Supplemental Digital Content is available in the text.
Keywords: antibiotic resistance, surveillance, pediatric, ocular, methicillin resistance, multidrug resistance
Abstract
Background:
The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study is a nationwide longitudinal antibiotic resistance surveillance program specific to bacterial pathogens commonly encountered in ocular infections. We evaluated in vitro resistance rates and trends among isolates obtained from pediatric patients (≤17 years of age).
Methods:
Clinical centers across the United States were invited to submit ocular isolates of Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Haemophilus influenzae and Pseudomonas aeruginosa to a central laboratory. Minimum inhibitory concentrations for various antibiotic classes were determined by broth microdilution per Clinical and Laboratory Standards Institute guidelines and interpreted as susceptible, intermediate or resistant based on available breakpoints. Longitudinal trends were analyzed using a Cochran-Armitage test for linear trends in a proportion.
Results:
Of 4829 isolates collected from January 2009 to December 2016, 995 isolates, sourced primarily from hospitals and referral centers, were obtained from pediatric patients (n = 286 H. influenzae, n = 284 S. aureus, n = 213 CoNS, n = 150 S. pneumoniae and n = 62 P. aeruginosa). With few exceptions, P. aeruginosa and H. influenzae were generally susceptible to the antibiotics tested. Of S. aureus and CoNS isolates, respectively, 56% and 72% were resistant to azithromycin and 24% and 47% were methicillin-resistant (MR); concurrent resistance to other drug classes and multidrug resistance (≥3 drug classes) were prevalent among MR staphylococci. Of S. pneumoniae isolates, 38% and 35% demonstrated resistance to azithromycin and penicillin, respectively. Besifloxacin had the lowest minimum inhibitory concentration against the Gram-positive isolates.
Conclusions:
These in vitro data suggest antibiotic resistance is common among staphylococcal and pneumococcal isolates collected from pediatric patients with ocular infections. Methicillin resistance was prevalent among staphylococci with many strains demonstrating multidrug resistance. These findings may not be representative of resistance trends in community-based practices.
Key Points
Recent findings from the nationwide (United States) ARMOR study showed high, but apparently stabilizing, rates of in vitro antibiotic resistance, including multidrug resistance, among staphylococcal isolates sourced from ocular infections in patients 0–99 years of age.
The current analysis focused specifically on isolates obtained from patients ≤17 years of age in the ARMOR study.
This 8-year longitudinal analysis shows in vitro antibiotic resistance to be common, but generally not increasing, among staphylococcal and pneumococcal isolates collected from pediatric-sourced ocular infections.
These data should be useful for clinicians tasked with choosing empiric therapy for pediatric ocular infections.
BACKGROUND
Bacterial eye infections are frequently encountered in pediatric medical care. Children can develop various types of ocular surface infections including keratitis, dacryocystitis, blepharokeratoconjunctivitis and conjunctivitis.1–3 Gram-positive organisms, particularly coagulase-negative staphylococci (CoNS) and Staphylococcus aureus, and the Gram-negative organism, Pseudomonas aeruginosa, are frequently implicated in pediatric keratitis.1–4 Staphylococci are common pathogens in pediatric blepharokeratoconjunctivitis and dacryocystitis.1,5–7 Common bacterial conjunctivitis pathogens in children include Haemophilus influenzae, Streptococcus pneumoniae and less frequently, Moraxella catarrhalis,8–12 with S. aureus and S. epidermidis considered causative when present above quantitative thresholds.13–20 Before initiating antibacterial therapy in any eye infection, identification of the causative bacterium along with its corresponding antibiotic resistance profile is ideal for guiding therapy. However, in real-world clinical practice, the utility of culture and sensitivity testing is lessened because of the delay involved in getting results. Thus, it is common for ocular infections to be treated empirically, a practice both possibly contributing to, and complicated by, the problem of antibiotic resistance among ocular bacterial pathogens.
Increased microbial resistance to antibiotic therapy has been a growing concern over recent decades, including among ocular pathogens.21–25 There have been only 2 nationwide, prospective surveillance studies that have monitored resistance data among ocular pathogens specifically: the Ocular Tracking Resistance in US Today (TRUST) study, conducted between 2005 and 2008,25–27 and the ongoing Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study initiated in 2009.28–30 Both studies evaluated antibiotic resistance among bacterial species common to ocular infections in general, namely S. aureus, CoNS (S. epidermidis in ocular TRUST), H. influenzae, S. pneumoniae and P. aeruginosa (ARMOR only), and both reported high rates of in vitro antibiotic resistance as well as multidrug resistance among bacterial isolates from clinically significant ocular infections, particularly among S. aureus and CoNS.26,28–30
While the ARMOR surveillance study is not specific to the pediatric population, there is no limit as to the age of the patient from whom isolates are obtained. The isolate database therefore includes a significant number of isolates obtained from ocular infections in pediatric patients (≤17 years of age). Herein, we report antibiotic resistance profiles and trends for the 995 ocular isolates obtained exclusively from pediatric patients in the ARMOR surveillance study as of December 2016.
METHODS
Study Design
The ARMOR surveillance study design and methods have been described in detail elsewhere.28 Here, we report antibiotic resistance among ocular isolates collected from January 1, 2009, to December 31, 2016, exclusively from pediatric patients ≤17 years of age. Briefly, participating eye care centers, community hospitals and academic or university hospitals across the United States were invited to submit a defined number of isolates: up to 65 ocular isolates (including 20 S. aureus, 20 CoNS, 5 S. pneumoniae, 5 H. influenzae and 15 P. aeruginosa) from 2009 to 2013 and up to 50 ocular isolates (with no more than 12 isolates per species) from 2014 to 2016. Sites were requested to submit clinically significant (ie, presumed causative) bacterial isolates from any eye infection sources. Not all sites submitted samples throughout all 8 years. Informed consent and institutional review board approval were not required for study conduct at any of the clinical sites, and Health Insurance Portability and Accountability Act compliance did not apply given initial ocular samples were taken as part of routine medical care unrelated to this study and no patient identifying information was provided.
Antibacterial Susceptibility Testing
Isolates were sent to an independent central laboratory [Eurofins Medinet, Chantilly, VA (2009–2013); IHMA, Schaumburg, IL (2014–2016)] for species confirmation and determination of the antibiotic resistance profile. Minimum inhibitory concentration (MIC) profiles were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) defined methodology31–33 using frozen antimicrobial microtiter panels (Thermo Fisher Scientific, Walthom, MA). Representative antibiotics from 12 different antibiotic classes (fluoroquinolone, macrolide, aminoglycoside, lincosamide, cephalosporin, penicillin, carbapenem, dihydrofolate reductase inhibitor, polypeptide, amphenicol, tetracycline and glycopeptide) were tested against isolates as appropriate based on species. Not all antibiotics were tested in each year. Where breakpoints were available, isolate MICs were interpreted as susceptible, intermediate or resistant according to the CLSI interpretive criteria in use during the collection year for a particular combination of species and antibiotic.34–41 Staphylococci were classified as methicillin-resistant (MR) or methicillin-susceptible (MS) based on susceptibility to oxacillin. Susceptibility and resistance of S. pneumoniae isolates to penicillin were determined using the breakpoint for oral penicillin. For reporting purposes, resistant isolates were classified as those with intermediate or full resistance to an antibiotic. Multidrug resistance among isolates was defined as resistance to at least 3 classes of antibiotics.
Statistical Analysis
Logistic regression models for concurrent resistance to each antibiotic were used to obtain odds ratios based on MR. Confidence intervals for antibiotic resistance rates were computed using the Wilson score with a correction for continuity42 using Statistix 10 software (Analytic Software, Tallahassee, FL). Changes in resistance rates over time were evaluated using a Cochran-Armitage test for linear trends in a proportion43 with statistical significance defined as P < 0.05.
RESULTS
Isolates
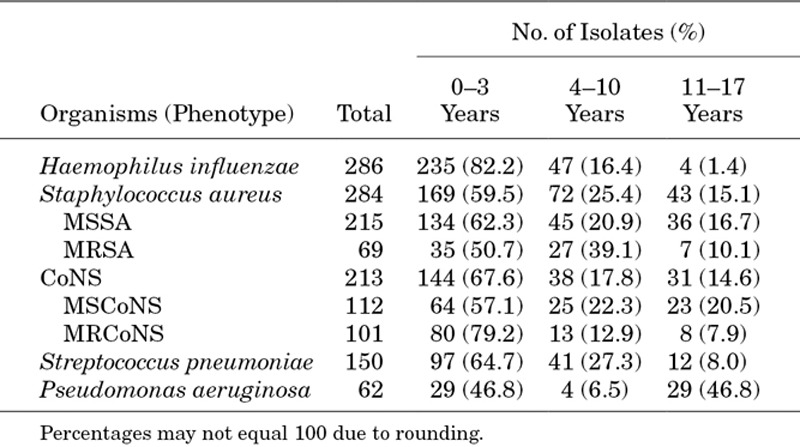
A total of 4829 ocular isolates were collected from January 2009 to December 2016. Of these, 995 were specific to pediatric patients with ocular infections and were obtained from 67 sites (34 community hospitals, 24 university hospitals, 7 specialty centers, 2 reference laboratories). These included 286 H. influenzae, 284 S. aureus, 213 CoNS, 150 S. pneumoniae and 62 P. aeruginosa isolates. The 213 CoNS isolates included 154 S. epidermidis, 25 S. hominis, 12 unspeciated CoNS, 8 S. capitis, 6 S. warneri, 2 each S. haemolyticus and S. lugdunensis and 1 each S. caprae, S. equorum, S. saprophyticus and S. simulans.
Of the pediatric patients from whom samples were obtained, 429 (43.1%) were female, 527 (53.0%) were male and gender was not reported for 39 (3.9%). Stratification by patient age (Table 1) showed that the majority of H. influenzae, S. aureus, CoNS and S. pneumoniae isolates were obtained from pediatric patients ≤3 years of age, whereas approximately half of P. aeruginosa isolates were obtained from adolescent patients 11–17 years of age. The precise anatomical source of the ocular isolates was reported for only 373 (37.5%) isolates. In the subset of isolates with known ocular sources, the conjunctiva was the most common anatomical source for H. influenzae, S. aureus, CoNS and S. pneumoniae isolates, with 94.7%, 90.1%, 82.9% and 88.3% of isolates collected from that tissue, respectively. The most common anatomical source for P. aeruginosa isolates was the cornea (65.5%).
TABLE 1.
Bacterial Isolates Collected From Pediatric Patients (January 2009 Through December 2016)
In Vitro Antibiotic Resistance Rates
Resistance among H. influenzae isolates was minimal. Except for 2 isolates resistant to azithromycin and 3 isolates resistant to chloramphenicol, all H. influenzae isolates were susceptible to all antibacterials tested. The MIC that inhibits visible growth of 90% of isolates (MIC90) against H. influenzae was no greater than 0.06 μg/mL for any fluoroquinolone tested, 2 μg/mL for azithromycin, 0.5 μg/mL for chloramphenicol, 0.03 μg/mL for ceftriaxone and 1 μg/mL for imipenem.
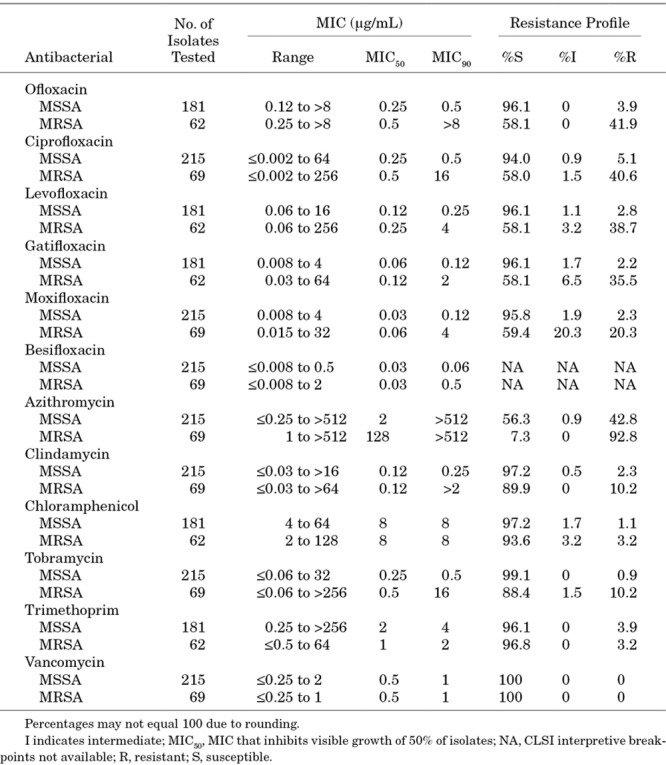
Of the 284 S. aureus isolates, 69 (24.3%) were resistant to oxacillin (MR S. aureus, MRSA), 42 (14.8%) were resistant to ciprofloxacin and 158 (55.6%) were resistant to azithromycin. All S. aureus isolates were susceptible to vancomycin, and only a small proportion was resistant to clindamycin (13 of 284 [4.6%]), chloramphenicol (9 of 243 [3.7%]), tobramycin (10 of 284 [3.5%]) or trimethoprim (9 of 243 [3.7%]). Table 2 presents the MICs and resistance profiles for S. aureus isolates by MR phenotype. Among MRSA isolates, resistance was high for fluoroquinolones (range 40.6%–42.0%) and azithromycin (92.8%). Among MS S. aureus (MSSA), resistance was high only for azithromycin (43.7%).
TABLE 2.
Minimum Inhibitory Concentrations and Resistance Profiles for Staphylococcus aureus Isolates From Ocular Infections in Pediatric Patients 0–17 Years of Age
Of the 213 CoNS isolates, 101 (47.4%), 33 (15.5%) and 153 (71.8%) were resistant to oxacillin, ciprofloxacin and azithromycin, respectively. Like S. aureus, all CoNS were susceptible to vancomycin, and all but one were susceptible to chloramphenicol. However, higher proportions were resistant to clindamycin (70 of 213 [32.9%]), tobramycin (34 of 213 [16.0%]) and trimethoprim (37 of 182 [20.3%]). Consistent with S. aureus, resistance was higher in the subset of CoNS isolates that were MR (MRCoNS) (Table 3), with 26.7%–30.8% resistant to fluoroquinolones and 88.1% resistant to azithromycin. Resistance among MRCoNS was also higher for clindamycin (43.6%), tobramycin (32.7%) and trimethoprim (34.6%).
TABLE 3.
Minimum Inhibitory Concentrations and Resistance Profiles for CoNS Isolates From Ocular Infections in Pediatric Patients 0–17 Years of Age
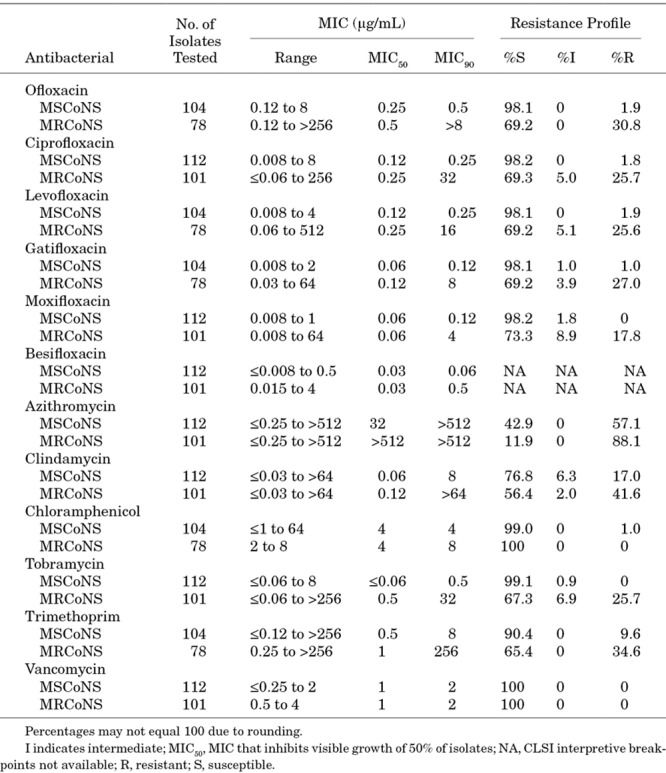
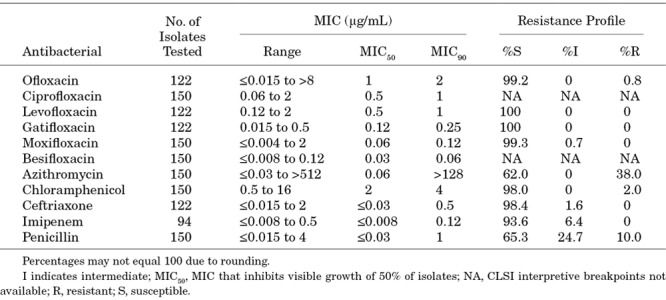
Table 4 presents the MICs and resistance profiles for S. pneumoniae isolates. S. pneumoniae isolates appeared highly susceptible to the antibiotics tested with the exceptions being azithromycin and penicillin (resistance rates of 38.0% and 34.7%, respectively).
TABLE 4.
Minimum Inhibitory Concentrations and Resistance Profiles for S. pneumoniae isolates From Ocular Infections In Pediatric Patients 0–17 Years of Age
All P. aeruginosa isolates were susceptible to the fluoroquinolones tested; all but two isolates were susceptible to tobramycin, and all but one isolate was susceptible to polymyxin B (data provided in Additional File 1, Supplemental Digital Content 1, http://links.lww.com/INF/D305). Notably, about one fifth of P. aeruginosa isolates demonstrated resistance to the beta-lactam imipenem (10 of 52 [19.2%]). The MIC90 against P. aeruginosa was no greater than 2 μg/mL for the fluoroquinolones, 1 μg/mL for tobramycin, 4 μg/mL for imipenem and 2 μg/mL for polymyxin B.
Multidrug Resistance
MR staphylococcal isolates were more likely to be concurrently resistant to another drug class when compared with MS staphylococcal isolates, with P ≤ 0.018 for resistance to representative antibiotics from the macrolide, fluoroquinolone, lincosamide and aminoglycoside classes (Fig. 1). The percentage of multidrug resistance among staphylococcal isolates is presented in Figure 2. Among all S. aureus and all CoNS isolates, rates of multidrug resistance (resistance to ≥3 classes of antibiotics) were 13.0% (37 of 284) and 36.6% (78 of 213), respectively, whereas among MRSA and MRCoNS rates were 49.3% (34 of 69) and 70.3% (71 of 101), respectively.
FIGURE 1.
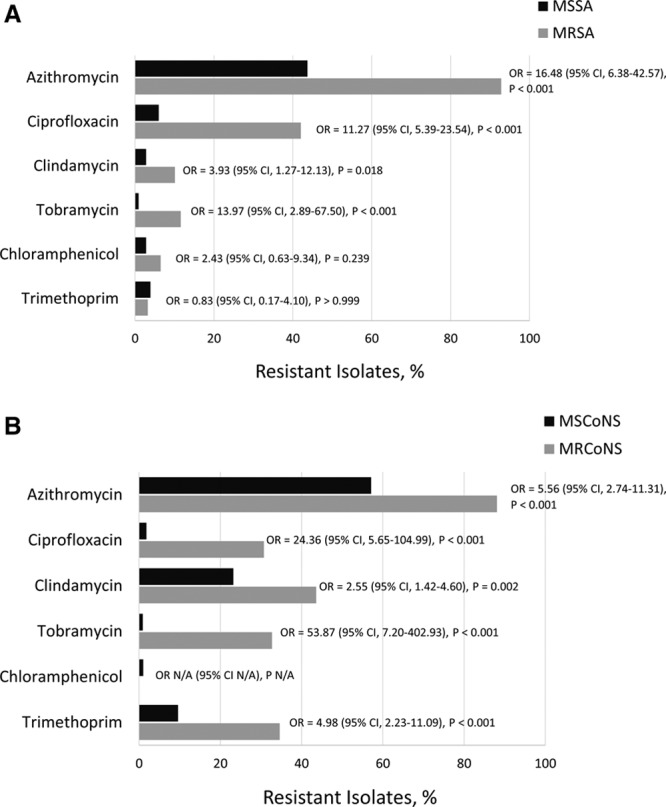
Resistance to antibacterial agents against staphylococcal isolates from pediatric patients collected in the ARMOR study. Staphylococcus aureus (A) and CoNS (B) isolates were tested for resistance to representative macrolide (azithromycin), fluoroquinolone (ciprofloxacin), lincosamide (clindamycin), dihydrofolate reductase inhibitor (trimethoprim) and aminoglycoside (tobramycin) antibacterials. Odds ratios (OR) and P values were obtained from logistic regression models.
FIGURE 2.
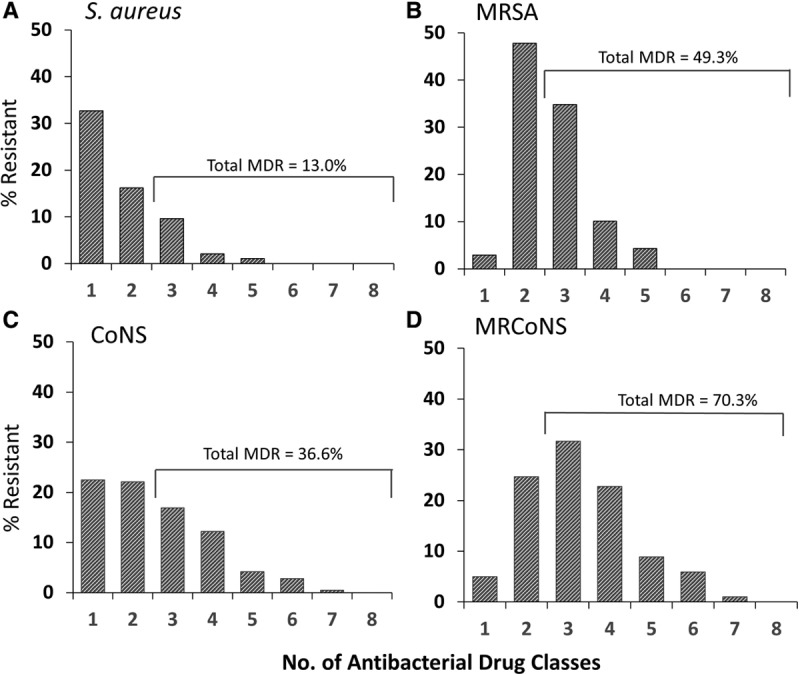
Multidrug resistance among ocular isolates collected from pediatric patients in the ARMOR study. Isolates were tested against ciprofloxacin, azithromycin, clindamycin, chloramphenicol, tobramycin, oxacillin, vancomycin, tetracycline and trimethoprim. The percentage of resistance includes intermediate resistance. MDR indicates multidrug resistant.
Resistance Rates Over Time
There were only two significant changes observed in resistance rates over the 8-year study period; among S. aureus, the percentage of isolates resistant to oxacillin decreased significantly from 17.1% in 2009 to 11.6% in 2016 (P = 0.014) and ciprofloxacin resistance also decreased significantly from 17.1% in 2009 to 4.7% in 2016 (P = 0.001). No other longitudinal trends were observed for any other ocular species/antibiotic class combination.
DISCUSSION
Surveillance data on antibiotic resistance patterns among common ophthalmic pathogens can inform clinical treatment decisions, particularly when instituting empiric therapy in the absence of culture and sensitivity data. The current analysis is the first to specifically report on in vitro antibiotic resistance among isolates of H. influenzae, S. aureus, CoNS, S. pneumoniae and P. aeruginosa cultured from ocular infections specific to the pediatric population from a nationwide surveillance study. The analysis included data from nearly 1000 ocular isolates obtained from pediatric patients across 67 U.S. clinical sites. Although the precise ocular tissue source of the isolates was reported for only about one-third of isolates, where reported, the majority was collected from the conjunctiva suggesting a significant proportion of isolates evaluated may have originated from bacterial conjunctivitis cases. By organism, the majority of isolates were obtained from patients 0–3 years of age, the one exception being P. aeruginosa for which equal numbers of isolates were obtained from patients 0–3 years of age and patients 11–17 years of age. This observation likely reflects the fact that contact lens wear is a known risk factor for P. aeruginosa ocular infections (typically keratitis) and would be frequent among adolescent patients.44–46
Results of antibiotic resistance testing showed low resistance among ocular H. influenzae from pediatric patients to the antibiotics tested, consistent with reports of low level resistance against these antibiotics among H. influenzae from other sources (ear, nose and/or throat).47–49 However, we did not test for ampicillin resistance, which has been shown among both ocular50–52 and nasopharyngeal49,52–54 isolates. In contrast, we found significant in vitro resistance among staphylococci. Approximately 1 in 2 S. aureus and 3 in 4 CoNS isolates demonstrated in vitro resistance to azithromycin; as well, approximately 1 in 4 S. aureus and 1 in 2 CoNS isolates demonstrated in vitro resistance to oxacillin (ie, MR). Among S. pneumoniae, approximately 2 in 5 isolates demonstrated resistance to azithromycin and to penicillin, whereas little in vitro antibiotic resistance was noted among P. aeruginosa, with the exception of some resistance to imipenem. Of note, nearly identical rates of resistance were found when data from the subset of isolates obtained from pediatric patients 0 to 3 years of age were analyzed (data not shown), emphasizing that within the pediatric population as a whole, antibiotic resistance patterns were consistent regardless of age subgrouping.
In general, our findings specific to ocular isolates from pediatric patients were consistent with findings for the 2009–2015 full ARMOR dataset30 with few exceptions. Notable among these was the finding of an approximately 2-fold higher resistance to imipenem among pediatric P. aeruginosa isolates and a lower resistance to methicillin among pediatric S. aureus isolates (24.3% vs. 36.6%, respectively), which was expected given the reported greater prevalence of ocular MRSA among older patients.29,51 Again, consistent with prior reporting,30 all staphylococcal isolates in the pediatric dataset were susceptible to vancomycin; this finding is reassuring given that compounded vancomycin is commonly utilized by ophthalmologists when treating particularly resistant ocular infections. As reported previously, MR significantly increases the likelihood of concurrent resistance to other antibiotic classes.29 However, for reasons that are unclear, the percentage of multidrug-resistant S. aureus isolates was lower in the pediatric isolate subset as compared with the full ARMOR dataset (13.0% vs. 33.5%, respectively) as was the percentage of multidrug-resistant MRSA (49.3% vs. 76.4%, respectively).30
The fluoroquinolone besifloxacin was developed exclusively for topical ocular administration, and as such, no systemic susceptibility breakpoints are available to interpret bacterial isolates as resistant or susceptible to besifloxacin. However, besifloxacin demonstrated the lowest MIC90 values of all ophthalmic antibiotics tested against S. aureus and CoNS, including MR isolates and S. pneumoniae. This is consistent with previous studies where the MIC90 of besifloxacin was lower compared with other fluoroquinolones for these species, including those with substantial resistance13,55,56 and suggests a potential for improved efficacy with besifloxacin, especially against MR staphylococcal infections on the ocular surface. Besifloxacin had an MIC90 comparable to that of other newer generation fluoroquinolones tested against P. aeruginosa (MIC90 = 2), while the older generation fluoroquinolone ciprofloxacin had the lowest MIC90 in this class against that organism (MIC90 = 0.25).
The longitudinal nature of this study allowed for analysis of resistance trends over the 8-year study period. Contrary to the reported increase in antibiotic resistance observed among ocular bacteria in the years before 2009, and most notably for MR among staphylococci,25–27,57 there were no significant increases in resistance rates of any ocular bacterial species to the tested antibiotics in the current dataset, consistent with findings in the full ARMOR dataset. In fact, there was a small but significant decrease in in vitro resistance to oxacillin and ciprofloxacin among S. aureus pediatric isolates. Consistent with the decrease in MRSA prevalence over time that was observed in the current study, a previous retrospective analysis of S. aureus isolates from infections (not limited to any anatomical site, mostly skin and soft tissue) in over 39,000 pediatric patients found that oxacillin resistance declined from 41% in 2005 to 32% in 2014 (P < 0.001).58 Further longitudinal data are needed to determine whether this decreasing trend persists among pediatric ocular isolates. Nonetheless, the lack of any increases in resistance over time is a welcome finding, and one that hopefully will be sustained and even improved upon over coming years. To this end, careful adherence to treatment guidelines and implementation of antibiotic stewardship strategies such as those advocated by the Pediatric Infectious Diseases Society59 and the American Academy of Pediatrics60 may minimize antimicrobial resistance development among bacteria causing ocular infections. Empiric coverage against MRSA is encouraged by many experts as long as prevalence of the organism exceeds 10%–15%.61,62
The pediatric ARMOR data are subject to several limitations. The isolate collection was limited to U.S. sites, thus possibly limiting generalizability of the data to other global regions. There is potential for sampling biases as ocular pathogens are not routinely cultured in community-based practices. Indeed the majority of participating centers were hospitals and referral centers, and investigators were instructed to supply isolates for ocular infections that were considered to be of clinical significance, thus the pediatric isolate dataset analyzed here may be skewed toward more difficult cases and may not reflect resistance patterns in community-based practices. The precise ocular source was known for only one-third of isolates, and information on diagnosis was not collected, limiting application of findings to specific ocular infections. Relevant ophthalmic antibiotics were included, but not all agents could be tested. Chloramphenicol, no longer available as a topical formulation in the United States because of its apparent association with systemic adverse drug reactions, is still widely used in other countries as the first line for the treatment of bacterial conjunctivitis and was therefore included63; vancomycin was also included as it is often compounded for ophthalmic use and may be available in a commercial formulation for use in bacterial conjunctivitis in the future.64 Finally, bacterial susceptibility and resistance to tested antibiotics were interpreted using MIC data for systemic breakpoints, which may be of limited value for determining ocular susceptibility given the expected differences in achievable drug concentrations in the eye compared with systemic administration. Nevertheless, if concentrations in ocular tissues after topical administration are assumed to be at least equal to that found in systemic tissues after systemic administration, application of systemic breakpoints is an appropriate method to compare antibiotic susceptibilities among ocular bacterial pathogens.
In summary, in vitro antibiotic resistance appears common among staphylococcal and pneumococcal isolates collected from pediatric patients with ocular infections, but such resistance does not appear to be increasing. MR was prevalent among staphylococci with many strains demonstrating multidrug resistance. Despite limitations in MIC interpretation for topically administered ocular antibiotics, these data may be of interest to clinicians tasked with choosing empiric therapy for pediatric ocular infections and can serve as a benchmark for future surveillance of resistance among pediatric ocular isolates.
ACKNOWLEDGMENTS
The authors thank Thomas R. Sexton, PhD, Stony Brook University (Stony Brook, NY) for performing statistical analyses and funded by Bausch & Lomb Incorporated. The authors also thank Rachel Hathcock, RN, BSN, and Sandra Westra, PharmD of Churchill Communications (Maplewood, NJ) for providing writing and editorial assistance, also funded by Bausch & Lomb Incorporated.
Supplementary Material
Footnotes
Supported by Bausch & Lomb Incorporated.
S.J.A. is on the speaker’s bureau for Pfizer, Inc. P.A.A. has served as an advisory board member for Bausch & Lomb Incorporated, the funding body for this study. C.M.S. and H.H.D. are employees of Bausch & Lomb Incorporated.
S.J.A. and P.A.A. were involved in data acquisition/collection (as both have appointments at clinical centers which contributed isolates to the ARMOR surveillance study). C.M.S. and H.H.D. were responsible for data analysis and study concept/design/supervision. All authors were involved in data interpretation, contributed important content, critically reviewed the article and approved the final version.
Presented in part at the 2016 Annual Meeting of the American Academy of Pediatrics; October 21–25, 2016; San Francisco, CA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Wong VW, Lai TY, Chi SC, et al. Pediatric ocular surface infections: a 5-year review of demographics, clinical features, risk factors, microbiological results, and treatment. Cornea. 2011;30:995–1002.. [DOI] [PubMed] [Google Scholar]
- 2.Song X, Xu L, Sun S, et al. Pediatric microbial keratitis: a tertiary hospital study. Eur J Ophthalmol. 2012;22:136–141.. [DOI] [PubMed] [Google Scholar]
- 3.Amato M, Pershing S, Walvick M, et al. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17:243–247.. [DOI] [PubMed] [Google Scholar]
- 4.Lee YS, Tan HY, Yeh LK, et al. Pediatric microbial keratitis in Taiwan: clinical and microbiological profiles, 1998-2002 versus 2008-2012. Am J Ophthalmol. 2014;157:1090–1096.. [DOI] [PubMed] [Google Scholar]
- 5.Hammersmith KM. Blepharokeratoconjunctivitis in children. Curr Opin Ophthalmol. 2015;26:301–305.. [DOI] [PubMed] [Google Scholar]
- 6.Ali MJ. Pediatric acute dacryocystitis. Ophthalmic Plast Reconstr Surg. 2015;31:341–347.. [DOI] [PubMed] [Google Scholar]
- 7.Baskin DE, Reddy AK, Chu YI, et al. The timing of antibiotic administration in the management of infant dacryocystitis. J AAPOS. 2008;12:456–459.. [DOI] [PubMed] [Google Scholar]
- 8.Teweldemedhin M, Gebreyesus H, Atsbaha AH, et al. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel PB, Diaz MC, Bennett JE, et al. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med. 2007;14:1–5.. [DOI] [PubMed] [Google Scholar]
- 10.Comstock TL, Paterno MR, Usner DW, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trials. Paediatr Drugs. 2010;12:105–112.. [DOI] [PubMed] [Google Scholar]
- 11.Block SL, Hedrick J, Tyler R, et al. Increasing bacterial resistance in pediatric acute conjunctivitis (1997-1998). Antimicrob Agents Chemother. 2000;44:1650–1654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buznach N, Dagan R, Greenberg D. Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance era. Pediatr Infect Dis J. 2005;24:823–828.. [DOI] [PubMed] [Google Scholar]
- 13.DeLeon J, Silverstein BE, Allaire C, et al. Besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis in adults and children. Clin Drug Investig. 2012;32:303–317.. [DOI] [PubMed] [Google Scholar]
- 14.Chen FV, Chang TC, Cavuoto KM. Patient demographic and microbiology trends in bacterial conjunctivitis in children. J AAPOS. 2018;22:66–67.. [DOI] [PubMed] [Google Scholar]
- 15.Abelson M, Protzko E, Shapiro A, et al. A randomized trial assessing the clinical efficacy and microbial eradication of 1% azithromycin ophthalmic solution vs tobramycin in adult and pediatric subjects with bacterial conjunctivitis. Clin Ophthalmol. 2007;1:177–182.. [PMC free article] [PubMed] [Google Scholar]
- 16.Cavuoto K, Zutshi D, Karp CL, et al. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115:51–56.. [DOI] [PubMed] [Google Scholar]
- 17.Cagle G, Davis S, Rosenthal A, et al. Topical tobramycin and gentamicin sulfate in the treatment of ocular infections: multicenter study. Curr Eye Res. 1981;1:523–534.. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112(suppl 4):29S–33S.. [PubMed] [Google Scholar]
- 19.Bertino JS., Jr Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol. 2009;3:507–521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland EJ, McDonald MB, Parekh JG, et al. Antibiotic resistance in acute postoperative endophthalmitis. Ophthalmology. 2014;121(11 suppl):S1–S9; quiz S10.. [DOI] [PubMed] [Google Scholar]
- 21.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34:296–302.. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S. Antibiotic resistance in ocular bacterial pathogens. Indian J Med Microbiol. 2011;29:218–222.. [DOI] [PubMed] [Google Scholar]
- 23.Gentile RC, Shukla S, Shah M, et al. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: a 25-year review. Ophthalmology. 2014;121:1634–1642.. [DOI] [PubMed] [Google Scholar]
- 24.Chang VS, Dhaliwal DK, Raju L, et al. Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20-year review. Cornea. 2015;34:698–703.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asbell PA, Sahm DF, Shedden A. Ocular TRUST 3: Ongoing Longitudinal Surveillance of Antimicrobial Susceptibility in Ocular Isolates. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 7, 2009; San Francisco, CA. [Google Scholar]
- 26.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–958.. [DOI] [PubMed] [Google Scholar]
- 27.Asbell PA, Sahm DF. Longitudinal Nationwide Antimicrobial Susceptibility Surveillance in Ocular Isolates: Results for Ocular TRUST 2. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 28, 2008; San Diego, CA. [Google Scholar]
- 28.Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152:567–574.e3.. [DOI] [PubMed] [Google Scholar]
- 29.Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance among ocular pathogens in the united states: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133:1445–1454.. [DOI] [PubMed] [Google Scholar]
- 30.Asbell PA, Sanfilippo CM. Antibiotic resistance trends among ocular pathogens in the US—cumulative results from the antibiotics resistance monitoring in ocular microorganisms (ARMOR) surveillance study. US Ophthalmic Rev. 2017;10:35–38.. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 2009. 8th ed Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M7-A8. [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 2012. 9th ed Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M7-A9. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 2015. 10th ed Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M7-A10. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement. 2009. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S19. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. 2010. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S20. [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement. 2011. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S21. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement. 2012. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S22. [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement. 2013. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S23. [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement. 2014. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S24. [Google Scholar]
- 40.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement. 2015. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S25. [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Sixth Informational Supplement. 2016. Wayne, PA: Clinical & Laboratory Standards Institute; CLSI document M100-S26. [Google Scholar]
- 42.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212.. [Google Scholar]
- 43.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386.. [Google Scholar]
- 44.Dutta D, Cole N, Willcox M. Factors influencing bacterial adhesion to contact lenses. Mol Vis. 2012;18:14–21.. [PMC free article] [PubMed] [Google Scholar]
- 45.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–278.. [DOI] [PubMed] [Google Scholar]
- 46.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27.. [DOI] [PubMed] [Google Scholar]
- 47.Olzowy B, Kresken M, Havel M, et al. ; Working Party ‘Antimicrobial Resistance’ of the Paul-Ehrlich-Society for Chemotherapy. Antimicrobial susceptibility of bacterial isolates from patients presenting with ear, nose and throat (ENT) infections in the German community healthcare setting. Eur J Clin Microbiol Infect Dis. 2017;36:1685–1690.. [DOI] [PubMed] [Google Scholar]
- 48.Bae S, Lee J, Lee J, et al. Antimicrobial resistance in Haemophilus influenzae respiratory tract isolates in Korea: results of a nationwide acute respiratory infections surveillance. Antimicrob Agents Chemother. 2010;54:65–71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–389.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller D. Update on the Epidemiology and Antibiotic Resistance of Ocular Infections. Middle East Afr J Ophthalmol. 2017;24:30–42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oydanich M, Dingle TC, Hamula CL, et al. Retrospective report of antimicrobial susceptibility observed in bacterial pathogens isolated from ocular samples at Mount Sinai Hospital, 2010 to 2015. Antimicrob Resist Infect Control. 2017;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dabernat H, Delmas C. Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001-2008: a retrospective database analysis. Eur J Clin Microbiol Infect Dis. 2012;31:2745–2753.. [DOI] [PubMed] [Google Scholar]
- 53.Tyrstrup M, Melander E, Hedin K, et al. Children with respiratory tract infections in Swedish primary care; prevalence of antibiotic resistance in common respiratory tract pathogens and relation to antibiotic consumption. BMC Infect Dis. 2017;17:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H, Wang A, Tong J, et al. Nasopharyngeal carriage and antimicrobial susceptibility of Haemophilus influenzae among children younger than 5 years of age in Beijing, China. BMC Microbiol. 2015;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas W, Pillar CM, Hesje CK, et al. Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. J Antimicrob Chemother. 2010;65:1441–1447.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanfilippo CM, Hesje CK, Haas W, et al. Topoisomerase mutations that are associated with high-level resistance to earlier fluoroquinolones in Staphylococcus aureus have less effect on the antibacterial activity of besifloxacin. Chemotherapy. 2011;57:363–371.. [DOI] [PubMed] [Google Scholar]
- 57.Asbell PA, Sahm DF, Shaw M, et al. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg. 2008;34:814–818.. [DOI] [PubMed] [Google Scholar]
- 58.Sutter DE, Milburn E, Chukwuma U, et al. Changing susceptibility Staphylococcus aureus of in a US pediatric population. Pediatrics. 2016;137:e20153099. [DOI] [PubMed] [Google Scholar]
- 59.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33:322–327.. [DOI] [PubMed] [Google Scholar]
- 60.Kimberlin DW, Brady MT, Jackson MA, et al. Red Book: 2015 Report of the Committee on Infectious Diseases. 201530th ed Elk Grove Village, IL, American Academy of Pediatrics. [Google Scholar]
- 61.Gorwitz RJ, Jernigan DB, Powers JH; participants in the Centers for Disease Control and Prevention -convened Experts’ Meeting on Management of MRSA in the Community. Strategies for Clinical Management of MRSA in the Com munity: Summary of an Experts’ Meeting Convened By the Centers for Disease Control and Prevention, March 2006. Available at: https://www.cdc.gov/mrsa/pdf/MRSA-Strategies-ExpMtgSummary2006.pdf. Accessed May 2, 2018.
- 62.Kaplan SL. Staphylococcus aureus infections in children: The implications of changing trends. Pediatrics. 2016;137:e20160101. [DOI] [PubMed] [Google Scholar]
- 63.Fraunfelder FW, Fraunfelder FT. Restricting topical ocular chloramphenicol eye drop use in the United States. Did we overreact? Am J Ophthalmol. 2013;156:420–422.. [DOI] [PubMed] [Google Scholar]
- 64.Safety and Efficacy of Vancomycin Ophthalmic Ointment in Patients with Moderate to Severe Bacterial Conjunctivitis (NCT02432807). Kurobe LLC, May 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02432807?term=vancomycin&cond=Bacterial+Conjunctivitis&rank=1. Accessed March 21, 2018.


