Supplemental Digital Content is available in the text.
Keywords: anamnestic, inactivated quadrivalent influenza vaccine, primed, children
Abstract
Background:
It has not yet been demonstrated whether 2 doses of inactivated quadrivalent influenza vaccine (IIV4) prime a booster response in infants. We evaluated the anamnestic immune response to an IIV4 in children 17−48 months of age.
Methods:
Children were randomized to 2 doses of IIV4 or control in the primary phase III study (NCT01439360). One year later, in an open-label revaccination extension study (NCT01702454), a subset of children who received IIV4 in the primary study (primed group) received 1 IIV4 dose and children who received control in the primary study (unprimed) received 2 IIV4 doses 28 days apart. The primary objective was to evaluate hemagglutination inhibition (HI) antibody titers 7 days after first IIV4 vaccination in the per-protocol cohort (N = 224 primed; N = 209 unprimed). Neutralizing and antineuraminidase antibodies were also measured. Safety was analyzed in the total vaccinated cohort (N = 241 primed; N = 229 unprimed).
Results:
An anamnestic response was observed in primed children relative to unprimed controls, measured by age-adjusted geometric mean HI titer ratios against strains homologous (A/H1N1: 9.0; B/Victoria: 3.9) and heterologous (A/H3N2: 2.7; B/Yamagata: 6.7) to those in the primary vaccination series. The anamnestic response in primed children included increases in neutralizing antibodies (mean geometric increase: 5.0–10.6) and antineuraminidase antibodies (4.9–8.8). No serious adverse events related to vaccination were reported.
Conclusions:
In this study, 2-dose priming with IIV4 induced immune memory that was recalled with 1-dose IIV4 the following year to boost HI, antineuraminidase and neutralizing antibodies, even though the IIV4 strain composition partially changed.
Influenza has a high incidence and burden of disease in children1–3 and vaccination is recommended by the World Health Organization.4 Suboptimal vaccine protection may occur if there is a mismatch between the circulating virus strains and the strains contained in the vaccine. This can be a particular problem with regard to the vaccine B strains, because 2 antigenically distinct lineages of influenza B circulate worldwide, the Yamagata lineage and the Victoria lineage. Mismatch between the circulating lineage and the vaccine lineage reduces the degree of protection offered by the vaccine.5–8
Until recently, vaccination strategies used a trivalent influenza vaccine containing 2 influenza A strains (H1N1 and H3N2 subtypes) and 1 influenza B strain. Quadrivalent influenza vaccines containing B strains from both lineages offer broader protection and lessen the problem of mismatching because of B lineage. They may be particularly useful in children because, although vaccinated adults show moderate cross-reactive antibody responses against the alternative B lineage,9 the responses of children show poor cross-reactivity.10,11 Indeed, a meta-analysis of vaccine trials in young children found that efficacy was substantially reduced against influenza B strains of the alternative lineage to that contained in the vaccine compared with the same lineage.8
The World Health Organization recommends that children less than 9 years of age are given 2 doses of influenza vaccine during their first season of vaccination to optimize the immune response.4 Thereafter, children are considered to be primed and require only 1 dose of influenza vaccine per season. This strategy relies on the ability of influenza vaccine given in 2 doses to establish immune memory and subsequently drive an acceptable anamnestic response when boosted with a single dose the following year. However, published evidence on immune memory and anamnestic response elicited by inactivated quadrivalent influenza vaccine (IIV4) in children is lacking. We therefore conducted the present study to evaluate the humoral anamnestic response to a candidate IIV4 in children 17–48 months of age. This IIV4 (Fluarix Quadrivalent) is licensed in the United States and Europe for use in adults and children from 6 months of age. The primary objective of the study was to assess the anamnestic immune response to the IIV4 in terms of hemagglutination inhibition (HI) antibody titer in children 17–48 months of age.
MATERIALS AND METHODS
The trial was sponsored by GlaxoSmithKline Biologicals SA and approved by independent ethics committees and/or institutional review boards, conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and all applicable regulatory requirements. Parents provided written informed consent before participation of their child. The trial was registered with clinicaltrials.gov: NCT01702454.
Study Design and Participants
The present revaccination study was an extension of the first seasonal cohort (northern hemisphere influenza season 2011–2012) of a primary phase III study12 designed to evaluate the efficacy of the candidate IIV4, in which healthy children (6–35 months of age) were randomized 1:1 to receive IIV4 or noninfluenza control vaccine (NCT01439360) (Text, Supplemental Digital Content 1, http://links.lww.com/INF/D312).
The revaccination study (2012–2013 season) enrolled a convenience sample of children who had received 2 doses of study vaccine (IIV4 or control) in the primary study during the previous year. Children were 17–48 months of age at enrollment into the revaccination study (stratified into 17–29 and 30–48 months). Because more children in the older age group (30−48 months) participated in the first cohort of the primary study, to ensure an adequate balance between age groups, parents of children in the younger group (17−29 months) were contacted first to invite their children to participate in the revaccination study. Parents of older children were contacted in a second wave. All parents were contacted in the same order as the randomization list of the primary study.
In the open-label revaccination study, children retained their randomly allocated treatment group from the primary study. Children who were randomly allocated to the IIV4 group in the primary study and had received 2 IIV4 doses were given 1 dose of IIV4 (the primed group); children who were randomly allocated to the control vaccine group in the primary study and had received 2 doses of the control vaccine were given 2 doses of IIV4 28 days apart (the unprimed group). The IIV4 used in the primary study and in the revaccination study was administered intramuscularly in a 0.5 mL dose. Thirty-three centers in the Czech Republic, Poland, Spain and the United Kingdom participated in the study.
The IIV4 (Fluarix Quadrivalent, GSK, Dresden, Germany) was prepared from influenza viruses propagated in embryonated chicken eggs. Each of the 4 viruses was purified by zonal centrifugation using a linear sucrose density gradient solution containing detergent to split the virions, further purified by diafiltration and inactivated by the consecutive effects of sodium deoxycholate and formaldehyde. The IIV4 was formulated to contain 15 μg hemagglutinin antigen per strain of the following recommended influenza strains: A/Christchurch/16/2010 (A/H1N1; an A/California/7/2009-like strain), A/Victoria/361/2011 (A/H3N2), B/Brisbane/60/2008 (B/Victoria) and B/Hubei-Wujiagang/158/2009 (B/Yamagata). Two strains were updated between the primary study and the revaccination study: the 2 different strains in the primary study were A/Victoria/210/2009 (A/H3N2; an A/Perth/16/2009-like virus) and B/Brisbane/3/2007 (B/Yamagata; a B/Florida/4/2006-like virus).
Study Endpoints
Immunogenicity
Blood samples were taken before and at day 7 after administration of the first IIV4 dose in the revaccination study [the first dose of IIV4 ever for unprimed children and the third dose for primed children (after an interval of approximately 1 year)]. Immunogenicity was evaluated at day 7 because an anamnestic response is characterized by an early and sharp rise in antibody titers. All samples were tested by HI assay and a random subset was tested by microneutralization (MN) assay and neuraminidase inhibition (NI) assay (Text, Supplemental Digital Content 2, http://links.lww.com/INF/D313).
The following parameters were derived from HI titers: (1) geometric mean titer (GMT); (2) seropositivity rate; (3) seroconversion rate (SCR); (4) seroprotection rate (SPR); (5) mean geometric increase (MGI). The seropositivity rate was defined as the percentage of children with HI titer equal to or above the assay cut-off value. The SCR was defined as the percentage of children with either (1) prevaccination titer <1:10 and a postvaccination titer ≥1:40 or (2) prevaccination titer ≥1:10 and a minimum 4-fold increase in postvaccination titer. Although there is no accepted criterion for seroprotection in children, the SPR was defined as the percentage of children with HI titer ≥1:40 that is usually accepted as indicating protection in 50% of adult vaccinees.13 The MGI was defined as the fold increase in HI GMTs postvaccination compared with prevaccination. The GMT and MGI were also calculated for neutralizing and antineuraminidase antibody titers.
Safety
Parents recorded solicited injection site reactions (pain, redness and swelling) and solicited systemic reactions (drowsiness, fever, irritability/fussiness, loss of appetite) in a diary card every day up to day 7 after the first vaccination. They recorded other adverse events (spontaneously reported AEs) up to day 28 after the first vaccination. Medically attended AEs and serious AEs were reported throughout the study until the final telephone contact at approximately day 180.
Statistics
The study was planned to enroll a sufficient number of children to assess the relative immune response of the vaccine-primed participants versus vaccine-unprimed participants, with at least 80% power in terms of HI GMT ratio (primed/unprimed). Assuming a standard deviation of 0.8 for HI titer in logarithm base 10 for both primed and unprimed groups, and assuming that all 4 strains in the revaccination vaccine were homologous to those in the primary study vaccine, a total of 184 evaluable subjects for each group gave a global power of at least 80% to detect a difference in terms of GMT ratio (ie, GMT ratio = 1 under null hypothesis) at day 7 at the 2.5% significance level, by assuming the observed difference is 2-fold (by PASS 2005, 1-sided 2-sample t test for a difference of means, 1-sided α = 2.5%).
The objectives were to evaluate after 1 dose of IIV4 at day 7: (1) GMTs, SCRs, SPRs and MGIs in terms of HI titers; (2) priming effect via the GMT ratios of influenza vaccine-primed versus unprimed children and the difference in SCR and SPR between primed and unprimed children (based on HI titers); (3) GMTs and MGIs in terms of neutralizing and antineuraminidase antibody titers.
A seronegative participant was defined as having an antibody titer below the assay cut-off value; a seropositive participant was defined as having a titer greater than or equal to the assay cut-off value (Text, Supplemental Digital Content 2, http://links.lww.com/INF/D313). GMT calculations were performed by taking the antilog of the log titer transformations. Antibody titers below the assay cut-off value were given an arbitrary value of half the cut-off value for the GMT calculation.
The Clopper-Pearson exact 95% confidence interval (CI) for a proportion within a group was calculated.14 The 95% CI for the mean of log-transformed titer was first obtained assuming that log-transformed values were normally distributed with unknown variance. The 95% CI for the GMTs was then obtained by exponential transformation of the 95% CI for the mean of log-transformed titer. The group GMT ratio was obtained using an Analysis of covariance model on the logarithm-transformed titers that included the vaccine group as fixed effect and age as a regressor. The GMT ratio and its 95% CI were derived as exponential transformation of the corresponding group contrast in the model. The standardized asymptotic 95% CI for the group difference in proportion was based on Method 6 as described by Newcombe.15
The primary immunogenicity analysis was based on the per-protocol cohort which included all children who met the eligibility criteria, complied with the procedures and vaccination intervals specified, did not receive a product or have a medical condition leading to elimination from the per-protocol cohort and who had data available for immunogenicity endpoints against at least 1 vaccine strain. An exploratory immunogenicity analysis was performed excluding children who had experienced a reverse transcription polymerase chain reaction (RT-PCR)–confirmed influenza infection in the primary study the year before. The safety analysis was based on the total vaccinated cohort which included all children who received at least 1 vaccine dose.
RESULTS
The parents of 665 children of 1777 children from the first seasonal cohort of the primary study were contacted regarding participation in the revaccination study, of whom the parents of 473 children agreed and 192 declined. Three children were allocated to a study group but were not vaccinated. Enrollment took place between October and November 2012, and the last visit took place in June 2013.
The total vaccinated cohort included 470 (241 primed and 229 unprimed) children; the per-protocol cohort included 433 (224 primed and 209 unprimed) children (Fig. 1). Three primed and 8 unprimed children did not complete the study (Fig. 1). In the primed group, the mean age was 33.2 months, 47.3% were female and 97.9% were Caucasian (total vaccinated cohort). Corresponding values in the unprimed group were 32.5 months, 41.9% female and 97.8% Caucasian. Demographics were considered to be representative of the original study cohort enrolled 1 year earlier. A total of 183 and 250 children were included in the 17–29 and 30–48 months age strata, respectively, in the per-protocol cohort.
FIGURE 1.
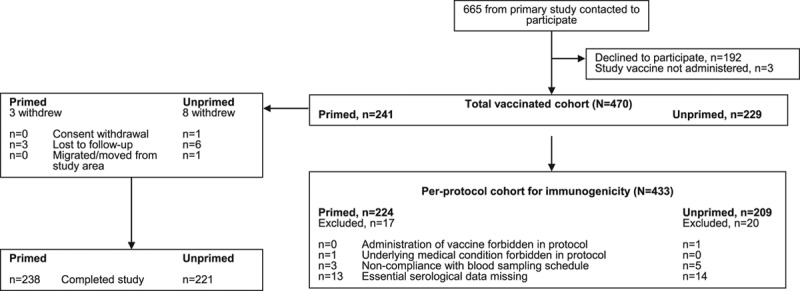
Participant disposition.
The exploratory immunogenicity analysis excluding children who experienced an RT-PCR–confirmed influenza infection in the primary study comprised 392 children; 11 children were excluded from the primed group (who had received 2 doses of IIV4 in the primary study) and 30 children were excluded from the unprimed group (who had received 2 doses of the control vaccine in the primary study). In the primed group, all 11 children had experienced an infection with influenza A/H3N2; in the unprimed group, 1 child had an infection with influenza A/H1N1, 27 children with A/H3N2, 1 child with B/Yamagata and 1 child with an unknown subtype or lineage.
Immunogenicity in the Per-protocol Cohort
HI Antibody Titers
More primed than unprimed children were seropositive to a vaccine strain before vaccination in the revaccination study (Table, Supplemental Digital Content 3, http://links.lww.com/INF/D314), and prevaccination antibody titers were higher in primed than unprimed children except for A/H3N2 (Fig. 2).
FIGURE 2.
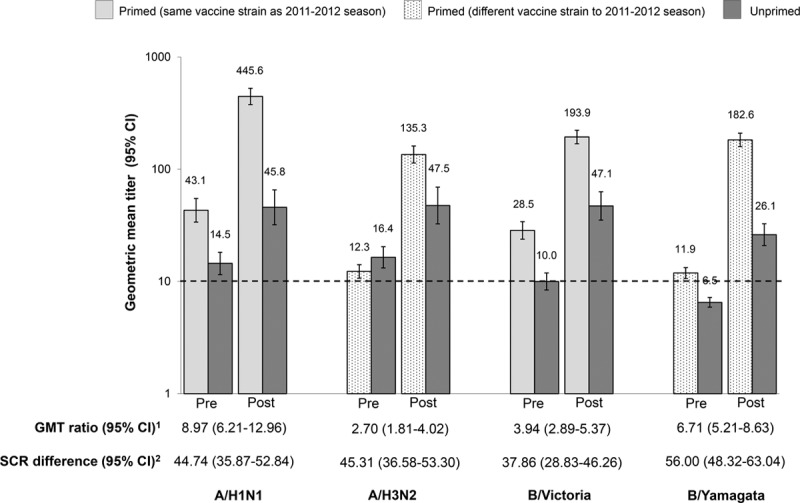
GMT prevaccination and 7 days after first vaccination, GMT ratio and SCR difference for HI antibodies in the revaccination study (per-protocol cohort). 1GMT ratio adjusted for age (primed/unprimed); 2difference in SCR (primed minus unprimed). Dotted line represents the assay cut-off (10 1/dil). GMT values are shown above the bars. Post indicates 7 days following vaccination; Pre, prevaccination. CI indicate 95% confidence interval; GMT, geometric mean titer; HI, hemagglutination inhibition; SCR, seroconversion rate.
The primed group mounted an anamnestic response that was detected 7 days after the booster dose of IIV4, with a rise in GMTs for strains that were unchanged from the 2011–2012 season (A/H1N1 and B/Victoria) and strains that had changed compared with the 2011–2012 season (A/H3N2 and B/Yamagata) (Fig. 2). It was observed that the lower limit of the 95% CI of the GMT ratio (primed/unprimed) was above 1 for each vaccine strain (Fig. 2). The between-group difference in the anamnestic response was also observed in the SCR difference (primed minus unprimed), with the lower limit of the 95% CIs being above zero for all vaccine strains (Fig. 2), and in the SPR difference (Table, Supplemental Digital Content 4, http://links.lww.com/INF/D315). The highest postvaccination GMTs were observed for the A/H1N1 strain (Fig. 2) and in children 30–48 months of age (Figures, Supplemental Digital Content 5 and 6, http://links.lww.com/INF/D316 and http://links.lww.com/INF/D317).
After 1 dose of IIV4, 76.5%–94.1% of primed children seroconverted per vaccine strain compared with 32.2%–38.6% of unprimed children (Table, Supplemental Digital Content 7, http://links.lww.com/INF/D318). There was little difference in SCR between primed children 17–29 months of age versus 30–48 months of age, but a higher proportion of unprimed children in the older age group seroconverted versus the younger unprimed children (Table, Supplemental Digital Content 7, http://links.lww.com/INF/D318). A similar pattern was observed for SPR (Table, Supplemental Digital Content 8, http://links.lww.com/INF/D319). Higher MGIs were observed in primed children than in unprimed children (Table, Supplemental Digital Content 9, http://links.lww.com/INF/D320).
Neutralizing and Antineuraminidase Antibody Titers
GMTs for neutralizing and antineuraminidase antibodies rose after vaccination in both primed and unprimed children (Fig. 3A, B). GMTs were higher in primed children for the A/H1N1, B/Victoria and B/Yamagata strains. However, there was almost no difference between groups in terms of GMTs for the A/H3N2 strain, although the MGI values were higher in the primed group (Fig. 3A, B; Tables, Supplemental Digital Content 9, http://links.lww.com/INF/D320).
FIGURE 3.
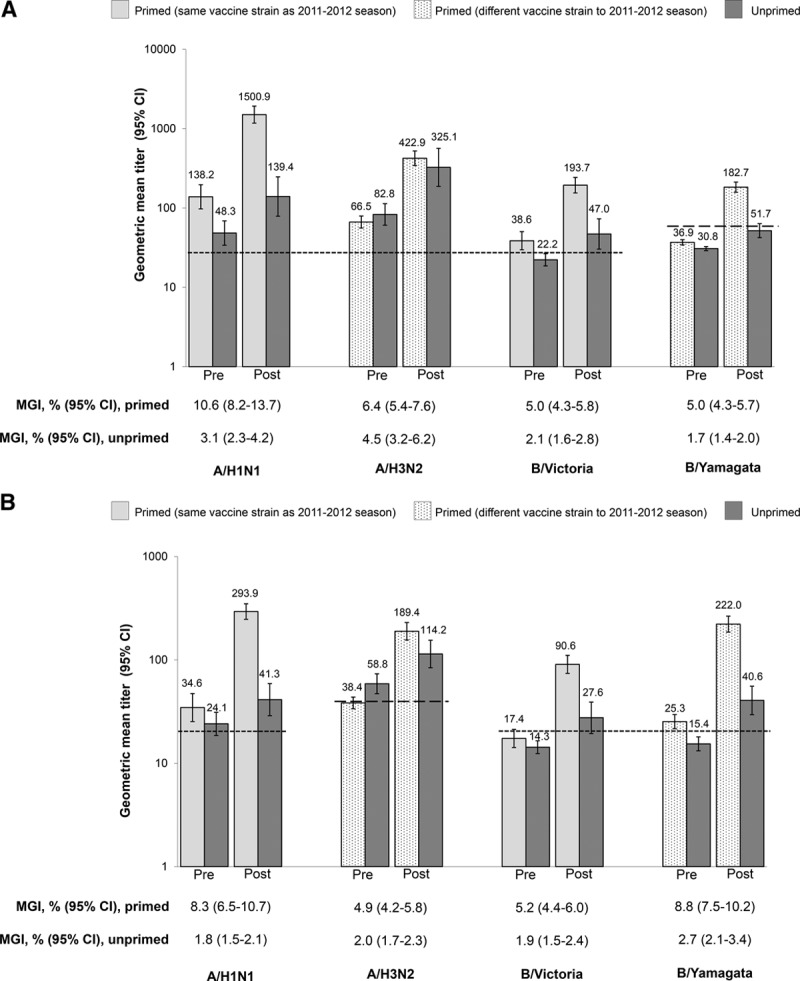
GMT prevaccination and 7 days after first vaccination and MGI for neutralizing and antineuraminidase antibodies in the revaccination study (per-protocol cohort). A: Neutralizing antibodies. Dotted line represents the assay cut-off for the A/H1N1, A/H3N2 and B/Victoria strains (28 1/dil) and the B/Yamagata strain (57 1/dil). GMT values are shown above the bars. B: Antineuraminidase antibodies. Dotted line represents the assay cut-off for the A/H1N1, B/Victoria and B/Yamagata strains (20 1/dil) and the A/H3N2 strain (40 1/dil). GMT values are shown above the bars. Post indicates 7 days following vaccination; Pre, prevaccination. GMT indicates geometric mean titer; MGI, mean geometric increase.
Exploratory Immunogenicity Analysis Excluding Children With RT-PCR–Confirmed Influenza Infection in the Primary Study
In the analysis excluding children with a RT-PCR–confirmed influenza infection in the primary study, primed children mounted a similar anamnestic response to those in the overall per-protocol cohort. GMTs for HI antibodies were similar to the overall per-protocol cohort in both primed and unprimed children (Figure, Supplemental Digital Content 10, http://links.lww.com/INF/D321). For each vaccine strain, the lower limit of the GMT ratio (primed/unprimed) was above 1 and the lower limits of the SCR and SPR difference (primed minus unprimed) were above 0 (Figure, Supplemental Digital Content 10, http://links.lww.com/INF/D321). Likewise, SPR and SCR were comparable in this exploratory analysis to those in the overall per-protocol cohort (Table, Supplemental Digital Content 11, http://links.lww.com/INF/D322). A similar pattern was observed for neutralizing and antineuraminidase antibodies (Figures, Supplemental Digital Content 12 and 13, http://links.lww.com/INF/D323; http://links.lww.com/INF/D324).
Safety
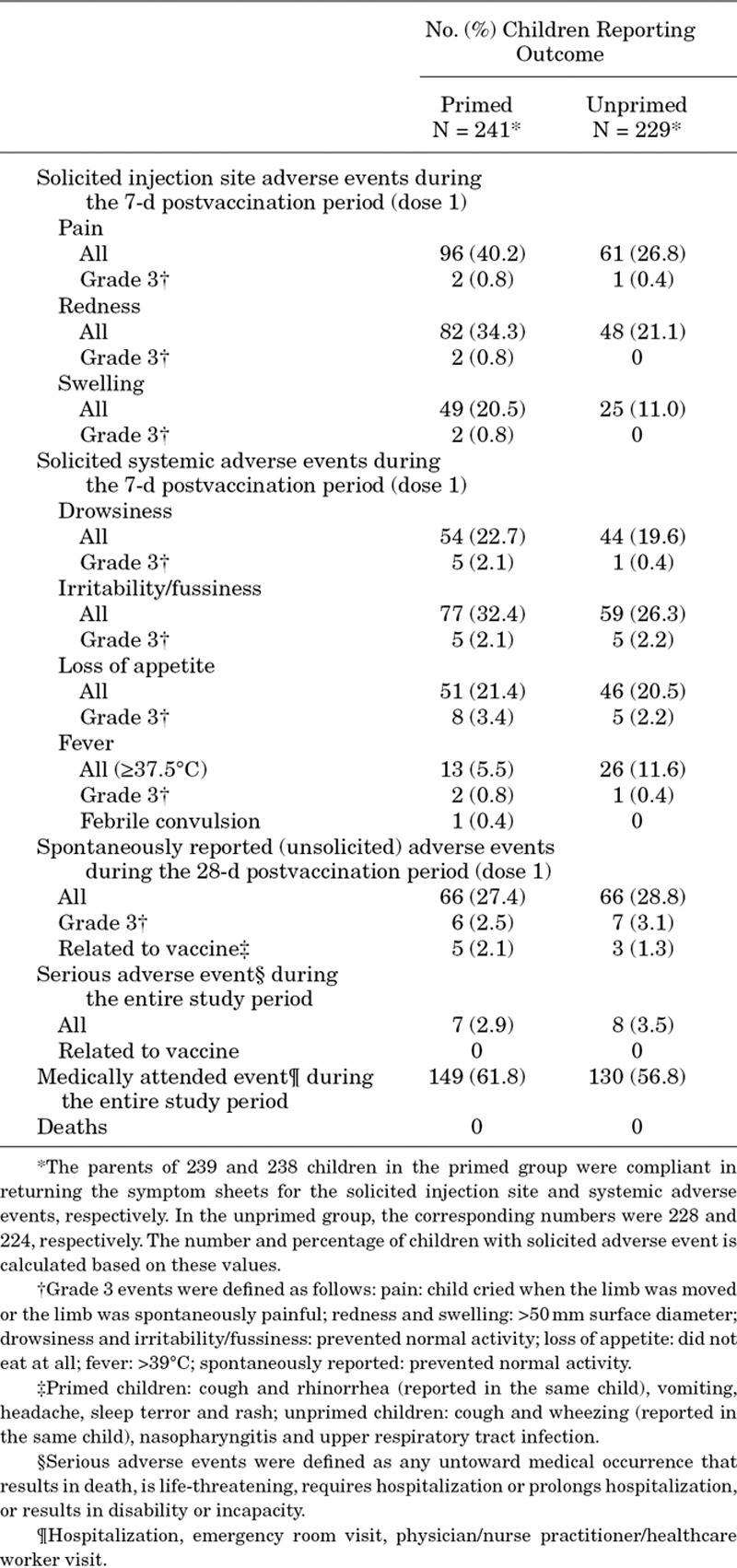
Safety outcomes are shown in Table 1. More children in the primed group experienced injection site AEs compared with the unprimed group. Fever (temperature ≥37.5°C by any route) during the 7 days postvaccination period was observed more often in unprimed (11.6%) than primed (5.5%) children. A febrile convulsion was reported for a primed child 100 days after vaccination and was not considered to be causally related to the study vaccine by the investigator. The frequency of spontaneously reported safety endpoints was similar between groups. No serious AEs related to vaccination occurred during the study and there were no deaths.
TABLE 1.
Safety Outcomes Reported Throughout Study (Total Vaccinated Cohort)
DISCUSSION
This is the first randomized study in children 17−48 months of age to demonstrate an anamnestic immune response to a booster dose of IIV4 in terms of HI, neutralizing and antineuraminidase antibodies. The immune response 7 days after the booster dose was higher than the immune response after the first dose in influenza vaccine naïve children. An anamnestic immune response was observed in both age strata, with little or no difference between children 17–29 and 30–48 months of age.
Immunogenicity of IIV4 has now been widely evaluated in children.12,16–22 Compared with inactivated trivalent influenza vaccine (IIV3), studies show that IIV4 produces a similar immune response to the common vaccine strains and a superior response to the B lineage not contained in the IIV3. In these previous studies, following a full vaccination course of IIV4, SCRs varied between 74% and 92% for A/H1N1, 70% and 88% for A/H3N2, 65% and 85% for B/Victoria and 66% and 94% for B/Yamagata in children from 6 months to 17 years of age.16–21 SCRs in the present study were consistent with these previous studies, with values of 77%, 81%, 77% and 94% observed for A/H1N1, A/H3N2, B/Victoria and B/Yamagata, respectively, in the primed group. Efficacy of the IIV4 in the prevention of mild and moderate-to-severe influenza in children has also been shown.12,22
Most primed children were seropositive to a vaccine strain before first vaccination, and prevaccination titers were higher in primed than unprimed children, except for influenza A/H3N2. The high prevaccination seropositivity and antibody titers in the primed group reflect the persistence of the immune response to the vaccine given during the previous season in the primary study. It is unclear why no difference between primed and unprimed children in prevaccination titers against A/H3N2 was observed in both analyses including and excluding children with a previous influenza illness. It may be related to the update of the A/H3N2 vaccine strain between the 2011−2012 influenza season and the 2012–2013 season; the A/Victoria/210/2009 virus, an A/Perth/16/2009-like virus, was updated to the A/Victoria/361/2011 virus. The B/Yamagata virus was also updated between these seasons; the B/Brisbane/3/2007 virus, a B/Florida/4/2006-like virus, was updated to the B/Hubei-Wujiagang/158/2009 virus. The A/Victoria/361/2011 virus had a 16-fold reduced titer by virus neutralization assay compared with the A/Perth/16/2009 virus.23 The B/Wisconsin/1/2010 virus, which is the reference strain for the B/Hubei-Wujiagang/158/2009 virus, had an 8-fold reduced HI titer compared with the B/Florida/4/2006 virus.23 Despite the update, there was an anamnestic response to the A/H3N2 and B/Yamagata strains, indicating that the prior year’s strains induced a priming response. This is a relevant observation because the vaccine strains in the seasonal vaccine change frequently and therefore booster influenza vaccination must be able to drive an effective anamnestic response to newly introduced vaccine strains or even newly emerging drifted strains not present in the vaccine. Two previous studies of IIV3 in children 6–23 months of age showed an anamnestic immune response after priming with heterologous vaccine strains, although the response was lower compared with priming with homologous strains.10,24
The demonstration of immunogenicity in terms of both HI and antineuraminidase antibodies is important, as it confirms the functional breadth of the immune response to surface proteins of the vaccine. Antineuraminidase antibody has been shown to be an independent predictor of immunity to naturally occurring influenza.25 Overall, the immune response followed the same pattern with the 3 different assays (HI, MN and NI). The booster response appeared to be particularly high for A/H1N1 with the MN assay (reaching titers of 1500). Although the HI and NI assays measure the functional immune response toward the surface proteins of the vaccine, the MN assay may detect a broader range of neutralizing antibodies.26
Excluding children with RT-PCR–confirmed influenza infection in the primary study had no significant impact on the analysis of the immune response in either primed or unprimed children. As expected, more children in the unprimed group, who did not receive influenza vaccination in the primary study, experienced an influenza illness than the primed group (30 vs. 11 children, respectively). Influenza A/H3N2 was by far the most commonly detected virus in children with influenza illness with all 11 children in the primed group and 27 of 30 children in the unprimed group experiencing influenza associated with this virus. Prior exposure to infection and natural antibody production in the unprimed group would be expected to mask differences between the primed and unprimed groups. Excluding children with a previous illness may therefore be expected to increase the difference between the primed and unprimed groups. This was indeed observed for A/H3N2, for which the GMT ratio increased from 2.7 in the analysis of all children to 4.2 in the analysis excluding children with a previous illness.
The IIV4 used in the study was given at a dose of 15 µg per antigen (0.5 mL volume), rather than the lower dose of 7.5 µg per antigen (0.25 mL volume) traditionally used in young children. The lower dose was introduced in young children during the 1970s as a response to the high reactogenicity experienced by this age group to the whole virus vaccines available at the time.27 However, young children mount a variable immune response to the 7.5 µg dose,10 and current split virus vaccines are much better tolerated than whole virus vaccines.28 Use of a 15 µg dose has been shown to improve the immune response in young children compared with the 7.5 µg dose.20,21 The 15 µg dose was shown to be well tolerated in the present study, with a safety profile in line with other studies; the higher antigen content of the IIV4 resulting from the additional B lineage antigen and the 15 µg dose does not appear to adversely affect tolerability in children, including the very young.12,16–22
Our study had some limitations. First, the traditional measure of immunogenicity according to European and US licensure criteria is the immune response determined on blood samples collected 1 month after vaccination. Here, we chose to evaluate immunogenicity 1 week after vaccination to measure the anamnestic response which is characterized by an early and sharp rise in antibody titers. To limit the number of blood samples drawn in these young children, no immunogenicity analysis was planned at 1 month. Second, although participants were randomized at baseline of the primary study to IIV4 or control vaccine, a convenience sample was enrolled in the revaccination study, with no randomization of the primed and unprimed groups. A relatively small number of children from the first of the 5 seasonal cohorts of the primary study participated in the revaccination study. Third, children who participated in the revaccination study may or may not have been part of the immunogenicity subset of the primary study, as this comprised a convenience sample consisting of approximately 250 children out of a total of approximately 1800 enrolled.
In conclusion, the present study has shown that the IIV4 induces anamnestic immune responses in IIV4-primed children to the 2 major surface proteins of the influenza virus that are important for protection against infection, for strains that are antigenically like the vaccine strains administered in the previous year and for drift variants. The findings indicate the capacity of an annual booster of IIV4 to enhance immunity after primary vaccination of infants and toddlers, with an acceptable safety profile. These data support extending to IIV4 the current use of a 2-dose IIV series followed by 1 dose in subsequent years in very young vaccine-naïve children who are at increased risk of poor outcomes associated with influenza.
ACKNOWLEDGMENTS
The authors are indebted to the participating study volunteers and their parents. The authors thank the investigators who provided support and cared for study participants; the staff members of the study centers, in particular, P. Heath, R. Tomlinson, B. Bodalia and M. Wheeler for their contribution to the study and A. Finn for his contribution to the study and review of the manuscript. The authors also thank the global and regional teams of GSK, in particular, D. Daehnick (Scientific Writer), F. Bieswal (Study Delivery Lead), M. Heynderickx (Clinical Regulatory Affairs) and Allen Izu (Lead Statistician). Finally, the authors thank R. Ray (GSK) and E. Neumeier (GSK) for review of the manuscript, M. L. Greenacre (An Sgriobhadair, UK, on behalf of GSK) for providing medical writing services, and Business & Decision Life Sciences platform (on behalf of GSK) for editorial assistance and manuscript coordination. B. Dumont coordinated the manuscript development and provided editorial support.
Supplementary Material
Footnotes
Supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA paid for all costs associated with the trial and development of this manuscript.
C.C., V.C., D.F., T.O., J.S. and A.S. are employees of the GSK group of companies. P.L. reports she was employed by the GSK group of companies at the time of the study and is currently employed by Pfizer. B.L.I. reports he was employed by the GSK group of companies at the time of the study. V.K.J. reports she was employed by the GSK group of companies at the time of the study. C.C, A.S., P.L. and V.K.J. hold shares in the GSK group of companies. A.J.P. reports grants from the GSK group of companies for the submitted work, and previous grants from Okairos, and Pfizer outside the submitted work in the last 36 months. A.J.P. also reports he is chair of the UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination and Immunisation (JCVI) and a member of WHOs SAGE. The views expressed in this manuscript do not necessarily reflect the views of DHSC or JCVI. F.M.-T. reports grants to his institution from the GSK group of companies for this study and reports grants from the GSK group of companies, Pfizer, Sanofi Pasteur, MSD, Janssen, Ablynx and Novartis, outside the submitted work, his research activities are funded by Instituto Carlos III (Intensificación de la actividad investigadora) and Fondo de Investigación Sanitaria (FIS, PI13/02382) of the Plan Nacional de I+D+I and Fondos FEDER. J.D.-D. declares his institution received payments from the GSK group of companies for the conduct of this study, and is the principal investigator of clinical trials and observational studies for the GSK group of companies, as well as a member of a GSK group of companies’ advisory board, outside the submitted work. J.G.-S. reports his institution received payments from the GSK group of companies for the conduct of this study and reports grants from the GSK group of companies, Pfizer, Sanofi Pasteur and Novartis outside the submitted work. J.M.B. received grants from the GSK group of companies for the conduct of this study and grants for lectures including services on speakers bureaux from Sanofi Pasteur, MSD, the GSK group of companies and Pfizer, grants for board membership from Sanofi Pasteur, MSD and grants as principal investigator in clinical trials from Pfizer and the GSK group of companies, outside the presented work. M.D.S. declares that his institution received grants from the GSK group of companies during the conduct of this study and reports that his institution has received payment from the GSK group of companies to attend conferences, outside the submitted work. M.D.S. also reports that, outside of the submitted work, his institution received funding to conduct research from other vaccine manufacturers including Sanofi Pasteur, MSD, Medimmune, Novavax and Pfizer, and that before 2017 his institution received funding for Dr. Snape’s attendance at advisory committees and to speak at industry-sponsored symposium. R.P. reports grants and personal fees from the GSK group of companies during the conduct of this study and received grants and personal fees from Sanofi, Novartis and Baxter, outside the submitted work. S.N.F. reports his institution received grants per-patient fee for research costs associated with clinical trials from the GSK group of companies, his institution also received payments for advisory board membership from the GSK group of companies, Pfizer, Novartis and Sanofi, and for travel support from the GSK group of companies, outside the submitted work. A.U. has received payments from the GSK group of companies to conduct clinical trials through her institution and a grant from Pfizer for a lecture outside the submitted work. The other authors have no conflicts of interest to disclose.
Ping Li, PhD, is currently at the Pfizer VRD, Collegeville, Pennsylvania.
Bruce L. Innis, MD, is currently at the PATH, Washington, DC.
Varsha K. Jain, MD, MPH, is currently at Bill & Melinda Gates Foundation, Seattle, Washington.
All authors participated in the design or implementation or analysis, interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Fluarix Quadrivalent is a trademark of the GSK group of companies.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Cromer D, van Hoek AJ, Jit M, et al. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68:363–371.. [DOI] [PubMed] [Google Scholar]
- 2.Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014;173:265–276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowlkes A, Steffens A, Temte J, et al. ; Influenza Incidence Surveillance Project Working Group. Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009-13. Lancet Respir Med. 2015;3:709–718.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–476.. [PubMed] [Google Scholar]
- 5.Skowronski DM, Masaro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005-2006 season of dual A and B vaccine mismatch in Canada. Vaccine. 2007;25:2842–2851.. [DOI] [PubMed] [Google Scholar]
- 6.Skowronski DM, De Serres G, Dickinson J, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006-2007. J Infect Dis. 2009;199:168–179.. [DOI] [PubMed] [Google Scholar]
- 7.Belongia EA, Kieke BA, Donahue JG, et al. ; Marshfield Influenza Study Group. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199:159–167.. [DOI] [PubMed] [Google Scholar]
- 8.Belshe RB, Coelingh K, Ambrose CS, et al. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–2156.. [DOI] [PubMed] [Google Scholar]
- 9.Barr IG, Komadina N, Durrant C, et al. Circulation and antigenic drift in human influenza B viruses in SE Asia and Oceania since 2000. Commun Dis Intell Q Rep. 2006;30:350–357.. [PubMed] [Google Scholar]
- 10.Englund JA, Walter EB, Gbadebo A, et al. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics. 2006;118:e579–e585.. [DOI] [PubMed] [Google Scholar]
- 11.Levandowski RA, Regnery HL, Staton E, et al. Antibody responses to influenza B viruses in immunologically unprimed children. Pediatrics. 1991;88:1031–1036.. [PubMed] [Google Scholar]
- 12.Claeys C, Zaman K, Dbaibo G, et al. ; Flu4VEC Study Group. Prevention of vaccine-matched and mismatched influenza in children aged 6-35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2:338–349.. [DOI] [PubMed] [Google Scholar]
- 13.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138.. [DOI] [PubMed] [Google Scholar]
- 14.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–413.. [Google Scholar]
- 15.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872.. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg DP, Robertson CA, Landolfi VA, et al. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J. 2014;33:630–636.. [DOI] [PubMed] [Google Scholar]
- 17.Langley JM, Carmona Martinez A, Chatterjee A, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis. 2013;208:544–553.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langley JM, Wang L, Aggarwal N, et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012-2013: a randomized, double-blind, controlled, multicenter, multicountry, clinical trial. J Pediatric Infect Dis Soc. 2015;4:242–251.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domachowske JB, Pankow-Culot H, Bautista M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis. 2013;207:1878–1887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Chandrasekaran V, Domachowske JB, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine in US children 6-35 months of age during 2013-2014: results from a phase II randomized trial. J Pediatric Infect Dis Soc. 2016;5:170–179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain VK, Domachowske JB, Wang L, et al. Time to change dosing of inactivated quadrivalent influenza vaccine in young children: evidence from a phase III, randomized, controlled trial. J Pediatric Infect Dis Soc. 2017;6:9–19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369:2481–2491.. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. 2012. Available at: http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf?ua=1.http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf?ua=1. Accessed October 14, 2015.
- 24.Walter EB, Neuzil KM, Zhu Y, et al. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics. 2006;118:e570–e578.. [DOI] [PubMed] [Google Scholar]
- 25.Couch RB, Atmar RL, Franco LM, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207:974–981.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardelid P, Andrews NJ, Hoschler K, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess. 2010;14:115–192.. [DOI] [PubMed] [Google Scholar]
- 27.Wright PF, Thompson J, Vaughn WK, et al. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136(suppl):S731–S741.. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein DI, Zahradnik JM, DeAngelis CJ, et al. Clinical reactions and serologic responses after vaccination with whole-virus or split-virus influenza vaccines in children aged 6 to 36 months. Pediatrics. 1982;69:404–408.. [PubMed] [Google Scholar]


