, and efinaconazole, were defined according to those used for VRCZ. Endpoints for echinocandins were defined by the minimal effective concentration. Candida parapsilosis ATCC22019 and Candida krusei ATCC6258 were used as quality control strains. LLCZ, lanoconazole, and efinaconazole were provided by Nihon Nohyaku Co, Ltd (Tokyo, Japan), and ravuconazole was provided by Seren Pharmaceuticals Inc (Tokyo, Japan), with all other agents purchased from Sigma–Aldrich (St. Louis, MO).
RESULTS
Species Identification and Phylogenetic Tree Analysis of Fusarium Isolates
The DNA sequence-based species identification revealed that the 18 total Fusarium isolates belonged to 5 species complexes; Fusarium solani species complex (FSSC; n = 13), Fusarium oxysporum species complex (FOSC; n = 2), Fusarium chlamydosporum species complex (FCSC; n = 1), Fusarium incarnatum-equiseti species complex (FIESC; n = 1), and Fusarium fujikuroi species complex (FFSC; n = 1). The 13 FSSC strains were further divided into 3 clades; FSSC5 (n = 8), FSSC3 + 4 (n = 4), and FSSC9-a (n = 1) (Table 1 and Fig. 1).
FIGURE 1.
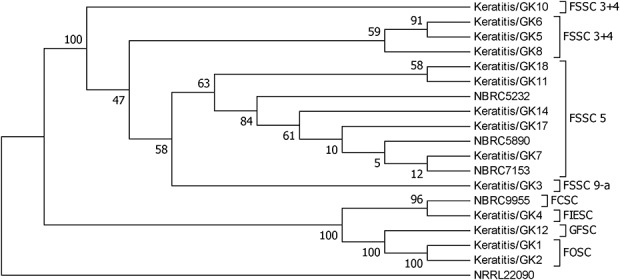
Phylogenetic diversity of Fusarium species isolates included in this study based on elongation factor-1α (EF-1α) sequence data. The percentages of replicate trees in which associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. Evolutionary distances were computed using the p-distance method and are presented in units of base differences per site. The sequence of Fusarium illudens NRRL22090 was used as an out-group.
Growth Temperatures and Colony Morphologies
All tested Fusarium isolates grew well at 30°C, but exhibited variable growth at 37°C in accordance with the strain (Table 1). Eight strains of FSSC grew at 37°C. Of these, only one strain GK10 (FSSC3 + 4) grew a colony that was larger than 10 mm in diameter. All other strains exhibited colonies that were smaller than 10 mm. None of the strains belonging to species complexes other than FSSC grew at 37°C. The strain GK14, which belongs to FSSC5, showed a villous colony appearance, whereas all other FSSC strains showed a felt-like cream-colored colony appearance. The GK12 (FFSC) colony strain also showed a villous appearance. Purplish colonies were observed in both FOSC strains.
Antifungal Susceptibility Testing
Table 1 presents results of the antifungal susceptibility test and MICs/MECs for all strains. Table 2 shows the MIC50, MIC90, and MIC ranges for polyenes and azoles against the Fusarium species. LLCZ showed the lowest MIC90 (0.06 μg/mL) against the Fusarium species, followed by lanoconazole (0.12 μg/mL). The MIC90 for NAT and VRCZ was 4 and 8 μg/mL, respectively. VRCZ and efinaconazole exhibited moderate MICs for the Fusarium species, whereas they had low MICs against the nonFusarium strains. Posaconazole and ravuconazole exhibited high MICs against the Fusarium species, especially for FSSC (Table 1). Cumulative MIC curves for the in vitro testing showed that LLCZ was most effective for the Fusarium species among all antifungal drugs examined in this study (Fig. 2). MICs for the Fusarium species of amphotericin B and NAT were low regardless of the species. Fusarium species were not susceptible to the Echinocandins (Table 1). LLCZ also exhibited the lowest MICs against other filamentous fungi including A. flavus, A. alternata, P. lilacinum, B. bassiana, and S. apiospermum (Table 1). However, the antifungal activity of LLCZ for the Candida species was lower than that for echinocandins and triazoles, such as efinaconazole, VRCZ, posaconazole, and ravuconazole.
TABLE 2.
Comparison of Antifungal Susceptibility of Polyenes and Azoles Against Fusarium Species
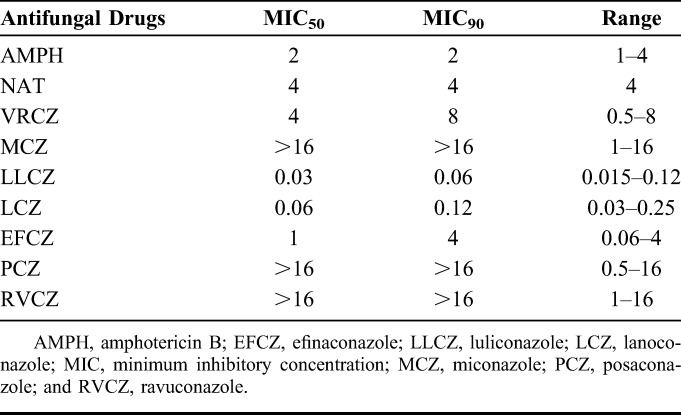
FIGURE 2.
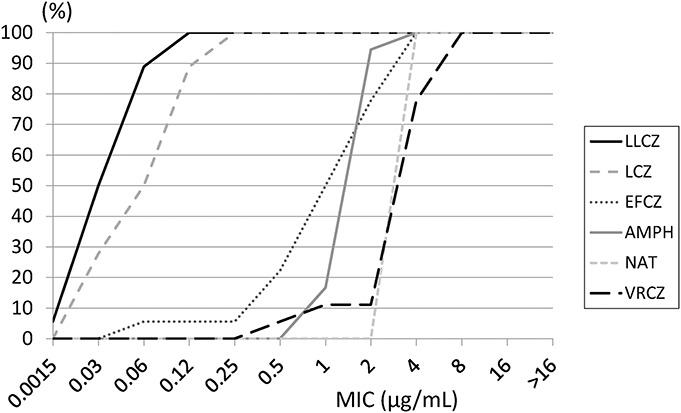
Cumulative minimum inhibitory concentration curves of antifungal drugs tested against the Fusarium species (n = 18). The 18 total isolates included 14 clinical strains from keratitis and 4 strains from a culture collection. The vertical axis shows the percentage of isolates (%), whereas the horizontal axis shows the minimum inhibitory concentrations (MICs, μg/mL). Luliconazole (LLCZ) exhibited the lowest MIC among all antifungal drugs tested. LCZ, lanoconazole; EFCZ, efinaconazole; AMPH, amphotericin B; NAT, natamycin; and VRCZ, voriconazole.
DISCUSSION
This study tested the in vitro activity of LLCZ, a new imidazole antifungal, against various fungi including the keratitis-derived Fusarium species. Results showed that LLCZ had lowest MICs for a broad-range of filamentous fungi among all tested antifungal agents. Notably, the MIC90 for the Fusarium species was 0.06 μg/mL, which was far lower than that observed for NAT (4 μg/mL) and VRCZ (8 μg/mL) (Table 2). If further studies including corneal penetration, absorption, tissue compatibility studies, and stability of drugs are completed, LLCZ could potentially be a new medical treatment option for fungal keratitis.
Although initial medical treatments for fungal keratitis are usually empirical, management of the disease is difficult, as there has yet to be any single agent found that is universally effective.28 For Fusarium keratitis, NAT has been shown to be more effective than VRCZ.21 However, in Aspergillus keratitis, more cases treated with NAT were reported to develop perforation or required therapeutic keratoplasty than VRCZ-treated cases.22 Furthermore, ulcers caused by Aspergillus were more refractory to primary treatments with topical 5% NAT monotherapy compared with those caused by Fusarium.29 P. lilacinum is also one of the important species of fungal keratitis that is known to be resistant to polyenes and sensitive only to VRCZ in most of the strains.13,30 The findings of all previous studies suggest that there is a strong need to create a new antifungal drug for potential use as a first-line topical drug.
About frequency and severity of fungal keratitis, the Fusarium species remain the most important cause of this disease. The genus Fusarium is composed of several species complexes, which indicate there are groups of closely related species. There have been 7 species complexes previously shown to be related to human infections and they include: Fusarium dimerum species complex (FDSC), FSSC, FOSC, FFSC, FIESC, FCSC, and the complex including Fusarium sporotrichoides (FSAMSC).31 Among these, FSSC is the most dominant species complex in keratitis, accounting for 75% to 88% of all Fusarium keratitis cases.19,31 FSSC has been further divided into several phylogenetic groups by phylogenetic tree analysis. Hassan et al reported that FSSC3 + 4 (also called Fusarium falciforme) was predominantly isolated from keratitis,6 whereas Muraosa et al32 reported that FSSC3 + 4 was the most frequent isolate collected from patients with superficial fusariosis in humans, followed by FSSC5. In our present study, 10 keratitis-derived FSSC isolates were distributed among the FSSC5 (n = 5), FSSC3 + 4 (n = 4), and FSSC9 (n = 1) complexes (Table 1). Our current results do not contradict any of the aforementioned reports. All FSSC isolates in our study showed high MICs for VRCZ, with the exception for the GK10 strain (FSSC3 + 4) with low azole MICs. Moreover, this strain also showed differences in growth at 37°C from the other 3 strains belonging to FSSC3 + 4 (GK10, GK6, and GK5). To differentiate these strains, additional molecular identification by sequencing the RNA polymerase II largest subunit (RPB1) and RNA polymerase II second largest subunit (RPB2) will need to be undertaken.
LLCZ is a novel imidazole antifungal compound that was originally synthesized in Japan33 and which is now widely used as a 1% cream, 1% solution, and a 1% ointment in the topical treatment of onychomycosis and dermatophytosis.34,35 Furthermore, our present study also showed that LLCZ is active not only against dermatophytes but also against a broad-range of filamentous fungi that includes the Fusarium species, A. flavus, P. lilacinum, B. bassiana, and S. apiospermum, which cover most of the isolates from fungal keratitis. In addition, the advantage of using LLCZ is its small molecular weight (MW), as the MWs of LLCZ, VRCZ, NAT, amphotericin B, and micafungin are 354, 349, 665, 924, and 1270 Da, respectively. Because of the small MW and lipophilicity, topical VRCZ exhibits good drug penetration into the deep corneal stroma and anterior chamber.36 Thus, topical LLCZ may be a potential candidate as a new antifungal ophthalmic solution with a broad-spectrum, strong antifungal activity, and high corneal penetration. Although efinaconazole exhibited a slightly lower MIC90 against FSSC (4 μg/mL) versus VRCZ (8 μg/mL), its potential activity was less than that observed for LLCZ. Posaconazole and ravuconazole were not active against FSSC (Table 1). However, the disadvantage of using LLCZ is that MICs against the Candida species were higher than those for VRCZ and echinocandins. Thus, it should be noted that LLCZ is a less effective drug when used in the treatment of candidiasis.
The major limitation of this study is that we only tested the efficacy of LLCZ in vitro. Thus, corneal toxicity, corneal permeability, and drug stability when using LLCZ in an ophthalmic solution will need to be examined in the future. Another limitation is that we failed to include several important fungi such as Curvularia and Exerohilum species in antifungal susceptibility testing. These fungi also should be tested in future studies.
In conclusion, we showed that LLCZ exhibited the strongest antifungal activity among all existing antifungals against a broad-range of filamentous fungi including FSSC, which is the most important cause of fungal keratitis. LLCZ could potentially be a new medical treatment option for fungal keratitis.
Footnotes
Supported by JSPS KAKENHI Grant 16K11317.
K. Inagaki is an employee of Nihon Nohyaku Co, Ltd, Tokyo, Japan. Luliconazole, lanoconazole and efinaconazole used in this study were provided by Nihon Nohyaku Co, Ltd. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81:622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopinathan U, Sharma S, Garg P, et al. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller D, Galor A, Alfonso EC. Fungal keratitis. In: Mannis MJ, Holland EJ, eds. Cornea. Amsterdam, Netherlands: Elsevier; 2017:964–975. [Google Scholar]
- 4.Nath R, Baruah S, Saikia L, et al. Mycotic corneal ulcers in upper Assam. Indian J Ophthalmol. 2011;59:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. [DOI] [PubMed] [Google Scholar]
- 6.Hassan AS, Al-Hatmi AM, Shobana CS, et al. Antifungal susceptibility and phylogeny of opportunistic members of the genus Fusarium causing human keratomycosis in south India. Med Mycol. 2016;54:287–294. [DOI] [PubMed] [Google Scholar]
- 7.Marangon FB, Miller D, Giaconi JA, et al. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–825. [DOI] [PubMed] [Google Scholar]
- 8.Sunada A, Asari S, Inoue Y, et al. Multicenter prospective observational study of fungal keratitis—identification and susceptibility test of fungi [in Japanese]. Nippon Ganka Gakkai Zasshi. 2016;120:17–27. [PubMed] [Google Scholar]
- 9.Mehta H, Mehta HB, Garg P, et al. Voriconazole for the treatment of refractory Aspergillus fumigatus keratitis. Indian J Ophthalmol. 2008;56:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa A, Matsumoto Y, Yaguchi T, et al. Successful treatment of Beauveria bassiana fungal keratitis with topical voriconazole. J Infect Chemother. 2016;22:257–260. [DOI] [PubMed] [Google Scholar]
- 11.Ozbek Z, Kang S, Sivalingam J, et al. Voriconazole in the management of Alternaria keratitis. Cornea. 2006;25:242–244. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Jain A, Ta CN. Aspergillus fumigatus keratitis following laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:1806–1807. [DOI] [PubMed] [Google Scholar]
- 13.Todokoro D, Yamada N, Fukuchi M, et al. Topical voriconazole therapy of Purpureocillium lilacinum keratitis that occurred in disposable soft contact lens wearers. Int Ophthalmol. 2014;34:1159–1163. [DOI] [PubMed] [Google Scholar]
- 14.Wu PC, Lai CH, Tan HY, et al. The successful medical treatment of a case of Paecilomyces lilacinus keratitis. Cornea. 2010;29:357–358. [DOI] [PubMed] [Google Scholar]
- 15.Lalitha P, Sun CQ, Prajna NV, et al. In vitro susceptibility of filamentous fungal isolates from a corneal ulcer clinical trial. Am J Ophthalmol. 2014;157:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taj-Aldeen SJ, Salah H, Al-Hatmi AM, et al. In vitro resistance of clinical Fusarium species to amphotericin B and voriconazole using the EUCAST antifungal susceptibility method. Diagn Microbiol Infect Dis. 2016;85:438–443. [DOI] [PubMed] [Google Scholar]
- 17.Guevara-Suarez M, Cano-Lira JF, de Garcia MC, et al. Genotyping of Fusarium isolates from onychomycoses in Colombia: detection of two new species within the Fusarium solani species complex and in vitro antifungal susceptibility testing. Mycopathologia. 2016;181:165–174. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, et al. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother. 2015;70:1068–1071. [DOI] [PubMed] [Google Scholar]
- 19.Oechsler RA, Yamanaka TM, Bispo PJ, et al. Fusarium keratitis in Brazil: genotyping, in vitro susceptibilities, and clinical outcomes. Clin Ophthalmol. 2013;7:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, et al. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–809. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Das S, Virdi A, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99:1190–1195. [DOI] [PubMed] [Google Scholar]
- 22.Prajna VN, Lalitha PS, Mascarenhas J, et al. Natamycin and voriconazole in Fusarium and Aspergillus keratitis: subgroup analysis of a randomised controlled trial. Br J Ophthalmol. 2012;96:1440–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16:730–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezaei-Matehkolaei A, Khodavaisy S, Alshahni MM, et al. In vitro antifungal activity of novel triazole efinaconazole and five comparators against dermatophyte Isolates. Antimicrob Agents Chemother. 2018;62:e02423–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell K, Sutton DA, Fothergill A, et al. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol. 2008;46:2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI. M38-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 28.Lalitha P, Shapiro BL, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–793. [DOI] [PubMed] [Google Scholar]
- 29.Lalitha P, Prajna NV, Kabra A, et al. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113:526–530. [DOI] [PubMed] [Google Scholar]
- 30.Ali TK, Amescua G, Miller D, et al. Contact-lens-associated Purpureocillium keratitis: risk factors, microbiologic characteristics, clinical course, and outcomes. Semin Ophthalmol. 2017;32:157–162. [DOI] [PubMed] [Google Scholar]
- 31.Van Diepeningen AD, Al-Hatmi AMS, Brankovics B, et al. Taxonomy and clinical spectra of Fusarium species: where do we stand in 2014?. Curr Clin Micro Rpt. 2014;1:10–18. [Google Scholar]
- 32.Muraosa Y, Oguchi M, Yahiro M, et al. Epidemiological study of Fusarium species causing invasive and superficial fusariosis in Japan. Med Mycol J. 2017;58:E5–e13. [DOI] [PubMed] [Google Scholar]
- 33.Uchida K, Nishiyama Y, Yamaguchi H. In vitro antifungal activity of luliconazole (NND-502), a novel imidazole antifungal agent. J Infect Chemother. 2004;10:216S–219. [DOI] [PubMed] [Google Scholar]
- 34.Gupta AK, Foley KA, Versteeg SG. New antifungal agents and new formulations against dermatophytes. Mycopathologia. 2017;182:127–141. [DOI] [PubMed] [Google Scholar]
- 35.Scher RK, Nakamura N, Tavakkol A. Luliconazole: a review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57:389–393. [DOI] [PubMed] [Google Scholar]
- 36.Thiel MA, Zinkernagel AS, Burhenne J, et al. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob Agents Chemother. 2007;51:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]