Abstract
Background
Halophytes tolerate external salt concentrations of 200 mm and more, accumulating salt concentrations of 500 mm and more in their shoots; some, recretohalophytes, excrete salt through glands on their leaves. Ions are accumulated in central vacuoles, but the pathway taken by these ions from the outside of the roots to the vacuoles inside the cells is poorly understood. Do the ions cross membranes through ion channels and transporters or move in vesicles, or both? Vesicular transport from the plasma membrane to the vacuole would explain how halophytes avoid the toxicity of high salt concentrations on metabolism. There is also a role for vesicles in the export of ions via salt glands.
Scope and Methods
We have collected data on the fluxes of sodium and chloride ions in halophytes, based on the weight of the transporting organs and on the membrane area across which the flux occurs; the latter range from 17 nmol m–2 s–1 to 4.2 μmol m–2 s–1 and values up to 1 μmol m–2 s–1 need to be consistent with whatever transport system is in operation. We have summarized the sizes and rates of turnover of vesicles in plants, where clathrin-independent vesicles are 100 nm or more in diameter and can merge with the plasma membrane at rates of 100 s–1. We gathered evidence for vesicular transport of ions in halophytes and evaluated whether vesicular transport could account for the observable fluxes.
Conclusions
There is strong evidence in favour of vesicular transport in plants and circumstantial evidence in favour of the movement of ions in vesicles. Estimated rates of vesicle turnover could account for ion transport at the lower reported fluxes (around 20 nmol m–2 s–1), but the higher fluxes may require vesicles of the order of 1 μm or more in diameter. The very high fluxes reported in some salt glands might be an artefact of the way they were measured.
Keywords: Salt tolerance, endocytosis, exocytosis, pinocytosis, Na+ flux, ion transport, vesicle
INTRODUCTION
All plants can tolerate some NaCl in the medium in which they grow, but the concentration varies considerably, from under 50 mm to over 1 m (see Flowers et al., 2010; Moir-Barnetson et al., 2016). While there is a continuous spectrum of salt tolerance, it is useful to separate the less tolerant glycophytes from the more tolerant halophytes and there have been various suggestions (see Huchzermeyer and Flowers, 2013) of where to draw a rather arbitrary dividing line between the two groups – from about 90 mm (Aronson, 1989) to 200 mm (Flowers and Colmer, 2008). Here we adopt the higher concentration, the arguments for which were put forward in Flowers and Colmer (2008). Halophytes are remarkable in that not only do they grow in external salt concentrations lethal to most plants, but they also accumulate high concentrations of salts in their leaves (in excess of 500 mm in some species; see Flowers, 1985): some species (recretohalophytes) secrete salt from their leaves. However, despite much research, the molecular mechanisms by which halophytes transport and sequester salts within tissues and secrete salts from leaves are still largely unknown.
This Viewpoint explores the idea that vesicles might be involved in salt transport. Vesicular transport of ‘particles’ was suggested in the 1950s (see below) and remains an attractive but controversial topic for discussion and research into transport processes in plants. Historically, the concept of vesicular ion transport was based on microscopic observations and preceded ideas about the existence of ion channels and transporters (Behrends, 2012). However, the rapid rise of research into membrane transport proteins and the intensive accumulation of knowledge of ion channels pushed ideas on vesicular ion transport into the shadows, virtually suppressing and abolishing them. Nevertheless, MacRobbie, in a series of papers published between 1966 and 1977 (see MacRobbie, 1999), drew attention to the fact that the kinetics of Cl– movement between the cytoplasm and vacuole in algae with giant cells suggested transport in ‘salt-filled vesicles’. That ions may be moved in vesicles gains support from the fact that vesicular transport is widespread throughout eukaryotic organisms, with the mechanisms of endo- and exocytosis being conserved (Cucu et al., 2017). The idea we examine is that, in addition to ion channels and transporters, halophytes use vesicles to move salts through the cytoplasm (endocytosis and exocytosis) and to expel excess salts through salt glands (exocytosis). A consequence of vesicular transport is that Na+ and Cl–, which are toxic at the concentrations found in the cells of halophytes, would not come into direct contact with the proteins that they have been shown to disrupt in vitro. Using the recent literature on ion transport and vesicular trafficking in plants, we test the feasibility of vesicular transport as an integral part of salt tolerance in halophytes. Baral et al. (2015) have previously argued that the stress of salinity modulates endo- and exocytosis in glycophytes.
Na+ and Cl– toxicity in the cytosol
Plants growing in saline soils take up large quantities of salt for osmotic adjustment. Perhaps the leading theory to account for this uptake of Na+ and Cl– is that it occurs passively according to thermodynamic gradients or using secondary active transport, since no pumps for Na+ (Pedersen and Palmgren, 2017) or Cl– are known in higher plants. In halophytes, Na+ and Cl– are sequestered in central vacuoles of cells (Flowers et al., 1977, 1986) and it has been presumed that cytosolic concentrations of Na+ are kept within a reasonable range through the activity of Na+/H+ antiporters, other transporters and ion channels in the vacuolar and plasma membranes in both root and leaf cells (Fig. 1; see also Volkov, 2015a, cf. fig. 2 in Maathuis, 2014). Consequently, it has been argued that enzymes in the cytosol (which is not a simple aqueous solution of proteins; Cheeseman, 2013; Yu et al., 2016) are not exposed to concentrations of salt higher than 100–200 mm (Flowers et al., 2015). However, vesicular transport of Na+ and Cl– would allow large fluxes of Na+ and Cl– through the cytosol while avoiding exposing enzymes to toxic concentrations of transiting ions.
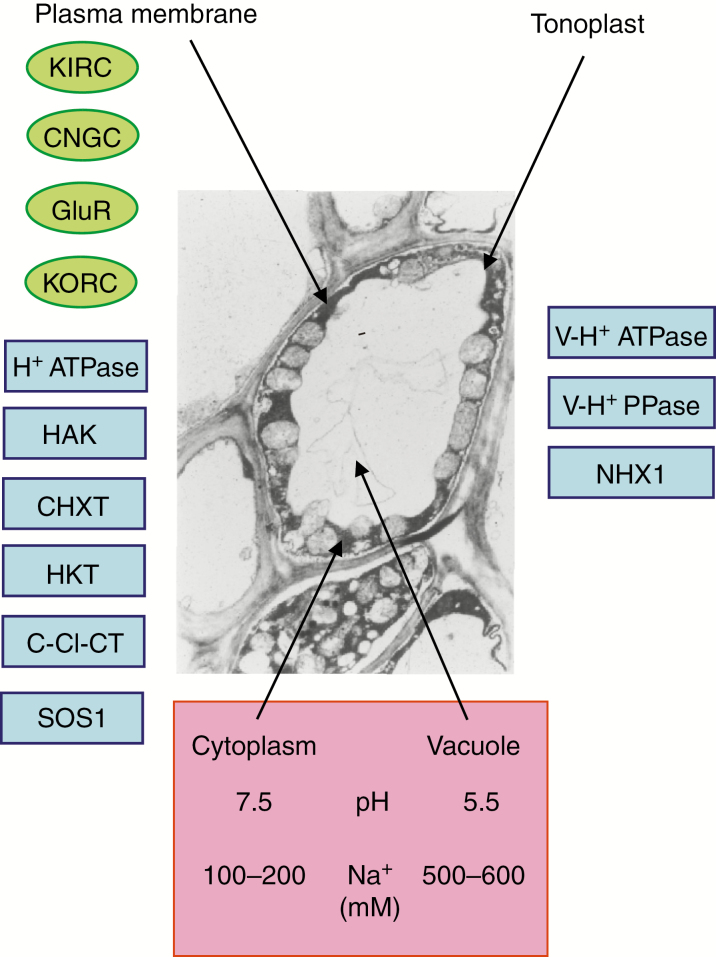
Fig. 1.
The transporters that might be found in the membranes of a halophyte cell (after Volkov, 2015a). The estimated concentration of Na+ is that of a mesophyll cell of Suaeda maritima and the pH values are taken from the literature: abbreviations enclosed by ovals are ion channels, and those within rectangles are transporters. Plasma membrane: KIRC, inward rectifying potassium channels; CNGC, cyclic-nucleotide gated ion channels; GluR, glutamate receptors; KORC, outward rectifying potassium channels; H+ATPase, plasma membrane proton pump; HAK, high affinity potassium transporter; CHXT, cation H+ exchange transporters; HKT, high affinity K+ transporters; C-Cl-CT, cation–chloride co-transporters; SOS1, sodium–proton antiporter. Tonoplast: V-H+ ATPase, tonoplast proton pump; V-H+ PPase, tonoplast pyrophosphatase; and NHX1, tonoplast sodium (cation)/proton antiporter.
A further argument in favour of vesicular transport of ions is that the mechanisms are completely different from those involved in transport by ion channels and transporters. Vesicular ion transport depends more on intracellular machinery, cell metabolism and cytoskeletal activity than on trans-membrane electric potential and ion concentrations, and hence could be a safety valve for directing intracellular ion fluxes. Another important point is that while the selectivity of ion transport via ion channels and transporters is determined by the molecular protein structure of the ion channels and transporters (e.g. Volkov et al., 2004), for vesicular ion transport the selectivity of trapped ion cargo would depend on local ion concentrations near forming vesicles and on electric charges of vesicular membranes. Here is another mechanism to generate selective (or semi-selective) ion fluxes.
Transport of Na+ and Cl– in plants
Much more is known about ion transport in glycophytes than in halophytes. For example, in Arabidopsis thaliana about 5 % of around 25 000 genes are likely to encode transport proteins in membranes, with >150 predicted from their nucleotide sequences to be cation channels or transporters (see Mäser et al., 2001; http://aramemnon.uni-koeln.de/). Of these, non-selective cation channels (NSCCs), including cyclic nucleotide gated ion channels and probably glutamate receptors, have been assumed to be the major pathway for Na+ entry into root cells of A. thaliana (Davenport, 2002; Demidchik and Tester, 2002; Demidchik et al., 2002; Essah et al., 2003) and in some other plants (reviewed in Maathuis et al., 2014). However, in a critical review of the transport of Na+ into plants, Kronzucker and Britto (2011) concluded that the involvement of NSCCs was promulgated prematurely and that evidence for their involvement was highly speculative. There is, however, strong evidence in support of the role of Na+,K+/H+ exchangers (NHXs; see Pilar Rodriguez-Rosales et al., 2009 for information on NHX transporters in plants) in the transport of Na+, both across the plasma membrane (SOS1; see Ji et al., 2013) and into vacuoles (Volkov, 2015a). Interestingly, the aquaporin AtPIP2 also has non-selective cation channel activity and could provide a link between water and Na+ transport (Byrt et al., 2017). Chloride channels are also known for plants (see Ward et al., 2009) and inhibitor studies with bumetanide suggest that cation–chloride cotransporters may be involved in the transport of Na+ and Cl– (Zhang et al., 2010). For halophytes, 18 or more transporters may potentially be involved in the transport of Na+, K+ and Cl– into cells (Flowers and Colmer, 2008; Fig. 1), but, in spite of >80 years of research (see Huchzermeyer and Flowers, 2013), we still do not know the precise identity of the proteins involved. Low-affinity cation transporter 1 (LCT1), AKT-type ion channels, K+ transporters from the KUP/HAK/KT family as well as members of the HKT1 and HKT2 classes of transporters possibly participate in Na+ transport across the plasma membrane (see, for example, Wang et al., 2007; Almeida et al., 2013), but the exact means by which Na+ and Cl– enter the cells of halophytes remains uncertain. Before reviewing evidence for vesicular transport of ions, first suggested in the 1950s and developed for ion transport in the 1970s, we evaluate the magnitude of the fluxes involved.
ION FLUXES BASED ON ROOT WEIGHT
In attempting to ascertain the feasibility of vesicular transport, as opposed to transport through channels or transporters, we next assess the magnitude of fluxes of Na+ and Cl– so that we can calculate required rates of membrane turnover to deliver these measured fluxes. The effects of external concentration of K+ and its flux into roots have been analysed over many years and often viewed as separable into a high-affinity system saturating at external concentrations of around 1 mm and a low-affinity system where the flux increases with external concentration >1 mm (recently reviewed in Nieves-Cordones et al., 2016). The magnitude of influxes has generally been determined from measurements of the uptake of isotopes, which it has been suggested may be difficult to separate from concurrent efflux (Coskun et al., 2016), so the values may need to be viewed with caution.
High- and low-affinity systems are presumed to occur for Na+ as well as K+ fluxes through roots (see Nieves-Cordones et al., 2016). Halophytes are normally exposed to high concentrations of NaCl in the medium in which they grow, certainly in the low-affinity range as, for example, seawater contains about 480 mm Na+ and 560 mm Cl–. Salt concentrations around the roots could be higher than in any bulk solution, as salts tend to accumulate in the rhizosphere following water uptake by the roots, when salts are left behind in the soil. For example, plants of the halophyte Tamarix ramosissima had soil-solution salinities of 280–620 mm NaCl in the capillary fringe above the water table from which they extracted water, when aquifer salinities were 54–172 mm NaCl (Glenn et al., 2013); Salicornia bigelovii irrigated daily with 670 mm seawater at 2.25 times the potential evaporation rate had soil-solution salinities in the range 1228–1315 mm (mostly NaCl) while still maintaining high productivity of biomass and seeds (Glenn et al., 1997). To maintain such high growth rates requires high, but controlled, fluxes of ions from roots to shoots (see, for example, Yeo and Flowers, 1986).
For halophytes, we have collected the values of Na+ fluxes (Table 1; see also Flowers and Colmer, 2008; Kronzucker and Britto, 2011), where Na+ concentrations were in the low-affinity range (>25 mm and often >200 mm). Influx values were determined from short-time (a few minutes) uptake of labelled isotope (22Na+ or 24Na+) and steady-state values generally from changes in chemical content over days. Influx values range from 4 to 110 nmol g–1 f. wt root s–1 and steady-state (net) fluxes from about 3 to 11 nmol g–1 f. wt root s–1; net fluxes are generally lower than influxes since part of the initial uptake could be expelled by, for example, Na+/H+ antiporters (see, for example, Wang et al., 2006). For the halophyte Suaeda maritima, Zhang et al. (2013) recorded a short-term influx of 28 nmol g–1 f. wt root s–1 in 200 mm NaCl (this is equivalent to 295 nmol g–1 d. wt root s–1, calculated from the fresh weight figure assuming a f. wt:d. wt ratio of 10.4; Yeo and Flowers, 1980) and a very similar net flux of 25 nmol g–1 f. wt root s–1 over 12 h (equivalent to 260 nmol g–1 d. wt root s–1). While the high influx of 28 nmol g–1 f. wt root s–1 (Zhang et al., 2013) and the value of 110 nmol g–1 f. wt root s–1 recorded for Spergularia marina (Lazof and Cheeseman, 1986, 1988) may be subject to the objections raised by Coskun et al (2016), a sustained (over 7 d) net flux of around 11 nmol g–1 f. wt root s–1 (110 nmol g–1 d. wt root s–1; Yeo and Flowers, 1986) has been measured through the roots of S. maritima plants growing in 340 mm NaCl (calculated from the f. wt:d. wt ratio in roots of S. maritima of 10.4; Yeo and Flowers, 1980): this is a net flux of ions from roots to shoots determined from changes in chemical content (Table 1) and so not subject to objections raised by Coskun et al. (2016). An additional feature of this net flux (measured over 5 d) through the roots of S. maritima to the shoots is that it does not saturate as the external concentration of Na+ rises from 20 to 200 and 400 mm; Al-Zahrani, 1990; Table 1).We will return below to the very high influx value calculated for S. marina (Lazof and Cheeseman, 1986).
Table 1.
A summary of the magnitude of Na+ fluxes in plants, reported on the basis of fresh (nmol g-1 f. wt s-1) or dry (nmol g-1 d. wt s-1) weight
| Summary or species | NaCl (mm) | Period of measurement and direction | Flux | Reference |
|---|---|---|---|---|
| Reported on the basis of f. wt | ||||
| Spergularia marina | 25 | Influx 0 min | 13 | Lazof and Cheeseman (1988) |
| Spergularia marina | 90 | Influx 0 min | 110 | Lazof and Cheeseman (1986) |
| Suaeda maritima | 25 | Influx 2 min | 3.8 | Wang et al. (2007) |
| Suaeda maritima | 200 | Influx 2 min | 30 | Wang et al. (2007) |
| Suaeda maritima | 200 | Influx 2 min | 25 | Zhang et al. (2013) |
| Suaeda maritima | 200 | Influx 2 min | 60 | Zhang et al. (2013) |
| Hordeum vulgare | 80 | Influx 5 min | 15–39* | Chen et al. (2007) |
| Arabidopsis thaliana | 50 | Influx 2 min | 31 | Essah et al. (2003) |
| Arabidopsis thaliana | 100 | Influx 3 min | 11 | Wang et al. (2006) |
| Arabidopsis thaliana | 100 | Efflux 10 min | 7.3–8.5 | Wang et al. (2006) |
| Eutrema halophilum | 100 | Influx 3 min | 5.2 | Wang et al. (2006) |
| Eutrema halophilum | 100 | Efflux 10 min | 1.4–4.0 | Wang et al. (2006) |
| Spergularia marina | 25 | Steady state 10–15 min | 2.9 | Lazof and Cheeseman (1988) |
| Spergularia marina | 90 | Linear influx 1–20 min | 1.2 | Lazof and Cheeseman (1986) |
| Suaeda maritima | 25 | Net | 3.3 | Wang et al. (2007) |
| Suaeda maritima | 150 | Net | 9.3 | Wang et al. (2007) |
| Arabidopsis thaliana | 50 | Net 21 d | 0.5 | Wang et al. (2007) |
| Median influx | Approx. 25 | |||
| Reported on the basis of d.wt | ||||
| Arabidopsis thaliana | 100 | 6 h | 28.2 | Wang et al. (2006) |
| Eutrema halophilum | 100 | 6 h | 4.3–9.5 | Wang et al. (2006) |
| Suaeda maritima | 100 | 24 h | 31 | Yeo and Flowers (1986) |
| Suaeda maritima | 300 | 24 h | 62 | Yeo and Flowers (1986) |
| Arabidopsis thaliana | 100 | 25 h | 56 | Volkov et al. (2004) |
| Arabidopsis thaliana | 100 | 25 h | 16.2–20.2 | Wang et al. (2006) |
| Eutrema halophilum | 100 | 25 h | 16.0 | Volkov et al. (2004) |
| Eutrema halophilum | 100 | 25 h | 5.3 | Wang et al. (2006) |
| Suaeda maritima | 340 | Net 7 d | 110 | Yeo and Flowers (1980) |
| Suaeda maritima | 20 | Net 5 d | 36 | Al-Zahrani (1990) |
| Suaeda maritima | 200 | Net 5 d | 67 | Al-Zahrani (1990) |
| Suaeda maritima | 400 | Net 5 d | 80 | Al-Zahrani (1990) |
| Tamarix ramosissima | 242 | Net 30 d | 4.6 | Glenn et al. (2012) |
| Arabidopsis thaliana | 50 | 42 d | 2.0 | Wang et al. (2006) |
| Elymus × oliveri | 75 | 33 d | 5.5 | Ansari (1982) |
| Elymus × oliveri | 150 | 33 d | 5.6 | Ansari (1982) |
| Eutrema halophilum | 100 | 42 d | 0.63 | Wang et al. (2006) |
| Puccinellia maritima | 100 | 63 d | 3.3 | Ansari (1982) |
| Puccinellia maritima | 200 | 63 d | 3.5 | Ansari (1982) |
Influx data represent uptake into the roots (estimated from flux analysis or isotope uptake over minutes); net fluxes were gathered over longer periods (hours, days or weeks) and are fluxes into the whole plant.
*Dependent on the external calcium concentration.
Conclusion
Influx of Na+ through the roots of halophytes is about 25 nmol g–1 f. wt root s–1; net fluxes measured over days can reach values close to 10 nmol g–1 f. wt root s–1 or >100 nmol g–1 d. wt root s–1, setting values that have to be accounted for by the transport systems that operate through membranes in these plants.
FLUXES OF IONS BASED ON MEMBRANE AREA
While fluxes calculated on the basis of fresh or dry weight are often the best data available for higher plants, they do not provide values for the fluxes of ions across membranes per se – although they do provide a context by which to judge such membrane fluxes as are available. A flux based on membrane area is a crucial parameter for determining whether vesicular transport of salts could be feasible and, as so few are available (note fluxes based on the use of vibrating microelectrodes external to a root provide estimates based on the area of the plane in which the electrode vibrates, not on a membrane area; see, for example, Bose et al., 2015), we have collected data from both freshwater and saltwater species.
Fluxes in algae
Fluxes have been estimated for freshwater algae, where cell dimensions can be calculated; for those growing in ‘pond’ water (0.1 mm K, 1.0 mm Na, 0.1 mm Ca and 1.3 mm Cl). MacRobbie (1971a) concluded that fluxes ranged from 10 to 20 nmol m–2 s–1 (fluxes: K+, 10 nmol m–2 s–1; Na+, 5 nmol m–2 s–1; Cl–, 10–20 nmol m–2 s–1), similar to the values of tracer fluxes of 2–35 nmol m–2 s–1 for freshwater algae summarized by Raven (1976; external K+ from 0.1 to 0.2 mm and fluxes from 2 to 20 nmol m–2 s–1; external Na+ from 1 to 2 mm, fluxes 15–30 nmol m–2 s–1; external Cl– from 1 to 2 mm, flux 30–35 nmol m–2 s–1). The tracer fluxes for marine algae (Raven, 1976), where the external concentrations of monovalent ions were in the low-affinity range (K+, 10 mm; Na+ and Cl–, 550 mm) were significantly higher: K+, flux up to 2.1 μmol m–2 s–1; Na+ flux up to 1.1 μmol m–2 s–1; and Cl– flux up to 10 μmol m–2 s–1 (Table 2).
Table 2.
A summary of the magnitude of Na+ fluxes in algae and plants, based on area of the membrane
| NaCl (mm) | Na flux (nmol m–2 s–1) | ||
|---|---|---|---|
| Freshwater algae | 0.1 | 5 | MacRobbie (1971a) |
| Freshwater algae | 1–2 | 1–20* | Raven (1976) |
| Marine algae | 550 | 170 | Raven (1976) |
| Suaeda maritima roots | 340 | 262 | Yeo and Flowers (1986) |
| Suaeda maritima leaves | 66† | 17 | Flowers and Yeo (1986) |
| Arabidopsis thaliana root protoplasts§ | 100 | 700 | Volkov and Amtmann (2006) |
| Eutrema halophilum root protoplasts§ | 100 | 150 | Volkov and Amtmann (2006) |
| Cynodon dactylon gland | 75$ | 408 | Oross et al. (1985) |
| Limonium perezii gland | 300 | 4200‡ | Faraday and Thomson (1986a) |
*Flux across the plasma membrane in the light.
†Estimated xylem concentration (Clipson and Flowers, 1987).
‡Efflux was, in the words of the authors (Faraday and Thomson 1986a), calculated as though ‘evenly distributed across the non-transfusion zone plasma membrane of the secreting gland’.
$Fed to the cut shoots.
Membrane fluxes in salt glands
In order to effect osmotic adjustment, ions are transported across roots and then via the xylem to the shoots where they are accumulated. However, since continued import into the transpiration stream follows from the need for open stomata necessary for continued carbon fixation, halophytes require some mechanism to avoid excess levels of Na+ and Cl– building up in their shoots. Halophytes generally reduce transpiration over time (Flowers et al., 1986; Koyro et al., 2013; Rakhmankulova et al., 2014), thus reducing excessive ion import and balancing import with growth. Salts can be removed by leaf shedding (Albert, 1975; Cram et al., 2002; Ibrahim, 2013), accumulation in external bladders (Thomson and Healey, 1984; Shabala et al., 2014) or excretion via glandular structures (Thomson and Healey, 1984; Flowers and Colmer, 2008; Shabala and Mackay, 2011; Roy and Chakraborty, 2014; Yuan et al., 2016b), an attribute that has evolved >10 times (Dassanayake and Larkin, 2017). Not all halophytes excrete salts, but, in those that do, the mass of excreted salt can exceed those retained in the plant for osmotic adjustment. For example, Rozema and Gude (1981) found that excreted salts accounted for 60 % of total uptake by Spartina anglica, while Glenn et al. (2013) found that 80 % of salt uptake was excreted by Tamarix ramosissima growing in salinized soil.
Glands and bladders have been reported in 117 halophytes (eHALOPH; https://www.sussex.ac.uk/affiliates/halophytes/index.php) and are mainly found within the Amaranthaceae (29 species, largely salt bladders in the genus Atriplex), the Plumbaginaceae (29 species) and the Poaceae (29 species): their structures and function have been the subject of a number of reviews (Lüttge, 1975; Thomson, 1975; Hill and Hill, 1976; Fahn, 1988; Thomson et al., 1988; Salama et al., 1999; Shabala and Mackay, 2011; Tyerman, 2013; Shabala et al., 2014; Yuan et al., 2016b; Dassanayake and Larkin, 2017). Salt glands are two- to multicelled epidermal structures (Fig. 2; a unique exception seems to be the single-celled glands of Porteresia coarctata; Flowers et al., 1990) that are responsible for the movement of salt to an external aerial surface: salt bladders are epidermal cells that accumulate salt, and may burst, releasing the salt solution contained in a large vacuole. Here we are concerned not with the mechanism of secretion per se (see Yuan et al., 2016b; Dassanayake and Larkin, 2017 for recent discussions of salt glands and the mechanism of salt secretion), but with any evidence that vesicles and exocytosis might be involved in a process where Na+ and Cl– fluxes are high (Table 1).
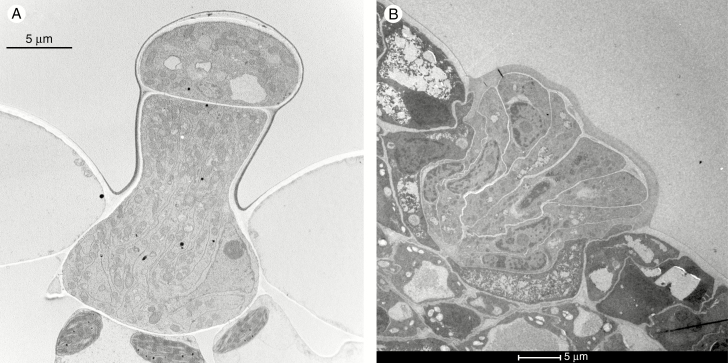
Fig. 2.
Examples of transverse sections of salt glands seen by transmission electron microscopy. (A) A bicellular gland from the abaxial leaf surface of Rhodes grass (Chloris gayana) and (B) a multicellular gland on a leaf of sea lavender (Limonium bicolor). (A) Courtesy of Takao Oi of Nagoya University: more detail can be found in Oi et al. (2012). (B) Courtesy of Bao-Shan Wang, Shandon Normal University, China; detail can be found in Feng et al. (2014).
The simplest of glands are the bicellular trichomes (Fig. 2A) found on the leaves of most grasses (with the exception of the Pooideae; Amarasinghe and Watson, 1988). Some of these trichomes (in the subfamily Chloridoideae) are able to secrete salt (Amarasinghe and Watson, 1989; Ceccoli et al., 2015) and, in one case, fluxes based on membrane area have been estimated. For the glands of a hybrid, Cynodon dactylon × C. transvalensis, Oross et al. (1985) were able to calculate a flux of Na+ of 408 nmol m–2 s–1 (see Table 2).
More complex salt-secreting glands are found in five halophytic species of the family Plumbaginaceae (Aegialitis, Armeria, Limoniastrum, Limonium and Plumbago; Fig. 2B). The glands consist of 16 secretory cells surrounded by a cuticle that isolates most of the apoplast of the gland from the rest of the leaf (there is some apoplastic continuity between what are termed sub-basal cells lying next to the mesophyll, the transfusion zone and the innermost gland cells; Thomson et al., 1988). There is also symplastic continuity via plasmodesmata between the mesophyll cells and the gland cells. For the glands of Limonium perezii, detailed analysis of membrane areas has been carried out, allowing fluxes of Na+ across the plasma membrane that lines wall protuberances to be calculated (Faraday and Thomson, 1986a; see also Table 3). Values were dependent upon the area over which efflux was deemed to occur, but the maximum fluxes of Na+ and Cl– were 70 and 85 μmol m–2 s–1, respectively, if the flux occurred across the plasma membrane of the transfusion zone. Even the lowest fluxes estimated – for fluxes across the plasma membrane lining wall protuberances – were 13 μmol m–2 s–1 for Na+ and 16 μmol m–2 s–1 for Cl–; the flux for Na+ is about 32 times the value reported for the glands of C. dactylon.
Table 3.
The presence of numerous small vesicles has been described in some but not all ultrastructural studies of glands
| Genus | Species | Micro-vesicles | Wall protuberances | Reference |
|---|---|---|---|---|
| (a) | ||||
| Armeria | maritima | Yes | Heumann (2002) – for the uptake of Zn | |
| Atriplex | halimus | Yes | Yes | Smaoui et al. (2011) |
| Atriplex | spongiosa | Yes | Osmond et al. (1969) | |
| Avicennia | marina | Yes | Drennan et al. (1987) | |
| Limonium | bicolor | Yes | Feng et al. (2015); Yuan et al. (2015) | |
| Lysimachia | maritima | Yes | Yes | Rozema et al. (1977) |
| Odyssea | paucinervis | Yes | Yes | Somaru et al. (2002) |
| Porteresia | coarctata | No* | Flowers et al. (1990) | |
| Spartina | foliosa | Yes | Yes | Levering and Thomson (1971) |
| Tamarix | aphylla | Yes | Thomson et al. (1969) | |
| (b) | ||||
| Aegiceras | corniculatum | Cardale and Field (1971) | ||
| Avicennia | germinans | Balsamo and Thomson (1993) | ||
| Atriplex | semibaccata | Campbell et al. (1974) | ||
| Cynodon | dactylon | Oross and Thomson (1982) | ||
| Distichlis | spicata | Oross and Thomson (1982) | ||
| Frankenia | pauciflora | Olesen, 1979 | ||
| Frankenia | salina | Balsamo and Thomson (1993) | ||
| Frankenia | grandifolia | Campbell and Thomson (1976a, b) | ||
| Limonium | platyphyllum | Vassilyev and Stepanova (1990); Campbell et al. (1974) | ||
| Limonium | perezii | Faraday and Thomson (1986a); Faraday et al. (1986) | ||
| Limonium | sinuatum | Faraday and Thomson (1986b) | ||
| Limonium | vulgare | Ziegler and Lüttge (1967) | ||
| Plumbago | auriculata | Faraday and Thomson (1986b) | ||
| Tamarix | aphylla | Thomson and Liu (1967); Campbell et al. (1974) | ||
| Tamarix | usneoides | Wilson et al. (2017a) | ||
The data in (a) include only cases where plants growing in saline media were investigated and exclude those where cut stems were exposed to higher salt concentrations than might be expected in the xylem (see text). These excluded papers are listed in (b).
*The glands are unlike the bicellular glands of other grasses (Type 4 in Dassanayake and Larkin, 2017).
Membrane fluxes through roots of halophytes
Membrane fluxes have been estimated for the roots of one halophyte, S. maritima, an annual species within the Amaranthaceae (a family with 24 % of known halophytes; https://www.sussex.ac.uk/affiliates/halophytes/index.php?content=plantStats). Plants of S. maritima grow optimally in 200–300 mm NaCl, when the sustained net flux of Na+ through the roots is about 100 nmol g–1 d. wt s–1 over a period of 7 d (Yeo and Flowers, 1986; Table 1). Using estimates of the membrane area per unit dry weight of roots and their component cells from the data of Hajibagheri et al. (1985), Yeo and Flowers (1986) calculated fluxes of Na+ to be 763 nmol m–2 s–1 across the epidermal plasmalemma, 262 nmol m–2 s–1 across the combined area of epidermal and cortical plasmalemma, and 6.25 μmol m–2 s–1 across the endodermal surface area. They (Yeo and Flowers, 1986) argued a flux across epidermal and cortical cells to be the most likely, based on its size: loading of the xylem, however, required a flux of 1.18 μmol m–2 s–1.
Ions passing through the roots of S. maritima are transferred to the xylem, where the concentration of Na+ ions in these plants growing in 200 mm NaCl (around the optimum salinity for growth) is about 66 mm (Clipson and Flowers, 1987); this results in the delivery of 74 mol Na+ m–3 shoots d–1 (Flowers and Yeo, 1986). Using the stereological data of Hajibagheri et al. (1984), Flowers and Yeo (1986b) calculated that the supply of Na+ to the leaf would average about 17 nmol m–2 plasma membrane s–1, similar to values in the range reported for freshwater algae (see Table 2).
Fluxes have also been calculated from electrophysiological data. Volkov and Amtmann (2006) obtained values of 700 and 150 nmol m–2 s–1 (with an external concentration of 100 mm NaCl and –80 mV applied to the interior of the plasma membrane) of protoplasts of the glycophyte A. thaliana and the halophyte Eutrema halophilum (syn. Thellungialla halophila), respectively (values could vary with the NaCl concentration and applied voltages).
Fluxes in other systems
There have been other estimates of the magnitude of solute fluxes in plants – of K+ and of sugars. For example, K+ influx into protoplasts isolated from roots of Zea mays from a 1 mm K+ concentration was 99 nmol m–2 s–1 (Gronwald and Leonard, 1982), while the flux of K+ into guard cells of Commelina communis was estimated to be up to 1.9 μmol m–2 s–1 (Penny and Bowling, 1974). For the roots of rye, K+ flux across the epidermal cells (White and Lemtiri-Chlieh, 1995) was calculated to lie between 14 and 27 nmol m–2 s–1. By way of comparison, oocytes of the African clawed toad Xenopus laevis, which are used for heterologous expression and overexpression of membrane ion channels and transporters from different organisms, can provide the range of maximal potential fluxes via these membrane proteins. These artificial systems may give ion currents of around 10 μA (sometimes slightly higher) per oocyte for high-level expression, which is about 30 μmol m–2 s–1 (e.g. Volkov, 2015a).
Turning to non-electrolytes, estimated fluxes of sugars for plants range between 3 nmol m–2 s–1 and 157 μmol m–2 s–1. Although not charged and so different from Na+ or Cl–, the magnitude of the fluxes of sucrose does illustrate the capacity of vesicular transport in plants. Phloem transport of sugars ranges from 20 to 200 nmol m–2 s–1 (MacRobbie, 1971b), while a flux of sucrose through plasmodesmata in the nectaries of Abutilon megapotamicum var. anviegaturn was calculated to be 157 μmol m–2 s–1, about three orders of magnitude greater than for other fluxes (Gunning and Hughes, 1976). Using data in Etxeberria et al (2005b), we calculate that the transport rate of sugar in Citrus reticulata is about 90 nmol m–2 plasma membrane s–1 over 24 h. This transport process, which was sufficient to support the rapid growth of citrus fruits from fruit set to ripening at a sucrose import rate of 2.5 nmol g–1 d. wt s–1, is within the range of Na+ uptake rates listed for halophytes in Table 1.
Conclusions
Membrane fluxes of Na+ in halophytes are commonly in the range 150–1000 nmol m–2 s–1. There are, however, instances of higher fluxes, such as that of K+ across stomata (1.9 μmol m–2 s–1), Na+ in Limonium salt glands (4 μmol m–2 s–1), Na+ and Cl– fluxes in marine algae (up to 1.1 and 10 μmol m–2 s–1, respectively), sucrose fluxes in A. megapotamicum (157 μmol m–2 s–1) and fluxes across oocyte membranes under artificial conditions (up to 30 μmol m–2 s–1). The high value of 4 μmol m–2 s–1 that has been estimated for Limonium salt glands depends on an assessment of their morphology and judgement of the area of available membranes or contact zones (via membranes or plasmodesmata) between cells in the gland. A concerning feature of the Limonium estimates, which used cut shoots and leaf disks to measure fluxes, is the difference in secretion rates from those calculated from intact shoots of another species in the same genus. The flux of Na+ estimated by sequential rinsing of leaf tissues of L. vulgare growing in 200 mm NaCl by Rozema et al. (1981) was around 1 pmol per gland per hour, dramatically lower than the 230–390 pmol per gland per hour reported for glands on disks punched from detached leaves of L. perezii floated on 300 mm NaCl or detached whole leaves of L. perezii with their petioles in 300 mm NaCl (Faraday et al., 1986). This suggests to us that ions were reaching the apoplast at higher concentrations than might be expected in an intact transpiring leaf and that this influenced secretion rates, raising doubts over the very high fluxes estimated and requiring additional assessments and checks. While we do not have an explanation for the very high symplastic fluxes via plasmodesmata in the Abutilon gland, the high fluxes across oocyte membranes are artificially created with a very high density of heterologously expressed membrane proteins. We conclude that fluxes of the order of 150–1000 nmol m–2 s–1 need to be accounted for in halophytes. The question is, could such fluxes occur though vesicular transport?
VESICULAR TRANSPORT IN PLANTS
Vesicle trafficking in cells involves both exo- and endocytosis (sometimes called pinocytosis), the first being the fusion of vesicles produced by the Golgi apparatus with the plasmalemma and the latter being the recovery of membrane material from the plasma membrane (Fig. 3). There is now strong evidence that both endo- and exocytosis occur in plants (Battey et al., 1999). Early evidence of endocytosis came from the uptake of the membrane-impermeable dye, Lucifer Yellow, and of electron-dense tracers by plant protoplasts (see Gradmann and Robinson, 1989; Low and Chandra, 1994). More recently, the use of patch clamp to measure electric capacitance has allowed detection of vesicle fusions (see Homann and Tester, 1997; Thiel and Battey, 1998) and the uptake of nanoparticles by plants has been established (reviewed in Anjum et al., 2016), including gold nanoparticles in tomato and rice (Li et al., 2016). Evidence for exocytosis has been gathered from studies of tissues such as pollen tubes and nectaries (Battey et al., 1999; Grebnev et al., 2017).
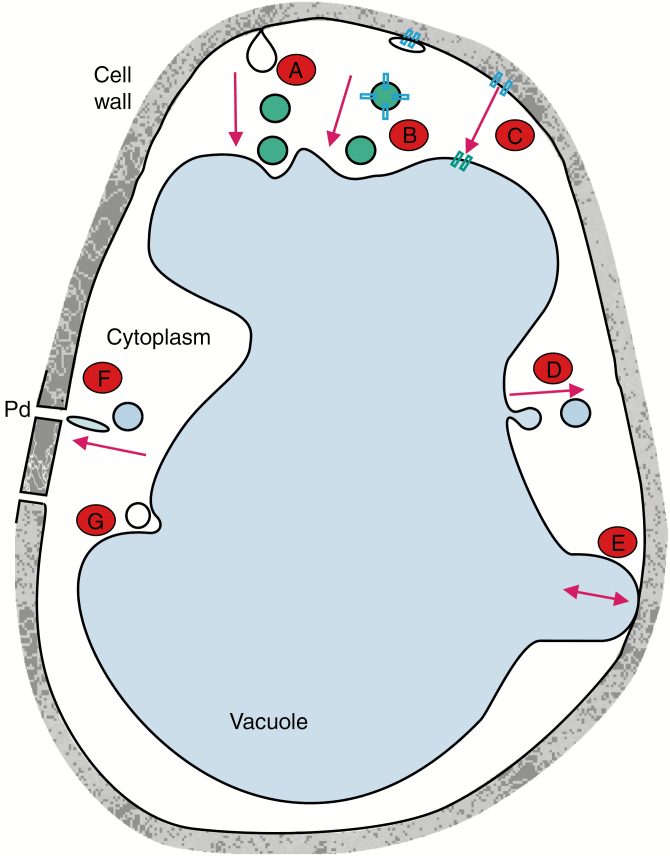
Fig. 3.
Hypothetical vesicular transport of Na+ ions in halophytes. The figure illustrates a hypothetical cell from a halophyte. The average Na+ concentrations in the cytoplasm and vacuole are about 150–200 mm (Flowers et al., 2015) and 500 mm (Flowers, 1985), respectively; the turgor pressure is low for a plant cell at about 0.2 MPa (Flowers et al., 2015), due to high apoplastic ion concentrations (Harvey et al., 1981). In the figure, we hypothesize six possible transport scenarios, none of which excludes combination with other possibilities. A, clathrin-dependent or clathrin-independent (more likely due to the size of the vesicles) endocytosis with vesicles either fusing with the vacuole or moving from cell to cell via plasmodesmata (F). B, a microdomain of the plasma membrane-rich in transporters invaginates to form a vesicle that is loaded with Na+ from the cell wall or cytoplasm; the fate of the vesicle is as in A. C, non-vesicular ion transport through transporters and channels. D, exocytosis of vesicles originating from the tonoplast allows export of ions (Echeverria, 2000; see also Mellander et al., 2014 for possible mechanisms). E, junctional complexes (Vassililyev and Stepanova, 1990); in the words of Echeverria (2000), ‘Juxtaposition of membrane-bound carriers located at the tonoplast and the plasmalemma. These allow the direct transfer of metabolites to the apoplast’. F, vesicular transport through plasmodesmata (Wilson et al., 2017). G, formation of a multivesicular body (MVB; see Balnokin et al., 2007 and text above). pd, plasmodesma.
Although there is now strong evidence in favour of endo- and exocytosis in plants (see Echeverria, 2000), in 1980 Cram wrote in the abstract of his review on ‘Pinocytosis in plants’: ‘Thus it appears not only that pinocytosis does not occur, but also that it could not occur in plant cells.’ He (Cram, 1980) raised objections on the grounds of selectivity of uptake, depolarization of the membrane, turgor, energetics, the rate of endocytosis in relation to measured rates of uptake and the density of putative binding sites. However, the energy required for plasmalemma recycling has been estimated to be a very small (0.06 %) fraction of the total energy required for biochemical synthetic processes in cells (Raven, 1987, appendix II) and sufficient for receptor-mediated endocytosis (RME), since the vesicles are small (Saxton and Breidenbach, 1988). As far as turgor is concerned, Gradmann and Robinson (1989) concluded that endocytotic vesicles could only form where turgor was less than about 0.1 MPa. More recently, however, a thermodynamic analysis has shown that endocytosis is feasible if the tension in the membrane is low and vesicles are flat with little volume as they detach from the plasma membrane (Fricke et al., 2000). The thermodynamic approach sets a biophysical framework for the processes and has been applied assuming membranes have a homogeneous structure and surface tension. However, adjustments may be necessary for real biological membranes, where, for example, micro-domains with localized ion channels have been described for plasma membranes (e.g. Mongrand et al., 2004; Sutter et al., 2006; reviewed in Malinsky et al., 2013) demonstrating their complex structure in plant cells. More recent modelling suggests that vesiculation can occur even under high membrane tensions (Hassinger et al., 2017). Furthermore, it is reasonable to consider that all the membrane enzymes and ion channels in plants operate at the pressures found in their cells (approx. 0.5 MPa), so there could be a variety of unknown effects at the molecular level with extra weak molecular forces involved (e.g. Van der Waals forces and London forces), which may outweigh the turgor pressure. The forces may act non-linearly and could even be negative depending on the distance between interacting molecules (e.g. Israelachvili, 2011). So, while the thermodynamic approach remains valid for the whole cells and larger systems, it may need refining for individual parts of systems and for their membrane domains. A further complication lies in the fact that the physical properties of membranes and their behaviour are influenced by the underlying cytoskeleton: myosin-I controls cell membrane tension for living animal epithelial cells and fibroblasts (e.g. Nambiar et al., 2009), while for fungal cells of Aspergillus nidulans (which has cell walls and high turgor pressures), a mutated version of myoA (the same class I myosin gene) constitutively activated endocytosis (Yamashita and May, 1998). Myosin from Chara corallina moves actin filaments an order of magnitude faster than myosin from skeletal muscle (Ito et al., 2007). Cytoplasmic streaming, which is driven by myosin motors (Kurth et al., 2017), was thought to be a possible limiting factor in the movement of Na+ (and Cl–) across the roots of S. maritima (Yeo and Flowers, 1986).
In spite of early scepticism, there is now good evidence for endocytosis in plants and its role in solute transport (Echeverria, 2000). For example, a sucrose-induced endocytosis of sucrose into the vacuoles of cultured cells of sycamore and juice cells of Citrus (Etxeberria et al., 2005a) has been clearly demonstrated. Where sucrose or other sugars are transported, they are delivered in high concentration, typically 250 mm or more, to the apoplast of the receiving cells via unloading from the phloem. For citrus juice cells, uptake of sucrose into the cells does occur via transporters as well as by relatively large endocytotic vesicles (up to 7 μm in diameter) that form at the plasma membrane (Etxeberria et al., 2005b, 2007, 2009). Transport via an endocytic system allows uptake to bypass both the plasmalemma and tonoplast transporters and to incorporate sucrose directly from the apoplast to the vacuole (Etxeberria et al., 2005a, b). The process has been followed by labelling the outside lamella of the plasma membrane with vital fluorescent stains, transport of which equates quantitatively with the formation and transport of sucrose into vacuoles. In protoplasts of Citrus aurantifolia (sweet lime), fluid-phase endocytosis transports sucrose from the medium to the vacuoles in vesicle populations of two sizes, one about 1 μm in diameter and the other 1–7 μm in diameter (Etxeberria et al., 2009). Labelling with fluorescent dyes suggested that the larger vesicles were the primary transport system for fluid-phase endocytosis (Etxeberria et al., 2012) and independent of the endosome.
Although lagging behind information available for animals, much is now known about the endosome in plants and about the mechanism of endocytosis (Valencia et al., 2016). During endocytosis, parts of the plasma membrane enclose extracellular substances in vesicles that then fuse with membranes originating from the Golgi apparatus, the endosome. This endosome is comprised of the trans-Golgi network (TGN), which as an early endosome receives the contents of vesicles containing a cargo; these early endosomes mature to multivesicular endosomes that can fuse with lysosomes and vacuoles (Valencia et al., 2016). The process of trafficking (most often investigated in terms of protein as the cargo; see, for example, Uemura, 2016) involves a protein coat (Hwang and Robinson, 2009), which prevents fusion of the vesicle membrane with other membranes: coated pits have long been known to be present in plant cells (see Robinson and Hillmer, 1990). A key feature of the transport process is the presence of micro-domains in the plasma membrane surface that are able to recognize and concentrate the cargo. It is the cargo that is postulated to bind to proteins that in turn binds to other proteins that cause the membrane to form a pit and then, in a complex process, a vesicle. These pits may be coated by a protein, clathrin (clathrin-mediated endocytosis, CME; see also Hassinger et al., 2017), or vesiculation may be clathrin independent (Fan et al., 2015). In plants, there is evidence that flotillins (membrane proteins that function in endocytosis; see Otto and Nichols, 2011; Danek et al., 2016) are involved in clathrin-independent endocytosis (CIE). These vesicles involved in CIE have a diameter of 65–200 nm in yeast (median value 115 nm; Cucu et al., 2017) and 100 nm in A. thaliana; clathrin-coated vesicles appear to be of similar size (Beevers, 1996; Li et al., 2012; Baral et al., 2015). Danek et al (2016) speculated that for the same cargo, CME may be constitutive while CIE may be induced by stress or signalling. The large (up to 7 μm in diameter) clathrin-independent vesicles seen in sweet lime are probably the primary pathway for fluid-phase endocytosis (Etxeberria et al., 2012). Interestingly, it is CIE, some kinds of which involve flotillins, whose function is associated with membrane sterols (Danek et al., 2016). Membrane sterols are normally a small component of plant membranes, but are relatively enriched in the halophyte S. maritima (Leach et al., 1990). Chloroplast detergent-resistant membrane fractions from the halophyte Salicornia perennans contained a higher concentration of sterols than the less tolerant Artemisia santonicum (Nesterov et al., 2017).
Endocytosis requires exocytosis to recycle the membrane material and water that accompanies the solutes (Robinson and Hillmer, 1990; Thiel and Battey, 1998; Battey et al., 1999; Etxeberria et al., 2013). In beet storage cells, rapid endocytosis was balanced by the formation of vacuole-derived vesicles which transiently counter-balanced the addition of surplus endocytic membrane (Etxeberria et al., 2013). However, as far as we are aware, little is known of how the lipid composition is modified or of the role of flippases (see, for example, Sahu and Gummadi, 2008; Lopez-Marques et al., 2012; Underwood et al., 2017) and how they work under turgor pressure. Exocytosis is also the mechanism by which plants secrete a range of substance – from nectar, lipophilic materials, resins, gum and the cellulose and other polysaccahrides for cell wall synthesis (e.g. Yoneda and Doering, 2006) to salts, for which there is, at least, circumstantial evidence for the role of vesicular transport (Fahn, 1988). Mechanical energy is released when vesicles at high pressure in the cytoplasm fuse with the membrane at the lower pressure of the cell wall, so that turgor favours exocytosis (but not endocytosis; Fricke et al., 2000).
Might ions be transported in vesicles?
Although theoretically possible, the question remains as to whether plants and particularly halophytes use vesicular transport for the movement of Na+ and Cl – into and out of cells. Bennett (1956) hypothesized that ‘membrane flow may be an important part of a type of active transport mechanism carrying particles, including ions, along, within, into and out of cells’, a concept promulgated for plants by Sutcliffe (1962). Evidence for and against the presence of micro-vesicles and their involvement in ion uptake as well as the radial transport of ions was reviewed by Baker and Hall (1973), at a time when kinetic analysis of influx and efflux in plants was much in vogue. They (Baker and Hall, 1973) emphasized the notion of a dynamic cell, where endocytotic vesicles, which incorporated membrane-bound carriers, formed at the plasma membrane. Once formed, the vesicles would release ions either in the cytoplasm by breakdown of the membrane or in a larger vacuole by fusion with the tonoplast. An immediate question that arose was identifying any vesicle involved in transport from other small vacuoles. Hall (1970) had earlier provided evidence of the presence of small (average diameter of 130 nm) vesicles in the meristematic and cortical cells of maize roots that stained heavily for ATPase and were associated with the plasma membrane. Treating the roots with KCl increased the proportion of cell sections with vesicles. These data were taken as providing significant evidence in favour of the role of endocytosis in the uptake of K+ into roots. Evidence in favour of vesicular transport of ions now comes from a variety of experiments. (see Echeverria, 2000).
Endocytosis has been implicated in the uptake of toxic elements (uranyl and lead) into oats, barley and maize roots (Wheeler and Hanchey, 1971; Wheeler et al., 1972; Malone et al., 1974) and in the glycophyte Raphanus sativus, where treatment with uranyl acetate induced vesiculation and uranyl phosphate was visible in both the apoplast and vesicles (Romanenko et al., 1986). NaCl-induced vesiculation was seen in the halophyte Salicornia europaea and the glycophytes Hordeum vulgare and Phaseolus vulgaris (Nassery and Jones, 1976a). Treatment of samples containing Cl– with a soluble salt of Ag leads to precipitation of AgCl, which has very low solubility and is electron dense, so visible under an electron microscope. Electron-dense deposits of Cl as its Ag salt have been demonstrated in the halophytes Limonium vulgare, Salicornia pacifica, Tamarix aphylla, Aegiceras corniculata and Petrosimina triandra (Ziegler and Lüttge, 1967; Hess et al., 1975; Campbell and Thomson, 1975; Van Steveninck et al., 1976b; Kurkova et al., 1992) and the glycophytes Pinus radiata, Phaseolus vulgaris and Hordeum vulgare (Nassery and Jones, 1976b; Van Steveninck et al., 1976a, b; Foster and Sands, 1977). Support has also come from the localization of La3+ in the bladders of Atriplex halimus, where, after submergence of excised leaves in a solution of La(NO3)3, La3+ is present in small vesicles (Smaoui et al., 2011). While the presence of electron-dense deposits of AgCl argues in favour of the role of vesicles in the transport of Cl–, it was clear from the early work of Harvey et al (1976) that there is a significant loss of Cl– during preparation of tissue for electron microscopy. So, localization, especially in small vesicles, could be compromised and remains a technical challenge, especially as side-scattering of electrons means the minimal area that can be resolved is >100 nm2 (see Ralle and Lutsenko, 2009). Losses of Cl– can be reduced by the use of freeze substitution, and precipitates of AgCl have been seen in the cytoplasm of the halophyte Suaeda maritima prepared using this procedure (Harvey et al., 1979), although with this technical improvement the resolution in unstained tissues was insufficient to localize Cl to small vesicles. Overall, however, the presence of Ag deposits in vesicles is supportive of their role in ion transport.
In studies of the halophyte Suaeda altissima, Balnokin and colleagues (Kurkova et al., 1992; Kurkova and Balnokin, 1994) suggested that vesicles they could see in electron micrographs were composed of more than one membrane and led to the view (see Balnokin et al., 2007) that these structures were composed of plasmalemma and tonoplast. AgCl was identifiable in Ag-treated material, but could not be unequivocally located to the large vesicles seen in glutaraldehyde-fixed material. These vesicles, which were 2–3 μm in diameter, are not consistent with the endo- and exocytotic vesicles described above, but could be examples of multivesicular bodies, (MVBs) about which much has been discovered over recent years (Cui et al., 2016; Schoneberg et al., 2017; Wang et al., 2017), including a role in response to salt stress (Wang et al., 2015a). The evidence from S. altissima suggests that these MVBs are involved in Cl– transport, although the evidence is again compromised by the potential movement of Cl– during fixation. However, Shuvalov et al. (2015) have provided evidence that a Cl–/H+ antiporter is associated with a Golgi membrane fraction prepared from the roots of S. altissima.
Attempts have also been made to use the electron opacity of Rb+ in order to identify its localization within cells as it is accumulated by plants and has been used as a tracer for K+. Following exposure of roots of S. maritima to RbCl, electron-dense deposits can been seen in the central vacuoles (Hall et al., 1974). However, significant quantities of Rb are lost during conventional fixation for microscopy (Hall et al., 1974), raising questions about the quality of the evidence for endocytosis in K+ (Rb+) uptake. Nevertheless, the presence of Rb-containing vesicles is suggestive of a role in ion transport. Further evidence in favour of vesicular transport in halophytes has been obtained from the study of salt glands.
The salt glands of species of the genus Tamarix consist of eight cells: six secretory cells and two basal cells (Thomson and Liu, 1967; Bosabalidis and Thomson, 1984; Fig. 2). Secretion, which takes place through four secretory pores (Feng et al., 2014), is greater during the day than at night (Imada et al., 2012) and reflects the salt concentration around the roots (Thomson et al., 1969; Sookbirsingh et al., 2010). Circumstantial evidence in favour of the involvement of vesicles in transport (Table 3) comes from electron microscopic investigations of Tamarix aphylla that found micro-vesicles associated with the plasma membrane (Thomson and Liu, 1967; Thomson et al., 1988; Wilson et al., 2017). After exposure of cut twigs to RbSO4, these vesicles contained electron-dense material (Thomson et al., 1969). Faraday and Thomson (1986b) looked at the anatomy and ultrastructure of 11 species and six genera of Plumbaginaceae. They noted that ‘While microvacuoles and vesicles were often present, they were not prominent, and there was no apparent correlation between their presence or absence and whether the glands were secreting or non-secreting’. They (Faraday and Thomson, 1986b) went on to conclude that the absence of numerous vesicles in the glands of Limonium perezii was evidence of a trans-membrane rather than a vesicle-mediated pathway. Nevertheless, there are genes associated with vesicle formation that are upregulated in L. bicolor when plants are exposed to salinity (Yuan et al., 2015).
As well as the species listed in Table 3a, there have been many other species (Table 3b) where vesicles have been described, but where plants were grown on garden soil and in some cases where the ends of cut shoots were inserted into salt solutions much higher than might be expected in the xylem. For example, Campbell and Thomson (1976b) induced salt secretion in glands of Frankenia grandifolia by immersing cut stems into NaCl (513 mm). Secretion began 4 h after treatment, and after 15 h the authors noted micro-vesicles scattered through the cytoplasm, which they speculated arose from the endoplasmic reticulum (ER). They (Campbell and Thomson, 1976b) suggested ‘transport components, perhaps synthesized in the ER, might feasibly be functional while being transferred to the plasma membrane in microvesicular form’. The treatment, however, may have supplied the leaf apoplast with a much higher salt concentration (>500 mm) than that normally experienced, as all plants, including halophytes, prevent a significant proportion of the ions present in the external solution from reaching the xylem (Munns, 2005). For example, in the halophyte S. maritima, the mean Na+ concentration in the xylem of plants growing in 200 mm NaCl was just 66 mm (Clipson and Flowers, 1987). The data from the experiments listed in Table 3b have not been included in our analysis.
In addition to the microscopic evidence for the presence of vesicles in plant cells, there is also physiological evidence consistent with vesicular transport. MacRobbie (1971a) reviewed analyses of influx and efflux kinetics of ions in algae and concluded that for Nitella transluscens, an alga with giant cells, there were within the cytoplasmic and vacuolar phases rapid entry components that were consistent with movement of ions in vesicles. Subsequently, Lazof and Cheeseman (1986) provided kinetic evidence in the roots of the halophyte Spergularia marina for a small (1 %) rapidly exchanging compartment of the cytoplasm, which they could not identify, but would be consistent with a vesicular pool (evidence in the literature which they discussed in some detail). In a later review, MacRobbie (1999) suggested it would be timely to reinvestigate the transfer of Cl–and Br– between the cytoplasm and vacuole of giant algal cells using methods that would test whether vesicular transport is involved in this transport process and in the rapid movement of K+ and sucrose in stomatal closure as well as the rapid movement of water in the leaf of species of Mimosa. There is now evidence for endocytosis of K+ in guard cells of Vicia faba, where turgor pressures are high, obtained using a styryl dye, FM4-64 (Meckel et al., 2004; see also below). In the future, the use of FM dyes (lipophilic styryl dyes that bind to membranes; see, for example, Bolte et al., 2004; Malinska et al., 2014; Rigal et al., 2015) and other fluorescent probes may aid localization of vesicular transport of ions; for example, the use of Di-4-ANEPPDHQ (Zhao et al., 2015) and of filipin (a sterol-specific marker; Klima and Foissner, 2008) to label membrane domains.
Evidence from molecular biology
There is molecular evidence pointing towards a role for vesicular transport in the tolerance of plants to salt (reviewed in Baral et al., 2015).
(1) In A. thaliana, overexpression of a gene, AtRabG3e, that regulates vesicle trafficking increased vesicular endocytosis and raised Na+ contents and salt tolerance in transgenic plants compared with control plants (Mazel et al., 2004): the authors suggested that salinity tolerance was associated with an increase in vacuolar volume and the ‘process requires delivery of membrane material to the vacuole by an intracellular vesicle trafficking system, which is in line with the predicted function of AtRabG3e’. Salt stress (150 mm NaCl) induced the production of reactive oxygen species (ROS) and increased endocytosis (monitored with FM dyes) in the root tips of A. thaliana, leading to the suggestion that ROS act as a signal in the response to salt (Leshem et al., 2007). In separate experiments, treatment of roots of A. thaliana with high (200 mm) salt concentrations resulted in accumulation of Na+ in small vesicles (Hamaji et al., 2009). However, whether this is an adaptive or a pathological response is not clear, as the treatment with 200 mm NaCl is likely to be lethal for A. thaliana (Inan et al., 2004, Attia et al., 2008).
(2) In habanero pepper exposed to NaCl, Na+ was associated with vacuoles and small intracellular compartment in a relatively tolerant genotype, whereas this was not so in a relatively salt-sensitive genotype (Bojorquez-Quintal et al., 2014).
(3) The NHX antiporter genes AtNHX5 and AtNHX6 are located in the endosome (see Wang et al., 2015b) and expressing AtNHX5 in transgenic soybean raised its tolerance to salt compared with wild-type plants and increased the concentration of Na+ in leaves and roots, suggesting a role for endosomal transport in the efficient movement of Na+ (Wu et al., 2016).
(4) Annexins (widely conserved Ca2+- and phospholipid-binding proteins) are thought to have a role in membrane trafficking as well as in exocytosis due to their ability to bind to membranes and position membrane structures in a Ca-dependent way (see the review by Konopka-Postupolska and Clark, 2017). Since annexins also appear to play a role in stress tolerance in plants through ROS-induced Ca2+ signalling (see Shabala et al., 2015; Wilkins et al., 2016), there is a possible link between salt stress, the generation of ROS in the apoplast by NADPH oxidases and vesicular transport, perhaps involving MVBs, where annexins may also play a role (Konopka-Postupolska and Clark, 2017). Such a link may prove to be part of the explanation of the long-established importance of Ca2+ in salt tolerance (Greenway and Munns, 1980). There is also molecular evidence for the trafficking of plasma membrane transporters (Fan et al., 2015; see Valencia et al., 2016), of their clustering in membrane micro-domains (Fan et al., 2015) and regulation of alkali metal transporters through their recycling from the plasma membrane in yeasts, plants and mammals (Miguel Mulet et al., 2013).
(5) The A. thaliana flotillin genes (Aflot) are induced under salt stress, and Aflot2 binds to a cation hydrogen exchanger (CHX), CPA2, suggesting the flotillins may be involved in the uptake of monovalent cations (see Danek et al., 2016 for further discussion).
(6) Evidence has been presented supporting the view that K+ channels are added and removed in clusters to and from the plasma membrane of isolated guard cell protoplasts of V. faba during pressure-driven volume changes (Hurst et al., 2004) and that the hormone abscisic acid initiates the endocytosis of the K+ channel KAT1 in A. thaliana (Sutter et al., 2007).
(7) Other transporters have also been shown to be located in vesicular membranes. For example, the eight NHX transporters found in A. thaliana are divided between vacuolar, endosomal and plasma membranes (see Qiu, 2016), with AtNHX5 and AtNHX6 being localized in the Golgi and TGN, where they regulate endosomal pH (Bassil et al., 2011a, b; Reguera et al., 2015) as well as playing a role in K+ transport (see Wang et al., 2015b).
(8) The cation–chloride co-transporters, from both A. thaliana and Vitis vinifera have also been shown located to the Golgi and TGN with a potential role in Na+ and Cl– transport and salt tolerance (Henderson et al., 2015).
Membrane fluxes in halophytes
While molecular mechanisms for salt excretion through salt bladders can be postulated based on transporters alone (Shabala et al., 2014), recent reviews of multicellular salt glands have included a role for vesicular cell to cell transport followed by exocytosis onto leaves (Feng et al., 2014; Zouhaier et al., 2015; Yuan et al., 2016a; Wilson et al., 2017). Whether or not vesicular transport of ions is a feasible means of transport depends, at least in part, on the rate at which vesicles fuse with the tonoplast (for internal accumulation) or the plasma membrane (for secretion), on the concentration of ions in vesicles (see Table 4) and on movement of vesicles. Estimates for rates of movement of vesicles vary widely (three orders of magnitude) in different organisms. For yeasts, vesicles move at 150 nm s–1 (Toshima et al., 2006), while in Characean algae, the rate of cytoplasmic streaming is 50 μm s–1 (Tominaga et al., 2013). The rate of movement of clathrin-coated vesicles in yeast has been estimated at 5 μm s–1 (Ehrlich et al., 2004). So, finally, we evaluate whether rates of membrane turnover match the fluxes – and whether the fluxes recorded in halophytes in Table 2 could feasibly occur via vesicles.
Table 4.
Diameters of vesicles reported from eukaryotic cells
| Vesicle type | Vesicle diameter (nm) | Characteristics | References | |
|---|---|---|---|---|
| Endocytotic | 80 | Time to internalize an area equivalent to plasma membrane area (min) | 20–115 | See Steer (1988) |
| Exocytotic | 70–400 | Productivity/unit volume dictyosome (μm2 μm–3 min–1) | 0.65–3.6 | See Steer (1988) |
| Exocytotic | 80–150 | Arrival rate number s–1 | 100 | See Thiel and Battey (1998) |
| Exocytotic (yeast) | 100 | Fusions min–1 during growth | 18 | Cucu et al. (2017) |
| Endocytotic (Arabidopsis) | 30–100 | Clathrin mediated | Beevers (1996); Dhonukshe et al. (2007),Baral et al. (2015) | |
| Endocytotic (glucose uptake) | 80–220 | Clathrin independent | Bandmann and Homann (2012) | |
| Endocytotic (nano beads) | 20–1000 | Clathrin independent | Bandmann et al. (2012) | |
| Endocytotic | 100 | Clathrin independent, flotillin mediated | Li et al. (2012) | |
Although there are few data on membrane fluxes for halophytes, there are examples that cover movement of ions across roots and leaves and excretion of ions from salt glands. In order to model fluxes, we have used data on vesicular sizes taken from the literature (Table 4), i.e. vesicles with a diameter of 100 nm arriving at the plasma membrane at rates of up to 100 s–1. Because of their size, this vesicular transport may be clathrin independent and probably involves flotillins (cf Table 4).
In our first example, we evaluate transport in the halophyte S. maritima. The flux of Na+ across the root has been estimated to be between 262 and 6250 nmol m–2 s–1 (see above). A vesicle of 100 nm diameter containing a concentration of 500 mm NaCl would contain 2.62 × 10–10 nmol of Na+, so a flux of 262 nmol m–2 s–1 would require 1012 vesicles m–2 s–1. This number of vesicles would have a surface area of 0.03 m2, so 32 × 1012 vesicles merging per second would replace the membrane area of 1 m2. For an average cell (diameter 28 μm and area about 2500 μm2) this would mean around 2500 vesicles fusing per second. Larger vesicles of 400 nm diameter containing a similar concentration of ions would require 40 vesicles per cell per second and, if the diameter was 1 μm (Balnokin et al., 2007; Klima and Foissner, 2008; Etxeberria et al., 2009; Table 4), just three vesicles every second. A similar argument could be made for the flux of approx. 1000 nmol m–2 s–1 that crosses into the xylem (Yeo and Flowers, 1986; see also above) where fusing nine 1 μm diameter vesicles containing 500 mm Na+ every second over the area of an average xylem parenchyma cell would deliver a flux of 1000 nmol m–2 s–1 or 100 nmol g–1 d. wt roots s–1.
The flux for leaves of S. maritima is 17 nmol m–2 s–1, where mesophyll cells have an average radius of 27 μm (volume of 80 × 10–15 m3; see Flowers and Yeo, 1986). With a vesicle diameter of 100 nm containing a solution of 500 mm Na+ (close to that in the vacuoles of plants growing in 340 mm NaCl (Harvey et al., 1981), a flux of 17 nmol m–2 s–1 could be delivered when the surface area is replaced every 16 min. It appears entirely feasible that the flux of ions from the xylem to cell vacuole could occur within vesicles. Flowers and Yeo (1986) also calculated fluxes for different leaves, which were expanding at different rates (between 2 and 10 m3 m–3 s–1) and for cells of different sizes. The flux required to maintain an internal salt concentration of 350 mm (for plants growing in 200 mm NaCl, the measured range was between 420 and 380 mm as a leaf doubled in weight) was <20 nmol m–2 s–1, again well within the range of membrane fluxes reported in Table 2 and feasible as a vesicular flux. Vesicles, however, carry water as well as ions, but water transport is hard to evaluate.
When endocytosis occurs the solution would be sampled from the cell wall, not the external medium. In Suaeda maritima, this would be between 100 and 200 mol m–3 cell wall volume for plants growing in 200 and 340 mm NaCl (Flowers, 1985; Hajibagheri and Flowers, 1989). If the cell wall has a water content of about 35 % solute available space (Flowers, 1985), then this would represent approx. 300–500 mm Na+. However, the concentration of ions in solution and not bound to fixed charges in the cell wall is not known. In our calculations of fluxes, we used a figure of 500 mm as the ion concentration entering the vesicle. Water will enter along with ions, but we argue it will also leave during the balancing of membrane area via exocytosis. However, while we make assumptions of net water movement, we do not know of the detailed water relations between vesicles and the surrounding cytoplasm. These will be a function of reflection coefficients and osmotic potential differences, but complicated by the pressure, which in a droplet is determined by radius and surface tension – so, for a 300 nm vesicle, this might mean an internal pressure of 9.6 MPa depending on water interaction with lipid membrane, well above that in the cell as a whole. Ions remaining in the apoplast will serve to reduce cellular turgor (see Yeo and Flowers, 1986), which although unknown for root cells is low in the leaves of S. maritima, i.e, between 0.05 and 0.3 MPa (Clipson et al., 1985). High elasticity in the cell wall will also mitigate against a turgor rise, as would a continuous slow increase in volume – as seen in the leaves of halophytes (Flowers, 1985).
Other important factors that are intrinsically related to vesicular transport are the speed of vesicles in the cytoplasm and the molecular motors for any vesicular transport (specific types of actin–myosins or dynein–kinesins). Several types of myosins participate in vesicular transport (e.g. Buss et al., 2001; Peremyslov et al., 2013; Kurth et al., 2017); kinesins running on tubulin microtubules could also be involved (e.g. reviewed in Endow et al., 2010). Here there are still many questions about how the size and type/molecular surface of the vesicles are linked to the speed of vesicular transport via the cytoplasm and the particular molecular motors involved. Evidently, a thicker layer of cytoplasm requires more time and energy for the transport, while the estimates for the speed of transport differ by orders of magnitude and need more study. Developing methods that allow manipulation of the speed of cytoplasmic streaming and intracellular flows (Mittasch et al., 2018) could be a way to estimate quantitatively the role of vesicular transport and the particular roles of specific vesicles in net salt transport in halophytes.
CONCLUSIONS
We have recorded a considerable range for the fluxes of Na+ and Cl– into and out of the organs of halophytes, with many values lying between 150 and 1000 nmol m–2 s–1. Although the number of estimates is small, we take confidence that this range is reasonable from the fact that the values are derived from examples where fluxes calculated on a mass basis lie within what might be described as a ‘normal range’. We have then shown that such fluxes could be accounted for by rates of membrane turnover calculated for endo- and exocytosis, although there are too many uncertainties in the calculations to inspire real confidence in vesicles as a means of transport. In Fig. 3, we summarize possible pathways for the transport of ions into and out of cells. Endocytosis could transfer ions from the cell wall to the vacuole (A in Fig. 3), and ion transporters and channels may function as part of the vesicular membrane (B) or independently of vesicles (C). Vacuolar contents may be transferred to the apoplast via exocytosis (D), through so-called junctional complexes (E) or move to neighbouring cells through plasmodesmata (F); finally there is the possibility of movement through multivesicular bodies (G).
A key question is whether vesicular transport can support the range of fluxes needed for transport of NaCl into and out of halophytes as they grow. As we have shown, many assumptions are currently needed to attempt to answer this question definitively at the molecular, cellular and tissue levels of organization. What is now required for analysis of transport and membrane properties is the use of fluorescent dyes in the tissues of halophytes. At the molecular level, rates of exocytosis might be measured using fluorescence (Luo et al., 2016), while the role of the myosin–MyoB complex in cytoplasmic streaming and its association with ion transport in halophytes is worthy of investigation (Kurth et al., 2017).
It is not clear whether trans-membrane fluxes operate in parallel or in series with vesicular transport, nor how selectivity might be maintained during vesicular transport. However, as pointed out by Etxeberria et al. (2005a) for sucrose, transport through both membrane proteins and vesicles could occur simultaneously. As we mentioned earlier, vesicular transport is another mechanism to generate selective ion fluxes. While the selectivity of ion transport via ion channels and transporters is determined by the molecular protein structure of the ion channels and transporters, for vesicular ion transport the selectivity of trapped ion cargo would depend on local ion concentrations near forming vesicles and on electric charges of vesicular membranes. Variation of K+/Na+ selectivity amongst halophytes is worthy of further examination as are the energetics of the two processes, molecular vs. vesicular transport. Vesicular transport is likely to be energetically cheap (0.06 %; see above and Raven, 1987), while the energy costs of ion transport are high (30–50 % of a cells energy; Volkov, 2015b). However, plants may not be limited by energy due to that available through photosynthesis. For halophytes, the energetics of transport and compartmentation remain uncertain (see discussion in Flowers and Colmer, 2008).
The underlying issue in terms of large fluxes of ions across cells is how cytosolic ion concentrations compatible with metabolism are maintained – issues recently discussed by Flowers et al. (2015) and Munns et al. (2016). Constraining potentially toxic ions in vesicles would avoid interactions between damaging ion concentrations and the metabolic machinery of the cytosol. It is difficult to know if such vesicles would ‘leak’ but, if they had similar properties to the plasma and tonoplast membranes, then this is unlikely: ions do not leak through lipid bilayers (Hauser et al., 1972) but will cross membranes through channel proteins (e.g. Demidchik and Tester, 2002; Demidchik et al., 2002). Membranes of the halophyte S. maritima have been shown to have a relatively high cholesterol content with low leakage of Na+ (Leach et al., 1990). ‘Leaks’ occur through channels or transporters and so are subject to gradients in electrochemical potentials with mechanisms to control transport through the protein (and there is evidence that Na+-permeable channels are closed in the tonoplast in S. maritima (Maathuis et al., 1992). In summary, ions could accumulate to high concentration in vesicles, creating protected compartments that may be moved through the cell to fuse with the vacuole. Movement from the vacuole to the apoplast could involve the reverse process – loading of a protected compartment that then moves to fuse with the plasma membrane, releasing ions to the apoplast. A high ion concentration in the apoplast would reduce turgor to a value that would not prevent vesicular transport (cf. Gradmann and Robinson, 1989). Low turgor pressure can, and does, occur in halophytes (Clipson et al., 1985), where ions accumulate in the cell walls – and has been seen as a means to regulate cell turgor (James et al., 2006; see also discussion in Flowers et al., 2015). As far as the cell wall is concerned, its structure should not limit the movement of ions, although charge on the walls will alter the concentration of soluble ions through Donnan equilibria (Briggs et al., 1961); only the movement of substances with dimensions greater than about 5–20 nm would be likely to be constrained by the wall structure (Carpita et al., 1979; Anjum et al., 2016).
So, what should physiologists and breeders pursue in defining the key features of salt tolerance? Certainly, as suggested almost 20 years ago (MacRobbie, 1999), the role of vesicular transport in euhalophytes requires further investigation and, if proven to be an important aspect of salt tolerance, this would influence the choices of genes that might be transformed into plants in the quest for salt-tolerant crops.
LITERATURE CITED
- Albert R. 1975. Salt regulation in halophytes. Oceologia 21: 57–71. [DOI] [PubMed] [Google Scholar]
- Almeida P, Katschnig D, de Boer AH. 2013. HKT transporters – state of the art. International Journal of Molecular Sciences 14: 20359–20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe V, Watson L. 1988. Comparative ultrastructure of microhairs in grasses. Botanical Journal of the Linnean Society 98: 303–379. [Google Scholar]
- Amarasinghe V, Watson L. 1989. Variation in salt secretory activity of microhairs in grasses. Australian Journal of Plant Physiology 16: 219–229. [Google Scholar]
- Al-Zahrani HSM. 1990. Effect of environmental factors on the physiology of the halophyte Suaeda maritima L. Dum. PhD thesis, University of Sussex, Brighton, Sussex, UK. [Google Scholar]
- Anjum NA, Rodrigo MAM, Moulick A, et al. . 2016. Transport phenomena of nanoparticles in plants and animals/humans. Environmental Research 151: 233–243. [DOI] [PubMed] [Google Scholar]
- Ansari R. 1982. Salt tolerance studies in some halophytes. PhD thesis, University of Sussex, Brighton, UK. [Google Scholar]
- Aronson JA. 1989. Salt-tolerant plants of the world. Tucson, AZ: University of Arizona. [Google Scholar]
- Attia H, Arnaud N, Karray N, Lachaal M. 2008. Long-term effects of mild salt stress on growth, ion accumulation and superoxide dismutase expression of Arabidopsis rosette leaves. Physiologia Plantarum 132: 293–305. [DOI] [PubMed] [Google Scholar]
- Baker DA, Hall JL. 1973. Pinocytosis, ATP-ase and ion uptake by plant cells. New Phytologist 72: 1281–1291. [Google Scholar]
- Balnokin YV, Kurkova EB, Khalilova LA, Myasoedov NA, Yusufov AG. 2007. Pinocytosis in the root cells of a salt-accumulating halophyte Suaeda altissima and its possible involvement in chloride transport. Russian Journal of Plant Physiology 54: 797–805. [Google Scholar]
- Balsamo RA, Thomson WW. 1993. Ultastructural features associated with secretion in the salt glands of Frankenia grandifolia (Frankeniaceae) and Avicennia germinans (Avicenniaceae). American Journal of Botany 80: 1276–1283. [Google Scholar]
- Bandmann V, Homann U. 2012. Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. The Plant Journal 70: 578–584. [DOI] [PubMed] [Google Scholar]
- Bandmann V, Muller JD, Kohler T, Homann U. 2012. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Letters 586: 3626–3632. [DOI] [PubMed] [Google Scholar]
- Baral A, Shruthi KS, Mathew MK. 2015. Vesicular trafficking and salinity responses in plants. Iubmb Life 67: 677–686. [DOI] [PubMed] [Google Scholar]
- Bassil E, Ohto M-A, Esumi T, et al. . 2011. a The Arabidopsis intracellular Na+/H+ antiporters nhx5 and nhx6 are endosome associated and necessary for plant growth and development. The Plant Cell 23: 224–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang Y-C, et al. . 2011. b The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. The Plant Cell 23: 3482–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenland AJ, Brownlee C. 1999. Exocytosis and endocytosis. The Plant Cell 11: 643–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L. 1996. Clathrin-coated vesicles in plants. International Review of Cytology 167: 1–35. [Google Scholar]
- Behrends JC. 2012. Evolution of the ion channel concept: the historical perspective. Chemical Reviews 112: 6218–6226. [DOI] [PubMed] [Google Scholar]
- Bennett HS. 1956. The concepts of membrane flow and membrane vesiculation as mechanisms for active transport and ion pumping. Journal of Biophysical and Biochemical Cytology 2: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojorquez-Quintal E, Velarde-Buendia A, et al. . 2014. Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Frontiers in Plant Science 5: 605. doi: 10.3389/fpls.2014.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B. 2004. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. Journal of Microscopy 214: 159–173. [DOI] [PubMed] [Google Scholar]
- Bosabalidis AM, Thomson WW. 1984. Light microscopical studies on salt gland development in Tamarix aphylla L. Annals of Botany 54: 169–174. [Google Scholar]
- Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, Shabala S. 2015. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Annals of Botany 115: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs GE, Hope AB, Robertson RN. 1961. Electrolytes and plant cells. Oxford: Blackwell Scientific Publications. [Google Scholar]
- Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J. 2001. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO Journal 20: 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Zhao MC, Kourghi M, et al. . 2017. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant, Cell and Environment 40: 802–815. [DOI] [PubMed] [Google Scholar]
- Campbell N, Thomson WW. 1975. Chloride localization in leaf of Tamarix. Protoplasma 83: 1–14. [Google Scholar]
- Campbell N, Thomson WW. 1976a Ultrastructural basis of chloride tolerance in leaf of Frankenia. Annals of Botany 40: 687–693. [Google Scholar]
- Campbell N, Thomson WW. 1976b Ultrastructure of Frankenia salt-glands. Annals of Botany 40: 681–686. [Google Scholar]
- Campbell N, Thomson WW, Platt K. 1974. Apoplastic pathway of transport to salt-glands. Journal of Experimental Botany 25: 61–69. [Google Scholar]
- Cardale S, Field CD. 1971. The structure of the salt gland of Aegiceras corniculatum. Planta 99: 183–191. [DOI] [PubMed] [Google Scholar]
- Carpita N, Subularse D, Montezinos D, Delmer DP. 1979. Determination of the pore size of of cell walls of living plant cells. Science 205: 1144–1147. [DOI] [PubMed] [Google Scholar]
- Ceccoli G, Ramos J, Pilatti V, et al. . 2015. Salt glands in the poaceae family and their relationship to salinity tolerance. Botanical Review 81: 162–178. [Google Scholar]
- Cheeseman JM. 2013. The integration of activity in saline environments: problems and perspectives. Functional Plant Biology 40: 759–774. [DOI] [PubMed] [Google Scholar]
- Chen Z, Pottosin II, Cuin TA, et al. . 2007. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology 145: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipson NJW, Flowers TJ. 1987. Salt tolerance in the halophyte Suaeda maritima (L) Dum – the effect of salinity on the concentration of sodium in the xylem. New Phytologist 105: 359–366. [DOI] [PubMed] [Google Scholar]
- Clipson NJW, Tomos AD, Flowers TJ, Jones RGW. 1985. Salt tolerance in the halophyte Suaeda maritima L Dum – the maintenance of turgor pressure and water-potential gradients in plants growing at different salinities. Planta 165: 392–396. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Kochian LV, Kronzucker HJ. 2016. How high do ion fluxes go? A re-evaluation of the two-mechanism model of K+ transport in plant roots. Plant Science 243: 96–104. [DOI] [PubMed] [Google Scholar]
- Cram WJ. 1980. Pinocytosis in plants. New Phytologist 84: 1–17. [Google Scholar]
- Cram WJ, Torr PG, Rose DA. 2002. Salt allocation during leaf development and leaf fall in mangroves. Trees - Structure and Function 16: 112–119. [Google Scholar]
- Cucu B, Degreif D, Bertl A, Thiel G. 2017. Vesicle fusion and fission in plants and yeast. Cell Calcium 67: 40–45. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shen J, Gao C, Zhuang X, Wang J, Jiang L. 2016. Biogenesis of plant prevacuolar multivesicular bodies. Molecular Plant 9: 774–786. [DOI] [PubMed] [Google Scholar]
- Danek M, Valentova O, Martinec J. 2016. Flotillins, Erlins, and HIRs: from animal base camp to plant new horizons. Critical Reviews in Plant Sciences 35: 191–214. [Google Scholar]
- Dassanayake M, Larkin JC. 2017. Making plants break a sweat: the structure, function, and evolution of plant salt glands. Frontiers in Plant Science 8: 406. doi: 10.3389/fpls.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R. 2002. Glutamate receptors in plants. Annals of Botany 90: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. 2002. Nonselective cation channels in plants. Annual Review of Plant Biology 53: 67–107. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. 2002. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology 128: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, et al. . 2007. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Current Biology 17: 520–527. [DOI] [PubMed] [Google Scholar]
- Drennan PM, Berjak P, Lawton JR, Pammenter NW. 1987. Ultrastructure of the salt glands of the mangrove, Avicennia marina (Forssk.) Vierh., as indicated by the use of selective membrane staining. Planta 172: 176–183. [DOI] [PubMed] [Google Scholar]
- Echeverria E. 2000. Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiology 123: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, van Oijen A, et al. . 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118: 591–605. [DOI] [PubMed] [Google Scholar]
- Endow SA, Kull FJ, Liu H. 2010. Kinesins at a glance. Journal of Cell Science 123: 3420–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M. 2003. Sodium influx and accumulation in Arabidopsis. Plant Physiology 133: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria E, Baroja-Fernandez E, Munoz FJ, Pozueta-Romero J. 2005a Sucrose-inducible endocytosis as a mechanism for nutrient uptake in heterotrophic plant cells. Plant & Cell Physiology 46: 474–481. [DOI] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P, Pozueta-Romero J. 2005b Sucrose transport into citrus juice cells: evidence for an endocytic transport system. Journal of the American Society for Horticultural Science 130: 269–274. [Google Scholar]
- Etxeberria E, Gonzalez P, Pozueta J. 2007. Fluid-phase endocytosis in Citrus juice cells is independent from vacuolar pH and inhibited by chlorpromazine, an inhibitor of PI-3 kinases and clathrin-mediated endocytosis. Journal of Horticultural Science & Biotechnology 82: 900–907. [Google Scholar]
- Etxeberria E, Gonzalez P, Pozueta J. 2009. Evidence for two endocytic transport pathways in plant cells. Plant Science 177: 341–348. [Google Scholar]
- Etxeberria E, Pozueta-Romero J, Fernandez B. 2012. Fluid-phase endocytosis in plant cells. In: Samaj, J, ed. Endocytosis in plants. Berlin, Heidelberg: Springer, 107–122. [Google Scholar]
- Etxeberria E, Gonzalez P, Pozueta-Romero J. 2013. Architectural remodeling of the tonoplast during fluid-phase endocytosis. Plant Signaling & Behavior 8: e24793. doi: 10.4161/psb.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn A. 1988. Secretory tissues in vascular plants. New Phytologist 108: 229–257. [DOI] [PubMed] [Google Scholar]
- Fan L, Li R, Pan J, Ding Z, Lin J. 2015. Endocytosis and its regulation in plants. Trends in Plant Science 20: 388–397. [DOI] [PubMed] [Google Scholar]
- Faraday CD, Thomson WW. 1986a Morphometric analysis of Limonium salt-glands in relation to ion efflux. Journal of Experimental Botany 37: 471–481. [Google Scholar]
- Faraday CD, Thomson WW. 1986b Structural aspects of the salt glands of the Plumbaginaceae. Journal of Experimental Botany 37: 461–470. [Google Scholar]
- Faraday CD, Quinton PM, Thomson WW. 1986. Ion fluxes across the transfusion zone of secreting Limonium salt-glands determined from secretion rates, transfusion zone areas and plasmodesmatal frequencies. Journal of Experimental Botany 37: 482–494. [Google Scholar]
- Feng ZT, Sun QJ, Deng YQ, Sun SF, Zhang JG, Wang BS. 2014. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiologiae Plantarum 36: 2729–2741. [Google Scholar]
- Feng Z-T, Deng Y-Q, Zhang S-C, et al. . 2015. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Science 238: 286–296. [DOI] [PubMed] [Google Scholar]
- Flowers TJ. 1985. Physiology of halophytes. Plant and Soil 89: 41–56. [Google Scholar]
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR. 1986. Ion relations of plants under drought and salinity. Australian Journal of Plant Physiology 13: 75–91. [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR. 1977. The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology 28: 89–121. [Google Scholar]
- Flowers TJ, Hajibagheri MA, Clipson NJW. 1986. Halophytes. Quarterly Review of Biology 61: 313–337. [Google Scholar]
- Flowers TJ, Flowers SA, Hajibagheri MA, Yeo AR. 1990. Salt tolerance in the halophytic wild rice, Porteresia coarctata Tateoka. New Phytologist 114: 675–684. [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. 2010. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology 37: 604–612. [Google Scholar]
- Flowers TJ, Munns R, Colmer T. 2015. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany 115: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RC, Sands R. 1977. Response of radiata pine to salt stress. II Localization of chloride. Australian Journal of Plant Physiology 4: 863–875. [Google Scholar]
- Fricke W, Jarvis MC, Brett CT. 2000. Turgor pressure, membrane tension and the control of exocytosis in higher plants. Plant, Cell and Environment, 23: 999–1003. [Google Scholar]
- Glenn E, Miyamoto S, Moore D, Brown JJ, Thompson TL, Brown P. 1997. Water requirements for cultivating Salicornia bigelovii Torr with seawater on sand in a coastal desert environment. Journal of Arid Environments 36: 711–730. [Google Scholar]
- Glenn EP, Morino K, Nagler PL, Murray RS, Pearlstein S, Hultine KR. 2012. Roles of saltcedar (Tamarix spp.) and capillary rise in salinizing a non-flooding terrace on a flow-regulated desert river. Journal of Arid Environments 79: 56–65. [Google Scholar]
- Glenn EP, Nagler PL, Morino K, Hultine KR. 2013. Phreatophytes under stress: transpiration and stomatal conductance of saltcedar (Tamarix spp.) in a high-salinity environment. Plant and Soil 371: 655–672. [Google Scholar]
- Gradmann D, Robinson DG. 1989. Does turgor prevent endocytosis in plant-cells. Plant Cell and Environment 12: 151–154. [Google Scholar]
- Grebnev G, Ntefidou M, Kost B. 2017. Secretion and endocytosis in pollen tubes: models of tip growth in the spot light. Frontiers in Plant Science 8, 154. doi: 10.3389/fpls.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway M, Munns R. 1980. Mechanisms of salt tolerance in non-halophytes. Annual Review of Plant Physiology 31: 149–190. [Google Scholar]
- Gronwald JW, Leonard RT. 1982. Isolation and transport-properties of protoplasts from cortical-cells of corn roots. Plant Physiology 70: 1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Hughes JE. 1976. Quantitative assessment of symplastic transport of pre nectar into the trichomes of Abutilon megapotamicum-var-variegatum nectaries. Australian Journal of Plant Physiology 3: 619–637. [Google Scholar]
- Hajibagheri MA, Flowers TJ. 1989. X-ray microanalysis of ion distribution within root cortical cells of the halophyte Suaeda maritima (L.) Dum. Planta 177: 131–134. [DOI] [PubMed] [Google Scholar]
- Hajibagheri MA, Hall JL, Flowers TJ. 1984. Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum. Journal of Experimental Botany 35: 1547–1557. [Google Scholar]
- Hajibagheri MA, Yeo AR, Flowers TJ. 1985. Salt tolerance in Suaeda maritima (L.) Dum. Fine structure and ion concentrations in the apical region of roots. New Phytologist 99: 331–343. [Google Scholar]
- Hall JL. 1970. Pinocytotic vesicles and ion transport in plant cells. Nature 226: 1253–1254. [DOI] [PubMed] [Google Scholar]
- Hall JL, Yeo AR, Flowers TJ. 1974. Uptake and localization of rubidium in the halophyte Suaeda maritima. Zeitschrift für Pflanzenphysiologie 71: 200–206. [Google Scholar]
- Hamaji K, Nagira M, Yoshida K, et al. . 2009. Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant & Cell Physiology 50: 2023–2033. [DOI] [PubMed] [Google Scholar]
- Harvey DMR, Flowers TJ, Hall JL. 1976. Localization of chloride in leaf-cells of halophyte Suaeda maritima by silver precipitation. New Phytologist 77: 319–323. [Google Scholar]
- Harvey DMR, Hall JL, Flowers TJ, Kent B. 1981. Quantitative ion localization within Suaeda maritima leaf mesophyll cells. Planta 151: 555–560. [DOI] [PubMed] [Google Scholar]
- Harvey DMR, Flowers TJ, Hall JL. 1979. Precipitation procedures for sodium, potassium and chloride localization in leaf cells of the halophyte Suaeda maritima. Journal of Microscopy 116: 213–226. [Google Scholar]
- Hassinger JE, Oster G, Drubin DG, Rangamani P. 2017. Design principles for robust vesiculation in clathrin-mediated endocytosis. Proceedings of the National Academy of Sciences, USA 114: e1118–e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H, Stubbs M, Phillips MC. 1972. Ion permeability of phospholipid bilayers. Nature 239: 342–344. [DOI] [PubMed] [Google Scholar]
- Henderson SW, Wege S, Qiu J, et al. . 2015. Grapevine and Arabidopsis cation–chloride cotransporters localize to the Golgi and trans-Golgi network and indirectly influence long-distance ion transport and plant salt tolerance. Plant Physiology 169: 2215–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WM, Hansen DJ, Weber DJ. 1975. Light and electron-microscopy localization of chloride-ions in cells of Salicornia pacifica var utahensis. Canadian Journal of Botany-Revue Canadienne de Botanique 53: 1176–1187. [Google Scholar]
- Heumann HG. 2002. Ultrastructural localization of zinc in zinc-tolerant Armeria maritima ssp halleri by autometallography. Journal of Plant Physiology 159: 191–203. [Google Scholar]
- Hill AE, Hill BS. 1976. Mineral ions. In: Lüttge U, Pitman MG, eds. Transport in plants. II. Part B. Tissues and organs. Berlin: Springer Verlag, 224–243. [Google Scholar]
- Homann U, Tester M. 1997. Ca2+-independent and Ca2+/GTP-binding protein-controlled exocytosis in a plant cell. Proceedings of the National Academy of Sciences, USA 94: 6565–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchzermeyer B, Flowers TJ. 2013. Putting halophytes to work – genetics, biochemistry and physiology. Functional Plant Biology 40: v–viii. [DOI] [PubMed] [Google Scholar]
- Hurst AC, Meckel T, Tayefeh S, Thiel G, Homann U. 2004. Trafficking of the plant potassium inward rectifier KAT1 in guard cell protoplasts of Vicia faba. The Plant Journal 37: 391–397. [DOI] [PubMed] [Google Scholar]
- Hwang I, Robinson DG. 2009. Transport vesicle formation in plant cells. Current Opinion in Plant Biology 12: 660–669. [DOI] [PubMed] [Google Scholar]
- Ibrahim AH. 2013. Tolerance and avoidance responses to salinity and water stresses in Calotropis procera and Suaeda aegyptiaca. Turkish Journal of Agriculture and Forestry 37: 352–360. [Google Scholar]
- Imada S, Acharya K, Yamanaka N. 2012. Short-term and diurnal patterns of salt secretion by Tamarix ramosissima and their relationships with climatic factors. Journal of Arid Environments 83: 62–68. [Google Scholar]
- Inan G, Zhang Q, Li PH, et al. . 2004. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiology 135: 1718–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili JN. 2011. Intermolecular and surface forces. Amsterdam: Elsevier, 674. [Google Scholar]
- Ito K, Ikebe M, Kashiyama T, Mogami T, Kon T, Yamamoto K. 2007. Kinetic mechanism of the fastest motor protein, Chara myosin. Journal of Biological Chemistry 282: 19534–19545. [DOI] [PubMed] [Google Scholar]
- James JJ, Alder NN, Muhling KH, et al. . 2006. High apoplastic solute concentrations in leaves alter water relations of the halophytic shrub, Sarcobatus vermiculatus. Journal of Experimental Botany 57: 139–147. [DOI] [PubMed] [Google Scholar]
- Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X. 2013. The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Molecular Plant 6: 275–286. [DOI] [PubMed] [Google Scholar]
- Klima A, Foissner I. 2008. FM dyes label sterol-rich plasma membrane domains and are internalized independently of the cytoskeleton in Characean internodal cells. Plant & Cell Physiology 49: 1508–1521. [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G. 2017. Annexins as overlooked regulators of membrane trafficking in plant cells. International Journal of Molecular Sciences 18: 863. doi: 10.3390/ijms18040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyro HW, Hussain T, Huchzermeyer B, Khan MA. 2013. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environmental and Experimental Botany 91: 22–29. [Google Scholar]
- Kronzucker HJ, Britto DT. 2011. Sodium transport in plants: a critical review. New Phytologist 189: 54–81. [DOI] [PubMed] [Google Scholar]
- Kurkova EB, Balnokin YV. 1994. Pinocytosis and its possible role in ion-transport in the salt-accumulating organs of halophytes. Russian Journal of Plant Physiology 41: 507–511. [Google Scholar]
- Kurkova EB, Balnokin YV, Myasoedov NA. 1992. Some characteristics of cell ultrastructure and accumulation of Na+ and Cl– in tissues of the halophyte Petrosimonia triandra. Soviet Plant Physiology 39: 17–22. [Google Scholar]
- Kurth EG, Peremyslov VV, Turner HL, et al. . 2017. Myosin-driven transport network in plants. Proceedings of the National Academy of Sciences, USA 114: E1385–E1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof D, Cheeseman JM. 1986. Sodium transport and compartmentation in Spergularia marina. Partial characterization of a functional symplasm. Plant Physiology 81: 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof D, Cheeseman JM. 1988. Sodium and potassium compartmentation and transport across the roots of intact Spergularia marina. Plant Physiology 88: 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RP, Wheeler KP, Flowers TJ, Yeo AR. 1990. Molecular markers for ion compartmentation in cells of higher plants. II. Lipid composition of the tonoplast of the halophyte Suaeda maritima (L.) Dum. Journal of Experimental Botany 41: 1089–1094. [Google Scholar]
- Leshem Y, Seri L, Levine A. 2007. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. The Plant Journal 51: 185–197. [DOI] [PubMed] [Google Scholar]
- Levering CA, Thomson WW. 1971. The ultrastructure of the salt gland of Spartina foliosa. Planta 97: 183–196. [DOI] [PubMed] [Google Scholar]
- Li H, Ye X, Guo X, Geng Z, Wang G. 2016. Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. Journal of Hazardous Materials 314: 188–196. [DOI] [PubMed] [Google Scholar]
- Li RL, Liu P, Wan YL, et al. . 2012. A membrane microdomain-associated protein, Arabidopsis flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. The Plant Cell 24: 2105–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Marques RL, Poulsen LR, Palmgren MG. 2012. A putative plant aminophospholipid flippase, the Arabidopsis P4 ATPase ALA1, localizes to the plasma membrane following association with a beta-subunit. PLoS One 7: e33042. doi: 10.1371/journal.pone.0033042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Chandra S. 1994. Endocytosis in plants. Annual Review of Plant Physiology and Plant Molecular Biology 45: 609–631. [Google Scholar]
- Luo N, Yan A, Yang Z. 2016. Measuring exocytosis rate using corrected fluorescence recovery after photoconversion. Traffic 17: 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 1975. Salt glands. In: Baker DA, Hall JL, eds. Ion transport in plant cells and tissues. Amsterdam: North Holland Publishing Company, 335–376. [Google Scholar]
- Maathuis FJM. 2014. Sodium in plants: perception, signalling, and regulation of sodium fluxes. Journal of Experimental Botany 65: 849–858. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Flowers TJ, Yeo AR. 1992. Sodium chloride compartmentation in leaf vacuoles of the halophyte Suaeda maratima (L.) Dum. and its relation to tonoplast permeability. Journal of Experimental Botany 43: 1219–1223. [Google Scholar]
- Maathuis FJM, Ahmad I, Patishtan J. 2014. Regulation of Na+ fluxes in plants. Frontiers in Plant Science 5: 467. doi: 10.3389/fpls.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EA. 1971a Fluxes and compartmentation in plant cells. Annual Review of Plant Physiology 22: 75–96. [Google Scholar]
- MacRobbie EA. 1971b Phloem translocation. Biological Reviews 46: 429–481. [Google Scholar]
- MacRobbie EAC. 1999. Vesicle trafficking: a role in trans-tonoplast ion movements?Journal of Experimental Botany 50: 925–934. [Google Scholar]
- Malinska K, Jelinkova A, Petrasek J. 2014. The use of FM dyes to analyze plant endocytosis. Methods in Molecular Biology 1209: 1–11. [DOI] [PubMed] [Google Scholar]
- Malinsky J, Opekarova M, Grossmann G, Tanner W. 2013. Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annual Review of Plant Biology 64: 501–529. [DOI] [PubMed] [Google Scholar]
- Malone C, Koeppe DE, Miller RJ. 1974. Localization of lead accumulated by corn plants. Plant Physiology 53: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, et al. . 2001. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology 126: 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel A, Leshem Y, Tiwari BS, Levine A. 2004. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiology 134: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U. 2004. Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K+-channel KAT1. The Plant Journal 39: 182–193. [DOI] [PubMed] [Google Scholar]
- Mellander LJ, Kurczy ME, Najafinobar N, Dunevall J, Ewing AG, Cans AS. 2014. Two modes of exocytosis in an artificial cell. Scientific Reports 4: 3847. doi: 10.1038/srep03847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel Mulet J, Llopis-Torregrosa V, Primo C, Carmen Marques M, Yenush L. 2013. Endocytic regulation of alkali metal transport proteins in mammals, yeast and plants. Current Genetics 59: 207–230. [DOI] [PubMed] [Google Scholar]
- Mittasch M, Gross P, Nestler M, et al. . 2018. Non-invasive perturbations of intracellular flow reveal physical principles of cell organization. Nature Cell Biology 20: 344–351. [DOI] [PubMed] [Google Scholar]
- Moir-Barnetson L, Veneklaas EJ, Colmer TD. 2016. Salinity tolerances of three succulent halophytes (Tecticornia spp.) differentially distributed along a salinity gradient. Functional Plant Biology 43: 739–750. [DOI] [PubMed] [Google Scholar]
- Mongrand S, Morel J, Laroche J, et al. . 2004. Lipid rafts in higher plant cells – purification and characterization of triton X-100-insoluble microdomains from tobacco plasma membrane. Journal of Biological Chemistry 279: 36277–36286. [DOI] [PubMed] [Google Scholar]
- Munns R. 2005. Genes and salt tolerance: bringing them together. New Phytologist 167: 645–663. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Gilliham M, Flowers TJ, Colmer TD. 2016. Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Functional Plant Biology 43: 1103–1113. [DOI] [PubMed] [Google Scholar]
- Nambiar R, McConnell RE, Tyska MJ. 2009. Control of cell membrane tension by myosin-I. Proceedings of the National Academy of Sciences, USA 106: 11972–11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassery H, Jones RL. 1976a Salt-induced pinocytosis in barley and bean. Journal of Experimental Botany 27: 358–367. [Google Scholar]
- Nassery H, Jones RL. 1976b Salt retention by bean hypocotyl. Journal of Experimental Botany 27: 1279–1289. [Google Scholar]
- Nesterov VN, Nesterkina IS, Rozentsvet OA, Ozolina NV, Salyaev RK. 2017. Detection of lipid–protein microdomains (rafts) and investigation of their functional role in the chloroplast membranes of halophytes. Doklady Biochemistry and Biophysics 476: 303–305. [DOI] [PubMed] [Google Scholar]
- Nieves-Cordones M, Martinez V, Benito B, Rubio F. 2016. Comparison between arabidopsis and rice for main pathways of K+ and Na+ uptake by roots. Frontiers in Plant Science 7: 992. doi: 10.3389/fpls.2016.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi T, Taniguchi M, Miyake H. 2012. Morphology and ultrastructure of the salt glands on the leaf surface of Rhodes grass (Chloris gayana Kunth). International Journal of Plant Sciences 173: 454–463. [Google Scholar]
- Olesen P. 1979. Ultrastructral observations on the cuticular envelope in salt glands of Frankenia pauciflora. Protoplasma 99: 1–9. [Google Scholar]
- Oross JW, Thomson WW. 1982. The ultrastructure of the salt glands of Cynodon and Distichlis (Poaceae). American Journal of Botany 96: 939–949. [Google Scholar]
- Oross JW, Leonard RT, Thomson WW. 1985. Flux rate and a secretion model for salt-glands of grasses. Israel Journal of Botany 34: 69–77. [Google Scholar]
- Osmond CB, Luttge U, West KR, Pallaghy CK, Shacher-Hill B. 1969. Ion absorption in Atriplex leaf tissue. II. Secretion of ions to epidermal bladders. Austalian Journal of Biological Sciences 22: 797–814. [Google Scholar]
- Otto GP, Nichols BJ. 2011. The roles of flotillin microdomains – endocytosis and beyond. Journal of Cell Science 124: 3933–3940. [DOI] [PubMed] [Google Scholar]
- Pedersen TJ, Palmgren MG. 2017. Why do plants lack sodium pumps and would they benefit from having one?Functional Plant Biology 44: 473–479. [DOI] [PubMed] [Google Scholar]
- Penny MG, Bowling DJF. 1974. Study of potassium gradients in epidermis of intact leaves of Commelina communis L in relation to stomatal opening. Planta 119: 17–25. [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Morgun EA, Kurth EG, Makarova KS, Koonin EV, Dolja VV. 2013. Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles. The Plant Cell 25: 3022–3038.23995081 [Google Scholar]
- Pilar Rodriguez-Rosales M, Galvez FJ, Huertas R, et al. . 2009. Plant NHX cation/proton antiporters. Plant Signaling & Behavior 4: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q-S. 2016. Plant endosomal NHX antiporters: activity and function. Plant Signaling & Behavior 11: e1147643. doi: 10.1080/15592324.2016.1147643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhmankulova ZF, Voronin PY, Shuyskaya EV, et al. . 2014. Effect of NaCl and isoosmotic polyethylene glycol stress on gas exchange in shoots of the C-4 xerohalophyte Haloxylon aphyllum (Chenopodiaceae). Photosynthetica 52: 437–443. [Google Scholar]
- Ralle M, Lutsenko S. 2009. Quantitative imaging of metals in tissues. Biometals 22: 197–205. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1976. Transport in algal cells. In: Lüttge U, Pitman MG, eds. Transport in plants II part A. Cells. Berlin, Heidelberg, New York: Springer-Verlag, 129–188. [Google Scholar]
- Raven JA. 1987. The role of vacuoles. New Phytologist 106: 357–422. [Google Scholar]
- Reguera M, Bassil E, Tajima H, et al. . 2015. pH Regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. The Plant Cell 27: 1200–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigal A, Doyle SM, Robert S. 2015. Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in arabidopsis root cells. Methods in Molecular1 Biology 1242: 93–103. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hillmer S. 1990. Endocytosis in plants. Physiologia Plantarum 79: 96–104. [Google Scholar]
- Romanenko AS, Kovtun GY, Salyaev RK. 1986. Effect of metabolic-inhibitors on pinocytosis of uranyl ions by radish root-cells – probable mechanisms of pinocytosis. Annals of Botany 57: 1–10. [Google Scholar]
- Roy S, Chakraborty U. 2014. Salt tolerance mechanisms in salt tolerant grasses (STGs) and their prospects in cereal crop improvement. Botanical Studies 55: 31. doi: 10.1186/1999-3110-55-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozema J, Riphagen I, Sminia T. 1977. Light and electron-microscopical study on structure and function of salt-gland of Glaux maritima L. New Phytologist 79: 665–671. [Google Scholar]
- Rozema J, Gude H, Pollack G. 1981. An ecophysical study of the salt secretion of four halophytes. New Phytologist 89: 201–217. [Google Scholar]
- Sahu SK, Gummadi SN. 2008. Flippase activity in proteoliposomes reconstituted with Spinacea oleracea endoplasmic reticulum membrane proteins: evidence of biogenic membrane flippase in plants. Biochemistry 47: 10481–10490. [DOI] [PubMed] [Google Scholar]
- Salama FM, El-Naggar SM, Ramadan T. 1999. Salt glands of some halophytes in Egypt. Phyton-Annales Rei Botanicae 39: 91–105. [Google Scholar]
- Saxton MJ, Breidenbach RW. 1988. Receptor-mediated endocytosis in plants is energetically possible. Plant Physiology 86: 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneberg J, Lee I-H, Iwasa JH, Hurley JH. 2017. Reverse-topology membrane scission by the ESCRT proteins. Nature Reviews Molecular Cell Biology 18: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Mackay A. 2011. Ion transport in halophytes. In: Turkan, I, ed. Plant responses to drought and salinity stress: developments in a post-genomic era. London: Academic Press Ltd–Elsevier Science Ltd, 151–199. [Google Scholar]
- Shabala S, Bose J, Hedrich R. 2014. Salt bladders: do they matter?Trends in Plant Science 19: 687–691. [DOI] [PubMed] [Google Scholar]
- Shabala S, Wu HH, Bose J. 2015. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Science 241: 109–119. [DOI] [PubMed] [Google Scholar]
- Shuvalov AV, Orlova JV, Khalilova LA, et al. . 2015. Evidence for the functioning of a Cl–/H+ antiporter in the membranes isolated from root cells of the halophyte Suaeda altissima and enriched with Golgi membranes. Russian Journal of Plant Physiology 62: 45–56. [Google Scholar]
- Smaoui A, Barhoumi Z, Rabhi M, Abdelly C. 2011. Localization of potential ion transport pathways in vesicular trichome cells of Atriplex halimus L. Protoplasma 248: 363–372. [DOI] [PubMed] [Google Scholar]
- Somaru R, Naidoo Y, Naidoo G. 2002. Morphology and ultrastructure of the leaf salt glands of Odyssea paucinervis (Stapf) (Poaceae). Flora 197: 67–75. [Google Scholar]
- Sookbirsingh R, Castillo K, Gill TE, Chianelli RR. 2010. Salt separation processes in the saltcedar Tamarix ramosissima (Ledeb.). Communications in Soil Science and Plant Analysis 41: 1271–1281. [Google Scholar]
- Steer MW. 1988. Plasma membrane turnover in plant cells. Journal of Experimental Botany 39: 987–996. [Google Scholar]
- Sutcliffe JF. 1962. Mineral salts absorption in plants. Oxford: Pergamon Press. [Google Scholar]
- Sutter JU, Campanoni P, Tyrrell M, Blatt MR. 2006. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. The Plant Cell 18: 935–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J-U, Sieben C, Hartel A, Eisenach C, Thiel G, Blatt MR. 2007. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Current Biology 17: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Thiel G, Battey N. 1998. Exocytosis in plants. Plant Molecular Biology 38: 111–125. [PubMed] [Google Scholar]
- Thomson WW. 1975. The structure and function of salt glands. In: Poljakoff-Mayber A, Gale J, eds. Ecological studies. Berlin: Springer Verlag, 118–146. [Google Scholar]
- Thomson WW, Healey PL. 1984. Cellular basis of trichome secretion. In: Rodriguez, E, ed. Biology and chemistry of plant trichomes. New York: Plenum Press, 95–111. [Google Scholar]
- Thomson WW, Liu LL. 1967. Ultrastructural features of salt gland of Tamarix aphylla L. Planta 73: 201–220. [DOI] [PubMed] [Google Scholar]
- Thomson WW, Berry WL, Liu LL. 1969. Localization and secretion of salt by the salt glands of Tamarix aphylla. Proceedings of the National Academy of Sciences, USA 63: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WW, Faraday CD, Oross JW. 1988. Salt glands. In: Baker DA, Hall JL, eds. Solute transport in plant cells and tissues. Harlow: Longman Scientific and Technical, 498–537. [Google Scholar]
- Tominaga M, Kimura A, Yokota E, et al. . 2013. Cytoplasmic streaming velocity as a plant size determinant. Developmental Cell 27: 345–352. [DOI] [PubMed] [Google Scholar]
- Toshima JY, Toshima J, Kaksonen M, Martin AC, King DS, Drubin DG. 2006. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proceedings of the National Academy of Sciences, USA 103: 5793–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD. 2013. The devil in the detail of secretions. Plant, Cell and Environment 36: 1407–1409. [DOI] [PubMed] [Google Scholar]
- Uemura T. 2016. Physiological roles of plant post-Golgi transport pathways in membrane trafficking. Plant & Cell Physiology 57: 2013–2019. [DOI] [PubMed] [Google Scholar]
- Underwood W, Ryan A, Somerville SC. 2017. An arabidopsis lipid flippase is required for timely recruitment of defenses to the host–pathogen interface at the plant cell surface. Molecular Plant 10: 805–820. [DOI] [PubMed] [Google Scholar]
- Valencia JP, Goodman K, Otegui MS. 2016. Endocytosis and endosomal trafficking in plants. In: Merchant, SS, ed. Annual Review of Plant Biology 67: 309–335. [DOI] [PubMed] [Google Scholar]
- Van Steveninck RFM, Ballment B, Peters PD, Hall TA. 1976a Ultrastructural localization of ions. Part II: X-ray analytical verification of silver precipitation products and distribution of chloride in mesophyll cells of barley seedlings. Australian Journal of Plant Physiology 3: 359–365. [Google Scholar]
- Van Steveninck RFM, Van Steveninck ME, Peters PD, Hall TA. 1976b Ultrastructural localization of ions. Part IV: localization of chloride and bromide in Nitella translucens and X-ray energy spectroscopy of silver precipitation products. Journal of Experimental Botany 27: 1291–1312. [Google Scholar]
- Vassilyev AE, Stepanova AA. 1990. The ultrastructure of ion-secreting and non-secreting salt glands of Limonium platphyllum. Journal of Experimental Botany 41: 41–46. [Google Scholar]
- Volkov V. 2015a Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Frontiers in Plant Science 6: 873. doi: 10.3389/fpls.2015.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V. 2015b Quantitative description of ion transport via plasma membrane of yeast and small cells. Frontiers in Plant Science 6: 425. doi: 10.3389/fpls.2015.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V, Amtmann A. 2006. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. The Plant Journal 48: 342–353. [DOI] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A. 2004. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant, Cell and Environment 27: 1–14. [Google Scholar]
- Wang B, Davenport RJ, Volkov V, Amtmann A. 2006. Low unidirectional sodium influx into root cells restricts net sodium accumulation in Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana. Journal of Experimental Botany 57: 1161–1170. [DOI] [PubMed] [Google Scholar]
- Wang H-J, Hsu Y-W, Guo C-L, Jane W-N, Wang H, Jiang L, Jauh G-Y. 2017. VPS36-dependent multivesicular bodies are critical for plasmamembrane protein turnover and vacuolar biogenesis. Plant Physiology 173: 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Yang Y, Wang Z, Zhou J, Fan B, Chen Z. 2015a A critical role of lyst-interacting protein5, a positive regulator of multivesicular body biogenesis, in plant responses to heat and salt stresses. Plant Physiology 169: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu X, Liu Y, Qiu Q-S. 2015b AtNHX5 and AtNHX6 control cellular K+ and pH homeostasis in arabidopsis: three conserved acidic residues are essential for K+ transport. PLoS One 10: e0144716. doi: 10.1371/journal.pone.0144716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SM, Zhang JL, Flowers TJ. 2007. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiology 145: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Mäser P, Schroeder JI. 2009. Plant ion channels: gene families, physiology, and functional genomics analyses. Annual Review of Physiology 71: 59–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H, Hanchey P. 1971. Pinocytosis and membrane dilation in uranyl-treated plant roots. Science 171: 68–71. [DOI] [PubMed] [Google Scholar]
- Wheeler H, Baker BL, Hanchey P. 1972. Pinocytosis in root cap cells exposed to uranyl salts. American Journal of Botany 59: 858–868. [Google Scholar]
- White PJ, Lemtiri-Chlieh F. 1995. Potassium currents across the plasma-membrane of protoplasts derived from rye roots – a patch-clamp study. Journal of Experimental Botany 46: 497–511. [Google Scholar]
- Wilkins KA, Matthus E, Swarbreck SM, Davies JM. 2016. Calcium-mediated abiotic stress signaling in roots. Frontiers in Plant Science 7: 1296. doi: 10.3389/fpls.2016.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H, Mycock D, Weiersbye IM. 2017. The salt glands of Tamarix usneoides E. Mey. ex Bunge (South African Salt Cedar). International Journal of Phytoremediation 19: 587–595. [DOI] [PubMed] [Google Scholar]
- Wu XX, Li J, Wu XD, et al. . 2016. Ectopic expression of Arabidopsis thaliana Na+(K+)/H+ antiporter gene, AtNHX5, enhances soybean salt tolerance. Genetics and Molecular Research 15: doi: 10.4238/gmr.15027483. [DOI] [PubMed] [Google Scholar]
- Yamashita RA, May GS. 1998. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. Journal of Biological Chemistry 273: 14644–14648. [DOI] [PubMed] [Google Scholar]
- Yeo AR, Flowers TJ. 1980. Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: evaluation of the effect of salinity upon growth. Journal of Experimental Botany 31: 1171–1183. [Google Scholar]
- Yeo AR, Flowers TJ. 1986. Ion transport in Suaeda maritima: its relation to growth and implications for the pathway of radial transport of ions across the root. Journal of Experimental Botany 37: 143–159. [Google Scholar]
- Yoneda A, Doering TL. 2006. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Molecular Biology of the Cell 17: 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Mori T, Ando T, Harada R, Jung J, Sugita Y, Feig M. 2016. Biomolecular interactions modulate macromolecular structure and dynamics in atomistic model of a bacterial cytoplasm. Elife 5: pii: e19274. doi: 10.7554/eLife.19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Lyu MJA, Leng BY, et al. . 2015. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant, Cell and Environment 38: 1637–1657. [DOI] [PubMed] [Google Scholar]
- Yuan F, Leng B, Wang B. 2016a Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt?Frontiers in Plant Science 7: 977. doi: 10.3389/fpls.2016.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Lyu MJA, Leng BY, Zhu XG, Wang BS. 2016b. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Molecular Biology 91: 241–256. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Flowers TJ, Wang SM. 2010. Mechanisms of sodium uptake by roots of higher plants. Plant and Soil 326: 45–60. [Google Scholar]
- Zhang JL, Flowers TJ, Wang SM. 2013. Differentiation of low-affinity Na+ uptake pathways and kinetics of the effects of K+ on Na+ uptake in the halophyte Suaeda maritima. Plant and Soil 368: 629–640. [Google Scholar]
- Zhao XY, Li RL, Lu CF, Baluska F, Wan YL. 2015. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiology and Biochemistry 87: 53–60. [DOI] [PubMed] [Google Scholar]
- Ziegler H, Lüttge U. 1967. The salt-glands of Limonium vulgare. II. The localisation of chloride. Planta 74: 1–17. [DOI] [PubMed] [Google Scholar]
- Zouhaier B, Abdallah A, Najla T, et al. . 2015. Scanning and transmission electron microscopy and X-ray analysis of leaf salt glands of Limoniastrum guyonianum Boiss. under NaCl salinity. Micron 78: 1–9. [DOI] [PubMed] [Google Scholar]