Abstract
Background and Aims
Substantial evidence supports the hypothesis that morphophysiological dormancy (MPD) is the basal kind of seed dormancy in the angiosperms. However, only physiological dormancy (PD) is reported in seeds of the ANA-grade genus Nymphaea. The primary aim of this study was to determine the kind of dormancy in seeds of six species of Nymphaea from the wet–dry tropics of Australia.
Methods
The effects of temperature, light and germination stimulants on germination were tested on multiple collections of seeds of N. immutabilis, N. lukei, N. macrosperma, N. ondinea, N. pubescens and N. violacea. Embryo growth prior to hypocotyl emergence was monitored.
Key Results
Germination was generally <10 % after 28 d in control treatments. Germination percentage was highest at 30 or 35 °C for seeds exposed to light and treated with ethylene or in anoxic conditions in sealed vials of water, and it differed significantly between collections of N. lukei, N. macrosperma and N. violacea. Seeds of N. pubescens did not germinate under any of the conditions. Embryo growth (8–37 % in length) occurred before hypocotyl emergence (germination) in seeds of the five species that germinated.
Conclusions
Fresh seeds were dormant, and the amount of pregermination embryo growth in seeds of N. lukei and N. immutabilis was relatively small, while in seeds of N. macrosperma, N. ondinea and N. violacea it was relatively large. Thus, seeds of N. lukei and N. immutabilis had PD and those of N. macrosperma, N. ondinea and N. violacea had MPD. Overall, we found that seeds in the most phylogenetically derived clades within Nymphaea have MPD, suggesting that PD is the most likely basal trait within the Nymphaeales. This study also highlights the broad range of dormancy types and germination strategies in the ANA-grade angiosperms.
Keywords: Aquatic plants, basal angiosperms, embryo growth, morphophysiological dormancy, Nymphaeaceae, physiological dormancy, seed germination, wet–dry tropics
INTRODUCTION
Seed dormancy, defined as the restriction of germination under otherwise favourable conditions (Baskin and Baskin, 2004), allows seeds to survive periods of environmental stochasticity, maximizes the chances of seedling survival and reduces competition between siblings (Fenner and Thompson, 2005; Baskin and Baskin, 2014). Of the five classes of seed dormancy described by Baskin and Baskin (2004), morphophysiological dormancy (MPD) is considered to be the most ancestral type within the angiosperms (Baskin and Baskin, 2004; Willis et al., 2014; Fogliani et al., 2017). Seeds with morphological dormancy (MD) contain embryos that are differentiated, i.e. cotyledons and radicle can be distinguished, but they are underdeveloped and must grow prior to radicle emergence (Baskin and Baskin, 2004). Furthermore, in addition to MD seeds with MPD also have physiological dormancy (PD) (Baskin and Baskin, 2004).
Embryo morphology and development are ultimately linked to the different kinds of seed dormancy, and it is generally accepted that the rudimentary embryo is a basal seed trait in angiosperms and that embryo size has increased over geological time (Martin, 1946; Nikolaeva, 1977; Forbis et al., 2002; Finch-Savage and Leubner Metzger, 2006; Baskin and Baskin, 2014; Willis et al., 2014; Friis et al., 2015). Studies on seeds of both extant and extinct members of the ANA-grade angiosperms (Amborella, Nymphaeales and Austrobaileyales) revealed that seeds of Amborella and Austrobaileyales have underdeveloped embryos, and thus MPD or MD. However, except for seeds of Trithuria (Hydatellaceae) that possess an undifferentiated embryo and a specialized type of MPD (Rudall et al., 2009; Tuckett et al., 2010b; Fogliani et al., 2017; Losada et al., 2017), seeds of species of the Nymphaeales have been exclusively reported as having a fully developed (broad) embryo and PD (Martin, 1946; Baskin and Baskin, 2007).
Nonetheless, with the exception of the study by Baskin and Baskin (2007), empirical studies investigating whether embryos of seeds from the Nymphaeaceae grow prior to radicle emergence are absent. Baskin and Baskin (2007) examined seeds of nursery-grown plants of five members of Nymphaea (N. capensis var. zanzibariensis, N. immutabilis, N. mexicana, N. micarantha and the hybrid N. ‘Albert Greenburg’) for evidence of embryo growth. Seeds of the three species that germinated contained differentiated embryos that increased only a little in length (3–14 %) prior to germination, which was not considered sufficient to warrant classifying the embryos as underdeveloped. Thus, the authors concluded that seeds of Nymphaea have only PD. These data were also considered in a recent study investigating the evolutionary context of MPD in Amborella (Fogliani et al., 2017), which proposed that the transition from MPD to PD occurred in the common ancestor of the Cambombaceae and Nymphaeaceae, while also confirming support for MPD as basal.
The five taxa of Nymphaea studied by Baskin and Baskin (2007) represent <5 % of the total species richness within the genus Nymphaea, from across a small part of the otherwise wide geographical range of this cosmopolitan genus (Conard, 1905; Löhne et al., 2008b). Thus, defining the dormancy and germination traits of species of Nymphaea more broadly and including species from environmentally unpredictable habitats where dormancy is central to regenerative success will strengthen our understanding of the evolution of seed dormancy in ANA-grade angiosperms.
In Australia, 18 species of Nymphaea occur throughout the wet–dry tropics in the northern part of the continent (Löhne et al., 2009; Jacobs and Hellquist, 2011). This region is characterized by hot, wet monsoonal summers and warm, dry winters (Finlayson, 1999). Rainfall in the region is influenced by sporadic, tropical low-pressure systems and cyclones, which yield intermittent and often unpredictable amounts of precipitation (Taylor and Tulloch, 1985; Bureau of Meteorology, 2011). Therefore, many wetlands in the wet–dry tropics are transient or ephemeral and experience significant fluctuations in water level and temperature (Cowie et al., 2000). Nymphaea species occupy a diverse range of these habitats in northern Australia, including perennial and ephemeral wetlands (Dalziell, 2016).
Our aim in this study was to identify the kind of dormancy and the germination requirements of representative species of Nymphaea from Australia to determine whether MD and/or MPD are present in extant members of the genus. Specifically, we addressed the following questions: (1) Are seeds water-permeable? (2) What are the optimal conditions for germination? (3) Do embryos grow under conditions considered optimal for germination? (4) Do seeds of the same species collected in different locations (collections) exhibit the same dormancy and germination responses?
MATERIALS AND METHODS
Species selection, seed collection and assessment of seed characteristics
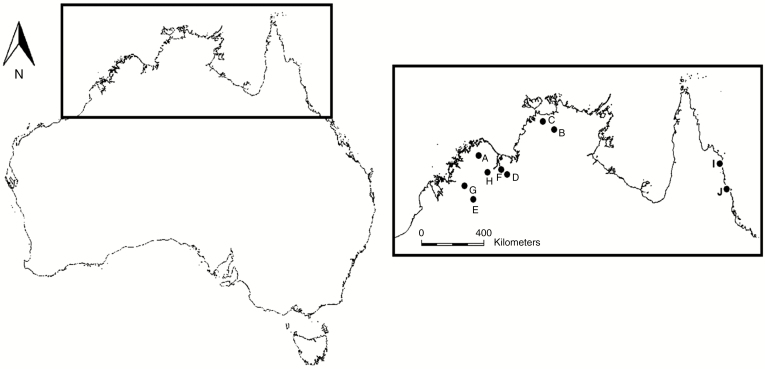
Seeds of N. immutabilis, N. lukei, N. macrosperma, N. ondinea, N. pubescens and N. violacea were collected from wild populations over a 2-year period between March 2011 and August 2013 from multiple locations across northern Australia (Fig. 1). These species represent three of the four subgenera of Nymphaea (Anecphya, Confluentes and Lotos) in Australia and the diversity of habitat types across the entire distribution of Nymphaea in Australia (Table 1). For example, N. ondinea is restricted to a small area of north-west Australia in ephemeral, sandstone-based creeks of ultra-oligotrophic water, while N. violacea is found across northern Australia and was collected from both ephemeral and permanent waterways comprising a range of water qualities. Multiple collections were made of N. lukei, N. macrosperma and N. violacea to examine among-population variation (if any) in dormancy traits and germination responses.
Fig. 1.
Collection locations for Nymphaea seeds used in this study: (A) N. ondinea (NO1), N. violacea (NV1, NV2, NV3, NV6), (B) N. pubescens (NP1), N. violacea (NV4), (C) N. macrosperma (NM1), (D) N. violacea (NV5), (E) N. lukei (NL1), (F) N. macrosperma (NM2, NM3), N. violacea (NV7), (G) N. lukei (NL2), (H) N. violacea (NV8, NV9), (I) N. immutabilis (NI1), and (J) N. violacea (NV10).
Table 1.
Seed collection details for all seeds used in experiments in this study
| Species | Subgenus | Endemism | Collection | State | Collection location (GDA 94) | Habitat | Date | Dried | Mean rainfall (mm, ± s.e.) | Mean minimum temperature (°C, ±s.e.) | Mean maximum temperature (°C, ±s.e.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nymphaea immutabilis | Anecphya | Cosmopolitan | NI1 | QLD | S15°25′35.7″, E145°9′14.5″ | Large, permanent swamp (Barrett’s Lagoon) | Jul-13 | Y | 47.8 ± 25.2 | 21.5 ± 0.9 | 26.8 ± 0.4 |
| Nymphaea lukei | Confluentes | Endemic WA | NL1 | WA | S17°34′47.6″ E126°04′59.6″ | Small, ephemeral, sandstone based creek | May-12 | N | 90.5 ± 90.5 | 18.3 ± 3.3 | 33.9 ± 1.2 |
| NL2 | WA | S16°43′0.2″, E125°27′35.8″ | Small, semi-permanent, sandstone-based creek | Jun-13 | N | 31.4 ± 16.9 | 17.9 ± 1.6 | 31.0 ± 0.9 | |||
| Nymphaea macrosperma | Anecphya | Cosmopolitan | NM1 | NT | S12°42′47.9″, E131°38′17.7″ | Permanent river oxbow (Mary River) | Jun-11 | Y | 55.9 ± 55.9 | 20.1 ± 1.9 | 31.9 ± 0.8 |
| NM2 | WA | S15°35′35.7″, E128°16′47.9″ | Ephemeral creek running into permanent swamp | May-12 | N | 98.2 ± 97.0 | 21.7 ± 2.3 | 33.9 ± 0.8 | |||
| NM3 | WA | S15°35′35.7″, E128°16′47.9″ | Ephemeral creek running into permanent swamp | May-13 | N | 40.1 ± 20.1 | 23.5 ± 1.3 | 36.1 ± 0.7 | |||
| Nymphaea ondinea | Confluentes | Endemic WA | NO1 | WA | S14°48′30.1″, E126°33′01.1″ | Small, ephemeral, sandstone-based creek | Mar-11 | N | 485.0 ± 159.5 | 22.4 ± 0.1 | 30.8 ± 0.2 |
| Nymphaea pubescens | Lotos | NT, QLD | NP1 | NT | S12°53′27.8″, E132°30′59.7″ | Large permanent swamp (Yellow Water, Kakadu National Park) | Jun-11 | Y | 55.9 ± 55.9 | 20.1 ± 1.9 | 31.9 ± 0.8 |
| Nymphaea violacea | Confluentes | Cosmopolitan | NV1 | WA | S14°46′33.1″ E126°30′52.6″ | Small permanent swamp | May-11 | N | 151.6 ± 77.8 | 17.7 ± 2.9 | 29.7 ± 0.4 |
| NV2 | WA | S14°47′18.8″, E126°31′47.5″ | Ephemeral creek | May-11 | N | 151.6 ± 77.8 | 17.7 ± 2.9 | 29.7 ± 0.4 | |||
| NV3 | WA | S14°48′23.9″, E126°31′47.5″ | Ephemeral swamp | May-11 | N | 151.6 ± 77.8 | 17.7 ± 2.9 | 29.7 ± 0.4 | |||
| NV4 | NT | S12°53′27.8″, E132°30′59.7″ | Large permanent swamp (Yellow Water, Kakadu National Park) | Jun-11 | N | 38.4 ± 38.4 | 15.7 ± 2.7 | 29.7 ± 1.1 | |||
| NV5 | WA | S15°56′46.5″, E128°56′56.6″ | Ephemeral creek | Jun-11 | N | 55.9 ± 55.9 | 20.1 ± 1.9 | 31.9 ± 0.8 | |||
| NV6 | WA | S14°46′33.1″, E126°30′52.6″ | Small permanent swamp | Mar-12 | N | 336.1 ± 92.0 | 17.4 ± 2.8 | 31.1 ± 0.9 | |||
| NV7 | WA | S15°35′35.7″, E128°16′47.9″ | Ephemeral creek running into permanent swamp | May-12 | N | 98.2 ± 97.0 | 21.8 ± 2.1 | 33.3 ± 0.9 | |||
| NV8 | WA | S15°51′39.8″, E127°14′20.8″ | Small, ephemeral creek | Jun-13 | N | 34.1 ± 18.3 | 17.9 ± 1.6 | 31.0 ± 0.9 | |||
| NV9 | WA | S15°51′39.8″, E127°14′21.0″ | Small, ephemeral creek | Jun-13 | N | 34.1 ± 18.3 | 17.9 ± 1.6 | 31.0 ± 0.9 | |||
| NV10 | QLD | S16°54′55.3″, E145°22′20.6 | Small, ephemeral wetland adjacent to roadside | Jul-13 | Y | 8.9 ± 3.1 | 16.7 ± 0.8 | 25.7 ± 0.6 |
Cosmopolitan species are common throughout Australia, while endemic species are restricted to Western Australia (WA), the Northern Territory (NT) or Queensland (QLD). The majority of seed collections were kept damp during transport, except collections NI1 and NV10, which were spread in a thin layer and sun dried for 10 d, and NM1, NP1 and NV4, which were dried over silica gel for 14 d prior to transport. Rainfall and temperature data were obtained from the nearest Bureau of Meteorology weather station and averaged for the three months prior to seed collection.
Mean monthly minimum and maximum temperatures and rainfall for each collection site were obtained from the nearest Australian Bureau of Meteorology weather station for the three months prior to seed collection (Bureau of Meteorology, 2005, 2011, 2013). Mature seeds were collected at the time of natural dispersal, i.e. the pedicle had coiled and receded below the water line, fruits were dehiscing, and individual seeds were dark in colour with a fully intact aril. Unless otherwise stated (Table 1), whole seed heads were collected, kept moist and transported to Perth, Western Australia, in Glad® Snap Lock® bags. Upon arrival, seed heads were placed in shallow pans of water at room temperature (c. 24 °C) to allow fruits to dehisce naturally, except for collections of seeds that were cleaned in the field and dried for transport, which remained dry prior to experimentation. Sieves were used to separate the seeds from remaining plant material. Seeds were then blotted dry with paper towel, and initial seed fill was determined by X-ray analysis (Faxitron Specimen Radiography System MX-20 Cabinet, Tucson, AZ, USA). Seed viability was confirmed via a cut test (ISTA, 2017), whereby three replicates of 20 seeds were cut lengthways with a scalpel blade and visually inspected. Seeds with a fully formed, turgid, white embryo (lacking any conspicuous browning or necrotic areas) and endosperm/perisperm were considered viable. The remaining seeds were then passed through an aspirator (SELECTA BV Gravity Seed Separator ‘Zig Zag’, Netherlands) to separate filled and empty seeds. Once this operation was complete, seeds were placed back into pans of water at room temperature until the start of experiments (up to 2 weeks). Seed length and width were measured on 50 randomly chosen seeds of each collection using a dissecting microscope (Olympus SZX16, Japan) equipped with a camera and digital micrometer. Five replicates of 50 filled, intact seeds were weighed to estimate individual seed mass, which was then multiplied by 1000 to obtain 1000-seed mass.
Water uptake (imbibition)
To determine if seeds were water-permeable, three replicates of ≥0.05 g of pre-dried small seeds (or 15–25 individual large seeds) of each species were weighed (initial seed mass, at t0) and then placed on a glass filter paper (84 mm; Advantec, Japan) inside a Petri dish under standard laboratory temperatures (approx. 22 °C). The filter paper was irrigated with reverse-osmosis (RO) water, and at each pre-determined time interval (1, 2, 5 and 30 min and 1, 2, 4, 6, 8, 24, 48, 72 and 96 h) seeds were removed from the Petri dish, blotted dry, weighed and placed back onto the moistened filter paper. Percentage moisture uptake was determined using the following equation:
where Wi is the mass of imbibed seeds at any given time (tx) and Wd is the initial mass at t0. For an additional three replicates of ≥0.05 g of seeds, the seed coat of each seed from each collection was scarified with a scalpel along the longitudinal axis and subjected to the same imbibition procedure.
Seed germination
The exogenous application of gibberellins to seeds with PD may be used to distinguish between non-deep and deep PD, whereby germination is either stimulated or not, respectively (Baskin and Baskin, 2004; Fogliani et al., 2017). Assuming seeds of Nymphaea have a PD component to their dormancy, the application of gibberellic acid (GA3) may promote germination. Additionally, the application of ethylene has been shown to promote germination in seeds of Nymphaea and is postulated to be produced when many individual seeds are crowded together under anoxic conditions (Else and Riemer, 1984). To determine the type of dormancy and assess the germination responses in the 18 collections of the six species of Nymphaea, we constructed a full-factorial experimental design to test the effects of GA3, ethephon and crowding vs. no treatment on germination in light/dark (12/12 h) at 10, 15, 20, 25, 30, 35, 40 °C and 20/35 °C (12/12 h). Additionally, seeds of N. immutabilis (NI1), N. lukei (NL2), N. macrosperma (NM1), N. ondinea (NO1), N. pubescens (NP1) and N. violacea (NV3) were subjected to the same germination stimulant × temperature design in total darkness for the duration of the trial. Each treatment consisted of four replicates of 25 seeds, except for the crowding treatment which consisted of one tube of 50 seeds. All seeds were used in germination experiments within 28 d of collection.
Prior to testing, fresh seeds were sterilized in a 2 % (w/v) solution of calcium hypochlorite (Ca(OCl)2) with two drops of surfactant (Tween 80) for 30 min under alternating vacuum (10 min on/off/on) and then rinsed three times in sterile RO water. For control treatments, seeds were sown in 90-mm-diameter, sterile Petri dishes containing sterile 0.7 % (w/v) water agar, while the GA3 treatment consisted of water agar containing 0.29 mm GA3 (Sigma, Australia). The ethephon treatment was administered by placing seeds inside a nylon mesh bag and immersing it in a 20 mm solution (Ethrel® Bayer Crop Science, Australia) for 24 h (at room temperature, approx. 24 °C) prior to sowing the seeds on 0.7 % (w/v) water agar. All Petri dishes were wrapped four times in polyethylene film to prevent excessive evaporation. To test the effect of crowding on germination, seeds were placed in a 10-mL plastic tube (TechnoPlas, Australia) filled with 10 mL of sterile RO water. For dark treatments, the Petri dishes or the 10-mL tubes containing seeds were immediately doubly wrapped with aluminium foil and placed in a light-excluding box for the duration of the experiment. For the light treatment, seeds were incubated in alternating light/dark conditions under a daily 12-h photoperiod of 30 μmol m−−2 s−1, 400–700 nm, cool white fluorescent light.
Seeds of N. immutabilis (NI1), N. lukei (NL2), N. macrosperma (NM1), N. ondinea (NO1), N. pubescens (NP1) and N. violacea (NV3) incubated in the presence of light were scored daily for germination for the first 2 weeks and weekly thereafter for a total of 100 d. Dark treatments were only scored after 8 weeks. All other species and collections were scored daily for germination for the first 2 weeks and weekly thereafter for an additional 6 weeks. Seeds were considered to have germinated upon hypocotyl (sensuHaines and Lye, 1975) emergence ≥2 mm. At the conclusion of each experiment, all non-germinated seeds were subjected to a cut test to assess viability (ISTA, 2017). Seeds with a fully formed, turgid, white embryo, perisperm and endosperm were considered to be viable. Empty, necrotic or soft seeds were considered to be non-viable and excluded from further analysis. As such, germination data presented here are based on the number of viable seeds.
Embryo morphology
To confirm that the classification of Nymphaea embryos was broad (Martin, 1946) and that they were differentiated, 20 seeds each of N. immutabilis (NI1) N. lukei (NL1 and NL2), N. macrosperma (NM3), N. ondinea (NO1), N. pubescens (NP1) and N. violacea (NV7–NV10) were kept for 24 h on moist filter paper. The embryo was then excised under a microscope using a scalpel and a pair of fine forceps. Seeds and embryos were visually assessed for shape and differentiation.
Embryo growth
Embryo growth was measured for all species but due to limited seed numbers only for the following collections: N. immutabilis (NI1), N. lukei (NL1 and NL2), N. macrosperma (NM3), N. ondinea (NO1), N. pubescens (NP1) and N. violacea (NV3, NV8–NV10). To determine whether embryo growth occurred prior to hypocotyl emergence, 100 seeds of each species/collection were placed in 10-mL plastic tubes filled with 10 mL of sterile RO water and incubated at either 30 or 35 °C (previously determined optimal temperature for germination of each seed collection) under light/dark conditions (described above). After approximately 6 h, 20 seeds from each collection were removed, their embryos were excised and total embryo length and the internal length of the seed (the seed coat excluded) were measured; these served as ‘fresh seed’ measurements (Baskin and Baskin, 2007). The remaining seeds were inspected daily for 10 weeks, and any seeds with a split in the seed coat (indicating imminent germination) were removed and measured. Seeds where the hypocotyl had protruded (germinated) were removed from the vials and excluded from further analysis.
Statistical analyses
The germination data followed a binomial distribution and were analysed with binomial logistic regressions (GLMs), using the ‘glm’ function fitted with a logit-link function, followed by a likelihood ratios test in R (R Core Team, 2017). To determine the effects of temperature (continuous factor), light vs. constant dark (categorical factor) and germination stimulant (categorical factors), germination data were analysed across N. immutabilis (NI1), N. lukei (NL2), N. macrosperma (NM1), N. ondinea (NO1), N. pubescens (NP1) and N. violacea (NV3). To determine the effects of temperature and germination stimulant within species, germination data were analysed between each accession of N. lukei (NL1 and NL2), N. macrosperma (NM1–NM3) and N. violacea (NV1–NV10). All statistical effects are shown for main factors only. Embryo length and embryo to seed length ratio (E: S) were compared between fresh seeds and seeds that showed a split in the seed coat and were analysed with linear models using the ‘lm’ function in R (R Core Team, 2017). Prior to analysis all embryo growth data were checked for homogeneity to satisfy model assumptions via a graphical analysis of the residuals. When the data did not meet the model assumptions, the data were log-transformed. Pairwise comparisons (Holm’s) were then used to determine significance (P < 0.05) between fresh and split seeds (imminent germination).
RESULTS
Seed characteristics and quality assessment
Nymphaea immutabilis and N. macrosperma (subgen. Anecphya) had the largest (length and width) seeds and N. violacea and N. ondinea (subgen. Confluentes) the smallest. However, some variation within species (between collections) was noted (Table 2). Seed mass was also variable within species, particularly for collections of N. macrosperma, which varied between 7.96 and 18.07 g (1000 seeds)−1. Initial seed fill of each species ranged from 64 to 100 % (Table 2).
Table 2.
Mean seed length, width, 1000-seed mass and initial seed fill of all collections of the six species of Nymphaea.
| Species | Collection | Seed length (mm) (mean ± s.e.) | Seed width (mm) (mean ± s.e.) | 1000-seed mass (g) | Seed fill (%) |
|---|---|---|---|---|---|
| Nymphaea immutabilis | NI1 | 4.64 ± 1.30 | 2.74 ± 0.07 | 18.89 | 99 |
| Nymphaea lukei | NL1 | 2.54 ± 0.05 | 1.96 ± 0.03 | 3.84 | 97 |
| NL2 | 2.35 ± 0.06 | 1.67 ± 0.05 | 3.47 | 98 | |
| Nymphaea macrosperma | NM1 | 4.05 ± 0.07 | 3.35 ± 0.06 | 18.07 | 92 |
| NM2 | 3.00 ± 0.10 | 2.61 ± 0.07 | 7.96 | 95 | |
| NM3 | 3.38 ± 0.07 | 2.85 ± 0.07 | 11.16 | 98 | |
| Nymphaea ondinea | NO1 | 1.81 ± 0.04 | 1.46 ± 0.03 | 0.81 | 64 |
| Nymphaea pubescens | NP1 | 1.93 ± 0.06 | 1.61 ± 0.06 | 2.49 | 99 |
| Nymphaea violacea | NV1 | 1.90 ± 0.06 | 1.58 ± 0.05 | 1.89 | 74 |
| NV2 | 1.73 ± 0.06 | 1.21 ± 0.05 | 1.45 | 98 | |
| NV3 | 1.68 ± 0.03 | 1.23 ± 0.03 | 1.6 | 100 | |
| NV4 | 1.52 ± 0.13 | 0.91 ± 0.08 | 3.19 | 80 | |
| NV5 | 1.58 ± 0.07 | 1.15 ± 0.06 | 1.11 | 95 | |
| NV6 | 1.19 ± 0.01 | 0.80 ± 0.01 | 3.8 | 88 | |
| NV7 | 1.60 ± 0.05 | 1.13 ± 0.04 | 0.75 | 68 | |
| NV8 | 1.62 ± 0.06 | 1.19 ± 0.05 | 1.14 | 99 | |
| NV9 | 1.57 ± 0.04 | 1.10 ± 0.04 | 1.1 | 99 | |
| NV10 | 1.56 ± 0.05 | 1.08 ± 0.03 | 0.94 | 98 |
Water uptake (imbibition)
Seeds of all six species took up water readily over the 96-h period of imbibition, and average moisture uptake after 96-h was 45.6 % (± 1.5 %) for N. immutabilis, 38.8 % (± 1.6 %) for N. lukei, 47.1 % (± 1.2 %) for N. macrosperma, 49.0 % (± 1.6 %) for N. ondinea, 45.9 % (± 2.1 %) for N. pubescens and 48.7 % (± 7.1 %) for N. violacea. For seeds scarified prior to imbibition, the final percentage moisture uptake was not significantly different from that of non-scarified seeds (P = 0.9, data not shown).
Seed germination
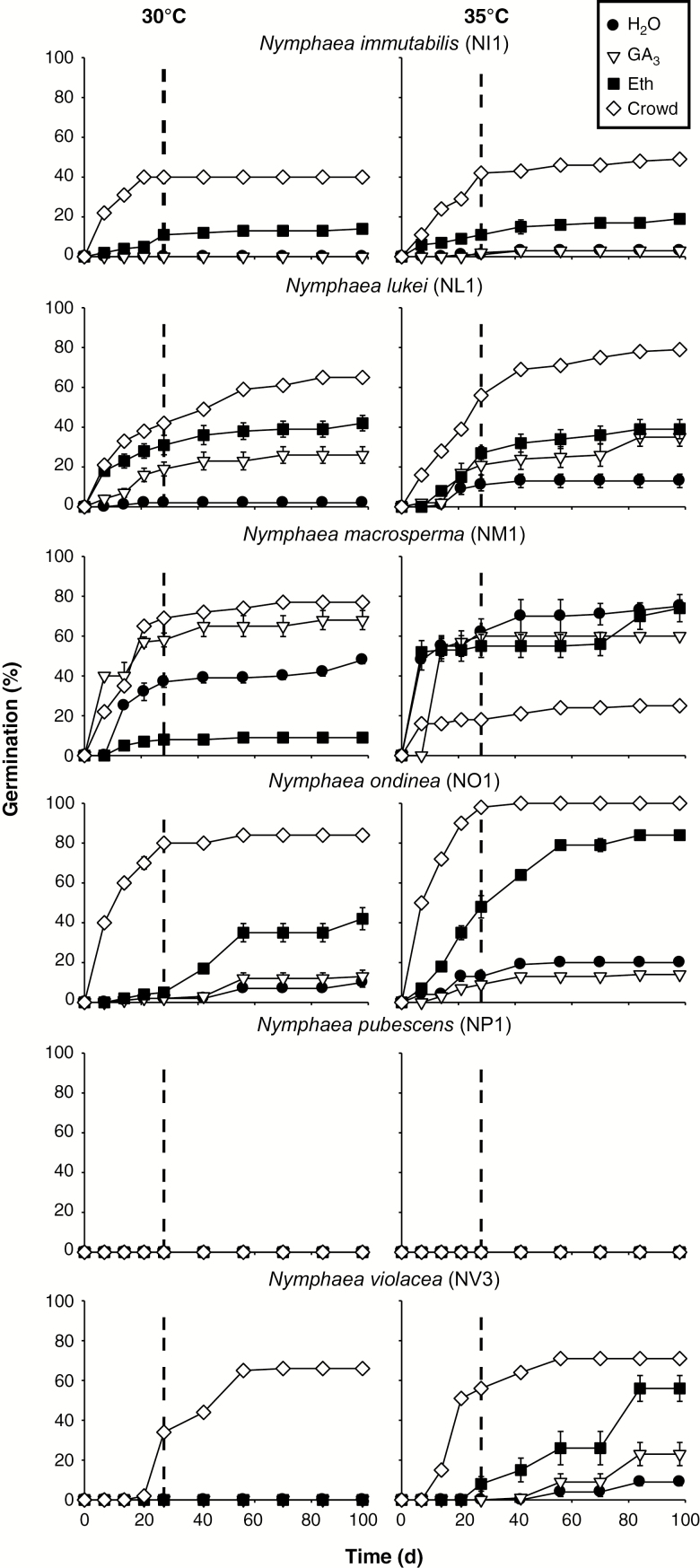
Under dark conditions, overall percentage germination was low (0–19 %) in all species and collections, and most of the germination occurred in treatments with light (Fig. 2; Supplementary Data Table S1, P < 0.001 for all species). Seeds of N. pubescens did not germinate in any of the treatment combinations tested. Temperature had a significant effect on germination (P < 0.001) as no seeds of any species germinated in light at <25 °C and germinated mostly at 30 and 35 °C. Seeds of N. ondinea and N. macrosperma germinated at 40 °C (<60 %). No seeds of any species germinated at 20/35 °C. Germination of N. immutabilis, N. lukei and N. ondinea seeds in the control treatment (H2O) never exceeded 20 %, whereas germination of N. macrosperma seeds reached a maximum of 70 % at 35 °C.
Fig. 2.
Effect of H2O (control), gibberellic acid (GA3; 0.29 μm), ethephon (Eth; 20 mm) and crowding (Crowd) on total germination percentage (mean ± s.e.) of six Nymphaea species exposed to light/dark or full dark conditions at six temperatures after 8 weeks.
The crowding treatment produced the highest germination (P < 0.001 for all species) at 30 °C (N. macrosperma 74 %) or 35 °C (N. immutabilis 46 %, N. lukei 71 %, N. ondinea 99 % and N. violacea 71 %). Seeds of N. lukei (at 30 and 35 °C) treated with GA3 germinated to higher percentages (23 and 25 %, respectively) than those in the control. However, the application of GA3 did not significantly increase germination of N. immutabilis, N. macrosperma, N. ondinea or N. violacea seeds (P > 0.05). Overall, at optimal temperatures, ethephon and GA3 significantly increased germination percentages (P < 0.001) in seeds of N. immutabilis, N. lukei, N. ondinea and N. violacea. Maximum germination after 28 d of incubation at the apparent optimal temperatures (30–35 °C) was about 45–75 % for all species, except for seeds of N. ondinea, which germinated to 80–100 % in the crowding treatment (Fig. 3).
Fig. 3.
Effect of H2O (control), gibberellic acid (GA3; 0.29 μm), ethephon (Eth; 20 mm) and crowding (Crowd) on cumulative germination percentage (mean ± s.e.) of six Nymphaea species exposed to 12/12-h light/dark regime at optimum temperature (30 °C and 35 °C) range. Dashed vertical line indicates 28 d of incubation, used to classify dormancy type.
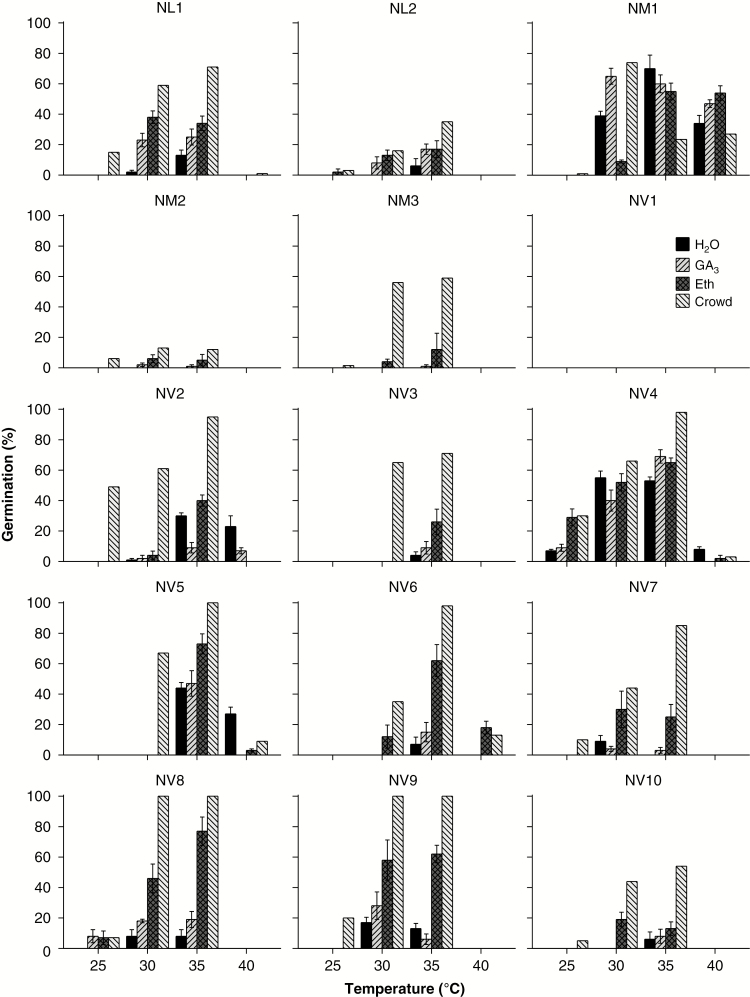
Intraspecific differences in germination responses were evident between different within-seed collections of N. lukei, N. macrosperma and N. violacea (Fig. 4; Table S2). Germination percentage of N. lukei seeds was highest at 35 °C for both collections in the crowding treatment. However, seeds of NL1 germinated to 70 %, whereas those of NL2 germinated to <40 %. Seeds of N. macrosperma from the NM1 collection in the control germinated to 70 % at 35 °C, whereas no germination occurred in controls for the NM2 and NM3 collections. Germination in the crowded treatments was highest at 30 °C for NM2 (12 %) and at 35 °C for NM3 (58 %). For seeds of N. violacea, the NV1 collection did not germinate at any temperature regardless of the addition of a germination stimulant, whereas seeds of NV4 readily germinated at all temperatures and with most stimulants. With the exception of seeds from NV1, germination percentages of NV collections were highest in the crowding treatments at 30 or 35 °C. In collections NV5, NV8 and NV9, germination in crowded treatments was 100 % after 8 weeks at 35 °C, while germination in the control never exceeded 40 %.
Fig. 4.
Effect of H2O (control), gibberellic acid (GA3; 0.29 μm), ethephon (Eth; 20 mm) and crowding (Crowd) on total germination percentage (mean ± s.e.) of multiple collections of Nymphaea lukei (NL1, NL2), N. macrosperma (NM1–NM3) and N. violacea (NV1–NV10) after 8 weeks of incubation in light at 25, 30, 35 or 40 °C.
Embryo morphology
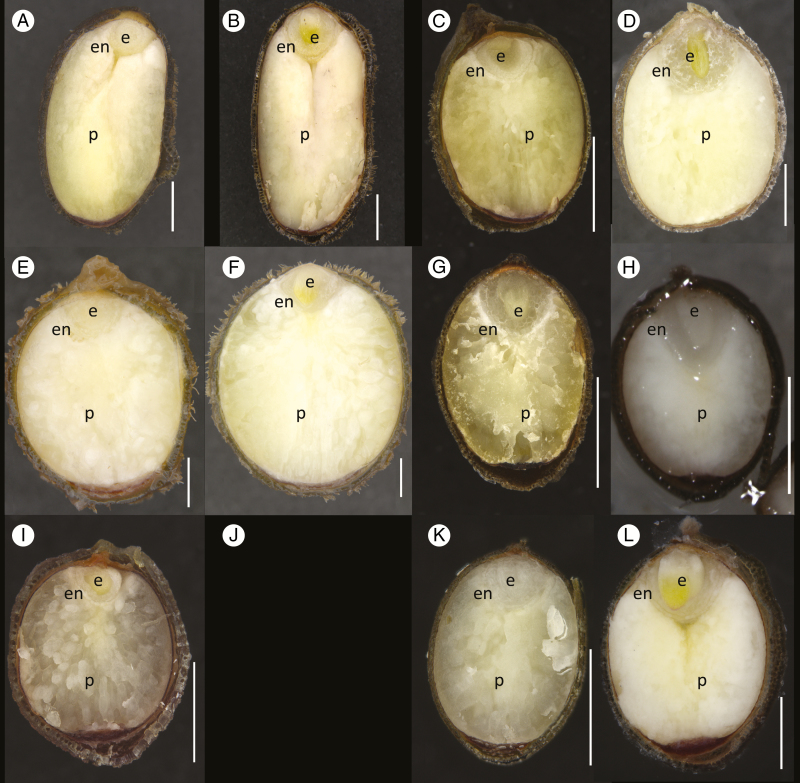
Embryos of all six species were wider than long, and they were surrounded by a thin layer of endosperm, which in turn was surrounded by abundant perisperm. The embryos were thus considered to be broad, in accordance with Martin’s (1946) classification system. Embryos generally took up less than 25 % of seed (minus seed coat thickness) length (Fig. 5). Embryos were fully differentiated; the hypocotyl and cotyledons could be distinguished.
Fig. 5.
Longitudinal sections of seeds of Nymphaea species prior to (A, C, E, G, I and K) and after a period of incubation (B, D, F, H and L), showing evidence of embryo growth: N. immutabilis (A and B), N. lukei (C and D), N. macrosperma (E and F), N. ondinea (G and H), N. pubescens (I and J: seeds did not germinate) and N. violaceae (K and L). e = embryo, en = endosperm, p = perisperm. Scale bar (white vertical line in each image) = 1 mm.
Embryo growth
Prior to germination, embryos in seeds of N. immutabilis, N. lukei and N. macrosperma exhibited a small but significant (P < 0.001) increase in length of 10, 8 and 21 %, respectively, while embryos of N. ondinea and N. violacea increased by 37 and 32 %, respectively (P < 0.001) (Table 3; Fig. 5). E: S ratios increased significantly (P < 0.01) in all species and collections during incubation, except for NL2. The change in E: S ratio was greatest for embryos of N. ondinea seeds, which increased from 0.29 to 0.38.
Table 3.
Information on fresh seed embryos and embryos prior to hypocotyl emergence of six Nymphaea species; embryo type classified as per Martin (1946)
| Species | Collection | Embryo type | Embryo differentiated? | Mean embryo length (mm) of fresh seed (mean ± s.e.) | Embryo length (mm) at hypocotyl emergence (mean ± s.e.) | E: S ratio of fresh seed (mean ± s.e.) | E: S ratio at hypocotyl emergence (mean ± s.e.) |
|---|---|---|---|---|---|---|---|
| Nymphaea immutabilis | NI1 | Broad | Y | 0.78 ± 0.02a | 0.86 ± 0.01b | 0.19 ± 0.01a | 0.22 ± 0.002b |
| Nymphaea lukei | NL1 | Broad | Y | 0.53 ± 0.02a | 0.61 ± 0.01b | 0.22 ± 0.01a | 0.27 ± 0.003b |
| NL2 | Broad | Y | 0.58 ± 0.02a | 0.59 ± 0.01a | 0.24 ± 0.01a | 0.26 ± 0.01a | |
| Nymphaea macrosperma | NM2 | Broad | Y | 0.71 ± 0.02a | 0.86 ± 0.01b | 0.22 ± 0.005a | 0.28 ± 0.002b |
| Nymphaea ondinea | NO1 | Broad | Y | 0.49 ± 0.01a | 0.67 ± 0.01b | 0.29 ± 0.01a | 0.38 ± 0.004b |
| Nymphaea pubescens | NP1 | Broad | Y | 0.35 ± 0.01 | NA | 0.20 ± 0.01 | NA |
| Nymphaea violacea | NV7 | Broad | Y | 0.43 ± 0.01a | 0.55 ± 0.01b | 0.25 ± 0.01a | 0.30 ± 0.01b |
| NV8 | Broad | Y | 0.38 ± 0.01a | 0.48 ± 0.01b | 0.23 ± 0.01a | 0.31 ± 0.003b | |
| NV9 | Broad | Y | 0.38 ± 0.01a | 0.47 ± 0.01b | 0.23 ± 0.01a | 0.31 ± 0.004b | |
| NV10 | Broad | Y | 0.33 ± 0.01a | 0.50 ± 0.01b | 0.22 ± 0.004a | 0.32 ± 0.003b |
Letters in superscript denote significant differences at P < 0.05.
DISCUSSION
The primary aim of this study was to determine whether MD and/or MPD is/are present in species of the Nymphaeaceae from the wet–dry tropics of Australia. We also aimed to define the range of conditions suitable for germination and to determine whether seed dormancy and germination responses varied between different populations of the same species. Because seeds of all Nymphaea species we tested readily imbibed water, the presence of physical dormancy was excluded. Seeds of all species and collections, except N. macrosperma NM1, not treated with a germination stimulant (i.e. controls), germinated to ≤13 % in 28 d; the seeds of N. macrosperma germinated to 70 %. Furthermore, germination in the controls was <17 % over the 8-week test period for all collections, with the exception of NM1 (70 %) and three of ten collections of N. violacea (30, 55 and 44 %), indicating that seeds within most populations were dormant.
Embryos in seeds were broad and fully differentiated, and embryo length in five of the six species that germinated increased 8–37 % prior to hypocotyl emergence (germination). Baskin and Baskin (2007) proposed that the small increase in embryo length (≤14 %) in three species of nursery-grown Nymphaea was not enough to consider the embryos as being underdeveloped. For seeds of N. immutabilis and N. lukei, in which embryo growth was only 10 and 8 %, respectively, we likewise conclude that the embryos are not underdeveloped and that the seeds have PD. Compared to seeds of most other species that have MPD/MD, this amount of embryo growth is small. For example, underdeveloped linear embryos in members of the Campanulaceae (Baskin et al., 2005) increased 87–179 % prior to radicle emergence. On the other hand, embryos in seeds of Viburnum odoratissimum (Adoxaceae) (Baskin et al., 2008) increased by approx. 300 %, and those in seeds of the basal angiosperm Schisandra arisanensis increased by 360 % (Chien et al., 2011) prior to germination. However, in some seeds with MD/MPD, there is a relatively small amount of embryo growth. For example, in a recent study of the basal angiosperm Austrobaileya scandens, embryo development and growth continues following seed dispersal and prior to germination, and longitudinal sections of the seed shows that embryo growth is minor prior to the emergence of the radicle (Losada et al., 2017). Furthermore, Erickson et al. (2017) found that embryos in seeds of Wahlenbergia tumidifructa (Campanulaceae) increased in size by 27.5 % prior to radicle emergence, and thus were considered to have MD (Erickson et al., 2016).
In seeds of N. ondinea and N. violacea in the present study, the increase in embryo length prior to germination was 37 and 32 %, respectively, with growth occurring over a period of several days (Dalziell, 2016). Thus, we conclude that these embryos are underdeveloped and have MPD. We can further conclude that the level of MPD present in seeds is non-deep simple MPD, i.e. embryo growth occurs at temperatures suitable for warm stratification. Temperatures in northern Australian rarely fall below 10 °C (Bureau of Meteorology, 2005), and germination occurs only at high temperatures (30–35 °C), indicating that embryos only grow at temperatures suitable for dormancy break during warm (≥15 °C) stratification (Baskin and Baskin, 2004, 2014). The embryos of seeds of N. macrosperma (accession NM2) increased in length by 21 % prior to germination, indicating that seeds of this species also have some morphological component to their dormancy. As total germination in two of three collections of N. macrosperma (NM2 and NM3) was low without the addition of a germination-stimulating treatment, we conclude that the majority of seeds in these collections have non-deep simple MPD. Given the higher germination observed in the NM1 accession of N. macrosperma seeds, it seems this population of seeds either has morphological (MD) dormancy only or the seeds are non-dormant.
The five species of Nymphaea that germinated in our study did so at significantly higher percentages in light than in darkness. This result is congruent with the findings of a range of studies showing that the majority of aquatic plants require light to germinate (Baskin and Baskin, 2014), including those that grow in temporary pools in the Mediterranean region (Carta et al., 2013), south-west Western Australia (Tuckett et al., 2010a) and the monsoon tropical Kimberley region in Australia (Cross et al., 2015). The requirement for light may ensure that germination occurs at shallow enough depths of water to allow sufficient light penetration to support subsequent seedling establishment, or allows for germination of seeds in the soil seed bank in response to disturbance (Grime et al., 1981; Pons, 1991). The five germinating species of Nymphaea in our study germinated to the highest percentages at 30 and 35 °C; these are similar to the mean maximum temperatures (Table 1) seeds experience during maturation and dehiscence. Furthermore, N. macrosperma and N. ondinea germinated to >50 % at 40 °C in some treatments. Outside the optimal temperature range, particularly at lower temperatures, germination did not occur, or was reduced significantly in all species. Interestingly, no germination was recorded for any species when seeds were incubated at 20/35 °C. The suppression of germination under such a diurnal variation in temperature may allow seeds to avoid unseasonal germination during the onset of the dry season, when night temperatures and water availability decrease (Bureau of Meteorology, 2013). At optimal temperatures, crowding (sensuElse and Riemer, 1984) elicited the greatest germination response in the five species (and most collections) and the addition of ethephon also tended to increase germination. The increased germination response in the crowding treatment has previously been attributed to the endogenous production of ethylene by seeds, which stimulates germination (Else and Riemer, 1984; Yin et al., 2009). In the natural environment, ethylene is produced under waterlogged or anaerobic conditions in both permanently (Smith and Restall, 1971; Zeikus and Winfrey, 1976) and ephemerally (Cross et al., 2014) inundated wetland soils. The region of northern Australia from which our collections were made experiences summer monsoonal rainfall, and summer temperatures frequently exceed 35 °C with high overnight temperatures (McQuade et al., 1996; Bureau of Meteorology, 2011). The strong germination response to high temperatures and ethylene suggests that seeds of these Nymphaea are cued to germinate and establish in the summer wet season, when water availability is at its peak. In ephemeral creeks, where water turbidity is low, and visibility is high (i.e. allowing for observations to be made), seedlings of N. lukei, N. ondinea and N. violacea often can be seen during the late wet season (February). While this region of northern Australia reliably receives rainfall during the summer wet season, the timing, duration and amount of precipitation is highly variable and often related to cyclonic events (Taylor and Tulloch, 1985; Peel et al., 2007; Bureau of Meteorology, 2011), which has a significant impact on the hydrological regime of wetlands in the region (Cowie et al., 2000). Thus, seed dormancy delays germination for a period of time, which ensures that water is available for establishment either in the same or in a subsequent wet season (Tuckett et al., 2010a, b; Cross et al., 2015).
Depth of dormancy varied both between and within the collected populations of seeds of the Nymphaea species we tested. No seeds of N. pubescens germinated, suggesting that they were deeply dormant, whereas seeds of the other five species germinated. Furthermore, germination responses differed substantially between seed collections of N. lukei, N. macrosperma and N. violacea. Spatial and temporal variation in the degree of dormancy and germination requirements among seed populations is well established (Andersson and Milberg, 1998; Donohue, 2009; Baskin and Baskin, 2014). Nymphaea violacea seeds collected from the same location in different years (NV1 and NV6) had very different responses; seeds of NV1 did not germinate under any conditions (despite being deemed viable), while seeds of NV6 displayed 100 % germination under optimal conditions. Similarly, some seeds of N. macrosperma NM1 appear to be ND (i.e. those of NM1), but only a very low germination percentage was obtained for seeds of other accessions (NM2 and NM3). These responses may be attributed to a number of factors, including maternal/genetic or ecotypic effects between seed populations (see Baskin and Baskin, 2014, and references therein). Given the inter-seasonal variability and unpredictable nature of rainfall in the wet–dry tropics of Australia, along with the range of sizes of permanent and ephemeral wetlands Nymphaea species inhabit (Table 1), it seems likely that seeds from environments with greater unpredictability may produce more dormant seeds than those in predictable environments, thus ensuring population survival during adverse conditions. For example, seeds of NV4 collected from plants growing in a large, permanent water body were the least dormant of the N. violacea collections, while the majority of the other N. violacea collections came from ephemeral wetlands and these seeds were more dormant.
Our study highlights some diversity in the dormancy and germination traits in tropical, Australian Nymphaea species, particularly those occurring in unpredictable wetland ecosystems. Overall, our results lead to the conclusion that the ANA-grade angiosperms encompass a range of seed dormancy classes, including MPD in Amborella trichopoda (Fogliani et al., 2017) and Austrobaileya scandens (Losada et al., 2017); the specialized form of MPD in Trithuria austinensis and T. submersa (Tuckett et al., 2010b); PD in seeds of Nymphaea immutabilis, N. Albert Greenburg and N. capensis var. zanzibariensis (Baskin and Baskin, 2007); and the range of PD, MPD, MD and ND we have found here in N. immutabilis, N. lukei, N. macrosperma, N. ondinea, N. pubescens and N. violacea. However, there is still a significant lack of information about the kinds of dormancy in other extant genera within the Nymphaeales. To our knowledge, no studies have specifically investigated the kind of dormancy in seeds of Cabomba, Brasenia (Cabombaceae), Barclaya, Euryale, Nuphar or Victoria (Nymphaeaceae). Previous reports of PD in Cabomba and Nuphar have been based on inference from reports of germination observations or experiments (e.g. see Tarver and Sanders, 1977; Smits et al., 1990, 1995). Furthermore, the current phylogenetic tree of the Nymphaeaceae is incomplete; this is a task made particularly challenging by the complex, reticulate patterns of evolution and high frequency of hybridity within certain groups of Nymphaea, and there being a general lack of information regarding the distribution and ecology of species (Löhne et al., 2008a, 2009; Borsch et al., 2011). As such, further research is required to resolve the evolution of PD/MPD within the Nymphaeaceae.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Summary statistics (z-statistics and P-values) from binomial logistic regressions to determine the effects of GA3, ethephon, crowding, light and temperature on germination of the six Nymphaea species studied. Table S2: Summary statistics (z-statistics and P-values) from binomial logistic regressions to determine the effects of GA3, ethephon, crowding and temperature on germination of individual collections of Nymphaea lukei, N. macrosperma and N. violacea.
ACKNOWLEDGEMENTS
We thank Christine Best, Matthew Barrett, Todd Erickson, Wolfgang Lewandrowski, Myles Menz, Ryan Phillips, Alison Ritchie and Sean Tomlinson for their assistance with fieldwork, and Wolfgang Lewandrowski for his assistance with statistical analysis. We are grateful to a number of landholders, conservation agencies and other groups including the Myers family (particularly Cecelia at Theda Station), Wavelength Nominees (particularly Christine Simpson-Stokes and Stephen Bartlett), the Australian Wildlife Conservancy (Mornington Station and Charnley River) and the staff at Parry Creek Farm and Kakadu National Park for their assistance and allowing access to their properties for field collections. The authors also thank two anonymous reviewers for the comments that improved an earlier version of the manuscript. This work was supported by research grants awarded to E.L.D. from the Friends of Kings Park; The Graduate Women of Western Australia (Mary Walters Bursary); and the ANZ Trustees Holsworth Wildlife Research Endowment. During the research, E.L.D. was supported by an Australian Postgraduate Award and more recently the Australian Research Council (ARC LP160100381).
LITERATURE CITED
- Andersson L, Milberg P. 1998. Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research 8: 29–38. [Google Scholar]
- Baskin CC, Baskin JM. 2007. Nymphaeaceae: a basal angiosperm family (ANITA grade) with a fully developed embryo. Seed Science Research 17: 293–296. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds. Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd edn. San Diego: Academic Press, Elsevier. [Google Scholar]
- Baskin CC, Baskin JM, Yoshinaga A. 2005. Morphophysiological dormancy in seeds of six endemic lobelioid shrubs (Campanulaceae) from the montane zone in Hawaii. Canadian Journal of Botany 83: 1630–1637. [Google Scholar]
- Baskin CC, Chien CT, Chen SY, Baskin JM. 2008. Germination of Viburnum odoratissimum seeds: a new level of morphophysiological dormancy. Seed Science Research 18: 179–184. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Borsch T, Löhne C, Mbaye MS, Wiersema JH. 2011. Towards a complete species tree of Nymphaea: shedding further light on subg. Brachyceras and its relationships to the Australian water-lilies. Telopea 13: 193–217. [Google Scholar]
- Bureau of Meteorology 2005. Average annual and monthly maximum, minimum, and mean temperature Available at http://www.bom.gov.au/jsp/ncc/climate_averages/temperature/index.jsp (April 2015).
- Bureau of Meteorology 2011. Monthly summaries archive Available at http://www.bom.gov.au/climate/current/statement_archives.shtml (April 2015).
- Bureau of Meteorology 2013. Climate Data Online Available at http://www.bom.gov.au/climate/data/ (April 2013)
- Carta A, Bedini G, Muller JV, Probert RJ. 2013. Comparative seed dormancy and germination of eight annual species of ephemeral wetland vegetation in a Mediterranean climate. Plant Ecology 214: 339–349. [Google Scholar]
- Chien C-T, Chen S-Y, Baskin JM, Baskin CC. 2011. Morphophysiological dormancy in seeds of the ANA grade angiosperm Schisandra arisanensis (Schisandraceae). Plant Species Biology 26: 99–104. [Google Scholar]
- Conard H. 1905. The waterlilies. A monograph of the genus Nymphaea. Baltimore: Lord Baltimore Press. [Google Scholar]
- Cowie ID, Short PS, Osterkamp Madsen M. 2000. Floodplain Flora: A Flora of the Coastal Floodplains of the Northern Territory, Australia. Darwin: Australian Biological Resources Study. [Google Scholar]
- Cross AT, Cawthray GR, Merritt DJ, Turner SR, Renton M, Dixon KW. 2014. Biogenic ethylene promotes seedling emergence from the sediment seed bank in an ephemeral tropical rock pool habitat. Plant and Soil 380: 73–87. [Google Scholar]
- Cross AT, Turner SR, Renton M, Baskin JM, Dixon KW, Merritt DJ. 2015. Seed dormancy and persistent sediment seed banks of ephemeral freshwater rock pools in the Australian monsoon tropics. Annals of Botany 115: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziell EL. 2016. Seed biology and ex situ storage behaviour of Australian Nymphaea (water lilies): Implications for conservation. PhD Thesis, University of Western Australia, Australia. [Google Scholar]
- Donohue K. 2009. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society. Biological Sciences 364: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else MJ, Riemer DN. 1984. Factors affecting germination of seeds of fragrant waterlily (Nymphaea odorata). Journal of Aquatic Plant Management 22: 22–25. [Google Scholar]
- Erickson TE, Merritt DJ, Turner SR. 2016. Seed dormancy and germination of arid zone species. In: Erickson TE, Barrett RL, Merritt DJ, Dixon KW, eds. Pilbara Seed Atlas and Field Guide: Plant Restoration in Australia’s Arid Northwest. Victoria, Australia: CSIRO Publishing. [Google Scholar]
- Erickson TE, Muñoz-Rojas M, Kildisheva OA, et al. . 2017. Benefits of adopting seed-based technologies for rehabilitation in the mining sector: a Pilbara perspective. Australian Journal of Botany 65: 646–660. [Google Scholar]
- Fenner M, Thompson K. 2005. The Ecology of Seeds. Cambridge: Cambridge University Press. [Google Scholar]
- Finch-Savage WE, Leubner Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Finlayson CM. 1999. Wetland types and their distribution in northern Australia. In: Finlayson CM, Spiers AG, eds. A Compendium of Information for Managing and Monitoring Wetlands in Tropical Australia. Canberra: Supervising Scientist Group. [Google Scholar]
- Fogliani B, Gâteblé G, Villegente M, et al. . 2017. The morphophysiological dormancy in Amborella trichopoda seeds is a pleisiomorphic trait in angiosperms. Annals of Botany 119: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbis TA, Floyd SK, Queiroz A. 2002. The evolution of embryo size in angiosperms and other seed plants: implications for the evolution of seed dormancy. Evolution 56: 2112–2125. [DOI] [PubMed] [Google Scholar]
- Friis EM, Crane PR, Pedersen KR, Stampanoni M, Marone F. 2015. Exceptional preservation of tiny embryos documents seed dormancy in early angiosperms. Nature 528: 551–555. [DOI] [PubMed] [Google Scholar]
- Grime JP, Mason G, Curtis AV, Rodman J, Band SR. 1981. A comparative study of germination characteristics in a local flora. Journal of Ecology 69: 1017–1059. [Google Scholar]
- Haines RW, Lye KA. 1975. Seedlings of Nymphaeaceae. Botanical Journal of the Linnean Society 70: 255–265. [Google Scholar]
- ISTA 2017. International Rules for Seed Testing. Bassersdorf: International Seed Testing Association. [Google Scholar]
- Jacobs SWL, Hellquist CB. 2011. New species, possible hybrids and intergrades in Australian Nymphaea (Nymphaeaceae) with a key to all species. Telopea 13: 233–243. [Google Scholar]
- Löhne C, Borsch T, Jacobs SWL, Hellquist CB, Wiersema JH. 2008a Nuclear and plastid DNA sequences reveal complex reticulate patterns in Australian water-lilies (Nymphaea subgenus Anecphya, Nymphaeaceae). Australian Systematic Botany 21: 229–250. [Google Scholar]
- Löhne C, Yoo M-J, Borsch T, et al. . 2008. b Biogeography of Nymphaeales: extant patterns and historical events. Taxon 57: 1123–1146. [Google Scholar]
- Löhne C, Wiersema JH, Borsch T. 2009. The unusual Ondinea, actually just another Australian water-lily of Nymphaea subg. Anecphya (Nymphaeaceae). Willdenowia-Annals of the Botanic Garden and Botanical Museum Berlin-Dahlem 39: 55–58. [Google Scholar]
- Losada JM, Bachelier JB, Friedman WE. 2017. Prolonged embryogenesis in Austrobaileya scandens (Austrobaileyaceae): its ecological and evolutionary significance. New Phytologist 215: 851–864. [DOI] [PubMed] [Google Scholar]
- Martin AC. 1946. The comparative internal morphology of seeds. The American Midland Naturalist 36: 513–660. [Google Scholar]
- McQuade CV, Arthur JT, Butterworth IJ. 1996. Climate and hydrology. In: Finlayson CM, von Oertzen I, eds. Landscape and Vegetation Ecology of the Kakadu Region, Northern Australia. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Nikolaeva MG. 1977. Factors controlling the seed dormancy pattern. In: Kahn AA, ed. The Physiology and Biochemistry of Seed Dormancy and Germination. Amsterdam: North-Holland. [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11: 1633–1644. [Google Scholar]
- Pons TL. 1991. Induction of dark dormancy in seeds: its importance for the seed bank in the soil. Functional Ecology 5: 669–675. [Google Scholar]
- R Core Team 2017. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rudall PJ, Eldridge T, Tratt J, et al. . 2009. Seed fertilization, development, and germination in Hydatellaceae (Nymphaeales): implications for endosperm evolution in early angiosperms. American Journal of Botany 96: 1581. [DOI] [PubMed] [Google Scholar]
- Smith K, Restall S. 1971. The occurrence of ethylene in anaerobic soil. Journal of Soil Science 22: 430–443. [Google Scholar]
- Smits AJM, van Avesaath PH, Van der Velde G. 1990. Germination requirements and seed banks of some nymphaeid macrophytes: Nymphaea alba L., Nuphar lutea (L.) Sm. and Nymphoides peltata (Gmel.) O. Kuntze. Freshwater Biology 24: 315–326. [Google Scholar]
- Smits A, Schmitz G, Vand der Velde G, Voesenek L. 1995. Influence of ethanol and ethylene on the seed germination of three nymphaeid water plants. Freshwater Biology 34: 39–46. [Google Scholar]
- Tarver DP, Sanders Sr DR. 1977. Selected life cycle features of fanwort. Journal of Aquatic Plant Management 15: 18–22. [Google Scholar]
- Taylor JA, Tulloch D. 1985. Rainfall in the wet-dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983 inclusive. Australian Journal of Ecology 10: 281–295. [Google Scholar]
- Tuckett R, Merritt D, Hay F, Hopper S, Dixon K. 2010a Dormancy, germination and seed bank storage: a study in support of ex situ conservation of macrophytes of southwest Australian temporary pools. Freshwater Biology 55: 1118–1129. [Google Scholar]
- Tuckett RE, Merritt DJ, Rudall PJ, et al. . 2010. b A new type of specialized morphophysiological dormancy and seed storage behaviour in Hydatellaceae, an early-divergent angiosperm family. Annals of Botany 105: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CG, Baskin CC, Baskin JM, et al. ; the NESCent Germination Working Group. 2014. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203: 300–309. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang C, Chen Y, Yu C, Cheng Y, Li W. 2009. Cold stratification, light and high seed density enhance the germination of Ottelia alismoides. Aquatic Botany 90: 85–88. [Google Scholar]
- Zeikus JG, Winfrey MR. 1976. Temperature limitation of methanogenesis in aquatic sediments. Applied and Environmental Microbiology 31: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.