Abstract
Background and Aims
The pathways whereby foliar-applied nutrients move across the leaf surface remain unclear. The aim of the present study was to examine the pathways by which foliar-applied Zn moves across the sunflower (Helianthus annuus) leaf surface, considering the potential importance of the cuticle, stomata and trichomes.
Methods
Using synchrotron-based X-ray florescence microscopy and nanoscale secondary ion mass spectrometry (NanoSIMS), the absorption of foliar-applied ZnSO4 and nano-ZnO were studied in sunflower. The speciation of Zn was also examined using synchrotron-based X-ray absorption spectroscopy.
Key Results
Non-glandular trichomes (NGTs) were particularly important for foliar Zn absorption, with Zn preferentially accumulating within trichomes in ≤15 min. The cuticle was also found to have a role, with Zn appearing to move across the cuticle before accumulating in the walls of the epidermal cells. After 6 h, the total Zn that accumulated in the NGTs was approx. 1.9 times higher than in the cuticular tissues. No marked accumulation of Zn was found within the stomatal cavity, probably indicating a limited contribution of the stomatal pathway. Once absorbed, the Zn accumulated in the walls of the epidermal and the vascular cells, and trichome bases of both leaf sides, with the bundle sheath extensions that connected to the trichomes seemingly facilitating this translocation. Finally, the absorption of nano-ZnO was substantially lower than for ZnSO4, with Zn probably moving across the leaf surface as soluble Zn rather than nanoparticles.
Conclusions
In sunflower, both the trichomes and cuticle appear to be important for foliar Zn absorption.
Keywords: Bundle sheath extension, cuticle, foliar absorption, stomata, trichomes, Zn foliar fertilizer, sunflower
INTRODUCTION
It has been known since the 19th century that nutrients can be absorbed by plant leaves (Mayer, 1874). Since then, foliar fertilization has become increasingly widespread in its usage (Alexander, 1986; Fernández and Eichert, 2009; Fernández et al., 2013). Although the movement of nutrients into root systems is well understood, comparatively little is known about nutrient absorption by above-ground tissues. Indeed, the cuticle, stomata and trichomes have all been proposed as potential pathways for the movement of foliar-applied nutrients, but their relative contributions to overall foliar absorption are uncertain (Fernández et al., 2013), with this being the focus of the present study.
For the cuticle, there are two proposed different cuticular penetration pathways: (1) the lipophilic pathway for non-ionic, apolar and lipophilic compounds (e.g. pesticides and herbicides); and (2) the hydrophilic pathway for ionic, polar, hydrophilic substances (e.g. mineral nutrients) (Schönherr, 2006; Stagnari, 2007; Schreiber and Schönherr, 2009). In the present study, the hydrophilic pathway is of particular interest, although comparatively little is known regarding this pathway (Schönherr, 1976, 2006). Fernández et al. (2017) proposed the ‘dynamic aqueous continuum’ model, which suggested that the cuticle has highly tortuous, relative humidity-dependent, dynamic sorption and desorption of water connections associated with hydrophilic domains existing between the leaf surface and interior tissues. However, due to the complex chemical composition and structure of the cuticle, the mechanisms and importance of the cuticle in the foliar absorption of mineral nutrients remain unclear (Fernández et al., 2017).
Turrell (1947) reported that water could not penetrate the stomata of citrus (Citrus reticulatus) whereas oil could. Subsequently, Schönherr and Bukovac (1972) suggested that spontaneous infiltration of stomata cannot occur unless pressure is applied or surfactants are added to reduce surface tension. Interestingly, Eichert et al. (1998) used leaf epidermal strips and found that the penetration of uranine through stomata occurred without the addition of either surfactants or pressure. Furthermore, Eichert et al. (2008) observed that hydrophilic polystyrene particles with a diameter of 43 nm suspended in water can enter leaves through stomata, although only <10 % of the stomata beneath the droplet were penetrated. In addition, Burkhardt et al. (2012) found that the hygroscopic leaf surface particles potentially promote stomatal penetration. Thus, although it has been shown that the movement of foliar-applied nutrients through the stomata is possible, both the mechanisms by which this occurs and the overall contribution of stomata to foliar nutrient absorption are unclear.
The role of trichomes in absorption of foliar nutrients has received comparatively little attention (Fernández et al., 2013). Schlegel and Schönherr (2001) used Ag to visualize foliar absorption through the precipitation of AgCl, finding that there was a substantial accumulation of Ag in trichomes of broad bean (Vicia faba) and maize (Zea mays). Absorption of water from dew (Pina et al., 2016) or fog (Ju et al., 2012) through trichomes has been reported in some species as a drought resistance mechanism, thereby indicating the possibility that absorption of foliar-applied nutrients may potentially occur through trichomes in some plants. However, trichomes are markedly diverse in their shape, structure and function (Huchelmann et al., 2017), and hence it is possible that the potential contribution of trichomes to absorption of foliar-applied nutrients varies amongst plant species. Of particular interest in the present study is sunflower (Helianthus annuus), which has been reported to accumulate excess Mn in the non-glandular trichomes (NGTs) when the Mn is supplied in the rooting medium (Blamey et al., 1986, 2015).
The aim of the present study was to examine the pathways whereby foliar-applied Zn moves across the sunflower leaf surface, considering the potential importance of the cuticle, stomata and trichomes. In addition, given the increasing interest in using nanotechnology in plant production systems (De Rosa et al., 2010; Wang et al., 2016), we compared absorption of a traditional Zn foliar fertilizer (ZnSO4) with a nano-foliar fertilizer (nano-ZnO). To do this, we first examined leaf structure and surface morphology using scanning electron microscopy (SEM) and light microscopy. Next, we examined the lateral distribution of foliar-absorbed Zn in situ within hydrated, intact leaves using synchrotron-based X-ray fluorescence microscopy (µ-XRF). We then examined the distribution of the foliar-absorbed Zn within leaf cross-sections at tissues and subcellular tissue levels using µ-XRF and nanoscale secondary ion mass spectrometry (NanoSIMS), respectively. Finally, the speciation of foliar-absorbed Zn within the leaves was examined using synchrotron-based X-ray absorption spectroscopy (XAS). It was hypothesized that the cuticle, stomata and trichomes would all contribute as pathways for the movement of foliar-applied nutrients across the leaf surface. It was hoped that this study would help to identify the mechanisms of foliar nutrient absorption, and hence provide important information for improving the efficacy of foliar fertilizers.
MATERIALS AND METHODS
Plant growth and examination of leaf morphology
Sunflower plants were grown in nutrient solution at The University of Queensland (St Lucia, Australia) as described by Li et al. (2017). Briefly, seeds (‘Hyoleic’) were germinated for 3 d using paper towel moistened with tap water. Seedlings were transplanted to 11-L black containers, with one plant placed in each of the four holes in the lids. The containers were filled with nutrient solution which contained (µm): 910 N (94 % NO3− and 6 % NH4+), 20 P, 475 K, 1126 Ca, 227 Mg, 1251 Cl, 556 S, 25 Fe(III)EDTA, 3 B, 0.5 Mn, 0.5 Zn, 0.2 Cu and 0.01 Mo (Blamey et al., 2015). The nutrient solution was continuously aerated, room temperature was maintained at 25 °C, and light was provided using high-pressure sodium lamps (photon flux density of 1500 μmol m–2 s–1) for 12 h d−1. Nutrient solutions were changed after the first 7 d then every 4 d thereafter. After 10 d, 5 mL of 44 mm KH2PO4 was added to each 11-L container every other day to replenish P. Nutrient solution pH was unadjusted and was approx. 5.5.
After 16 d of growth, the youngest fully expanded leaves (YFELs) were used to examine leaf morphology and structure using SEM following processing as described by Li et al. (2017). The adaxial leaf surface was examined using a JEOL NEOSCOPE scanning electron microscopy at 10 kV accelerating voltage. For examining leaf structure, the fresh YFELs were cut into pieces approx. 1 mm × 4 mm and fixed, dehydrated and embedded in Epon resin as described by Li et al. (2017). Leaf sections (1 µm thick) were cut using an ultramicrotome (Leica EM UC6), stained with Toluidine blue (0.5 % in 1 % borax) and observed using light microscopy (O’Brien et al., 1964). Leaf structure was also examined using fresh leaf tissues. For this, fresh leaf segments were embedded in a 4 % agar solution at 30 °C. Once the agar solidified, the block was glued to the sample holder of a vibratome (Leica VT 1000s) using super glue, then 80-µm leaf sections were prepared without any buffering solution. Sections were immediately imaged using an Aperio XT Slide Scanner without staining.
Laterally-resolved examination of Zn distribution in leaf tissues using µ-XRF
The 16-d-old sunflower plants were transported to the Australian Synchrotron (Clayton, Australia) where they continued to be grown under the same conditions as at The University of Queensland. Two types of Zn fertilizers were prepared at 1000 mg L−1 Zn (15.4 mm), being ZnSO4.7H2O (pH 5.2) and nano-ZnO (approx. 30 nm diameter, pH 7.1) [see Li et al. (2018) for scanning electron micrograph and dynamic light scattering results of the nano-ZnO]. To prepare 50 mL of 1000 mg L−1 nano-ZnO, nano-ZnO was placed into a 50-mL Falcon tube, 30 mL water was first added and mixed for 15 min using an ultrasonic processor, then water was added to 50 mL. The suspension was shaken and mixed thoroughly before use. The solutions of both types of Zn compounds contained 0.05 % Tween 20 (Sigma-Aldrich) as a surfactant.
We first performed a time series experiment to investigate the Zn absorption process using intact, hydrated leaves for which only ZnSO4 was used. Specifically, we investigated six foliar application times, 0 (control), 0.25, 0.5, 1, 3 and 6 h. Two 5-µL droplets of the ZnSO4 solution were applied to the adaxial surface of the YFELs. The whole leaf (still attached to the plant) was then placed inside a Petri dish (the dish had a hole in the side wall to allow the petiole to pass through) with moistened filter paper in the bottom of the dish to maintain humidity. The temperature within the Petri dish was 30 °C and the relative humidity increased rapidly to 98 %. The growth lamps remained switched on during the incubation period and the droplets remained as a liquid (i.e. did not dry out) during the experimental period. After incubation for the appropriate length of time, the leaves were excised and rinsed using 2 % HNO3, 3 % ethanol and deionized water (Vu et al., 2013). For each specimen, the rinsed leaf was blotted dry and mounted on a sample holder between two layers of Ultralene film (4 µm thick). Samples were then immediately analysed using µ-XRF at the XFM beamline of the Australian Synchrotron. This beamline has been described previously (Paterson et al., 2011), including for the in situ analysis of hydrated plant tissues (Kopittke et al., 2011). For these scans, a 384-element Maia detector system in a backscatter position was used to collect the X-ray fluorescence emitted by the specimen. We used an incident energy of 12.9 keV with a total photon flux of approx. 1.5 × 109 photons s−1. Initially, a ‘survey scan’ was used to obtain a comparatively rapid image of the leaf from which the area for a detailed scan could be identified. For this initial survey scan, the transit time per virtual pixel was 7.5 ms and the horizontal stage velocity was 4 mm s−1, resulting in a virtual pixel size of 30 µm. The scan area was often approx. 10 × 10 mm, with these scans taking approx. 15 min. After the survey scan, an area entirely underneath where the Zn droplet had been applied was selected, and a detailed scan was conducted. For the detailed scan, the transit time was 1 ms and the horizontal stage velocity was 1 mm s−1, resulting in a virtual pixel size of 1 µm. The detailed scans were often approx. 4 × 5 mm in size and took approx. 75 min to complete. Next, we compared the absorption of ZnSO4 and nano-ZnO. For this experiment, 5-µL droplets of ZnSO4 and nano-ZnO were applied next to each other on the adaxial surface of the same YFEL for either 3 h or 3 d. The leaf was rinsed as described above before being analysed using µ-XRF.
For the next experiment, we examined the distribution of Zn within cross-sections of leaf tissues. All tissues examined within the cross-sections were obtained from tissues that had been entirely underneath the Zn-containing droplets. This was done by applying a larger droplet (40 µL) and excising only those tissues that had been underneath these droplets. A time series was prepared, 0 (control), 0.25, 0.5, 1, 3 and 6 h from the time of application. Cross-sections of each sample were then prepared from tissues located beneath where the Zn droplets had been applied. Two types of cross-sections were prepared. First, freeze dried specimens were prepared in advance at The University of Queensland. For these samples, leaf tissues were excised with a razor blade and leaf segments were sectioned (150 µm thick) using a vibratome as outlined earlier. The sections were then carefully sealed in Ultralene film held onto XRF sample cups (SC-8047, Premier Lab Supply) and freeze dried after small holes had been punctured in the film. Secondly, we prepared fresh (hydrated) cross-sections at the Australian Synchrotron in order to allow comparison with the freeze dried samples. Specifically, using plants growing at the Australian Synchrotron (the plants were grown under the same conditions as at The University of Queensland), the tissues underneath where the droplet had been applied were excised and placed in 4 % agar solution, transported to Monash University (Clayton, Australia) whilst being held on ice (transfer from the Australian Synchrotron to Monash University took approx. 10 min), and sectioned (150 µm) using a vibratome as outlined earlier. These hydrated (fresh) sections were then sealed between two layers of Ultralene film held using XRF sample cups and immediately transported back to the Australian Synchrotron (on ice) for examination.
The leaf cross-sections were then examined using µ-XRF. We first examined samples that had been incubated for either 3 or 6 h, comparing the fresh and freeze dried samples. It was found that freeze drying did not alter the distribution of Zn in the leaf tissues (see below). Thus, hereafter, unless otherwise noted, all cross-sections that were examined were freeze dried. To analyse these samples using µ-XRF, the sections were held between two layers of Ultralene film stretched over a Perspex frame magnetically mounted on the motion stage under a cryo-stream (operated at −100 °C, while the actual temperature on the sample is estimated to be below −50 °C). A beam of 15.8-keV X-rays was focused to a spot of around 2 µm diameter, and a silicon drift-diode detector (Vortex) located in the 90° geometry was used to collect X-ray fluorescence emitted by the specimen. The photon flux was approx. 2 × 109 photons s−1. First, a quick ‘survey scan’ was conducted, with the transit time being 50 ms and the horizontal stage velocity being 1 mm s−1, resulting in a virtual pixel size of 50 µm. The area scanned was generally approx. 5 × 0.15 mm, taking approx. 3 min to complete. Next, a ‘detailed scan’ was conducted, with the transit time being 3.33 ms and the horizontal stage velocity being 0.6 mm s−1, yielding a virtual pixel size of 2 µm. The detailed scans took approx. 100 min. To compare the foliar absorption of ZnSO4 and nano-ZnO, a 6 h nano-ZnO exposure cross-section was also examined using µ-XRF. All µ-XRF data were analysed using the CSIRO Dynamic Analysis method in GeoPIXE (http://www.nmp.csiro.au/dynamic.html) (Ryan and Jamieson, 1993; Ryan, 2000).
To obtain measurements of bulk Zn concentrations, bulk leaves (four replicates) were analysed by inductively coupled plasma mass spectrometry (ICP-MS) to compare foliar absorption of ZnSO4 and nano-ZnO. A half-leaf loading method was used (Vu et al., 2013), with half of each leaf receiving either 60 droplets (5 µL per droplet) of 1000 mg L−1 ZnSO4 or nano-ZnO and the other half receiving 60 droplets (5 µL per droplet) of deionized water for 6 or 24 h (both the Zn solution and the deionized water contained 0.05 % Tween 20). The leaves were cut in half along the mid-vein after incubation in the Petri dishes, then rinsed, oven dried (60 °C), digested using a 5: 1 mixture of nitric acid and perchloric acid, and analysed using ICP-MS. Blanks and reference materials were included to ensure accuracy.
Examining subcellular distribution of foliar-absorbed Zn in leaf tissues using NanoSIMS
To supplement the µ-XRF scans of the leaf cross-sections, we used NanoSIMS due to its higher resolution (approx. 100 nm). For NanoSIMS analyses, Zn distribution was examined only in leaf cross-sections from two samples: 0 (control) and 6 h application of 1000 mg L−1 ZnSO4. Using 16-d-old sunflower, a 40-µL droplet of 1000 mg L−1 ZnSO4 with 0.05 % Tween 20 was applied to the adaxial surface of a YFEL. Another YFEL had a 40-µL droplet of deionized water (also containing 0.05 % Tween 20) applied to the adaxial leaf surface as the control. The leaves were incubated in Petri dishes for 6 h with the growth lamps switched on. Afterwards, the leaves were rinsed and the area beneath the droplets was excised with a steel razor blade and cut into 2 × 3 mm segments. These segments were placed in planchettes filled with hexadecane, frozen in a high-pressure freezer (Bal-Tec HPM010) and processed as described by Kopittke et al. (2015). Next, 1-μm-thick sections were cut using an ultramicrotome (Leica EM UC6) and placed on substrates for NanoSIMS analysis.
NanoSIMS analyses were done similarly as described before (Blamey et al., 2018). Areas of interest were identified by optical microscopy in the NanoSIMS instrument. Signals of 12C14N− and 64Zn16O− secondary ions and secondary electron (SE) signal were collected to give morphological information and show the subcellular distribution of Zn. A large aperture (D1 = 1) and high primary current (~1 nA) was used to presputter the areas of interest to achieve the steady state for secondary ions. Imaging was done with a smaller aperture (D1 = 2) with a primary current of 10 pA with total dwell time of 56 ms per pixel for 256 × 256 pixels. Image analysis used the ImageJ plugin OpenMIMS (MIMS, Harvard University, Cambridge, MA, USA; https://nano.bwh.harvard.edu).
Examining the speciation of foliar-absorbed Zn in bulk leaf tissues using XAS
We used synchrotron-based XAS analysis to examine the chemical speciation of Zn in YFELs to which 1000 mg L−1 ZnSO4 and nano-ZnO had been applied for 6 h. The XAS analyses were performed at the XAS beamline at the Australian Synchrotron as described by Kopittke et al. (2011). To prepare these samples, 60 droplets (5 µL) of 1000 mg L−1 ZnSO4 or nano-ZnO were placed evenly across the entire whole leaf, using separate YFELs (four replicates) of 16-d-old sunflower plants. Leaves were rinsed after 6 h (as described above), frozen in liquid nitrogen, and ground using an agate mortar and pestle maintained under liquid nitrogen. The homogeneous specimen was then placed in a sample holder, sealed with Kapton tape, and immediately transferred to the cryostat (15 K) for analysis. At no time were the frozen (hydrated) samples allowed to thaw. In addition to the samples, we also prepared 11 standard reference compounds. First, we prepared three solid compounds, ZnO (Sigma Aldrich, 93632), Zn3(PO4)2 (Sigma Aldrich, 587583) and [ZnCO3]2·[Zn(OH)2]3 (Sigma Aldrich, 96466). These three solid compounds were ground and then diluted to a Zn concentration of 100 mg kg−1 using cellulose. We also examined eight aqueous compounds, Zn oxalate, Zn pectin, Zn citrate, Zn histidine, Zn(NO3)2, Zn phytate, Zn cysteine and Zn glutathione. The Zn(NO3)2 standard had a final Zn concentration of 4 mm, while the other seven standards were made by mixing of 4 mm Zn(NO3)2 with 20 mm oxalate acid, pectic acid, citric acid, histidine, phytic acid, cysteine and glutathione. Where appropriate, pH was then adjusted to approx. 6 using 0.1 m NaOH. The eight aqueous standards were then analysed following mixing with 30 % glycerol solutions (except Zn glutathione) which limits ice-crystal formation during cooling. Where possible, modelling using GEOCHEM-EZ (Shaff et al., 2010) indicated that >99 % of Zn was complexed with citric acid, oxalic acid and histidine, and >96 % was with cysteine. Furthermore, for the Zn(NO3)2 standard, >99.9% of the Zn was present as free Zn2+. Thus, hereafter, the Zn(NO3)2 standard is referred to as ‘Zn2+’.
The Zn Kα-edge X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectra were obtained in fluorescence mode using a 100-element solid-state Ge detector. Two replicate spectra were collected for all samples and standards. Each spectrum was calibrated by simultaneous measurement in transmission mode of a metallic Zn foil. The two replicates were merged and normalized using Athena (version 0.8.056), and linear combination fitting (LCF) was performed with a maximum of three standards.
RESULTS
Leaf properties of sunflower
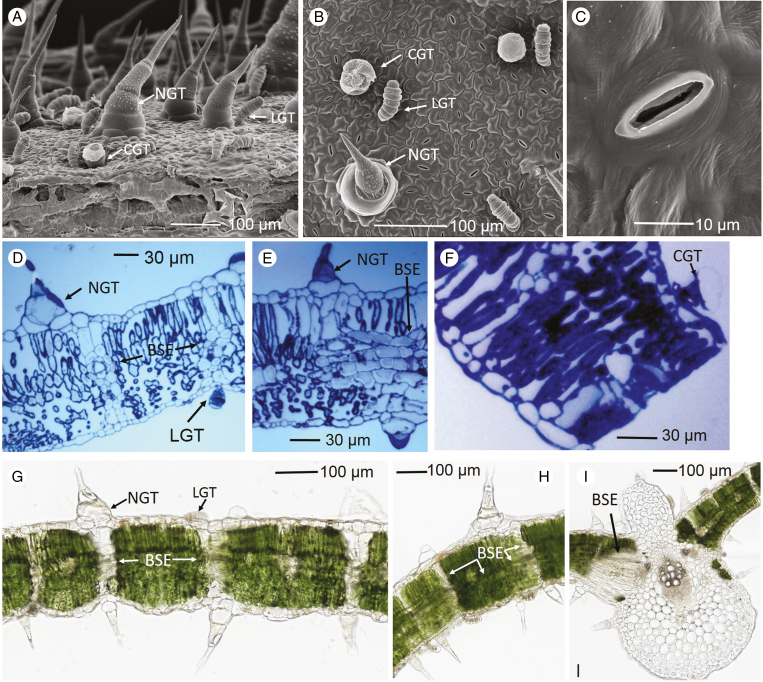
Three types of trichomes were observed on the surface of sunflower leaves (Fig. 1), namely a type of NGT, linear glandular trichome (LGT) and capitate glandular trichome (CGT) according to the classification of Aschenbrenner et al. (2013). The NGTs were approx. 0.1–0.8 mm in length, 0.05–0.1 mm in width at the base and consisted of multi-stalk cells and base cells. The LGTs were approx. 0.05 mm in length and consisted of 6–11 linearly arranged cells (Fig. 1A, B, D). Finally, the CGTs were approx. <0.05 mm in length and consisted of one short stalk cell and a round glandular cell on the tip (Fig. 1A, B, F). The total trichome density on sunflower adaxial leaf surface was 1600 cm−2, with the NGTs accounting for approx. 55 % of the total trichomes (and hence it can be estimated that the NGTs accounted for approx. 17 % of the leaf area), LNGs for 33 % and CGTs for 12 % (Fig. 1). The stomatal density of the adaxial leaf side was 22 900 cm−2, with the size of the stomatal opening being approx. 14 × 3 µm (Fig. 1B, C). Secondly, as expected, the leaf consisted of upper epidermal cells, palisade mesophyll cells, spongy mesophyll cells and a layer of lower epidermal cells. However, it was also noted that the NGTs and LGTs were generally connected to the bundle sheath extensions (BSEs) (Wylie, 1952), with the BSEs either directly beneath the NGTs and LGTs or connecting with the edge of trichome bases (Fig. 1D, E, G, H). It was also noted that the BSEs extended horizontally in the leaf tissues (Fig. 1G) and that they connected with vascular tissues (Fig. 1I).
Fig. 1.
Trichomes on the sunflower leaf surface, with the NGTs (non-glandular trichomes), LGTs (linear glandular trichomes) and CGTs (capitate glandular trichomes) labelled accordingly. The bundle sheath extensions (BSEs) are also labelled. (A–C) Scanning electron micrographs of the leaf surface. (D–F) Light micrographs showing leaf cross-sections (1 µm thick), with the sections stained using toluidine blue. (G–I) Light micrographs (80 µm thick) of fresh leaf cross-sections without staining.
The distribution of Zn in intact leaves using µ-XRF
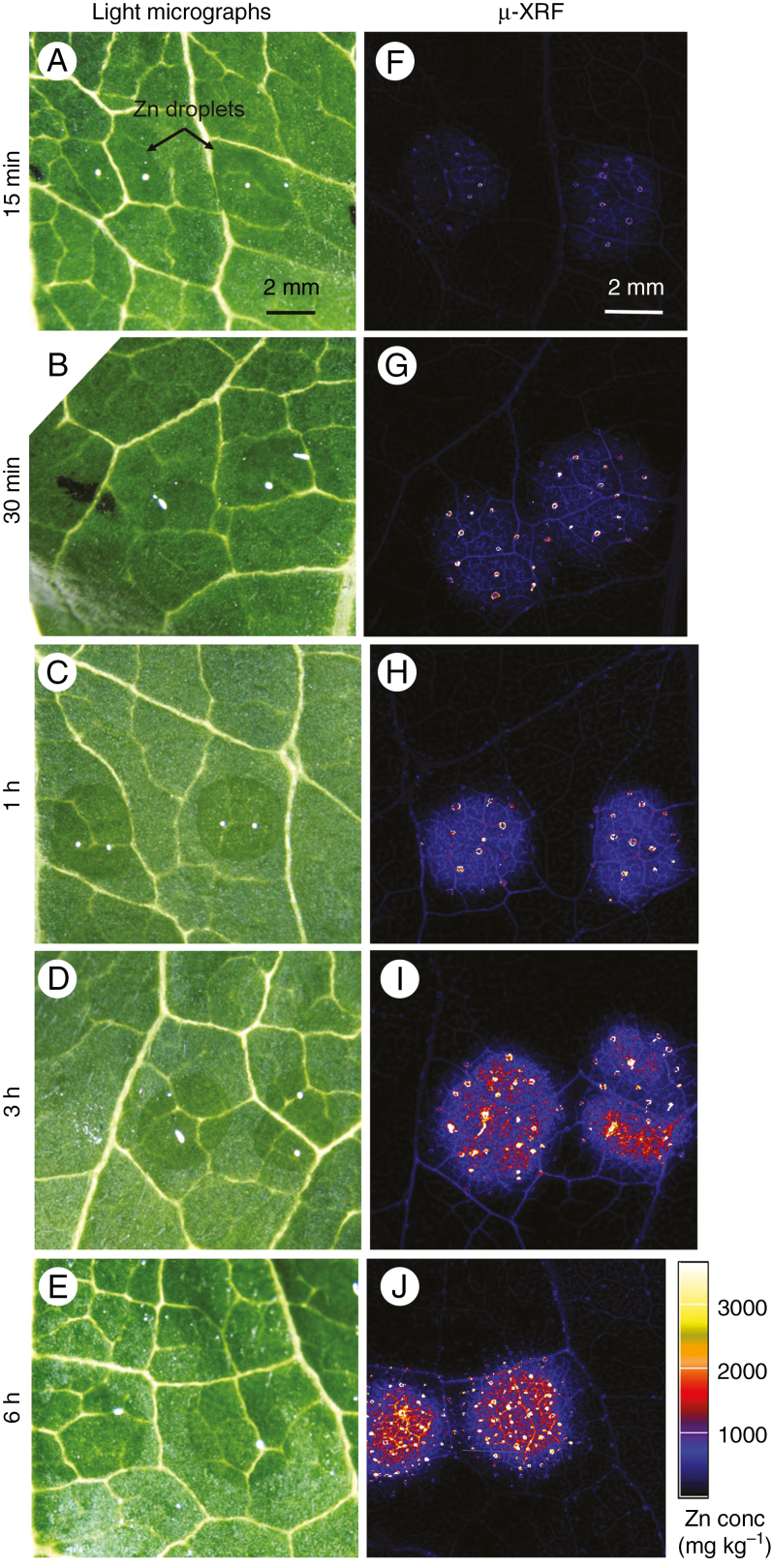
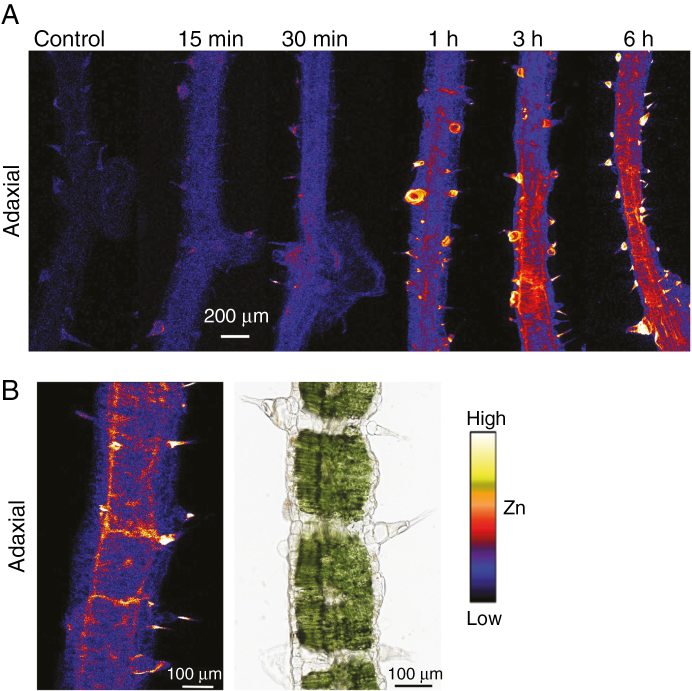
The distribution of Zn in the leaves was examined in situ using synchrotron-based µ-XRF after foliar application for 15 min, 30 min, 1 h, 3 h and 6 h. First, we conducted survey scans which examined larger areas of the leaves (Fig. 2), with Zn detected in the leaf tissues underneath the droplets within only 15 min (Fig. 2F), and with the tissue Zn concentration increasing as the exposure time increased. However, for all treatments, the Zn was observed to accumulate primarily underneath the Zn droplets, suggesting the Zn that had moved across the leaf surface had only limited translocation away from the site of application. Surprisingly, it was also observed that there were some bright spots (i.e. high Zn concentrations), approx. 0.05–0.1 mm in size, with the frequency and the intensity of these spots increasing with exposure time (Fig. 2).
Fig. 2.
(A–E) Light micrographs showing the Zn-containing droplets (ZnSO4) on the sunflower leaf surfaces before being removed and rinsed. (F–J) Images from the µ-XRF survey scans showing the distribution of Zn. All Zn maps are presented on the same concentration scale (mg kg–1), with brighter colours corresponding to higher Zn concentrations. Droplets were left on the leaf surface for 15 min, 30 min, 1 h, 3 h or 6 h. The scale bar in A applies to A–E, and the scale bar in F applies to F–J.
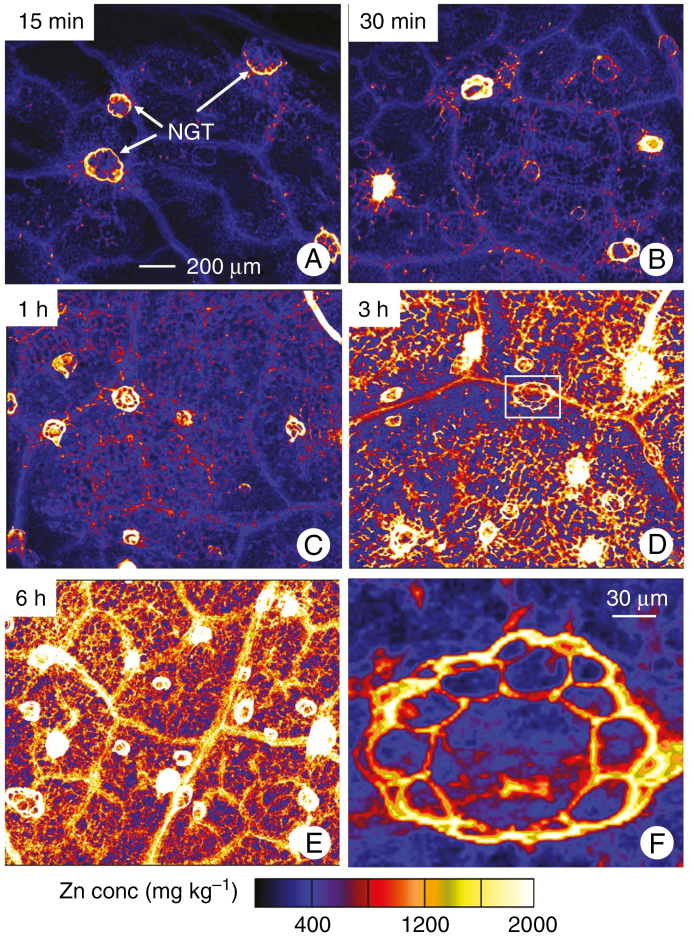
Based on these survey scans, we conducted detailed scans to examine smaller areas entirely underneath the droplets (Fig. 3). Using tri-colour maps of Ca–Mn–Zn, it was found that the ‘bright spots’ identified from the survey scans were bases of NGTs (Supplementary Data Fig. S1). Indeed, within only 15 min, Zn was observed to accumulate at the base of some of the NGTs, with some Zn also found to accumulate within the veins (Figs. 2F and 3A). As the exposure time increased, the number of NGTs that had high concentrations of Zn in their basal cells also increased. Indeed, at the final period of exposure (6 h), the highest Zn concentrations were found in the basal cells of the NGT, with Zn also accumulating (to a lesser extent) in the vascular tissues (Fig. 3).
Fig. 3.
Detailed µ-XRF scans showing the distribution of Zn in hydrated leaves of sunflower to which ZnSO4 had been applied for 15 min (A), 30 min (B), 1 h (C), 3 h (D) or 6 h (E). (F) High magnification of the white box in D. All Zn maps are presented on the same concentration scale (mg kg–1), with brighter colours corresponding to higher Zn concentrations. All of the areas imaged were entirely underlying where the Zn-containing droplet had been applied. The scale bar in A applies to A–E.
Distribution of Zn in cross-sections examined using NanoSIMS and µ-XRF
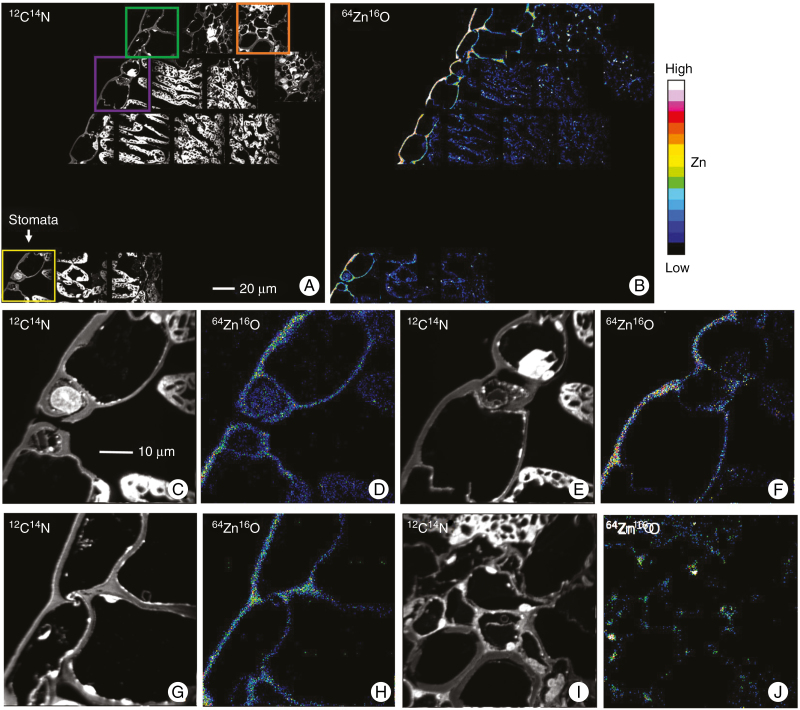
Next, the distribution of Zn was examined in cross-sections (1 µm thick) using NanoSIMS in order to obtain information at a subcellular level. We examined both a control and a leaf exposed to ZnSO4 for 6 h. By comparing with the control (Fig. S2), for the leaf exposed to ZnSO4 for 6 h, it was found that the entire abaxial surface contained elevated concentrations of Zn. It appeared that Zn had moved across the entire leaf surface rather than only at specific locations (such as stomata). Indeed, Zn appeared to have penetrated through the entire cuticle area underneath the Zn droplet (Fig. 4). Of particular importance, there was no marked accumulation of Zn within the stomatal cavity and surrounding cells, indicating that there did not appear to be preferential movement of Zn through the stomata (compare Fig. 4C, D and Fig. 4E, F). In addition, where the epidermis connected with the palisade cells, Zn accumulated primarily within the epidermal cell walls (Fig. 4A, B). In contrast, where the epidermis connected with vascular tissues, Zn was observed to not only accumulate in the cell walls of the epidermal cells, but also in the cell walls of the vascular cells (Fig. 4A, B, I, J). Unfortunately, we were only able to examine a small area using NanoSIMS, and the analyses did not include any NGTs.
Fig. 4.
NanoSIMS images showing the distribution of Zn in a cross-section of a sunflower leaf that had been exposed to ZnSO4 for 6 h. The 12C14N images are in greyscale for identification of the cellular and subcellular structures (A, C, E, G and I). The 64Zn16O images are given as a 16-colour scale showing Zn distribution (B, D, F, H, and J). (C–D) The Zn distribution for a stomata, which correspond to the yellow rectangle in A. (E–F) The Zn distribution in the epidermis, which correspond to the purple rectangle in A. (G–F) The Zn distribution in vascular tissues, which correspond to the green rectangle (G–H) and orange rectangle (I–L) in A. The scale bar in A applies to B, and the scale bar in C applies to D–J.
We then examined the distribution of Zn using µ-XRF in cross-sections (150 µm thick) of leaves that had been exposed to ZnSO4 for 15 min, 30 min, 1 h, 3 h and 6 h. Initially, we compared fresh and freeze dried cross-sections that had been exposed for 3 or 6 h to ZnSO4, finding that the freeze drying process did not appear to alter Zn distribution (Figs 5 and S3). Hence, hereafter we examined freeze dried samples (Fig. 5). Firstly, as observed from the µ-XRF analyses of the intact leaves (Figs 2 and 3), Zn was again found to accumulate rapidly (in ≤15–30 min) in the bases of the NGTs on the adaxial surface (Figs 5A and S4). After exposure for ≥1 h, not only did the Zn concentration in the basal cells of the NGTs of the adaxial surface increase, but Zn was also detected in the inner leaf tissues, as well as in epidermal cells and NGTs on both leaf surfaces (adaxial and abaxial) (Fig. 5A).
Fig. 5.
(A) µ-XRF scans showing Zn distribution in cross-sections of sunflower leaves (150 µm, freeze dried) underlying ZnSO4 droplets that had been applied for 0 min (control), 15 min, 30 min, 1 h, 3 h, or 6 h. (B) A close-up of a portion of a cross-section (3 h exposure) showing the movement of Zn through the bundle sheath extensions from the adaxial (Zn applied side) to the inner leaf tissues and abaxial side. Note that the µ-XRF image and light micrograph in B are not from the same sample. Rather, the light micrograph is simply provided for reference. Brighter colours correspond to higher Zn concentrations, but colours are not comparable between these Zn maps as their Zn concentrations differed too much to show using the same colour threshold.
From these µ-XRF scans, it is possible to compare the amount of Zn within the various tissues. Analysis of the data from the µ-XRF scans showed that normalized counts for Zn in the NGTs was on average approx. five times higher than the cuticular areas (including cuticle, stomata and the rest of the trichome areas) after 15 min and approx. nine times higher than the cuticular areas after 6 h (Fig. S5). As noted earlier, the NGTs accounted for approx. 17 % of the leaf area, and hence after exposure for 6 h, it is estimated that the total Zn accumulation in the NGTs was 1.9-fold higher than the Zn that accumulated in the cuticular tissues between the NGTs.
Of particular interest, it is known that BSEs generally connect the NGTs on opposite sides of the leaf (Fig. 1), with the µ-XRF analyses of cross-sections appearing to show that Zn moved readily from the NGT through the BSEs to the opposite leaf surface (Fig. 5B). Given (1) the penetrating nature of X-rays, and (2) that the sections examined by µ-XRF were 150 µm thick (approx. 10 cellular layers, see Fig. 1), the exact cellular and subcellular distribution of Zn is not clear from the µ-XRF analyses.
Comparing the foliar absorption of nano-ZnO and ZnSO4
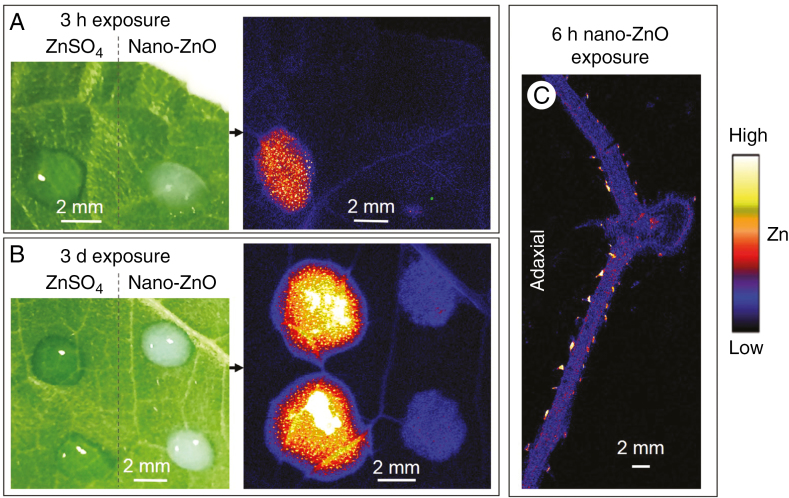
First, droplets of 1000 mg L−1 nano-ZnO and ZnSO4 were applied to the same leaf before being scanned using µ-XRF after either 3 h or 3 d (Fig. 6A, B). It was found that the absorption of nano-ZnO was substantially lower than that of ZnSO4 regardless of exposure time, with this result agreeing with measurements of bulk Zn (Fig. S6). For example, foliar absorption of ZnSO4 was 92-fold higher than that of nano-ZnO after 6 h exposure (Fig. S6). However, the localization of the foliar-absorbed Zn was highly limited for both forms of Zn, with the Zn accumulating almost exclusively in the tissues underlying where the droplet had been applied, even after 3 d (Fig. 6B). Although the concentration of Zn in the tissues underlying the nano-ZnO droplet was substantially lower than that of the ZnSO4 exposure leaf, the distribution of Zn within the tissues was similar, with the highest Zn concentrations again found in the basal cells of the NGTs (Figs 5 and 6C).
Fig. 6.
Comparison the foliar absorption of nano-ZnO and ZnSO4 in leaves of sunflower after exposure for either 3 h (A) or 3 d (B). In A and B, images on the left are light micrographs showing the Zn droplets on leaves (before being removed), while the images on the right are µ-XRF survey scans showing the distribution of Zn. (C) A µ-XRF scan showing the distribution of Zn in a cross-section of a sunflower leaf (150 µm thick, freeze dried) underlying the Zn droplets after 6 h exposure of nano-ZnO. Brighter colours correspond to higher Zn concentrations, but colours are not comparable between these Zn maps as their Zn concentrations differed too much to show using the same colour threshold.
Speciation of Zn within leaf tissues
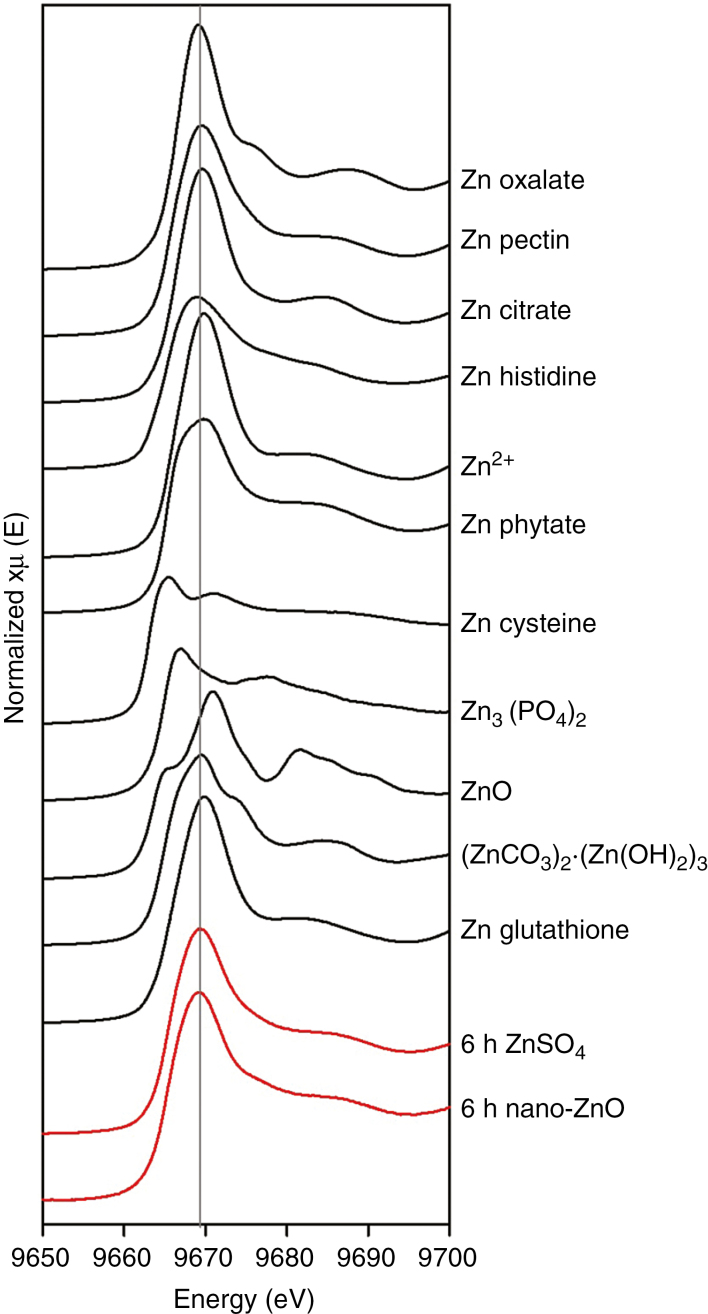
To compare the speciation of the foliar-absorbed Zn, leaves exposed to 1000 mg L−1 nano-ZnO or ZnSO4 for 6 h were examined using XAS (Fig. 7). First, we compared the spectra of the 11 standard compounds (black lines in Fig. 7). There were clear differences between the various spectra, differing not only in the energy of the white line peak and the magnitude of the peak, but also differing in other spectral features. For example, compounds in which Zn is complexed with carboxyl groups (oxalate, pectin, citrate) or is present as free Zn2+ [Zn(NO3)2] tended to have a white line peak at 9669 eV, while Zn3(PO4)2 had a peak at 9665 eV and ZnO at 9671 eV. Other differences were also evident between the standard compounds, with Zn phytate and Zn cysteine tending to have broader peaks compared to compounds in which Zn is complexed with carboxyl groups.
Fig. 7.
Normalized K-edge XANES spectra of 11 standard Zn compounds (black lines) and sunflower leaf tissues exposed to two different forms of Zn (red lines).
Next, we compared the spectra from the two leaf tissue samples which had been exposed to either ZnSO4 or nano-ZnO for 6 h (red lines in Fig. 7). Although the standard spectra for the two fertilizers differed markedly (compare black lines for Zn2+ and ZnO in Fig. 7), the spectra for leaf tissues were visually similar, despite the tissues being exposed to the two different fertilizers (red lines in Fig. 7). Of particular importance, it was noted that the spectra of Zn in leaves exposed to nano-ZnO differed markedly from the spectra of ZnO (compare black line for ZnO with red line for 6 h nano-ZnO, Fig. 7). Using LCF, it was modelled that 40–50 % Zn within the leaf tissues of both treatments was present as Zn phytate, with 30–40 % present as Zn oxalate (Table S1). However, given the similarity in the spectra of many of the standard compounds (e.g. Fig. S7), it was not possible to unequivocally identify the exact forms of Zn within the leaf tissues.
DISCUSSION
In the present study, we examined the importance of the cuticle, stomata and trichomes in foliar absorption of Zn. We found that the trichomes appear to be of particular importance, with Zn rapidly (≤15 min) accumulating within the basal cells of NGTs (Figs 3, 5 and S1). The cuticle was also found to be important, with Zn accumulating in the walls of all epidermal cells underlying the Zn-containing droplet (Fig. 4). Interestingly, there was no apparent accumulation of Zn in the stomatal cavity or the surrounding cells (Fig. 4), indicating that the stomata were probably not an important pathway for the foliar absorption of Zn in sunflower. We also compared nano-ZnO and ZnSO4, finding that the absorption of nano-ZnO was substantially lower than for ZnSO4 (Figs 6 and S6) despite the pattern of absorption being similar (Figs 6 and 7). Finally, although the absorbed Zn had only limited subsequent transportation from the application site (Figs 2 and 6), our analyses of Zn distribution appeared to indicate that the Zn moved through the BSEs and other vascular tissues to the opposite leaf side (Figs 4 and 5).
Role of trichomes in foliar Zn absorption
In the present study investigating sunflower, we have shown that trichomes appear to be an important organ for foliar-applied Zn across the leaf surface, with the NGTs containing the highest Zn concentrations within the tissues to which the Zn was applied (Figs. 2–3, 5–6, and Figs S2 and S5). Specifically, Zn accumulated rapidly (≤15 min) in the basal cells of the NGTs (Figs 3 and 5) and the total amount of Zn accumulation in the NGTs for 6 h exposed leaves was approx. 1.9 times higher than that in the cuticular area. There are several possible reasons why Zn moved rapidly into the base of NGTs. First, it is possible that the area surrounding the NGTs had a higher wettability than the surrounding interveinal tissues. Secondly, it is possible that the chemical composition and structure of the NGTs’ cuticle differs from the cuticle covering the main portion of the leaf surface, and it could be more permeable to solutes. For example, Bahamonde et al. (2018) showed that the major veins of the adaxial leaf surface of beech (Fagus sylvatica) are more permeable than the other leaf areas. Thirdly, it is also possible that sunflower has a specialized mechanism for the absorption of water through trichomes, as has been noted for other plant species adapted to drought stress (Ohrui et al., 2007; Winkler and Zotz, 2010; Vitarelli et al., 2016), with this resulting in the concomitant absorption of Zn in the present experiment. However, it is known that trichomes are highly diverse (Werker, 2000; Ohrui et al., 2007), and it is likely that not all plant species would have trichomes that are important for foliar nutrient absorption. For example, Li et al. (2018) found that trichomes of tomato (Solanum lycopersicum) and soybean (Glycine max) are not important in foliar Zn absorption. Thus, the role of trichomes in foliar nutrient absorption depends upon the ecological conditions, species and the property of the specific trichome.
The observation that the NGTs contained elevated concentrations of Zn following its foliar application is similar to that observed in other studies where elevated levels of metals are supplied in the rooting medium. For example, Blamey et al. (2015) found that high concentrations of Mn accumulated in these NGTs of sunflower when exposed to elevated levels of Mn in the rooting medium [also see Sarret et al. (2009) in Arabidopsis halleri and Arabidopsis lyrata, Broadhurst et al. (2009) in Alyssum murale and Alyssum corsicum, McNear and Kupper (2014) in Alyssum murale, and Tomasi et al. (2014) in Cucumis sativus]. Furthermore, the prediction that 30–40 % of the foliar absorbed Zn is associated with oxalate (Table S1) is presumably associated with the accumulation of Zn in the NGTs, with Zn potentially substituting for Ca in the Ca-containing compounds of the NGTs. This is similar to that reported by Sarret et al. (2006) in tobacco (Nicotiana tabacum), with these authors finding that when exposed to toxic levels of Zn, the Zn preferentially accumulated in trichomes, with Zn-substituted calcite excreted as a mechanism of detoxification. However, whether the Zn that accumulates within the NGTs can subsequently be translocated to other parts of the plant requires further study. In a similar manner, it is necessary to better clarify the accumulation of Zn within trichomes that we have observed in the present study. In particular, it is important to understand whether the rapid movement of Zn into the NGT is a specific absorption mechanism, or whether it occurs indirectly due to absorption of water as a drought stress mechanism. In a related manner, it is necessary to understand differences between plant species, including why the trichomes of some plant species do not absorb foliar-applied nutrients (Li et al., 2018) but others do. Finally, it would also be useful to assess the impact of growth conditions (such as water stress) on the absorption of nutrients by trichomes.
Role of the cuticle and stomata in foliar Zn absorption
Apart from the important role of trichomes in the movement of Zn across the leaf surface, we also found that Zn appeared to move across the leaf cuticle. The importance of the cuticle was demonstrated by the subcellular distribution of Zn examined using NanoSIMS (Fig. 4). To the best of our knowledge, this is the first time that NanoSIMS has been used for the examination of foliar nutrient absorption. We found that Zn concentrations were increased and appeared to be comparatively uniform along the epidermal cells of the adaxial surface (Figs 4 and S2), indicating the movement of Zn across the cuticle. Note, however, that the relative humidity during foliar Zn application in the present study was 98 %, and this could promote the cuticular penetration (Fernández and Eichert, 2009; Schönherr, 2001). Furthermore, cuticular permeability differs between the various plant species (Schreiber and Riederer, 1996; Schreiber and Schönherr, 2009), depending upon the chemical composition of the cuticle, including cutin (Sadler et al., 2016; Huang et al., 2017) and intracuticular wax (Zeisler-Diehl et al., 2018), with the permeability enhanced greatly by adjuvants such as Tween 20 (Arand et al., 2018). However, much remains unknown regarding the structure, composition, formation and physicochemical properties of the cuticle (Fernández et al., 2017; Khanal and Knoche, 2017; Ingram and Nawrath, 2017; Schuster et al., 2017), as do mechanisms by which Zn moves across the cuticle.
We found no evidence that the stomata played an important role in the foliar absorption of Zn. Rather, concentrations of Zn in the stomatal cells were similar to those observed for other epidermal cells on the adaxial surface, with no distinct accumulation of Zn in the stomata or the stomatal cavity (Fig. 4A–F). This observation is consistent with that of Eichert et al. (2008) who visually observed that hydrophilic polystyrene particles with a diameter of 43 nm suspended in water entered leaves through the stomata by diffusion along the walls of the pore other than from the opening cavity, but less than 10 % of all stomata participated in this pathway. The role of hygroscopic leaf surface particles in promoting stomatal uptake can also not be neglected (Burkhardt et al., 2012). Despite the potential hydrophobility of the stomatal cavity and its geometry limiting solution infiltration (Schönherr and Bukovac, 1972), the accumulation of hygroscopic particles in the pore may lead to the formation of a continuous liquid film connecting along the cuticular walls of the stomata between the interior and the outer surface, with this process called ‘hydraulic activation of stomata’ (Burkhardt et al., 2009; Burkhardt, 2010). The activation of this process is required separately for each stomata and could be relevant to the composition or structure of the cuticle above the guard cells or the pore wall cells (Burkhardt et al., 2012), modifications of the pore such as deposition of hygroscopic particles, and microbes growing in the pore which increased wettability (Burkhardt et al., 2012; Eichert and Fernández, 2012). However, major differences in the chemical composition and structure of the pore walls and stomatal surfaces can be found among species and this is likely to affect the process of stomatal penetration and the significance and contribution of this pathway to the overall process of foliar absorption as noted by Fernández et al. (2017).
Role of bundle sheath extensions in movement of foliar-absorbed Zn
In the present study, we have identified an important role of BSEs, with little currently known regarding these tissues (Wylie, 1952; Buckley et al., 2011). In sunflower, the BSEs extend vertically and horizontally within the leaf (Fig. 1). We noted that following the accumulation of Zn at the NGT bases, the Zn appeared to move comparatively rapidly into BSEs, and as a result, although Zn was applied to the adaxial surface, it also moved rapidly to the abaxial surface (Fig. 5). Thus, we propose that in sunflower, BSEs are involved in the movement of foliar-applied Zn from the trichome into other leaf tissues. However, here we examined changes in Zn distribution rather than Zn movement per se. Although the changes in distribution reflect the underlying movement of Zn, further studies are required to examine the redistribution of the absorbed Zn.
Comparing the absorption of nano-ZnO and ZnSO4
There is increasing interest in the use of nanomaterials in agricultural production systems (De Rosa et al., 2010; Wang et al., 2016). We found that the foliar absorption of nano-ZnO was substantially lower than that of ZnSO4 regardless of the exposure time, with the two types of Zn having a similar pattern of absorption (Figs 6 and S6). Furthermore, we found that the Zn within the leaf tissues that had moved across the leaf surface was present as the same chemical species, regardless of whether the Zn was supplied as nano-ZnO or ZnSO4 (Fig. 7). These findings indicate that nano-ZnO was probably absorbed in the same form as ZnSO4 – soluble Zn ions rather than as intact ZnO particles. This is in agreement with previous studies, including a similar study of Li et al. (2018) in soybean, and Alexander and Hunsche (2016) found that absorption of insoluble foliar applied nutrients was limited (even for nano-scale particles). Therefore, solubility is an important factor to consider when designing foliar fertilizers. However, this slower release of Zn from nano-ZnO is potentially advantageous, as it may reduce any potential ‘burning’ observed from highly soluble Zn foliar fertilizers (Drissi et al., 2015). In this regard, it would be useful to identify how sparingly soluble forms of Zn may be retained on the leaf surface as a slow-release fertilizer.
CONCLUSIONS
We have demonstrated that trichomes are important for the absorption of foliar-applied Zn for leaves of sunflower. The cuticle was also found to be important, with Zn appearing to move across the cuticle before accumulating in the walls of underlying cells. No marked accumulation of Zn was found within the stomatal cavity, indicating that movement of Zn through the stomata was not likely to be important. After absorption, Zn was located in the epidermal cells on both sides of the leaf. In this regard, the BSEs which connected to the trichomes appear to be important for the movement of Zn away from the overlying trichomes. Finally, the absorption of nano-ZnO was substantially lower than for ZnSO4, with Zn probably moving across the leaf surface as soluble Zn rather than as nanoparticles. It is hoped that the information from this study will assist in improving the efficacy of foliar fertilizers as required to improve the nutrition of both crop plants and humans.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Tri-colour image from µ-XRF analyses showing the distribution of Ca, Zn and Mn in a sunflower leaf directly beneath where a ZnSO4 droplet had been applied for 6 h. Fig. S2: NanoSIMS analyses showing Zn distribution in leaves of sunflower exposed to ZnSO4 for 6 h and a control leaf. Fig. S3: µ-XRF analyses showing the distribution of Zn in fresh (hydrated) cross-sections of sunflower leaves, to which ZnSO4 had been applied for either 3 or 6 h. Fig. S4: Tri-colour image from µ-XRF analyses showing the distribution of Ca, Zn and Mn in cross-sections of a sunflower leaf. Fig. S5: µ-XRF image of a leaf treated with ZnSO4 for 15 min and 6 h, with the upper µ-XRF images showing Zn distribution and the bottom graphs showing the normalized concentration of Zn along the transect (white lines) shown in the upper images. Fig. S6: Comparison of foliar Zn absorption of ZnSO4 and nano-ZnO in sunflower using inductively coupled plasma mass spectrometry. Fig. S7: Normalized K-edge XANES spectra illustrating the close similarity of four Zn compounds which prevented differentiation during linear combination fitting. Table S1: Results of linear combination fitting of K-edge XANES data for sunflower leaves to which Zn had been applied for 6 h.
ACKNOWLEDGEMENTS
This work was supported by Sonic Essentials. Funding was also provided by the Australian Research Council (ARC) through the Linkage Projects funding scheme (LP130100741), the Future Fellowship scheme (FT120100277, Peter Kopittke), and the Discovery Early Career Researcher Award scheme (DE160100429, Antony van der Ent). Cui Li is supported by the China Scholarship Council and The University of Queensland (UQ). The authors acknowledge use of the facilities and technical assistance of both the Monash Histology Platform (Department of Anatomy and Developmental Biology, Monash University), the Queensland Brain Institute (UQ) and the Centre for Microscopy and Microanalysis (UQ). Much of this research was undertaken on the XFM and XAS beamlines at the Australian Synchrotron, part of ANSTO. The assistance of both Chris Glover (XAS beamline, Australian Synchrotron) and Victoria Fernández is gratefully acknowledged.
LITERATURE CITED
- Alexander A. 1986. Foliar Fertilization Proceedings of the First International Symposium on Foliar Fertilization, Organized by Schering Agrochemical Division, Special Fertilizer Group, Berlin (FRG) March 14–16, 1985. Dordrecht: Springer Netherlands. [Google Scholar]
- Alexander A, Hunsche M. 2016. Influence of formulation on the cuticular penetration and on spray deposit properties of manganese and zinc foliar fertilizers. Agronomy 6: 39. [Google Scholar]
- Arand K, Asmus E, Popp C, Schneider D, Riederer M. 2018. The mode of action of adjuvants–Relevance of physicochemical properties for effects on the foliar application, cuticular permeability and greenhouse performance of Pinoxaden. Journal of Agricultural and Food Chemistry 66: 5770–5777. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner A-K, Horakh S, Spring O. 2013. Linear glandular trichomes of Helianthus (Asteraceae): morphology, localization, metabolite activity and occurrence. AoB Plants 5: plt028. [Google Scholar]
- Bahamonde HA, Fernandez V, Gil L. 2018. Surface properties and permeability to calcium chloride of Fagus sylvatica and Quercus petraea leaves of different canopy heights. Frontiers in Plant Science 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey F, Joyce D, Edwards D, Asher C. 1986. Role of trichomes in sunflower tolerance to manganese toxicity. Plant and Soil 91: 171–180. [Google Scholar]
- Blamey FPC, Hernandez-Soriano MC, Cheng M, et al. . 2015. Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiology 169: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey FPC, McKenna BA, Li C, et al. . 2018. Manganese distribution and speciation help to explain the effects of silicate and phosphate on manganese toxicity in four crop species. New Phytologist 217: 1146–1160. [DOI] [PubMed] [Google Scholar]
- Broadhurst CL, Tappero RV, Maugel TK, Erbe EF, Sparks DL, Chaney RL. 2009. Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum. Plant and Soil 314: 35–48. [Google Scholar]
- Buckley TN, Sack L, Gilbert ME. 2011. The role of bundle sheath extensions and life form in stomatal responses to leaf water status. Plant Physiology 156: 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. 2010. Hygroscopic particles on leaves: nutrients or desiccants?Ecological Monographs 80: 369–399. [Google Scholar]
- Burkhardt J, Hunsche M, Pariyar S. 2009. Progressive wetting of initially hydrophobic plant surfaces by salts–a prerequisite for hydraulic activation of stomata? In: Proceedings 16th International Plant Nutrition Colloquium: Davis, USA https://escholarship.org/uc/item/2m09483m [Google Scholar]
- Burkhardt J, Basi S, Pariyar S, Hunsche M. 2012. Stomatal penetration by aqueous solutions–an update involving leaf surface particles. New Phytologist 196: 774–787. [DOI] [PubMed] [Google Scholar]
- De Rosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y. 2010. Nanotechnology in fertilizers. Nature Nanotechnology 5: 91. [DOI] [PubMed] [Google Scholar]
- Drissi S, Houssa AA, Bamouh A, Benbella M. 2015. Corn silage (Zea mays L.) response to zinc foliar spray concentration when grown on sandy soil. Journal of Agricultural Science 7: 68–79. [Google Scholar]
- Eichert T, Fernández V. 2012. Uptake and release of elements by leaves and other aerial plant parts. In: Marschner P, ed. Marschner’s mineral nutrition of higher plants, 3rd edn. San Diego: Academic Press. [Google Scholar]
- Eichert T, Goldbach H, Burkhardt J. 1998. Evidence for the uptake of large anions through stomatal pores. Botanica Acta 111: 461–466. [Google Scholar]
- Eichert T, Kurtz A, Steiner U, Goldbach HE. 2008. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum 134: 151–160. [DOI] [PubMed] [Google Scholar]
- Fernández V, Eichert T. 2009. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Critical Reviews in Plant Sciences 28: 36–68. [Google Scholar]
- Fernández V, Sotiropoulos T, Brown P. 2013. Foliar fertilization: scientific principles and field practices. Paris, France: International Fertilizer Industry Association (IFA). [Google Scholar]
- Fernández V, Bahamonde HA, Javier Peguero-Pina J, et al. . 2017. Physico-chemical properties of plant cuticles and their functional and ecological significance. Journal of Experimental Botany 68: 5293–5306. [DOI] [PubMed] [Google Scholar]
- Huang H, Burghardt M, Schuster A-C, Leide J, Lara I, Riederer M. 2017. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. Journal of Agricultural and Food Chemistry 65: 8790–8797. [DOI] [PubMed] [Google Scholar]
- Huchelmann A, Boutry M, Hachez C. 2017. Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiology 175: 6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram G, Nawrath C. 2017. The roles of the cuticle in plant development: organ adhesions and beyond. Journal of Experimental Botany 68: 5307–5321. [DOI] [PubMed] [Google Scholar]
- Ju J, Bai H, Zheng Y, Zhao T, Fang R, Jiang L. 2012. A multi-structural and multi-functional integrated fog collection system in cactus. Nature Communications 3: 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal BP, Knoche M. 2017. Mechanical properties of cuticles and their primary determinants. Journal of Experimental Botany 68: 5351–5367. [DOI] [PubMed] [Google Scholar]
- Kopittke PM, Menzies NW, de Jonge MD, et al. . 2011. In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiology 156: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Moore KL, Lombi E, et al. . 2015. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiology 167: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang P, Menzies NW, Lombi E, Kopittke PM. 2017. Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Annals of Botany 120: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang P, Lombi E, et al. . 2018. Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. Journal of Experimental Botany 69: 2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. 1874. Ueber die Aufnahme von Ammoniak durch oberirdische Pflanzentheile. Winter. [Google Scholar]
- McNear DH, Kupper JV. 2014. Mechanisms of trichome-specific Mn accumulation and toxicity in the Ni hyperaccumulator Alyssum murale. Plant and Soil 377: 407–422. [Google Scholar]
- O’Brien T, Feder N, McCully ME. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373. [Google Scholar]
- Ohrui T, Nobira H, Sakata Y, et al. . 2007. Foliar trichome-and aquaporin-aided water uptake in a drought-resistant epiphyte Tillandsia ionantha Planchon. Planta 227: 47–56. [DOI] [PubMed] [Google Scholar]
- Paterson D, De Jonge M, Howard D, et al. . 2011. The X-ray fluorescence microscopy beamline at the Australian Synchrotron. In: The 10th International Conference on X-ray Microscopy. Melville, NY: AIP Publishing. [Google Scholar]
- Pina AL, Zandavalli RB, Oliveira RS, Martins FR, Soares AA. 2016. Dew absorption by the leaf trichomes of Combretum leprosum in the Brazilian semiarid region. Functional Plant Biology 43: 851–861. [DOI] [PubMed] [Google Scholar]
- Ryan C. 2000. Quantitative trace element imaging using PIXE and the nuclear microprobe. International Journal of Imaging Systems and Technology 11: 219–230. [Google Scholar]
- Ryan C, Jamieson D. 1993. Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 77: 203–214. [Google Scholar]
- Sadler C, Schroll B, Zeisler V, Waßmann F, Franke R, Schreiber L. 2016. Wax and cutin mutants of Arabidopsis: quantitative characterization of the cuticular transport barrier in relation to chemical composition. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1861: 1336–1344. [DOI] [PubMed] [Google Scholar]
- Sarret G, Harada E, Choi Y-E, et al. . 2006. Trichomes of tobacco excrete zinc as zinc-substituted calcium carbonate and other zinc-containing compounds. Plant Physiology 141: 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret G, Willems G, Isaure MP, et al. . 2009. Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytologist 184: 581–595. [DOI] [PubMed] [Google Scholar]
- Schlegel T, Schönherr J. 2001. Selective permeability of cuticles over stomata and trichomes to calcium chloride. In: International Symposium on Foliar Nutrition of Perennial Fruit Plants, Leuven, Belgium: International Society for Horticultural Science (ISHS), 594. [Google Scholar]
- Schönherr J. 1976. Water permeability of isolated cuticular membranes: the effect of pH and cations on diffusion, hydrodynamic permeability and size of polar pores in the cutin matrix. Planta 128: 113–126. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 2001. Cuticular penetration of calcium salts: effects of humidity, anions, and adjuvants. Journal of Plant Nutrition and Soil Science-Zeitschrift für Pflanzenernahrung und Bodenkunde 164: 225–231. [Google Scholar]
- Schönherr J. 2006. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany 57: 2471–2491. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Bukovac MJ. 1972. Penetration of stomata by liquids dependence on surface tension, wettability, and stomatal morphology. Plant Physiology 49: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Riederer M. 1996. Determination of diffusion coefficients of octadecanoic acid in isolated cuticular waxes and their relationship to cuticular water permeabilities. Plant, Cell & Environment 19: 1075–1082. [Google Scholar]
- Schreiber L, Schönherr J. 2009. Water and solute permeability of plant cuticles. Measurement and data analysis. Berlin: Springer-Verlag. [Google Scholar]
- Schuster A-C, Burghardt M, Riederer M. 2017. The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions?Journal of Experimental Botany 68: 5271–5279. [DOI] [PubMed] [Google Scholar]
- Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV. 2010. GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant and Soil 330: 207–214. [Google Scholar]
- Stagnari F. 2007. A review of the factors influencing the absorption and efficacy of lipophilic and highly water-soluble post-emergence herbicides. European Journal of Plant Science and Biotechnology 1: 22–35. [Google Scholar]
- Tomasi N, Mimmo T, Terzano R, et al. . 2014. Nutrient accumulation in leaves of Fe-deficient cucumber plants treated with natural Fe complexes. Biology and Fertility of Soils 50: 973–982. [Google Scholar]
- Turrell F. 1947. Citrus leaf stomata: structure, composition, and pore size in relation to penetration of liquids. Botanical Gazette 108: 476–483. [Google Scholar]
- Vitarelli NC, Riina R, Cassino MF, Meira RMSA. 2016. Trichome-like emergences in Croton of Brazilian highland rock outcrops: evidences for atmospheric water uptake. Perspectives in Plant Ecology, Evolution and Systematics 22: 23–35. [Google Scholar]
- Vu DT, Huang L, Nguyen AV, et al. . 2013. Quantitative methods for estimating foliar uptake of zinc from suspension-based Zn chemicals. Journal of Plant Nutrition and Soil Science 176: 764–775. [Google Scholar]
- Wang P, Lombi E, Zhao F-J, Kopittke PM. 2016. Nanotechnology: a new opportunity in plant sciences. Trends in Plant Science 21: 699–712. [DOI] [PubMed] [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- Winkler U, Zotz G. 2010. ‘And then there were three’: highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Annals of Botany 106: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie RB. 1952. The bundle sheath extension in leaves of dicotyledons. American Journal of Botany 39: 645–651. [Google Scholar]
- Zeisler-Diehl V, Müller Y, Schreiber L. 2018. Epicuticular wax on leaf cuticles does not establish the transpiration barrier, which is essentially formed by intracuticular wax. Journal of Plant Physiology. DOI: org/ 10.1016/j.jplph.2018.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.