Abstract
Background and Aims
Structures that simultaneously perform many functional roles are likely to show a variety of morphological solutions to these demands, and thus probably exhibit high morphological disparity. In contrast, specialization for a few simple functions should result in a more limited suite of morphologies. We explore this idea using lycopsid reproductive structures, which, throughout their history, have performed a limited set of functional roles compared with the reproductive structures of other plant groups such as seed plants.
Methods
We scored living and fossil lycopsid taxa for 18 discrete character measurements and several continuous traits, including sporangium size, supporting axis diameter, and strobilus length and width. We used the discrete characters to construct a multivariate morphospace for lycopsid reproductive morphology through time, and the continuous characters to test whether fossil and extant lycopsids show similar patterns of tissue allocation within reproductive structures.
Results
Lycopsids occupy similar areas of reproductive morphospace and show similar patterns of tissue allocation over most of their history, alternating between diffuse fertile zones with leaf-like sporophylls and compact strobili with specialized sporophylls that allow sporangia to be closely packed while also protected during their development. Growth habit also plays an important role in lycopsid reproductive evolution, broadly influencing the size and shape of reproductive structures.
Conclusions
Lycopsid reproductive structures are primarily specialized for densely packaging sporangia, and are consistent with the idea that performing limited functional roles is associated with reduced morphological disparity. Morphologies similar to lycopsid strobili are also found in other groups with simple, wind-dispersed propagules, suggesting that the same processes occur across plant lineages.
Keywords: Macroevolutionary patterns, reproductive allocation, strobilus, pteridophyte, lycopsid
INTRODUCTION
Form and function relationships in plants can provide key insights into their evolution, not only in terms of specific morphologies (e.g. Darwin, 1877; Niklas, 1992) but also with regard to macroevolutionary and macroecological patterns and trends (e.g. Fenster et al., 2004; Boyce et al., 2009; Feild et al., 2011). Functionality in a more general sense, such as the number and types of biological roles that plant organs perform (see Bock and von Wahlert, 1965), may also be important in structuring evolutionary patterns. For example, Niklas (1994, 1999, 2004) used computer simulations to suggest that such functional diversity could lead to the evolution of increased morphological disparity, because it is difficult simultaneously to optimize the performance of functional demands such as mechanical stability, light interception and propagule dispersal in a single structure or a single body plan. The resulting complex fitness landscape should lead to the evolution of a diverse set of sub-optimal morphologies that balance this complex set of demands (Niklas, 1994).
Functional diversity may then play an important role in shaping broad patterns of morphological evolution, but this idea has rarely been directly investigated. Leslie (2011) suggested that the greater morphological diversity of conifer seed cones relative to pollen cones reflects the more diverse set of functional roles that these structures perform (including pollination, seed protection and seed dispersal), but such relationships can be explored further using other plant groups. Pteridophyte, or free-sporing, vascular plant lineages are useful systems in which to test whether limited functional diversity is associated with limited morphological disparity, because their reproductive structures are relatively simple and primarily serve to protect developing sporangia and release spores. Pteridophytes lack ovules that need to be pollinated, and, subsequently, seeds that need to be protected and dispersed, so their reproductive structures perform fewer functional roles than those of seed plants. Pteridophyte lineages might then be expected to show reduced reproductive diversity over their history relative to seed plants.
Lycopsids, which are the sister clade to all other extant vascular plants (Kenrick and Crane, 1997; Wickett et al., 2014), are a particularly good pteridophyte group in which to explore this idea because of their long fossil record and significant extant diversity. Lycopsids first appear in the Late Silurian around 427 million years ago (Baragwanathia; Rickards, 2000) and were major components of terrestrial ecosystems for the next 150 million years, peaking in Pennsylvanian coal swamps where they formed trees up to 50 m high (Phillips and DiMichele, 1992; Taylor et al., 2009; Hetherington et al., 2016). Lycopsids declined in ecological importance over the Permian (DiMichele et al., 2006; Montañez et al., 2007), but surviving lineages remain species rich even today, with around 1300 extant species of aquatic, terrestrial and epiphytic herbs (Christenhusz and Byng, 2016). In addition to their wide range of growth habits, lycopsids also exhibit some basic differences in reproductive biology and morphology that could potentially influence morphological evolution; fossil and living members include homosporous and heterosporous species and show a range of reproductive structures, from specialized strobili to more diffuse fertile zones consisting of sporangia born on undifferentiated vegetative axes (see Pigg, 2001; Taylor et al., 2009; Field et al., 2016).
Lycopsids are therefore diverse in aspects of their reproductive biology, reproductive ecology and vegetative growth habit, but their reproductive structures nonetheless perform the same limited suite of basic functions as those of other pteriodophytes. If functional diversity is key in the evolution of reproductive diversity, then we might expect lycopsid reproductive structures to exhibit relatively low disparity and show fewer shifts through time when compared with seed plant reproductive structures, which perform a greater number of functional roles. In this study, we quantify basic patterns in lycopsid morphological diversity through time and we show that multiple lycopsid clades evolve a similar basic suite of reproductive morphologies, and that they largely maintain them over their evolutionary history. These morphologies appear to be primarily adapted for the efficient packing and protection of sporangia, and are also found in other pteridophyte groups such as horsetails and even in the pollen cones of gymnosperms and staminate catkins of some flowering plants. These patterns of convergent evolution suggest that the diversity of functional roles, or the lack thereof, play an important role in reproductive evolution across plants.
MATERIALS AND METHODS
We used both discrete and continuous traits to characterize the reproductive disparity of lycopsids through time. We compiled data for both types of traits from published literature sources (for fossils) and from direct observations of herbarium specimens (for extant taxa). The total data set includes 152 fossil lycopsid taxa ranging from the Late Silurian [Ludlow; 427–423 million years ago (Mya)] through the Late Triassic (approx. 200 Mya), although it is focused on Devonian, Carboniferous and Triassic species and not all measurements were possible in every taxon. Fossil lycopsid taxa are also known from the Jurassic and Cretaceous (e.g. Skog, 1992; Pigg, 2001), but they are generally similar to extant taxa and were not included in the analysis. Measurements of living taxa were based on collections housed at the Harvard University Herbaria, and include 122 species from 15 genera of Lycopodiales and Selaginellales.
The discrete character data set includes 18 unordered binary and multistate morphological characters that describe basic aspects of how reproductive organs are arranged on the plant, how they are attached to supporting structures such as sporophylls, and the morphology of these supporting structures (see Fig. 1 for illustration of the most important scored features; for a full character list and discussion, see Supplementary Data File S1). For a number of widespread or diverse fossil genera, such as Lepidostrobus, Pleuromeia and Cyclostrobus, we chose to score exemplar taxa rather than attempting to score every described species (most described species would also fall within the same character scoring). Similarly, for the most diverse living genera such as Phlegmariurus and Selaginella, we scored representative species that we could directly observe (see Supplementary Data File S1). The total number of scored taxa therefore includes 35 Silurian and Devonian taxa, 41 Carboniferous taxa, 13 Triassic taxa and 27 extant taxa, although a few of these taxa have similar basic reproductive morphology and score identically.
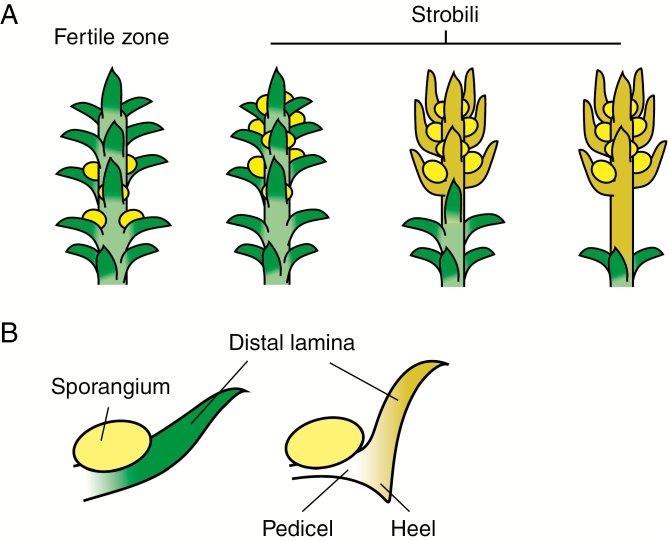
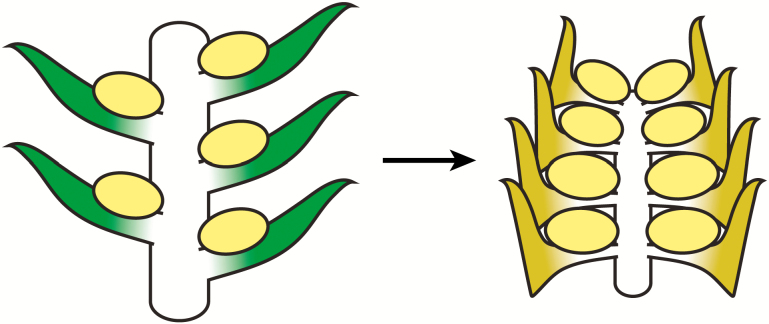
Fig. 1.
Schematic of selected reproductive features examined in this study. (A) Four different potential arrangements of sporangia (yellow ellipses) with increasing specialization from left to right: fertile zone with unmodified sporophylls similar to vegetative leaves; strobilus with unmodified sporophylls; strobilus with specialized sporophylls; and strobilus with specialized sporophylls borne on a dedicated fertile branch. (B) Illustration of major features of an unmodified sporophyll (left) and a specialized sporophyll (right). The specialized sporophyll shows a distal lamina, an expanded blade-like distal portion and a pedicel, a narrow stalk-like proximal portion.
We assembled the discrete character data into a matrix (see Supplementary Data File S1) and calculated the morphological distance, or dissimilarity, between pairs of taxa as the number of character mismatches divided by the total number of applicable or non-missing characters. Dissimilarities from all pairs of taxa were then assembled into a single matrix and analyzed using principal co-ordinate analysis (PCoA), a method with a long history of use in analysing morphological evolution (see Foote, 1994; Lupia, 1999; Boyce and Knoll, 2002; Leslie, 2011). For PCoA, the initial dissimilarity matrix was transformed to set the centroid of the dissimilarity distribution to zero (Gower, 1966), and eigenvalues and eigenvectors were then calculated from the transformed matrix. The eigenvectors form new axes (the principal co-ordinate or PCO axes) that summarize multidimensional morphological data, and the scores of individual taxa on these axes can be used to ordinate them for visualization and further analysis. We also calculated correlations between specific PCO axes and individual characters, as well as correlations among the characters, using Spearman’s rank correlation.
The continuous character data set includes measurements of sporangium length and width, the diameter of the axes subtending the sporangia, and the length and width of strobili in taxa that produced them. For sporangia, for strobili and for the axes of strobili, we estimated their total volumes as ellipsoids using length and width as minor and major axes, respectively. For fossil sporangia that were greatly flattened (such as in Pleuromeia and some other Triassic isoetaleans), we also recorded sporangium height and used it to better estimate volume. We defined a strobilus as a determinate aggregation of sporangia that terminates a single shoot; in contrast, a fertile zone was defined as a region of sporangia occurring on an indeterminate vegetative shoot (Fig. 1A). Some extant Phlegmariurus species in the Lycopodiales have distal dichotomizing shoot systems that are covered in sporangia even up to their apices and are typically described as strobili, but for the purpose of this study we considered them to represent a type of fertile zone because they are branching systems. Either strobili or fertile zones in lycopsids may consist entirely of microsporangia (microsporangiate strobili), entirely of megasporangia (megasporangiate strobili) or have both types (bisporangiate strobili).
For interpreting results of both discrete and continuous analyses, we broadly grouped lycopsids by their overall growth habit. We divided lycopsids into herbaceous taxa, sub-arborescent taxa and arborescent taxa. Herbaceous taxa show no evidence of secondary tissues and are found in multiple clades, including a variety of early diverging Devonian lineages, extinct and extant Lycopodiales, and extinct and extant Selaginellales. Arborescent taxa are found within the Isoetales and may exhibit an extensive branched rooting system called the stigmarian rhizomorph (see Hetherington et al., 2016), a trunk that produces a highly branched canopy and secondary tissues throughout the plant body (see Taylor et al., 2009). Sub-arborescent taxa in this study refer to lycopsids with some secondary growth but which are <3 m in height. It includes ‘pseudoherbaceous’ taxa (sensuBateman, 1992) and ‘sub-arborescent’ taxa (e.g. Bek et al., 2009). We further divided sub-arborescent taxa into those that produce multibranched aerial trunks and those that consist of an unbranched (or once-branched) trunk, such as Triassic Pleuromeia or modern Isoetes. Most sub-arborescent taxa belong to the Isoetales, although the relationships of some Devonian taxa are unclear.
We used standardized major axis regression on log-transformed data to evaluate scaling relationships among the continuous characters. Regression analyses were performed using the package lmodel2 (Legendre, 2014) in R, the open source statistical software R (version 3.4.0; R Core Team, 2018). The lmodel2 package reports estimates and 95 % confidence intervals (CIs) for key regression parameters including intercept and slope. All other statistical analyses in this study, including PCoA and Spearman’s rank correlations, were also performed in R.
RESULTS
Discrete character analyses
The first two PCO axes (Fig. 2) broadly separate lycopsids based on the structure of their fertile region, the presence of specialized sporophylls and whether the fertile region is monosporangiate or bisporangiate. Specifically, PCO axis 1 is most strongly and positively correlated with having a strobilus and with producing sporophylls that have a differentiated pedicel and are borne at 90° to the main strobilus axis, while PCO axis 2 is most strongly positively correlated with having a bisporangiate fertile region and most negatively correlated with sporophylls that are differentiated into a pedicel as well as an abaxial heel (see Fig. 1). These sporophyll traits are generally associated with closely packed, horizontally stacked sporangia covered by some kind of distal lamina tissue.
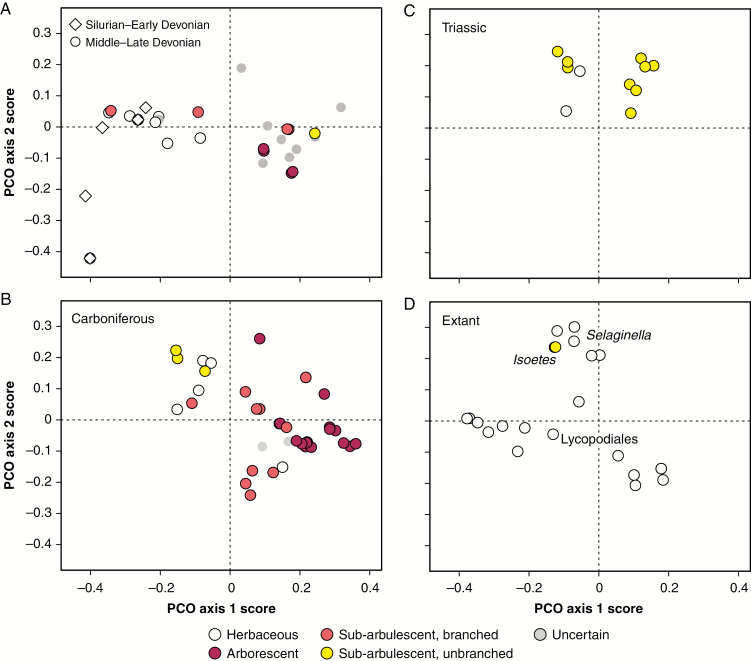
Fig. 2.
Ordination of lycopsid taxa on the first two principal co-ordinate (PCO) axes. PCO axis 1 is positively correlated with the presence of strobili and specialized sporophyll morphologies, while PCO axis 2 is positively correlated with bisporangiate reproductive structures and negatively correlated with specialized sporophyll morphologies. (A) Late Silurian to Early Devonian (427–393 Mya) and Middle to Late Devonian (393–359 Mya) taxa. Note outlier taxa in the lower left quadrant, which are unlike other lycopsids in having stalked sporangia that are not associated with specific sporophylls (includes Asteroxylon, Drepanophycus and Hueberia). (B) Carboniferous (359–299 Mya) taxa. (C) Triassic (252–201 Mya) taxa. (D) Extant taxa, with the positions of representatives from the major surviving lineages indicated. The apparent contraction of morphospace occupation in the Triassic probably represents a bias in either preserved or described reproductive structures, as Lycopodiales were present during this time period.
The earliest known lycopsids from the Late Silurian through the Early Devonian (426–398 Mya) occupied a relatively restricted area of the total morphospace formed by PCO axes 1 and 2 (Fig. 2A); these taxa were herbaceous and produced fertile zones of reniform to ovoid sporangia either borne on, or in association with, relatively unmodified leaf-like sporophylls. Some early lycopsid lineages (e.g. Asteroxylon and Drepanophycus) produced sporangia that were not associated with sporophylls but were instead borne directly on the axis (Fig. 2A, taxa in the lower left quandrant). Beginning in the Middle Devonian (398–385 Mya) and continuing over the Late Devonian (385–359 Mya), lycopsids evolved well-defined strobili and specialized sporophylls that were clearly different from vegetative leaves, therefore expanding the total area of reproductive morphospace occupation along PCO axis 1. These taxa include arborescent forms (e.g. Sublepidodendron), sub-arborescent forms with a branching crown (e.g. Minostrobus), sub-arborescent forms with a single (or once-branched) trunk (Clevelandodendron) and a large number of taxa whose growth habit is not clear (Fig. 2A).
Strobili with closely packed sporangia and specialized sporophylls were common over the Carboniferous (359–299 Mya), and were present in herbaceous (e.g. Carinostrobus), sub-arborescent (e.g. Chaloneria and Spencerites) and arborescent taxa (e.g. Achlamydocarpon Flemingites, Lepidocarpon and Mazocarpon; see Fig. 2B). Carboniferous taxa in various growth habits also produced bisporangiate strobili, which tend to plot more positively on PCO axis 2 if they are also associated with relatively unspecialized sporophylls (e.g. Chaloneria, Flemingites arctuatus and Selaginella; Fig. 2B). Observed morphospace occupation contracted from the Permian through the Triassic, and is restricted to Selaginella-like herbaceous forms and surviving isoetalean sub-arborescent taxa with single trunks such as Pleuromeia (Fig. 2C). Extant taxa (Fig. 2D) exhibit a range of morphospace occupation that is similar to Devonian and Carboniferous taxa, reflecting living Lycopodiales with fertile zones (e.g. Huperzia) that are not unlike Early Devonian lycopsids, as well as taxa with specialized strobili and sporophylls (e.g. Diphasiastrum).
Continuous character analyses
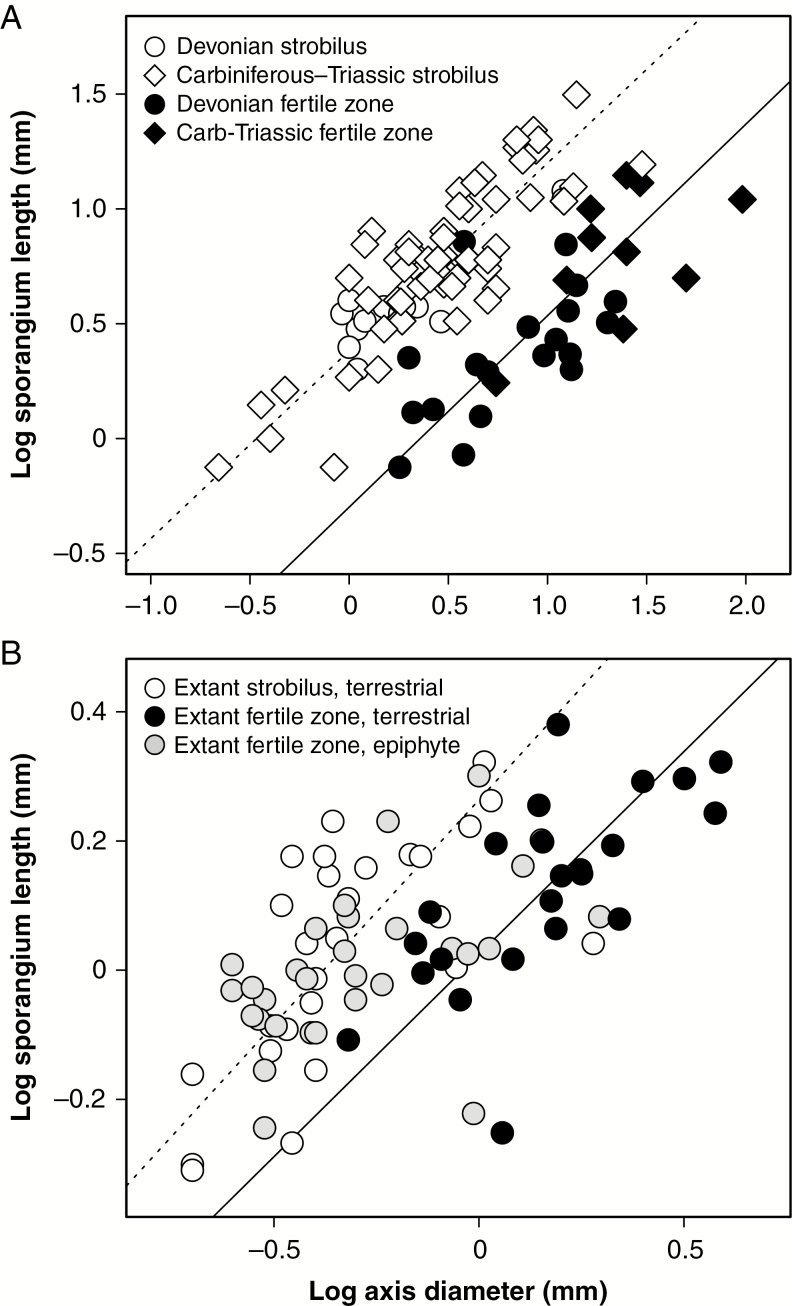
Fossil lycopsids with fertile zones and those with strobili show different scaling relationships in the size of sporangia and the axes supporting them (Fig. 3A). Sporangia-bearing axes in fossil taxa with strobili are typically smaller than fertile zone axes bearing the same size sporangia (Fig. 3A), as shown by their different regression intercepts (–0.31, 95 % CI –0.53 to –0.07; and 0.38, 95 % CI 0.32–0.44, respectively), but otherwise show a similar positive relationship with sporangium (slope = 0.84, 95 % CI 0.65–1.07 in strobilus taxa; and 0.82, 95 % CI 0.73–0.92 in fertile zone taxa). Scaling relationships are more complicated among extant herbaceous lycopsids (Fig. 3B). As in fossil taxa, extant species with fertile zones tend to bear sporangia on proportionally larger axes (Fig. 3B; intercept 0.02, 95 % CI –0.04 to 0.09 vs. 0.26, 95 % CI 0.18–0.35). However, these relationships hold only among ground-dwelling species; epiphytic species show a range of sporangium and axis sizes and can produce specialized fertile zones characterized by thin axes (i.e. the ‘tassles’ of Phlegmariurus) that scale like strobili.
Fig. 3.
Relationship between sporangium size, as measured by maximum length, and the diameter of the axis supporting them. Fossil taxa (A) show a clear distinction between taxa with strobili and those with fertile zones, with the exception of a few Devonian forms [Monilistrobus (Wang and Berry, 2003) and Wuxia (Berry et al., 2003)]. (B) Extant taxa show a more complicated set of relationships that depend on growth habit; ground-dwelling or terrestrial taxa show a similar pattern to fossil lycopsids but epiphytic taxa exhibit a wide range of morphologies. In both panels, the dotted line represents the best-fit regression line for terrestrial taxa with strobili, while the black line represents the best-fit regression line for terrestrial taxa with fertile zones (see text for parameter estimates).
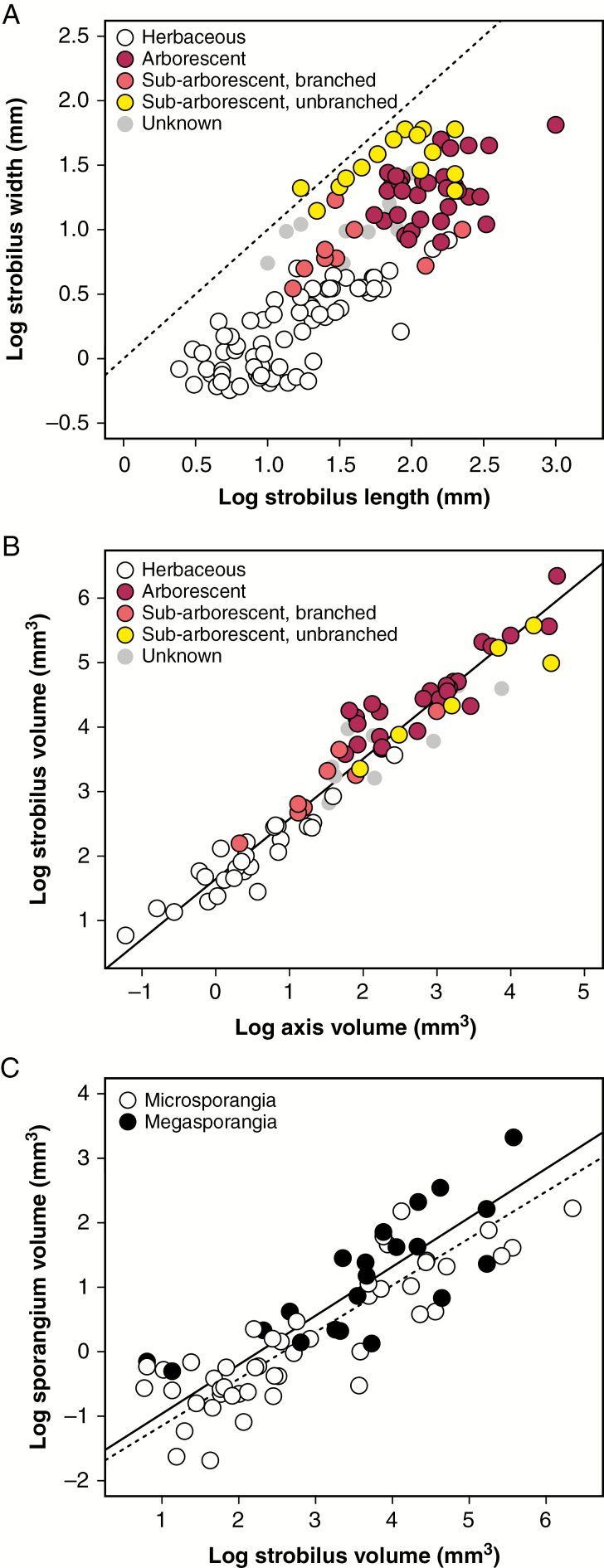
Among taxa with strobili, lycopsids exhibit wide variation in overall strobilus size (Fig. 4A) from those <1 cm in length in some extant Selaginella to those up to 1 m long in some Carboniferous arborescent isoetaleans (e.g. Lepidostrobus sternbergii). Growth habit influences strobilus size and sometimes shape; small herbaceous lycopsids produce small strobili, whereas arborescent taxa produce large strobili, with the smaller sub-arborescent taxa in between (Fig. 4A). Sub-arborescent taxa with a single large trunk tend to produce strobili that are both large and proportionally wide, unlike other lycopsid taxa. Although not shown for clarity, there are no obvious differences in strobilus size and shape between microsporangiate, megasporangiate or bisporangiate strobili after accounting for growth habit.
Fig. 4.
Strobilus construction and tissue allocation among lycopsids. (A) Length and width of lycopsid strobili across different clades and growth habits. The dotted line is a one-to-one line for comparison. (B) Relationship between estimated strobilus axis volume and estimated sporangium volume across clades and growth habits. The line represents the best-fit slope of a standardized major axis (SMA) regression (slope = 0.93, R2 = 0.93, P < 0.001). (C) Relationship between estimated strobilus volume and estimated sporangium volume. Best-fit regression slopes by SMA are similar for microsporangiate (dotted line; slope = 0.73, R2 = 0.75, P < 0.001) and megasporangiate strobili (solid line; slope = 0.76, R2 = 0.63, P < 0.001).
Despite differences in size and shape among lycopsid strobili, their total estimated volume scales nearly linearly with their estimated axis volume across different growth habits and across different clades (Fig. 4B; regression slope is 0.93 with 95 % CI 0.88–0.99), suggesting that they have a similar basic structure and resource allocation. Individual sporangium size also scales with strobilus volume across different clades and growth habits (Fig. 4C), and the relationship is similarly allometric for both microsporangia (regression slope 0.73, 95 % CI 0.64–0.83) and megasporangia (slope 0.76, 95 % CI 0.60–0.97). Sporangium volume also scales similarly to total strobilus volume regardless of whether the strobilus is mono- or bisporangiate and regardless of plant growth habit.
DISCUSSION
Since the Late Devonian, lycopsid reproductive structures have occupied a similar total range of morphospace and shown similar allocation to sporangial and supporting axis tissues. The shifts in morphospace occupation that did occur primarily reflect the appearance of taxa with strobili and specialized sporophylls with either an upturned distal lamina, a projecting abaxial heel, a thin pedicel borne around 90° to the supporting axis or some combination of these features. These traits, in conjunction with the relatively thin axes characteristic of strobili, allow sporangia to be densely packed while also creating a protective layer of imbricated or interlocking sporophyll laminae (Fig. 5). The evolution of lycopsid reproductive morphology therefore primarily appears to reflect adaptations for the efficient packaging and protection of sporangia prior to spore release, although laminae in modern lycopsid sporophylls are green for at least part of their ontogeny, and it is conceivable that they also function to provide some photosynthate to developing cones.
Fig. 5.
Illustration of major patterns in lycopsid strobilus and sporophyll morphology. Specialized sporophylls with thin stalks, upturned distal lamina and abaxial heels allow for dense packaging and protection of developing sporangia, while dedicated strobilus axes allow plants to invest less in supporting tissues relative to vegetative axes that must also support leaves and the plant body.
However, lycopsid reproductive structures may have performed a more diverse set of reproductive functions than just sporangium packaging in some cases; for example, derived arborescent isoetaleans such as Lepidodendron, Diaphorodendron and Lepidophloios (which produced Achlamydocarpon takhtajanii-type megasporophylls, A. various-type megasporophylls and Lepidocarpon cones, respectively) shed their sporangia and sporophylls as a single unit. The distal lamina in these taxa has been proposed to function as a seed wing for aerial sporangium dispersal, as a device to orient the unit as it floated in standing water or both (Phillips, 1979; Thomas, 1981; Phillips and DiMichele, 1992; Wang et al., 2014). Some arborescent isotealeans [as well as the enigmatic Carboniferous herbaceous lycopsid Miadesmia (Benson, 1908)] also produced additional flaps of sporophyll tissue that in the most derived members (e.g. Lepidocarpon and Miadesmia) nearly covered the megasporangium (Ramanujam and Stewart, 1969; Phillips, 1979), leaving only a narrow opening. These flaps probably protected the megasporangium after dispersal and may have also facilitated the collection of microspores, perhaps in a manner analogous to the seed integument (see Thomas, 1981).
Yet despite the potentially complex reproductive biology of some lycopsids, their basic strobilus structure and sporophyll morphology is not fundamentally different from that of other lycopsids. Derived arborescent isoetaleans, including Lepidocarpon, do not show different patterns of tissue allocation within the strobilus nor do they occupy substantially different areas of reproductive morphospace from other lycopsids that have compact strobili and specialized sporophylls, such as modern Lycopodiales. Furthermore, microsporangiate, megasporangiate and bisporangiate reproductive structures all show similar suites of strobilus and sporophyll traits. In general, differences in reproductive biology among lycopsids are not associated with fundamental differences in reproductive morphology, but rather with more subtle modifications to a common structural plan.
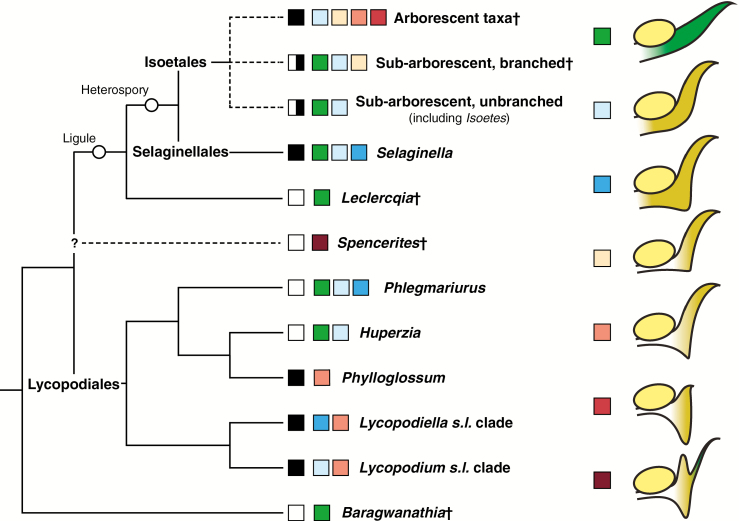
Specialized lycopsid strobili and sporophylls are likely to have evolved multiple times, further suggesting that efficient packaging and protection of developing sporangia have been an important selective filter in their history. In particular, specialized sporophylls with pedicels, upturned distal lamina and projecting abaxial heels in some extant Lycopodiales and extinct arborescent isoetaleans are convergent (Fig. 6; topology adapted from Kenrick and Crane, 1997; Wikström, 2001; Korall and Kenrick, 2004; Field et al., 2016). Such morphologies may have evolved independently even within the Isoetales clade, depending on the exact phylogenetic relationships of lineages whose sporangia were borne in essentially unmodified fertile zones (e.g. Chaloneria; Pigg and Rothwell, 1983). If these lineages represent ancestral morphologies, strobili with specialized sporophylls probably evolved several times across isoetaleans (see Fig. 6), although more phylogenetic studies that include fossils (e.g. Bateman, 1992; Xue, 2011) are necessary to understand these transitions. The Carboniferous sub-arborescent genus Spencerites may represent yet another independent origin of specialized protective sporophylls, as theirs possessed a uniquely inflated distal lamina base (Fig. 6; see also Leisman and Stidd, 1967; Bek et al., 2009).
Fig. 6.
Schematic outline of possible phylogenetic relationships among representative lycopsids, illustrating reproductive morphologies associated with each group or taxon as well as the position of two potentially important synapomorphies (ligules and heterospory). White boxes indicate fertile zones, black boxes indicate strobili and a white/black box indicates that both arrangements are present. Coloured boxes indicate sporophyll morphologies; green represents an unmodified sporophyll identical to vegetative leaves, blue colours indicate modified sporophylls that lack a differentiated stalk or pedicel, and brown/orange colours indicate specialized sporophylls with stalks and upturned distal laminae (tan), abaxially projecting heels (orange/red) or adaxial ridges of tissue distal to the sporangium (brown). Sporangium is indicated in yellow, but specific details of its shape and attachment differ among the clades and are not indicated in the figure. The position of Spencerites is unknown and relationships among various fossil Isoetales are also uncertain; for simplicity and consistency, they are shown here by growth habit even though it is unlikely that the different growth habits form clades in all cases. A dagger indicates an extinct lineage.
Sporangium packaging appears to have exerted a pervasive influence on lycopsid reproductive evolution, but other factors were important as well. Growth habit, for example, controls how reproductive tissue is arrayed in lycopsids and therefore influences the overall size and organization of fertile structures. For example, herbaceous taxa such as modern Lycopodiales produce small and generally narrow strobili because they are borne on thin distal branches, while single-trunked sub-arborescent isoetaleans (e.g. Pleuromeia and Tomiostrobus) often produce large and wide strobili because they occur on a squat trunk (e.g. Retallack, 1997; Naugolnykh, 2013). Such a linkage between reproductive morphology and growth architecture is present in many plants (see Primack, 1987; Niklas, 1993; Diggle, 1995; Leslie et al., 2014), but may be particularly strong in lycopsids because they show little fundamental distinction between vegetative and fertile shoots; for example, many clades include taxa with fertile zones as well as those with strobili (Fig. 6).
The extant genus Phlegmariurus (Lycopodiales) in particular illustrates how growth habit can influence the evolution of reproductive morphology. Phlegmariurus generally produces sporangia in branching fertile zones towards the tips of its shoots, and includes both ground-dwelling terrestrial herbs and pendent epiphytes (see Field et al., 2016). In most terrestrial species, sporangia occur in indistinct fertile zones with leaf-like sporophylls on otherwise unmodified vegetative axes. In contrast, many epiphytic species produce highly modified fertile shoots (or ‘tassels’) that have clearly differentiated sporophylls and proportionally thin axes (see Fig. 3B). Because these plants are epiphytic and their fertile axes need not contribute to the mechanical or hydraulic support of the plant body, mutations or other changes in development that result in tighter sporangial packing and proportionally smaller axes would lead to a more efficient use of resources and would not be structurally detrimental. It is not surprising then that some epiphytic species have highly modified fertile zones with reduced supporting axes and compact sporophylls, much like the strobili of other Lycopodiales.
In a broader sense, the repeated evolution and persistence of a limited suite of lycopsid strobilus and sporophyll morphologies, after controlling for the effects of growth habit, is consistent with the idea that limited functional demands generate a simple adaptive landscape with a few optimal morphological solutions (Niklas, 1994, 1999). Further supporting this idea, reproductive structures that perform similar functional roles in other groups of vascular plants are similar to those of lycopsids. For example, free-sporing and wind-dispersed lineages such as horsetails (see Taylor et al., 2009) and the enigmatic extinct noeggerathialeans (Wang et al., 2009; Wang et al., 2017) both produce thin, compact strobili, as do wind-pollinated conifers in their pollen cones and some angiosperm groups (e.g. Betulaceae) in their staminate catkins. Although these reproductive structures are not homologous and are diverse in their specific morphologies, they have all converged on at least some of the characteristic elements of lycopsid reproductive structures, including closely packed sporangia that are oriented perpendicular to the cone axis, sporophylls with a distal covering like a lamina, and thin pedicels or equivalent features. Some of these reproductive organs, such as the pollen cones of conifers, exhibit all of these features and show a remarkable degree of morphological convergence with lycopsid strobili.
Lycopsid evolutionary history therefore suggests that functional diversity plays an important role in the evolution of plant reproductive disparity. These effects are most apparent at the largest scales of plant evolution, as in the divergent histories of reproductive disparity among different plant lineages. For example, pteridophyte groups such as horsetails and lycopsids independently evolved some of the same basic kinds of strobilus structures over the Devonian and appear to have maintained them over their evolutionary history, while, in contrast, seed plants show continued diversification of their reproductive structures, particularly in their ovulate organs (see Leslie, 2011). Seed plant ovulate organs must satisfy a complex set of functional demands, including capturing pollen, protecting fertilized seeds over potentially long time periods and dispersing them via wind, water or animals. Not only are there many potential ways to balance these functions at any given time, but the potential set of solutions must often change in response to shifts in the physical and biotic environment. At the most basic level then, the incredible diversification of seed plant ovulate structures from the Late Devonian onwards, compared with the relative stability of lycopsid strobili, fern sori and horsetail cones, reflects the virtually limitless number of possible solutions to the complex set of functional demands that they must meet.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of File S1: table of discrete character data used in this study and discrete character key.
ACKNOWLEDGEMENTS
We would like to thank Anthony Brach and the Harvard University Herbaria for access to extant lycopsid specimens. We would also like to thank William DiMichele and an anonymous reviewer for helpful comments and suggestions on the manuscript.
LITERATURE CITED
- Bateman RM. 1992. Morphometric reconstruction, palaeobiology and phylogeny of Oxroadia gracilis Alvin emend. and O. conferta sp. nov.: anatomically-preserved rhizomorphic lycopsids from the Dinantian of Oxroad Bay, SE Scotland. Palaeontographica Abteilung B 228: 29–103. [Google Scholar]
- Bateman RM, DiMichele WA, Willard DA. 1992. Experimental cladistic analysis of anatomically preserved arborescent lycopsids from the Carboniferous of Euramerica: an essay on paleobotanical phylogenetics. Annals of the Missouri Botanical Garden 79: 500–559. [Google Scholar]
- Bek J, Libertín M, and Drábková J. 2009. Spencerites leismanii sp. nov., a new sub-arborescent compression lycopsid and its spores from the Pennsylvanian of the Czech Republic. Review of Palaeobotany and Palynology 155: 116–132. [Google Scholar]
- Benson M. 1908. X. Miadesmia membranacea, Bertand; a new Palœozoic lycopod with a seed-like structure. Philosophical Transactions of the Royal Society B: Biological Sciences 199: 409–425. [Google Scholar]
- Berry CM, Yi W, Chongyang C. 2003. A lycopsid with novel reproductive structures from the Upper Devonian of Jiangsu, China. International Journal of Plant Sciences 164: 263–273. [Google Scholar]
- Bock WJ, Wahlert G. 1965. Adaptation and the form–function complex. Evolution 19: 269–299. [Google Scholar]
- Boyce CK, Knoll AH. 2002. Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants. Paleobiology 28: 70–100. [Google Scholar]
- Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proceedings of the Royal Society B: Biological Sciences 276: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz MJ, Byng JW. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261: 201–217. [Google Scholar]
- Darwin CR. 1877. The various contrivances by which orchids are fertilised by insects, 2nd edn. London: John Murray. [Google Scholar]
- Diggle PK. 1995. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology, Evolution, and Systematics 26: 531–552. [Google Scholar]
- DiMichele WA, Tabor NJ, Chaney DS, Nelson WJ. 2006. From wetlands to wet spots: environmental tracking and the fate of Carboniferous elements in Early Permian tropical floras. Special Paper of Geological Society of America 399: 223–248. [Google Scholar]
- Feild TS, Brodribb TJ, Iglesias A, et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proceedings of the National Academy of Sciences, USA 108: 8363–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. [Google Scholar]
- Field AR, Testo W, Bostock PD, Holtum JA, Waycott M. 2016. Molecular phylogenetics and the morphology of the Lycopodiaceae subfamily Huperzioideae supports three genera: Huperzia, Phlegmariurus and Phylloglossum. Molecular Phylogenetics and Evolution 94: 635–657. [DOI] [PubMed] [Google Scholar]
- Foote M. 1994. Morphological disparity in Ordovician–Devonian crinoids and the early saturation of morphological space. Paleobiology 20: 320–344. [Google Scholar]
- Gower JC. 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53: 325–338. [Google Scholar]
- Hetherington AJ, Berry CM, Dolan L. 2016. Networks of highly branched stigmarian rootlets developed on the first giant trees. Proceedings of the National Academy of Sciences, USA 113: 6695–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. 1997. The origin and early diversification of land plants: a cladistic study. Washington DC: Smithsonian Institute Press. [Google Scholar]
- Korall P, Kenrick P. 2004. The phylogenetic history of Selaginellaceae based on DNA sequences from the plastid and nucleus: extreme substitution rates and rate heterogeneity. Molecular Phylogenetics and Evolution 31: 852–864. [DOI] [PubMed] [Google Scholar]
- Legendre P. 2014. lmodel2: Model II Regression. R package version 1.7–2. [Google Scholar]
- Leisman GA, Stidd BM. 1967. Further occurrences of Spencerites from the Middle Pennsylvanian of Kansas and Illinois. American Journal of Botany 54: 316–323. [Google Scholar]
- Leslie AB. 2011. Shifting functional roles and the evolution of conifer pollen-producing and seed-producing cones. Paleobiology 37: 587–602. [Google Scholar]
- Leslie AB, Beaulieu JM, Crane PR, Donoghue MJ. 2014. Cone size is related to branching architecture in conifers. New Phytologist 203: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Lupia R. 1999. Discordant morphological disparity and taxonomic diversity during the Cretaceous angiosperm radiation: North American pollen record. Paleobiology 25: 1–28. [Google Scholar]
- Montañez IP, Tabor NJ, Niemeier D, et al. 2007. CO2-forced climate and vegetation instability during Late Paleozoic deglaciation. Science 315: 87–91. [DOI] [PubMed] [Google Scholar]
- Naugolnykh SV. 2013. The heterosporous lycopodiophyte Pleuromeia rossica Neuburg, 1960 from the Lower Triassic of the Volga River basin (Russia): organography and reconstruction according to the ‘whole plant’ concept. Wulfenia 20: 1–6. [Google Scholar]
- Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago, IL: University of Chicago Press. [Google Scholar]
- Niklas KJ. 1993. The allometry of plant reproductive biomass and stem diameter. American Journal of Botany 80: 461–467. [Google Scholar]
- Niklas KJ. 1994. Morphological evolution through complex domains of fitness. Proceedings of the National Academy of Sciences, USA 91: 6772–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. 1999. Evolutionary walks through a land plant morphospace. Journal of Experimental Botany 50: 39–52. [Google Scholar]
- Niklas KJ. 2004. Computer models of early land plant evolution. Annual Review of Earth and Planetary Science 32: 47–66. [Google Scholar]
- Phillips TL. 1979. Reproduction of heterosporous arborescent lycopods in the Mississippian–Pennsylvanian of Euramerica. Review of Palaeobotany and Palynology 27: 239–289. [Google Scholar]
- Phillips TL, DiMichele WA. 1992. Comparative ecology and life-history biology of arborescent lycopsids in Late Carboniferous swamps of Euramerica. Annals of the Missouri Botanical Garden 79: 560–588. [Google Scholar]
- Pigg KB. 2001. Isoetalean lycopsid evolution: from the Devonian to the present. American Fern Journal 91: 99–114. [Google Scholar]
- Pigg KB, Rothwell GW. 1983. Chaloneria gen. nov.; heterosporous lycophytes from the Pennsylvanian of North America. Botanical Gazette 144: 132–147. [Google Scholar]
- Primack RB. 1987. Relationships among flowers, fruits, and seeds. Annual Review of Ecology, Evolution, and Systematics 18: 409–430. [Google Scholar]
- R Core Team. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Ramanujam CG, Stewart WN. 1969. A Lepidocarpon cone tip from the Pennsylvanian of Illinois. Palaeontographica Abteilung B 16: 159–167. [Google Scholar]
- Retallack GJ. 1997. Earliest Triassic origin of Isoetes and quillwort evolutionary radiation. Journal of Paleontology 71: 500–521. [Google Scholar]
- Rickards RB. 2000. The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia. Geological Magazine 137: 207–209. [Google Scholar]
- Skog JE, Hill CR. 1992. The Mesozoic herbaceous lycopsids. Annals of the Missouri Botanical Garden 79: 648–675. [Google Scholar]
- Taylor EL, Taylor TN, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, 2nd edn. New York: Academic Press. [Google Scholar]
- Thomas BA. 1981. Structural adaptations shown by the Lepidocarpaceae. Review of Palaeobotany and Palynology 32: 377–388. [Google Scholar]
- Wang D, Meng M, Xue J, Basinger JF, Guo Y, Liu L. 2014. Changxingia longifolia gen. et sp. nov., a new lycopsid from the Late Devonian of Zhejiang Province, South China. Review of Palaeobotany and Palynology 203: 35–47. [Google Scholar]
- Wang J, Pfefferkorn HW, Bek J. 2009. Paratingia wudensis sp. nov., a whole noeggerathialean plant preserved in an earliest Permian air fall tuff in Inner Mongolia, China. American Journal of Botany 96: 1676–1689. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Bateman RM, Spencer AR, Wang J, Shao L, Hilton J. 2017. Anatomically preserved ‘strobili’ and leaves from the Permian of China (Dorsalistachyaceae, fam. nov.) broaden knowledge of Noeggerathiales and constrain their possible taxonomic affinities. American Journal of Botany 104: 127–149. [DOI] [PubMed] [Google Scholar]
- Wang Y, Berry CM. 2003. A novel lycopsid from the Upper Devonian of Jiangsu, China. Palaeontology 46: 1297–1311. [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences, USA 111: E4859–E4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström N. 2001. Diversification and relationships of extant homosporous lycopods. American Fern Journal 91: 150–165. [Google Scholar]
- Xue J. 2011. Phylogeny of Devonian lycopsids inferred from Bayesian phylogenetic analyses. Acta Geologica Sinica 85: 569–580. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.