Abstract
Background and Aims
Rapid evolutionary divergence and reticulate evolution may result in phylogenetic relationships that are difficult to resolve using small nucleotide sequence data sets. Next-generation sequencing methods can generate larger data sets that are better suited to solving these puzzles. One major and long-standing controversy in conifers concerns generic relationships within the subfamily Cupressoideae (105 species, approx. 1/6 of all conifers) of Cupressaceae, in particular the relationship between Juniperus, Cupressus and the Hesperocyparis–Callitropsis–Xanthocyparis (HCX) clade. Here we attempt to resolve this question using transcriptome-derived data.
Methods
Transcriptome sequences of 20 species from Cupressoideae were collected. Using MarkerMiner, single-copy nuclear (SCN) genes were extracted. These were applied to estimate phylogenies based on concatenated data, species trees and a phylogenetic network. We further examined the effect of alternative backbone topologies on downstream analyses, including biogeographic inference and dating analysis.
Results
Based on the 73 SCN genes (>200 000 bp total alignment length) we considered, all tree-building methods lent strong support for the relationship (HCX, (Juniperus, Cupressus)); however, strongly supported conflicts among individual gene trees were also detected. Molecular dating suggests that these three lineages shared a most recent common ancestor approx. 60 million years ago (Mya), and that Juniperus and Cupressus diverged about 56 Mya. Ancestral area reconstructions (AARs) suggest an Asian origin for the entire clade, with subsequent dispersal to North America, Europe and Africa.
Conclusions
Our analysis of SCN genes resolves a controversial phylogenetic relationship in the Cupressoideae, a major clade of conifers, and suggests that rapid evolutionary divergence and incomplete lineage sorting probably acted together as the source for conflicting phylogenetic inferences between gene trees and between our robust results and recently published studies. Our updated backbone topology has not substantially altered molecular dating estimates relative to previous studies; however, application of the latest AAR approaches has yielded a clearer picture of the biogeographic history of Cupressoideae.
Keywords: Single-copy nuclear genes, transcriptome, Cupressoideae, Hesperocyparis, Cupressus, Juniperus, Xanthocyparis, Callitropsis
INTRODUCTION
It can be challenging to reconstruct deep phylogenetic relationships accurately within groups that experienced rapid evolutionary divergence, incomplete lineage sorting and/or reticulate evolution, especially with small data sets (Maddison, 1997; Dunn et al., 2008; Jian et al., 2008; Zeng et al., 2014; Ruhsam et al., 2015). Rapid evolutionary divergence may lead to short internodal distances and soft polytomies (Weisrock et al., 2005; Whitfield and Lockhart, 2007; Jian et al., 2008; Pyron et al., 2014; Leaché et al., 2016). In addition, incomplete lineage sorting, which involves mis-sorting of ancestral polymorphisms relative to the species tree, or reticulate evolution, which involves the combination or transmission of genetic material between divergent evolutionary lineages due to hybridization and introgression, may both cause inaccurate or conflicting species tree inference (Beiko et al., 2008; Sun et al., 2015).
Next-generation sequencing approaches, which generate large amounts of DNA sequence data from throughout the genome, are transforming phylogenetic inference (e.g. Dunn et al., 2008; Lee et al., 2011; Faircloth et al., 2012; Zeng et al., 2014). This is especially true where rapid evolutionary events resulted in few fixed substitutions between divergent species, yielding gene trees that are usually unresolved with respect to the true species tree, when only a few loci are used (Whitfield and Lockhart, 2007). A larger amount of sequence data is likely to capture such species-specific substitutions, potentially resulting in improved phylogenetic resolution (Jian et al., 2008; Zeng et al., 2014). In the case of incomplete lineage sorting, many independent gene trees from throughout the genome can be used to estimate a credible species tree by reconciling genealogical discordance between loci (Edwards, 2009; Lemmon and Lemmon, 2013). Therefore, phylogenetic estimation of species trees based on genomic data sets might resolve branches that were poorly supported in smaller data sets (Rokas et al., 2003; Dunn et al., 2008). For example, phylogenetic analyses using as few as 29 and 59 low-copy nuclear genes have resulted in well-resolved deep phylogenetic estimates for ferns (Rothfels et al., 2015) and flowering plants (Zeng et al., 2014), respectively.
Two main methods have recently been proposed to construct species trees from large data sets (Liu et al., 2009a, b, 2015). One method uses the multiple-species coalescent model as implemented in the program *BEAST (Heled and Drummond, 2010), which estimates gene trees and the species tree at the same time. However, this method is computationally intensive (Edwards et al., 2007; Pyron et al., 2014), and may result in poor convergence if the data set is large (O’Neill et al., 2013). The other method uses a two-step approach when estimating species trees. In the first step, gene trees are generated using software such as RaxML (Stamatakis et al., 2014), and in the second step they are summarized under the coalescent model as implemented in the software MP-EST (Liu et al., 2010) and STAR (Liu et al., 2009a). This method reduces computation time considerably when compared with analyses based on the multiple species coalescent model (Liu et al., 2009b). In addition, a recently developed two-step approach, ASTRAL-II (Mirarab et al., 2015; Sayyari and Mirarab, 2016), has been shown to run much faster and to be less sensitive than MP-EST to the effects of gene tree errors, when estimating a species tree based on large data sets (e.g. hundreds of taxa and thousands of genes). The accuracy of ASTRAL remains high when a small number of genes is adopted and a moderate level of incomplete lineage sorting is assumed, whereas its local posterior probabilities of quartet branches are conservative; this leads to very few false positives that have high support, at the cost of missing some true positives (Mayyari and Mirarab, 2016).
Cupressaceae, also known as the cypress family, contains >160 species in 32 genera, of which 17 are monotypic (Farjon, 2005; Mao et al., 2012; Yang et al., 2012; Adams, 2014; Wang and Ran, 2014). They occur in many different habitats on all continents except Antarctica (Farjon, 2005). Cupressoideae, which contains >100 species in 13 genera, is the largest of the seven subfamilies of Cupressaceae (Gadek et al., 2000; Mao et al., 2012; Yang et al., 2012). This subfamily occurs throughout the Northern Hemisphere and contains many ecologically important and dominant species especially in mountainous and arid or semi-arid regions (Farjon, 2005; Adams, 2014). It contains many economically important timber species (e.g. Calocedrus, Chamaecyparis, Cupressus and Thuja) and ornamental trees (e.g. Chamaecyparis, Juniperus, Platycladus and Thuja) (Farjon, 2005). Phylogenetic analyses suggest that this subfamily is monophyletic (Gadek et al., 2000; Mao et al., 2012; Yang et al., 2012) and comprises four clades (Gadek et al., 2000; Little, 2006; Mao et al., 2012; Yang et al., 2012) which have been treated as separate tribes by some authors (Gadek et al., 2000). However, taxonomic treatment at the generic level and intergeneric relationships within the subfamily remain controversial (Little et al., 2004; Little, 2006; Mill and Farjon, 2006; Rushforth, 2007; Adams et al., 2009; Christenhusz et al., 2011; Dörken et al., 2017), especially for Cupressus sensu lato (s.l.), which comprises 30 species (Little, 2006; Christenhusz et al., 2011; Dörken et al., 2017). Cupressus s.l. may be divided into four genera: Cupressus sensu stricto (s.s.) and Xanthocyparis s.s. in the Old World, and Hesperocyparis and Callitropsis s.s. in the New World (Adams et al., 2009; Mao et al., 2010; Christenhusz et al., 2011) (see Table 1 for a summary of taxonomic treatment history). From here on, if not stated otherwise, ‘Cupressus’, ‘Xanthocyparis’ and ‘Callitropsis’ refer to Cupressus s.s., Xanthocyparis s.s. and Callitropsis s.s., respectively. Although the monophyly of Cupressus and the Hesperocyparis–Callitropsis–Xanthocyparis clade (the HCX clade; Mao et al., 2010) is well defined (Little et al., 2004; Little, 2006; Mao et al., 2010, 2012; Yang et al., 2012), the phylogenetic relationship between Cupressus, the HCX clade and Juniperus remains uncertain. All possible phylogenetic topologies among these three clades have been supported by different studies with different data sets and analyses, as follows: (Cupressus, (Juniperus, HCX)) topology was recovered by Xiang and Li (2005), Adams et al. (2009) and Terry and Adams (2015); (Juniperus, (Cupressus, HCX)) topology by Mao et al. (2010); (HCX, (Cupressus, Juniperus)) topology by Little (2006) and Yang et al. (2012); and a trichotomy (HCX, Cupressus, Juniperus) by Mao et al. (2012). From here on, these topologies are referred to as Cu(HCX,Ju), Ju(Cu,HCX), HCX(Cu,Ju) and (HCX,Cu,Ju), respectively, for simplicity. A recent phylogenomic study based on the whole plastid genomes of 22 species of Cupressaceae and accounting for long branch attraction (e.g. Felsenstein, 1978; Hendy and Penny, 1989) supported the Ju(Cu,HCX) topology (Qu et al., 2017). However, all of these studies used either no more than four bi-parentally inherited nuclear loci (e.g. Little, 2006; Adams et al., 2009) or plastid DNA (ptDNA), the latter of which, despite the use of nine ptDNA regions (Mao et al., 2010), 11 ptDNA regions (Terry and Adams, 2015) or even the whole plastid genome (Qu et al., 2017), can be considered to be a single locus due to its lack of recombination.
Table 1.
A brief summary of alternative taxonomic treatments of Juniperus, Cupressus, Hesperocyparis, Callitropsis and Xanthocyparis since the description of Xanthocyparis vietnamensis in 2002. Underlined taxa are either monophyletic or monotypic. The abbreviations in brackets after common names are in accordance with Fig. 1.
| (A) | Common names | Junipers | Old world cypresses (OWC) | New world cypresses (NWC) | Alaska cedar (A.) | Vietnamese golden cypress (V.) |
|---|---|---|---|---|---|---|
| (B) | This study; Adams et al. (2009); Mao et al. (2010, 2012) | Juniperus | Cupressus (s.s.) | Hesperocyparis | Callitropsis (s.s.) | Xanthocyparis (s.s.) |
| (C) | Farjon et al. (2002); Farjon (2005) | Juniperus | Cupressus sensu Farjon | Cupressus sensu Farjon | Xanthocyparis s. l. | Xanthocyparis s. l. |
| (D) | Little et al. (2004) | N/A | Cupressus sensu Farjon | Cupressus sensu Farjon | Callitropsis sensu Little (2004) | Callitropsis sensu Little (2004) |
| (E) | Little (2006) | N/A | Cupressus (s.s.) | Callitropsis s.l. | Callitropsis s.l. | Callitropsis s.l. |
| (F) | Christenhusz et al. (2011) | Juniperus | Cupressus s.l. | Cupressus s.l. | Cupressus s.l. | Cupressus s.l. |
The aim of the current study is to resolve this long-standing controversy and to reconstruct the phylogenetic relationship between Cupressus, Juniperus and the HCX clade based on a number of single- or low-copy nuclear loci from transcriptome data using 17 species representing major lineages within these three clades, plus three outgroups. Specifically, we (a) investigate the evolutionary relationship between the three major lineages using a phylotranscriptomic approach; (b) compare and explain the discordance and agreement between the current species tree topology and phylogenetic topologies that were gained in previous studies, and characterize the impact of different topologies of the three major lineages on (c) molecular dating of this group and (d) the inference of its biogeographic history.
MATERIALS AND METHODS
Provenance of samples
Fresh leaf samples from a total of 18 species (including the outgroup species Microbiota decussata) were collected for transcriptome sequencing. Fourteen samples were collected from the living collection of the Royal Botanic Garden Edinburgh (RBGE), three were collected in the field in Yunnan, China (Cupressus duclouxiana) and Xizang, China (Cupressus gigantea and Juniperus microsperma), and one (Cupressus funebris) was a cultivated individual from the campus of Sichuan University, Chengdu, China (Table 2). Additionally, we used transcriptome data for three outgroup species (Calocedrus decurrens, M. decussata and Thuja plicata) from the one thousand transcriptome project (‘1000 plant project’, 1KP) (Table 2). All species were represented by a single accession, apart from M. decussata (n = 2).
Table 2.
Accessions used for RNA extraction, transcriptome assembly and subsequent phylogenetic analyses
| Species | Collecting number (identifier) | Latitude/Longitude | Country |
|---|---|---|---|
| Callitropsis nootkatensis | 19941704B (RBGE) | 49°24’N/123°11’W | Canada |
| Calocedrus decurrens | FRPM (1kp) | n/a | Cultivated (UBC) |
| Cupressus duclouxiana | MSZ-49-01 (SZ) | 27º00’N/100º14’E | China |
| C. funebris | Mao-CF (SZ) | n/a | Cultivated (SZ) |
| C. gigantea | MSZ-24–03 (SZ) | 29º00’N/93º14’E | China |
| C. sempervirens | 19752308 (RBGE) | 45°12’N/13°36’E | Croatia |
| Hesperocyparis arizonica | 19921324*C (RBGE) | 30°50’N/115°16’W | Mexico |
| H. bakeri | 19851378*B (RBGE) | 41°57’N/123°18’W | USA |
| H. macrocarpa | 20090071 (RBGE) | 36°31’N/121°56’W | USA |
| Juniperus drupacea | 20100261 (RBGE) | 37°55’N/36°34’E | Turkey |
| J. flaccida | 19922158*C (RBGE) | 25°17’N/100°26’W | Mexico |
| J. indica | 19790193*A (RBGE) | 27°13’N/88°02’E | India |
| J. microsperma | MSZ-11 (SZ) | 29º37’N/96º20’E | China |
| J. oxycedrus | 19921237A (RBGE) | 37°54’N/2°52’W | Spain |
| J. phoenicea | 19921233*A (RBGE) | 37°54’N/2°52’W | Spain |
| J. procera | 20080832*J (RBGE) | 00°19’N/36°58’E | Kenya |
| J. scopulorum | 20081601 (RBGE) | 39°39’N/105°12’W | USA |
| Microbiota decussata | 19881678*A (RBGE) | n/a | Cultivated (RBGE) |
| M. decussata | XQSG (1kp) | n/a | Cultivated (UBC) |
| Thuja plicata | VFYZ (1kp) | n/a | Cultivated (UBC) |
| Xanthocyparis vietnamensis | 20030523 (RBGE) | 23°06’N/105°01’E | Vietnam |
(RBGE) and (SZ) refer to material collected from the wild held at the Royal Botanic Garden Edinburgh and Sichuan University, respectively; (1kp) refers to transcriptome data downloaded from the ‘1000 plant project’ (http://www.onekp.com/samples/ list.php) with vouchers held at the University of British Columbia (UBC).
Transcriptome sequencing, assembly and alignment
Transcriptomes were either generated in Edinburgh/UK (RBGE; Table 2) or in Chengdu/China (SZ; Table 2) apart from three downloaded from the 1KP project (http://www.onekp.com/samples/list.php; labelled as ‘1kp’ in Table 2). RNA was extracted using the Spectrum Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, USA) following protocol A with a few minor modifications (2–3 times the amount of lysis buffer, 750 µL of binding buffer and three final washes). Library preparation and sequencing were outsourced to Edinburgh Genomics (Edinburgh, UK) and Novogene (Beijing, China) for RBGE and SZ samples, respectively (Table 2). Transcriptomes were sequenced on Illumina HiSeq platforms generating 2 × 100 bp paired-end reads. Raw reads were prepared for assembly using Trimmomatic (Bolger et al., 2014) with the parameters ‘LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36’ and cutadapt (Martin, 2011) to remove adaptors and low quality sequences. Reads for each taxon were then assembled into contigs with SOAPdenovo-trans (Xie et al., 2014) using SOAPdenovo-Trans-31mer with ‘-K 29 -L 100’. The program Cd-hit (Li and Godzik, 2006), software for clustering and comparing protein or nucleotide sequences, was used to retrieve only unique contigs from the SOAPdenovo-trans analysis with the command cd-hit-est and default values. The output of Cd-hit was then fed into MarkerMiner v1.0 (Chamala et al., 2015) with parameters ‘-singleCopyReference Athaliana -minTranscriptLen 900’. MarkerMiner identifies and aligns putative orthologous single- or low-copy nuclear genes in a set of transcriptome assemblies, using a reciprocal BLAST search against a reference database (Chamala et al., 2015). The alignments of genes included for further analyses were visually checked with minimal editing and trimming either side of the sequence where missing sites accounted for more than half of all available taxa. In subsequent analyses, data from the two Microbiota accessions (Table 2) were amalgamated to represent one sample in order to minimize the amount of missing data for that species.
Phylogenetic analyses
Alignments of putative single-copy loci from MarkerMiner v1.0 (Chamala et al., 2015) were used to compile two sets of data, the first comprising individual genes, in which each locus is treated independently, and the second a concatenated data set in which all chosen loci were combined into one ‘super locus’. First, we used three conventional methods, maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI), to infer phylogenetic trees based on the concatenated data set. Analyses based on such a data set could lead to species tree misinference if there is sufficient conflict between gene trees, but these concatenation-based methods often recover the same tree that other species tree estimation methods recover (e.g. Wickett et al., 2014) and are commonly done to compare with other species tree estimation methods, e.g. MP-EST (Liu et al., 2010), STAR (Liu et al., 2009a) and ASTRAL (Mirarab et al., 2014). MP analysis was performed using PAUP*4.0b10 (Swofford, 2003), with gaps treated as missing data and polymorphic states as uncertain. A ‘branch and bound’ search with MulTrees on was carried out for both data sets. Branch support was estimated via bootstrapping with 1000 bootstrap replicates using heuristic searches (Felsenstein, 1985). We also used RAxML v8 (Stamatakis, 2014) to estimate a ML tree and ML bootstrap values, by applying the parameters ‘-f a -m GTRGAMMA -p 12345 -x 12345 -# 1000’ where the GTRGAMMA model and 1000 bootstrap replicates were applied (see the RAxML manual for detailed parameter settings). A BI analysis was also performed using MrBayes v. 3.1.2 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003) with the GTR + I + G model, which was selected using MrModelTest v. 2.3 (Nylander, 2004) under the Akaike information criterion. The analysis was run for 2 million generations with four MCMC (Markov chain Monte Carlo) chains in two independent parallel analyses, with one tree sampled every 500 generations. The average standard deviation of split frequencies was 0.00000 at the end of the run. TRACER v1.5 (Rambaut and Drummond, 2009) was used to assess the quality of the MCMC simulations and suggested a high degree of convergence between runs. The effective sample size (ESS) values, i.e. the number of effectively independent draws from the posterior, were >500 for all parameters, indicating that sufficient sampling occurred.
From the individual gene data set, we constructed individual ML gene trees for each locus using the software RAxML v8 (Stamatakis, 2014) applying the parameters as for the concatenated data set. The topology of each gene tree was then manually examined, looking in particular for well-supported alternative relationships that might indicate gene tree conflict. Then, based on these gene trees, we generated a species tree based on the multispecies coalescent model in ASTRAL 5.6.1 (Mirarab et al., 2014; Sayyari and Mirarab, 2016), which estimates species trees from unrooted gene trees, and maximizes the number of quartet trees shared between the gene trees and the species tree. ASTRAL-II estimates branch lengths for internal branches (not terminal branches) in coalescent units, and branch support values measure the support for a quadripartition (the four clusters around a branch) and not bipartition, as is commonly done. The species tree was fully annotated using the ‘-t 4’ option, which calculates the measurements for each branch, including quartet support (q), total number of quartet trees in all the gene trees (f), and the local posterior probabilities (pp) for the main topology and the first and second alternatives, total number of quartets defined around each branch (QC) and the effective number of genes for the branch (EN).
Conservative pp scores cause some true positives to be overlooked in ASTRAL (Mayyari and Mirarab, 2016); moreover, average positive branch rates, which represent the proportion of the estimated species tree in which a certain branch is successfully recovered, may be lower in ASTRAL than in STAR and MP-EST (Liu et al., 2015). Therefore, we also conducted STAR and MP-EST analyses based on gene trees to reduce the chance of missing any true positives, and to improve the average positive branch rates. Hence the rooted ‘best tree’ RAxML output for each gene plus bootstrap values for each gene tree using 1000 replicates was then uploaded to ‘The Species TRee Analysis Webserver’ STRAW (Shaw et al., 2013) to estimate the species tree using STAR (Liu et al., 2009a) and MP-EST (Liu et al., 2010). Both programs apply the multispecies coalescent model (Rannala and Yang, 2003) to obtain estimates of the species tree from gene trees. STAR (Liu et al., 2009a) uses the average ranks of coalescences, whereas MP-EST (Liu et al., 2010) uses a pseudo-likelihood function of the species tree, and both of them generate bootstrap support values using non-parametric bootstrap techniques (Liu et al., 2009a, 2010). Both methods are based on summary statistics calculated across all gene trees, with the effect that a small number of genes that significantly deviate from the coalescent model will have relatively little effect on the ability to infer the species tree accurately.
Because there was some well-supported conflict among gene trees (see the Results), we conducted two additional analyses to investigate this further. First, we applied MulRF (Chaudhary et al., 2013, 2015) to estimate the best species tree, i.e. the one that minimizes the overall Robinson–Foulds (RF) distance between each candidate species tree and the individual gene trees. This software is also able to calculate the MulRF score of a given tree topology, which is the RF distance between this given tree and all gene trees. In a soft polytomy where relationship among three clades are difficult to resolve, this function may be used to compare the compatibility of each of the three dichotomy candidate species trees with all gene trees.
Finally, we used the NeighborNet method implemented in SplitsTree 4.11.3 (Huson and Bryant, 2006) to reconstruct phylogenetic networks based on the concatenated alignment of all 73 nuclear genes. For distance calculations, we excluded insertions/deletions (indels) and used the K2P model (Kimura, 1980). The relative robustness of the clades was estimated by performing 1000 bootstrap replicates, and a confidence network was generated with a 95 % threshold (Huson and Bryant, 2006). This analysis can summarize how homoplasy that might include hybridization or incomplete lineage sorting might have affected the phylogenetic reconstruction.
Molecular dating
To investigate the impact of topological differences on the evolutionary divergence time scale in Cupressoideae, we conducted molecular dating analyses. We tried to adopt the eight fossil calibration points as in Mao et al. (2010), but only three of them could be used for the dating of our 20-taxon data set, whereas the remaining five could only be attached to apparently deeper nodes, relative to those in the phylogeny of Mao et al. (2010). As too few calibration points and/or assigning fossils to deeper nodes (due to sparse sampling) has been shown to bias the estimates of node ages (e.g. Linder et al., 2005; Mao et al., 2012; Wang and Mao, 2016), we adopted a hybrid strategy to reconstruct the evolutionary divergence time scale of Cupressoideae. Hence dating was carried out on our previous ptDNA data set comprising nine ptDNA fragments from 84 species (Mao et al., 2010), but with the relationship between the three main clades constrained to the topology from the current study based on transcriptome data (see below). The original ptDNA data set comprising 92 accessions was slightly reduced by removing multiple accessions of six species, resulting in a final data set of 86 accessions representing 84 species in Cupressoideae (referred to as the ‘86-accession data set’ from here on). Three parallel molecular dating analyses were carried out, one constraining to the HCX(Cu,Ju) topology, another constraining to the Cu(HCX,Ju) topology, and the third was unconstrained, allowing it to retain the Ju(Cu,HCX) topology from Mao et al. (2010). We adopted eight calibration fossils from Mao et al. (2010), seven of which were used as minimum age constraints with uniform priors, and one was set as a fixed age constraint with a normal prior (see Table 1 in Mao et al., 2010 for details).
BEAST version 1.8.0 (Drummond and Rambaut, 2007) was used to estimate topology, substitution rates and node ages simultaneously by employing a Bayesian MCMC chain. BEAST parameter settings, including fossil calibration settings, were all the same as in Mao et al. (2010), except that two independent MCMC analyses of 100 000 000 generations were conducted, sampled every 2000 generations, with 20 % burn-in. The program Tracer 1.5.1 (Rambaut and Drummond, 2007) was employed to check effective sample size, and the program TreeAnnotator 1.8.0 (part of the BEAST 1.8.0 package) was used to summarize the output results. Finally, a tree with ages for each node and their 95 % highest posterior density intervals (95 % HPDs) was displayed and formatted in FigTree 1.4.0 (Rambaut, 2012).
Ancestral area reconstruction
We conducted an ancestral area reconstruction using the BioGeoBEARS packages as implemented in RASP 4.0 (Yu et al., 2015). Four operational geographic areas (A, North America; B, Africa; C, Asia; and D, Europe), were defined for our analyses, following those in Mao et al. (2010). A total of 400 trees, which were resampled from the output trees of the BEAST analysis, and the BEAST summary tree, were imported into RASP 4.0, along with the distribution information of each species. BioGeoBEARS allows the testing of six models (DIVALIKE, DIVALIKE +J, DEC, DEC +J, BAYAREALIKE and BAYAREALIKE +J) (Matzke, 2013, 2014). Of the six models, the DIVALIKE model (Ronquist, 1997) is an event-based approach that adopts a simple biogeographic model; it does not consider general area relationships or branch lengths of the input tree, and it applies different costs to vicariance, duplication, dispersal and extinction to construct ancestral distributions (Ronquist, 1997; Yu et al., 2015). The DEC model (Ree and Smith, 2008) allows dispersal, extinction and cladogenesis as fundamental processes, accommodates differing dispersal probabilities among areas across different time periods, and can integrate branch lengths, divergence times and geological information (Ree and Smith, 2008; Yu et al., 2015). In contrast to the former two models, which accept only bifurcating trees, the BAYAREALIKE model (Landis et al., 2013) allows polytomies. It considers distribution area to be a ‘trait’ of a species, and hence reconstructs ancestral ‘traits’ using BI; furthermore, it does not define the dispersal rate, constrain the maximum number of areas at each node or exclude widespread and unlikely ancestral areas before analysis (Landis et al., 2013; Yu et al., 2015). The other three models with the ‘+J’ suffix (i.e. DIVALIKE +J, DEC +J and BAYAREALIKE +J) allow founder event speciation, in contrast to the three original models (Matzke, 2014).
We conducted model testing, and two models (the best and the second best model, as given in the Results) were employed to reconstruct the ancestral area for every node in the phylogeny based on 100 trees that were randomly selected from 400 BEAST trees. At most two areas were allowed for any node in any tree. An among-area dispersal probability matrix, which is the same as in Mao et al. (2012), was coded to define different dispersal probabilities in five time periods, 0–5, 5–30, 30–45, 45–70 and 70–115 million years ago (Mya). The ancestral area reconstruction results were optimized in the Treeview window of the RASP program.
RESULTS
Phylogenetic analyses
A total of 581 putative single- or low-copy genes were detected by MarkerMiner. About 50 % of these were shared by five or fewer samples. To minimize the impact of missing data on the ability to resolve phylogenetic relationships confidently, for further phylogenetic analyses we only included genes that did not have more than two ingroup and two outgroup taxa missing. This resulted in 73 putatively single- or low-copy genes yielding an alignment of 208 484 nuclear base pairs for 20 taxa (Supplementary Data Table S1). The DNA sequences have been deposited in NCBI GenBank (accession numbers are shown in Supplementary Data Table S2).
For six of the 73 genes chosen by MarkerMiner (Chamala et al., 2015), a secondary transcript that passed the BLAST filtering process was reported for one of the included taxa (Supplementary Data Table S1). These secondary transcripts, defined as the one of the two from a given taxon that received a lower BLAST score, may represent splice isoforms, putative paralogues or partially assembled transcripts (Chamala et al., 2015). However, as removing the taxon from the particular alignment for which a secondary transcript was detected did not change the phylogenetic results in any way, for these species (data not shown) the transcript for each taxon with the higher BLAST score was included in all analyses.
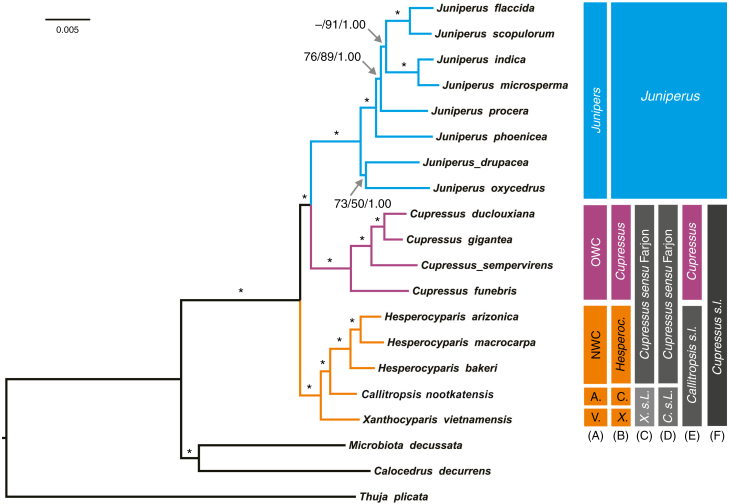
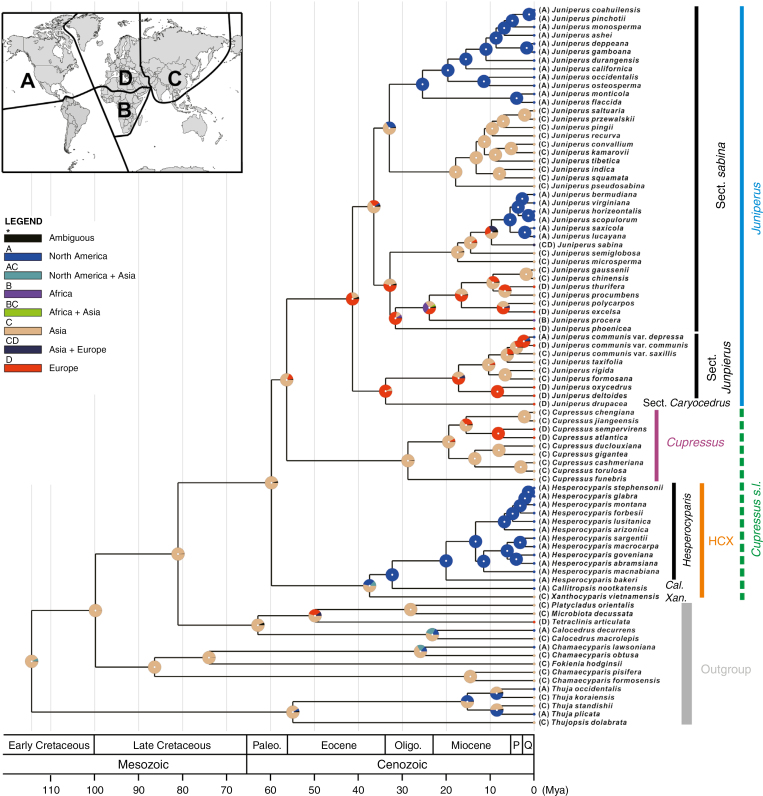
In this matrix consisting of 73 genes, 183 022 (87.8 %) characters were constant, 16 405 (7.9 %) were variable but parsimony uninformative, and 9057 (4.3 %) were parsimony informative. The same topology was retrieved regardless of the bifurcate tree-building method used (including MP, ML, BI, MP-EST, STAR, ASTRAL-II and MulRF), with an HCX clade (comprising a monophyletic Hesperocyparis, plus Callitropsis and Xanthocyparis) as the sister group of a clade consisting of Cupressus (monophyletic) and Juniperus (also monophyletic) (Figs 1 and 2). Branch support was high for the MP, ML and BI analyses of the concatenated data set and for the MP-EST, STAR and ASTRAL-II analyses using a coalescent approach. Individual trees produced by Bayesian analysis and the three coalescent approaches were identical. The MP tree topology differed from these in only one respect: here (J. procera (J. indica, J. microsperma)) was sister to (J. flaccida, J. scopulorum) (Supplementary Data Fig. S1), whereas in the other analyses J. procera was sister to ((J. indica, J. microsperma) (J. flaccida, J. scopulorum)) (Figs 1 and 2). However, two branches concerning this relationship were weakly or moderately supported by the bootstrap analysis in the MP analysis [bootstrap support (BS) = 55 and 76 %, respectively; Supplementary Data Fig. S1]. The Juniperus clade was also the only ingroup clade where some of the internal relationships did not receive maximum branch support; this was true for all six analyses methods used (MP, BI, ML, MP-EST, STAR and ASTRAL-II; Figs 1 and 2).
Fig. 1.
Bayesian tree based on 73 concatenated nuclear genes (208.484 bp). Numbers or asterisks above branches are statistical support values for maximum parsimony/maximum likelihood/Bayesian inference analyses, respectively, with * denoting maximum support in all three analyses. Colour and grey scale bars to the right of the cladogram illustrate (A) common names of all included taxa [OWC = Old World cypresses; NWC = New World cypresses; A. = Alaska cedar; V. = Vietnamese golden cypress; C. = Callitropsis; X. = Xanthocyparis; s.L. = sensuLittle (2004)] and taxonomic treatments adopted (B) in this study, Adams et al. (2009) and Mao et al. (2010, 2012), (C) by Farjon et al. (2002) and Farjon (2005), (D) by Little et al. (2004), (E) by Little (2006) and (F) by Christenhusz et al. (2011). The scale bar indicates the estimated number of mutations per site.
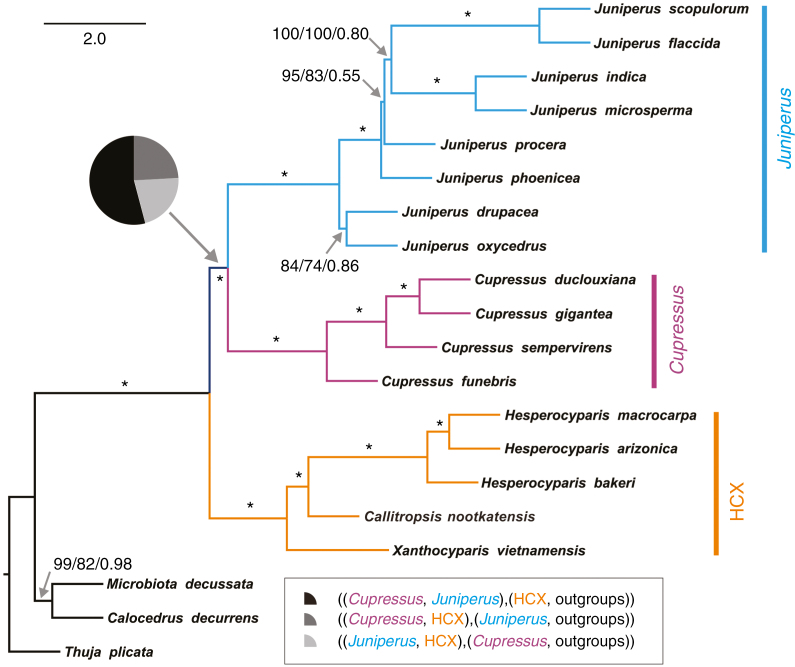
Fig. 2.
Species tree generated using ASTRAL-II based on 73 nuclear genes (208 484 bp). Numbers or asterisks above branches are branch support values for MP-EST, STAR and ASTRAL-II analyses, respectively, with asterisks denoting maximum support in all three analyses. ASTRAL-II measures branch lengths in coalescent units (the scale bar shown corresponds to two coalescent units) for internal branches and not terminal branches (branch lengths of terminal branches are therefore arbitrary and meaningless). The pie chart shows respective quartet support for the main topology, the first and the second alternative topology (as shown in the inset). Note that in the inset, the tree formulae in parentheses were presented in the sense of an unrooted quadripartition (four-taxon) tree, where the central piece of the tree is an internode branch between the two pairs of partitions. The first case shown ((Cupressus, Juniperus), (HCX, outgroups)) is consistent with HCX being sister to (Cupressus, Juniperus) in an outgroup-rooted tree.
The HCX(Cu,Ju) topology of our species tree based on single-copy nuclear (SCN) genes conflicts with the Ju(Cu,HCX) topology based on the ptDNA data from Mao et al. (2010) (Supplementary Data Fig. S2). Quartet support analyses in ASTRAL-II suggest that the HCX(Cu,Ju), Ju(Cu,HCX) and Cu(HCX,Ju) topologies are supported by 54.16, 24.24 and 21.60 % of the gene trees, respectively (Fig. 2). A manual check of gene trees (Supplementary Data Fig. S3) which were generated in RAXML using maximum likelihood bootstrapping (MLBS) indicated similar proportions of gene trees supporting these three topologies [i.e. most supporting the HCX(Cu,Ju) topology, which is equivalent to the Cupressus–Juniperus sister topology in Supplementary Data Table S1], except that a few MLBS gene trees are unresolved (Supplementary Data Table S3). We also calculated the MulRF score (the total RF topological distance of all 73 gene trees against the candidate species tree) for each of the above three topologies concerning Cupressus, Juniperus and HCX (other relationships remain the same). The HCX(Cu,Ju), Ju(Cu,HCX) and Cu(HCX,Ju) topologies received MulRF scores of 744 (= closest and therefore favoured), 786 and 790 (= furthest), respectively.
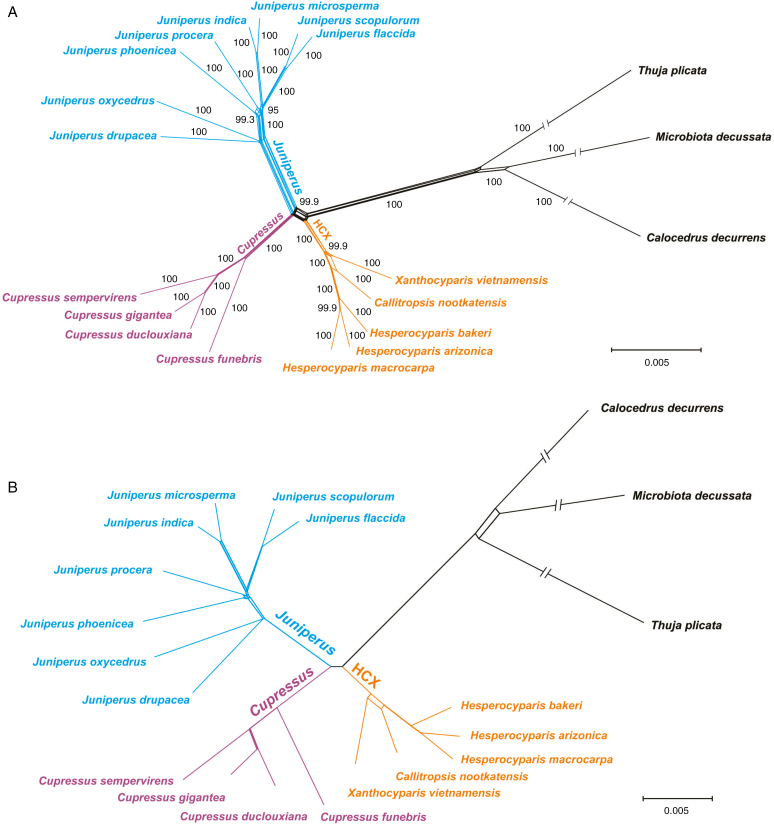
Finally, NeighborNet analyses provide 100 % bootstrap support for the quartet branch that links (Juniperus, Cupressus) and (HCX, outgroups), although the length of this branch is relatively short (Fig. 3A). Very few strongly supported relationships that might have suggested hybridization or incomplete lineage sorting (BS >95 %) were recovered in the NeighborNet confidence network; these were mainly found within Juniperus, but also once within Cupressus [the branch leading to (C. gigantea, C. duclouxiana)], at the basal position of the HCX clade, and among the three outgroups (Fig. 3B).
Fig. 3.
Neighbour-Net networks based on 73 concatenated nuclear genes (208 484 bp) using SplitsTree. (A) A Neighbour-Net network with bootstrap support values and (B) a consensus Neighbour-Net network using a 95 % threshold based on 1000 bootstrap replicates. In (A), numbers next to ‘branches’ are bootstrap support values. Note that in both (A) and (B), the branches leading to the three outgroup taxa are truncated so as to show more details of ingroup relationships.
Molecular dating
The BEAST analysis based on the two phylogenetic topologies, HCX(Cu,Ju) and Ju(Cu,HCX), yielded effective sample sizes that were well above 200 (>800) for branch lengths, topology and clade posteriors and all other relevant parameters, indicating adequate sampling of the posterior distribution. However, the BEAST analysis based on the Cu(HCX,Ju) topology failed, despite being identical to other analyses in all but enforcement of topology, because every one of >20 attempts returned an error message that the log likelihood of the initial tree is negative infinity.
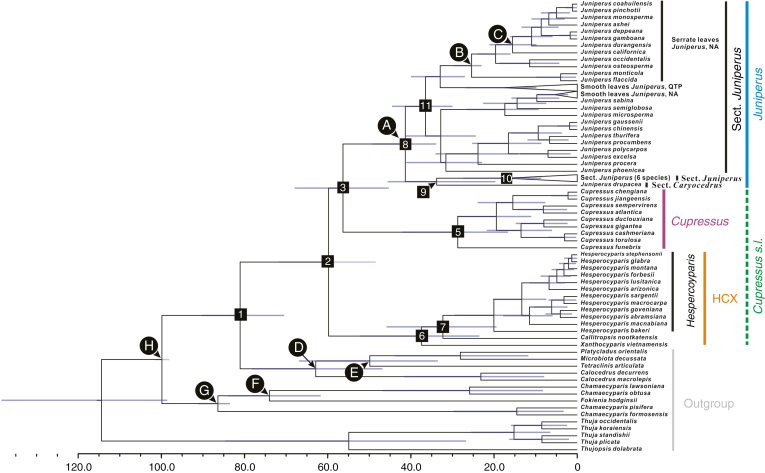
Based on the HCX(Cu,Ju) topology that was supported by SCN genes, we estimate that the most recent common ancestor (MRCA) of Cupressus, Juniperus and the HCX clade diverged from the MRCA of Platycladus, Microbiota, Calocedrus and Tetraclinis 81.06 Mya [70.50–90.40] (from here on, the 95 % HPD range of age estimation are shown in square brackets), the HCX clade diverged from the MRCA of Cupressus and Juniperus 59.80 Mya [48.45–71.74] and Juniperus diverged from Cupressus 56.33 [45.30–67.95] Mya. The crown ages of the HCX clade, Cupressus and Juniperus were estimated to be 37.45 Mya [23.54–52.30], 28.73 Mya [16.65–42.15] and 41.34 Mya [29.99–44.63], respectively (Fig. 4; Table 3).
Fig. 4.
Evolutionary divergence time scale of Cupressoideae based on the ptDNA data set (86 accessions from Mao et al., 2010) with the imposed constraint of the nuclear species tree (HCX(Cu,Ju)) topology from this study using BEAST. Blue bars represent 95 % HPD (highest posterior density) for each node, and white trianges with a black outline represent compressed clades. Letters in black circles represent fossil calibration points (corresponding to those in Table 1 in Mao et al., 2010), and numbers in black squares indicate numbers for nodes of interest (see Table 3). ‘HCX’ stands for the Hesperocyparis–Callitropsis–Xanthocyparis clade.
Table 3.
Estimates for the divergence times for nodes within Juniperus and Cupressus (s.s.) and the Hesperocyparis–Callitropsis–Xanthocyparis clade (HCX), based on the ptDNA data set of Mao et al. (2010) using the constraint of the nuclear species tree topology from this study [HCX(Cu,Ju) topology] or without any constraint [i.e. ptDNA tree topology, Ju(Cu,HCX) topology] employing a relaxed molecular dating approach in BEAST
| Node no. | Description of node | HCX(Cu,Ju) topology | Ju(Cu,HCX) topology |
|---|---|---|---|
| 1 | Stem of the MRCA of the three clades | 81.06 (70.45–90.40) | 80.96 (71.07–89.75) |
| 2 | Crown of the MRCA of the three clades | 59.80 (48.45–71.74) | 59.44 (47.54–71.24) |
| 3 | Split between Cupressus and Juniperus | 56.33 (45.30–67.95) | Equal to Node 2 |
| 4 | Split between Cupressus and the HCX clade | Equal to node 2 | 54.09 (41.29–67.03) |
| 5 | Crown of Cupressus | 28.73 (11.65–42.15) | 28.44 (16.57–42.03) |
| 6 | Crown of the HCX clade | 37.45 (23.54–52.30) | 36.41 (22.73–50.81) |
| 7 | Split between Callitropsis (s.s.) and Hesperocyparis | 32.30 (19.40–45.86) | 31.58 (19.13–44.84) |
| 8 | Crown of genus Juniperus | 41.34 (33.90–49.45) | 41.79 (33.91–50.80) |
| 9 | Split: sects. Juniperus–Caryocedrus | 33.80 (19.68–45.58) | 34.12 (20.28–46.87) |
| 10 | Crown of sect. Juniperus | 17.20 (8.63–27.41) | 17.16 (8.34–27.05) |
| 11 | Crown of sect. Sabina | 36.50 (29.99–44.53) | 36.80 (29.49–44.86) |
Based on the Ju(Cu,HCX) topology that was supported by the ptDNA tree, age estimation for all nodes overlapped with the above estimation (see Table 3) except that the HCX clade diverged from Cupressus 54.09 Mya (95 % HPD 41.29–67.03). A comparison of age estimation of major nodes in BEAST analyses based on each of the above two topologies is shown in Table 3.
Ancestral area reconstruction
Model tests in the BioGeoBEARS package, based on either the HCX(Cu,Ju) or the Ju(Cu,HCX) topology, suggested that DIVALIKE +J is the best-performing model [AICc_wt values: HCX(Cu,Ju) topology = 0.65, Ju(Cu,HCX) topology = 0.60], whereas the DEC +J model is the second best model [AICc_wt values: HCX(Cu,Ju) topology = 0.33, Ju(Cu,HCX) topology = 0.39].
Based on the HCX(Cu,Ju) topology, the DIVALIKE +J model and the 86-accession data set, Cupressus, Juniperus and the HCX clade share a common ancestor whose ancestral distribution area is probably Asia (approx. 0.96), whereas Cupressus and Juniperus shared a common ancestor whose ancestral distribution area is likely to be Asia (approx. 0.82) or less likely Europe (approx. 0.16). The ancestral area for the MRCA of the HCX clade is inferred to be Asia (approx. 0.54), North America (approx. 0.33) or a combination of these two (approx. 0.13). Within this clade, the common ancestor of all New World cypresses (Callitropsis plus Hesperocyparis) most probably migrated to and diversified in North America later (Fig. 5). The ancestral area for Cupressus is probably Asia (approx. 0.99), and Cupressus semperivens and its close allies dispersed to Europe around the middle Miocene (Fig. 5). Furthermore, the ancestral area for Juniperus is inferred to be Europe (approx. 0.72), or possibly Asia (approx. 0.23). The common ancestor of sect. Juniperus was inferred to be in Europe (approx. 0.56), Asia (approx. 0.36) or a combination of both, whereas that of sect. Sabina was probably in Asia (approx. 0.65) and possibly in Europe (approx. 0.25) or Africa (approx. 0.05); overall, Juniperus is most likely to have diversified within Eurasia, with three separate dispersal events to North America and one to Africa. BioGeoBEARS analysis based on the DEC +J model yielded highly similar results (not shown), especially concerning major nodes in the phylogeny.
Fig. 5.
Ancestral area reconstruction based on BEAST trees constrained using nuclear species tree (HCX(Cu,Ju)) topology and the DIVALIKE +J model in BioGeoBEARS, as implemented in RASP 4.0. The inset shows the division of the distribution area of the five genera into four operational areas (North America, Africa, Asia and Europe). The pie at each node represents the reconstructed ancestral area; different colours of circular sectors in a pie represent the relative probabilities of different ancestral areas at a node.
Based on the Ju(Cu,HCX) topology, either the DIVALIKE +J or the DEC +J model and the 86-accession data set, the ancestral area for nearly all nodes is highly similar to the HCX(Cu,Ju) topology, except for the node of the common ancestor of Cupressus and the HCX clade, which does not exist in the HCX(Cu,Ju) topology. The ancestral area for this node in the Ju(Cu,HCX) topology is likely to be Asia (DIVALIKE +J, approx. 0.97; DEC +J, 0.95) (Supplementary Data Fig. S4).
DISCUSSION
Rapid evolutionary divergence and inference of phylogenetic relationships among the three major clades in Cupressoideae
The main aim of this study is to resolve and explain the long-standing controversy of generic and intergeneric relationships between the three major lineages in Cupressoideae, Cupressus, the Hesperocyparis–Callitropsis–Xanthocyparis (HCX) clade and Juniperus. Our phylogenetic analyses using MP, ML and BI analyses of concatenated data and species tree analyses (MP-EST, STAR and ASTRAL), based on 73 putative SCN genes totalling >200 000 bp, all show a maximally supported sister relationship of Cupressus and Juniperus, and that their common ancestor is sister to the HCX clade (Figs 1 and 2). Although only weakly or moderately supported, the Ju(Cu,HCX) topology based on ptDNA (Supplementary Data Fig. S2; Mao et al., 2010) conflicts with the HCX(Cu,Ju) topology here, as well as several published phylogenies (Xiang and Li, 2005; Little et al., 2006; Adams et al., 2009; Yang et al., 2012; Terry and Adams, 2015). This incongruence may have been caused by incomplete lineage sorting due to rapid evolutionary divergence and/or hybridization and introgression between the three clades during their early evolutionary history. Yang et al. (2012) constructed a reticulate network using two nuclear loci and, because relationships of the three subclades were incongruent among different data sets, suggested that Cupressus ‘might have originated through hybridization between Juniperus and the ancestor of Hesperocyparis–Callitropsis–Xanthocyparis’ (Yang et al., 2012; p. 462). However, although hybridization and introgression during earlier history is a possibility, the main cause of the phylogenetic pattern among the three clades appears to be a combination of rapid evolutionary divergence and incomplete lineage sorting.
First, all phylogenetic analyses that we conducted using 73 loci support the HCX(Cu,Ju) topology. As we have shown above, the species tree constructed using MP-EST, STAR, ASTRAL or trees built using MP, ML or BI based on concatenated data show 100 % support for the HCX(Cu,Ju) topology. The species tree estimate from MulRF also supports the HCX(Cu,Ju) topology: in particular, the RF distance between all gene trees to the HCX(Cu,Ju) topology is closer than to either the Ju(Cu,HCX) topology or the Cu(HCX,Ju) topology. In addition, the Neighbor-Net tree based on the concatenated data set also supports the HCX(Cu,Ju) topology, with 100 % bootstrap support (Fig. 3A), and the ‘reticulate’ pattern among the three clades that Yang et al. (2012) reported was not detected (Fig. 3).
Secondly, the gene tree topology frequency we found here may fit better with incomplete lineage sorting as an explanation of conflicting gene trees. The maximum support value for nodes in the species tree does not necessarily mean that there is no conflict between the 73 gene trees. In our ASTRAL analyses, for example, the HCX(Cu,Ju), Ju(Cu,HCX) and Cu(HCX,Ju) topologies received quartet support values of 54.16, 24.24 and 21.60 %, respectively (equivalent to 39.54, 17.69 and 15.77 gene trees, respectively). We further checked the MLBS tree for each of the 73 genes and found that 38, 15 and 14 gene trees support the above three topologies, respectively; if only MLBS values >70 % are considered (corresponding to a moderately well supported branch), then 31, 11 and 7 gene trees supported the three topologies, respectively. If conflict between gene trees is caused by incomplete lineage sorting, which is always a close companion of rapid evolutionary divergence (e.g. Maddison, 1997), then we would expect one high-frequency topology and two lower frequency topologies. Conversely, if the conflict between gene trees is caused by hybridization and introgression [e.g. the hypothesis that Cupressus is a hybrid clade between Juniperus and HCX that Yang et al. (2012) have put forward], one might expect two major (and equivalent) frequency gene tree topologies (e.g. if the branch was a result of hybrid speciation) or some other set of frequencies (e.g. if a sub-set of the genome introgressed at this point). To conclude, the pattern of gene tree topology frequencies we found above is more consistent with the scenario of incomplete lineage sorting than the hybridization and introgression scenario.
Thirdly, the internode branch lengths between the three clades are consistently short in both our species tree (Fig. 2) and trees based on concatenated data (Fig. 1), and the molecular dating suggests that the interval between the MRCA of HCX–Juniperus–Cupressus (approx. 59.8 Mya) and the MRCA of Juniperus–Cupressus (approx. 56.3 Mya) is also relatively short [approx. 3.5 million years (Myr)], consistent with rapid evolutionary divergence (and presumably a substantial chance of retaining some conflicting ancestral polymorphisms, as documented for our individual gene trees; Supplementary Data Fig. S3). This has also been the case in previous phylogenies of Cupressoideae. For example, using the whole plastid genome, the inferred internode branch length is short, regardless of whether the HCX(Cu,Ju) or Ju(Cu,HCX) topology is recovered (Qu et al., 2017), and based on six ptDNA regions the phylogenetic relationship of these three clades remained unresolved (Mao et al., 2012). However, there is one exception to the pattern, which is that the internode branch length between the MRCA of the three clades and the MRCA of Cupressus and Juniperus is relatively long based on the nuclear gene NEEDLY (Yang et al., 2012).
Thus, although we cannot exclude reticulate evolution in shaping the current phylogenetic pattern of the three clades within Cupressoideae, rapid evolutionary divergence better explains the pattern we found. This inference is different from another case in this subfamily where reticulate evolution is clearly indicated among Thuja species (Peng and Dan, 2008).
Transcriptomic data provide strong support for a four-genus taxonomic treatment in Cupressus s.l.
Previous studies suggested four possible phylogenetic topologies concerning these three clades. Phylogenetic analyses based on either three or six ptDNA markers show that these three clades are part of a trichotomy (Little et al., 2006; Mao et al., 2012), whereas nine ptDNA markers provide moderate support for the Ju(Cu,HCX) topology (Mao et al., 2010). A recent study based on whole plastid genomes supported the clustering of Juniperus and Cupressus, while a filtered data set, which was meant to reduce or elucidate long branch artefacts, supported the clustering of Cupressus and the HCX clade (Qu et al., 2017). The Cu(HCX,Ju) topology was supported by a series of studies: based on a nuclear ribosomal internal transcribed spacer (nrITS) region alone (Xiang and Li, 2005), a combined data set that included nrITS, two ptDNA markers and 56 morphological characters (Little et al., 2004); a combined data set that included one ptDNA region and three nuclear regions (nrITS, ABI3 and 4CL) (Adams et al., 2009); and a combined data set that included 11 ptDNA regions and two nuclear regions (nrITS and NEEDLY) (Terry and Adams et al., 2015). Phylogenetic analyses based on a single nuclear region, NEEDLY (MP support value: 100 %), and a combined data set that included three ptDNA regions, two nuclear regions (ITS and NEEDLY) and 88 organismal characters (MP support value: 100 %; Little, 2006) supported a HCX(Cu,Ju) topology, in agreement with our SCN gene results (Fig. 1).
One important implication of our results is that Cupressus s.l. is paraphyletic, and should be divided into four genera (see Fig. 1 and Table 1 for a clarification of taxon names). Nearly all published molecular phylogenetic analyses support the monophyly of both Cupressus s.s. and the HCX clade, yet the sister relationship between them is rarely supported (Mao et al., 2010). Hence, Little (2006) proposed to call the HCX clade Callitropsis s.l., where Xanthocyparis s.l. was merged into Callitropsis s.l., yet such a treatment is not universally accepted. Considering a proposal of Mill and Farjon (2006) to conserve the genus name Xanthocyparis, which was ratified by the International Botanical Congress in 2011 (Barrie, 2011), and that Xanthocyparis s.l. is not monophyletic, Adams et al. (2009) proposed to place all New World cypresses (Cupressus sensu Farjon species in North America) in the new genus Hesperocyparis and keep both Xanthocyparis s.s. and Callitropsis s.s. as monotypic genera. Our results support this, showing that both Cupressus s.l. and Xanthocyparis s.l. are paraphyletic, while each of Cupressus, the HCX clade and Hesperocyparis is monophyletic. Hence, our nuclear-based results strongly support the division of Cupressus s.l. into four genera: Cupressus, Hesperocyparis, Xanthocyparis s.s. and Callitropsis s.s. (Adams et al., 2009; Mao et al., 2010) and rejects both the combination of these four genera under Cupressus s.l. (Christenhusz et al., 2011; Table 1) and the combination of Xanthocyparis s.s. and Callitropsis s.s. under either Xanthocyparis s.l. or Callitropsis sensuLittle (2004). Our data, as well as those of many others (e.g. Little, 2006; Mao et al., 2010), would also be consistent with combining Xanthocyparis s.s., Callitropsis s.s. and Hesperocyparis under Callitropsis s.l., yet Hesperocyparis is morphologically distinct enough to deserve recognition as a distinct genus (Adams et al., 2009).
An updated evolutionary divergence time scale and biogeographic history of Cupressus, the HCX clade and Juniperus
Rerunning the molecular dating on the ptDNA data set from Mao et al. (2010), while constraining it with the nuclear species tree HCX(Cu,Ju) topology of our results, suggests that the split between the Cupressus–Juniperus clade and the HCX clade occurred (48.45–) 59.80 (–71.74) Mya, with a split of the former clade into Cupressus and Juniperus happening only 3.47 Myr later, (45.30–) 56.33 (–67.95) Mya. Comparing this with the Ju(Cu,HCX) topology that was supported by ptDNA data (Mao et al., 2010), the only difference is that Juniperus diverges first (47.54–) 59.44 (–71.24) Mya, followed by the divergence of Cupressus from HCX (41.29–) 54.09 (–67.03) Mya, in the Ju(Cu,HCX) topology. All other nodes occur in both topologies and differ in age between topologies by no more than 1.04 Myr, a difference dwarfed by HPD error ranges (Fig. 4; Table 3). This indicates that, in our case, a single topological difference, even in the deep nodes in a phylogeny, had a very limited effect on node age estimates. A possible reason for this may be that this particular topological difference did not alter the phylogenetic position of fossil calibration points, and barely affected the total length between any given node and the root of the tree (Sauquet et al., 2012; Wang and Mao, 2016).
We also reran the ancestral area reconstruction analyses for both the HCX(Cu,Ju) and Ju(Cu,HCX) topologies using BioGeoBEARS, and four parallel analyses were conducted for each of the two topologies based on two different models (DIVALIKE +J and DEC +J). Apart from the MRCA of Cupressus and Juniperus, and the MRCA of Cupressus and the HCX clade, that are specific to the HCX(Cu,Ju) and Ju(Cu,HCX) topologies, respectively, the relative probabilities of the ancestral area for all other nodes in all four parallel analyses are highly similar. We therefore discuss the reconstructed biogeographic history of the HCX clade, Cupressus and Juniperus based on the HCX(Cu,Ju) topology and the best model (DIVALIKE +J model). Our ancestral area reconstruction (AAR) analysis inferred that both the MRCA of the HCX clade, Cupressus and Juniperus, and the MRCA of Cupressus and Juniperus, most probably originated in Asia. Likewise, the HCX clade most probably originated in Asia and then dispersed once to North America and diversified there (Fig. 5). This fits a pattern of directional migration from the north-west to the south-east in North America in New World Cypresses (Callitropsis and Hesperocyparis), which may have been caused by climate cooling and aridification in the latter half of the Cenozoic (Terry et al., 2016). Cupressus probably originated in Asia, and then dispersed to Europe (and northern Africa) around the middle Miocene (Fig. 5). The genus Juniperus and sect. Juniperus most probably originated in Europe, whereas sect. Sabina originated in Asia; three independent migrations from Eurasia to North America and one migration from Eurasia to Africa were inferred (Fig. 5).
Comparing these results with the previous AAR analysis based on S-DIVA (Mao et al., 2010), the AAR analysis based on BioGeoBEARS yielded a clearer resolution, especially concerning the ancestral area of the MRCA of the HCX clade, the MRCA of Juniperus, the MRCA of Juniperus sect. Juniperus and sect. Caryocedrus, the MRCA of Juniperus sect. Juniperus and the MRCA of Clade I (Juniperus pseudosabina plus all Himalayan/Qinghai-Tibet Plateau species except J. microsperma and J. gausenii) and Clade II (serrate-leaved junipers of North America) (Mao et al., 2010). BioGeoBEARS tends to infer a single area as the ancestral area, whereas S-DIVA usually infers the combination of two disjunct areas as the ancestral area. The integration of dispersal probability among areas during different time periods in the past, and the use of a model test to seek the best performing model are likely to have improved the resolution of AAR in BioGeoBEARS compared with S-DIVA.
Conclusion
Phylogenetic relationships among Cupressus, Hesperocyparis–Callitropsis–Xanthocyparis (HCX) and Juniperus have been a contentious issue since the discovery of the Golden Vietnamese Cypress, Xanthocyparis vietnamensis. Our species tree based on 73 nuclear loci yielded 100 % support for a (HCX, (Cupressus, Juniperus)) topology which is in agreement with previous phylogenies based on two nuclear loci (LEAFY and NEEDLY; Yang et al., 2012) and a combined data set including both morphological characters and a molecular data set (Little, 2006), but contradicts many others. This indicates that Cupressus s.l. (Christenhusz et al., 2011; Table 1) is paraphyletic, and can be considered instead as two monophyletic genera, Cupressus (s.s.) and Hesperocyparis, and two monotypic genera, Callitropsis (s.s.) and Xanthocyparis (s.s.). Rapid evolutionary divergence and incomplete lineage sorting may have been the major cause for the minor conflicts observed among gene trees. Molecular dating based on the nuclear species tree HCX(Cu,Ju) topology suggests that the three clades underwent two evolutionary splits in a time period as short as approx. 3.47 Myr. The split between Cupressus + Juniperus and the HCX occurred approx. 59.80 Mya (95 % HPD 48.45–71.74 Mya), and the split between Cupressus and Juniperus occurred approx. 56.33 Mya (95 % HPD 45.30–67.95 Mya). Ancestral area reconstruction analyses suggest that the MRCA of Juniperus probably occurred in Europe, whereas the MRCAs of HCX, Cupressus, Cupressus + Juniperus and HCX + Cupressus + Juniperus all most probably occurred in Asia. Therefore, the common ancestor of these three clades most probably originated in Asia and then diversified and dispersed to Europe, North America and Africa. Our study shows that combining low-copy nuclear genes collected using next-generation sequencing and coalescent-based species tree estimation methods is a powerful approach that provides more refined phylogenetic estimates of deep nodes in conifer phylogeny that were controversial based on small data sets.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: detailed information for the 73 single-/low-copy nuclear genes used in phylogenetic analyses. Table S2: NCBI GenBank accession numbers for the 73 genes of 20 taxa that were used in phylogenetic analyses. Table S3: a summary of the topology and the maximum likelihood (ML) bootstrap support value (MLBS) for each of the 73 nuclear genes used to construct the species tree. Figure S1: maximum parsimony (MP) tree based on 73 concatenated nuclear genes (208.484 bp). Figure S2: phylogenetic relationships and posterior probability of major clades in Cupressoideae. Figure S3: maximum likelihood bootstrap tree for each of the 73 nuclear genes as constructed in RaxML based on 1000 bootstrap replicates. Figure S4: ancestral area reconstruction based on ptDNA tree (Ju(Cu,HCX)) topology and the DIVALIKE +J model in BioGeoBEARS as implemented in RASP 4.0.
ACKNOWLEDGEMENTS
We are grateful to Dr Jun Wen for her constructive suggestions on early ideas of this work. This study was funded by the National Natural Science Foundation of China (grant nos. 31590821, 31622015 and 31370261), the Department of Science and Technology of Sichuan Province (grant no. 2015JQ0018) and Sichuan University. The Royal Botanic Garden Edinburgh is supported by the Scottish Government’s Rural and Environment Science and Analytical Services Division. K.M., M.R. and J.L. designed the research; K.M., M.R., Y.M., S.G., J.L., P.T., R.I.M. and P.M.H. performed the research; M.R., K.M. and Y.M. analysed the data; K.M., M.R., S.G., P.T. and P.M.H. procured specimens; K.M., M.R. and R.I.M. wrote the manuscript, all authors revised the manuscript; and K.M., M.R., S.G. and R.I.M. finalized the manuscript.
LITERATURE CITED
- Adams R, Bartel J, Price R. 2009. A new genus, Hesperocyparis, for the cypresses of the Western Hemisphere (Cupressaceae). Phytologia 91: 160–185. [Google Scholar]
- Adams RP. 2014. Junipers of the world: the genus Juniperus. Vancouver, BC: Trafford Publishing. [Google Scholar]
- Barrie FR. 2011. Report of the General Committee: 11. Taxon 60: 1211–1214. [Google Scholar]
- Beiko RG, Doolittle WF, Charlebois RL. 2008. The impact of reticulate evolution on genome phylogeny. Systematic Biology 57: 844–856. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamala S, Garcia N, Godden GT, et al. . 2015. Markerminer 1.0: a new application for phylogenetic marker development using angiosperm transcriptomes. Applications in Plant Sciences 3: 1400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary R, Burleigh JG, Fernández-Baca D. 2013. Inferring species trees from incongruent multi-copy gene trees using the Robinson–Foulds distance. Algorithms for Molecular Biology 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary R, Fernández-Baca D, Burleigh JG. 2015. MulRF: a software package for phylogenetic analysis using multi-copy gene trees. Bioinformatics 31: 432–433. [DOI] [PubMed] [Google Scholar]
- Christenhusz MJM, Reveal JL, Farjon A, Gardner MF, Mill RR, Chase MW. 2011. A new classification and linear sequence of extant gymnosperms. Phytotaxa 19: 55–70. [Google Scholar]
- Dörken VM, Nimsch H, Jagel A. 2017. Morphology, anatomy and morphogenesis of seed cones of Cupressus vietnamensis (Cupressaceae) and the taxonomic and systematic implications. Flora 230: 47–56. [Google Scholar]
- Drummond AJ, Rambaut A, Shapiro B, Pybus OG. 2005. Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular Biology and Evolution 22: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Dunn CW, Hejnol A, Matus DQ, et al. . 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452: 745–749. [DOI] [PubMed] [Google Scholar]
- Edwards SV. 2009. Is a new and general theory of molecular systematics emerging?Evolution 63: 1–19. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Liu L, Pearl DK. 2007. High-resolution species trees without concatenation. Proceedings of the National Academy of Sciences, USA 104: 5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faircloth BC, McCormack JE, Crawford NG, Harvey MG, Brumfield RT, Glenn TC. 2012. Ultraconserved elements anchor thousands of genetic markers spanning multiple evolutionary timescales. Systematic Biology 61: 717–726. [DOI] [PubMed] [Google Scholar]
- Farjon A. 2005. A monograph of Cupressaceae and Sciadopitys. Kew, UK: Royal Botanic Gardens, Kew. [Google Scholar]
- Felsenstein J. 1978. Cases in which parsimony or compatibility methods will be positively misleading. Systematic Zoology 27: 401–410. [Google Scholar]
- Felstenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gadek PA, Alpers DL, Heslewood MM, Quinn CJ. 2000. Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. American Journal of Botany 87: 1044–1057. [PubMed] [Google Scholar]
- Heled J, Drummond AJ. 2010. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution 27: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy MD, Penny D. 1989. A framework for quantitative study of evolutionary trees. Systematic Zoology 38: 297–309. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Jian SG, Soltis PS, Gitzendanner MA, et al. . 2008. Resolving an ancient, rapid radiation in Saxifragales. Systematic Biology 57: 38–57. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Landis MJ, Matzke NJ, Moore BR. 2013. Bayesian analysis of biogeography when the number of areas is large. Systematic Biology 62: 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leache AD, Banbury BL, Linkem CW, de Oca ANM. 2016. Phylogenomics of a rapid radiation: is chromosomal evolution linked to increased diversification in North American spiny lizards (Genus Sceloporus)?BMC Evolutionary Biology 16: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Cibrian-Jaramillo A, Kolokotronis SO, et al. . 2011. A functional phylogenomic view of the seed plants. PLoS Genetics 7: e1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon EM, Lemmon AR. 2013. High-throughput genomic data in systematics and phylogenetics. Annual Review of Ecology, Evolution, and Systematics 44: 99–121. [Google Scholar]
- Li WZ, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659. [DOI] [PubMed] [Google Scholar]
- Linder HP, Hardy CR, Rutschmann F. 2005. Taxon sampling effects in molecular clock dating: an example from the African Restionaceae. Molecular Phylogenetics and Evolution 35: 569–582. [DOI] [PubMed] [Google Scholar]
- Little DP. 2006. Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus). Systematic Botany 31: 461–480. [Google Scholar]
- Little DP, Schwarzbach AE, Adams RP, Hsieh CF. 2004. The circumscription and phylogenetic relationships of Callitropsis and the newly described genus Xanthocyparis (Cupressaceae). American Journal of Botany 91: 1872–1881. [DOI] [PubMed] [Google Scholar]
- Liu L, Yu LL, Pearl DK, Edwards SV. 2009a Estimating species phylogenies using coalescence times among sequences. Systematic Biology 58: 468–477. [DOI] [PubMed] [Google Scholar]
- Liu L, Yu LL, Kubatko L, Pearl DK, Edwards SV. 2009b Coalescent methods for estimating phylogenetic trees. Molecular Phylogenetics and Evolution 53: 320–328. [DOI] [PubMed] [Google Scholar]
- Liu L, Yu LL, Edwards SV. 2010. A maximum pseudo-likelihood approach for estimating species trees under the coalescent model. BMC Evolutionary Biology 10: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wu SY, Yu LL. 2015. Coalescent methods for estimating species trees from phylogenomic data. Journal of Systematics and Evolution 53: 380–390. [Google Scholar]
- Maddison MP. 1997. Gene trees in species trees. Systematic Biology 46: 523–536. [Google Scholar]
- Mao K, Hao G, Liu J, Adams RP, Milne RI. 2010. Diversification and biogeography of Juniperus (Cupressaceae): variable diversification rates and multiple intercontinental dispersals. New Phytologist 188: 254–272. [DOI] [PubMed] [Google Scholar]
- Mao K, Milne RI, Zhang L, Peng Y, Liu J, Thomas P, Mill RR, Renner SS. 2012. Distribution of living Cupressaceae reflects the breakup of Pangea. Proceedings of the National Academy of Sciences, USA 109: 7793–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal 17: 10–12. [Google Scholar]
- Matzke NJ. 2013. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Frontiers of Biogeography 5: 242–248. [Google Scholar]
- Matzke NJ. 2014. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic Biology 63: 951–970. [DOI] [PubMed] [Google Scholar]
- Mirarab S, Warnow T. 2015. ASTRAL-II: coalescent-based species tree estimation with many hundreds of taxa and thousands of genes. Bioinformatics 31: i44–i52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirarab S, Reaz R, Bayzid MS, Zimmermann T, Swenson MS, Warnowet T. 2014. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics 30: i541–i548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill RR, Farjon A. 2006. (1710) Proposal to conserve the name Xanthocyparis against Callitropsis Oerst. (Cupressaceae). Taxon 55: 229–231. [Google Scholar]
- Nylander J. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre Uppsala University. [Google Scholar]
- O’Neill EM, Schwartz R, Bullock CT, et al. . 2013. Parallel tagged amplicon sequencing reveals major lineages and phylogenetic structure in the North American tiger salamander (Ambystoma tigrinum) species complex. Molecular Ecology 22: 111–129. [DOI] [PubMed] [Google Scholar]
- Peng D, Wang XQ. 2008. Reticulate evolution in Thuja inferred from multiple gene sequences: implications for the study of biogeographical disjunction between eastern Asia and North America. Molecular Phylogenetics and Evolution 47: 1190–1202. [DOI] [PubMed] [Google Scholar]
- Pyron RA, Hendry CR, Chou VM, Lemmon EM, Lemmon AR, Burbrink FT. 2014. Effectiveness of phylogenomic data and coalescent species-tree methods for resolving difficult nodes in the phylogeny of advanced snakes (Serpentes: Caenophidia). Molecular Phylogenetics and Evolution 81: 221–231. [DOI] [PubMed] [Google Scholar]
- Qu XJ, Jin JJ, Chaw SM, Li DZ, Yi TS. 2017. Multiple measures could alleviate long-branch attraction in phylogenomic reconstruction of Cupressoideae (Cupressaceae). Scientific Reports 7: 41005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. 2012. Figtree version 1.4.0 Available from http://tree.bio.ed.ac.uk/software/figtree.
- Rambaut A, Drummond A. 2009. Tracer v1.5, available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Rannala B, Yang ZH. 2003. Bayes estimation of species divergence times and ancestral population sizes using DNA sequences from multiple loci. Genetics 164: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree RH, Smith SA. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- Rokas A, Williams BL, King N, Carroll SB. 2003. Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature 425: 798–804. [DOI] [PubMed] [Google Scholar]
- Ronquist F. 1997. Dispersal–vicariance analysis: a new approach to the quantification of historical biogeography. Systematic Biology 46: 195–203. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rothfels CJ, Li FW, Sigel EM, et al. . 2015. The evolutionary history of ferns inferred from 25 low-copy nuclear genes. American Journal of Botany 102: 1089–1107. [DOI] [PubMed] [Google Scholar]
- Ruhsam M, Rai HS, Mathews S, et al. . 2015. Does complete plastid genome sequencing improve species discrimination and phylogenetic resolution in Araucaria?Molecular Ecology Resources 15: 1067–1078. [DOI] [PubMed] [Google Scholar]
- Rushforth K. 2007. Notes on the Cupressaceae in Vietnam. Vietnam Journal of Biology 29: 32–39. [Google Scholar]
- Sayyari E, Mirarab S. 2016. Fast coalescent-based computation of local branch support from Quartet Frequencies. Molecular Biology and Evolution 33: 1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauquet H, Ho SYW, Gandolfo MA, et al. . 2012. Testing the impact of calibration on molecular divergence times using a fossil-rich group: the case of Nothofagus (Fagales). Systematic Biology 61: 289–313. [DOI] [PubMed] [Google Scholar]
- Shaw TI, Ruan Z, Glenn TC, Liu L. 2013. STRAW: Species TRee Analysis Web server. Nucleic Acids Research 41: W238–W241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Soltis DE, Soltis PS, Zhu XY, Burleigh JG, Chen ZD. 2015. Deep phylogenetic incongruence in the angiosperm clade Rosidae. Molecular Phylogenetics and Evolution 83: 156–166. [DOI] [PubMed] [Google Scholar]
- Swofford D. 2003. PAUP*: phylogenetic analysis using parsimony, version 4.0b10. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Terry RG, Adams RP. 2015. A molecular re-examination of phylogenetic relationships among Juniperus, Cupressus, and the Hesperocyparis–Callitropsis–Xanthocyparis clades of Cupressaceae. Phytologia 97: 67–75. [Google Scholar]
- Wang XQ, Ran JH. 2014. Evolution and biogeography of gymnosperms. Molecular Phylogenetics and Evolution 75: 24–40. [DOI] [PubMed] [Google Scholar]
- Weisrock DW, Harmon LJ, Larson A. 2005. Resolving deep phylogenetic relationships in salamanders: analyses of mitochondrial and nuclear genomic data. Systematic Biology 54: 758–777. [DOI] [PubMed] [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, et al. . 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences, USA 111: E4859–E4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Lockhart PJ. 2007. Deciphering ancient rapid radiations. Trends in Ecology & Evolution 22: 258–265. [DOI] [PubMed] [Google Scholar]
- Xiang Q, Li J. 2005. Derivation of Xanthocyparis and Juniperus from within Cupressus: evidence from sequences of nrDNA internal transcribed spacer region. Harvard Papers in Botany 9: 375–382. [Google Scholar]
- Xie YL, Wu GX, Tang JB, et al. . 2014. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 30: 1660–1666. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Ran JH, Wang XQ. 2012. Three genome-based phylogeny of Cupressaceae s.l.: further evidence for the evolution of gymnosperms and Southern Hemisphere biogeography. Molecular Phylogenetics and Evolution 64: 452–470. [DOI] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, Blair C, He XJ. 2015. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Molecular Phylogenetics and Evolution 87: 46–49. [DOI] [PubMed] [Google Scholar]
- Zeng LP, Zhang Q, Sun RR, Kong HZ, Zhang N, Ma H. 2014. Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times. Nature Communications 5: 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccon A, Zuccon D. 2014. MrEnt: an editor for publication-quality phylogenetic tree illustrations. Molecular Ecology Resources 14: 1090–1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.