Abstract
Background and Aims
Changes in the arrangement of cell wall components determine cell wall properties (integrity, stiffness), thereby affecting the macro-scale properties of fruits, which are important for consumers and industry. Arabinogalactan proteins (AGPs) are ubiquitous components of the plant cell, in which they have various functions. Currently, AGPs are considered to be one of the less well-known, enigmatic proteoglycans, a consequence of their heterogeneous structure and unclear mechanism of activity.
Methods
An immunocytochemical study was conducted to elucidate the distribution of AGPs and pectic polysaccharides contained in apple (Malus × domestica) fruit during senescence. De-esterified homogalacturonan (LM19), methyl-esterified homogalacturonan (LM20), processed arabinan (LM16) and three AGP epitopes (JIM13, JIM15, MAC207) were identified in the fruit at three stages: fresh fruit, and fruit at 1 and 3 months of post-harvest storage.
Key Results
Microscopy revealed spatio-temporal changes in the localization of all examined epitopes. Changes of fruit cell wall assembly and its degradation were confirmed by determination of the galacturonic acid content in the WSP (water soluble pectins), CSP (chelator soluble pectins) and DASP (dilute alkali soluble pectins) fractions.
Conclusions
The results revealed dependencies between AGPs, arabinan and homogalacturonan distribution in apple fruit, which are correlated with changes in microstructure during senescence. We propose that AGPs are involved in establishment of the cell wall – plasma membrane continuum.
Keywords: Arabinogalactan proteins, cell wall, fruit senescence, Malus × domestica, pectin, plasma membrane
INTRODUCTION
The cell wall of fruit plays a significant biological function and determines crop quality, which is important for both the grower and the consumer. The prevailing structural model of the cell wall consists of a cellulose–hemicellulose network, an amorphous matrix composed of pectins, and small amounts of proteins and phenolic compounds (Bidhendi and Geitmann, 2016; Guillon et al., 2017). Among many factors, the distribution of the above constituents, their mutual interactions, cross-linking and multidomain organization determine the mechanical properties of fruit such as firmness (Chen et al., 2009; Geitmann, 2010; Zhang et al., 2010; Jarvis, 2011). Numerous studies describe the presence of de-esterified homogalacturonan (HG) at the corners of intercellular spaces; their presence is related to their function in cell-to-cell contact. Moreover, the correlation with Ca2+ cross-linking suggests a role of de-esterified HG in the mechanism of intercellular adhesion (Ng et al., 2013). Also, methyl-esterified HG epitopes generally occur at cell junctions and in the middle lamella (Christiaens et al., 2011; Zamil and Geitmann, 2017). By contrast, an entirely different distribution is shown by rhamnogalacturonan type I (RG-I) epitopes, which are restricted to a region close to the plasma membrane, associated with its role in wall rearrangements during cell growth (Guillon et al., 2017).
Proteomic research on dynamic changes occurring during apple fruit ripening and storage indicated that proteins exert an impact on specific metabolic pathways and in this way have an influence on complex physiological changes, e.g. reduced firmness reduction and cell wall degradation during post-harvest storage. Proteins that are differentially expressed in apple fruit during maturation and storage were characterized by Shi et al. (2014). Moreover, it has been established that specific proteins are involved in cell wall synthesis, contributing to the stabilization of the actin cytoskeleton, and are associated with changes in the content of soluble solids (Shi et al., 2014). Furthermore, one of the structural proteins of the plant extracellular matrix, arabinogalactan protein (AGP), is involved in the response to abiotic factors, i.e. mechanical wounding. Studies of AGP gene expression profiles confirmed its involvement in oxygen deficiency adaptation in fruit. Moreover, constitutive expression during developmental processes indicates AGPs functions during fruit ripening (Fragkostefanakis et al., 2012). In addition, as dynamic cell wall components, AGPs are up-regulated by plant growth regulators, and interact with auxin and cytokinin (Seifert and Roberts, 2007).
AGPs are classified as a category of the superfamily of hydroxyproline-rich glycoproteins (HRGPs). Among the HRGPs, there are highly glycosylated members found in cells throughout the plant kingdom, acting as covalent cross-linkers in the polysaccharide–proteoglycan network. Identification of linkages between cell wall polymers provided evidence for the existence of the protein APAP1, composed of an AGP core, with attached RG-I, HG and arabinoxylan (Tan et al., 2013; Hijazi et al., 2014a). AGPs are distinguishable from other HRGPs (e.g. extensins) based on amino acid composition, the specific arrangement of Ala-Pro, Pro-Ala, Ser-Pro and Thr-Pro, various carbohydrate chains, and anchoring by glycosylphosphatidylinositol sequence to the plasma membrane (Showalter, 2001; Ma et al., 2017). Moreover, Lamport and Varnai (2013) and Lamport et al. (2014) described AGPs as a periplasmic Ca2+ capacitors involved in intramolecular Ca2+ binding in many biological processes. This feature of AGPs was also associated with their contribution to the generation of an adhesive film by cohesion of the adjacent AGP nanoparticles and Ca2+ movement in the extracellular space in root cells (Huang et al., 2016). We previously revealed the specific localization of AGPs in apple fruit tissue, and showed that the arrangement of the [βGlcA(1→3)-αGalA(1→2)Rha] epitope depends on the kind of tissue and the stage of post-harvest storage (Leszczuk et al., 2018). Although research on cell wall components is a very active field, little is known about associations between AGPs and other constituents of the cell wall; similarly, the contribution of AGPs to cell wall assembly is still poorly documented.
Therefore, the aim of present study was to describe the specific arrangement of AGPs and pectins with low vs. high levels of methyl esterification in the cell wall – plasma membrane continuum. Details of the localization of AGPs, processed arabinan, HG in the cell wall architecture, and a quantitative analysis of galacturonic acid (GalA) content allow us to make conclusions about changes in the assembly of fruit microstructure.
MATERIAL AND METHODS
Plant material and sample preparation
The apple (Malus × domestica) fruit of the cultivar ‘Gala Must’ were used in this study. Fruit were purchased from a local producer and had been harvested at the optimum maturity time for this cultivar. Samples were taken at three different stages: at the optimal harvest date (1), after storage in a cold room at 2 °C in a normal atmosphere for 1 month (2), and after 3 months of storage (3). The material was sampled from five apple fruit of each examined stage. From each apple, ten cube-shaped samples were taken from the outer part which included the skin and to a depth of approx. 2 cm under the skin. The epidermal and hypodermal layers were examined, as was the parenchymal tissue. For each immunohistochemical staining, a minimum of 20 tissue sections were analysed for each sample of fruit. The experiments were repeated several times for each antibody and typical image sets were selected and shown.
Fixation, resin embedding and tissue section
Prepared fruit segments were fixed in 2 % (w/v) paraformaldehyde and 2.5 % (v/v) glutaraldehyde in phosphate buffered saline (PBS; all from Sigma). PBS was prepared according to the manufacturer’s recommendations. One tablet dissolved in 200 mL of deionized water yields 0.01 m phosphate buffer, 0.0027 m potassium chloride and 0.137 m sodium chloride, pH 7.4, at 25 °C. After placement under a vacuum for 2 h and overnight incubation, the material was rinsed in PBS (three times, 15 min each). Dehydration (in a graded series of ethanol solutions, from 30, 50, 70, 90 to 96 % for 20 min) was carried out at room temperature. The samples were washed with 99.8 % ethanol twice. The ethanol was substituted with 3: 1, 1: 1 and 1: 3 mixtures of EtOH and LR White resin (Sigma) for 2 h and with pure LR White resin overnight. The polymerization was performed at 55 °C for 48 h in gelatin capsules. Semithin (1 µm) sections were obtained using an ultramicrotome (Leica Reichert Ultracut S) equipped with a glass knife. The sections were mounted on poly-l-lysine-coated glass slides (Sigma). The methods proposed by Wilson and Bacic (2012) were used to prepare samples for microscopy analysis.
Toluidine blue staining
After washing in water, the sections were stained with a 0.5 % (w/v) Toluidine blue aqueous solution at 55 °C for 30 s to visualize the anatomical changes in the fruit tissue during senescence. The observations were carried out and photographs were taken using an N-800M light microscope (Novel) with a Canon PowerShot A640 camera.
Yariv reagent staining to detect AGPs
To confirm the presence of AGPs in the fruit tissue, staining with Yariv reagent was performed (Biosupplies Australia). Yariv reagent (β-GlcY) is used as a cytochemical dye to perturb the functions of AGPs and also for the detection, quantification, purification and staining of AGPs (Popper, 2011; Kitazawa et al., 2013). β-GlcY selectively binds to AGPs, recognizing protein moieties and β-1,3-galactan chains (Huang et al., 2016).
The sections were incubated in a β-GlcY solution for 1 h at room temperature and examined by bright field microscopy. The working solution containing 2 mg of β-GlcY in 1 mL of 0.15 m NaCl was used following the instructions of the supplier.
Immunocytochemical analysis of cell wall glycans
A modified method described by Watanabe et al. (2011) and Rydahl et al. (2017) was used for immunofluorescence labelling. Slides prepared for immunochemistry reactions were circled with a liquid blocker (PAP Pen, Sigma). Sections adhering to poly-l-lysine slides were washed in PBS twice and treated with 1 % bovine serum albumin (BSA) in PBS for 30 min to avoid non-specific binding of antibodies. The sections were then incubated with the primary antibody present at a 1: 50 dilution in 0.1 % BSA at 4 °C for 24 h and washed four times with PBS. The experiment was conducted using monoclonal antibodies: JIM13, JIM15, MAC207 and LM16, LM19, LM20 (Table 1). The applied antibodies directed against AGPs and pectin are available at CarboSource Services (University of Georgia, USA) and PlantProbes (University of Leeds, UK). For visualization of the examined epitopes, incubation with anti-rat antibody conjugated with AlexaFluor 488 (Invitrogen Molecular Probes) was performed. The secondary antibody was diluted 1: 200 in 1 % BSA at 4 °C and left in darkness. After 24 h, the incubated sections were washed in PBS and deionized water, and finally enclosed in Dako Fluorescent Mounting Medium (Sigma). For better localization of the epitopes, some sections were counterstained with Calcofluor White (Fluka) for cellulose.
Table 1.
List of monoclonal antibodies used for the detection of AGP and pectin epitopes in fruit tissue
| Antibody name | Antigen | Reference/provider |
|---|---|---|
| JIM13 | Arabinogalactan protein βGlcA(1→3)-αGalA(1→2)Rha |
Yates et al. (1996)
CCRC, USA |
| JIM15 | Arabinogalactan protein Epitope: unknown |
Yates et al. (1996)
CCRC, USA |
| MAC207 | Arabinogalactan protein βGlcA(1→3)-αGalA(1→2)Rha |
Pattathil et al. (2010)
CCRC, USA |
| LM16 | Processed arabinan (1→5)-α-l-arabinan |
Verhertbruggen et al. (2009)
PlantProbes, UK |
| LM19 | De-esterified homogalacturonan α-GalA(1→4)α-GalA(1→4)α-GalA(1→4)-αGalA |
Verhertbruggen et al. (2009)
PlantProbes, UK |
| LM20 | Methyl-esterified homogalacturonan α-MeGalA(1→4)α-MeGalA(1→4) α-MeGalA(1→4)α-MeGalA |
Verhertbruggen et al. (2009)
PlantProbes, UK |
CCRC, Complex Carbohydrate Research Center.
Control reactions were carried out by omitting the primary antibody. The sections were also analysed for autofluorescence. All immunofluorescence images were examined by laser scanning confocal microscopy using an Olympus BX51 CLSM equipped with corresponding software FluoView v. 5.0. (Olympus Corporation). The excitation wavelength for the AlexaFluor 488 was 496 nm, and the emission wavelength was 519 nm. All parameters (i.e. laser intensity, gain) were kept constant for all experiments. Photographs and the scheme were edited using the CorelDrawX6 graphics program.
Cell wall material extraction
The cell wall material (CWM) for the study was obtained using the method of hot alcohol-insoluble solids with a few modifications as described by Renard (2005) and Szymańska-Chargot et al. (2015).
Determination of galacturonic acid content
The CWM (0.1 g) was stirred in deionized water (9 mL) overnight. It was then filtered and the supernatant containing water-soluble pectins (WSPs) was collected. The residue dissolved in water was stirred in 0.1 m CDTA (5 mL, pH 6.5) for 6 h, filtered, and again stirred in 0.1 m CDTA (5 mL, pH 6.5) for 2 h. The supernatants (with calcium chelator-soluble pectins, CSPs) from these two steps were collected together. The residue was then stirred in 0.05 m Na2CO3 with the addition of 20 mm NaBH4 (5 mL) overnight, filtered, and again stirred in 0.05 m Na2CO3 with the addition of 20 mm NaBH4 (5 mL) for 2 h. The supernatants (with dilute alkali-soluble pectins, DASPs) of these two steps were collected together.
Determination of the GalA content in the WSP, CSP, and DASP fractions was performed in triplicate with an automated wet chemistry analyser, known as a continuous flow analyser (San++, Skalar, Analytical). This is an automated procedure for colorimetric determination of GalA based on the total decomposition of the pectin sample in acidic medium (sulphuric acid). The products obtained are transformed into furfuric derivatives, which react with 3-phenyl phenol to form a coloured dye, the colour intensity of which is measured at 530 nm (Cybulska et al., 2015; Chylińska et al., 2016; Szymańska-Chargot et al., 2016). The pectic content is expressed as GalA content per mass of CWM (mg g–1).
Statistical analysis
The OriginPro 8.5 (Origin Lab v8.5 Pro) program was used for the calculation of average values and standard deviations for chemical compositional changes in the apples. For comparisons of means, an analysis of variance (one-way ANOVA) followed by post hoc Tukey’s honestly significant difference (HSD) test was used.
RESULTS
Iccurrence of arabinogalactan proteins in cell walls during fruit senescence
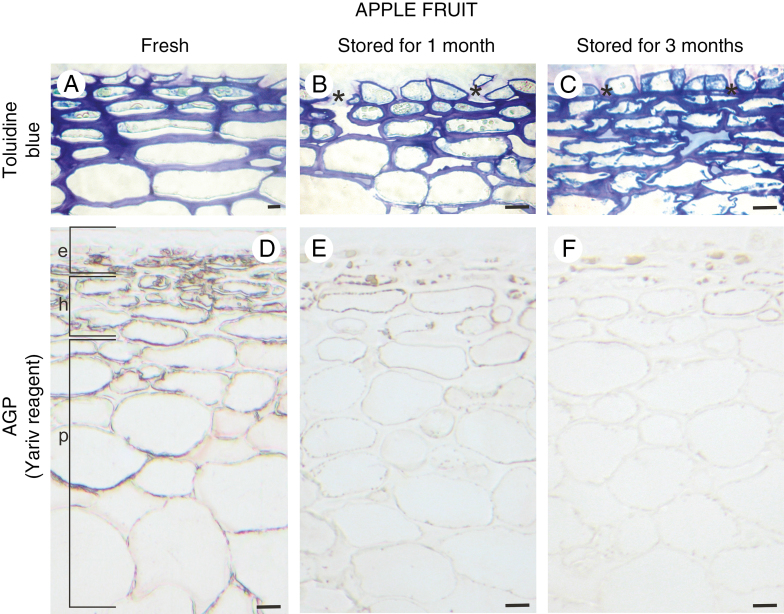
During post-harvest storage, microstructural and biochemical changes took place in all fruit compartments. The epidermal layer of fresh fruit at the optimal harvest date for ‘Gala Must’ is composed of cells that are flattened and stretched to the fruit surface. Intensively stained, thick epidermal cell walls of fruit at harvest maturity indicate an advanced stage of fruit ripening and beginning of fruit softening (Fig. 1A). Deep apertures in the epidermal and subepidermal layers, cuticle penetrating the anticlinal walls of the epidermis, disturbance in the integrity of the epidermal layer and numerous microcracks were visible (Fig. 1B and C). The differences in the morphological features of fresh and stored fruit correlated with the composition of the cell wall. Yariv reagent and monoclonal antibodies against AGPs were used to label fruit cell walls at every examined stage. In fresh fruit, red staining was noticeable in the epidermal layer (e), hypodermis (h) and parenchymatous cells (p) (Fig. 1D). Treatment with Yariv reagent revealed localization of AGPs along the cell wall, mainly in association with the cell membrane. Along with the ageing process in the fruit tissue, the colour reaction was less intense (Fig. 1E and F).
Fig. 1.
Histology of fruit tissue during senescence and its correlation with the presence of AGP. Toluidine blue-stained sections of apple fruit: fresh (A), after 1 month of storage (B), and after 3 months of storage (C). The deep apertures in the epidermal layer are asterisked in B and C. Red stain confirming the presence of AGPs in apple fruit: fresh (D), stored for 1 month (E), and stored for 3 months (F), after staining with Yariv reagent. Light microscopy. Scale bars = 10 µm (A–C), 20 µm (D–F). Abbreviations: e – epidermis, h – hypodermis, p – parenchymal cells.
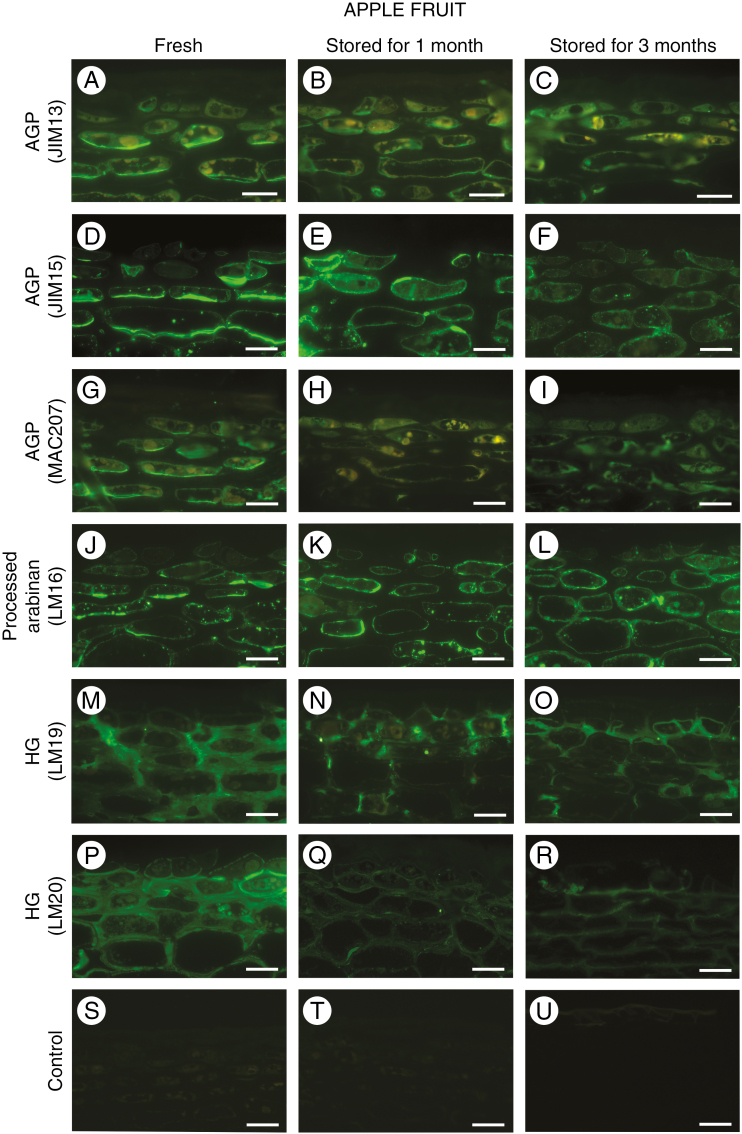
Distinctive arrangement of AGPs and pectin epitopes in the external layers of apple fruit
Most changes associated with fruit senescence and protection of the fruit from environmental conditions take place in the external layers and wax covering of the fruit. Immunofluorescence labelling allowed us to detect a changeable distribution of AGPs and pectins in the cell wall of the epidermal and hypodermal layers. The labelling pattern was similar for all of the antibodies against AGP epitopes in fresh apple tissue. Epitopes recognized by JIM13, JIM15 and MAC207 were found in the inner cell wall layer, in association with the plasma membrane. Moreover, AGP epitopes were absent in the central region of the cell wall (Fig. 2A, D, G). After 1 month of storage, fluorescence indicating the presence of AGPs was also visible in the cytoplasm endomembrane system of epidermal cells (Fig. 2B, E and H). Disorder in the localization of AGP epitopes was found in the fruit after 3 months of storage. Weak fluorescence was visible in the plasma membrane and in the adjacent cytoplasm compartments (Fig. 2C, F, I). The term ‘cytoplasm’ has been used here as a shortcut suggesting a cytoplasmic endomembrane system and/or vesicles of the endoplasmic reticulum and Golgi apparatus.
Fig. 2.
Changes in the localization of AGP and pectin epitopes in the fruit exocarp during senescence. AGP epitopes recognized by JIM13 antibody in apple fruit: fresh (A), stored for 1 month (B), and stored for 3 months (C). Similar immunofluorescence reaction with JIM15 antibody (D, E, F) and MAC207 (G, H, I). Reactions with LM16-recognized processed arabinan epitopes in apple fruit (J, K, L). Immunofluorescence labelling with LM19 antibody against de-esterified homogalacturonan epitopes (M, N, O) and LM20 antibody against methyl-esterified homogalacturonan (P, Q, R) in apple fruit. Control reactions omitting primary antibody (S, T, U). Confocal laser scanning microscopy. Scale bars = 20 µm (A–R), 40 µm (S–U).
Immunolocalization of processed arabinan (LM16) and HG (LM19 and LM20) showed different distributions of these epitopes. The peel of fresh apple fruit is rich in domains recognized by LM16 monoclonal antibody (mAb). Detection of the processed arabinan epitope in the adherent walls of the cells of epidermal and subepidermal layers, and its absence from the central region of the cell wall were visible in fresh fruit (Fig. 2J). After reaction with LM16 mAb in sections of fruit after storage, the fluorescence signal was unchanged and originated from the cell walls/plasma membranes (Fig. 2K and L).
The localization of de-esterified and methyl-esterified HG differs significantly from the distribution of previously described cell wall components. The level of esterification of the pectic HG influences its functional properties. LM19 recognizes de-esterified HG with a degree of esterification lower than 50 %. For LM20, the binding strength decreased upon de-esterification (Christiaens et al., 2011). LM19 epitopes are abundant in the thickened cell walls, especially at the corners (Fig. 2M). Within 3 months, the fruit exhibited a decrease in fluorescence intensity and the LM19 epitopes are concentrated in the zones of cell adhesion, at the corners and in the anticlinal walls of the epidermal layer (Fig. 2N and 2O). Epitopes of methyl-esterified HG recognized by LM20 were present along whole cell walls of fresh apple fruit, and in the cellular junctions (Fig. 2P). In fruit stored for between 1 and 3 months, the fluorescence signal decreased (Fig. 2Q and 2R). The control reaction performed without the presence of the primary antibody lacked any fluorescence signal (Fig. 2S, T, U).
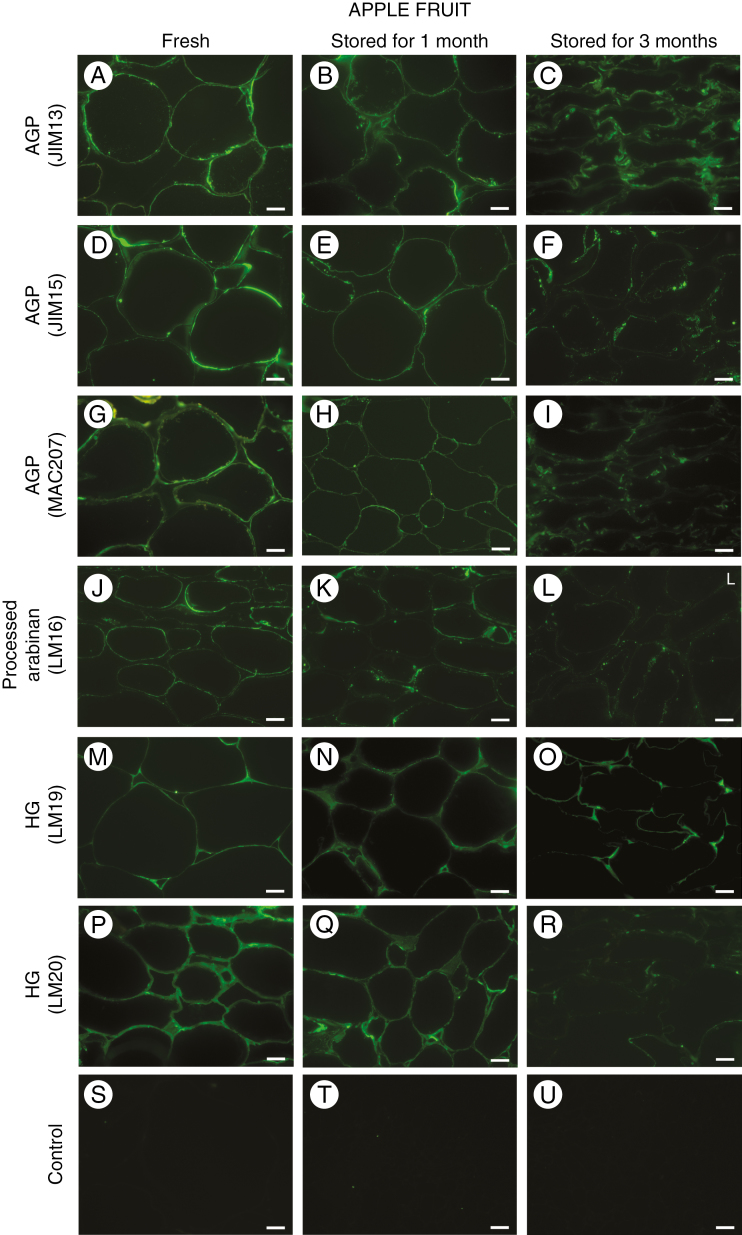
Distribution of AGP and pectin epitopes in the parenchymal tissue of apple fruit during storage
More differences between fresh and stored apple fruit cells were observed in the cells of fruit parenchyma (Fig. 3). The labelling signals after reactions with JIM13, JIM15 and MAC207 mAbs were at their most pronounced in fresh fruit, especially in the plasma membrane and in the border of the cell wall (Fig. 3A, D, G). In the pulp of fruit stored for 1 month, a decline in the fluorescence signal was observed but without significant changes in the localization of AGP epitopes (Fig. 3B, E, H). In fruit stored for 3 months, the AGP epitopes were restricted to damaged cell walls, plasma membrane fragments and cytoplasm compartments (Fig. 3C, F, I).
Fig. 3.
Changes in the localization of AGP and pectin epitopes in parenchymal cell walls of fruit during senescence. Immunofluorescence labelling for AGP epitopes recognized by JIM13 antibody (A, B, C), JIM15 antibody (D, E, F) and MAC207 antibody (G, H, I) in parenchymal tissue from fresh apple fruit and fruit after storage for 1 and 3 months. Immunocytochemistry reactions with LM16 antibody (J, K, L), LM19 antibody (M, N, O) and LM20 antibody (P, Q, R). Control reactions omitting primary antibody (S, T, U). Confocal laser scanning microscopy. Scale bars = 20 µm (A–U).
An epitope recognized by the LM16 antibody was distributed in a similar zone of parenchymous cell as the AGP epitopes. In fresh fruit, the LM16-processed arabinan epitope occurred in the cell wall – plasma membrane continuum (Fig. 3J). However, after storage, this epitope was distributed non-homogeneously in regions of the degraded cell walls and plasma membranes (Fig. 3K and L).
Labelling with the LM19 antibody indicated that de-esterified HG epitopes were located in primary cell walls and at the corners of the cell–cell adhesion zones (Fig. 3M). Moreover, after storage, de-esterified HG epitopes were present within the junctions (Fig. 3N) and after 3 months, they were visible only at the corners of cellular junctions (Fig. 3O).
A different distribution characterized the epitopes of HG with a higher degree of esterification, recognized by LM20. The whole surface of the cell wall of fresh parenchyma was abundant in these kinds of pectin. A strong signal was observed in both the central region of cell wall and the cellular junctions (Fig. 3P and Q). In apple fruit stored for 3 months, the LM20 epitope was dispersed throughout the cell wall and the fluorescence signal monitored was also weaker (Fig. 3R). The control reactions performed without the primary antibody lacked any fluorescence signal (Fig. 3S, T, U).
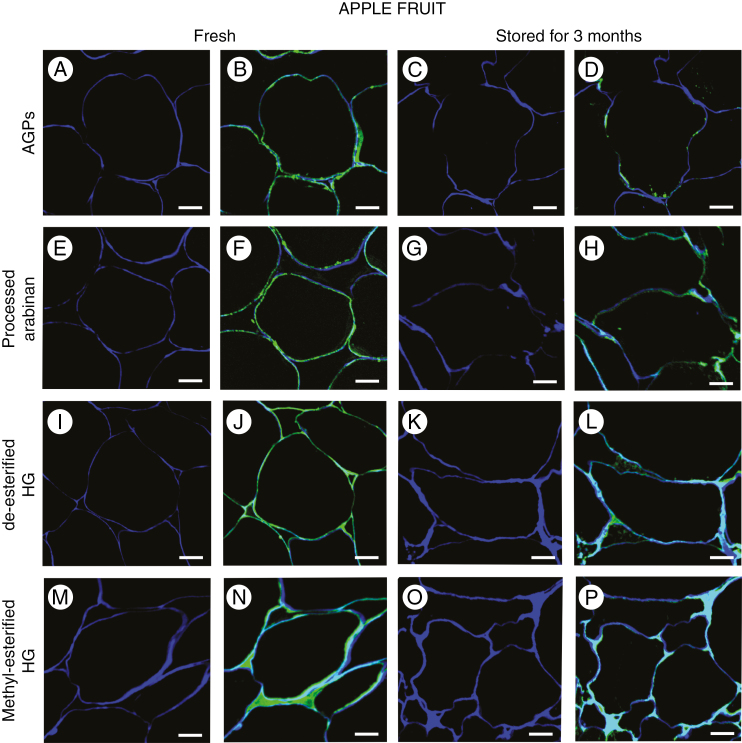
Differences in the location of AGPs, processed arabinan and HG in the cell wall were more noticeable after calcofluor counterstaining, especially in the regions of cell–cell contact (Fig. 4). AGPs and processed arabinan were located mainly in the plasma membrane and at the border of the cell wall (Fig. 4B and F). In stored fruit, both AGPs and arabinan epitopes occurred at lower amounts in the plasma membrane close to the disintegrated structure of the cell wall (Fig. 4D and H). Fluorescence imaging indicated the presence of HGs in intercellular spaces, which distinguishes them from AGPs and arabinan. Furthermore, de-esterified HGs were concentrated mainly at the corners (Fig. 4J and L), whereas methyl-esterified HGs filled the entire intercellular spaces. In apple fruit stored for 3 months, the fluorescence signal was weaker (Fig. 4N and P).
Fig. 4.
Localization of AGP and pectin epitopes in cells of fresh fruit and fruit stored for 3 months. Sections after reactions with antibodies and calcofluor counterstaining: JIM13 (A and B, C and D), LM16 (E and F, G and H), LM19 (I and J, K and L), and LM20 (M and N, O and P). Confocal laser scanning microscopy. Scale bars = 20 µm (A–P).
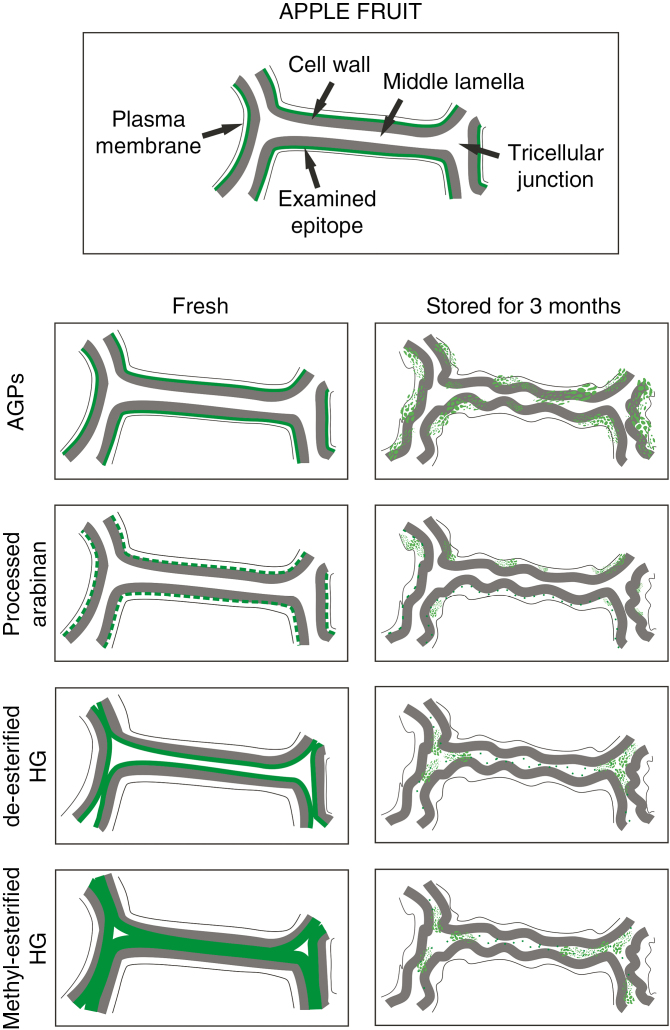
To visualize and summarize all of the results described, we created a schematic representation of the localization of the examined epitopes in fresh apple fruit and the changes which occur during fruit storage (Fig. 5).
Fig. 5.
Schematic representation of the localization of AGP, processed arabinan and HG epitopes in the cell wall of fresh and stored fruit.
Biochemical analysis of cell wall polysaccharides in fruit during storage
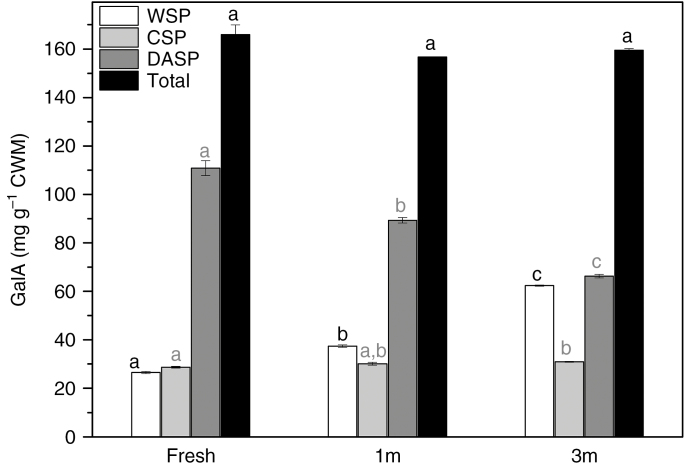
To explain the weaker fluorescence signal in the sections of the stored material, standard chemical analysis of the GalA content of the fruit was carried out for the three stages examined. During fruit storage, the amount of GalA in the WSP and DASP fractions was variable. The WSP fraction contained concentrations varying from 26.48 (±0.46) to 62.38 (±0.24) mg g–1 of GalA in CWM during storage. The level of GalA in the CSP fraction was stable, i.e. approx. 28.64 (±0.38) mg g–1 CWM in fresh fruit and 30.88 (±0.17) mg g–1 CWM in the stored material. These results reveal that the content of GalA in the DASP fraction was higher than in the other fractions examined and significantly decreased after 3 months of storage [from 110.87 (±3.07) to 66.28 (±0.74) mg g–1 CWM]. The observed changes in GalA content are shown in Fig. 6.
Fig. 6.
Content of galacturonic acid in different pectin fractions from apple fruit at three stages: fresh, stored for 1 month (1m) and 3 months (3m). Bars show standard deviation. Different letters in the same colour indicate significant differences between means (α = 0.05).
DISCUSSION
The cell wall is a network composed of polysaccharides and proteins. It is well known that the functions of the components depend on interlinks between them, but their specific distribution in the particular parts of the cell is also crucial for cell wall – plasma membrane properties (Bidhendi and Geitmann, 2016).
The localization of pectins in fruit has mainly been investigated using immunolabelling methods (Ng et al., 2013) and confocal Raman microscopy (Szymańska-Chargot et al., 2016; Chylińska et al., 2017). The present study has shown a distinctive pattern of AGPs, arabinan and HG localization in fresh and stored apple fruit. We have shown that AGP and de-esterified and methyl-esterified pectic polymers are located in different parts of the fruit cell. The AGP and arabinan epitopes have been labelled in the plasma membrane and in the border of the cell wall, and their absence in tricellular junctions excludes AGPs having role in intercellular adhesion in fruit tissue.
During the senescence process, the cell wall undergoes numerous modifications causing fruit softening. A change in the content of pectins in fruit is the major factor influencing cell wall properties and thereby the organization of intercellular spaces and texture of the whole fruit structure (Winisdorffer et al., 2015). The enzymatic degradation of pectins through increased pectin methylesterase expression is connected to the transformation of GalA (Chylińska et al., 2017). Chemical analysis performed on carrot (Cybulska et al., 2015) and apple (Szymańska-Chargot et al., 2016) showed that the GalA content in the DASP fraction decreased, whereas GalA extracted from the CSP and WSP fractions increased during fruit storage. Our results, obtained by calorimetric determination of GalA content, confirm the changes of pectin content in fruit at different times of post-harvest storage. There were substantial differences between the particular pectic fractions, i.e. covalently bonded in DASPs and HG from the middle lamella present in CSPs. The content of the pectin most closely associated with the cell wall (DASPs) decreases during storage. These results are in agreement with the observations of the pectin distribution in cell walls carried out using the immunolabelling technique. In sections of fruit after storage, the fluorescence signal was weaker. Moreover, less intensive fluorescence after JIM13, JIM15 and MAC207 labelling indicates that the glycan moiety of arabinose and galactose sugars of the AGP molecule also undergoes degradation during fruit senescence. The LM16 antibody is a determinant of arabinan metabolism and reveals the enzymatic removal of arabinan side chains from the RG-I backbone (Verhertbruggen et al., 2009). Thus, detection of the (1→5)-α-l-arabinan epitope recognized by the LM16 antibody in regions of senescing fruit tissue indicates that cell wall degradation is ongoing.
Current knowledge provides evidence that AGPs are interlinked with pectins (Immerzeel et al., 2006; Ma et al., 2017). AGP domains contribute to the cell wall architecture by reinforcing the polysaccharide scaffold (Lamport et al., 2006; Hijazi et al., 2014b). The interactions between AGPs and pectins are crucial for the regulation of cell expansion (Griffiths et al., 2014). As is well known, changes in the spatial arrangement of polysaccharides lead to softening of the fruits (Szymańska-Chargot et al., 2016). In our studies, we observed that the distribution of AGP, processed arabinan and HG changes in fruit tissue during post-harvest storage. The increasing irregularity in the content of pectins and in their localization during the senescence process was combined with alterations in the distribution of AGP and arabinan.
Interestingly, our studies show that the assembly of AGPs changed in various way to the distribution of HG. These results indicate the specific direction of AGP ‘movement’ in the senescing fruit tissue. Migration of AGPs through the cell wall has been the subject of many previous studies, but the mechanism of their release from the plasma membrane is still unclear (Knox, 2006; Lamport et al., 2006; Showalter and Basu, 2016; Olmos et al., 2017). The ‘movement’ of AGPs during plasma membrane – cell wall changes is associated with cell wall – membrane disintegration. We hypothesize that the ability of AGPs to detach the glycosylphosphatidylinositol (GPI) anchor from the plasma membrane and its penetration into the extracellular matrix as a signalling molecule may play a role in the maintenance of contact between the cell wall and the plasma membrane of fruit. Due to the breakdown of fruit microstructure, a non-homogeneous distribution of AGPs confirms disturbance to establishment of the cell wall – plasma membrane continuum. The specific arrangement of AGPs, essential for cell wall – plasma membrane links, is crucial for fruit tissue organization.
CONCLUSION
We have used an immunomicroscopy approach to examine cell wall changes at the cellular level based on epitope distribution using cell wall antibodies in fresh and stored apple tissue. The immunocytochemistry reactions with mAbs against AGPs, arabinan and HG showed that the examined polysaccharides and proteoglycans occurred in different zones of the plant cell. Our results are in accordance with the well-known function of pectin in cell–cell adhesion and allow us to propose that AGPs are good candidates for cell wall – plasma membrane anchors.
ACKNOWLEDGEMENTS
The study was partially supported under the project no. 2016/21/N/NZ9/01343 funded by the National Science Centre, Poland. A.L. designed and performed microscopic research, analysed data and wrote the paper, M.C. quantified GalA, and A.Z. helped with manuscript preparation. The authors have no conflicts of interest to declare.
LITERATURE CITED
- Bidhendi AJ, Geitmann A. 2016. Relating the mechanics of the primary plant cell wall to morphogenesis. Journal of Experimental Botany 67: 449–461. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhang L, An H, et al. . 2009. The nanostructure of hemicellulose of crisp and soft Chinese cherry (Prunus pseudocerasus L.) cultivars at different stages of ripeness. LWT: Food Science and Technology 42: 125–130. [Google Scholar]
- Christiaens S, van Buggenhout S, Ngouémazong ED, et al. . 2011. Anti-homogalacturonan antibodies: a way to explore the effect of processing on pectin in fruits and vegetables. Food Research International 44: 225–234. [Google Scholar]
- Chylińska M, Szymańska-Chargot M, Kruk B, Zdunek A. 2016. Study on dietary fibre by Fourier transform-infrared spectroscopy and chemometric methods. Food Chemistry 196: 114–122. [DOI] [PubMed] [Google Scholar]
- Chylińska M, Szymańska-Chargot M, Deryło K, Tchórzewska D, Zdunek A. 2017. Changing of biochemical parameters and cell wall polysaccharides distribution during physiological development of tomato fruit. Plant Physiology and Biochemistry 119: 328–337. [DOI] [PubMed] [Google Scholar]
- Cybulska J, Zdunek A, Kozioł A. 2015. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocolloids 43: 41–50. [Google Scholar]
- Fragkostefanakis S, Dandachi F, Kalaitzis P. 2012. Expression of arabinogalactan proteins during tomato fruit ripening and in response to mechanical wounding, hypoxia and anoxia. Plant Physiology and Biochemistry 52: 112–118. [DOI] [PubMed] [Google Scholar]
- Geitmann A. 2010. Mechanical modeling and structural analysis of the primary plant cell wall. Current Opinion in Plant Biology 13: 693–699. [DOI] [PubMed] [Google Scholar]
- Griffiths JS, Tsai AY, Xue H, et al. . 2014. SALT-OVERLY SENSITIVE5 mediates Arabidopsis seed coat mucilage adherence and organization through pectins. Plant Physiology 165: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon F, Moïse A, Quemener B, et al. . 2017. Remodeling of pectin and hemicellulose in tomato pericarp during fruit growth. Plant Science 257: 48–62. [DOI] [PubMed] [Google Scholar]
- Hijazi M, Velasquez S, Jamet E, Esteves JM, Albenne C. 2014a An update on post-translational modifications of hydroxyproline-rich glycoproteins: toward a model high lighting their contribution to plant cell wall architecture. Frontiers in Plant Science 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi M, Roujol D, Nguyen-Kim H, et al. . 2014. b Arabinogalactan proteins 31 (AGP31), a putative network-forming protein in Arabidopsis thaliana cell walls?Annals of Botany 114: 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang Y, Tan L, et al. . 2016. Nanospherical arabinogalactan proteins are a key component of the high-strength adhesive secreted by English ivy. Proceedings of the National Academy of Sciences USA 113: E3193–E3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immerzeel P, Eppink MM, De Vries SC, Schols HA, Voragen AGJ. 2006. Carrot arabinogalactan proteins are interlinked with pectins. Physiologia Plantarum 128: 18–28. [Google Scholar]
- Jarvis MC. 2011. Plant cell walls; supramolecular assemblies. Food Hydrocolloids 25: 257–262. [Google Scholar]
- Kitazawa K, Tryfona T, Yoshimi Y, et al. . 2013. β-Galactosyl Yariv reagent binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiology 161: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP. 2006. Up against the wall: arabinogalactan-protein dynamics at cell surfaces. New Phytologist 169: 443–445. [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Varnai P. 2013. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist 197: 58–64. [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Kieliszewski MJ, Showalter AM. 2006. Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyse AGP function. New Phytologist 169: 479–492. [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Varnai P, Seal CE. 2014. Back to the future with the AGP-Ca2+ flux capacitor. Annals of Botany 114: 1069–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczuk A, Szczuka E, Wydrych J, Zdunek A. 2018. Changes in arabinogalactan proteins (AGPs) distribution in apple (Malus x domestica) fruit during senescence. Postharvest Biology and Technology 138: 99–106. [Google Scholar]
- Ma Y, Yan C, Li H, et al. . 2017. Bioinformatics prediction and evolution analysis of arabinogalactan proteins in the plant kingdom. Frontiers in Plant Science 8: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JKT, Schröder R, Sutherland PW, et al. . 2013. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biology 13: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos E, De La Garma JG, Gomez-Jimenez MC, Fernandez-Garcia N. 2017. Arabinogalactan proteins are involved in salt0adaptation and vesicle trafficking in tobacco BY-2 cell cultures. Frontiers in Plant Science 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, et al. . 2010. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiology 153: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA. 2011. Extraction and detection of arabinogalactan proteins. The Plant Cell Walls: Methods and Protocols, Methods in Molecular Biology 715: 245–253. [DOI] [PubMed] [Google Scholar]
- Renard CMGC. 2005. Variability in cell wall preparations: Quantification and comparison of common methods. Carbohydrate Polymers 60: 512–522. [Google Scholar]
- Rydahl MG, Kracřun SK, Fangel JU, et al. . 2017. Development of novel monoclonal antibodies against starch and ulvan - implications for antibody production against polysaccharides with limited immunogenicity. Scientific Reports 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. 2007. The biology of arabinogalactan proteins. Annual Review of Plant Biology 58: 137–161. [DOI] [PubMed] [Google Scholar]
- Shi Y, Jiang L, Zhang L, Kang R, Yu Z. 2014. Dynamic changes in proteins during apple (Malus x domestica) fruit ripening and storage. Horticulture Research 1: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. 2001. Arabinogalactan-proteins: structure, expression and function. Cellular and Molecular Life Sciences 58: 1399–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Basu D. 2016. Extensin and arabinogalactan-protein biosynthesis: glycosyltransferases, research challenges, and biosensors. Frontiers in Plant Science 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymańska-Chargot M, Chylińska M, Kruk B, Zdunek A. 2015. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydrate Polymers 115: 93–103. [DOI] [PubMed] [Google Scholar]
- Szymańska-Chargot M, Chylińska M, Pieczywek PM, et al. . 2016. Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 243: 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, et al. . 2013. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. The Plant Cell 25: 270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP, 2009. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydrate Research 344: 1858–1862. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Punge A, Hollopeter G, et al. . 2011. Protein localization in electron micrographs using fluorescence nanoscopy. Nature Methods 8: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Bacic A. 2012. Preparation of plant cells for transmission electron microscopy to optimize immunogold labeling of carbohydrate and protein epitopes. Nature Protocols 7: 1716–1727. [DOI] [PubMed] [Google Scholar]
- Winisdorffer G, Musse M, Quellec S, et al. . 2015. Analysis of the dynamic mechanical properties of apple tissue and relationships with the intracellular water status, gas distribution, histological properties and chemical composition. Postharvest Biology and Technology 104: 1–16. [Google Scholar]
- Yates EA, Valdor J, Haslam SM, et al. . 1996. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139. [DOI] [PubMed] [Google Scholar]
- Zamil MS, Geitmann A. 2017. The middle lamella – more than glue. Physical Biology 14: 1–9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen F, Yang H, et al. . 2010. Changes in firmness, pectin content and nanostructure of two crisp peach cultivars after storage. LWT: Food Science and Technology 43: 226–32. [Google Scholar]