Abstract
Background and Aims
Bird pollination is rare among species in the genus Utricularia, and has evolved independently in two lineages of this genus. In Western Australia, the Western Spinebill, Acanthorhynchus superciliosus, visits flowers of Utricularia menziesii (section Pleiochasia: subgenus Polypompholyx). This study aimed to examine the micromorphology of U. menziesii flowers to assess traits that might be linked to its pollination strategy.
Methods
Light microscopy, histochemistry and scanning electron microscopy were used. Nectar sugar composition was analysed using high-performance liquid chromatography.
Key Results
The flowers of U. menziesii fulfil many criteria that characterize bird-pollinated flowers: red colour, a large, tough nectary spur that can withstand contact with a hard beak, lack of visual nectar guides and fragrance. Trichomes at the palate and throat may act as tactile signals. Spur nectary trichomes did not form clearly visible patches, but were more frequently distributed along vascular bundles, and were small and sessile. Each trichome comprised a single basal cell, a unicellular short pedestal cell (barrier cell) and a multicelled head. These trichomes were much smaller than those of the U. vulgaris allies. Hexose-dominated nectar was detected in flower spurs. Fructose and glucose were present in equal quantities (43 ± 3.6 and 42 ± 3.6 g L–1). Sucrose was only detected in one sample, essentially at the limit of detection for the method used. This type of nectar is common in flowers pollinated by passerine perching birds.
Conclusions
The architecture of nectary trichomes in U. menziesii was similar to that of capitate trichomes of insect-pollinated species in this genus; thus, the most important specializations to bird pollination were flower colour (red), and both spur shape and size modification. Bird pollination is probably a recent innovation in the genus Utricularia, subgenus Polypompholyx, and is likely to have evolved from bee-pollinated ancestors.
Keywords: Australian bladderwort, bird pollination, carnivorous plant, floral micro-morphology, HPLC, Lentibulariaceae, nectary structure, nectar composition, ornithophily, sect. Pleiochasia, spur, trichomes
INTRODUCTION
Most Lentibulariaceae species (genera Pinguicula, Genlisea and Utricularia) are pollinated by insects (Taylor, 1989; Zamora, 1999; Hobbhahn et al., 2006; Fleischmann, 2012; Clivati et al., 2014; Villegas and Alcalá, 2018; Aranguren et al., 2018; Cross et al., 2018; Płachno et al., 2018a). Bird pollination is rare and has probably evolved independently three times in this family; once in Pinguicula (two species from the same section) and twice in Utricularia (three species, two non-related sections). Pinguicula hemiepiphytica Zamudio & Rzed. and the related Pinguicula laueana Speta & F.Fuchs (both from the section Longitubus) have purple-red or red flowers with long spurs and are visited by hummingbirds, and most probably pollinated by them (Lampard et al., 2016). However, these observations can only be verified when the transfer of Pinguicula pollen grains by hummingbirds can be confirmed. According to Taylor (1989), the South American Utricularia quelchii N.E.Br. and Utricularia campbelliana Oliv. (sect. Orchidioides: subgenus Utricularia) have red flowers that can be pollinated by hummingbirds (see photo of U. quelchii flowers in Rodrigues et al., 2017). In the case of U. quelchii, there is no published evidence of hummingbird pollination, but Taylor (1989) inferred this from floral characters. However, in the case of U. campbelliana, there is information from herbarium material that flowers of this species were visited by hummingbirds.
According to Lowrie (2013), Dr Greg Keighery observed a bird, the Western Spinebill, Acanthorhynchus superciliosus Gould, hopping on the ground and visiting and pollinating Utricularia menziesii flowers. Pollen grains of U. menziesii were noted at the base of the bird’s beak. Lambers et al. (2014) also presented this information about bird pollination of U. menziesii as a personal observation by Dr Keighery. Utricularia menziesii is remarkable among species from the subgenus Polypompholyx, because of its red flowers and ‘robust’ spur as compared with insect-pollinated bladderworts (Taylor, 1989). According to Jobson et al. (2017, 2018), in the subgenus Polypompholyx, the red corolla has arisen once (in U. menziesii) from a violet ancestor.
This study aimed to examine the micromorphology of putatively bird-pollinated flowers of U. menziesii. We compared characters of U. menziesii flowers with flowers of insect-pollinated Utricularia species. This is the first report on the flower micro-morphology in U. menziesii.
MATERIALS AND METHODS
Plant material
Flowers of Utricularia menziesii R.Br. were collected from the Alison Baird Reserve (Yule Brook) in Western Australia (Fig. 1A–C) by H.L. and F.J.N. Flowers were fixed in a mixture of 2.5 % (v/v) glutaraldehyde with 2.5 % (v/v) formaldehyde in a 0.05m cacodylate buffer (Sigma; pH 7.2) and sent to Poland for morphological and histochemical studies.
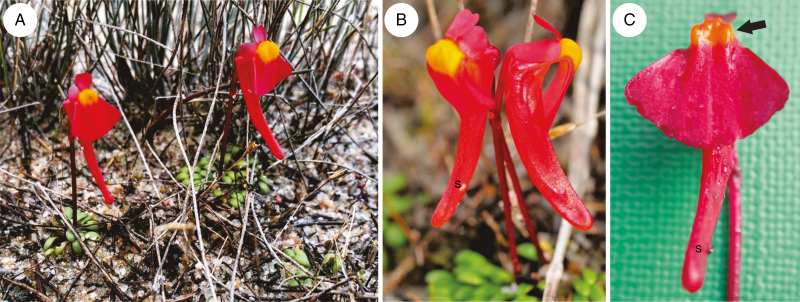
Fig. 1.
General floral morphology of Utricularia menziesii R.Br. (A and B) Utricularia menziesii in a natural habitat, Alison Baird Reserve, in Western Australia; nectar spur(s). (C) General floral morphology; palate (arrow), nectar spur (s).
Floral structure and histochemical investigations
The distribution of the secretory glandular trichomes was determined by examining whole flowers (corollas) using a Nikon SZ100 stereoscopic microscope (Nikon Instruments Europe B.V.). Floral parts, namely spurs, were examined using light microscopy and scanning electron microscopy. Fixed material was washed three times in a 0.1 m sodium cacodylate buffer and post-fixed in a 1 % (w/v) osmium tetroxide solution at room temperature for 1.5 h. Dehydration was achieved using a graded ethanol series, infiltration and embedding using an epoxy embedding medium kit (Fluka). Semi-thin sections (0.9–1.0 μm thick) were prepared for light microscopy, stained for general histology using aqueous methylene blue/azure II (MB/AII) for 1–2 min (Humphrey and Pittman, 1974) and examined with an Olympus BX60 light microscope (Tokyo, Japan).
The hand sections were immersed in water, and analysed with bright field and fluorescence microscopy. Material was tested for lipids, starch and mucilage, using a saturated ethanol solution of Sudan III, aqueous IKI (iodine–potassium iodide) solution and ruthenium red solution, respectively. Autofluorescence of the cuticle was observed under UV light, and the structure of the cuticle was studied on sections stained with auramine O (Gahan, 1984). For scanning electron microscopy, the representative floral parts were fixed (as above) and later dehydrated and subjected to critical-point drying using liquid CO2. Then, they were sputter-coated with gold and examined at an accelerating voltage of 20 kV using a Hitachi S-4700 scanning electron microscope (Hitachi, Tokyo, Japan), in the Institute of Geological Sciences, Jagiellonian University in Kraków.
The trichomes were counted using light microscopy on the inner surface of the spur in three flowers, using NIS elements software 4.0 (Nikon Instruments Europe B.V.). Since the trichomes were more densely distributed alongside vascular bundles, we counted them separately on ten random surfaces of 100 000 μm2 each, near the vascular bundle, and on ten similar surfaces in remaining areas. Subsequently, distribution of the trichomes was recalculated per 1 mm2
Nectar collection and analysis
Flowers from nine individual plants were picked, and nectar from spurs was expressed by gently squeezing up from the bottom of the spur then collecting with a 5 μL capillary tube. The volume of nectar collected was calculated by measuring the length of nectar fluid in the capillary tube (the nectar volume was about 3 μL, n = 9). Nectar was then expelled from the capillary tube onto a 5 mm diameter filter paper disc in a 2 mL Eppendorf tube with the capillary tube stored in the same Eppendorf tube. Samples were stored on dry ice for transport back to the laboratory, where they were transferred to –80 °C until high-performance liquid chromatography (HPLC) analysis.
Sample preparation involved adding 100 μL of Milli-Q water to each Eppendorf tube then rinsing the capillary tube five times before removal of the capillary tube. Tubes were then centrifuged at 8000 rpm for 5 min before transferring the sample to a HPLC vial for analysis of soluble sugars (fructose, glucose and sucrose) as per Slimestad and Vågen (2006). Standards were treated in a similar fashion to the samples and provided full recovery of sugars. The detection limit for this HPLC method, based on the dilutions made to wash sugars from the filter paper, was 4.0 g L–1 for all three sugars.
The HPLC system (Waters, Milford, MA, USA) consisted of a 600E pump, 717plus autosampler, 2998 photo-diode array detector and an Alltech (Deerfield, IL, USA) evaporative light scattering detector (ELSD). Separation was achieved at 25 ± 0.2 °C on a Prevail ES Carbohydrate column (250 × 4.6 mm id with 5 μm packing, Alltech) using a isocratic elution of 70 % acetonitrile in water at 1 mL min−1. Samples in the autoinjector were held at 10 °C, the ELSD drift tube was held at 90 °C and eluent nebulization was with high-purity nitrogen gas at a flow rate of 3.5 L min−1. The sample injection volume was 10 μL, with Empower™2 software (Waters) used for data acquisition and processing. Quantification was based on ELSD response, with calibration curves generated from the power relationship of peak area vs. mass of the standard analyte injected.
RESULTS
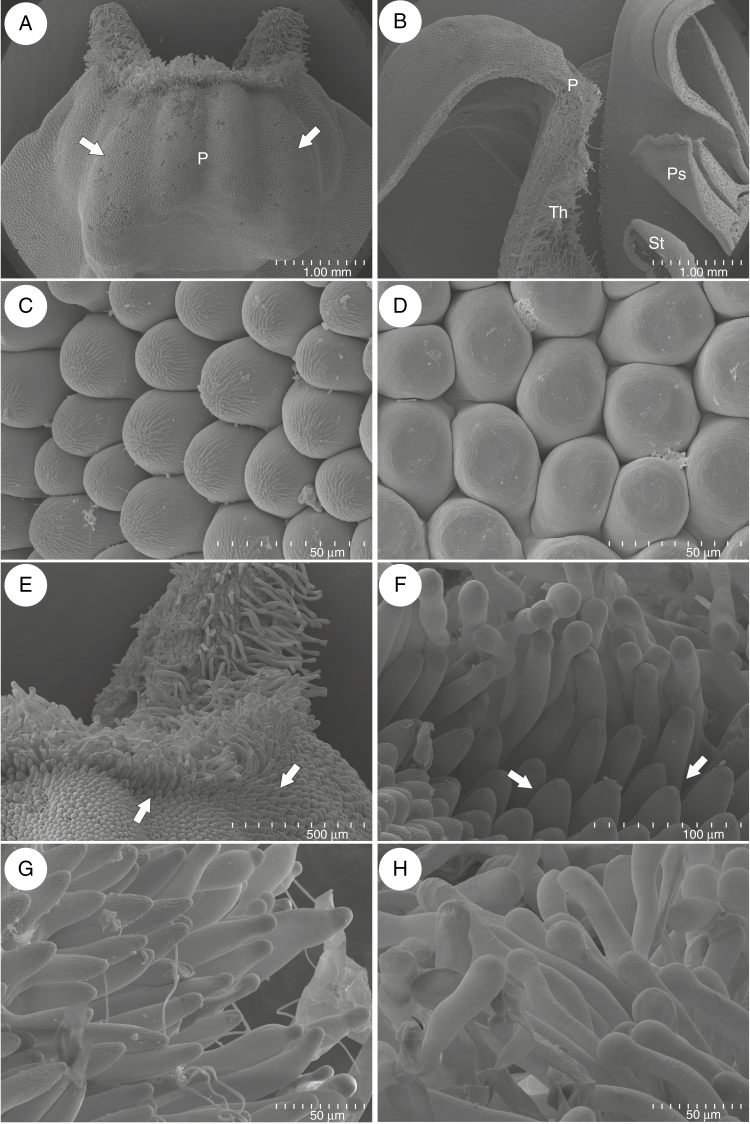
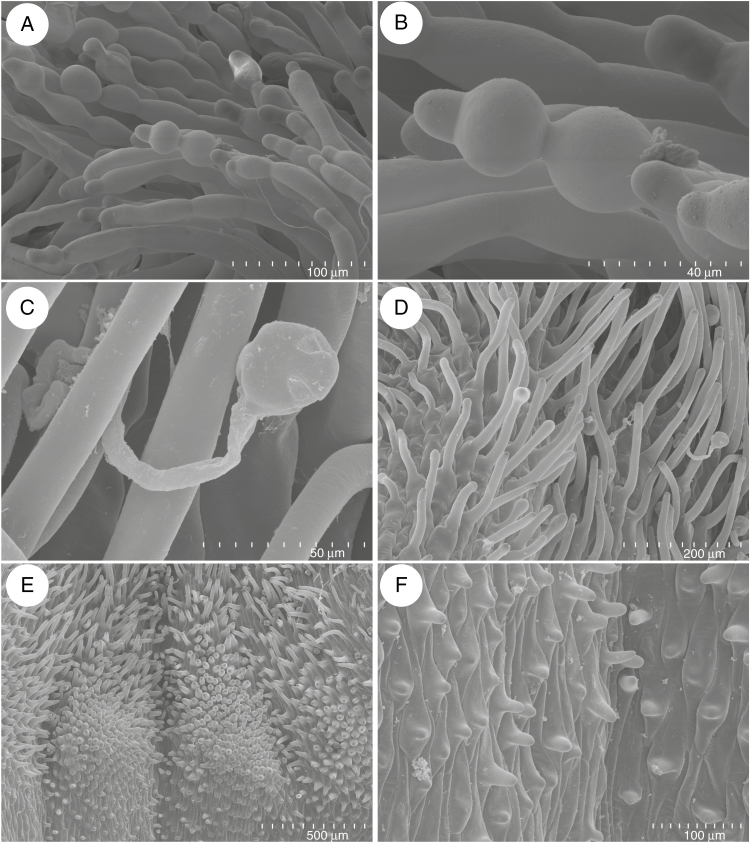
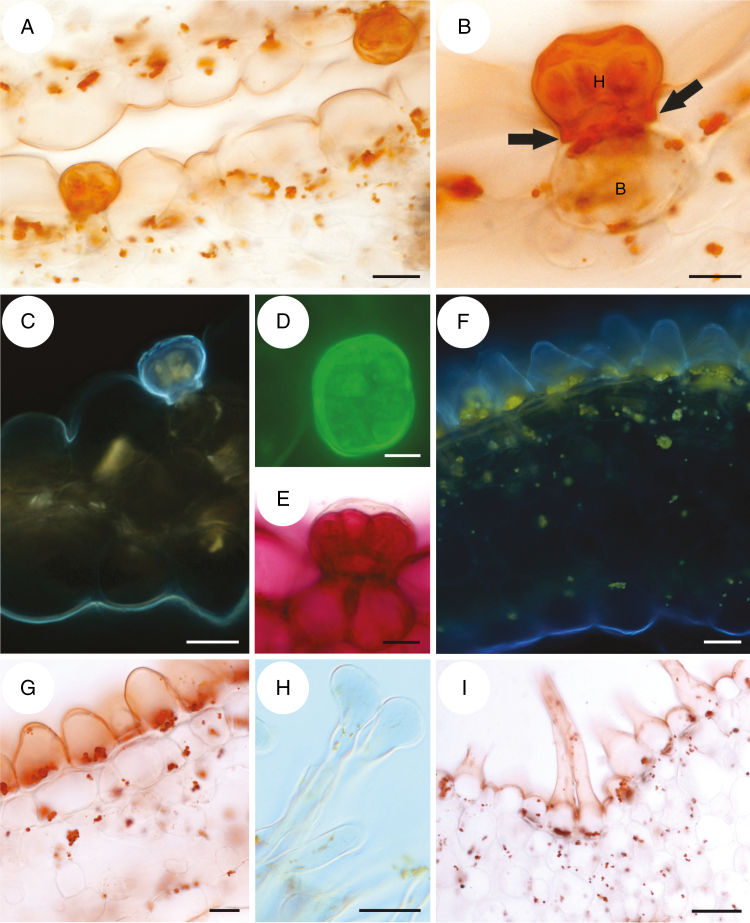
The corolla of Utricularia menziesii was red (Fig 1A–C). A large and prominent yellow/orange palate was at the corolla lower lip base (Figs 1B, C and 2A), which was bordered by a large throat where the pistil and stamens were located (Fig. 2B). The palate had clearly visible parallel ridges; the inner ridges were smaller than the outer ones (Fig. 2A). This part of the palate was covered by epidermal cells (adaxial epidermal surface) with cuticular striations on their apices (Fig. 2C), in contrast to epidermal cells of the remaining part of the lower lip, where these cells had a smooth surface (Fig. 2D). On the palate base, there were papillae (Fig. 2E, F) with cuticular striations on their apices (Fig. 2F, G). Papillae were longer and turned into unicellular trichomes (Fig. 2F, G). Trichomes (near papillae) had cuticular striations on their apices (Fig. 2G), but most of the trichomes had rounded apices and nearly smooth surfaces (Fig. 2H). There were also trichomes that possessed one or more constrictions; thus trichomes had diverse shapes (Fig. 3A, B). Germinating pollen grains were observed among trichomes (Fig. 3C). Trichomes also occurred in the throat (Fig. 3D); however, in the transition between the throat and spur, the trichomes were less abundant and shorter. In this part and in the basal part of the spur, they were transformed to the papillae, which were predominantly distributed on the parallel ridges (Fig. 3E). These papillae had smooth surfaces (Fig. 3F). The spur was the most prominent part of the flower, 2–4 times longer than the lower corolla lip (Figs 1B and 4A). The spur was directed down, more or less parallel to the lower corolla lip. The spur was cylindrical, but laterally compressed (Fig. 2B). In a transverse section, the wall of the spur comprised several cell layers: the internal epidermis, mesophyll and outer epidermis (Fig. 4B). The spur wall was about 99 μm thick (n = 18). The mesophyll cells were non-glandular. Relatively large intercellular spaces occurred between the mesophyll cells. Collateral vascular bundles occurred in the ground mesophyll (Fig. 4B), each containing both xylem and phloem elements. A part of the internal epidermis cells was slightly papillose (Fig. 4C, D). However, in apical parts of the spur, epidermal cells were not papillose (Fig. 4D, E); this is where we found the sessile nectary trichomes (Fig. 4B, F). These trichomes did not form clearly visible patches. Alongside vascular bundles, 58.9 trichomes were distributed, on average (s.d. 1.9), whereas on the remaining surfaces we noted 22.7 trichomes, on average (s.d. 0.9). The mean length of the trichomes was 35.5 μm (s.d. 2.5), and the diameter of the head was 30.4 μm, on average (s.d. 0.9). Each glandular spur trichome comprised a single basal cell, a unicellular short pedestal cell (barrier cell with a thick radial wall impregnated with cutin) and a multicelled head (Fig. 5A, B).
Fig. 2.
Micromorphology of Utricularia menziesii lower corolla lip. (A) Base of lower corolla lip with palate (P); note the clearly visible parallel ridges; the inner ridges were smaller than the outer ones (arrows); scale bar = 1 mm. (B) A part of a section through the flower showing: palate (P), throat (Th), pistil (Ps) and stamen (St); scale bar = 1 mm. (C). Micromorphology of epidermal cells of palate, note cuticular striations; scale bar = 50 μm. (D) Micromorphology of epidermal cells of the lower lip; scale bar = 50 μm. (E and D) Micromorphology of palate base, note papillae (arrows) and trichomes; scale bar = 500 μm and scale bar = 100 μm. (G–H) Trichomes of palate; scale bar = 50 μm.
Fig. 3.
Micromorphology of the palate and throat of a flower of Utricularia menziesii. (A and B) Trichomes that possessed one or more constrictions; scale bar = 100 and 40 μm. (C) Germinated pollen grains among trichomes; scale bar = 50 μm. (D) Trichomes in the throat; scale bar = 200 μm. (E) Accumulations of papillae on the parallel ridges; scale bar = 500 μm. (F) Papillae with smooth surface; scale bar = 100 μm.
Fig. 4.
Anatomy and morphology of Utricularia menziesii spur. (A) Section through flower showing: palate (arrow) and spur (s). (B) General anatomy of the spur showing vascular bundle and nectary trichome (arrow); scale bar = 50 μm. (C and D) Micromorphology of the middle part of a spur; note slightly papillose epidermal cells; scale bar = 500 μm, and scale bar = 100 μm, respectively. (E) Apical part of the spur with nectary trichomes; scale bar = 500 μm. (F) Nectary trichome (N) from the apical part of the spur; scale bar = 40 μm.
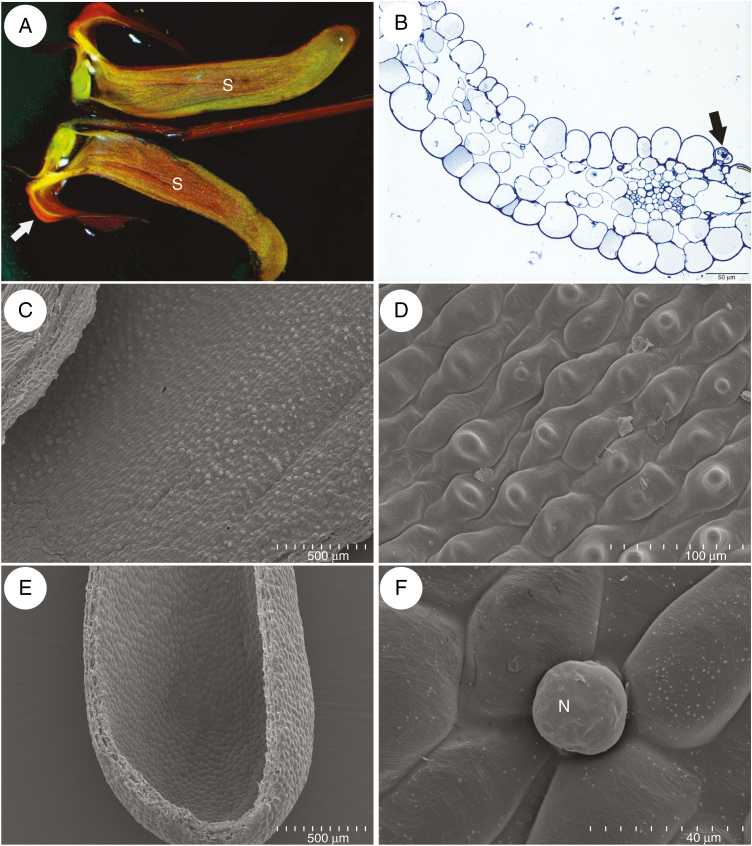
Fig. 5.
Histochemical tests of the spur, palate and throat of a flower of Utricularia menziesii. (A) Section of the apical part of the spur, stained with Sudan III; scale bar = 20 μm. (B) Detail of the structure of a trichome, showing a basal cell (B), barrier cell with cutinized lateral cell walls (arrows) and an eight-cellular head (H); scale bar = 10 μm. (C) Autofluorescence of the cuticle coating the epidermal cells and the trichome; scale bar = 20 μm. (D) Trichome head cells stained with auramine O; scale bar = 10 μm. (E) Trichome stained with ruthenium red; scale bar = 10 μm. (F) Section of the palate, autofluorescence of cuticle and chromoplasts; scale bar = 20 μm. (G) Palate, cuticle and lipids stained with Sudan III; scale bar = 20 μm. (H and I) Unicellular trichomes at the base of the palate. (H) Section showing apices of a trichome; scale bar = 20 μm. (I) Section stained with Sudan III; scale bar = 50 μm.
Histochemical tests indicated that epidermal cells lining the spur interior were coated with a thin layer of cuticle, whereas cell walls of the trichome head cells were covered by a thick cuticle layer (Fig. 5A–D). This cuticle frequently became distended and separated from the cell walls of the head cells of the trichomes (Fig. 5E). Similarly, cells of the external spur epidermis had a well-developed and thick cuticle (Fig. 5C). Epidermal cells contained starch, but starch was not observed in the parenchyma cells. Lipids were present in epidermal cells, in cells of the glandular trichomes and, in lesser amounts, in sub-epidermal parenchyma (Fig. 5A, B). On the surface of the epidermis and the trichomes, mucilage was absent, and ruthenium red stained cell walls exclusively (Fig. 5E). The cuticle coating epidermal cells of the palate and throat was also thin (Fig. 5F–I). These cells enclosed numerous chromoplasts and starch grains. Chromoplasts were less abundant in elongated unicellular trichomes (Fig. 5H, I).
Hexose-dominated nectar was detected in flower spurs. Fructose and glucose were present in equal quantities at 41.6 ± 3.61 g L–1 (n = 9), ranging from 28.9 to 66.4 g L–1 for fructose and from 29.2 to 66.4 g L–1 for glucose. Sucrose was detected in just one sample, but was essentially at the limit of detection with a reported concentration of 4.3 gL–1.
DISCUSSION
In Utricularia species pollinated by dipterans: U. macrorhiza (Honda, 2007), U. vulgaris (Płachno et al., 2018a), and hymenopterans: U. reniformis (Clivati et al., 2014), U. cornigera, U. nelumbifolia (Płachno et al., 2017a), U. foliosa (Płachno et al., 2018a) and U. babui (Chaudhary et al., 2018) there are clearly visible nectar guides (in the form of colour stripes) at the palates. These nectar guides also occur in Utricularia species whose flowers are visited and pollinated by various insects such as bees, butterflies, moths, hawk moths and dipterans (Hobbhahn et al., 2006). In contrast to these insect-pollinated species, Utricularia menziesii flowers lack visual nectar guides on the palate. According to Jobson et al. (2017), U. menziesii is nested in a monophyletic group with U. helix, U. volubilis, U. petertaylorii, U. violacea and U. inaequalis. Utricularia petertaylorii, U. violacea, U. inaequalis and U. helix have nectar guides at the palate (see flower photos in Lowrie, 2013). Utricularia menziesii has a yellow palate, which contrasts strongly with the red corolla. A yellow spot at the palate or a yellow palate is common in Utricularia species from the subgenus Polypompholyx; however, the palate of U. menziesii was very prominent in relation to the size of other parts of the lower lip. The red-coloured corolla of U. menziesii is unique among species from the subgenus Polypompholyx (Jobson et al., 2017, 2018). The red colour is a common character of bird-pollinated flowers (e.g. Proctor and Yeo, 1973; Proctor et al., 1996).
The palate of Lentibulariaceae may play a role as an area of osmophores; if so, there are glandular trichomes at the palate (e.g. U. uniflora and U. paulinae, Płachno et al., 2016; U. cornigera and U. nelumbifolia, Płachno et al., 2017a; U. bremii and U. minor, Płachno et al., 2017b; Genlisea hispidula, Płachno et al., 2018b). In the case of U. menziesii, there are no such glandular trichomes, so the palate in this species probably does not play a role in scent production. However, at the palate and throat of U. menziesii, there are many unicellular trichomes. Similar trichomes occur in U. uniflora and U. dichotoma (Płachno et al., 2016). The histochemical tests indicated that these trichomes did not produce mucilage or proteins, but had some chromoplasts containing lipids. Especially interesting is the occurrence of trichomes with constrictions. We surmise that these trichomes have a role as a tactile signal, but there is no experimental proof so far.
Commonly, bird-pollinated flowers have massive tissues (‘fleshy flowers’) and abundant sclerenchyma or collenchyma tissue, because the birds are potentially destructive pollinators (Cronk and Ojeda, 2008; e.g. occurrence of collenchyma tissue in flowers of bird-pollinated orchids, Stpiczyńska et al., 2004, 2011). Utricularia menziesii has a massive spur, which should be resistant to a bird beak; however, we did not find collenchyma in the spur. The general spur anatomy of U. menziesii is similar to the spur of insect-pollinated species (Płachno et al., 2017a, 2018a). Although the spur of U. menziesii appeared ‘robust’, the spur walls were similar in thickness to those of insect-pollinated Utricularia species, e.g. U. australis, U. bremii and U. foliosa (approx. 100 μm, see Płachno et al., 2018a, their figs 6–8). In both U. menziesii and insect-pollinated Utricularia species, there are collateral vascular bundles in the spurs. Flowers of U. menziesii do not serve as a perch for birds, and Acanthorhynchus superciliosus stays on the ground during flower penetration (Lowrie, 2013) which may explain the lack of collenchyma in the corolla.
In some bird-pollinated flowers, the corolla tube is curved downwards in a similar way to many bird beaks (Cronk and Ojeda, 2008). The U. menziesii spur is directed downwards, thus this fits to the curved A. superciliosus beak. Nectary trichomes of U. menziesii have a similar architecture to capitate nectary trichomes of the insect-pollinated species (Farooq, 1963; Farooq and Siddiqui, 1966; Płachno et al., 2016, 2017a, 2018a). In U. menziesii, as well as in all insect-pollinated Utricularia species examined to date, the nectary trichome had a barrier cell (pedestal cell) with a thick radial wall, which was impregnated with a lipophilic material (positive Sudan test). Because the cuticle became distended and separated from the cell walls of the trichome head cells, this suggests that nectar is accumulated in the subcuticular space. However, the localization of these trichomes in the spur apex might be connected with a mechanism to exclude illegitimate flower visitors. The nectar trichome heads of U. menziesii had a diameter of only 30.4 μm, much smaller than those of the U. vulgaris allies (42–47 μm; Płachno et al., 2018a). There was a different pattern of nectary trichome distribution at the spur surface in Utricularia species: U. vulgaris (on the internal abaxial side of the spur), U. australis (on both the abaxial and adaxial side), U. bremii (on the internal abaxial side) and U. foliosa (in two oblong patches) (Taylor, 1989; Płachno et al., 2018a). In U. menziesii, the nectar trichomes were more frequently distributed along vascular bundles. The close localization of the nectar trichomes near the vascular bundles was noted in U. foliosa (Taylor, 1989; Płachno et al., 2018a) and U. nelumbifolia spurs (Płachno et al., 2017a). Such a localization might be connected with shortening the route of sugar transport from the phloem to the nectary tissue, or to an increased water demand for the nectar production (Płachno et al., 2018a). However, in the spur of U. menziesii, nectary trichomes were quite rare when compared with those of the U. vulgaris allies (Płachno et al., 2018a: their figs 1c, 6c, 7a, 8b).
Abrahamczyk et al. (2017) estimated nectar sugar concentrations and composition for U. alpina Jacq. U. reniformis A.St.Hil. and U. nephrophylla Benj. (sect. Orchidioides); in the first species, only sucrose was found; however, in both U. reniformis and U. nephrophylla, hexose-dominated nectar was detected. All these species were classified by Abrahamczyk et al. (2017) as plants pollinated by bees and wasps. However, pollinators are known only for U. reniformis, i.e. Xylocopa sp. and Bombus sp. (Clivati et al., 2014). The occurrence of sucrose-dominant nectar in U. alpina suggests that large flowers of this species may be pollinated by bees or hummingbirds. Both bee- and hummingbird-pollinated flowers generally have sucrose-dominated nectar (Cronk and Ojeda, 2008). Utricularia menziesii has hexose-dominated nectar. Such a type of nectar was detected in insect-pollinated Utricularia species (U. reniformis); however, it also occurs in flowers pollinated by passerine perching birds (Cronk and Ojeda, 2008). Thus, occurrence of hexose-dominated nectar does not exclude insects as additional flower visitors (pollinators) of U. menziesii. However, there are no observations of insects visiting these flowers.
We showed that the U. menziesii spur exhibited papillae, except at its apex. These papillae had smooth surfaces. Spur papillae have been recorded in various insect-pollinated Utricularia species: papillae with striations were recorded in sect. Lasiocaules: U. dunlopii, sect. Pleiochasia: U. dichotoma (Płachno et al., 2016), sect. Orchidioides: U. reniformis (Clivati et al., 2014), U. nelumbifolia and U. cornigera (Płachno et al., 2017a). In sect. Utricularia, papillae with striations occur in U. bremii and U. foliosa, whereas exclusively smooth papillae occur in U. australis and U. vulgaris (Płachno et al., 2018a).
The bird–flower interactions are evolutionarily old. Mayr and Wilde (2014) described the fossil evidence of flower visiting by birds which is from the Middle Eocene (47 Ma). According to Jobson et al. (2017), the lineage containing U. menziesii and its common ancestor probably appeared in the Pliocene; thus, it seems that bird pollination is a young apomorphy in the genus Utricularia, subgenus Polypompholyx. Relatives of U. menziesii have flowers with characters adapted to bee pollination. Thus, we assume that the plesimorphic flower was bee pollinated and the U. menziesii lineage has evolved from this condition to a flower adapted to bird pollination.
Conclusion
The flowers of U. menziesii fulfilled many of the criteria that characterize bird-pollinated flowers: a red colour, a large, tough nectary spur, which can withstand contact with a hard beak, and a lack of visual nectar guides and fragrance. The architecture of the nectary trichomes in U. menziesii is similar to that of trichomes of insect-pollinated species in this genus; thus, the most important specializations to bird pollination in this species were flower colour change to red, and both spur shape and size modifications.
ACKNOWLEDGEMENTS
Sincere thanks are due to André Jardim for permission to use one of his photos. There is no conflict of interest.
LITERATURE CITED
- Abrahamczyk S, Kessler M, Hanley D, et al. . 2017. Pollinator adaptation and the evolution of floral nectar sugar composition. Journal of Evolutionary Biology 30: 112–127. [DOI] [PubMed] [Google Scholar]
- Aranguren Y, Płachno BJ, Stpiczyńska M, Miranda VFO. 2018. Reproductive biology and pollination of the carnivorous Genlisea violacea (Lentibulariaceae). Plant Biology 20: 591–60. [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Yadav SR, Tandon R. 2018. Delayed selfing ensures reproductive assurance in Utricularia praeterita and Utricularia babui in Western Ghats. Journal of Plant Research 131: 599–610. [DOI] [PubMed] [Google Scholar]
- Clivati D, Cordeiro GD, Płachno BJ, Miranda VFO. 2014. Reproductive biology and pollination of Utricularia reniformis A.St.-Hil. (Lentibulariaceae). Plant Biology 16: 677–682. [DOI] [PubMed] [Google Scholar]
- Cronk Q, Ojeda I. 2008. Bird-pollinated flowers in an evolutionary and molecular context. Journal of Experimental Botany 59: 715–727. [DOI] [PubMed] [Google Scholar]
- Cross AT, Davis AR, Fleischmann A, et al. . 2018. Reproductive biology and pollinator–prey conflicts. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 294–313. [Google Scholar]
- Farooq M. 1963. Trichomes on the floral parts of Utricularia. Journal of the Indian Botanical Society 45: 242–248 [Google Scholar]
- Farooq M, Siddiqui SA. 1966. Trichomes on the flowers of Utricularia. Beiträge zur Biologie der Pflanzen 42: 353–361 [Google Scholar]
- Fleischmann A. 2012. Monograph of the genus Genlisea. Poole, UK: Redfern Natural History. [Google Scholar]
- Gahan PB. 1984. Plant histochemistry and cytochemistry: an introduction. London: Academic Press. [Google Scholar]
- Hobbhahn N, Küchmeister H, Porembski S. 2006. Pollination biology of mass flowering terrestrial Utricularia species (Lentibulariaceae) in the Indian Western Ghats. Plant Biology 8: 791–804. [DOI] [PubMed] [Google Scholar]
- Honda M.2007. http://www.honda-e.com/CP_ARTICLES/UmacrorhizaPollinator.htm
- Humphrey C, Pittman G. 1974. A simple methylene blue–azure II–basic fuchsin for epoxy-embedded tissue sections. Stain Technology 49: 9–14. [DOI] [PubMed] [Google Scholar]
- Jobson RW, Baleeiro PC, Reut M. 2017. Molecular phylogeny of subgenus Polypompholyx (Utricularia; Lentibulariaceae) based on three plastid markers: diversification and proposal for new section. Australian Systematic Botany 30: 259–278. [Google Scholar]
- Jobson RW, Baleeiro PC, Guisande C. 2018. Systematics and evolution of Lentibulariaceae: III. Utricularia. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 89–104. [Google Scholar]
- Lambers H, Shane MW, Laliberté E, Swarts ND, Teste FP, Zemunik G. 2014. Plant mineral nutrition. In: Lambers, H, ed. Plant life on the sandplains in southwest Australia, a global biodiversity hotspot. Crawley: UWA Publishing, 101–127. [Google Scholar]
- Lampard S, Gluch O, Robinson A, et al. . 2016. Pinguicula of Latin America. Poole, UK: Redfern Natural History. [Google Scholar]
- Lowrie A. 2013. Carnivorous plants of Australia Magnum Opus. Poole, UK: Redfern Natural History. [Google Scholar]
- Mayr G, Wilde V. 2014. Eocene fossil is earliest evidence of flower-visiting by birds. Biology Letters 10: 20140223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Świątek P, Davies KL. 2016. Floral micromorphology of the Australian carnivorous bladderwort Utricularia dunlopii, a putative pseudocopulatory species. Protoplasma 253: 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Davies KL, Świątek P, Miranda VFO. 2017a. Floral ultrastructure of two Brazilian aquatic-epiphytic bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae). Protoplasma 254: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Krajewski Ł, Świątek P, Adamec L, Miranda VFO. 2017b. Flower palate structure of the aquatic bladderworts Utricularia bremii Heer and U. minor L. from section Utricularia (Lentibulariaceae). Protoplasma 254: 2007–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Adamec L, Miranda VFO, Świątek P. 2018a. Nectar trichome structure of aquatic bladderworts from the section Utricularia (Lentibulariaceae) with observation of flower visitors and pollinators. Protoplasma 255: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P, Stpiczyńska M, Miranda VFO. 2018b. Flower palate ultrastructure of the carnivorous plant Genlisea hispidula Stapf with remarks on the structure and function of the palate in the subgenus Genlisea (Lentibulariaceae). Protoplasma 255: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor M, Yeo P. 1973. The pollination of flowers. London: Collins. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Timber Press. [Google Scholar]
- Rodrigues FG, Marulanda NF, Silva SR, Płachno BJ, Adamec L, Miranda VFO. 2017. Phylogeny of the ‘orchid-like’ bladderworts (gen. Utricularia sect. Orchidioides and Iperua: Lentibulariaceae) with remarks on the stolon–tuber system. Annals Botany 120: 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimestad R, Vågen IM. 2006. Thermal stability of glucose and other sugar aldoses in normal phase high performance liquid chromatography. Journal of Chromatography A 1118: 281–284. [DOI] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. 2004. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Annals of Botany 93: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Kamińska M. 2011. Comparative anatomy of the nectary spur in selected species of Aeridinae (Orchidaceae). Annals of Botany 107: 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. 1989. The genus Utricularia: a taxonomic monograph. Kew Bulletin Additional Series 14: 1–724. [Google Scholar]
- Villegas SG, Alcalá R. 2018. Reproductive ecology of the carnivorous plant Pinguicula moranensis (Lentibulariaceae). Plant Biology 20: 205–212. [DOI] [PubMed] [Google Scholar]
- Zamora R. 1999. Conditional outcomes of interactions: the pollinator–prey conflict of an insectivorous plant. Ecology 80: 786–795. [Google Scholar]