Abstract
Background and Aims
Submergence is a severe stress for most plants. Melilotus siculus is a waterlogging- (i.e. root zone hypoxia) tolerant annual forage legume, but data were lacking for the effects of partial and full submergence of the shoots. The aim was to compare the tolerance to partial and full submergence of 15 M. siculus accessions and to assess variation in traits possibly contributing to tolerance. Recovery ability post-submergence was also evaluated.
Methods
A factorial experiment imposed treatments of water level [aerated root zone with shoots in air as controls, stagnant root zone with shoots in air, stagnant root zone with partial (75 %) or full shoot submergence] on 15 accessions, for 7 d on 4-week-old plants in a 20/15 °C day/night phytotron. Measurements included: shoot and root growth, hyponastic petiole responses, petiole gas-filled spaces, leaflet sugars, leaflet surface hydrophobicity, leaflet gas film thickness and phellem area near the base of the main root. Recovery following full submergence was also assessed.
Key Results
Accessions differed in shoot and root growth during partial and full shoot submergence. Traits differing among accessions and associated with tolerance were leaflet gas film thickness upon submergence, gas-filled spaces in petioles and phellem tissue area near the base of the main root. All accessions were able to re-orientate petioles towards the vertical under both partial and full submergence. Petiole extension rates were maintained during partial submergence, but decreased during full submergence. Leaflet sugars accumulated during partial submergence, but were depleted during full submergence. Growth resumption after full submergence differed among accessions and was positively correlated with the number of green leaves retained at desubmergence.
Conclusions
Melilotus siculus is able to tolerate partial and full submergence of at least 7 d. Leaflet surface hydrophobicity and associated gas film retention, petiole gas-filled porosity and root phellem abundance are important traits contributing to tolerance. Post-submergence recovery growth differs among accessions. The ability to retain green leaves is essential to succeed during recovery.
Keywords: Aerenchymatous phellem, messina, forage pasture legume, leaf gas films, leaf hydrophobicity, petiole elongation, petiole angle, petiole gas-filled porosity, leaf sugars, waterlogging, flooding stress, desubmergence
INTRODUCTION
Soil flooding affects >1700 Mha of land worldwide every year (Voesenek and Sasidharan, 2013), including grasslands and pastures containing forage legumes. The frequency and severity of floods are expected to increase in some regions (Hirabayashi et al., 2013) in which plants will need to cope with a variety of water excess conditions ranging from waterlogging (i.e. only roots immersed in water-saturated soil) to full submergence (i.e. shoots also under water). Plants exposed to these different water depth scenarios require various traits associated with tolerance to each condition (Colmer and Voesenek, 2009). For plants in waterlogged soil, the formation of aerenchyma in roots facilitates internal oxygen diffusion from shoots into, and along, roots, thereby allowing respiration (Armstrong, 1979). If water levels rise and submerge the shoots, in addition to aerenchyma, the hyponastic growth and extension of aerial organs (e.g. petioles) redirecting leaves upwards to maintain or restore air contact (Cox et al., 2003) can be essential to survive prolonged shallow floods. In contrast to this ‘escape strategy’, some species show a ‘quiescent strategy’ without shoot elongation to conserve carbohydrates to survive during submergence and then resume growth after the water subsides (Bailey-Serres and Voesenek, 2008).
Submerged shoots experience low oxygen at night due to respiratory consumption and slow inwards diffusion from surrounding water, and relatively high oxygen during the day as a product of underwater photosynthesis (Pedersen et al., 2009). Photosynthesis under water is, however, restricted due to low availability of carbon dioxide and/or light (Mommer and Visser, 2005; Colmer et al., 2011). Submerged plants face ‘carbohydrate starvation’ owing to the sugar consumption in catabolism and/or growth, exceeding the production by underwater photosynthesis (Bailey-Serres and Voesenek, 2008; Colmer and Voesenek, 2009). Underwater photosynthesis by submerged terrestrial plants can be aided by gas films retained on hydrophobic leaves (Colmer and Pedersen, 2008; Kurokawa et al., 2018).
Melilotus siculus (syn. M. messanensis) is a waterlogging- (and salt-) tolerant annual legume used for pastures in areas prone to floods with a Mediterranean climate (Nichols et al., 2008; Rogers et al., 2011; Striker and Colmer, 2016). The development of aerenchymatous phellem (a secondary aerenchyma) with high tissue porosity (up to 44–54 % gas-filled volume) in hypocotyl, taproot and older laterals contributes to waterlogging tolerance of M. siculus (Teakle et al., 2011, 2012; Verboven et al., 2012). The aerenchymatous phellem is a network of interconnected gas spaces that provide low resistance pathways for hypocotyl to root oxygen diffusion, which enhances root aeration (Teakle et al., 2011; Verboven et al., 2012), including to nodules (Konnerup et al., 2018). Some variation in porosity of both main and lateral roots, ranging from 15 to 33 % for plants in deoxygenated stagnant nutrient solution, was reported for 30 accessions of M. siculus (Rogers et al., 2011). A study of 15 accessions from Rogers et al. (2011) demonstrated a positive relationship between main root porosity and phellem cross-sectional area at maximum root diameter (Striker et al., 2015). In contrast to knowledge of waterlogging tolerance in M. siculus, little is known about responses to partial or full submergence of shoots, with one exception reported for full submergence of one accession (Teakle et al., 2014). Melilotus siculus survived, but did not grow, during 6 d of full submergence, and the leaflets were observed to retain gas films for the first few days under water, enabling underwater photosynthesis (Teakle et al., 2014). The present study evaluated the tolerance of 15 accessions of M. siculus to partial and complete shoot submergence, and assessed the hyponastic responses and extension of petioles, leaf lamina hydrophobicity and gas film thickness, petiole gas-filled porosity, leaf sugar concentrations, and shoot and root growth of 15 M. siculus accessions in response to partial or complete (full) submergence.
The ability to recover following submergence when water levels decline has been emphasized as crucial to define plant tolerance to water excess (Striker, 2012; Ismail et al., 2013). Rapid growth resumption after submergence would depend on the carbon fixation by the leaves present at desubmergence and by new leaves developed post-submergence during recovery. Retention of chlorophyll by submerged leaves, rather than senescence of leaves, can confer recovery ability (Fukao et al., 2006; Sarkar and Ray, 2016). The present study quantified the number of green leaves and tissue sugars at desubmergence, as well as recovery growth of roots and shoots during 7 d post-submergence, for the 15 accessions of M. siculus.
In summary, the main research questions addressed were as follows (1) Do M. siculus accessions differ in tolerance of partial or full submergence? (2) Do M. siculus accessions differ in recovery growth upon desubmergence? (3) Is variation in tolerance associated with traits (e.g. petiole porosity, petiole hyponastic growth, petiole extension, leaf surface hydrophobicity and gas film thickness, leaf sugars or root aerenchymatous phellem) when subjected to one or both of the shoot submergence depths?
MATERIALS AND METHODS
Experimental materials and growth conditions
Fifteen accessions of M. siculus were selected based on their differential responses to simulated waterlogging (stagnant deoxygenated agar nutrient solution), salinity or the combination thereof in previous experiments (Rogers et al., 2011; Teakle et al., 2011; Striker et al., 2015). Only one record was available for submergence tolerance of one accession (SA36983) when fully under water in saline or non-saline conditions (Teakle et al., 2014). Here, we tested for differences among accessions in tolerance to submergence at two water depths (partial or full shoot submergence) and assessed selected traits (see below) which can contribute to plant submergence tolerance. The experiment was in a naturally lit, temperature-controlled (20/15 °C day/night) phytotron in August–September 2017 in Perth, Western Australia (daily photoperiod of approx. 13 h).
Seeds of all accessions were scarified by rubbing with a fine sand-paper, washed in sodium hypochlorite (0.04 %, w/v), rinsed in deionized water and imbibed in Petri dishes containing filter paper moistened with 0.5 mm CaSO4 for 24 h, in darkness. Imbibed seeds were transferred onto mesh floating on aerated 10 % strength nutrient solution (composition given below) in darkness. Five days after imbibition, seedlings were transferred to aerated 25 % strength nutrient solution and exposed to light while still on the floating mesh. Nine days after imbibition, seedlings were individually transferred to 4.5 L pots containing aerated 50 % strength nutrient solution. Each seedling was held by foam in a lid of a pot. There were 53 pots and each contained eight seedlings (in total, 28 plants of each accession were raised). All pots were covered with aluminium foil to exclude light from the root zone. At 16 d after imbibition, nutrient solutions were changed to 100 % strength and continued to be aerated. The full-strength nutrient solution consisted of: 0.5 mm KH2PO4, 3 mm KNO3, 4 mm Ca(NO3)2, 1 mm MgSO4, 37.5 μm FeEDTA [EDTA iron(III) sodium salt], 23 μm H3BO3, 4.5 μm MnCl2, 4 μm ZnSO4, 1.5 μm CuSO4 and 0.05 μm MoO3 (as in Teakle et al., 2011). The solution was buffered with 2.5 mm MES and adjusted to pH 6.3 by the addition of KOH.
Twenty-one days after seed imbibition, a hypoxic pre-treatment was given to plants designated to the treatments of stagnant root zone with shoot in air, partially submerged or completely submerged (see below), by bubbling the nutrient solution with N2 for 30 min and then these pots were left without bubbling. After 24 h, the solution in these pots was changed to a deoxygenated stagnant 0.1 % (w/v) agar nutrient solution (composition as above, deoxygenated by overnight flushing with N2; the use of 0.1% agar is described in Wiengweera et al., 1997) and plants were grown for an additional 7 d prior to imposition of the shoot submergence treatments. Nutrient solution in pots designated to aerated controls was also renewed. During this stagnant root zone pre-treatment, the basal portions of the shoot and main root were observed to develop phellem (i.e. secondary aerenchyma). The plants were growing as a rosette at the time treatments were imposed and during the treatment period.
Submergence treatments
The experimental design was: 15 accessions × 4 treatments × 4 replicates. An extra batch of plants were also submerged (full submergence only, owing to space constraints) at the same time and with identical methods as this main experiment, and then desubmerged at the same time as plants in the main experiment, so that post-submergence recovery was assessed. In addition, an extra batch of plants was grown in aerated nutrient solution to evaluate leaflet surface hydrophobicity and leaflet gas film thickness (see below).
Twenty-eight days after seed imbibition, individual plants were carefully removed from the 4.5 L pots and the intact roots placed into a 250 mL black plastic bottle containing deoxygenated stagnant 0.1 % (w/v) agar nutrient solution or aerated nutrient solution depending on the previous 7 d conditions for each plant as assigned to either stagnant root zone and/or submergence treatments, or aerated root zone controls. A foam holder around the shoot base of each plant acted like a plug in the bottle neck. Each bottle contained seven glass marbles to weigh them down when submerged (controls also contained marbles). An initial harvest was taken (n = 4) and four treatments were imposed for 7 d: (1) aerated root zone with shoot in air (controls), air was supplied to the nutrient solution through a small tube inserted into each bottle; (2) deoxygenated stagnant root zone with shoot in air; (3) partial shoot submergence with a deoxygenated stagnant root zone; and (4) full shoot submergence with a deoxygenated stagnant root zone. Bottles each containing one plant were positioned inside clear 16.7 L Perspex cylinders, with eight bottles randomly assigned per cylinder; partial submergence was 75 % of the shoot height and full submergence was about 5 cm above the top of the plant (initial water depth of 23.6 ± 0.7 cm for full submergence). The submergence solution contained: 0.50 mm CaSO4, 0.25 mm MgSO4 and 1 mm KHCO3, at pH 7.5. As plants increased in height, additional submergence solution was added to the Perspex cylinders to maintain the levels in each of the two treatments at 75 % of shoot height (partial submergence) and 5 cm above the top of the shoots (full submergence). The pH of the submergence solution in each of the cylinders was checked daily and maintained at 7.5. Bottles containing plants in control and stagnant treatments, both with shoots in air, were placed in 4.5 L pots filled with submergence solution to just below the top of the bottles. In the case of full submergence, four extra plants of each accession were submerged as described above and then desubmerged at the end of the treatment period and allowed a ‘recovery period’ of 7 d with roots in deoxygenated stagnant nutrient solution and shoots in air.
Plant growth and morphology
Plant height, petiole lengths and angles (with respect to the horizontal) of the two youngest fully expanded leaves were measured at the beginning and at the end of the 7 d treatment period for each plant. The difference between final and initial values for petiole angles characterized the hyponastic growth responses of the petiole. The extension rate of petioles was calculated from the initial and final lengths. As petioles of both leaf ages measured behaved similarly, we present the results only for the youngest leaf (data for the second youngest leaf are given in Supplementary Data Fig. S1). In addition, the maximum main root diameter was measured with a digital caliper at the end of treatments. Root phellem – the spongy white tissue formed externally to the phellogen – was then removed and the diameter was re-measured. Diameters of the roots with and without phellem were used to calculate the cross-sectional area of phellem and also the area of the remaining tissues (hereafter for simplicity referred to as ‘stele’, which included phellogen and internal tissues such as the vascular cylinder) at the position of maximal diameter in the main root (assuming the root to be a cylinder) as in Striker et al. (2015).
Plants were sampled at the beginning (28 d after imbibition) and at the end of 7 d treatments (35 d after imbibition), and at the end of a 7 d recovery period only for plants subjected to full submergence (42 d after imbibition). At each sampling, shoots were separated from roots, oven-dried at 60 °C for at least 3 d, and weighed. In addition, at the end of the 7 d treatment period, the two youngest fully expanded leaves of each plant, which were measured to characterize hyponastic growth, were excised, and their leaflets separated, flash-frozen in liquid N2 and stored at –80 °C until freeze drying for subsequent measurements of tissue sugars (see description below). The dry weights of these leaflet samples, recorded after freeze-drying, were included in the total shoot dry weight.
The relative growth rates (RGRs) of shoots and roots were calculated following the approach of Hunt (1982), with the equation:
where W2 and W1 are the weights of shoots or roots of the corresponding treatment at times 2 and 1 (respectively), and t2 – t1 is the number of days between sampling times (i.e. 7 d).
Leaflet surface wettability or hydrophobicity and leaflet gas film thickness
The contact angle of water droplets (e.g. 5 μL) on a surface can be used to classify the hydrophobicity of surfaces where: <90° is hydrophilic, 90–150° is hydrophobic and >150° is superhydrophobic (Koch and Barthlott, 2009). Hydrophobic surfaces retain a gas film when submerged in water, a feature which enhances the gas exchange between the leaf and surrounding water and contributes to submergence tolerance in plants (Colmer and Pedersen, 2008; Kurokawa et al., 2018), as shown previously also for one accession of M. siculus (Teakle et al., 2014). The contact angles of water droplets on both abaxial and adaxial sides of leaflets were measured from digital pictures taken after holding leaflets flat using double-sided tape and placing a 5 μL deionized water droplet on the lamina, avoiding the midrib. There were four replicates per accession and leaflet side. The contact angle was measured using ImageJ free software (USA National Institutes of Health, Bethesda, MA, USA), as detailed in Konnerup et al. (2017).
The mean gas film thickness (height) on submerged leaves (the three leaflets without petiole) was estimated from measurements of gas film volumes using a buoyancy method (Raskin and Kende, 1983), employing equations as modified by Thomson et al. (1990), and of the double-sided surface area of the leaflets. Briefly, the fresh weight of the sample was measured, followed by the measurements of buoyancy of the sample with and without gas films present. Gas films were removed by brushing both sides of leaflets with 0.05 % Triton X-100 (non-ionic surfactant). Leaf gas film thickness was calculated as gas volume (m3) divided by twice the projected area (m2) of the leaflets given that both sides of the leaves were hydrophobic and possessed gas films when submerged. The projected areas of leaflets were measured from digital pictures (600 dpi) using ImageJ.
Leaflet soluble sugars
Freeze-dried leaf samples (leaflets of the two youngest fully expanded leaves per plant) were ground to fine powder using a ball-mill grinder (2010 Geno/Grinder®, SPEX SamplePrep, Metuchen, NJ, USA). Total sugars were extracted in 80 % ethanol by boiling in screw-cap vials for 20 min. Samples were then centrifuged for 20 min at 1500 rpm (Microfuge® 16 Centrifuge, Beckman Coulter®, Indianapolis, IN, USA) and supernatants were collected. The extraction was repeated twice. Total sugars (hexose equivalents) were determined by the anthrone method (Yemm and Willis, 1954) using a microplate reader (Multiscan® Spectrum Microplate Spectrophotometer, Thermo Electron Corporation, Vantaa, Finland).
Petiole tissue intercellular (i.e. gas-filled) spaces
Intercellular gas-filled spaces in petioles were quantified from cross-sections taken from 10 mm long segments excised from plants in control and partial submergence treatments (time did not permit such measurements also to be taken for the other treatments). The segments were excised from the middle part of the petiole of the youngest fully expanded leaf and stored in 1.7 % paraformaldehyde. Segments were then rinsed with deionized water, placed between two pieces of Styrofoam and cut using a vibrating microtome (Vibrotome 3000 Sectioning System, The Vibrotome Company, St. Louis, MO, USA). The sections were mounted in water and viewed under white light, and photographed using a Zeiss Axioskop2 Plus with a Zeiss AxioCam digital camera. Measurements were taken from digital images (600 dpi) of four cross-sections (each of a petiole from a different replicate plant) and processed using Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA). All pixels corresponding to intercellular spaces due to cubic arrangement of cells in the tissue along with isolated aerenchyma lacunae (when present) were coloured (i.e. ‘painted’); these areas (coloured pixels) were measured and divided by the area of the cross-section (total number of pixels in the section analysed) to obtain the percentage of intercellular spaces, which in fresh specimens would be the gas-filled spaces within these tissues.
Data analyses
Growth responses, plant morphological changes (plant height, petiole length and petiole angle), ‘stele’ and phellem area at maximum root diameter, leaflet soluble sugars (all of the preceding for control and the three treatments) and gas-filled spaces in petioles (control and partial submergence) were analysed through two-way analyses of variance (ANOVAs) with ‘accession’ and ‘submergence treatment’ as main factors. When significant interactions were detected, the least significant difference (LSD) was determined with Fisher’s protected LSD tests to allow the comparison of means for the treatment–accession combinations. When interactions were not significant, additional F-tests were carried out to compare the effects of the four treatments by pairs. Water droplet contact angle (i.e. leaf surface wettability–hydrophobicity) was analyzed using a two-way ANOVA with ‘leaf side’ (abaxial or adaxial) and ‘accession’ as main factors. Submerged leaflet surface gas film thickness was examined by one-way ANOVA to determine variation among accessions followed by a post-hoc Tukey test. The relationship between gas film thickness and average contact angle was explored, and a curvilinear quadratic equation had the best fit. Growth of shoots and roots during recovery from full submergence (7 d after desubmergence) was assessed by one-way ANOVA with ‘accession’ as factor followed by a post-hoc Tukey test. Relationships between growth parameters (RGR during submergence and recovery) and other measured traits (soluble sugars, leaf gas film thickness, petiole gas-filled spaces, root phellem) were explored through Pearson correlations. Normality and the homogeneity of variances were verified prior to each analysis. All results are presented as non-transformed means of four replicates ± s.e.
RESULTS
Shoot and root growth as affected by a stagnant root zone, and partial or full submergence of the shoots
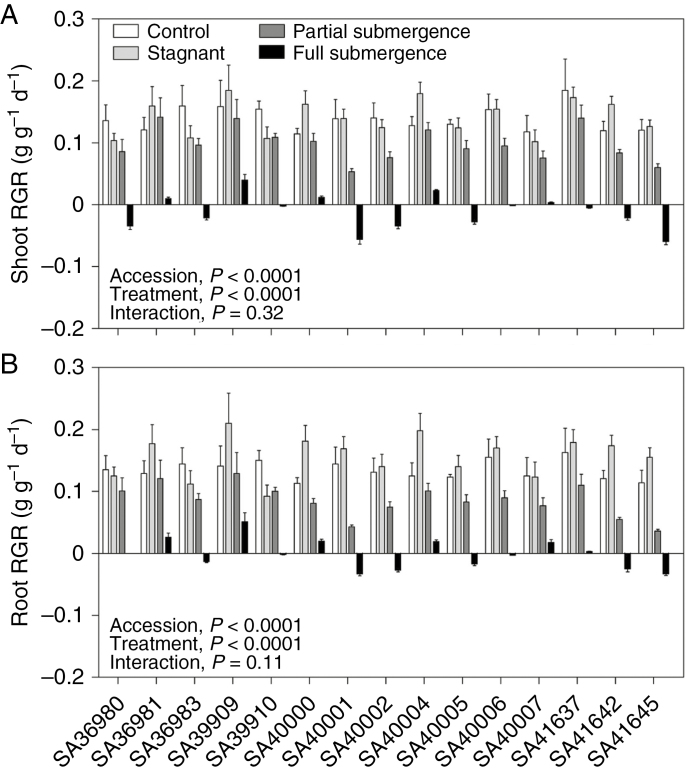
Average growth across Melilotus siculus accessions was not reduced in deoxygenated stagnant agar nutrient solution (average RGR of shoots was 102 % of that of controls, range 68–142 %; average RGR of roots was 116 % of that of controls, range 78–149 %; Fig. 1A, B; Tables 1 and 2), whereas growth was reduced for plants in both submergence treatments (Fig. 1A, B; see pairwise comparisons in Table 2). Partial submergence reduced RGR, on average, for shoots to 71 % (range 50–117 %) and for roots to 64 % (range 32–94 %) of that of controls (Fig. 1A, B) (Table 2). Full submergence drastically inhibited growth; only five out of 15 accessions were able to sustain some shoot and root growth, namely SA36981, SA39909, SA40000, SA40004 and SA40007, whereas ten accessions displayed negative values for shoot and root RGR, indicating loss of tissues/organs, such as detachment of some leaflets and/or death of distal portions of roots (Fig. 1A, B). Although the accessions differed in growth (‘accession’ effect P < 0.0001; Table 1), there was not a significant ‘accession’ × ‘treatment’ interaction (P > 0.11; Table 1). There was a strong positive relationship between shoot and root RGRs across the accessions within each submergence treatment (r > 0.90 within each treatment; Supplementary Data Fig. S2), indicating that the less affected accessions in shoot RGR were also the less impacted in root RGR.
Fig. 1.
Shoot (A) and root (B) relative growth rate (RGR) of 15 accessions of Melilotus siculus grown in nutrient solution for 7 d under control (aerated root zone), deoxygenated stagnant root zone, partial shoot submergence or full shoot submergence (the latter two treatments also with deoxygenated stagnant root zone). Plants subjected to partial submergence had an initial water column of 6.0 ± 0.9 cm above the root to shoot junction, so that 75 % of average shoot height submerged. Plants subjected to full submergence had a water column of 13.6 ± 0.8 cm above the root–shoot junction, which was approx. 5 cm of water above the top of plants. Plants were 28 d old when treatments were imposed. Values are means ± s.e. of four replicates. The significance of the main factors (‘accession’ and ‘treatment’) and the interaction of these two factors are shown in the P-values in the figure. Statistical analyses of these data are given in Tables 1 and 2.
Table 1.
F-values of two-way ANOVA (factors: ‘accessions’ and ‘submergence treatments’) for responses of growth, plant morphology, root basal phellem area and leaflet soluble sugars of 28-day-old plants of 15 accessions of Melilotus siculus grown in nutrient solution for an additional 7 d under control (aerated root zone), stagnant (deoxygenated stagnant root zone), partial submergence and full shoot submergence conditions (the latter two also with stagnant root zone); i.e. measurements taken on 35-day-old plants
| Plant responses | Main factors | Interaction | |
|---|---|---|---|
| Accession (A) | Treatment (T) | A × T | |
| Shoot RGR | 4.79*** | 220.6*** | 1.10n.s. |
| Root RGR | 3.43*** | 204.2*** | 1.31n.s. |
| Final plant height | 10.64*** | 122.0*** | 1.58* |
| Final angle youngest petiole | 5.43*** | 471.0*** | 2.12** |
| Extension rate youngest petiole | 4.14*** | 24.1*** | 1.89** |
| Phellem area at maximum root diameter | 3.19** | 191.6*** | 1.08 n.s. |
| ‘Stele’ area at maximum root diameter | 1.16 n.s. | 13.6*** | 0.72 n.s. |
| Leaflet soluble sugars | 3.68*** | 391.1*** | 0.99n.s. |
n.s., P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
Degrees of freedom for each source of variation were: 14 (‘Accession’), 3 (‘Treatment’), 42 (A × T) and 180 (‘error’).
Table 2.
Values of F-tests to compare treatments by pair for responses of growth, root basal phellem area, ‘stele’ area and leaflet soluble sugars of 28-day-old plants of 15 accessions of Melilotus siculus grown in nutrient solution for an additional 7 d under control (aerated root zone), stagnant (deoxygenated stagnant root zone), partial submergence and full shoot submergence conditions (the latter two also with stagnant root zone); i.e. measurements taken on 35-day-old plants
| Treatments comparison | Shoot RGR | Root RGR | Phellem area | ‘Stele’ area | Leaflet soluble sugars |
|---|---|---|---|---|---|
| Control vs. stagnant | 0.33n.s. | 3.34 n.s. | 22.17*** | 2.87 n.s. | 8.14*** |
| Control vs. partial submergence | 5.92** | 7.28** | 15.59*** | 2.06 n.s. | 27.90*** |
| Control vs. full submergence | 21.94*** | 20.41*** | 0.51 n.s. | 9.34*** | 10.02*** |
| Stagnant vs. partial submergence | 6.25** | 10.62*** | 6.58*** | 0.82n.s. | 19.76*** |
| Stagnant vs. full submergence | 22.27*** | 23.75*** | 21.66*** | 6.46** | 18.17*** |
| Partial submergence vs. full submergence | 16.02*** | 13.13*** | 15.07*** | 7.27** | 37.92*** |
n.s., P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
Degrees of freedom for each source of variation were: 3 (‘Treatment’) and 56 (‘error’).
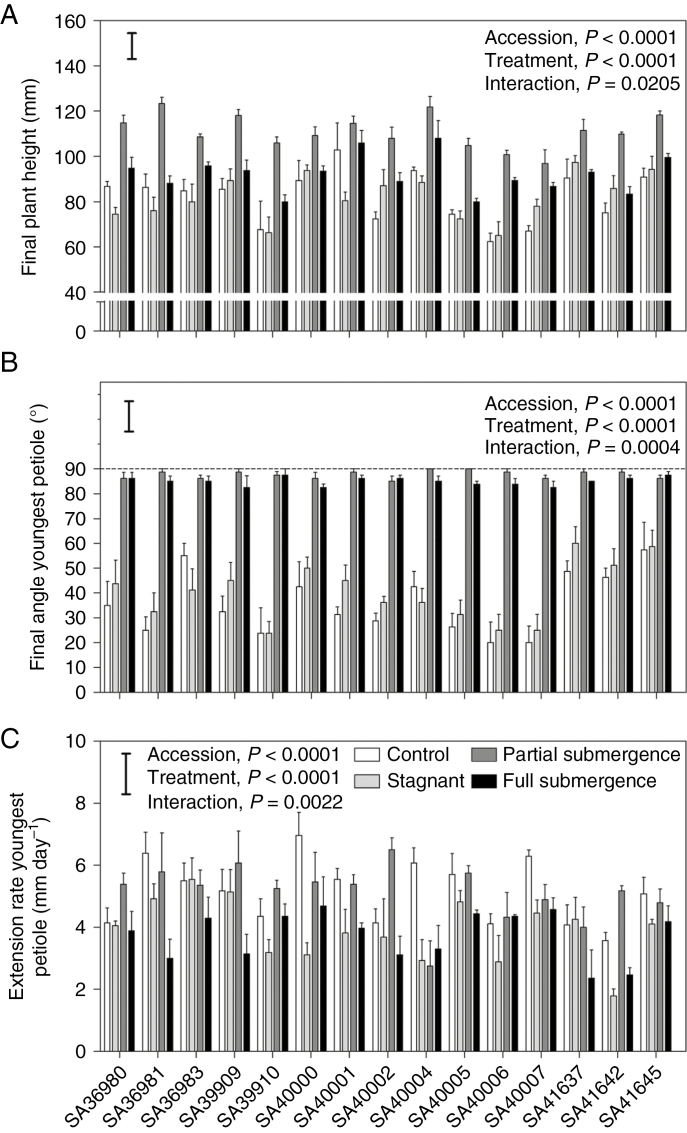
Plant height and petiole hyponastic responses
Average plant height was 82 ± 2.9 mm for controls (range 62–103 mm), 82 ± 2.6 mm in the stagnant treatment (range 65–97 mm), 111 ± 1.9 mm under partial submergence (range 97–123 mm) and 93 ± 2.1 mm under full submergence (range 80–108 mm) (Fig. 2A). Some accessions differed in response of plant height to the treatments (‘accession × treatment’, P < 0.05; Table 1). The tallest accessions under partial submergence were SA36981, SA39909, SA40004 and SA41645 (P < 0.05 compared with other accessions). Under full submergence, only three accessions (SA40004, SA40006 and SA40007) were taller than the respective controls; for all other accessions, plant height under full submergence did not differ from the controls (Fig. 2A; Table 1; P > 0.15 in all cases). The taller plants in submergence treatments, as compared with controls, were due to hyponastic growth resulting in more vertical petioles and not increased rates of petiole extension (next two paragraphs).
Fig. 2.
Final plant height (A), angle (B) and extension rate (C) of the youngest petiole of 15 accessions of Melilotus siculus grown in nutrient solution for 7 d under control (aerated root zone), deoxygenated stagnant root zone, partial shoot submergence or full shoot submergence (the latter two treatments also with deoxygenated stagnant root zone). Plants were 28 d old when treatments were imposed. Values are means ± s.e. of four replicates. The significance of the main factors (‘accession’ and ‘treatment’) and the interaction of these two factors are shown in the P-values in the figure (F-values of the two-way ANOVAs are given in Table 1). Plant height LSDinteraction= 13.45 mm; angle of youngest petiole LSDinteraction= 13.95º; extension rate of petiole LSDinteraction= 1.7 mm d–1. The bars represent the LSD (Fisher’s protected least significant difference at P = 0.05) for comparisons of means across accessions and treatments.
After 1 week of either partial or full submergence, plants of all accessions had the youngest petiole (Fig. 2B) and the second youngest petiole (Supplementary Data Fig. S1) in the vertical position (angles relative to the horizontal were between 82º and 90º; 36 ± 3º in control plants averaged across accessions). The interaction detected between ‘accession’ and ‘treatment’ (Table 1) was the result of differential ‘constitutive’ angles among accessions of petioles in control conditions (Fig. 2B). Plants in deoxygenated stagnant nutrient solution had petiole angles similar to those in the respective controls (Fig. 2B).
The extension rate of the youngest petiole was reduced, on average, from 5.1 ± 0.26 mm d–1 (controls) to 3.9 ± 0.26 mm d–1 (76 % of controls but ranging from 45 to 104 %) when in stagnant conditions, and to 3.7 ± 0.20 mm d–1 when under full submergence (73 % of controls but ranging from 47 to 106 %); partial submergence did not affect the average extension rate of petioles (5.1 ± 0.24 mm d1; range 45–157 % of controls; Fig. 2C). Interestingly, the five accessions with positive RGR (shoots and roots) during full submergence were among the accessions with the slowest petiole extension with reductions to 32–54 % of the controls (Fig. 2C), with a similar pattern of responses also for the second youngest petiole (Supplementary Data Fig. S1).
Responses of traits contributing to submergence tolerance: root base phellem area, leaflet soluble sugars, gas-filled spaces in petioles, leaflet surface hydrophobicity and gas film thickness
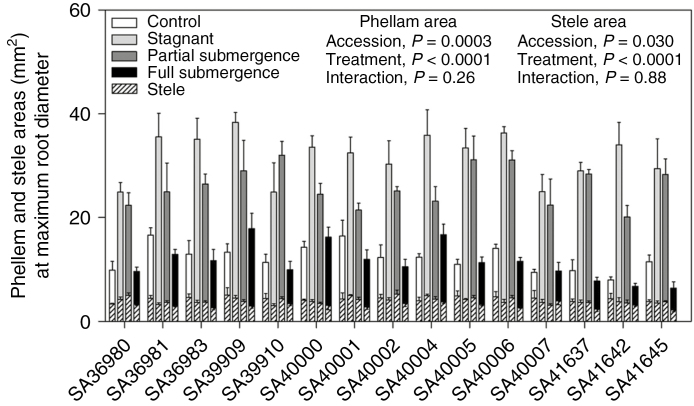
The phellem area at maximum root diameter was, on average, 7.9 ± 0.61 mm2 under control (range 3.6–12.1 mm2), 27.9 ± 1.10 mm2 under stagnant (range 20.7–33.9 mm2), 22.0 ± 0.96 mm2 under partial submergence (range 16.6 to –27.6 mm2) and 8.3 ± 0.86 mm2 under full submergence (range 3.6–14.9 mm2) (Fig. 3, ‘treatment’, P < 0.001; Tables 1 and 2). There were differences among accessions for the area of root phellem (‘accession’, P < 0.001; Table 1); the accessions which formed the greatest amounts of root phellem when in deoxygenated stagnant nutrient solution (i.e. above the average for all ‘accessions’) were SA36981, SA36983, SA39909, SA40000, SA40004 and SA40006 (Fig. 3; P < 0.05). Interestingly, three accessions with higher amounts of phellem were those also with a higher RGR during full submergence (SA39909, SA40000 and SA40004; compare Figs 1 and 3). The ‘stele’ area did not differ among accessions, but it was smaller for plants under full submergence (3.1 ± 0.12 mm2) than for the controls (4.3 ± 0.11 mm2) and plants in the two other treatments (stagnant 3.9 ± 0.14 mm2, partial submergence 4.1 ± 0.15 mm2) (Tables 1 and 2; Fig. 3).
Fig. 3.
Phellem and ‘stele’ areas (mm2) at maximum root diameter of 15 accessions of Melilotus siculus grown in nutrient solution for 7 d under control (aerated root zone), deoxygenated stagnant root zone, partial shoot submergence or full shoot submergence (the latter two treatments also with deoxygenated stagnant root zone). ‘Stele’ included phellogen and internal tissues such as the vascular cylinder. Plants were 28 d old when treatments were imposed. Values are means ± s.e. of four replicates. The significance of the main factors (‘accession’ and ‘treatment’) and the interaction of these two factors are shown in the P-values in the figure. Statistical analyses of these data are given in Tables 1 and 2.
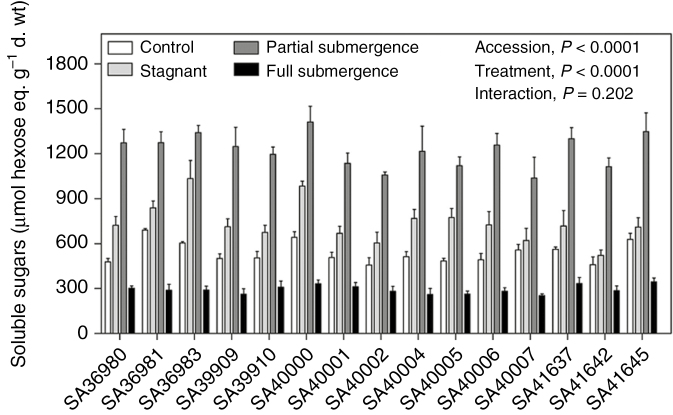
The concentration of soluble sugars in leaflets of the youngest two fully expanded leaves increased from 539 ± 18 μmol hexose eq. g–1 d. wt in controls to 738 ± 34 μmol hexose eq. g–1 d. wt when in stagnant conditions and to 1221 ± 28 μmol hexose eq. g–1 d. wt when under partial submergence (Fig. 4; ‘treatment’, P < 0.001; Tables 1 and 2). In contrast, the concentration of leaflet soluble sugars was 44 % lower under full submergence (293 ± 7 μmol hexose eq. g–1 d. wt) as compared with controls (Fig. 4; Table 2). Averaging across treatments, accessions with higher values of leaflet sugar concentrations were SA36981, SA36983, SA40000 and SA41645 (‘accession’, P < 0.001; Table 1).
Fig. 4.
Soluble sugars (μmol hexose eq. g–1 d. wt) in leaflets of young fully expanded leaves of 15 accessions of Melilotus siculus grown in nutrient solution for 7 d under control (aerated root zone), deoxygenated stagnant root zone, partial shoot submergence or full shoot submergence (the latter two treatments also with deoxygenated stagnant root zone). Plants were 28 d old when treatments were imposed. Values are means ± s.e. of four replicates. The significance of the main factors (‘accession’ and ‘treatment’) and the interaction of these two factors are shown in the P-values in the figure. Statistical analyses of these data are given in Tables 1 and 2.
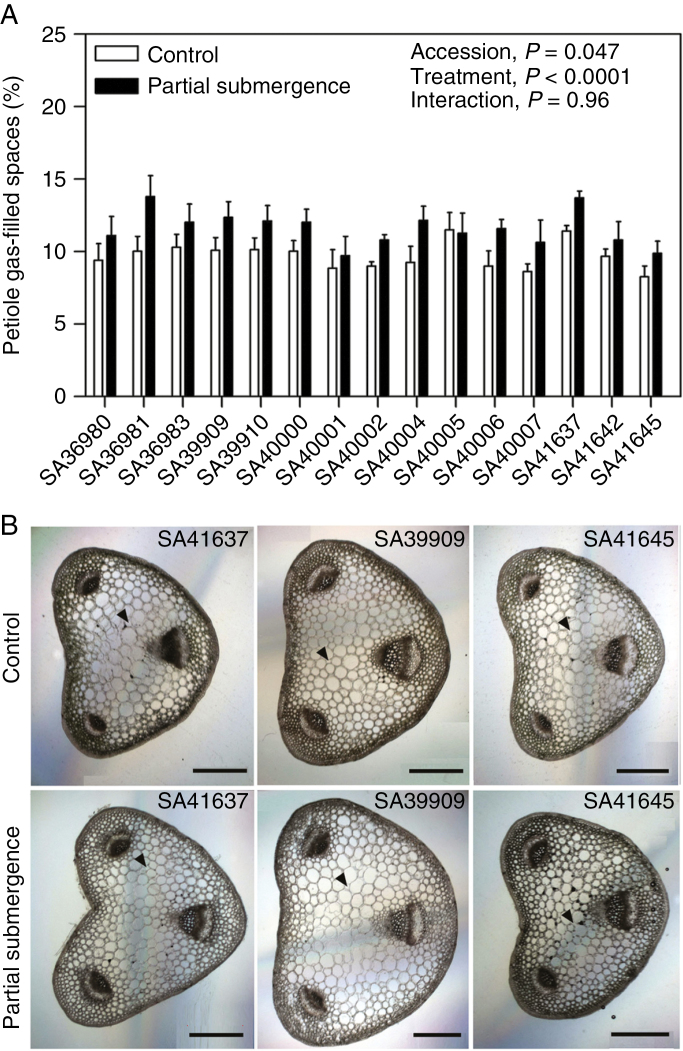
Average values for constitutive gas-filled spaces in petioles across accessions were 9.7 ± 0.34 % (range 8.3–11.5 %) due to a cubic packing of cells, mostly in the central tissues of the petioles and away from vascular bundles (Fig. 5). Under partial submergence, average values of this parameter increased to 11.6 ± 0.31 % (range 9.9–13.8 %) with similar patterns of cell packing in tissues and rarely accompanied by the presence of some isolated, small lacunae (‘treatment’, P < 0.0001; Fig. 5; cross-sections for all accessions are given in Supplementary Data Fig. S3).
Fig. 5.
Gas-filled spaces in petioles (A) from the youngest fully expanded leaves of 15 accessions of Melilotus siculus grown in nutrient solution for 7 d under control or partial submergence conditions, and representative cross-sections (B) taken from 10 mm segments from the middle part of petioles of accessions selected by having relatively high (SA41637), intermediate (SA39909) and low (SA41645) gas-filled spaces values (cross-sections of all accessions are available in Supplementary Data Fig. S2). Arrowheads in (B) indicate air spaces between cells. Plants were 28 d old when treatments were imposed; controls were in aerated nutrient solution with shoots in air, and partial submergence had roots in deoxygenated stagnant agar and shoots submerged to 75 % of the average shoots height (not assessed for plants in the stagnant or the completely submerged treatments). In (A), values are means ± s.e. of four replicates. The significance of the effects of the main factors (‘accession’ and ‘treatment’) and the interaction is shown. In (B), scale bars represent 500 μm.
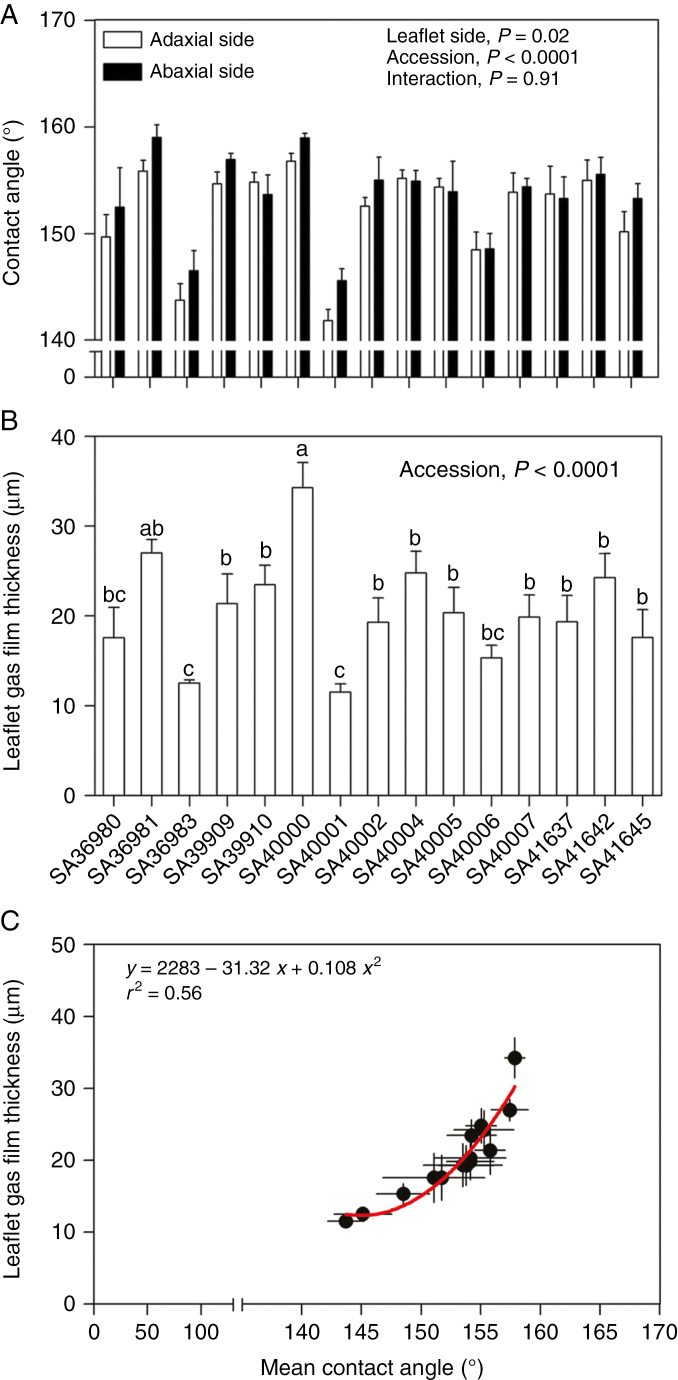
Leaflet surfaces were superhydrophobic (contact angle >150°) on both sides for 12 out of the 15 accessions with water droplet contact angles of 152–158° (‘accession’, P < 0.0001; Fig. 6A). The exceptions were accessions SA36983, SA40001 and SA40006, with hydrophobic leaflets as indicated by water droplet contact angles (averaged for both leaf sides) of 145, 144 and 148°, respectively. The abaxial (153.5°) and adaxial (152.0°) sides of leaflets hardly differed for average water droplet contact angles, but this small difference was statistically significant (‘leaf side’ P = 0.02; Fig. 6A).
Fig. 6.
Surface wettability or hydrophobicity of abaxial and adaxial leaflet sides determined as the contact angle of a water droplet (A) and leaflet gas film thickness upon initial submergence in water (B) of 15 accessions of 28-day-old plants of Melilotus siculus grown in aerated nutrient solution. In (C) is shown the curvilinear relationship between the leaf gas film thickness and the mean contact angle (averaged for both leaflet sides) as described by a fitted quadratic equation. The leaf lamina gas film thickness (i.e. height of the gas layer adjacent to the leaflet surface) was calculated from the total gas film volume for both sides and the two-sided surface area for the three leaflets of the youngest fully expanded leaf of each plant. Values are means ± s.e. of four replicates. In (A), the significance of the effects of the main factors (‘accession’ and ‘leaflet side’) and the interaction of a two-way ANOVA is shown. In (B), different letters above bars indicate differences among accessions based on Tukey test (P < 0.05).
Leaflet gas film thickness upon initial submergence differed among accessions (P < 0.001; Fig. 6B), with SA36981 and SA40000 possessing gas films of the greatest thickness (27 and 34 μm, respectively), and SA36983 and SA40001 having the thinnest ones (approx. 12 μm). Interestingly, these differences in gas film thickness were in accordance with the greater and lower surface hydrophobicities of the leaflet surfaces as evident by a positive curvilinear relationship (quadratic equation) between leaflet gas film thickness upon submergence and the mean water droplet contact angle averaged for both leaflet sides (r2 = 0.56; Fig. 6C).
Relationships between measured traits and growth
First, it was examined whether growth of the various accessions under full submergence (as the most stressful condition) was correlated with growth under the other various conditions. Both shoot and root RGR under full submergence were positively correlated with the RGR of accessions under partial submergence (r = 0.80 for both cases), but not with the RGR under control or stagnant conditions (Table 3; Supplementary Data Fig. S4). So, growth of accessions under either partial or full submergence was related, but was not simply a result of differences in vigour under control conditions. Secondly, potential relationships were explored between shoot and root RGR and measured traits potentially contributing to submergence tolerance, such as leaflet gas film thickness, gas spaces in petioles and root basal phellem area (Table 3; Supplementary Data Table S1). Under full submergence, leaflet gas film thickness correlated positively with shoot RGR (r = 0.57) and marginally with root RGR (r = 0.51; P = 0.06) (Table 3; Supplementary Data Fig. S5). Under partial submergence, the constitutive gas spaces in petioles (i.e. under control conditions) correlated positively with shoot RGR (r = 0.61) and root RGR (r = 0.52), and the correlations with both RGRs were stronger when the petiole gas space in partially submerged plants was used (r = 0.95 for shoot RGR and r = 0.84 for root RGR; Table 3; Supplementary Data Table S1; Supplementary Data Fig. S6). Neither constitutive nor flood-induced (partial submergence) gas spaces in petioles was correlated with growth of shoots or roots under control, stagnant root zone or full submergence conditions (Table 3; Supplementary Data Table S1; Supplementary Data Fig. S6). Phellem area at maximum root diameter correlated positively with both shoot and root RGR under stagnant root zone conditions and under full submergence conditions (r = 0.65–0.68 and r = 0.64–0.67, respectively), but it was not correlated with growth under control or, somewhat surprisingly, under partial submergence conditions (Supplementary Data Table S1; Supplementary Data Fig. S7).
Table 3.
Significance of relationships between plant growth (shoot and root RGR) among treatments across accessions and traits measured, for 15 accessions of Melilotus siculus under control (aerated root zone), stagnant (deoxygenated stagnant root zone), partial submergence or full shoot submergence (the latter two also with stagnant root zone)
| Assessed relationship | Pearson correlation coefficient | P-value | + or – trend | Source |
|---|---|---|---|---|
| Is growth under full submergence correlated with growth under the other treatments/growing conditions? | ||||
| Shoot RGR full submergence vs. shoot RGR control | 0.1 | 0.67 | n.s. | Supplementary Data Fig. S4 |
| Root RGR full submergence vs. root RGR control | 0.1 | 0.85 | n.s. | |
| Shoot RGR full submergence vs. shoot RGR stagnant | 0.47 | 0.06 | n.s. | |
| Root RGR full submergence vs. root RGR stagnant | 0.37 | 0.16 | n.s. | |
| Shoot RGR full submergence vs. shoot RGR partial submergence | 0.80 | <0.001 | + | |
| Root RGR full submergence vs. root RGR partial submergence | 0.81 | <0.001 | + | |
| Is the growth under full submergence conditions correlated with the initial leaf gas film thickness? | ||||
| Shoot RGR full submergence vs. leaflet gas film thickness | 0.57 | 0.026 | + | Supplementary Data Fig. S5 |
| Root RGR full submergence vs. leaflet gas film thickness | 0.51 | 0.06 | n.s. | |
| Is the shoot growth under partial submergence correlated with the amount of gas spaces (i.e. porosity) of petioles? | ||||
| Shoot RGR partial submergence vs. constitutive gas spaces in petioles | 0.61 | 0.015 | + | Supplementary Data Fig. S6 |
| Shoot RGR partial submergence vs. flood-induced gas spaces in petioles | 0.95 | <0.0001 | + | |
| Shoot RGR control vs. constitutive gas spaces in petioles | 0.30 | 0.27 | n.s. | |
| Shoot RGR control vs. flood-induced gas spaces in petioles | 0.37 | 0.17 | n.s. | |
| Is the recovery growth correlated with the growth during full submergence? | ||||
| Shoot RGR recovery vs. shoot RGR full submergence | 0.13 | 0.63 | n.s. | Supplementary Data Fig. S8 |
| Root RGR recovery vs. root RGR full submergence | 0.14 | 0.64 | n.s. | |
| Is the recovery growth correlated with the potential growth of accessions under control conditions? | ||||
| Shoot RGR recovery vs. shoot RGR control | 0.22 | 0.44 | n.s. | Supplementary Data Fig. S8 |
| Root RGR recovery vs. root RGR control | 0.31 | 0.26 | n.s. | |
| Is the recovery growth correlated with the number of green leaves maintained after full submergence? | ||||
| Shoot RGR recovery vs. number of green leaves | 0.89 | <0.0001 | + | Supplementary Data Fig. S9 |
| Root RGR recovery vs. number of green leaves | 0.77 | <0.001 | + | |
| Is the recovery growth correlated with the soluble sugars in leaves at the time of desubmergence? | ||||
| Shoot RGR recovery vs. soluble sugars in leaflets | 0.18 | 0.50 | n.s. | Supplementary Data Fig. S10 |
| Root RGR recovery vs. soluble sugars in leaflets | 0.15 | 0.58 | n.s. | |
| Is the recovery growth correlated with the initial leaf gas film thickness? | ||||
| Shoot RGR recovery vs. leaflet gas film thickness | 0.17 | 0.54 | n.s. | Supplementary Data Fig. S11 |
| Root RGR recovery vs. leaflet gas film thickness | 0.33 | 0.22 | n.s. | |
| Is the recovery growth correlated with the amount of gas spaces (i.e. porosity) of petioles? | ||||
| Shoot RGR recovery vs. constitutive gas spaces in petioles | 0.19 | 0.48 | n.s. | Supplementary Data Fig. S11 |
| Root RGR recovery vs. constitutive gas spaces in petioles | 0.14 | 0.61 | n.s. | |
| Is the recovery growth correlated with the developed phellem area? | ||||
| Shoot RGR recovery vs. phellem area full submergence | 0.02 | 0.94 | n.s. | Supplementary Data Fig. S11 |
| Root RGR recovery vs. phellem area full submergence | 0.09 | 0.74 | n.s. | |
Plants were 28 d old and exposed to treatments for an additional 7 d (and then the 35-day-old plants were measured or a set was desubmerged and measured after yet another 7 d).
Variation among accessions and traits aiding recovery following full submergence
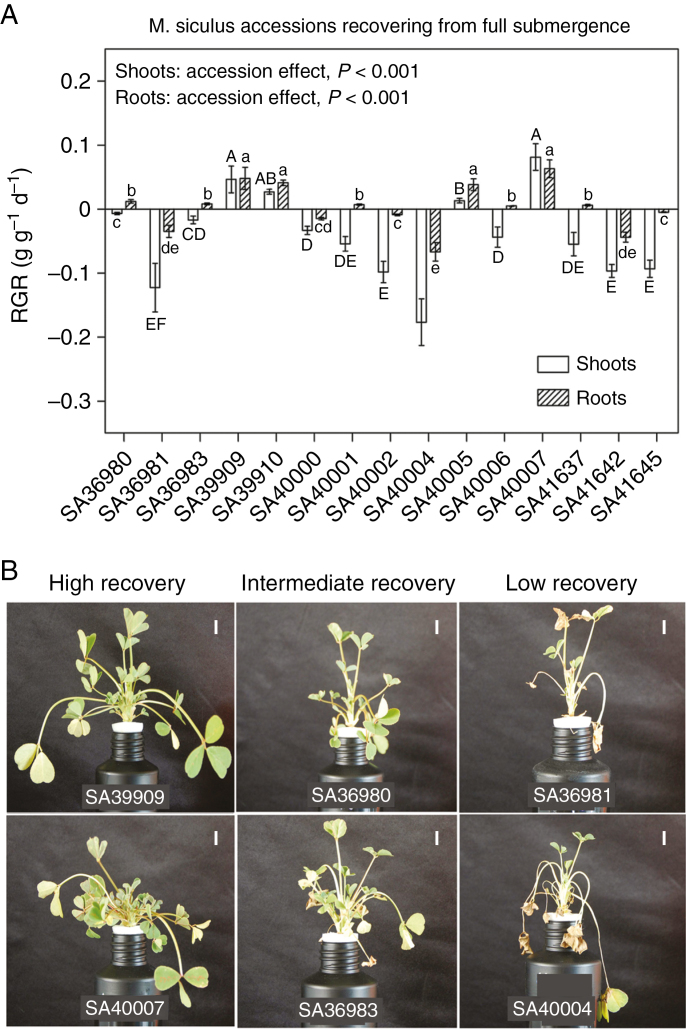
Accessions recovered from full submergence differentially (Fig. 7A, B). Out of 15 accessions, only four (SA39909, SA39910, SA40005 and SA40007) showed positive RGR values for shoots and roots during 1 week after desubmergence. Of these four accessions, only SA39909 and, to a lesser extent SA40007, showed positive RGR also during submergence (Fig. 1A, B). The other 11 accessions exhibited negative RGR for shoots and/or roots (Fig. 7A), indicating leaf senescence/drop after desubmergence (Fig. 7B) and/or death of root tissues.
Fig. 7.
Shoot and root relative growth rate (RGR; A) of 15 accessions of Melilotus siculus recovering from 7 d of full submergence of shoots (roots in stagnant nutrient solution) for the first 7 d after desubmergence. Plants were 35 d old when desubmerged and the 7 d recovery period commenced. Values are means ± s.e. of four replicates. The significance of the ‘accession’ effect on the shoot and root RGR from the one-way ANOVAs is shown. Different letters indicate significant differences (P < 0.05) in RGR among accessions based on Tukey test. Upper case letters allow comparison of means for shoot RGR among accessions; lower case letters allow comparison of means for root RGR among accessions. In (B) representative pictures of selected accessions with high (SA39909, SA40007), intermediate (SA36980, SA36983) and low (SA36981, SA40004) recovery ability are presented. Scale bars in (B) represent 1 cm.
Shoot and root RGRs of accessions upon desubmergence were not correlated with the RGRs during full submergence or in control conditions (Table 3; Supplementary Data Fig. S8). RGR of accessions (shoot and root) during recovery was strongly correlated (r = 0.89 for shoot and r = 0.77 for root) to the number of green leaves maintained after full submergence (Table 3; Supplementary Data Fig. S9). In contrast, RGR during recovery was not correlated to the concentration of soluble sugars in leaflets of the two youngest fully expanded leaves at the time of desubmergence (Table 2; Supplementary Data Fig. S10), or with the initial leaf gas film thickness, or constitutive gas spaces in petioles or phellem area under full submergence (Table 3; Supplementary Data Fig. S11).
DISCUSSION
The present study demonstrates variation amongst 15 Melilotus siculus accessions in tolerance to partial and full submergence. Variation among the accessions was also documented for root basal phellem area, gas-filled spaces in petioles, leaflet surface hydrophobicity and the associated gas film thickness, all traits which potentially contribute to submergence tolerance. Some of these traits correlated positively with plant growth across accessions depending on the water excess scenario (discussed below). Under partial submergence, all accessions reoriented their petioles towards the vertical position (i.e. 80–90º), and 13 out of 15 accessions maintained the petiole extension rate the same as in controls, so leading to taller plants. Under full submergence, petiole vertical reorientation was common for all M. siculus accessions, but petiole extension was on average 73 % of that of controls across accessions, so the plants remained under water. The fully submerged plants either lost dry mass (leaf detachment and/or death of some roots) or had only small increments in dry mass, and leaflet sugar levels decreased. Growth resumption after desubmergence differed among the accessions and only four of the 15 accessions had a positive RGR during the first week post-submergence. Post-submergence recovery growth was correlated with the ability to retain green leaves, as found also in rice (Fukao et al., 2006; Sarkar and Ray, 2016), but not with leaflet sugar concentrations at desubmergence, or with any other measured trait.
Under partial submergence, most accessions (14 out of 15) were moderately taller (compared with controls) due to the reorientation of petioles towards vertical; the details of such hyponastic growth of petioles in response to submergence and the associated signalling have been described for Rumex palustris (Cox et al., 2003). However, petiole extension in M. siculus (13 out of 15 accessions) was similar in partially submerged and control plants, i.e. partially submerged M. siculus lacked a shoot elongation ‘escape’ response as occurs for R. palustris and R. thyrsiflorus petioles of partially submerged plants (Banga et al., 1995) or for the shoots of the forage legume Lotus tenuis when partially covered by water (Manzur et al., 2009). The hyponastic response of M. siculus would be of adaptive value during shallow floods if this vertical reorientation is enough for plants to remain/re-establish contact with the air and/or if an increased proportion of leaf area above the water aids ‘snorkelling’ to aerate the submerged organs/tissues (cf. R. palustris, Herzog and Pedersen, 2014).
Root phellem and petiole gas-filled spaces were both increased in the partially submerged plants, which would enhance internal aeration of the roots (Armstrong, 1979; Pierik et al., 2009; Teakle et al., 2011; Verboven et al., 2012). Interestingly, we found a positive correlation between gas spaces in petioles (constitutive and flood-enhanced values) and the growth of shoots and roots across accessions when partially submerged (Table 3; Supplementary Data Fig. S6), but we did not find a correlation between root basal phellem area and growth (Supplementary Data Table S1; Supplementary Data Fig. S7). These results could be explained by the petioles being relatively low (9–14 %) in tissue gas spaces whereas the gas-filled porosity of the aerenchymatous phellem is much higher (44–54 %; Verboven et al., 2012), so that the petiole porosity might be a bottleneck for internal aeration of the roots of partially submerged plants. The possible limitation of internal aeration during partial shoot submergence could explain the 29–36 % reduction in growth of shoots and roots compared with controls. Moreover, the accumulation of sugars in leaflets of partially submerged plants (Fig. 4) supports the notion that internal aeration might have constrained growth of M. siculus as sugars were produced (i.e. leaves above water were photosynthesizing) but these sugars were not consumed by growth. The declines in growth of M. siculus during partial submergence (this study) contrast with the maintenance of growth in stagnant root zone conditions when the shoot was fully in air (Fig. 1A, B; see also Striker et al., 2015). The positive correlation between shoot (and marginally root) RGR and the area of root basal phellem across accessions when in stagnant conditions highlights the importance of this specialized tissue to facilitate internal aeration of the roots when almost the entire shoot is in air. The aerenchymatous phellem is present along the hypocotyl and older portions of the roots, and this high porosity tissue facilitates oxygen movement into and along the roots (Teakle et al., 2011; Verboven et al., 2012). Water levels above the uppermost aerenchymatous phellem when present on the hypocotyl (e.g. M. siculus, Teakle et al., 2011) or the stem base (e.g. soybean, Shimamura et al., 2010) clearly restrict plant internal aeration.
Under full submergence, hyponastic growth of petioles occurred for all M. siculus accessions (Fig. 2; one accession in Teakle et al., 2014), but the extension rate of petioles was reduced (ten accessions) or remained as for the controls (five accessions); so, M. siculus does not display a shoot elongation ‘escape response’ from submergence. All plants from all accessions survived the 7 d under the clear water, although 11 accessions ceased growth and four accessions had low RGRs (Fig. 1A, B). The ability to photosynthesize under water would aid survival, as sugars produced would delay any carbon starvation and, together with oxygen released, would provide substrates for respiration. Underwater photosynthesis can be facilitated by gas films retained by hydrophobic leaf surfaces; the thin gas layer increases gas exchange (O2 and CO2) between the leaf and surrounding water (Pedersen et al., 2009; Verboven et al., 2014). Leaflets of all accessions of M. siculus were hydrophobic (three accessions) or superhydrophobic (12 accessions) on both sides (sensuKoch and Barthlott, 2009). As a result, all accessions when submerged retained gas films on the leaflets and, interestingly, the variation amongst the accessions in gas film thickness showed a positive curvilinear relationship with the water droplet contact angle (a measure of hydrophobicity) (Fig. 6C). The significance of leaflet gas films as a trait associated with submergence tolerance was supported by a positive correlation between the thickness of these gas films and shoot RGR (Supplementary Data Fig. S5). Moreover, experimental removal of these gas films by application of a surfactant greatly reduced underwater photosynthesis by leaflets of M. siculus (Teakle et al., 2014). Together, these studies (this study and Teakle et al., 2014) point to the benefit of leaflet hydrophobicity and gas film retention for submergence tolerance of M. siculus, and the present data highlight the intraspecies variation for this leaf trait.
In fully submerged plants, root phellem development was restrained when compared with plants in stagnant and partial submergence treatments (exceptions were SA39909 and SA40004; Fig. 3). Nevertheless, this aerenchymatous root tissue was correlated with shoot and root RGR during submergence (Supplementary Data Fig. S7); this porous tissue would decrease the resistance along the path for diffusion of oxygen into and along the roots, whether the oxygen is produced by underwater photosynthesis or if it enters from the floodwater via the shoots. The mechanism explaining the phellem development arrest remains to be elucidated. However, the restriction in phellem formation could be related to the low concentration of sugars in leaflets of fully submerged plants (Fig. 4), thereby being unable to provide sufficient sugars to the hypocotyl and roots. This idea concurs with the study on waterlogged plants of Glycine max (soybean) where impeding the supply of sugars to the hypocotyl, by blocking phloem transport, inhibited aerenchymatous phellem and phellogen formation while sucrose application above the epicotyls of excised plants recovered phellem and phellogen formation (Takahashi et al., 2018).
Recovery following desubmergence differed amongst the M. siculus accessions (Fig. 7A, B). Only four out of 15 accessions showed shoot and root growth during the first 7 d after desubmergence, whereas the other 11 accessions ceased growth or even suffered leaf detachment or further decay of the roots (resulting in negative RGR values; Fig. 7A, B). The RGR during the recovery period was correlated with the number of green leaves retained upon desubmergence (Supplementary Data Fig. S9). Maintenance of green leaves, for example measured as chlorophyll retention, is a trait associated with growth resumption post-submergence in rice variety FR13A (Fukao et al., 2006; Sarkar and Ray, 2016), Arabidopsis thaliana accessions Bay-0 and Lp2-6 (Yeung et al., 2018), and wheat variety Jackson (Herzog et al., 2018). In contrast, neither leaf sugar levels (Supplementary data Fig. S10), innate growth ability (i.e. RGR under control conditions) nor growth during submergence was correlated with recovery growth across the M. siculus accessions (Table 3). One accession, SA39909, which had superhydrophobic leaves, developed phellem when submerged, and was one of the five accessions with reduced petiole extension rates under water as compared with the control; grew during both submergence and the recovery period. Therefore, future studies to elucidate further traits contributing to submergence tolerance and recovery could use accession SA39909, and this accession might also be useful as a parent in genetic studies and/or breeding programmes for submergence tolerance in M. siculus.
Conclusions
Melilotus siculus is able to tolerate partial and full submergence in clear water of at least 7 d, although some accessions differ in shoot and root growth under these conditions. Leaflet hydrophobicity and associated gas film retention, petiole gas-filled porosity and root phellem development are traits contributing to submergence tolerance. Petiole re-orientation towards the vertical occurs in all accessions under both partial and full submergence. However, petiole extension rates were maintained during partial submergence, but decreased during full submergence. Leaf sugars accumulated during partial submergence, but were depleted during full submergence. Together, these responses suggest that the underwater shoot elongation ‘escape’ strategy (cf. Bailey-Serres and Voesenek, 2008) is not present in this species. Post-submergence recovery growth differs among accessions, and the ability to retain green leaves is a trait contributing to recovery.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: significance of relationships between plant growth (shoot and root RGR) among treatments across accessions and traits measured for 15 accessions of Melilotus siculus. Figure S1: final angle and extension rate of the second youngest petiole of 15 accessions of Melilotus siculus. Figure S2: relationships of shoot RGR and root RGR for 15 accessions of Melilotus siculus. Figure S3: representative cross-sections of petioles taken from 10 mm segments excised from the middle part in 15 accessions of Melilotus siculus. Figure S4: relationships of shoot RGR and root RGR under full submergence vs. growth under all other conditions for 15 accessions of Melilotus siculus. Figure S5: relationships of shoot RGR and root RGR during full submergence vs. initial leaflet gas film thickness for 15 accessions of Melilotus siculus. Figure S6: relationships of shoot RGR and root RGR under control, stagnant, partial submergence or full submergence. Figure S7: relationships of shoot and root RGR under control, deoxygenated stagnant root zone, partial shoot submergence or full shoot submergence vs. phellem area at maximum root diameter for 15 accessions of Melilotus siculus. Figure S8: relationships of shoot RGR and root RGR during recovery vs. when under full submergence for 15 accessions of Melilotus siculus. Figure S9: relationships of shoot RGR and root RGR during recovery vs. number of green leaves per plant maintained after full submergence for 15 accessions of Melilotus siculus. Figure S10: relationships of shoot RGR and root RGR during recovery vs. soluble sugars in leaflets at the time of desubmergence for 15 accessions of Melilotus siculus. Figure S11: relationships of shoot RGR and root RGR during recovery vs. initial leaflet gas film thickness, constitutive gas spaces in petioles and phellem area at maximum root diameter under full submergence for 15 accessions of Melilotus siculus.
ACKNOWLEDGEMENTS
Travel funds from the A. W. Howard Memorial Trust for G.G.S. to visit The University of Western Australia are gratefully acknowledged. L.K. is supported by the ARC Industrial Research Hub for Legumes for Sustainable Development (Australian Research Council IH140100013 in partnership with the Grains Research and Development Corporation) at the UWA node with T.D.C. We thank Dr S. Hughes (Australian Pastures Genebank, SARDI) for providing seeds and Dr Phil Nichols for discussions related to this research. We thank two anonymous reviewers for comments which helped to improve the clarity of aspects of our manuscript.
LITERATURE CITED
- Armstrong W. 1979. Aeration in higher plants. In: Woolhouse, HW, ed. Advances in botanical research. London: Academic Press, 225–332. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Banga M, Blom CWPM, Voesenek LACJ. 1995. Flood-induced leaf elongation in Rumex species: effects of water depth and water movements. New Phytologist 131: 191–198. [Google Scholar]
- Colmer TD, Pedersen O. 2008. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177: 918–926. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36: 665–681. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Winkel A, Pedersen O. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 2011: plr030. doi: 10.1093/aobpla/plr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Millenaar FF, van Berkel YEM, Peeters AJM, Voesenek LACJ. 2003. Plant movement. Submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiology 132: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. 2006. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell 18: 2021–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Pedersen O. 2014. Partial versus complete submergence: snorkelling aids root aeration in Rumex palustris but not in R. acetosa. Plant, Cell and Environment 37: 2381–2390. [DOI] [PubMed] [Google Scholar]
- Herzog M, Fukao T, Winkel A, et al. . 2018. Physiology, gene expression and metabolome of two wheat cultivars with contrasting submergence tolerance. Plant, Cell and Environment 41: 1632–1644. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Mahendran R, Koirala S, et al. . 2013. Global flood risk under climate change. Nature Climate Change 3: 816–821. [Google Scholar]
- Hunt R. 1982. Plant growth analysis: second derivatives and compounded second derivatives of splined plant growth curves. Annals of Botany 50: 317–328. [Google Scholar]
- Ismail AM, Singh US, Singh S, Dar MH, Mackill DJ. 2013. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Research 152: 83–93. [Google Scholar]
- Koch K, Barthlott W. 2009. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 367: 1487–1509. [DOI] [PubMed] [Google Scholar]
- Konnerup D, Winkel A, Herzog M, Pedersen O. 2017. Leaf gas film retention during submergence of 14 cultivars of wheat (Triticum aestivum). Functional Plant Biology 44: 877–887. [DOI] [PubMed] [Google Scholar]
- Konnerup D, Toro G, Pedersen O, Colmer TD. 2018. Waterlogging tolerance, tissue nitrogen and oxygen transport in the forage legume Melilotus siculus: a comparison of nodulated and nitrate-fed plants. Annals of Botany 121: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa Y, Nagai K, Huan PD, et al. . 2018. Rice leaf hydrophobicity and gas films are conferred by a wax synthesis gene (LGF1) and contribute to flood tolerance. New Phytologist 218: 1558–1569. [DOI] [PubMed] [Google Scholar]
- Manzur ME, Grimoldi AA, Insausti P, Striker GG. 2009. Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Annals of Botany 104: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Visser EJW. 2005. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany 96: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols PGH, Craig AD, Rogers ME, et al. . 2008. Production and persistence of annual pasture legumes at five saline sites in southern Australia. Australian Journal of Experimental Agriculture 48: 518–535. [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. 2009. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58: 147–156. [DOI] [PubMed] [Google Scholar]
- Pierik R, Van Aken JM, Voesenek LACJ. 2008. Is elongation-induced leaf emergence beneficial for submerged Rumex species?Annals of Botany 103: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. 1983. How does deep water rice solve its aeration problem. Plant Physiology 72: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Colmer TD, Nichols PGH, et al. . 2011. Salinity and waterlogging tolerance amongst accessions of messina (Melilotus siculus). Crop and Pasture Science 62: 225–235. [Google Scholar]
- Sarkar RK, Ray A. 2016. Submergence-tolerant rice withstands complete submergence even in saline water: probing through chlorophyll a fluorescence induction OJIP transients. Photosynthetica 54: 275–287. [Google Scholar]
- Shimamura S, Yamamoto R, Nakamura T, Shimada S, Komatsu S. 2010. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Annals of Botany 106: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker GG. 2012. Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecological Research 27: 984–987. [Google Scholar]
- Striker GG, Colmer TD. 2016. Flooding tolerance of forage legumes. Journal of Experimental Botany 68: 1851–1872. [DOI] [PubMed] [Google Scholar]
- Striker GG, Teakle NL, Colmer TD, Barrett-Lennard EG. 2015. Growth responses of Melilotus siculus accessions to combined salinity and root-zone hypoxia are correlated with differences in tissue ion concentrations and not differences in root aeration. Environmental and Experimental Botany 109: 89–98. [Google Scholar]
- Takahashi H, Xiaohua Q, Shimamura S, Yanagawa A, Hiraga S, Nakazono M. 2018. Sucrose supply from leaves is required for aerenchymatous phellem formation in hypocotyl of soybean under waterlogged conditions. Annals of Botany 121: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teakle N, Armstrong J, Barrett-Lennard EG, Colmer TD. 2011. Aerenchymatous phellem in hypocotyl and roots enables O2 transport in Melilotus siculus. New Phytologist 190: 340–350. [DOI] [PubMed] [Google Scholar]
- Teakle NL, Bowman S, Barrett-Lennard EG, Real D, Colmer TD. 2012. Comparisons of annual pasture legumes in growth, ion regulation and root porosity demonstrate that Melilotus siculus has exceptional tolerance to combinations of salinity and waterlogging. Environmental and Experimental Botany 77: 175–184. [Google Scholar]
- Teakle NL, Colmer TD, Pedersen O. 2014. Leaf gas films delay salt entry and enhance underwater photosynthesis and internal aeration of Melilotus siculus submerged in saline water. Plant, Cell and Environment 37: 2339–2349. [DOI] [PubMed] [Google Scholar]
- Verboven P, Pedersen O, Herremans E, et al. . 2012. Root aeration via aerenchymatous phellem: three-dimensional micro-imaging and radial O2 profiles in Melilotus siculus. New Phytologist 193: 420–431. [DOI] [PubMed] [Google Scholar]
- Verboven P, Pedersen O, Ho QT, Nicolai BM, Colmer TD. 2014. The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant, Cell and Environment 37: 2433–2452. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Sasidharan R. 2013. Ethylene – and oxygen signalling – drive plant survival during flooding. Plant Biology 15: 426–435. [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. 1997. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80: 115–123. [Google Scholar]
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E, van Veen H, Vashisht D, et al. . 2018. A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 115: E6085–E6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.