Abstract
Relapses of Plasmodium dormant liver hypnozoites compromise malaria eradication efforts. New radical cure drugs are urgently needed, yet the vast gap in knowledge of hypnozoite biology impedes drug discovery. We previously unraveled the transcriptome of 6 to 7 day-old P. cynomolgi liver stages, highlighting pathways associated with hypnozoite dormancy (Voorberg-van der Wel et al., 2017). We now extend these findings by transcriptome profiling of 9 to 10 day-old liver stage parasites, thus revealing for the first time the maturation of the dormant stage over time. Although progression of dormancy leads to a 10-fold decrease in transcription and expression of only 840 genes, including genes associated with housekeeping functions, we show that pathways involved in quiescence, energy metabolism and maintenance of genome integrity remain the prevalent pathways active in mature hypnozoites.
Research organism: Other
Introduction
Plasmodium vivax malaria puts 35% of the world’s population at risk of disease (World Health Organization, 1987). A large barrier to P. vivax eradication is afforded by the parasite’s ability to cause relapsing disease weeks to months or even years after the primary mosquito-mediated infection (White, 2011). This aspect of P. vivax infection is caused by a dormant liver stage form of the parasite, named the hypnozoite. Hypnozoites can reactivate through unknown mechanisms, continue development into liver schizonts and cause renewed disease. While prophylactic drugs can prevent initial parasite development in the liver (Zeeman et al., 2016), there is a need for new radical cure compounds targeting established hypnozoites. There is a vast gap in knowledge surrounding hypnozoite biology that causes a significant setback in developing a novel radical cure drug that can eliminate hypnozoites. Such a drug is urgently needed to aid in malaria eradication because the current radical curative drugs cannot be used in all persons in endemic areas. For instance, primaquine and the recently FDA-approved tafenoquine cannot be administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, a common genetic disorder in malaria endemic countries, due to serious adverse side-effects and life-threatening drug-induced hemolysis (Mazier et al., 2009; Wells et al., 2010). For this reason, new and better drugs are urgently needed. One compound that appeared promising in in vitro screens, a phosphatidylinositol 4-kinase (PI4K) inhibitor (Zeeman et al., 2014), was shown to be active against hypnozoites in a prophylactic, but not radical cure dosing scheme (Zeeman et al., 2016). This indicates that ‘early’ hypnozoites differ from ‘established’ hypnozoites and suggests differences in active cellular pathways between the two forms. P. vivax hypnozoite biology research is hampered by the absence of an in vitro blood stage culture system, which makes the development of P. vivax research tools dependent on patient material as a source for sporozoites. The P. cynomolgi simian malaria, closely related to P. vivax, is a well-validated model for human P. vivax malaria useful to study the disease relapse caused by the reactivation of liver hypnozoites. P. cynomolgi in vitro liver stage cultures have been established and in combination with the established transfection technology for this parasite this provides unique opportunities for studies into hypnozoite biology. To investigate differences between different aged hypnozoites, we used genetically engineered fluorescent P. cynomolgi parasites (Voorberg-van der Wel et al., 2013), in vitro P. cynomolgi liver stage culture (Zeeman et al., 2014), cell-sorting and RNA-seq to expand the recently published liver stage transcriptomes (6 to 7 day-old in vitro cultured hypnozoites and replicating liver stages) (Voorberg-van der Wel et al., 2017) by transcriptionally profiling malaria parasite liver stages after 9 to 10 days of in vitro culture. The transcriptomic analysis we describe here, together with the previously published study show that hypnozoite maturation is accompanied by a 10-fold decrease in transcriptional activity and the expression of 840 genes of which only ~4% at higher levels (≥10 FPKM). Although established hypnozoites continue to express a subset of genes, a marker gene which specifically distinguishes hypnozoites from schizonts was not detected. Genes and pathways associated with quiescence, energy metabolism and maintenance of genome integrity remain prevalently expressed in mature hypnozoites, indicating that the hypnozoite stage may be defined by a network of regulatory factors cooperating in the maintenance of genome stability and in the epigenetic control of gene expression.
Results
Transcriptome analysis of malaria parasite liver stages after 9 to 10 days of in vitro culture
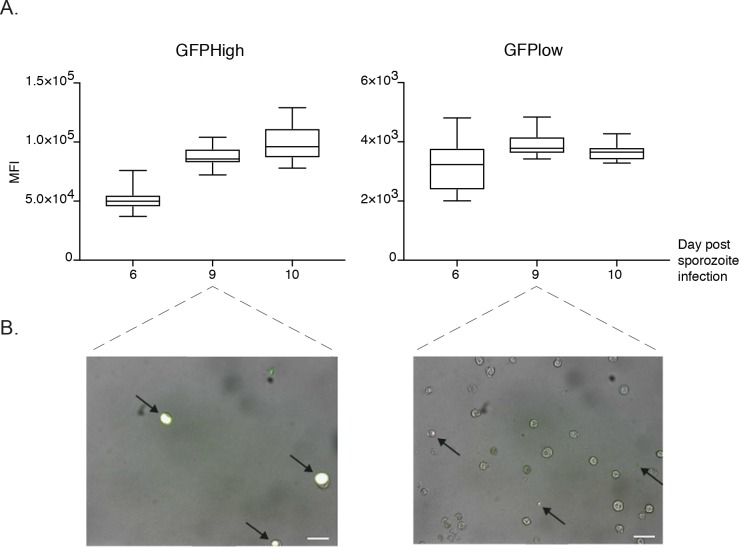
To gain further understanding of dormancy mechanisms described in hypnozoites at days 6 and 7, we FACS-purified hepatocytes containing GFP-expressing hypnozoites (GFPlow) and liver schizonts (GFPhigh) at later time points, 9 and 10 days after P. cynomolgi M strain sporozoite infection. Mean fluorescence intensities, a measure that depends on cell size and fluorescence intensity, slightly increased from day 6 to day 9 in the GFPlow samples, illustrating a small increase in the volume of hypnozoites over time (Mikolajczak et al., 2015). A more pronounced increase over time was observed in the GFPhigh samples, indicating significant parasite growth (Figure 1—figure supplement 1A). Microscopic analysis of quality control samples taken from the FACS-sorted parasites revealed advanced schizogony at day 9 (GFPhigh samples) and small forms that were, similar to day 6 sorted forms, in- or outside hepatocytes (GFPlow samples) (Figure 1—figure supplement 1B). The GFPlow samples contained substantial amounts of uninfected hepatocytes, possibly due to increased hepatocyte autofluorescence as a result of prolonged culture.
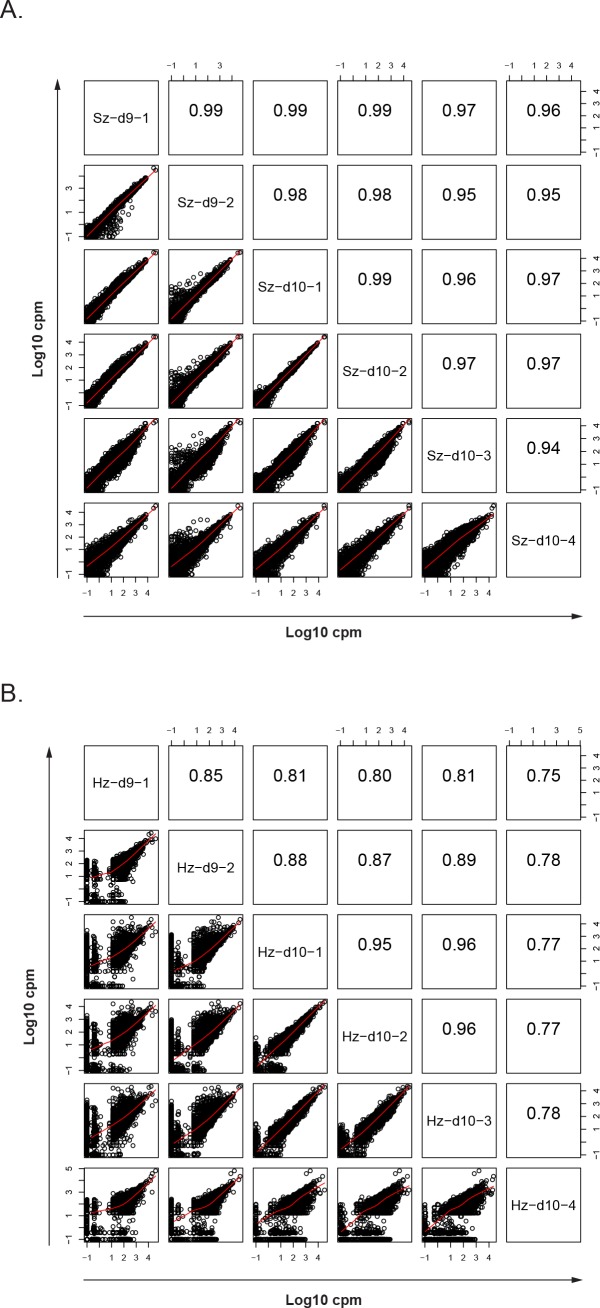
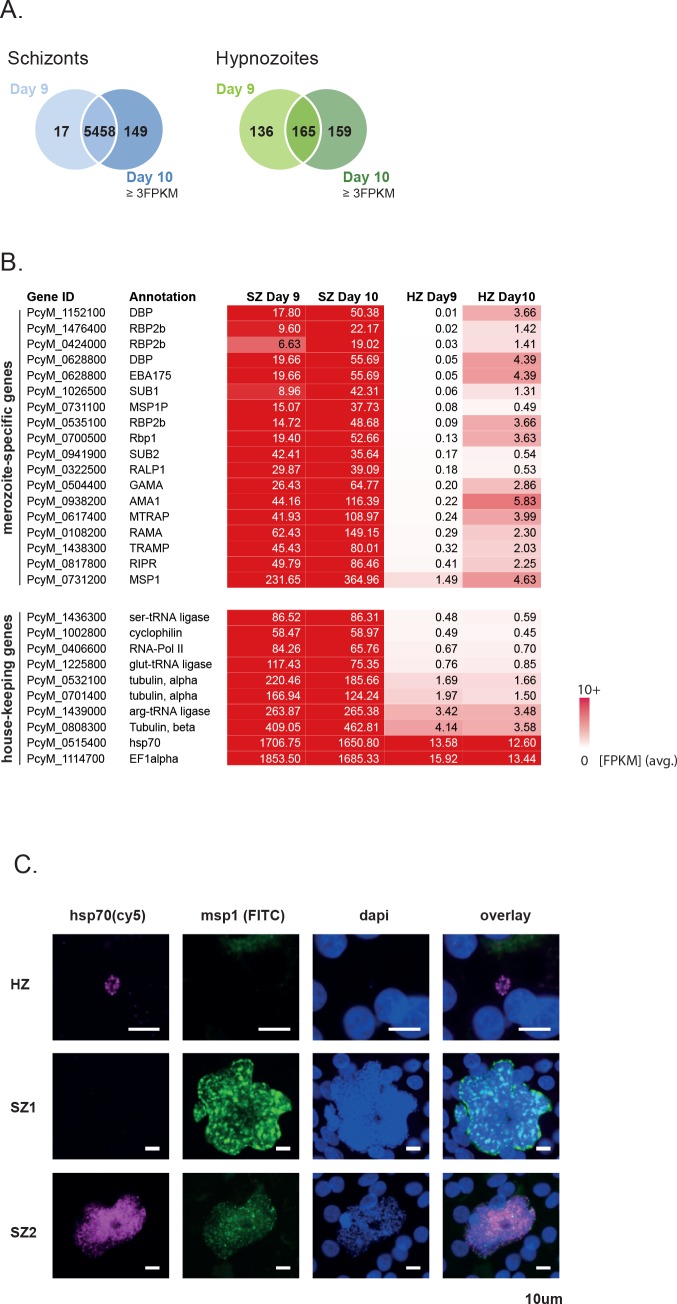
RNA-sequencing resulted in a dataset containing two independent schizont and hypnozoite samples at day 9, and four independent schizont and hypnozoite samples at day 10 (Supplementary file 1). Gene expression data showed high concordance between biological replicates (Figure 1—figure supplement 2). We observed a strong correlation between schizont (ρ = 0.99 at day 9 and ρ = 0.97 at day 10) and hypnozoite transcriptomes (ρ = 0.85 at day 9 and ρ = 0.87 at day 10), however the hypnozoite samples did not correlate as highly as schizonts between the time points (ρ = 0.82 vs ρ = 0.97). While schizonts revealed a large consensus between genes expressed at days 9 and 10, day 9 and day 10 hypnozoites showed little overlap because a significant number of genes showed low to no transcription levels at day 9 and high expression at day 10 (Figure 1—figure supplement 3A). These included genes involved in merozoite maturation (for example MSP1, AMA1 and SUB1) (Supplementary file 2) (Figure 1—figure supplement 3B). In contrast, housekeeping genes (e.g. hsp70) showed similar expression levels at these time points (Figure 1—figure supplement 3B). Given the presence of transcripts of genes involved in schizont maturation and merozoite invasion, we hypothesized and confirmed by RNA fluorescence in situ hybridization (FISH) a contamination of day 10 hypnozoites with schizonts and/or released merozoites. FISH with a probe against msp1 was positive for schizonts at day 10, while no transcripts were detected in hypnozoites at day 10 (Figure 1—figure supplement 3C). In contrast, transcripts for hsp70 were found both in schizonts and hypnozoites, except in fully mature schizonts (Figure 1—figure supplement 3C).
These results show that presence of schizont transcripts in the day 10 GFPlow samples requires careful in vitro validation for each transcript that is detected in hypnozoites. For this reason, we decided to exclude the day 10 timepoint from further analyses.
Late stage liver parasites reveal features of advanced schizogony and dormancy
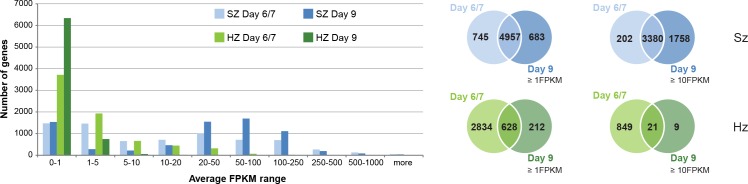
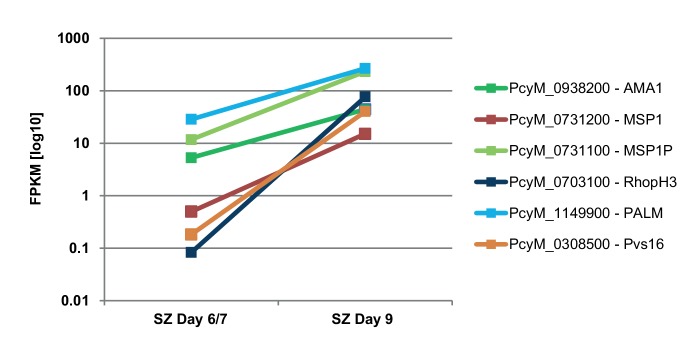
We found that later stage (day 9) liver schizonts express a similar number of genes as those observed at days 6/7 (5640 genes at day 9 vs 5702 at day 6/7, with FPKM ≥1). However at day 9 genes are expressed at higher levels (5138 genes at day 9 vs 3582 at day 6/7, with FPKM ≥10) (Figure 1). Most of the genes (94%, 3380 out of total 3582) showing already high expression level at day 6/7 (≥10 FPKM) remain highly expressed at day 9. However, at day 9 we find an additional 1758 genes with increased expression levels (≥10 FPKM) compared to day 6/7. These include genes that are involved in merozoite formation (PALM) or required by merozoites for red blood cell invasion (RhopH3, AMA1, MSP1) and that are already reported to be expressed in late stage liver schizogony (Sherling et al., 2017; Haussig et al., 2011). Moreover, the sexual stage-specific marker Pvs16, which was previously shown to be expressed in late stage P. vivax liver schizonts, also shows upregulation at day 9 compared to day 6/7 (Figure 1—figure supplement 4) (Roth et al., 2018). In addition to invasion pathways, cytoskeleton motor protein pathways showed increased transcriptional activity at day 9 (97%) compared to day 6 (69%) (Supplementary file 4). These actin-myosin motor proteins play crucial roles in apicomplexan host cell invasion (Bargieri et al., 2014). It is well known in blood stages that these genes exhibit peak expression in late schizont stage only, which correlates with schizont maturation (Bozdech et al., 2003). The high transcription level of these motor protein genes in day 9 liver schizonts suggests near to complete schizont maturation at this time point. Overall, the increased transcriptional activity observed in the later stage liver schizonts reveals molecular events associated with progressing schizogony.
Figure 1. Comparison of transcriptional activity in schizonts (SZ) and hypnozoites (HZ) at day 6/7 and day 9.
Left panel: Distribution of average gene expression values in the schizont samples at day 6/7 (light blue; n = 3) and at day 9 (dark blue; n = 2), and distribution of average gene expression values in the hypnozoites samples at day 6/7 (light green; n = 4) and at day 9 (dark green; n = 2). FPKM, Fragments per kilobase of transcript per million mapped reads. Upper right panel: Venn diagrams show the overlap of genes expressed ≥1 FPKM and ≥10 FPKM, respectively, at day 6/7 and at day 9 in the schizont samples. Lower right panel: Venn diagrams show the overlap of genes expressed ≥1 FPKM and ≥10 FPKM, respectively, at day 6/7 and at day 9 in the hypnozoite samples.
Figure 1—figure supplement 1. Quality check of FACS-sorted parasite cells.
Figure 1—figure supplement 2. Correlation of gene expression values between biological replicates.
Figure 1—figure supplement 3. Possible contamination of day 10 hypnozoites with schizont derived transcripts.
Figure 1—figure supplement 4. Diagram showing mean gene expression values (FPKM) of schizogony markers in the schizont samples at day 6/7 and day 9.
Figure 1—figure supplement 5. Expression levels of specific genes of interest.
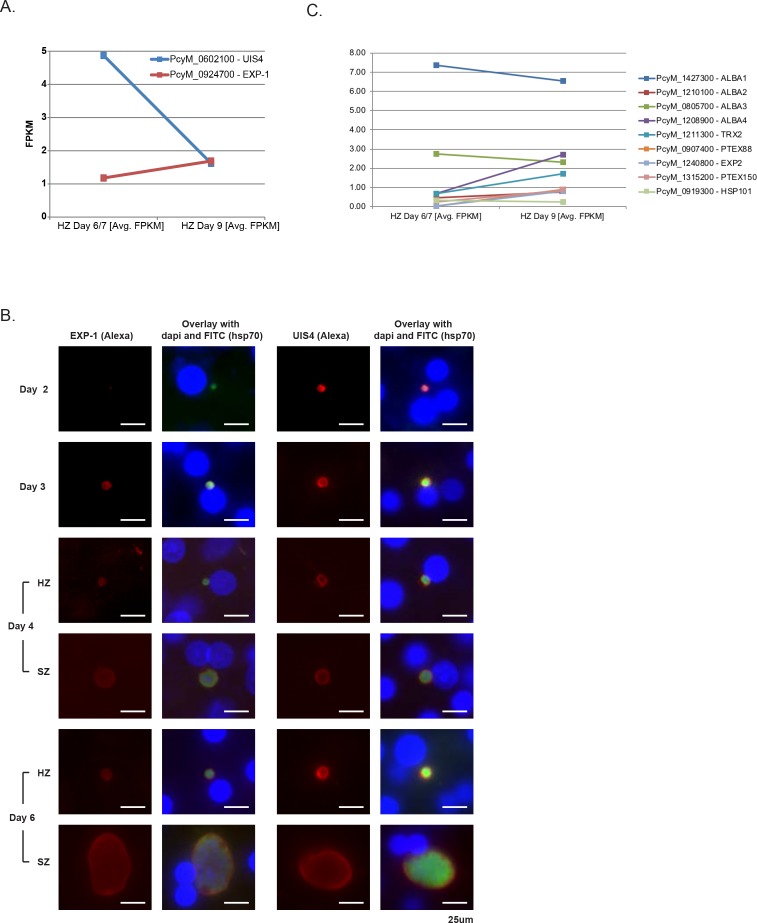
In contrast, day 9 hypnozoites showed a lower transcriptional activity compared to day 6/7 with only few genes expressed at higher levels (30 genes at day 9 vs 870 at day 6/7, with FPKM ≥10). This is also reflected in the significant 10-fold decrease of gene expression levels in hypnozoites (e.g. from 5.8 avg FPKM at day 6/7 down to 0.6 avg FPKM at day 9: Wilcoxon test p-value<0.02857). We observed that only 18% of genes expressed in days 6/7 hypnozoites (628 out of 3462 genes with FPKM ≥1) are as well expressed at day 9 (628 out of 840 genes with FPKM ≥1). This transcriptional decrease is even more pronounced for highly expressed genes where only 2.4% of genes expressed at day 6/7 (21 out of 870 genes with FPKM ≥10) show high transcriptional activity also at day 9 (21 out of 30 genes with FPKM ≥10) (Figure 1). The small number of highly expressed genes at day 9 is most likely the result of an overall reduced transcriptional activity ongoing during hypnozoite maturation. Indeed, the nine genes found to be highly expressed only in day 9 hypnozoites mostly encode ribosomal and histone proteins which are also expressed at day 6/7 but just below the cutoff of 10 FPKM. In contrast, over 70% of all genes expressed ≥10 FPKM at day 6/7 are expressed below 1 FPKM at day 9 (Supplementary file 3). These results provide for the first time a strong evidence that maturation of the dormant liver stage is associated with continued reduction of its transcriptional activity.
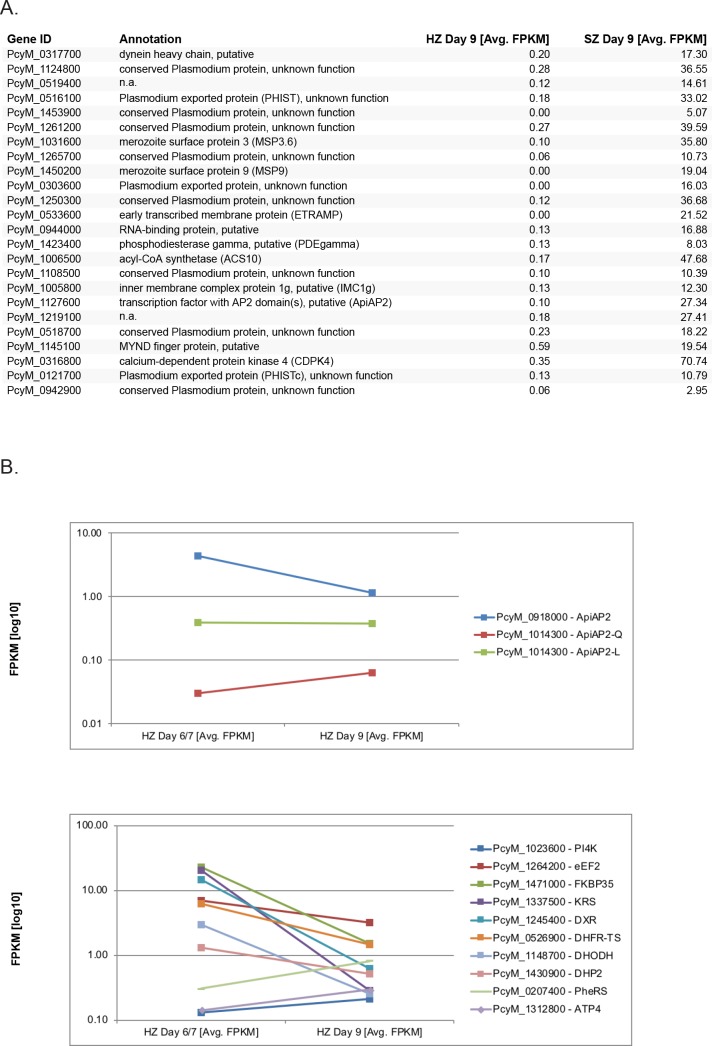
In the published dataset of day 6/7 a few hypnozoite-specific genes were identified with significantly higher expression levels compared to schizonts and could thus be hypnozoite markers (Voorberg-van der Wel et al., 2017). However, all these genes show expression levels <1 FPKM in hypnozoites at day 9, and higher transcriptional levels in schizonts (Figure 1—figure supplement 5A). We hence do not find any gene that is exclusively expressed in the hypnozoite population at day 9, concluding that there is no specific marker for dormant stages at the transcriptional level. Notably, the liver-stage-specific AP2-L protein, which is a member of the plant-derived Apicomplexan Apetala2 (ApiAP2) family of transcription factors, showed equivalent transcription levels in hypnozoites at day 6/7 and day 9 (Balaji et al., 2005; Iwanaga et al., 2012). In contrast, AP2-Q which has been proposed as a master regulator of transcription in hypnozoites (Cubi et al., 2017), showed only very low transcription levels in hypnozoites at day 9 (Figure 1—figure supplement 5B), which corroborates the results of the recently published P. vivax hypnozoite transcriptome (Cubi et al., 2017; Gural et al., 2018). Interestingly, in the P. vivax transcriptome, Gural et al. observed another ApiAP2 transcription factor (PV01_0916300) with high transcript abundance in P. vivax hypnozoites (Gural et al., 2018). Indeed, we also found transcription of this AP2-encoding gene (PcyM_0918000) in P. cynomolgi hypnozoites at day 6/7 and day 9 (Figure 1—figure supplement 5B).
To evaluate the potential to apply insights from the transcriptome data for drug discovery, we looked at the expression levels of clinically and chemically validated drug targets (Voorberg-van der Wel et al., 2017). For almost all drug targets transcription levels had dropped to low levels in day 9 hypnozoites (Figure 1—figure supplement 5B). Transcription levels of PI4K in hypnozoites at days 6/7 and day 9 are almost zero, which is in line with the proposed early mode of action that explains the lack of radical cure activity of the drug despite its strong prophylactic activity (Figure 1—figure supplement 5B) (Zeeman et al., 2016) and which aligns with the P. vivax hypnozoite data of Gural et al., 2018. In contrast, day 9 hypnozoites show still a significant number of transcripts for eukaryotic elongation factor 2 (eEF2), albeit at lower levels than at days 6/7, warranting further research into this target for potential radical cure.
Taken together, our data suggest that while late schizogony at day 9 is associated with increased transcriptional activity compared to days 6/7, continued dormancy at day 9 is associated with a decrease in gene transcription.
Dormancy in maturing hypnozoites is associated with a general metabolic shutdown
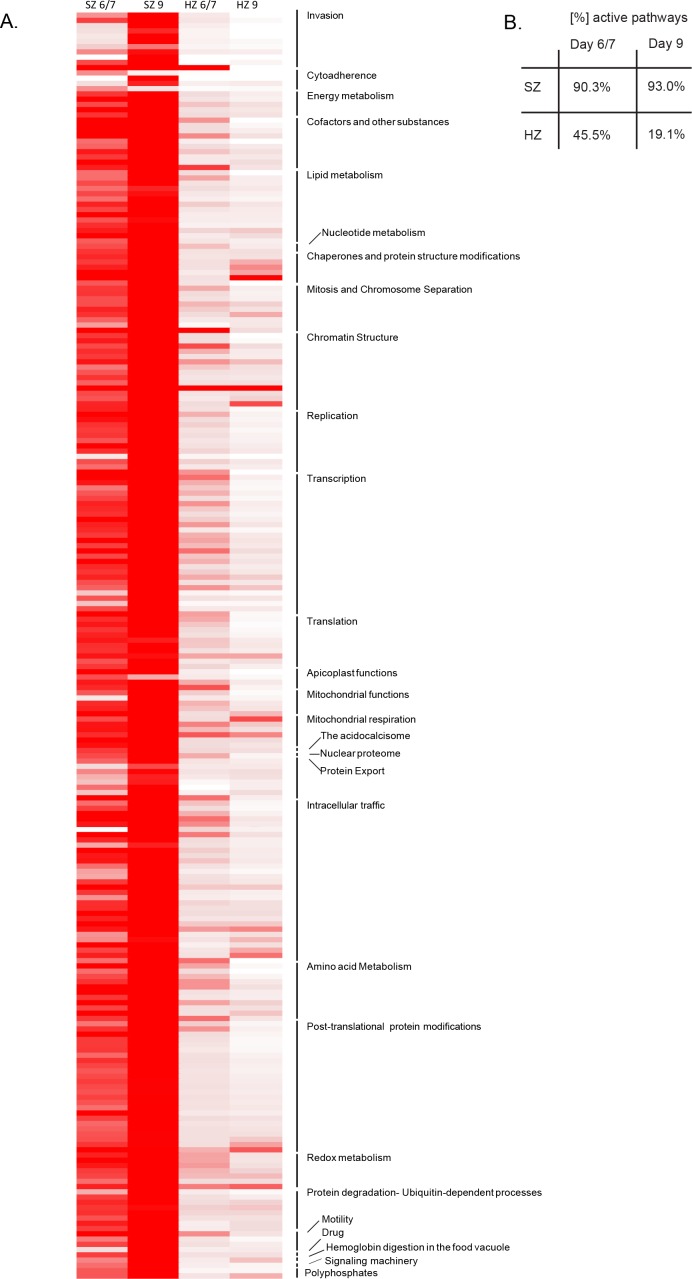
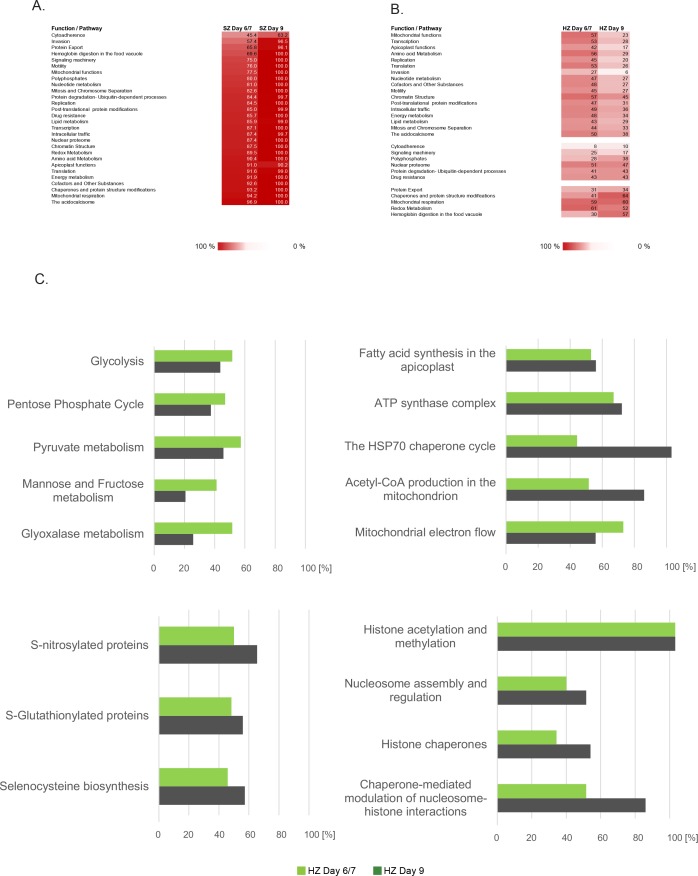
We previously reported that schizonts at days 6/7 express over 90% of the malaria pathways annotated in PlasmoDB (Voorberg-van der Wel et al., 2017). These included energy and glucose metabolism, such as pentose phosphate cycle enzymes, CoA biosynthesis and mannose/fructose metabolism, as well as some erythrocyte invasion pathways (Voorberg-van der Wel et al., 2017). In schizonts at day 9 this percentage is increased to 93% active pathways (Figure 2, Supplementary file 4). The only pathways with a decrease in gene activity compared to day 6/7 are involved in remodeling of the host erythrocyte, and expression of genes in the apicoplast. Indeed clustering of the pathways into higher level groups of similar functions (http://mpmp.huji.ac.il/; April, 2018) revealed that cytoadherence is the only function with expression of less than 70% of the genes (Figure 2—figure supplement 1A).
Figure 2. Pathway analysis of the malaria liver stages.
(A) Heat map representing expression of Plasmodium pathways in schizonts and hypnozoites at days 6/7 and 9. A total of 257 biological pathways annotated in P. falciparum were assigned to P. cynomolgi through orthology (see Materials and methods in Voorberg-van der Wel et al., 2017). Pathway expression is shown with a color gradient from white (where the fraction of genes detected above 1 FPKM is 0%) to red (where this fraction is 100%). (B) Overall percentage of active pathways in schizonts and hypnozoites at days 6/7 and 9.
Figure 2—figure supplement 1. Expression levels of selected pathways of interest.
In contrast, eight pathways with an increase of over 50% in gene activity at day 9 compared to day 6/7 represent almost exclusively functions involved in parasite invasion, DNA replication and homologous recombination (Supplementary file 4). Hence, we conclude that our data at day 9 reflects the maturation of merozoites in late schizogony.
In contrast to schizonts, we previously showed that day 6/7 hypnozoites express less than half of the annotated malaria pathways reflecting the quiescent state and low metabolism that may be expected in dormant forms (Voorberg-van der Wel et al., 2017). With only 49 out of 257 (19.1%) pathways expressing more than half of their constituent genes above 1 FPKM, the total number of active pathways is even lower in hypnozoites at day 9 (Figure 2).
For further analysis, the 257 pathways were clustered into higher-level groups of similar functions (http://mpmp.huji.ac.il/; April, 2018) and, for each group, the mean percentage of genes active in the pathways was calculated for hypnozoites at day 6/7 and day 9. This revealed lower transcription levels of genes involved in transcription, translation, replication and merozoite invasion in the day 9 hypnozoites. Functions such as chromatin structure and energy metabolism were less represented in hypnozoites at day 9, however, still showed a moderate expression level (Figure 2—figure supplement 1B).
A closer look at the energy metabolism in hypnozoites revealed that while glyoxalase, mannose and fructose metabolism are suppressed in hypnozoites at day 9, the pyruvate metabolism, the pentose phosphate cycle and glycolysis are expressed at similar levels as in hypnozoites at day 6/7, suggesting that these pathways represent the main energy source for dormant stages (Figure 2—figure supplement 1C). Moreover, histone acetylation and methylation as well as chaperone-mediated modulation of nucleosome-histone interactions show from 50% to 100% activation in day 6/7 and day nine hypnozoites, suggesting a possible role of these pathways in the maintenance of liver stage dormancy (Figure 2—figure supplement 1C).
Further, we show that functional processes such as cytoadherence were already expressed at a very low level in the hypnozoites at day 6/7 and stayed at a very low level at day 9. Interestingly, functions such as protein export (e.g. PTEX), chaperones and protein structure modifications (e.g. HSP70 cycle), mitochondrial respiration and redox metabolism showed either upregulation or sustained expression in day 9 hypnozoites, suggesting a key role in the maintenance of the quiescent state (Figure 2—figure supplement 1B, Supplementary file 4). Moreover, we show that genes required for fatty acid synthesis, genes coding for enzymes involved in post-translational modifications and genes required for the ATP homeostasis are relatively highly expressed in hypnozoites (Figure 2—figure supplement 1B, Supplementary file 4). The latter is of particular interest, since ATP homeostasis was shown to play an essential role in dormancy in other organisms, for example for the non-replicating dormant form of Mycobacterium tuberculosis (Rao et al., 2008).
To conclude, our data show that gene expression levels of housekeeping pathways in later stage, day 9 hypnozoites are dampened. Only pathways previously associated with quiescence and required for energy metabolism and maintenance of chromosome integrity remain expressed in hypnozoites.
Mature hypnozoites express genes involved in the export and transport of parasite proteins into the host-cell
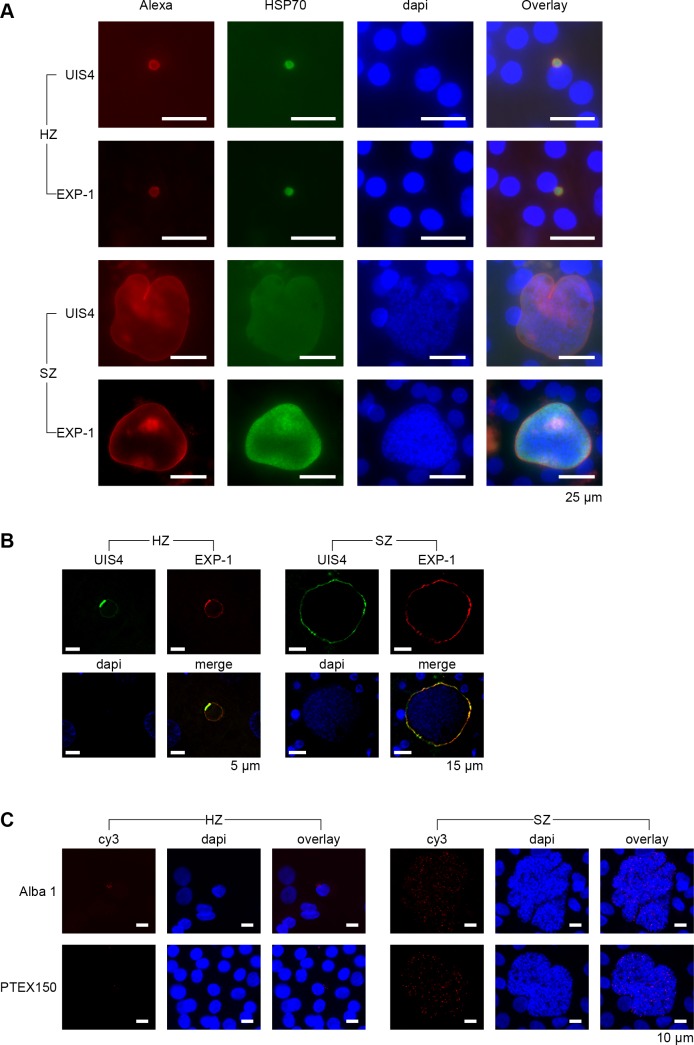
Although dormant, the hypnozoite may still interact with its host cell for survival. Two proteins at the parasite-host interface, the up-regulated in infective sporozoites gene 4 (UIS4) and the exported protein-1 (EXP-1), located at the site of the parasitophorous vacuole membrane (PVM), have indeed been shown to interact with host cell proteins in rodent malaria models (Sá E Cunha et al., 2017; Mueller et al., 2005; Petersen et al., 2017). In P. vivax, UIS4 was shown to be present in hypnozoites and schizonts throughout liver stage development, while EXP-1 expression was only observed in mid-stage schizonts (Mikolajczak et al., 2015). We found transcripts for those genes in both schizonts and hypnozoites in our day 9 transcriptome data and generated P. cynomolgi antibodies against these proteins for validation (Figure 3—figure supplement 1A). As expected, IFA with UIS4 antibodies showed signal in both hypnozoites and schizonts at day 9 (Figure 3A). However, we also observed EXP-1 staining in both parasite forms at day 9. Similar to P. vivax, EXP-1 staining was close to background at day 2, while UIS4 staining was clearly visible (Figure 3—figure supplement 1B). From day 3/4 onwards EXP-1 staining gradually increased over time and appeared to co-localize with the PVM, both in schizonts and hypnozoites. Further investigation of P. vivax liver stage parasites from a humanized mouse model confirmed the presence of EXP-1 in hypnozoites (Figure 3B, procedures were used as described in Mikolajczak et al. (2015)), indicating that our findings are not an artifact from cell culture. This shows that, while expression of most genes in the hypnozoite is decreased, other genes may be switched on during its maturation into true dormancy.
Figure 3. Validation of genes involved in the export and transport of parasite proteins.
(A) Immunofluorescent staining patterns of UIS4 (Alexa-594), EXP-1 (Alexa-594) and HSP70 (FITC) in day 9 P. cynomolgi hypnozoites (HZ) and liver schizonts (SZ). Scalebar 25 μm. (B) EXP-1 is expressed in P. vivax hypnozoites (HZ) and liver schizonts (SZ) at day 8 post sporozoite infection. Colocalization of EXP-1 and UIS4 suggests that EXP-1 is expressed on the PVM, in agreement with other Plasmodium species. DNA was localized with DAPI. Scalebar 5 μm (HZ) and 15 μm (SZ). (C) RNA-FISH staining of Alba1 (cy3) and PTEX150 (cy3) in day 10 P. cynomolgi hypnozoites (Hz) and liver schizonts (Sz). Scalebar 10 μm.
Figure 3—figure supplement 1. Validation of selected genes.
In the RNA-seq data set of day 9, we found relatively high transcript levels for members of the Alba gene family in hypnozoites, which is in concordance with the previously published data of days 6/7 (Figure 3—figure supplement 1C) (Voorberg-van der Wel et al., 2017). This gene family plays a crucial role in transcriptional and translational regulation during zygote development as well as in the blood stage of infection (Reddy et al., 2015; Mair et al., 2010). Indeed we found strong signals for Alba1 transcripts by RNA-FISH at days 6 (not shown) and day 10, both in hypnozoites and schizonts (Figure 3C), indicating that this gene may also fulfill important regulatory roles in liver stage development, including hypnozoites.
Further, we showed that protein export is upregulated in hypnozoites at day 9 compared to hypnozoites at day 6/7 (Figure 2—figure supplement 1B). This includes four out of the five known components of the Plasmodium translocon PTEX (Figure 3—figure supplement 1C). This complex composed of HSP101, EXP2, TRX2, PTEX150 and PTEX88 plays an essential role for trafficking exported parasite proteins into the erythrocyte cytoplasm or onto the erythrocyte surface during the blood stage of infection in P. falciparum (Elsworth et al., 2014). Intriguingly, however, it was shown that most known components of the PTEX translocon are expressed in Plasmodium liver stages, corroborating our data (Nyboer et al., 2018). RNA-FISH revealed that indeed PTEX150 transcripts were found both in day 6 (not shown) and day 10 schizonts and hypnozoites (Figure 3C). This confirms that PTEX is not unique to the blood stage of infection, but also is present in the liver stage of infection, including hypnozoites. However, PTEX functional role in liver-stages remains to be investigated and might differ from that of blood stage of infection (Vaughan et al., 2012; Kalanon et al., 2016; Matz et al., 2015).
Taken together, our data show that hypnozoites display lower transcriptional activity with progressing dormancy. With the markedly reduced transcription levels at day 9 post in vitro infection compared to day 6/7, we hypothesize that day 9 hypnozoites are the truly dormant parasites which new drugs need to be active against.
Discussion
These data add to the previously published transcriptome of day 6/7 parasites (Voorberg-van der Wel et al., 2017) and allows for the first time to study liver-stage schizont and hypnozoite maturation. Our analysis shows that maturation of liver schizonts is associated with a general increase in transcriptional activity. This includes genes previously shown to be essential for merozoite formation and red blood cell invasion (Roth et al., 2018; Swann et al., 2016). The in vivo staining patterns with immune-reagents used to characterize liver stage morphologies (Roth et al., 2018) confirmed proper liver stage development.
Amongst merozoite-specific genes, day 9 schizonts also revealed upregulation of Pvs16, a gametocyte-specific gene. Together with the data of a recent study, showing that a portion of P. vivax late-stage schizonts expresses the Pvs16 protein (Roth et al., 2018), this result suggests that some merozoites are already committed as gametocytes prior to the erythrocytic cycle.
In contrast to schizont maturation, we show that progressing dormancy is associated with a metabolic shutdown. Even though transcriptional levels are clearly reduced in day 9 hypnozoites compared to day 6/7 hypnozoites, there are still some specific pathways that show notable expression levels in the mature dormant stage. This includes functions such as energy metabolism, redox metabolism, mitochondrial respiration and chromatin maintenance. Interestingly, these are common features of dormancy found in other microorganisms (Rittershaus et al., 2013). Exposed to stress, many microorganisms enter a hardy, non-replicating state, which is able to tolerate adverse environmental conditions, immune responses and prolonged anti-microbial treatments. While cellular adaptations are not exactly the same for all organisms, recent research found some common features of quiescent cells (Rittershaus et al., 2013). In the following paragraph, these features are discussed in the light of the acquired data for Plasmodium dormant stages.
Our data propose a switch in energy metabolism, suggesting that pyruvate metabolism, the pentose phosphate cycle and glycolysis remain active in the dormant stage, while glyoxalase, mannose and fructose metabolism are suppressed. In other organisms, the switch to a latent lifestyle was indeed shown to be accompanied by a shift in energy metabolism (Rittershaus et al., 2013). Similar to our data, in Toxoplasma gondii the formation of latent bradyzoites is accompanied by upregulation of glycolysis (Jeffers et al., 2018; Sullivan and Jeffers, 2012). This is reflected by the prominent expression of pyruvate kinase and lactate dehydrogenase (LDH2) in the latent form as well as confirmed by a knockdown study, showing that LDH2 is essential for bradyzoite formation (Al-Anouti et al., 2004). Notably, pyruvate kinase (PcyM_1123400; 3.7 FPKM at day 6/7 (mean) and 1.3 FPKM at day 9 (mean)) and lactate dehydrogenase LDH (PcyM_1234100; 3.7FPKM at day 6/7 (mean) and 3 FPKM at day 9 (mean)) are as well expressed in our transcriptomic dataset of day 6/7 and day 9 hypnozoites, suggesting similar mechanisms.
Moreover, it was shown that Mycobacteria tuberculosis redirects acetyl-CoA from the TCA cycle into fatty acid synthesis to build up carbon storage in the latent stage (Baek et al., 2011). We show that genes required for fatty acid synthesis are constantly expressed at high levels in hypnozoites. This suggests that hypnozoites, similar to M. tuberculosis, accumulate lipids as carbon storage in preparation for long periods of inactivity.
Moreover, in M. tuberculosis, ATP homeostasis was shown to be critical for survival. In the latent form of M. tuberculosis, ATP level is slightly lower compared to proliferating cells, but it is maintained at a steady state. Depletion of ATP or inhibition of the F0F1 ATP synthase involved in ATP synthesis, results in cell death. This shows that de novo ATP synthesis is required to maintain the low ATP level in M. tuberculosis, and hence is critical for dormancy (Rao et al., 2008). Intriguingly, genes coding for ATP synthase complex are transcribed in day 6/7 hypnozoites and show upregulation in day 9 hypnozoites. It is hence tempting to speculate that similar mechanism as found in the dormant form of Mycobacteria are critical for dormancy in Plasmodium parasites.
In addition, in Toxoplasma and Mycobacteria it was shown that dormant stages are continually exposed to endogenous and exogenous reactive oxygen species (Rittershaus et al., 2013; Kehrer and Klotz, 2015). It is hence proposed that latent forms must be capable of dealing with long-term exposure to radicals and reactive metabolic byproducts. Indeed, T. gondii and M. tuberculosis induce a number of enzymes in the latent stage with roles metabolizing oxygen radicals to maintain balanced oxidation-reduction (Manger et al., 1998; Kumar et al., 2011). Similar to the data of Toxoplasma and Mycobacteria, we show that redox metabolism is highly active in day 6/7 hypnozoites and stays active with progressing dormancy. Identification of a mechanism which allows the hypnozoite to counter oxidoreductive stress is central for the development of effective intervention strategies, since its perturbation might either kill or awake the parasite.
Stress response pathways were previously observed to be associated with induction of dormancy. Apart from redox metabolism, we found the heatshock protein 70 (hsp70) chaperone cycle to be highly active in late stage hypnozoites. These data were validated by RNA-FISH and immunofluorescence assays. Intriguingly, in Toxoplasma expression of hsp70 is induced during bradyzoite differentiation and its inhibition can suppress bradyzoite development in vitro (Weiss et al., 1998). Since members of the HSP70 family are associated with stage transition in many different organisms, it is tempting to speculate that, similar to Toxoplasma, HSP70 plays a crucial role in the formation and maintenance of the dormant stage. Identification of interaction partners of HSP70 may yield information about the process of stage transition.
Interestingly, a recent study uncovered a complex ApiAP2 transcriptional network of repressors and activators controlling the switch between replicating tachyzoite and latent bradyzoite formation in Toxoplasma gondii (Lesage et al., 2018). The ApiAP2 family includes plant-like transcription factors that are key regulators of life cycle transition in Apicomplexan parasites. It is speculated that the AP2VIIa-1 is a master regulator which coordinates changes in the Toxoplasma transcriptome that leads to bradyzoite conversion (Radke et al., 2018). In Plasmodium spp. several ApiAP2 transcription factors were identified to play a crucial role in gametocytogenesis (Kafsack et al., 2014; Sinha et al., 2014; Yuda et al., 2015), ookinete development (Yuda et al., 2009), sporozoite formation (Yuda et al., 2010) and liver stage maturation (Iwanaga et al., 2012). So far, however, it remains elusive if ApiAP2 factors regulate hypnozoite formation, maturation and/or reactivation. Here, we show that ap2-l, an essential factor for liver stage schizont maturation (Iwanaga et al., 2012), is not only expressed in schizonts, but also in hypnozoites, suggesting an important role as well in hypnozoite development. In contrast, we could not confirm expression of the proposed quiescence ApiAP2 factor identified by (Cubi et al., 2017). Instead, we show that the ApiAP2 transcription factor PcyM_0918000 is transcribed in hypnozoites, corroborating the results of a recent transcriptome study in P. vivax (Gural et al., 2018). However, this factor is not exclusive for the dormant stage as suggested for a master regulator for hypnozoite conversion. It hence remains to be determined if indeed this ApiAP2 transcription factor and/or an ApiAP2 transcriptional network play a key role in regulating the hypnozoite fate.
Apart from ApiAP2 transcription factors as master regulators of hypnozoite fate, it is speculated that epigenetic control might be implicated in the maintenance of quiescence of hypnozoites (Malmquist et al., 2012). Indeed, exposure of dormant stages to an inhibitor of histone lysine methyltransferases induced an accelerated rate of hypnozoite activation (Dembélé et al., 2014). It is hence tempting to speculate that epigenetic processes such as methylation-dependent changes in transcription might control hypnozoite quiescence. Indeed, our data show that pathways of histone acetylation and methylation as well as chaperone-mediated modulation of nucleosome-histone interactions are highly expressed in days 6/7 and day 9 hypnozoites, suggesting a key role in maintaining dormancy.
To maintain genome fidelity is challenging for quiescent cells, since the low metabolic activity allows for only limited DNA repair mechanisms. One common strategy is to alter chromosomal structure to a more chemically stable form. In M. tuberculosis a histone-like protein Lsr2, mediates chromosome compaction and protection from reactive oxygen and nitrogen species (Summers et al., 2012). In our transcriptomic dataset, we found that the P. cynomolgi homologue of the bacterial histone-like protein (HU) (PcyM_0702400) is expressed in hypnozoites (3.5 FPKM at day 6/7 and 2.2 FPKM at day 9). It is hence tempting to speculate that this protein might play a role in chromosome condensation during dormancy. Moreover, we find Alba genes transcribed in hypnozoites. Members of the Alba family from protozoan parasites bind to both DNA and RNA and are implicated in transcriptional regulation, chromatin packaging, and translational control, as well as cellular differentiation and developmental processes (Goyal et al., 2016). In P. falciparum for example, Alba proteins were shown not only to drive translational repression in sporozoites, but also to be associated with subtelomeric DNA and epigenetic regulators, suggesting a role in heterochromatin formation (Vembar et al., 2015; Goyal et al., 2012). Further studies are needed to map epigenetic modifications in hypnozoites, which will hopefully shed light on the complex developmental pathway driving dormancy.
Finally, we show the translocon PTEX is actively transcribed in hypnozoites. So far, this complex was only described as essential for protein export in blood stage parasites (Elsworth et al., 2014). Whether this complex is indeed also important for nutrient acquisition in liver stage parasites, including hypnozoites, remains to be determined.
To conclude, although we do not identify a specific transcriptional marker for hypnozoites because dormancy is likely associated with a general metabolic shutdown, there are still several specific pathways that show notable expression levels, at least at the transcriptional level, in the mature dormant stage. Interestingly, these pathways were shown to be essential for dormancy in other organisms. Therefore specific targeting of these functions may be a productive strategy to identify novel effective therapies.
Materials and methods
Ethics statement
Nonhuman primates were used because no other models (in vitro or in vivo) were suitable for the aims of this project. The research protocol was approved by the local independent ethical committee conform Dutch law (BPRC Dier Experimenten Commissie, DEC, agreement number #708). Details are described by Voorberg-van der Wel et al. (2017).
Transgenic Plasmodium cynomolgi sporozoite production
P. cynomolgi M strain PcyC-PAC-GFPhsp70-mCherryef1α (Voorberg-van der Wel et al., 2013) sporozoites were produced as described previously (Voorberg-van der Wel et al., 2017).
Parasite liver stage culture and cell sorting
Procedures for liver cell isolation, liver stage culture and cell sorting were essentially as described previously (Voorberg-van der Wel et al., 2017). Briefly, transgenic P. cynomolgi salivary gland sporozoites were isolated and added to freshly isolated Macaca mulatta hepatocytes. Hepatocytes were seeded in 96-well collagen-coated plates at 90,000 hepatocytes per well and 2 days later 50,000 sporozoites were added per well. After 9 or 10 days of culture, infected cells were sorted with a BD FACSAria flowcytometer and fractions were collected in Trizol and stored at −80°C until RNA extraction. Median fluorescence intensity (MFI) values of GFPlow and GFPhigh samples were calculated using four separate recordings of 1 million cells per experiment (day 6, n = 4; days 9 and 10, n = 3).
Protein and antibody production
PcyM_0602100 (UIS4) aa20-166 and PcyM_0924700 (EXP-1) aa19-130 were expressed in E. coli, purified using a Ni-IMAC column followed by gel-filtration and used to immunize rats (Eurogentec, Belgium).
Immunofluorescence analysis (IFA)
IFA staining of methanol fixed liver stage parasites was carried out as before (Voorberg-van der Wel et al., 2017). Alternatively, parasites were fixed in 4% paraformaldehyde (30 min., room temperature), washed and incubated with primary and secondary antibodies diluted in 1% BSA/0.3% Triton X-100 in PBS.
RNAscope in situ hybridization
For RNA-FISH, P. cynomolgi liver stage cultures in 96-well collagen-coated CellCarrier plates (PerkinElmer) were washed once in PBS and fixed in 4% paraformaldehyde (Affymetrix) at room temperature for 30 min. Cultures were then washed, dehydrated and stored in 100% ethanol at −20°C until RNA-FISH was performed using a Tyramide Signal Amplification (TSA) based assay from Advanced Cell Diagnostics (RNAscope Multiplex Fluorescent Assay v2), essentially according to the manufacturer’s instructions. Following rehydration protease digestions were performed using pretreatment solution three from the kit at 1:10 dilution for 20 min. at room temperature. Hybridizations were 2 hr at 40°C. Probes used were directed against P. cynomolgi hsp70 (PcyM_0515400, region 606–1837 of XM_004221103.1), PTEX150 (PcyM_1315200 targeting 871–2360 of XM_004224250.1), Alba1 (PcyM_1427300; targeting 101–692 of PcyM_1427300) and MSP1 (PcyM_0731200 targeting 147–1127 of XM_004221774.1). After hybridization, TSA amplification steps were performed as described by the manufacturer. Following DAPI staining, cells were kept in PBS for imaging. Images were acquired with a Leica DMI6000B inverted fluorescence microscope equipped with a DFC365FX camera using a HC PL APO 63x/1.40–0.60 oil objective.
RNA sequencing
Total RNA was isolated using the Direct-zol RNA MiniPrep Kit (Zymo Research) including on-column DNase digestion according to the manufacturer’s instructions. The quality of the RNA samples was assessed with the High Sensitivity RNA kit using the TapeStation 4200 instrument (Agilent Technologies).
RNA samples were processed using the SMART-Seq v4 Ultra Low Input RNA Kit (Clontech) to generate high-quality cDNA. The results obtained were evaluated with the High Sensitivity DNA kit using the Bioanalyzer 2100 instrument (Agilent Technologies). The cDNA samples were then sheared to 200–500 bp length using a Covaris E220 instrument (Covaris) and subsequently processed with the Low Input Library Prep Kit v2 (Clontech) to generate sequencing libraries. The quality of the libraries was assessed with the D1000 TapeStation kit (Agilent Technologies). RNA-seq cDNA libraries were sequenced in paired-end mode, 2 × 76-base-pair (bp), using the Illumina HiSeq2500 platform. Read quality was assessed by running FastQC (version 0.10) on the FASTQ files. Sequencing reads showed high quality, with a mean Phred score higher than 30 for all base positions. The obtained 76-bp paired-end reads were used for the alignment to the reference genomes and used for the gene expression quantification with the Exon Quantification Pipeline (EQP) (Schuierer and Roma, 2016) as described in Voorberg-van der Wel et al. (2017).
To quantify parasite-specific expression for each P. cynomolgi gene, we determined the number of sequencing reads aligned to genes and computed gene expression values as the number of Fragments per Kilobase per Million fragments mapped (FPKM). For the normalization of the schizont expression values, we applied the previously developed method of normalization against the total number of host reads (Voorberg-van der Wel et al., 2017). For the hypnozoite expression values, we used a different normalization scheme since we detected uninfected host cells in the day 9/10 hypnozoite samples via microscopy. These contaminating uninfected host cells led to a higher content of monkey reads in the day 9/10 hypnozoite samples as compared to the day 6/7 hypnozoite samples which were described in Voorberg-van der Wel et al. (2017). So after discarding the samples of day 10 we decided to completely disregard the percentage of monkey reads in the hypnozoite samples and instead use a standard FPKM normalization which only takes the number of parasite reads into account (Love et al., 2014). Since we only compare schizont samples with each other and hypnozoite samples with each other, using two different normalization schemes still leads to consistent results. All data are based on FPKM values after such normalization which are provided in supplementary (Supplementary file 5).
Acknowledgements
We are grateful to the mosquito breeding facilities in Nijmegen for provision of Anopheles stephensi mosquitoes. We thank Francisca van Hassel for preparing graphical representations. We are grateful to the Kappe and Sattabongkot laboratories for continuous support and collaboration in the area of P. vivax liver stage research.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Clemens HM Kocken, Email: kocken@bprc.nl.
Guglielmo Roma, Email: guglielmo.roma@novartis.com.
Urszula Krzych, Walter Reed Army Institute of Research, United States.
Anna Akhmanova, Utrecht University, Netherlands.
Funding Information
This paper was supported by the following grants:
Bill and Melinda Gates Foundation to Thierry T Diagana, Clemens HM Kocken, Guglielmo Roma.
Wellcome to Thierry T Diagana, Clemens HM Kocken.
Medicines for Malaria Venture to Thierry T Diagana, Clemens HM Kocken.
Additional information
Competing interests
Employed by and/or shareholder of Novartis Pharma AG.
No competing interests declared.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Employed by and/or shareholder of Novartis Pharma AG.
Author contributions
Formal analysis, Investigation, Visualization, Writing—original draft, Project administration, Writing—review and editing.
Formal analysis, Validation, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Formal analysis, Investigation, Methodology.
Software, Formal analysis, Investigation, Methodology.
Software, Formal analysis, Investigation, Methodology.
Formal analysis, Investigation, Methodology.
Formal analysis, Investigation, Methodology.
Formal analysis, Validation, Investigation, Writing—review and editing.
Validation, Formal analysis, Methodology.
Formal analysis, Investigation, Methodology.
Formal analysis, Investigation, Methodology.
Formal analysis, Investigation, Methodology.
Formal analysis, Methodology.
Validation, Investigation, Writing—review and editing.
Validation, Investigation, Writing—review and editing.
Formal analysis, Investigation, Methodology.
Software, Formal analysis, Methodology, Writing—review and editing.
Resources, Investigation, Writing—review and editing.
Resources, Validation, Investigation.
Resources, Validation, Investigation.
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Investigation, Project administration, Writing—review and editing.
Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Ethics
Animal experimentation: Nonhuman primates were used because no other models (in vitro or in vivo) were suitable for the aims of this project. The research protocol was approved by the local independent ethical committee conform Dutch law (BPRC Dier Experimenten Commissie, DEC, agreement number #708). Details are described by (Voorberg-van der Wel et al., 2017).
Additional files
The table indicates if pathways are significantly up- or down-regulated over time.
Data availability
The raw RNA-sequencing reads are available in the NCBI Short Read Archive (https://www.ncbi.nlm.nih.gov/sra) under accession number SRP096160.
The following dataset was generated:
Guglielmo Roma. 2018. Malaria Liver Stages Transcriptome, '18. NCBI Short Read Archive. SRP096160
The following previously published dataset was used:
Guglielmo Roma. 2017. Malaria Liver Stages Transcriptome, '17. NCBI Short Read Archive. SRP096160
References
- Al-Anouti F, Tomavo S, Parmley S, Ananvoranich S. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. Journal of Biological Chemistry. 2004;279:52300–52311. doi: 10.1074/jbc.M409175200. [DOI] [PubMed] [Google Scholar]
- Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLOS Biology. 2011;9:e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Research. 2005;33:3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargieri D, Lagal V, Andenmatten N, Tardieux I, Meissner M, Ménard R. Host cell invasion by apicomplexan parasites: the junction conundrum. PLOS Pathogens. 2014;10:e1004273. doi: 10.1371/journal.ppat.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLOS Biology. 2003;1:e5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubi R, Vembar SS, Biton A, Franetich J-F, Bordessoulles M, Sossau D, Zanghi G, Bosson-Vanga H, Benard M, Moreno A, Dereuddre-Bosquet N, Le Grand R, Scherf A, Mazier D. Laser capture microdissection enables transcriptomic analysis of dividing and quiescent liver stages of Plasmodium relapsing species. Cellular Microbiology. 2017;19:e12735. doi: 10.1111/cmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembélé L, Franetich JF, Lorthiois A, Gego A, Zeeman AM, Kocken CH, Le Grand R, Dereuddre-Bosquet N, van Gemert GJ, Sauerwein R, Vaillant JC, Hannoun L, Fuchter MJ, Diagana TT, Malmquist NA, Scherf A, Snounou G, Mazier D. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nature Medicine. 2014;20:307–312. doi: 10.1038/nm.3461. [DOI] [PubMed] [Google Scholar]
- Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, Chan JA, Azevedo MF, Rogerson SJ, Beeson JG, Crabb BS, Gilson PR, de Koning-Ward TF. PTEX is an essential nexus for protein export in malaria parasites. Nature. 2014;511:587–591. doi: 10.1038/nature13555. [DOI] [PubMed] [Google Scholar]
- Goyal M, Alam A, Iqbal MS, Dey S, Bindu S, Pal C, Banerjee A, Chakrabarti S, Bandyopadhyay U. Identification and molecular characterization of an Alba-family protein from human malaria parasite Plasmodium falciparum. Nucleic Acids Research. 2012;40:1174–1190. doi: 10.1093/nar/gkr821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Banerjee C, Nag S, Bandyopadhyay U. The Alba protein family: Structure and function. Biochimica Et Biophysica Acta (BBA) - Proteins and Proteomics. 2016;1864:570–583. doi: 10.1016/j.bbapap.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Gural N, Mancio-Silva L, Miller AB, Galstian A, Butty VL, Levine SS, Patrapuvich R, Desai SP, Mikolajczak SA, Kappe SHI, Fleming HE, March S, Sattabongkot J, Bhatia SN. In vitro culture, drug sensitivity, and transcriptome of plasmodium vivax hypnozoites. Cell Host & Microbe. 2018;23:395–406. doi: 10.1016/j.chom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussig JM, Matuschewski K, Kooij TWA. Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Molecular Microbiology. 2011;81:1511–1525. doi: 10.1111/j.1365-2958.2011.07787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S, Kaneko I, Kato T, Yuda M. Identification of an AP2-family protein that is critical for malaria liver stage development. PLOS ONE. 2012;7:e47557. doi: 10.1371/journal.pone.0047557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers V, Tampaki Z, Kim K, Sullivan WJ. A latent ability to persist: differentiation in Toxoplasma gondii. Cellular and Molecular Life Sciences. 2018;75:2355–2373. doi: 10.1007/s00018-018-2808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, Cortés A, Llinás M. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanon M, Bargieri D, Sturm A, Matthews K, Ghosh S, Goodman CD, Thiberge S, Mollard V, McFadden GI, Ménard R, de Koning-Ward TF. The Plasmodium translocon of exported proteins component EXP2 is critical for establishing a patent malaria infection in mice. Cellular Microbiology. 2016;18:399–412. doi: 10.1111/cmi.12520. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Klotz LO. Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Critical Reviews in Toxicology. 2015;45:765–798. doi: 10.3109/10408444.2015.1074159. [DOI] [PubMed] [Google Scholar]
- Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Reviews in Molecular Medicine. 2011;13:e39. doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage KM, Huot L, Mouveaux T, Courjol F, Saliou JM, Gissot M. Cooperative binding of ApiAP2 transcription factors is crucial for the expression of virulence genes in Toxoplasma gondii. Nucleic Acids Research. 2018;46:6057–6068. doi: 10.1093/nar/gky373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Lasonder E, Garver LS, Franke-Fayard BM, Carret CK, Wiegant JC, Dirks RW, Dimopoulos G, Janse CJ, Waters AP. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLOS Pathogens. 2010;6:e1000767. doi: 10.1371/journal.ppat.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmquist NA, Moss TA, Mecheri S, Scherf A, Fuchter MJ. Small-molecule histone methyltransferase inhibitors display rapid antimalarial activity against all blood stage forms in Plasmodium falciparum. PNAS. 2012;109:16708–16713. doi: 10.1073/pnas.1205414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger ID, Hehl A, Parmley S, Sibley LD, Marra M, Hillier L, Waterston R, Boothroyd JC. Expressed sequence tag analysis of the bradyzoite stage of Toxoplasma gondii: identification of developmentally regulated genes. Infection and Immunity. 1998;66:1632–1637. doi: 10.1128/iai.66.4.1632-1637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz JM, Goosmann C, Brinkmann V, Grützke J, Ingmundson A, Matuschewski K, Kooij TW. The Plasmodium berghei translocon of exported proteins reveals spatiotemporal dynamics of tubular extensions. Scientific Reports. 2015;5:12532. doi: 10.1038/srep12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazier D, Rénia L, Snounou G. A pre-emptive strike against malaria's stealthy hepatic forms. Nature Reviews Drug Discovery. 2009;8:854–864. doi: 10.1038/nrd2960. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Vaughan AM, Kangwanrangsan N, Roobsoong W, Fishbaugher M, Yimamnuaychok N, Rezakhani N, Lakshmanan V, Singh N, Kaushansky A, Camargo N, Baldwin M, Lindner SE, Adams JH, Sattabongkot J, Prachumsri J, Kappe SH. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host & Microbe. 2015;17:526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. PNAS. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyboer B, Heiss K, Mueller A-K, Ingmundson A. The Plasmodium liver-stage parasitophorous vacuole: A front-line of communication between parasite and host. International Journal of Medical Microbiology. 2018;308:107–117. doi: 10.1016/j.ijmm.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Petersen W, Stenzel W, Silvie O, Blanz J, Saftig P, Matuschewski K, Ingmundson A. Sequestration of cholesterol within the host late endocytic pathway restricts liver-stage Plasmodium development. Molecular Biology of the Cell. 2017;28:726–735. doi: 10.1091/mbc.e16-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JB, Worth D, Hong D, Huang S, Sullivan WJ, Wilson EH, White MW. Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLOS Pathogens. 2018;14:e1007035. doi: 10.1371/journal.ppat.1007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SPS, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. PNAS. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BP, Shrestha S, Hart KJ, Liang X, Kemirembe K, Cui L, Lindner SE. A bioinformatic survey of RNA-binding proteins in Plasmodium. BMC Genomics. 2015;16:890. doi: 10.1186/s12864-015-2092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus ES, Baek SH, Sassetti CM. The normalcy of dormancy: common themes in microbial quiescence. Cell Host & Microbe. 2013;13:643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Maher SP, Conway AJ, Ubalee R, Chaumeau V, Andolina C, Kaba SA, Vantaux A, Bakowski MA, Luque RT, Adapa SR, Singh N, Barnes SJ, Cooper CA, Rouillier M, McNamara CW, Mikolajczak SA, Sather N, Witkowski B, Campo B, Kappe SHI, Lanar DE, Nosten F, Davidson S, Jiang RHY, Kyle DE, Adams JH. A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nature Communications. 2018;9:1837. doi: 10.1038/s41467-018-04221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá E Cunha C, Nyboer B, Heiss K, Sanches-Vaz M, Fontinha D, Wiedtke E, Grimm D, Przyborski JM, Mota MM, Prudêncio M, Mueller AK. Plasmodium berghei EXP-1 interacts with host Apolipoprotein H during Plasmodium liver-stage development. PNAS. 2017;114:E1138–E1147. doi: 10.1073/pnas.1606419114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuierer S, Roma G. The exon quantification pipeline (EQP): a comprehensive approach to the quantification of gene, exon and junction expression from RNA-seq data. Nucleic Acids Research. 2016;44:e132. doi: 10.1093/nar/gkw538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherling ES, Knuepfer E, Brzostowski JA, Miller LH, Blackman MJ, Ooij Cvan. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. eLife. 2017;6:e23239. doi: 10.7554/eLife.23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, Kafsack BFC, Williams AE, Llinás M, Berriman M, Billker O, Waters AP. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan WJ, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiology Reviews. 2012;36:717–733. doi: 10.1111/j.1574-6976.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers EL, Meindl K, Usón I, Mitra AK, Radjainia M, Colangeli R, Alland D, Arcus VL. The structure of the oligomerization domain of Lsr2 from Mycobacterium tuberculosis reveals a mechanism for chromosome organization and protection. PLOS ONE. 2012;7:e38542. doi: 10.1371/journal.pone.0038542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J, Corey V, Scherer CA, Kato N, Comer E, Maetani M, Antonova-Koch Y, Reimer C, Gagaring K, Ibanez M, Plouffe D, Zeeman AM, Kocken CH, McNamara CW, Schreiber SL, Campo B, Winzeler EA, Meister S. High-throughput luciferase-based assay for the discovery of therapeutics that prevent malaria. ACS Infectious Diseases. 2016;2:281–293. doi: 10.1021/acsinfecdis.5b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. Journal of Clinical Investigation. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar SS, Macpherson CR, Sismeiro O, Coppée JY, Scherf A. The PfAlba1 RNA-binding protein is an important regulator of translational timing in Plasmodium falciparum blood stages. Genome Biology. 2015;16:212. doi: 10.1186/s13059-015-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg-van der Wel A, Zeeman AM, van Amsterdam SM, van den Berg A, Klooster EJ, Iwanaga S, Janse CJ, van Gemert GJ, Sauerwein R, Beenhakker N, Koopman G, Thomas AW, Kocken CH. Transgenic fluorescent Plasmodium cynomolgi liver stages enable live imaging and purification of Malaria hypnozoite-forms. PLoS ONE. 2013;8:e54888. doi: 10.1371/journal.pone.0054888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg-van der Wel A, Roma G, Gupta DK, Schuierer S, Nigsch F, Carbone W, Zeeman AM, Lee BH, Hofman SO, Faber BW, Knehr J, Pasini E, Kinzel B, Bifani P, Bonamy GMC, Bouwmeester T, Kocken CHM, Diagana TT. A comparative transcriptomic analysis of replicating and dormant liver stages of the relapsing malaria parasite Plasmodium cynomolgi. eLife. 2017;6:e29605. doi: 10.7554/eLife.29605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infection and Immunity. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends in Parasitology. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malaria Journal. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Malaria Report 2015. Geneva: World Health Organization; 1987. http://www.who.int/malaria/publications/world-malaria-report-2015/en/ [Google Scholar]
- Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, Kato T, Kaneko I. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Molecular Microbiology. 2009;71:1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Molecular Microbiology. 2010;75:854–863. doi: 10.1111/j.1365-2958.2009.07005.x. [DOI] [PubMed] [Google Scholar]
- Yuda M, Iwanaga S, Kaneko I, Kato T. Global transcriptional repression: An initial and essential step for Plasmodium sexual development. PNAS. 2015;112:12824–12829. doi: 10.1073/pnas.1504389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman A-M, van Amsterdam SM, McNamara CW, Voorberg-van der Wel A, Klooster EJ, van den Berg A, Remarque EJ, Plouffe DM, van Gemert G-J, Luty A, Sauerwein R, Gagaring K, Borboa R, Chen Z, Kuhen K, Glynne RJ, Chatterjee AK, Nagle A, Roland J, Winzeler EA, Leroy D, Campo B, Diagana TT, Yeung BKS, Thomas AW, Kocken CHM. KAI407, a potent non-8-aminoquinoline compound that kills plasmodium cynomolgi early dormant liver stage parasites in vitro. Antimicrobial Agents and Chemotherapy. 2014;58:1586–1595. doi: 10.1128/AAC.01927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman A-M, Lakshminarayana SB, van der Werff N, Klooster EJ, Voorberg-van der Wel A, Kondreddi RR, Bodenreider C, Simon O, Sauerwein R, Yeung BKS, Diagana TT, Kocken CHM. PI4 kinase is a prophylactic but not radical curative target in plasmodium vivax-type malaria parasites. Antimicrobial Agents and Chemotherapy. 2016;60:2858–2863. doi: 10.1128/AAC.03080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]