Abstract
Converging evidence across species highlights the contribution of environmental stress to anhedonia (loss of pleasure and/or motivation). However, despite a clear link between stress and the emergence of anhedonic-like behavior in both human and animal models, the underlying biological pathways remain elusive. Here, we synthesize recent findings across multiple levels, from molecular signaling pathways through whole-brain networks, to discuss mechanisms through which stress may influence anhedonia. Recent work suggests the involvement of diverse systems that converge on the mesolimbic reward pathway, including medial-prefrontal cortical circuitry, neuroendocrine stress responses, homeostatic energy regulation systems, and inflammation. We conclude by emphasizing the need to disentangle the influences of key dimensions of stress on specific aspects of reward processing, taking into account individual differences that could moderate this relationship.
Keywords: Stress, Anhedonia, Reward Processing, Motivation, Psychiatric Illness, Dopamine, Mesolimbic Pathway
Stress, anhedonia, and psychiatric illness.
Our responses to environmental stressors help guide decision-making in an evolutionary balancing act that pits the pursuit of rewards—crucial for survival and reproduction (e.g., food and mating opportunities)—against potential threats (e.g., predators and pathogens) [1,2]. Stressors can tip this balance by decreasing reward-seeking behavior [3]. Seen through an evolutionary lens, decreased approach behavior in response to environmental threats may be highly adaptive in some contexts. For example, following physical harm or the threat of infectious disease, anhedonia (see Glossary) and social withdrawal may preserve resources for healing wounds and inhibit the spread of pathogens [4,5]. However, the adaptive value of a given trait or behavior is sensitive to both environmental context and the complexity of interactions across cognition, behavior, and genetics [2,6]. For some individuals with existing vulnerabilities, such as ruminative coping styles following a traumatic event [7], stress responses culminating in anhedonia can be maladaptive and even trigger the onset of psychiatric illness.
In this Review we highlight some of the diverse circuit-level and molecular mechanisms through which stress could lead to anhedonia. In doing so, we adopt a multi-system, multi-level approach, in which we examine how the effects of stress may echo across levels of analysis (e.g., molecular processes and functional circuits) and involve interactions between diverse systems (e.g., immune responses that alter brain reward functioning). We first discuss likely contributors to stress-induced anhedonia at the level of neurocircuitry, including systems that govern motivated behavior, neuroendocrine responses to stress, and energy homeostasis. Next, we review possible pathways to stress-induced anhedonia at the molecular level, with a particular focus on immune system signaling pathways. We conclude with a roadmap of promising future directions in the study of stress and anhedonia.
Impact of stress on anhedonia.
Research across species, including rodents and humans, has demonstrated a link between stress and anhedonic-like behaviors [3,8–10]. Here, we briefly review evidence of this relationship. For more details, including on cross-species comparisons, we direct readers to existing reviews on the topic [3,8–10].
Rodent studies have employed a variety of stress manipulations. These include social defeat stress, in which a rodent is placed in proximity to another, aggressive rodent and subjected to physical attack [11]; and chronic mild stress (CMS), in which rodents are exposed to an unpredictable series of stressors, including 24-hour constant illumination, food deprivation, and damp bedding [12,13]. Research groups employing these approaches have discovered associated decreases in reward-seeking behaviors, suggestive of decreased pleasure and/or motivation. Social defeat and CMS in rodents, for example, produce blunted sucrose preference and/or diminished social interaction [9,10,12].
Although considerably less work has focused on the study of stress and anhedonia in humans, at least in well-controlled settings, results are broadly consistent with the nonhuman animal literature [8,14]. Following naturally-occurring stressors (e.g., medical residence examinations [15]), individuals self-report decreased pleasure in daily activities [16] and exhibit lowered sensitivity to reward devaluation [15]. Laboratory studies using threat of shock as a stressor [17,18] have found decreased response bias toward rewarded outcomes [17] and diminished reward sensitivity [18]. In support of this experimental evidence, large, observational studies have established a link between life stress and phenotypes that are often marked by anhedonia, such as major depressive disorder (MDD) [19–21]. Notably, few large-scale studies have assessed the impact of stress on anhedonia per se (for an exception, see [22]), and more work is needed to examine individual symptoms. In all, converging evidence across species suggests that stress can produce anhedonic behavior.
Putative circuit-level mediators of stress-induced anhedonia.
Effects of stress on motivated behavior depend on the interplay of systems spanning the medial prefrontal cortex (mPFC), midbrain and striatum, amygdala, hypothalamus, brainstem, and other regions implicated in reward processing [9] (Box 1). Due to space constraints, we focus on the contributions of three brain systems: mesocorticostriatal reward circuits (mPFC, midbrain, and striatum); subcortical stress response circuits (including hypothalamus and extended amygdala); and brainstem-based energy homeostasis circuits (including GLP-1 neurons). We would like to note that although we discuss these three systems separately for the purposes of organization, the distinctions between them are partly arbitrary. The amygdala, for instance, is involved in reward processing [23]. Other reviews cover emerging research on the contributions of mu opioid systems [14] and the lateral habenula [24], and provide a more molecular-level focus on stress-induced changes in corticostriatal circuitry [9].
Box 1. Reward processing, dopamine, and the mesolimbic system.
Reward processing recruits diverse neural circuits spanning numerous brain regions, including the basal ganglia, medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), and amygdala [126]. Here, we provide a brief review focused on the dopaminergic mesolimbic pathway, given its well-established relation to anhedonia [9], although other circuits likely contribute as well. Many of the stress-initiated mechanisms described in this Review converge on the mesolimbic pathway (see Figure 1).
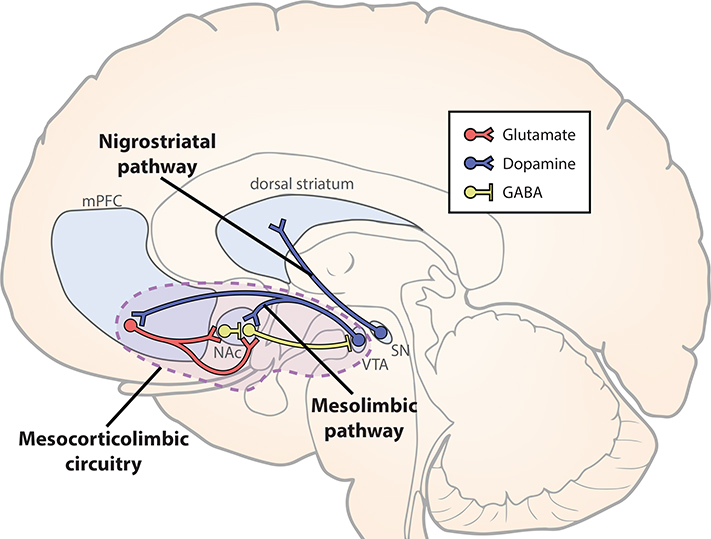
The mesolimbic pathway consists of dopaminergic neuronal projections from the ventral tegmental area (VTA) to nucleus accumbens (NAc) (Figure I). Dopamine neurons, including those of the mesolimbic pathway, play a key role in reward processing [127–129]. Their specific role may vary based on time [128] and on the particular population of dopamine neurons [e.g. 130]. For instance, researchers have argued that an initial component of the dopamine response is sensitive to stimulus salience, whereas a subsequent component encodes a reward prediction error (RPE) [128]. That is, the dopaminergic RPE signal spikes following unpredicted rewards, and is sensitive to reward value. As rewards become more predictable (e.g., due to association with predictive cues), dopaminergic firing spikes in response to reward-predicting cues and is suppressed when expected rewards are omitted [131]. Recent evidence suggests that mesolimbic dopaminergic firing encodes the value of working for a reward, and thus may signal reward value and influence motivation [132].
Distinct populations of dopamine neurons appear to differentially impact reward processing vs. locomotion, suggesting heterogeneity of dopamine subpopulations [130]. Dopamine also acts on functionally heterogeneous receptors: D1-type receptors, which are excitatory, and D2-type receptors, which inhibit neuronal firing. Spikes in mesolimbic dopamine activity (e.g., in response to unexpected rewards) excite the D1 receptor-expressing “direct” pathway, leading to behavioral reinforcement [133,134]. By contrast, suppressed dopaminergic firing disinhibits the D2 receptor-expressing “indirect” pathway to facilitate learning from non-reward/punishment [133,134].
Activity in the mesolimbic pathway appears key for reward learning and for anhedonic responses following stress (see Stress and the mesolimbic reward circuit). However, other dopaminergic projections also likely contribute to reward processing. For example, the nigrostriatal pathway consists of dopaminergic projections from substantia nigra to dorsal striatum [135]. Dorsal striatal activity appears to play a relatively larger role in goal-directed and habit-based reward learning, whereas ventral striatum (including NAc) predominantly contributes to associative learning [136].
mPFC is closely interconnected with mesolimbic regions [126] (Figure I). mPFC receives dopaminergic projections from VTA [137] and sends glutamatergic projections to NAc [138]. The vast majority of NAc neurons are GABAergic [139], including fast-spiking interneurons [140] and projections to VTA (both direct and indirect through the ventral pallidum) [141]. Connections among these regions form a mesocorticolimbic circuit, which is implicated the computation of reward value and motivation [39,40,136]. Thus, mPFC is ideally situated to influence mesolimbic dynamics, either directly (via glutamatergic synapses on NAc interneurons, or on NAc projections to VTA) or indirectly (via wide-ranging projections of mPFC). Notably, mesolimbic regions are connected with a host of other regions implicated in reward processing, including orbitofrontal cortex (OFC), amygdala, and hippocampus [126].
Stress and the mesolimbic reward circuit.
The mesolimbic reward circuit, which includes the ventral tegmental area (VTA) and nucleus accumbens (NAc), plays a key role in reward processing and motivated behavior (Box 1). A substantial literature has addressed whether the functioning of this circuit, in particular dopaminergic signaling, may mediate the effects of stress on subsequent anhedonic-like behavior [9,10,25].
Seemingly conflicting reports have emerged regarding VTA spontaneous dopaminergic firing and anhedonic-like responses to stress [10]. Several studies using social defeat stress have reported increased firing rates in “susceptible” mice—that is, those which exhibited decreased social interactions and decreased sucrose preference [26–28]. Yet other studies in rats found that CMS decreased the number of spontaneously firing neurons, leading to decreased mobility in the forced swim test, which is thought to represent anhedonic-like behavior [29,30]. These studies generally found no significant change in firing rates (except [29], Experiment 3). One possibility is that stress may induce increased firing rates in VTA dopamine neurons, but a decreased number of spontaneously active neurons.
Experimental manipulations of dopaminergic firing have also exerted apparently divergent effects on anhedonic behavior [13,31]. For example, induced phasic dopaminergic firing (via optogenetics) in VTA rescued decreases in sucrose preference caused by CMS [13]. Yet a study of social defeat stress found that optogenetically-induced phasic dopaminergic firing rendered mice more susceptible to anhedonic effects of stress, as reflected in reduced sucrose preference and decreased social interaction [31].
Numerous differences in study design could account for these apparently discrepant findings. As others have noted [10], several parameters varied across studies, including stressor type (social defeat vs. chronic mild stress), chronicity (10 days of social defeat vs. 4–6 weeks of CMS), and measure of dopaminergic activity (number of spontaneously active neurons vs. neuronal firing rate). It is possible that stress could decrease the number of spontaneously active dopamine neurons [29,30] while also increasing firing rates of the remaining, spontaneously active dopamine neurons [26–28] in susceptible animals. However, few studies report both measures. Also, studies of CMS often report main effects of stress, but rarely distinguish between susceptible and unsusceptible rodents.
Additionally, VTA contains phenotypically diverse dopamine neurons. Some VTA dopamine neurons co-release glutamate or GABA [32], and VTA dopamine neurons follow diverse projection pathways [32,33]. Thus, responses to stress may depend on the specific population of VTA neurons under study. For example, CMS produces a decrease in spontaneously active dopamine neurons in medial VTA (which primarily project to NAc) and central VTA, but not lateral VTA [30]; and susceptibility to social defeat increases after phasic stimulation of VTA projections to NAc, but not projections to mPFC [31].
Taken together, the existing literature suggests that stress impacts dopaminergic functioning, but the underlying mechanisms remain unclear. Further work may clarify which qualities of stressors affect dopaminergic firing rates vs. number of spontaneously active neurons, and whether such changes are necessary and sufficient to produce anhedonic behavior following stress.
Medial prefrontal cortex regulation of the mesolimbic circuit.
What is the mechanism through which stress impacts mesolimbic dopamine activity and perturbs reward functioning? Stress causes wide-ranging changes in brain structure and function, including in hippocampus, amygdala, and across PFC [34,35]. These interconnected regions may therefore mediate the effects of stress on anhedonia, especially given their roles in fear response and fear conditioning [36–38], as well as in guiding behavior based on incentive value [23,39,40]. To highlight some of the exciting recent work spanning rodents [30,41] and humans [42,43], this section focuses on the mPFC-mesolimbic circuit.
Stress may perturb mPFC-mesolimbic interactions via structural changes in the mPFC, for instance dendritic remodeling [44]. Specifically, in rats, chronic restraint stress or chronic immobilization cause dendritic shrinkage and spine loss in mPFC (prelimbic and infralimbic cortex) [44]. In humans, stress increases risk for several psychiatric disorders that commonly involve anhedonic symptoms: MDD, schizophrenia, and post-traumatic stress disorder (PTSD) [8,45,46]. These disorders are also marked by decreased mPFC gray matter volume (MDD and PTSD) [47–49] or accelerated gray matter loss in mPFC (schizophrenia) [50]. These gray matter differences are relatively subtle [2,48,50]. For instance, an estimated <1% annual change in cortical thickness characterizes converters to schizophrenia, although effect sizes for the comparison with high-risk non-converters and controls ranged from medium to large [50]. Additionally, mPFC volume may also relate to illness chronicity: As one example, left mPFC cortical thickness in patient populations is inversely related to number of depressive episodes [51]. Further work is needed to elucidate the mechanism through which these structural changes might give rise to alterations in mPFC function.
Given these stress-induced changes in mPFC structure, researchers have tested the hypothesis that stress produces anhedonia through mPFC hypofunction. In mice exhibiting anhedonic-like behavior (decreased sucrose preference and decreased social interaction) following social defeat stress, optogenetically-induced phasic firing in mPFC (prelimbic and infralimbic) attenuated these deficits [52]. This result could suggest that induced phasic firing compensated for a deficit in mPFC activity. In one study, following social defeat stress, the firing rate of VTA dopamine neurons projecting to mPFC decreased by about 80% [31], suggesting that decreased dopaminergic input to mPFC may contribute to mPFC hypoactivity. Work in humans also supports the mPFC hypofunction hypothesis: Perceived life stress [53] and stress induced by aversive video clips [54] are associated with decreased blood oxygenation level dependent (BOLD) activity in mPFC during reward anticipation and receipt. By itself, this evidence is consistent with the notion that stress leads to mPFC hypofunction and related decreases in motivated behavior.
Yet other findings suggest a more complex relation between mPFC-striatal activity and motivated behavior. In a key study, researchers employed optogenetic techniques in rats to stably increase excitability of mPFC (primarily infralimbic) neurons [41]. Increased mPFC excitability led to reversible reductions in reward seeking, as evidenced by decreased sucrose preference and social interaction. Furthermore, increased mPFC excitability led to blunted BOLD responses in dorsal striatum following dopaminergic midbrain excitation. This result suggests that heightening mPFC excitability caused decreased reward-seeking behavior by altering interactions between midbrain dopamine neurons and the striatum [41] through an unknown mechanism. This interpretation was supported by a recent study examining CMS and dopaminergic functioning in rats [30]. In non-stressed rats, pharmacological activation of mPFC (infralimbic) selectively inhibited dopamine neurons in medial VTA. CMS decreased the number of spontaneously firing dopamine neurons in medial and central, but not lateral, VTA. This decrease was rescued by pharmacological inactivation of mPFC (infralimbic) [30]. However, following a social defeat paradigm, the synaptic strength of mPFC-to-ventral striatal connections did not significantly differ between stress-susceptible and resilient mice [55], suggesting no contribution of this pathway to subsequent anhedonic behavior. This seemingly discrepant finding could be explained by indirect, rather than direct, influences of mPFC function on mesolimbic activation [30]. Alternately, different types of stress—e.g., CMS vs. social defeat stress—may exert different impacts on corticostriatal pathways (see Future directions in the study of stress and anhedonia). Nevertheless, when taken together, these results provide evidence that mPFC hyperactivity may contribute to anhedonia following stress.
Recent work in humans also suggests that mPFC-striatal connectivity may contribute to anhedonia in individuals with MDD and remitted MDD (rMDD). One study examined individuals with rMDD using spectral dynamic causal modeling of BOLD functional connectivity [43], an analytic approach that uses Bayesian inference to estimate directional interactions between neural systems. In response to a naturalistic positive mood induction, individuals with rMDD exhibited less reciprocal mPFC-ventral striatal connectivity and were characterized instead by mPFC modulation of ventral striatum (VS), relative to controls (see Box 1 for a discussion of corticostriatal structure and function). This pattern of functional connectivity in individuals with rMDD was accompanied by lower mood approximately 30 minutes following the induction [43].
A second study endeavored to characterize biological sub-phenotypes (“biotypes”) in large samples of individuals with MDD using BOLD functional connectivity data and MDD symptoms [42]. Hierarchical clustering analyses yielded four biotypes based on similar patterns of connectivity features. Hyperconnectivity in frontostriatal (and thalamic) networks characterized two of the biotypes, and this hyperconnectivity was associated with anhedonia and psychomotor retardation [42]. These results are consistent with the hypothesis that mPFC interaction with mesolimbic circuitry regulates motivation.
In summary, evidence suggests that a) stress causes dendritic shrinkage and spine loss in mPFC; b) mPFC activity alters mesolimbic dynamics; and c) mPFC may mediate the impact of stress on mesolimbic function and anhedonia. Notably, to fully capture the role of mPFC in stress-induced anhedonia, it may be necessary to examine how various subregions of mPFC coordinate with other brain regions implicated in stress.
Neuroendocrine stress responses and mesolimbic reward processing.
Neuroendocrine stress responses induce a variety of physiological changes to cope with threat, such as mobilizing stored energy for use by muscle [56]. In addition, neuroendocrine responses may inhibit or enhance reward seeking, depending on site of action and prior stress exposure (see below). Thus, neuroendocrine activity is also poised to mediate the link between stress and anhedonia. Much of the research on this possibility has focused on corticotropin-releasing factor (CRF), a key component of HPA axis functioning (see Box 2 and Figure 2). CRF is released by neurons in regions such as the hypothalamic paraventricular nucleus (PVN), the bed nucleus of the stria terminalis (BST), and central nucleus of the amygdala (CeA) [57], which contribute to the expression of fear and anxiety [38,58] and promote hormonal responses to threat [59]. CRF-releasing neurons from these regions project to VTA and NAc [60,61] (Figure 1, Key Figure), where CRF influences dopamine release and motivated behavior, but with divergent effects in either region [10], as discussed further below.
Box 2. Stress, inflammation, and the HPA axis.
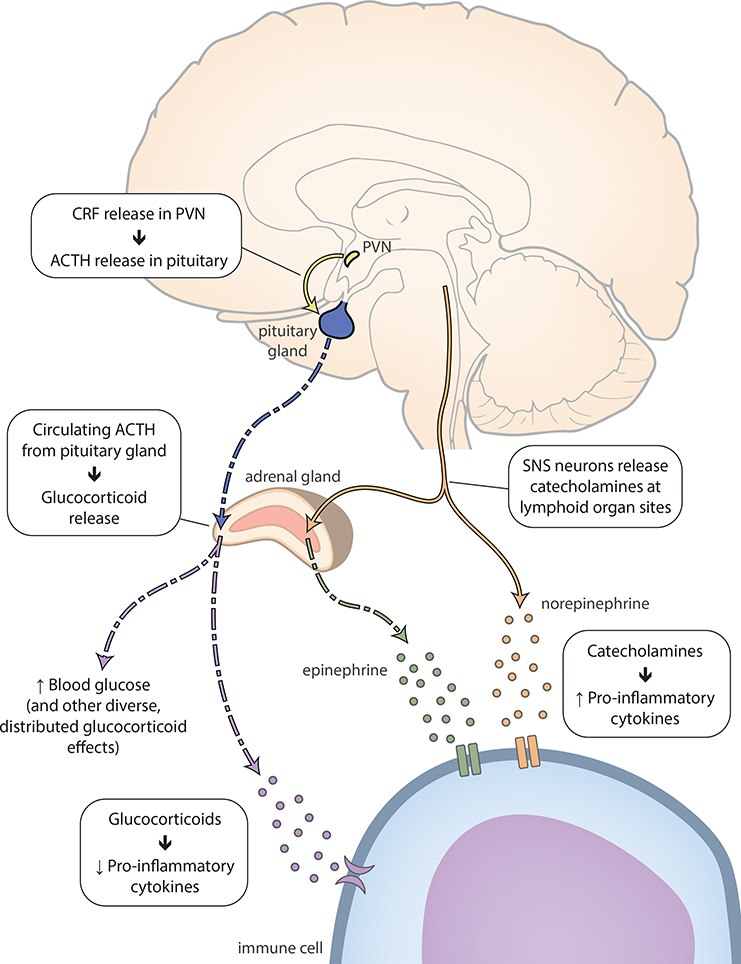
Although mammalian stress responses are complex, we provide a brief and schematized review of neuroendocrine and inflammatory responses here (for more detailed reviews, see [56,110,123]). Stress upregulates activity in two key systems: the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) [124]. HPA axis responses originate in the hypothalamic paraventricular nucleus (PVN), which releases corticotropin-releasing factor (CRF) into the median eminence (Figure 2). CRF binding in the pituitary gland causes the release of adrenocorticotropic hormone (ACTH) into the blood [123]. Circulating ACTH reaches binding sites in the adrenal cortex, causing the release of glucocorticoids (in humans, primarily cortisol) [123]. Glucocorticoids promote a variety of effects at numerous sites of action, including boosted glucose concentration in the bloodstream, which provides fast energy resources to cope with potential threat [56].
At the same time, sympathetic nervous system projections from the brainstem release catecholamines (including norepinephrine, epinephrine, and dopamine) at peripheral sites (including lymphoid organs, such as the thymus, spleen, and lymph nodes) (Figure 2) [124]. Cathecholamines then act on immune cell receptors at these sites, causing the release of cytokines that upregulate inflammation [125].
Broadly speaking, glucocorticoids suppress inflammatory cytokine activity [56] that might interfere with “fight or flight” behavioral coping [110]. However, as glucocorticoid responses wane, inflammation may produce “sickness behaviors” (e.g., decreased feeding and socializing) which are thought to facilitate recovery processes (e.g., wound healing) [4,5].
Figure 2. Neuroendocrine and inflammatory stress responses.
The PVN releases CRF, which reaches the pituitary gland through the median eminence. CRF binding in the pituitary gland causes ACTH release into the circulation [123]. Circulating ACTH reaches binding sites in the adrenal cortex, releasing glucocorticoids (in humans, primarily cortisol) [123]. Glucocorticoids act on immune cell receptors to downregulate pro-inflammatory cytokines, decreasing inflammation. At the same time, SNS terminals at release catecholamines at peripheral sites [124]. These neurotransmitters act on immune cell receptors, causing the release of cytokines that upregulate inflammation [125]. ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor; PVN, hypothalamic paraventricular nucleus; SNS, sympathetic nervous system.
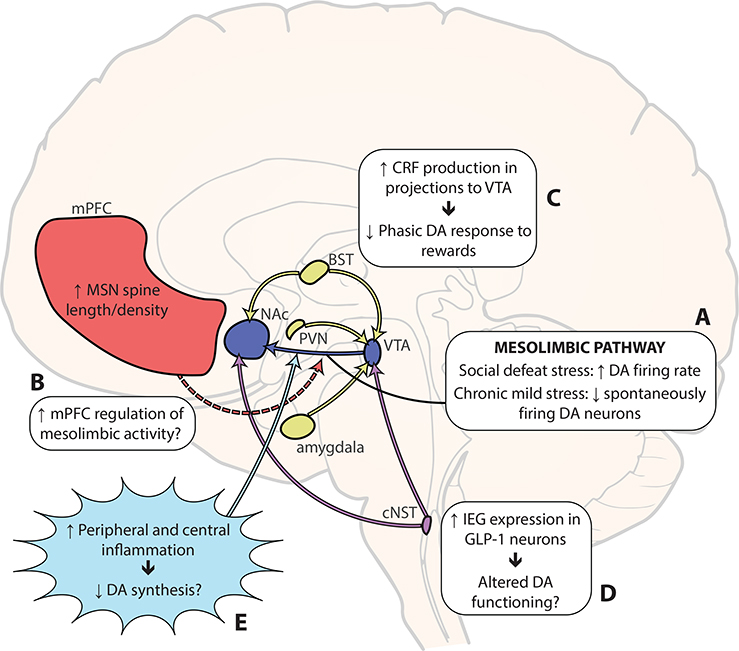
Figure 1, Key Figure. Putative circuit-level and molecular mechanisms of stressinduced anhedonia.
(A) In studies using social defeat stress in mice (applied over 10 days), an increase in the firing rate of VTA dopamine neurons was observed, but only in the “susceptible” group (those animals that developed anhedonic-like behaviors following stress) [26–28]. Chronic mild stress (4–7 weeks) decreased the number of spontaneously active VTA dopamine neurons [13,29,30]. (B) Some work suggests that increased mPFC excitability could suppress activity in the mesolimbic pathway [30,41]. (C) Endogenous CRF release in VTA seems to mediate the effect of restraint stress on motivation to work for food reward, likely by decreasing phasic dopamine responses to reward [64]. (D) GLP-1 signaling appears to mediate the hypophagic effects of restraint stress [77], likely by decreasing the rewarding value of food [82]. GLP-1 neurons in cNST project directly to VTA and NAc [83], where they appear to influence dopaminergic functioning, although the direction of the effect is unclear [85–88]. More research is needed to assess whether GLP-1 neurons (and other homeostatic energy systems) contribute to stress-induced anhedonia. (E) Inflammation may inhibit dopamine availability [100], either by inhibiting the function of enzymes in the dopamine biosynthetic pathway (see [93]) or by creating oxidative stress through increased kynurenine [106]. BST, bed nucleus of the stria terminalis; cNST, caudate nucleus of the solitary tract; CRF, corticotropin-releasing factor; DA, dopamine; GLP-1, glucagonlike peptide-1; mPFC, medial prefrontal cortex; MSN, medium spiny neuron; NAc, nucleus accumbens; PVN, hypothalamic paraventricular nucleus; VTA, ventral tegmental area.
In the rat VTA, uncontrollable foot-shock stress causes CRF release [62]. Injection of CRF into VTA dose-dependently increases the baseline firing rate of dopamine neurons [63], but decreases phasic dopamine response to food rewards (but not reward-predictive cues) [64]. Further, restraint stress decreases motivation to work for food reward in a progressive ratio task [64] and biases decision making towards low effort/low reward choices [65], which can be blocked by a CRF antagonist injected into VTA [64,65]. Thus, CRF release in VTA appears to decrease reward motivation following stress, likely via dopaminergic changes.
CRF activity in NAc seems to exert rather different effects on motivated behavior [66,67]. Injection of CRF into NAc increased the ability of Pavlovian reward cues to enhance lever-pressing for sucrose rewards [66] and caused conditioned place preference alongside increased dopamine release [67]. These results suggest that, unlike in VTA, CRF release in NAc enhances reward conditioning. However, severe forced-swim stress abolished the ability of CRF to increase dopamine release, and also switched the behavioral effect of CRF to conditioned place aversion [67]. Thus, impact of CRF release in NAc appears to be conditional on prior stress exposure.
CRF may exert its influence on mesolimbic functioning by gating the release of brain-derived neurotrophic factor (BDNF) following stress [27,68–70]. Numerous studies suggest that following 10-day social defeat stress, BDNF released from VTA acts on receptors in NAc to mediate the impact of stress on reward-seeking and social behavior [27,68,70]. Even briefer, “subthreshold” social defeat stress, when combined with phasic, optogenetic stimulation of VTA-NAc neurons, produced social interaction deficits and increased BDNF levels in NAc [69]. Importantly, CRF antagonism in NAc prior to subthreshold stress and optogenetic stimulation prevented this increase in BDNF and rescued effects of stress on social interaction [69]. Thus, CRF release may be necessary to produce stress-induced alterations in NAc BDNF and decreased social interaction.
Preliminary human work supports an effect of CRF on reward processing and behavior. One study examined a single-nucleotide polymorphism (SNP) in the CRHR1 gene that codes for the CRF receptor CRHR1 [71]. Homozygosity for the A allele of this SNP was associated with blunted neural response to rewards (indexed by scalp-recorded electrophysiology) under acute stress (threat of shock). A/A individuals also exhibited a decreased behavioral response bias under stress. Decreased response bias in this task has been previously associated with MDD status, and with increased anhedonia symptoms in individuals with MDD [72]. Though sample size in the genotyping study was relatively small (n=84) for detection of a gene by environment interaction, and the effects warrant replication, these results are concordant with animal literature suggesting an effect of stress-induced CRF on anhedonia.
In summary, neuroendocrine stress responses may recalibrate mesolimbic reward processing: CRF appears to decrease reward motivation via actions in the VTA, but enhances reward conditioning in NAc, except after prior severe stress exposure. CRF may exert these effects by moderating stress-induced BDNF release. More work in this domain will be crucial to understanding how HPA axis functioning and mesolimbic reward circuits coordinate during stress responses, and how dynamics in these systems contribute to stress-induced anhedonia.
Stress, energy homeostasis, and reward.
Maintaining energy homeostasis—e.g., by regulating feeding and satiety— requires flexible adjustments to reward seeking [73,74]. For example, rodents that have consumed food to satiety exhibit diminished motivation for food rewards [75]. To accomplish this, energy homeostasis systems coordinate with the mesolimbic reward system [76] (see below). Although energy homeostasis systems include diverse central and peripheral signaling pathways involving numerous peptides [73], for the purposes of this Review, we highlight the role of glucagon-like peptide-1 (GLP-1). GLP-1 signaling appears to decrease reward motivation [74], and these pathways are activated by stress [77] (see below). Thus, GLP-1 pathways and other energy homeostasis systems are well-positioned to mediate stress effects on anhedonia.
GLP-1-producing neurons originate almost exclusively in the caudal nucleus of the solitary tract (cNST) in the brainstem [78]. These projections play key roles in decreasing food intake both during satiety [79,80] and following stress [81]. GLP-1 signaling appears to decrease the rewarding value of food [82] through direct projections to NAc and VTA [83] (Figure 1). For instance, injection of a GLP-1 receptor agonist into rat VTA or NAc reduced lever pressing for food in a progressive ratio task [82]. Importantly, GLP-1-induced alterations in motivated behavior extend to alcohol and drug rewards [74]. For example, GLP-1 agonism in rats reduces the impact of alcohol reward on conditioned place preference [84]. Similar reports have emerged for other drugs, including cocaine and nicotine [74]. Several studies in rodents suggest that GLP-1 alters reward functioning by influencing dopaminergic mesolimbic circuitry, although some studies report decreases and some increases in dopamine activity [85– 88]. Taken together, these results suggest that GLP-1 signaling may decrease motivation for rewards in general.
GLP-1 signaling pathways are also activated by stress, including restraint and elevated platform stress [77]. Notably, peripheral inflammation in response to immune challenge also appears to activate GLP-1 neurons [89], highlighting the need for a multisystems approach to bridge work on immune responses and homeostatic energy regulation pathways following stress (see also Inflammation and reward processing, below). GLP-1 neurons projecting to the PVN also mediate HPA axis responses to stress (Figure 1), including release of adrenocorticotropic hormone (ACTH) and glucocorticoids [90] (see Box 2 and Figure 2). These results suggest that GLP-1 pathways could interact with other putative mechanisms of stress-induced anhedonia. Importantly, recent work in rats suggests that GLP-1 signaling is crucial for restraint stress to induce hypophagia (decreased food intake), as an intraventricular GLP-1 receptor antagonist attenuated the hypophagic effects of restraint stress [77]. Given the involvement of mesolimbic reward circuits in producing GLP-1 mediated hypophagia [83], this study suggests that GLP-1 could play a role in stress-induced alterations in reward behavior more generally. The possibility that GLP-1 signaling partially mediates the effect of stress on reward processing merits follow-up.
Not surprisingly, other energy homeostatic pathways may also contribute to stress-induced anhedonia. For instance, antagonism of a melanocortin receptor (MC4R) in NAc prevents anhedonic-like decreases in sucrose preference following chronic stress in mice [91]. Indeed, as research on the links between energy homeostasis systems and reward systems has expanded considerably in recent years [76], the list of possible stress-anhedonia mediators has also expanded. Notably, anhedonia is frequently accompanied by appetite and weight changes in humans diagnosed with MDD [92]. Thus, energy homeostasis systems in general are an exciting target for future research on stress-induced anhedonia.
Putative molecular signaling pathways to stress-induced anhedonia.
In addition to circuit-level mechanisms that could connect stress and anhedonia, researchers have also examined molecular signaling pathways contributing to this link [9,93]. Much recent work has focused on the cascade of inflammatory responses to stress (see Box 2) and possible effects on dopamine synthesis [93], as discussed next. Other molecular signaling pathways are covered elsewhere [9].
Inflammation and reward processing.
Numerous studies suggest that inflammation alters motivated behavior and mesolimbic function, possibly through dopaminergic changes. Although we highlight recent work in this domain, a more thorough treatment of this topic is available (see [93]).
A series of studies in mice suggests that the pro-inflammatory cytokine interleukin-6 (IL-6), in particular, may be a key contributor to stress-induced anhedonia [94–96]. Social defeat stress produces a ~27-fold increase in peripheral IL-6 [94]. Additionally, social defeat appears to weaken the blood-brain barrier by reducing levels of Cldn5, a cell adhesion molecule, allowing IL-6 to infiltrate NAc parenchyma and producing diminished social interaction [95]. These studies suggest that stress-induced IL-6 infiltration, in conjunction with synaptic remodeling in NAc (see [96]), contributes to the development anhedonic-like behavior [94–96]. Increases in IL-6 and social interaction deficits were both rescued by bone marrow infusions from IL-6 knockout mice, suggesting that these immune and behavioral changes are mediated by bone marrow-derived, peripheral immune cells [94]. Interestingly, administration of the plant metabolites dihydrocaffeic acid (DHCA) and malvidin-3’-O-glucoside (Mal-gluc) blunted IL-6 responses to social defeat stress and promoted resilience to anhedonic-like behaviors (blunted sucrose preference and decreased social interaction) [96]. DHCA inhibited IL-6 production via disrupted gene transcription, while Mal-gluc inhibited synaptic restructuring in NAc, and both changes were necessary to achieve therapeutic effects [96]. Taken together, this work suggests that inflammatory responses, together with neurovascular and synaptic changes, promote susceptibility to anhedonic behavior. Moreover, these exciting studies illustrate how research spanning multiple systems (e.g., immune response and brain reward systems) may produce key insights about pathways to stress-induced anhedonia.
Despite this evidence linking inflammatory responses to brain reward systems, rodent studies on inflammation and dopaminergic function specifically have yielded mixed results. Rodent studies measuring the impact of interferon-α (IFN-α, a proinflammatory cytokine) on dopamine release have reported either increases or decreases in dopamine/dopamine metabolites [93]. These divergent findings may be partly due to differences in dosing, chronicity, and timeframe of exposure [93]. However, some evidence suggests that recombinant human IFN-α does not bind to expected targets in rodents [97], and inconsistent use of species-specific IFN-α could therefore explain these mixed results in rodents [93]. By contrast, a recent study that administered IL-6 found decreased effortful responding for a preferred (vs. freely available) reward alongside decreased extracellular dopamine in NAc core, as assessed by microdialysis [98], suggesting that behavioral changes were mediated by alterations in dopamine release.
Two studies in rhesus monkeys have assessed in vivo dopamine release in response to inflammation. Chronic administration of IFN-α decreased effort-based, but not freely available, sucrose consumption [99] and in vivo microdialysis in these animals indicated decreased dopamine release in the caudate. Additionally, inflammation-induced deficits in dopamine release were abolished by administration of L-DOPA, the precursor to dopamine [100]. This finding suggests that IFN-α administration decreased dopamine release by reducing synthetic capacity.
In humans, a recent functional magnetic resonance imaging (fMRI) study of healthy female participants examined the effect of stress-induced inflammation on reward prediction errors (RPEs; see Box 1) in VS, which includes NAc [101]. First, participants completed a cold pressor task while performing serial subtraction in front of an experimenter. Blood levels of IL-6 were assessed before and after the stressor. During a second session, participants completed arithmetic problems of escalating difficulty while exposed to criticism from an unfriendly, impatient experimenter. They then completed a probabilistic reward task during an fMRI scan. Analyses revealed that stress-induced increases in IL-6 during the first session were associated with diminished VS BOLD responses to RPEs during the second session, although there was no main effect of stress on BOLD RPE signals, and no behavioral effects were detected [101].
An fMRI study of individuals with MDD investigated the association between resting-state functional connectivity, inflammatory markers, and anhedonic symptoms [102]. Connectivity between VS and ventromedial PFC (vmPFC) negatively correlated with blood levels of C-reactive protein (CRP), a marker of inflammation. Furthermore, decreased VS-vmPFC connectivity was associated with higher anhedonia scores [102]. These results suggest that resting-state fluctuations may capture alterations in the functional architecture of the corticomesolimbic circuit (see Box 1 and Medial prefrontal cortex regulation of the mesolimbic circuit) associated with inflammation and anhedonia.
Altogether, these findings suggest that inflammation impacts motivated behavior, possibly through changes in dopaminergic mesolimbic circuitry. Several plausible theories have been advanced to characterize these alterations at the molecular level.
Inflammation and dopamine synthesis.
Inflammation may decrease dopaminergic signaling by disrupting the biosynthetic pathway to dopamine [93]. Inflammation appears to decrease the availability of tetrahydrobiopterin (BH4), an enzyme cofactor that is critical at two stages of dopamine synthesis: a) conversion of phenylalanine to tyrosine, and b) conversion of tyrosine to L-3,4-dihydroxphenylalanine (L-DOPA), the precursor to dopamine (Box 3).
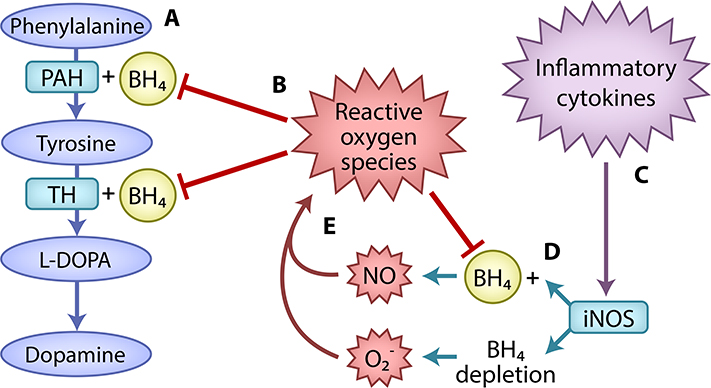
Box 3. Inflammation may inhibit dopamine synthesis via BH4 oxidation.
Inflammation may impede the biosynthetic pathway to dopamine. For a detailed review, see [93]. Briefly, dopamine synthesis requires the conversion of phenylalanine to tyrosine by phenylalanine hydroxylase (PAH). A second enzyme, tyrosine hydroxylase (TH), converts tyrosine to L-DOPA, the human precursor to dopamine. For these conversions, both enzymes require the cofactor BH4 (Figure I). BH4 also facilitates production of nitric oxide (NO) by nitric oxide synthases (NOSs). In the absence of BH4, NOSs increase production of a reactive oxygen species, superoxide (O2−). BH4 is susceptible to oxidation by O2−. Furthermore, NO and O2− react to produce peroxynitrite (ONOO−), a powerful oxidant, which may cause neuronal death in addition to BH4 oxidation [142]. Thus, initial BH4 depletion could cause still greater BH4 deficits through oxidative loss (Figure I).
Inflammation may drive BH4 oxidation by increasing the activity of inducible NOS (iNOS), an NOS type produced in peripheral macrophages and brain glial cells [143] (Figure I). Accordingly, a study in rats suggested that peripherally-administered IFN-α decreases brain levels of BH4 and dopamine through NO that is thought to cross the blood-brain barrier [105].
To test this hypothesis, researchers have examined cerebrospinal fluid (CSF) and blood concentrations of phenylalanine, tyrosine, and the phenylalanine/tyrosine (Phe/Tyr) ratio as indirect measures of dopamine synthesis. The Phe/Tyr ratio was elevated in individuals receiving IFN-α as a treatment for hepatitis C [103], suggesting that inflammation impeded the conversion of phenylalanine to tyrosine (see Box 3). Although promising, interpretation of these findings is limited by small sample size, especially in the control group (9 individuals). Additionally, in a sample of elderly persons with chronic low-grade inflammation, elevated tyrosine, but not phenylalanine or the Phe/Tyr ratio, was associated with reduced motivation [104]. However, tyrosine levels were non-significantly associated with inflammatory markers (IL-6 and CRP). Thus, the role of inflammation in impeding the conversion of tyrosine to L-DOPA (see Box 3) remained unclear. Taken together, this important research has yielded hints that inflammation disrupts dopamine synthesis by decreasing BH4 availability, consistent with some animal work [105]. Given the potential significance of these findings, additional follow-up is merited.
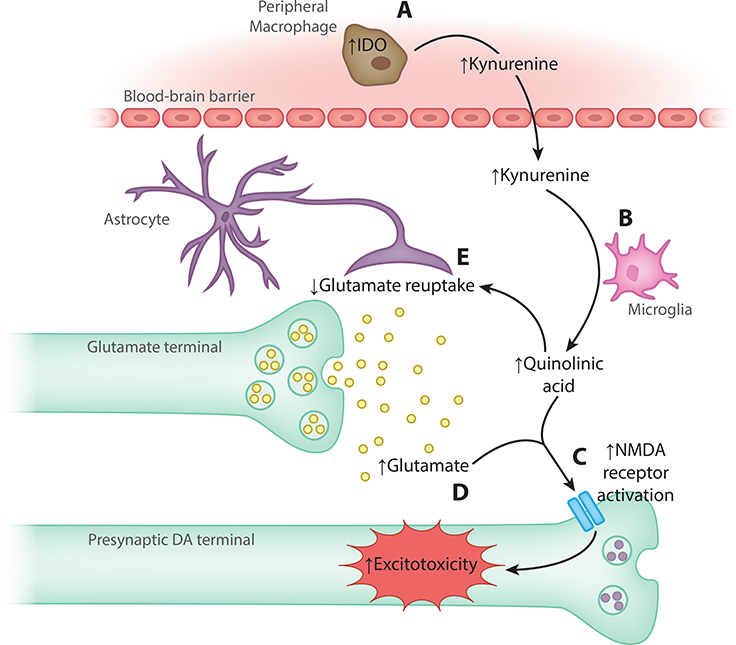
Inflammation may also decrease dopamine synthesis through the kynurenine pathway by increasing oxidative stress [106] (Figure 3; for more detailed discussion, see [93,106]). Importantly, dopamine neurons are especially vulnerable to inflammatory insult [107]. Moreover, xanthurenic acid, a kynurenine pathway metabolite, inhibits BH4 synthesis [108], suggesting that increased kynurenine production resulting from inflammation could interfere with dopamine synthesis through BH4 depletion. Consistent with the kynurenine pathway theory, increased quinolinic acid (QUIN) levels in CSF were associated with IFN-α treatment for hepatitis C [109] (see Figure 3).
Figure 3. Inflammation-induced excitotoxicity through the kynurenine pathway.
(A) Pro-inflammatory cytokines stimulate indoleamine 2,3-dioxygenase (IDO) in peripheral macrophages, resulting in production of kynurenine that crosses the blood-brain barrier. (B) Microglia convert kynurenine to quinolinic acid (QUIN). (C) In turn, QUIN exerts possibly neurotoxic effects, in part by (D) activating N-methyl-d-aspartate (NMDA) receptors, and (E) by decreasing glutamate reuptake, which increases glutamate release to potentially excitotoxic levels [106]. DA, dopamine; IDO, indoleamine 2,3-dioxygenase; NMDA, N-methyl-d-aspartate.
Altogether, evidence suggests that inflammation could interfere with dopamine synthesis, possibly by preventing enzymatic activity in the biosynthetic pathway or by increasing neurotoxicity. However, whether stress-induced inflammation causes disrupted dopamine synthesis remains equivocal. Some work has examined the impact of stress on dopamine depletion [25], although inflammation was not assessed. Moreover, it is not yet clear whether stress type (e.g., interpersonal stress; see [110]) may influence anhedonic responses via changes in inflammation.
Future directions in the study of stress and anhedonia.
Consistent with our view, recent work suggests that stress contributes to anhedonic behavior through perturbations across diverse systems and multiple levels of analysis. For example, pro-inflammatory signaling molecules may cross the blood-brain barrier to infiltrate brain reward systems directly [94–96], but could also affect motivated behavior through homeostatic energy regulation pathways [89]. However, despite much promising work to date on the relations linking stress and anhedonia, important gaps in our collective knowledge persist.
Anhedonia and key dimensions of stress.
Examinations of stress-induced anhedonia have implemented paradigms that vary considerably across studies in terms of stress chronicity, severity, controllability, and type [3], making it difficult to synthesize results across labs. As a result, the implications of variability in these dimensions of stress are incompletely understood.
Experiments also vary in the severity of their stress manipulations as a function of the species under study. In rodents, chronic mild stress commonly entails stressors such as “strobe light illumination for 1 to 16 h” or “dark cycle (continuous darkness for 24 to 36 h)” ([13], p. 542) twice per day for 8–12 weeks. Similar stressors would be unethical to administer to human participants. As a result, human stress inductions generally take place on the order of minutes, not hours (e.g., [17,101]) and accordingly are not matched for the chronicity (nor, likely, the severity) of many animal studies. Experimental work in humans often assumes that relatively mild and acute stressors will produce alterations in reward function that are reliably detectable and qualitatively similar to alterations produced by severe and/or chronic stress. Yet it is possible that the actual relation between stress and anhedonic processes in daily-life settings violates these core assumptions.
The impact of stressor chronicity also remains unclear. It is possible that stressors produce divergent effects on mesolimbic dopaminergic functioning depending on chronicity (see Stress and the mesolimbic reward circuit). Yet both acute stressors (e.g., death of a loved one) and chronic stress (e.g., ongoing financial difficulties) can lead to anhedonic symptoms [22]. The impact of these events on subsequent symptoms may depend in part on regulatory responses. For certain individuals, such as those with a ruminative response style [7], even acute stressors may provoke chronic stress responses, leading to long-lasting and relatively inflexible anhedonic states.
Observational studies of responses to naturally-occurring severe/chronic stressors could supplement the important experimental work reviewed above. Both avenues of research are necessary, given the trade-off between experimental control and strength of causal inference vs. ecological validity. Large observational studies in humans could distinguish the differential impact of numerous types of stressors. Indeed, preliminary evidence suggests that anhedonia may be especially prominent following interpersonal losses (e.g., death of a loved one or romantic loss) [22]. Notably, the aforementioned study assessed the severity of anhedonia relative to other dysphoric symptoms, rather than an “absolute” measure of anhedonia [22]. Thus, more work is needed to understand the impact of dimensions of stress, such as stressor chronicity, on anhedonia per se. A few important studies have compared the contributions of acute life events vs. chronic difficulties on broader phenotypes that may or may not include anhedonia, such as MDD [111], or on depressive symptom aggregates [112]. However, such studies rarely examine the links between stress and anhedonia specifically, or indeed any individual symptoms (for an exception, see [22]). More work in this domain could help researchers continue to increase the ecological validity of experimental models of stress and anhedonia.
Finally, anhedonia is a multifaceted construct. Although anhedonia is often defined with reference to loss of pleasure or motivation [92], researchers have recently conceptualized anhedonic behavior in terms of a broader array of motivational and reward processes. Because decision-making requires the weighing of potential rewards against expected costs [1,113], decreased reward seeking could result from changes to several facets of this process, e.g., reward devaluation and/or an increase in forecasted effort costs [114]. Future experiments can make use of paradigms that distinguish these facets of reward processing [113].
Variability in reward processing and motivated behavior following stress.
Researchers studying stress and anhedonia must account for a counterintuitive relationship: Under certain circumstances, stress increases reward motivation and sensitivity to reward [12,115]. Alcohol/substance use problems and obesity are linked with stress and (at least theoretically) involve increased reward seeking [116] (although increased sensation seeking may predispose individuals towards substance use [117], suggesting the importance of individual factors). How can we reconcile these apparent discrepancies?
One possibility is that certain individuals are more prone to seek out rewards rather than experience anhedonia in response to stress. Such between-individual variability is plausible from an evolutionary perspective, e.g. due to fluctuations in environmental demands that preclude the possibility of a single, “optimal” response profile [2,118]. Indeed, sex may be a meaningful individual difference for stress-induced changes in motivated behavior [119,120]. For instance, one study administered the balloon analogue risk task (BART) [121], in which participants increase the value of a potential reward by “inflating” a virtual balloon, while each “pump” increases the risk that the balloon will pop before the participant can “cash in” the reward for that trial. On average, men responded more quickly and “cashed in” more often, whereas women responded more slowly and “cashed in” less [119]. These differences only emerged following a cold pressor, in which participants hold their hands in cold water, suggesting that sex moderates stress-induced changes in reward responding [119]. Still, between-nindividual factors, including sex differences, are unlikely to fully account for variability in responses to stress. Some evidence in humans suggests that, within individuals, different events are likely to provoke more or less prominent anhedonia, and dissociable patterns of dysphoric symptoms in general [22]. A comprehensive model of stress and anhedonia should incorporate both stable tendencies that vary across individuals [122] and within-individual variability (e.g., in response to different types of stress) [6].
Concluding remarks.
Delineating an anhedonic phenotype rooted in etiology represents an important goal for psychiatric research. Such a phenotype could help to increase homogeneity in clinical research samples, and the accompanying increase in statistical power could make it easier to identify critical vulnerability factors for chronic, severe anhedonia. In turn, identifying vulnerability factors and proximal causes of anhedonia could improve predictions of conversion to disorder and suggest novel targets for treatment.
Cross-species work has made considerable progress in illuminating plausible mechanisms that could bridge the occurrence of stress and onset of anhedonia. Stress alters mesolimbic reward processing in humans and animals, and these changes are linked to anhedonic behavior. Stress also produces dendritic remodeling in mPFC, and co-occurring functional changes in the mPFC-mesolimbic circuit appear to contribute to anhedonic-like outcomes. CRF activity in the mesolimbic pathway also biases reward processing, possibly by gating the release of BDNF. GLP-1 neurons and other homeostatic energy regulation systems are also well positioned to decrease reward-seeking behavior following stress. Finally, following prolonged stress, pro-inflammatory cytokines appear to filter across the blood-brain barrier to interact with mesolimbic circuitry and increase susceptibility to anhedonic-like behavior, and inflammatory responses could also interfere with dopamine synthesis.
Yet seemingly contradictory patterns of results have emerged across lines of research. Better parsing heterogeneity in stressors and in reward processing could help to decipher puzzling results and yield crucial insights. Additionally, a unified model is needed to account for isolated findings across levels of analysis (e.g., molecular signaling, neural circuitry, behavior, subjective experience), including seemingly discrepant findings (e.g., stress decreases the number of spontaneously firing dopamine neurons in VTA, but increases firing rates).
Furthermore, the full realization of a multi-system, multi-level approach to psychopathology will require more inter-disciplinary collaboration, drawing on psychology, neuroscience, immunology, endocrinology, and economics, among other fields. Unraveling pathways to complex psychiatric phenotypes requires research that cuts across levels—from genetics to molecular signaling pathways, functional circuits, cognition, behavior, and culture. We believe that this approach holds the potential to yield much-needed answers for individuals experiencing debilitating psychiatric illness.
Box 1, Figure I. Dopaminergic and mesocorticolimbic circuitry.
GABA, γAminobutyric acid; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; SN, substantia nigra; VTA, ventral tegmental area.
Box 3, Figure I. Inflammatory disruption of dopamine synthesis through BH4 oxidation.
(A) Dopamine synthesis requires the conversion of phenylalanine to tyrosine by the enzyme PAH. A second enzyme, TH, converts tyrosine to LDOPA, the human precursor to dopamine. For these conversions, both enzymes require the cofactor BH4. (B) Reactive oxygen species contribute to BH4 oxidization, which inhibits dopamine synthesis. (C) Inflammation may drive BH4 oxidation by increasing the activity of the enzyme iNOS, which produces reactive oxygen species. (D) BH4 facilitates production of nitric oxide (NO). In the absence of BH4, iNOS increases production of a second reactive oxygen species, superoxide (O2−). (E) NO and O2− react to produce peroxynitrite (ONOO−), a powerful oxidant, further decreasing BH4 availability. BH4, tetrahydrobiopterin; iNOS, inducible nitric oxide synthase; L-DOPA, L-3,4-dihydroxyphenylalanine; PAH, phenylalanine hydroxylase; TH, tyrosine hydroxylase
Outstanding Questions.
Which types of experiences are most likely to produce anhedonia? Are individuals who experience chronic and/or severe stress at greater risk for anhedonic symptoms? Do chronic and/or severe stressful experiences produce additive effects, or do they interact? Do certain categories of stress (e.g., interpersonal losses) have greater effects on anhedonia?
How can one account for disparate effects of stress on mesolimbic dopaminergic functioning (e.g., changes in firing rates vs. number of spontaneously firing neurons)?
Which stages of reward processing (anticipation, consumption) or aspects of it (e.g., calculation of effort costs, estimates of reward value) are affected in anhedonic individuals? How do these changes map onto biological systems?
What are the within-individual factors that predispose certain people to develop anhedonic responses following stress? How do these factors interact with distinct classes of stressors?
Highlights.
Converging evidence across species suggests that environmental stressors contribute to the development of anhedonia (i.e., decreased motivation/responsiveness to reward), in part through alterations in the mesolimbic dopamine system.
At the circuit level, stress may disrupt mesolimbic reward functioning through a number of possible mechanisms: alternations in the input from medial prefrontal cortex; the actions of CRF secreted by neuroendocrine stress systems; or signals from homeostatic energy systems, including GLP-1 neurons.
At the molecular signaling level, inflammation in response to stress could disrupt mesolimbic reward functioning by interfering with dopamine synthesis, or via kynurenine-induced oxidative stress.
A thorough conceptualization of the relationship between stress and anhedonia should depict how different types of stress cause changes in molecular signaling and in neural circuits, leading to specific alterations in motivation and reward processing.
Acknowledgements.
This work was supported by the National Institute of Mental Health (grants K01MH099232 to A.J.H. and R01MH110750 to S.W.C.C.). We thank Daniel Kramer and Dana Allswede for their feedback on early versions of this manuscript.
Glossary.
- Agonist:
a chemical that binds to a receptor to produce a given effect; e.g., a GLP-1 agonist is any chemical that binds to and acts on GLP-1 receptors, including GLP-1 itself
- Antagonist:
a chemical that prevents another chemical from exerting its effects on a receptor; for example, α-helical CRF (a CRF antagonist) can reduce the behavioral effects of CRF by preventing it from binding to CRF receptors
- Anhedonia:
diminished pleasure or decreased motivation for rewards. Some researchers argue that a broader array of processes should be included here, for instance to account for the complex balancing of rewards vs. costs involved in decisionmaking
- Central and peripheral:
in this article, central refers to the central nervous system (brain and spinal cord) whereas peripheral refers to the rest of the body.
- Etiology:
the study of causation, often of a pathological condition
- Homeostasis:
a relatively stable equilibrium; in the context of this article, an equilibrium between energy intake and usage
- Homozygosity:
the state of having the two of the same form (or “allele”) of a gene (as opposed to heterozygosity, having two different forms of the gene
- Optogenetics:
a technique in which different colors of light are used to influence the firing of a neuron that has been genetically modified to express lightsensitive ion channels or ion pumps
- Parenchyma:
neurons and glial cells, in the context of the brain; in general, the characteristic tissue of an organ, as opposed to its supporting framework
- Phenotype:
a group of observable characteristics or traits used to classify organisms
- Progressive ratio task:
a task in which the response requirement for a reward increases on each trial. E.g., on the first trial 9 lever presses are required for a reward, then 12, then 15, then 20, etc
- Response bias:
in signal detection theory, the tendency to indicate that one has detected a stimulus, calculated using both the hit rate and false alarm rate for that stimulus (in the context of this Review, researchers measured the response bias to a stimulus for which responses were disproportionately rewarded, unbeknownst to the participant).
- Reward devaluation:
a process through which the reward value of a given stimulus is reduced; for example, feeding an animal to satiety reduces the value of food.
- Single-nucleotide polymorphism (SNP):
in a DNA sequence, a variant that differs based on a single nucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Lima SL (1998) Stress and decision making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav 27, 215–290 [Google Scholar]
- 2.Holmes AJ and Patrick LM (2018) The myth of optimality in clinical neuroscience. Trends Cogn. Sci DOI: 10.1016/j.tics.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisman H and Matheson K (2005) Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci. Biobehav. Rev 29, 525–546 [DOI] [PubMed] [Google Scholar]
- 4.Raison CL and Miller AH (2016) Pathogen-host defense in the evolution of depression: Insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology 42, 5–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AH and Raison CL (2016) The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingemanse NJ et al. (2010) Behavioural reaction norms: Animal personality meets individual plasticity. Trends Ecol. Evol 25, 81–89 [DOI] [PubMed] [Google Scholar]
- 7.Michael T et al. (2007) Rumination in posttraumatic stress disorder. Depress. Anxiety 24, 307–317 [DOI] [PubMed] [Google Scholar]
- 8.Pizzagalli DA (2014) Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol 10, 393–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo SJ and Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat. Rev. Neurosci 14, 609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollon NG et al. (2015) Stress effects on the neural substrates of motivated behavior. Nat. Neurosci 18, 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden SA et al. (2011) A standardized protocol for repeated social defeat stress in mice. Nat. Protoc 6, 1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willner P (2005) Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110 [DOI] [PubMed] [Google Scholar]
- 13.Tye KM et al. (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ironside M et al. (2018) Brain mechanisms mediating effects of stress on reward sensitivity. Curr. Opin. Behav. Sci 22, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares JM et al. (2012) Stress-induced changes in human decision-making are reversible. Transl. Psychiatry 2, e131s–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenbaum H and Connelly J (1993) The effect of stress on hedonic capacity. J. Abnorm. Psychol 102, 474–481 [DOI] [PubMed] [Google Scholar]
- 17.Bogdan R and Pizzagalli DA (2006) Acute stress reduces reward responsiveness: Implications for depression. Biol. Psychiatry 60, 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berghorst LH et al. (2013) Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci 7, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GW and Harris T (1978) Social origins of depression: A study of psychiatric disorder in women, Tavistock Publications. [Google Scholar]
- 20.Kendler KS et al. (1999) Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 [DOI] [PubMed] [Google Scholar]
- 21.Hammen C (2005) Stress and depression. Annu. Rev. Clin. Psychol 1, 293–319 [DOI] [PubMed] [Google Scholar]
- 22.Keller MC et al. (2007) Association of different adverse life events with distinct patterns of depressive symptoms. Am. J. Psychiatry 164, 1521–1529 [DOI] [PubMed] [Google Scholar]
- 23.Murray EA (2007) The amygdala, reward and emotion. Trends Cogn. Sci 11, 489–497 [DOI] [PubMed] [Google Scholar]
- 24.Yang Y et al. (2018) Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol 48, 90–96 [DOI] [PubMed] [Google Scholar]
- 25.Cabib S and Puglisi-Allegra S (2012) The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev 36, 79–89 [DOI] [PubMed] [Google Scholar]
- 26.Friedman AK et al. (2014) Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan V et al. (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 [DOI] [PubMed] [Google Scholar]
- 28.Cao J-L et al. (2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci 30, 16453–16458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CH and Grace AA (2014) Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry 76, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreines JL et al. (2017) Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42, 904–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhury D et al. (2012) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales M and Margolis EB (2017) Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci 18, 73–85 [DOI] [PubMed] [Google Scholar]
- 33.Ikemoto S (2007) Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev 56, 27–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwen BS et al. (2015) Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnsten AFT (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci 10, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phelps EA et al. (2004) Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43, 897–905 [DOI] [PubMed] [Google Scholar]
- 37.Milad MR and Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JE (2000) Emotion circuits in the brain. Annu. Rev. Neurosci 23, 155–184 [DOI] [PubMed] [Google Scholar]
- 39.Rangel A and Hare T (2010) Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol 20, 262–270 [DOI] [PubMed] [Google Scholar]
- 40.Kable JW and Glimcher PW (2007) The neural correlates of subjective value during intertemporal choice. Nat. Neurosci 10, 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferenczi EA et al. (2016) Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698–aac9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drysdale AT et al. (2017) Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 23, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Admon R and Pizzagalli DA (2015) Corticostriatal pathways contribute to the natural time course of positive mood. Nat. Commun 6, 10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEwen BS and Morrison JH (2013) The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nawijn L et al. (2015) Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev 51, 189–204 [DOI] [PubMed] [Google Scholar]
- 46.Barch DM and Dowd EC (2010) Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophr. Bull 36, 919–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bremner JD (2002) Neuroimaging studies in post-traumatic stress disorder. Curr. Psychiatry Rep 4, 254–263 [DOI] [PubMed] [Google Scholar]
- 48.Schmaal L et al. (2017) Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22, 900–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes AJ et al. (2012) Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J. Neurosci 32, 18087–18100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon TD et al. (2015) Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 77, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treadway MT et al. (2015) Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatry 77, 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covington HE et al. (2010) Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci 30, 16082–16090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treadway MT et al. (2013) Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front. Hum. Neurosci 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ossewaarde L et al. (2011) Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 55, 345–352 [DOI] [PubMed] [Google Scholar]
- 55.Christoffel DJ et al. (2015) Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat. Neurosci 18, 962–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sapolsky RM et al. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89 [DOI] [PubMed] [Google Scholar]
- 57.Kono J et al. (2017) Distribution of corticotropin-releasing factor neurons in the mouse brain: A study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct. Funct 222, 1705–1732 [DOI] [PubMed] [Google Scholar]
- 58.Shackman AJ and Fox AS (2016) Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36, 8050–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herman JP and Cullinan WE (1997) Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20, 78–84 [DOI] [PubMed] [Google Scholar]
- 60.Dabrowska J et al. (2016) Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J. Neuroendocrinol. 28, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodaros D et al. (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150, 8–13 [DOI] [PubMed] [Google Scholar]
- 62.Wang B et al. (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. J. Neurosci 25, 5389–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wanat MJ et al. (2008) Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of I h. J. Physiol 586, 2157–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wanat MJ et al. (2013) CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci 16, 383–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryce CA and Floresco SB (2016) Perturbations in effort-related decisionmaking driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacology 41, 2147–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peciña S et al. (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress? BMC Biol. 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemos JC et al. (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490, 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berton O et al. (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 [DOI] [PubMed] [Google Scholar]
- 69.Walsh JJ et al. (2014) Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci 17, 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koo JW et al. (2016) Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol. Psychiatry 80, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bogdan R et al. (2011) Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J. Neurosci 31, 13246–13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pizzagalli DA et al. (2008) Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J. Psychiatr. Res 43, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berthoud H-R (2012) The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc 71, 478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayes MR and Schmidt HD (2016) GLP-1 influences food and drug reward. Curr. Opin. Behav. Sci 9, 66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colwill RM and Rescorla RA (1985) Postconditioning devaluation of a reinforcer affects instrumental responding. J. Exp. Psychol. Anim. Behav. Process 11, 120–132 [Google Scholar]
- 76.Cassidy RM and Tong Q (2017) Hunger and satiety gauge reward sensitivity. Front. Endocrinol. (Lausanne). 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maniscalco JW et al. (2015) Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J. Neurosci 35, 10701–10714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merchenthaler I et al. (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol 403, 261–280 [DOI] [PubMed] [Google Scholar]
- 79.Maniscalco JW et al. (2012) Satiation and stress-induced hypophagia: Examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Front. Neurosci 6, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Bloemendaal L et al. (2014) Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol 221, T1–16 [DOI] [PubMed] [Google Scholar]
- 81.Maniscalco JW and Rinaman L (2017) Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiol. Behav 176, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dickson SL et al. (2012) The glucagon-like peptide 1 (GLP-1) analogue, Exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci 32, 4812–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alhadeff AL et al. (2012) GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shirazi RH et al. (2013) Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One 8, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mietlicki-Baase EG et al. (2013) The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. - Endocrinol. Metab 305, E1367–E1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mietlicki-Baase EG et al. (2014) Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci 34, 6985–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang XF et al. (2015) Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 12, 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortin SM and Roitman MF (2017) Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol. Behav 176, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaykema RP et al. (2009) Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res. 1294, 61–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghosal S et al. (2017) Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J. Neurosci 37, 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim BK et al. (2012) Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, (5th edn) American Psychiatric Publishing. [Google Scholar]
- 93.Felger JC and Treadway MT (2017) Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology 42, 216–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodes GE et al. (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci 111, 16136–16141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menard C et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 20, 1752–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J et al. (2018) Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loftis JM et al. (2006) Can rodents be used to model interferon-α-induced depressive symptoms? Prog. Neuro-Psychopharmacology Biol. Psychiatry 30, 1364–1365 [DOI] [PubMed] [Google Scholar]
- 98.Yohn SE et al. (2016) Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: Pharmacological and neurochemical characterization. Psychopharmacology (Berl). 233, 3575–3586 [DOI] [PubMed] [Google Scholar]
- 99.Felger JC et al. (2013) Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38, 2179–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Felger JC et al. (2015) Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol 18, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Treadway MT et al. (2017) Association between Interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol. Psychiatry 82, 570–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felger JC et al. (2015) Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Felger JC et al. (2013) Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain. Behav. Immun 31, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Capuron L et al. (2011) Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 70, 175–182 [DOI] [PubMed] [Google Scholar]
- 105.Kitagami T et al. (2003) Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: Role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 978, 104–114 [DOI] [PubMed] [Google Scholar]
- 106.Dantzer R (2016) Role of the kynurenine metabolism pathway in inflammationinduced depression: Preclinical approaches In Inflammation-associated depression: Evidence, mechanisms and implications (Dantzer R and Capuron L, eds), pp. 117–138, Springer International Publishing [Google Scholar]
- 107.Block ML et al. (2007) Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci 8, 57–69 [DOI] [PubMed] [Google Scholar]
- 108.Haruki H et al. (2016) Tetrahydrobiopterin biosynthesis as a potential target of the kynurenine pathway metabolite xanthurenic acid. J. Biol. Chem 291, 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raison CL et al. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: Relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Slavich GM and Irwin MR (2014) From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull 140, 774–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muscatell KA et al. (2009) Stressful life events, chronic difficulties, and the symptoms of clinical depression. J. Nerv. Ment. Dis 197, 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGonagle KA and Kessler RC (1990) Chronic stress, acute stress, and depressive symptoms. Am. J. Community Psychol 18, 681–706 [DOI] [PubMed] [Google Scholar]
- 113.Zald DH and Treadway MT (2017) Reward processing, neuroeconomics, and psychopathology. Annu. Rev. Clin. Psychol 13, 471–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Treadway MT and Zald DH (2011) Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev 35, 537–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mather M and Lighthall NR (2012) Risk and reward are processed differently in decisions made under stress. Curr. Dir. Psychol. Sci 21, 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Volkow ND and Wise RA (2005) How can drug addiction help us understand obesity? Nat. Neurosci 8, 555–560 [DOI] [PubMed] [Google Scholar]
- 117.Holmes AJ et al. (2016) Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. J. Neurosci 36, 4038–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dingemanse NJ et al. (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B Biol. Sci 271, 847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lighthall NR et al. (2012) Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci 7, 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lighthall NR et al. (2009) Acute stress increases sex differences in risk seeking in the Balloon Analogue Risk Task. PLoS One 4, e6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lejuez CW et al. (2002) Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). J. Exp. Psychol. Appl 8, 75–84 [DOI] [PubMed] [Google Scholar]
- 122.Sih A et al. (2004) Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol 19, 372–378 [DOI] [PubMed] [Google Scholar]
- 123.Smith SM and Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci 8, 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elenkov IJ et al. (2000) The sympathetic nerve -- An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev 52, 595–638 [PubMed] [Google Scholar]
- 125.Szelényi J (2001) Cytokines and the central nervous system. Brain Res. Bull 54, 329–338 [DOI] [PubMed] [Google Scholar]
- 126.Haber SN and Knutson B (2009) The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mirenowicz J and Schultz W (1994) Importance of unpredictability for reward responses in primate dopamine neurons. J. Neurophysiol 72, 1024–1027 [DOI] [PubMed] [Google Scholar]
- 128.Schultz W (2016) Dopamine reward prediction-error signalling: A two-component response. Nat. Rev. Neurosci 17, 183–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berridge KC et al. (2009) Dissecting components of reward: “liking”, “wanting”, and learning. Curr. Opin. Pharmacol 9, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Howe MW and Dombeck DA (2016) Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schultz W et al. (1997) A neural substrate of prediction and reward. Science 275, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 132.Hamid AA et al. (2016) Mesolimbic dopamine signals the value of work. Nat. Neurosci 19, 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hikida T et al. (2010) Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907 [DOI] [PubMed] [Google Scholar]
- 134.Kravitz AV et al. (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci 15, 816–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lynd-Balta E and Haber SN (1994) Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J. Comp. Neurol 345, 562–78 [DOI] [PubMed] [Google Scholar]
- 136.Liljeholm M and O’Doherty JP (2012) Contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn. Sci 16, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lidow MS et al. (1991) Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience 40, 657–671 [DOI] [PubMed] [Google Scholar]
- 138.Britt JP et al. (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meredith GE (1999) The synaptic framework for chemical signaling in nucleus accumbens. Ann. N. Y. Acad. Sci 877, 140–156 [DOI] [PubMed] [Google Scholar]
- 140.Pennartz CMA et al. (1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol 42, 719–761 [DOI] [PubMed] [Google Scholar]
- 141.Haber SN (2016) Corticostriatal circuitry. Dialogues Clin. Neurosci 18, 7–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma N and Nehru B (2015) Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int 87, 92–105 [DOI] [PubMed] [Google Scholar]
- 143.Galea E et al. (1992) Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci 89, 10945–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]