Abstract
Background and Aims
Fleshy structures that promote biotic dispersal by ingestion have evolved many times in seed plants. Within the yew family Taxaceae sensu lato (six genera, including Cephalotaxus), it remains controversial whether the characteristic fleshy structure surrounding the seed is interpreted as a novel outgrowth of the base of the ovule (i.e. an aril) or a fleshy seed coat that is entirely derived from the integument (i.e. a sarcotesta). This paper presents a detailed study of both wild-type and teratological seed cones of Pseudotaxus chienii, including morphology, anatomy and ontogeny.
Methods
Wild-type and teratological seed cones were investigated with the classical paraffin technique and subsequent astrablue/safranin staining and scanning electron microscopy.
Key Results
The wild-type seed cone of Pseudotaxus possesses a fleshy white aril that is cup-like and not entirely fused to the seed. In the teratological seed cones investigated, the aril was bilobed and consisted of two free halves. In both wild-type and teratological cones, the aril was initiated as two lateral primordia in a transverse plane, but in wild-type cones the two primordia became extended into a ring primordium, which grew apically, leading to the cup-like shape. The teratological seed cones lacked a ring primordium and the two lateral aril lobes remained free throughout their entire ontogeny, alternating with the scale-like leaves inserted below them on the same branch; in some cases, these leaves also became fleshy.
Conclusions
Based on the ontogeny and arrangement of the two fleshy aril lobes in the teratological seed cones of Pseudotaxus, we suggest that the typical aril of Taxaceae could be readily interpreted as a fused pair of strongly swollen leaves rather than a modified integument. Our investigations of the cup-like aril of Pseudotaxus demonstrate a similarity not only with other Taxaceae but also with relatively distantly related conifers such as Phyllocladus (Podocarpaceae).
Keywords: Aril, evolution, Pseudotaxus, seed cone, Taxaceae, teratology
INTRODUCTION
Reproductive structures of conifers are typically arranged in compact unisexual cones, either as ‘male’ (staminate) pollen cones or as ‘female’ (ovulate) seed cones. A characteristic feature of coniferous seed cones is the presence of a bract scale/seed scale complex, in which the bract scale is widely interpreted as a leaf that subtends a modified shoot, the axillary seed scale, though in some conifers the entire complex is so strongly modified that neither scale type is readily distinguishable (Coulter and Chamberlain, 1917; Florin, 1951, 1954; Farjon and Ortiz-Garcia, 2003; Jagel and Dörken, 2014, 2015a, b). Some conifer seeds possess associated fleshy structures that make the seed attractive to birds and animals and hence are implicated in biotic dispersal; these structures include the fleshy fused bracts of Juniperus (Cupressaceae), the fleshy seed scale (epimatium) of Podocarpus (Podocarpaceae) and the fleshy aril of the yew family Taxaceae (Tomlinson and Takaso, 2002; Contreras et al., 2017; Lovisetto et al., 2012). The seed of Taxus (Taxaceae) is toxic due to the presence of cyanogenic glycosides in the seed coat; however, the fleshy structure is edible and seeds pass through the bird undigested (Farjon, 2007; Dörken and Hetzel, 2017).
Within Taxaceae, it remains controversial whether the fleshy structure surrounding the seed is interpreted as an outgrowth of the funicle (an aril) or a fleshy seed coat derived from the integument (a sarcotesta), or indeed whether there exists a continuum between them. The fleshy structure is widely accepted as an aril in Austrotaxus, Taxus, Pseudotaxus and Torreya. Some authors also interpret the fleshy structure in Cephalotaxus as an aril (e.g. Stützel and Röwekamp, 1999; Mundry, 2000; Eckenwalder, 2009; Farjon, 2010), but others regard it as a sarcotesta (e.g. Page, 1990; Contreras et al., 2017). The fleshy structure in Amentotaxus was earlier interpreted as a specialized type of ovuliferous scale termed an epimatium (e.g. Krüssmann, 1955), a structure that is typically found in Podocarpaceae (e.g. Buchholz and Gray, 1948; Tomlinson, 1992; Vázquez-Lobo et al., 2007). However, based on comparative expression patterns of orthologues of the AG gene family, Englund et al. (2011) rejected a primary homology between the aril of Taxus globosa and the epimatium of Podocarpus reichei, which they considered homologous with the ovuliferous scale of Pinus. Although arillate Taxaceae seeds are known from the Triassic, comparative data from the fossil record are also difficult to interpret and do not conclusively resolve the issue (e.g. Meyen, 1987).
In this paper, we evaluate the controversial homologies and possible evolutionary origin of the aril structure in Taxaceae sensu lato using a detailed comparison of wild-type and teratological seed cones in Pseudotaxus chienii. No previous comparative studies of seed-cone ontogeny exist for Pseudotaxus, though descriptions exist for some other Taxaceae; for example, Mundry (2000) described the ontogeny of the fleshy structure surrounding the ovules/seeds in Taxus, Torreya and Cephalotaxus. However, comparison of mature seed cones shows that Pseudotaxus is very similar to Taxus, differing only in the number of scale leaves at the stalk of the seed cone and the colour of the aril: white in Pseudotaxus and red in Taxus (Florin 1948a; Krüssmann, 1983; Farjon, 2007, 2010; Eckenwalder, 2009). In both genera, the aril is strongly swollen, fleshy, cup-like and not fused to the seed, and the distal collar is unlobed. The similarities in their seed cones indicate a close relationship between the two genera, which is confirmed in morpho-anatomical (e.g. Ghimire and Heo, 2014) and genetic (e.g. Cheng et al., 2000) studies.
Taxaceae sensu lato consists of six extant genera, including two formerly segregated as Cephalotaxaceae (Amentotaxus, Cephalotaxus) and four ‘core’ Taxaceae: Austrotaxus, Pseudotaxus, Taxus and Torreya (e.g. Harris, 1976; Stevens, 2001; Eckenwalder, 2009; Lang et al., 2013; Ghimire et al., 2014). In many respects, cone morphology in Taxaceae is unique among living (extant) conifers. The seed cones of all Taxaceae lack a distinct bract scale/seed scale complex (Ghimire et al., 2014) and the pollen cones of Cephalotaxus and Pseudotaxus are interpreted as polyaxial, in contrast with other conifers, in which the entire pollen cone is interpreted as uniaxial (e.g. Coulter and Chamberlain, 1917; Wilde, 1944, 1975; Krüssmann, 1955, 1983; Mundry and Mundry, 2001; Dörken et al., 2011). Taxaceae (excluding Amentotaxus and Cephalotaxus) were formerly placed in a separate order, Taxales, but both morphological and molecular analyses demonstrate that they are true conifers, closely related to Cupressaceae (e.g. Chaw et al., 2000; Quinn et al., 2002; Burleigh and Matthews, 2004).
MATERIALS AND METHODS
Materials
Pseudotaxus chienii (Taxaceae) is a rare Chinese species of Taxaceae, which is rarely grown in cultivation. Taxus baccata is native to Europe and adjacent regions and frequently in cultivation. Both taxa are evergreen, dioecious (exceptionally monoecious) shrubs or small trees. Cones of both genders develop in axillary positions on lateral branchlets. Within Pseudotaxus two cone types were investigated here: (1) wild-type seed cones; and (2) teratological seed cones with anomalous strongly divided bilobed arils, mostly consisting of two entirely separate halves. Within Taxus only wild-type seed cones were investigated. All cones were collected by one of the authors (V.M.D.) in the extensive living collection of another author (H.N.) in St Ulrich, Bollschweil, Germany. Wild-type and teratological seed cones of Pseudotaxus were collected from two different trees that are cultivated as pot plants and placed outdoors in the summer and in a temperate glasshouse in the winter. Seed cones of Taxus were collected from a tree cultivated outdoors.
Methods
Freshly collected material was photographed, then fixed in formalin acetic alcohol (FAA: 70 % alcohol, formaldehyde solution and glacial acetic acid, in proportions 90:5:5) before being stored in 70 % ethanol. Cone anatomy was studied using the classical paraffin technique and subsequent astra blue/safranin staining (Gerlach, 1984). Macrophotography was accomplished using a digital camera (Canon PowerShot IS2) and microphotography with a digital microscope (Keyence VHX 500F) equipped with a high-precision VH mounting stand with an X–Y stage and bright-field illumination (Keyence VH-S5). For scanning electron microscope (SEM) analysis, FAA-fixed material was dehydrated in formaldehyde dimethyl acetal (FDA) for at least 24 h (Gerstberger and Leins, 1978) and critical-point dried, then mounted onto SEM stubs and sputter-coated using a sputter coater (Bal-Tec SCD 50, Balzers). Specimens were examined using an Auriga Zeiss TM SEM.
RESULTS
Pseudotaxus chienii: wild-type seed cones (Figs 1 and 2)
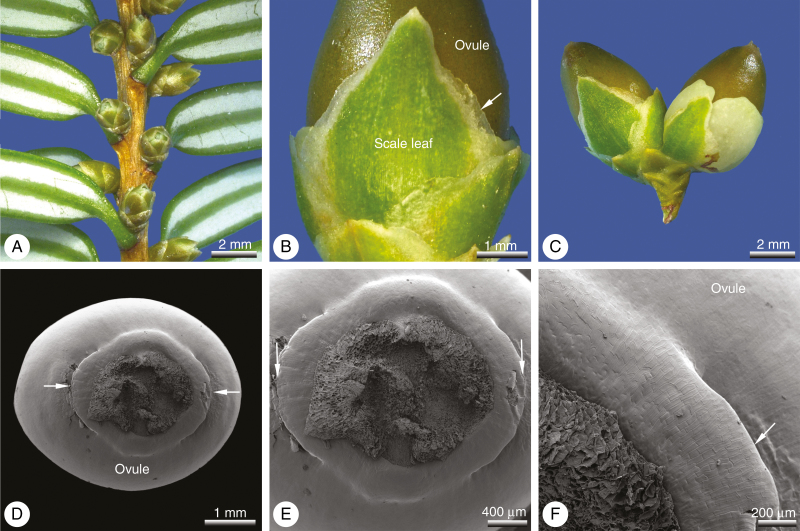
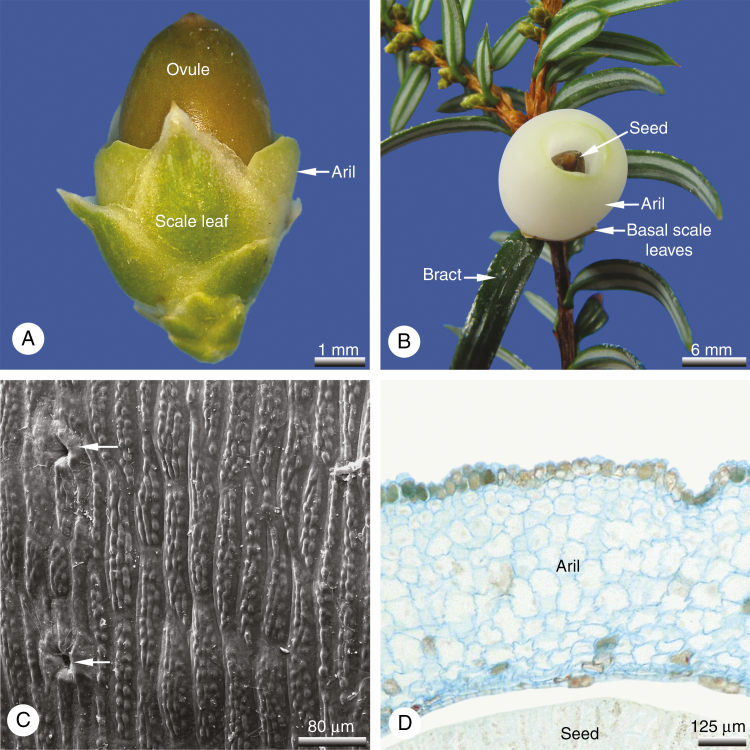
Seed cones develop on lateral shoots in the axil of a typical needle leaf, with a single seed cone per leaf (Figs 1A and 2B). At pollination time, the ovules and pollination drops are oriented in a strictly downward-facing position. Whereas typical foliage leaves on other branches are needle-like with a pointed tip and spiral arrangement (Fig. 1A), the leaves on the branchlet that bears the seed cones are scale-like and decussate. There are seven or eight pairs of such scale-like leaves per branchlet (Fig. 2A). Each leaf is keeled with a distinct hyaline margin (Fig. 1B) and supplied by a single collateral vascular strand. A single ovule is usually developed per seed cone (Fig. 2A), or exceptionally two (Fig. 1C). Two collateral vascular bundles supply the ovule. The aril develops relatively late and lacks vasculature entirely. At the time of pollination, the ovule is almost entirely surrounded by the decussate scale-like leaves; only the micropyle remains free (Fig. 1A). Aril formation commences after pollination with the initiation of two lateral primordia directly below the ovule, located in the transverse plane (Fig. 1D–F) and alternating with the lowermost pair of leaves. Subsequently, the broad bases of the two primordia become extended to form a ring primordium, which grows apically. Initially the ring primordium grows weakly; it first becomes visible externally ~2–3 months after pollination when the aril starts to exceed the surrounding leaves in length, though at this stage the aril is still green (Fig. 2A). After the aril has exceeded the leaves in length, it starts to grow more rapidly so that at maturity it is as long as (or sometimes longer than) the mature seed (Fig. 2B). During maturation, the tissues of the aril become strongly enlarged to form a succulent, fleshy, cup-like structure. The distal collar is roundish and unlobed. Its outer surface is strongly papillate, with cells arranged in vertical longitudinal rows (Fig. 2C). Some stomata are sunken in the outer epidermis of the aril (Fig. 2C). The inner tissue of the aril consists of fairly homogeneous isodiametric cells with thin walls and large vacuoles (Fig. 2D). The aril lacks vasculature entirely and remains unfused to the seed throughout ontogeny. At maturity, seeds are 7–8 mm long and 5–8 mm in diameter. The mature cup-like aril is ~8–10 mm long and 10–13 mm in diameter (Fig. 2B).
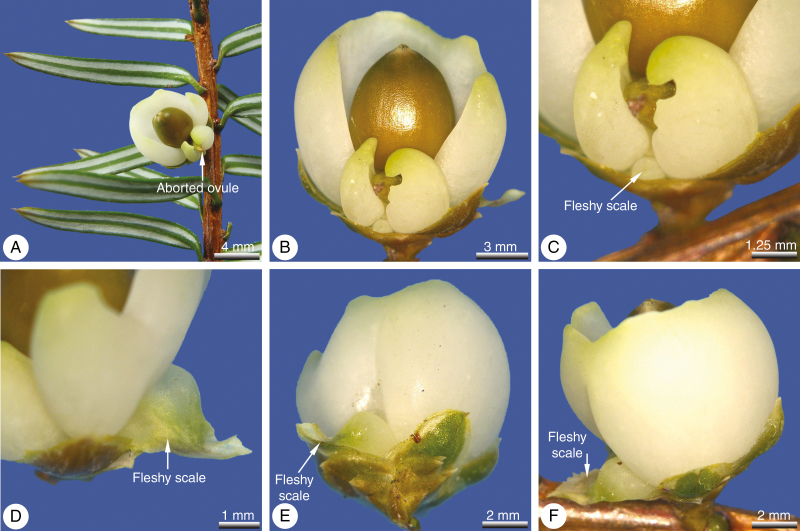
Fig. 1.
Pseudotaxus chienii, seed cones (wild-type). (A) Seed cones shortly before pollination. (B) Detail of decussate scale-like leaves that develop at the stalk of the seed cone and surround the juvenile ovule. (C) Seed cones with two ovules. (D) Young ovule from below; the aril is initiated by two lateral primordia (arrows) located in the transverse plane. (E) Detail of (D). (F) Detail of transitional zone between the transverse free aril initials (arrows) and the adjacent median part.
Fig. 2.
Pseudotaxus chienii, seed cone (wild-type). (A) Immature seed cone ~3 months after pollination; the aril is still green at this stage and starts to exceed the lower scale leaves. (B) Mature seed cone; seed surrounded by the fleshy, cup-like, white aril. (C) Outer epidermis of aril; cells axially oriented and with micropapillae on their surfaces; sparse sunken stomata (arrowed) are also present. (D) Transverse section of a mature aril showing tannin-filled epidermal cells surrounding parenchymatous internal tissue lacking vasculature.
Pseudotaxus chienii: teratological seed cones (Figs 3–6)
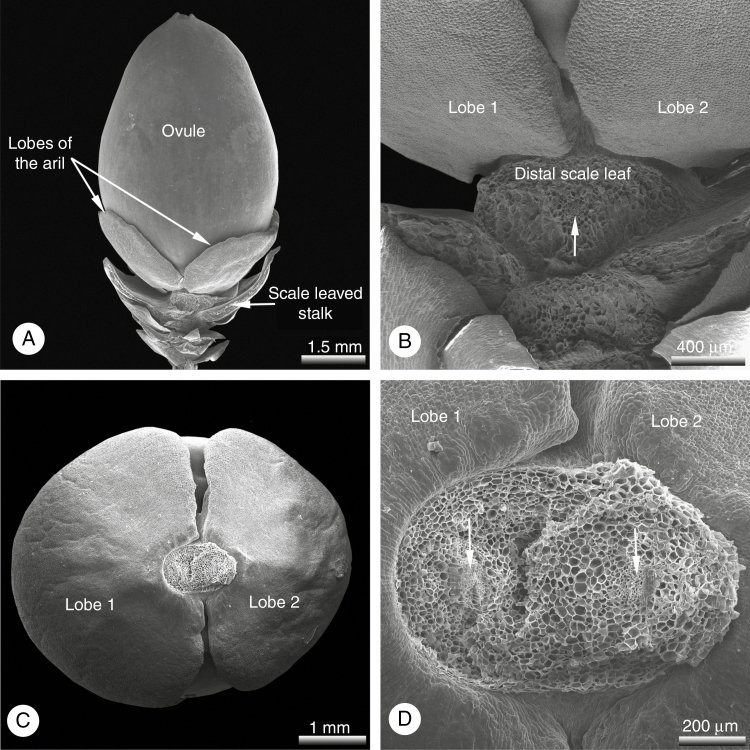
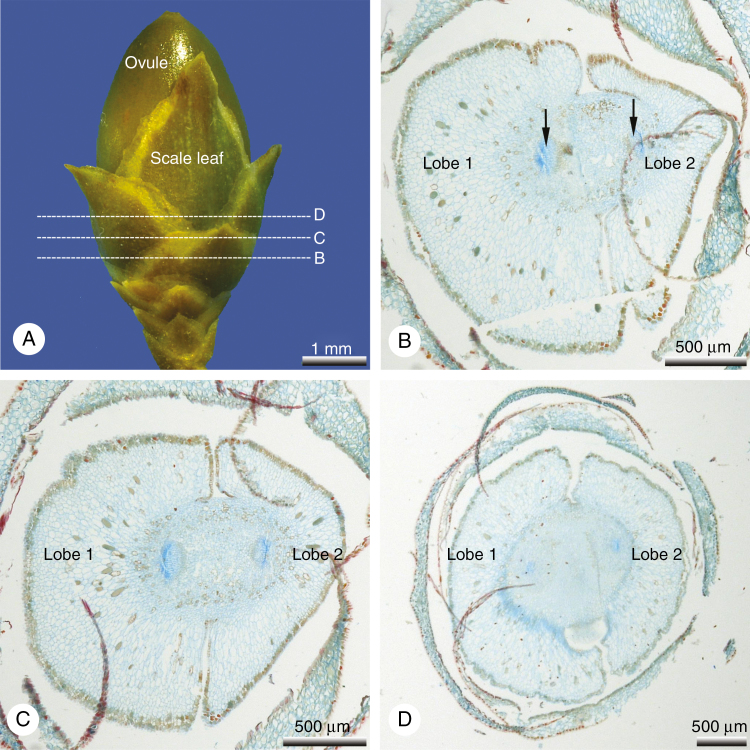
On one of the trees investigated, all the seed cones displayed an unusual strongly bilobed or entirely divided aril consisting of two free halves (Figs 3–6). In these teratological seed cones, the number and morpho-anatomical features of the scale-like leaves on the cone stalk resembled the wild-type. As in the wild-type, aril development commenced with two opposite primordia in the same plane, located directly below the ovule and precisely alternating with the distalmost pair of leaves. However, in these teratological seed cones the two lateral primordia did not fuse to each other (Figs 4 and 5) and never formed a ring primordium as in the wild-type; the two lobes remained independent from each other throughout their entire ontogeny, maintaining their initial location and orientation (Figs 3 and 4). As in the wild-type, the young lobes were green (Fig. 3B), maturing to white (Fig. 3C–F). At maturity, they represented an opposing pair of unfused distal lobes, which were as long as the seeds or slightly exceeded them in length (Fig. 3C–F); they still alternated with the distalmost pair of leaves on the stalk of the seed cone (Fig. 4A, B). The mature lobes were strongly swollen, fleshy (Fig. 3) and lacked vasculature entirely (Fig. 5).
Fig. 3.
Pseudotaxus chienii, mature teratological seed cone with a bilobed aril. (A) Branch with teratological seed cones. (B) Immature seed cone in top view; the two fleshy aril lobes (arrows) alternate with the lower pair of scale leaves. (C, D) Mature seed cone showing a bilobed aril in lateral view (C) and top view (D). (E) Mature seed surrounded by fleshy aril consisting of two separate, free lobes (lower stalk with scale leaves removed for a better overview). (F) Mature seed in top view; seed and aril not fused to each other.
Fig. 4.
Pseudotaxus chienii, SEM micrographs of a juvenile teratological seed cone shortly after pollination; the aril consists of two lateral free lobes located in the transverse plane (distal pair of decussate scale leaves removed for a better overview). (A) Lateral view of entire cone including stalk with scale-like leaves; the two lobes of the aril alternate with the lower scale-like leaves of the stalk. (B) Detail of (A); the lower scale leaves are supplied with a single collateral vascular bundle strand (arrow). (C) Young seed from below; the two transverse aril lobes are not fused to each other or to the ovule. (D) Detail of (C) showing funiculus with two vascular bundles supplying the ovule (arrows).
Fig. 5.
Pseudotaxus chienii, teratological seed cone with a bilobed aril ~3 months after pollination. (A) Ovule still enclosed by the basal pairs of scale leaves. (B–D) Series of transverse sections from basal to distal as marked in (A); the two lateral lobes of the aril are free and inserted at the same level below the ovule; the right lobe is slightly larger than the left one; the fleshy lobes are parenchymatous and lack vasculature; within the funiculus two vascular bundles (arrowed in B) supply the ovule.
Fig. 6.
Pseudotaxus chienii, mature teratological seed cone showing two ovules (rather than the more typical single ovule), one of them aborted. (A, B) Both ovules are surrounded by a fleshy, bilobed aril, consisting of two free lobes. (C–F) Below the ovules, several pairs of scale leaves are present; these are typically keeled and green, but some are strongly swollen and white, comparable with the lobes of the aril.
One of the teratological seed cones contained two ovules (Fig. 6) rather than the more typical single ovule, but one of the two ovules was aborted. The aril of each ovule consisted of two separate free lobes (Fig. 6A–C), which were identical to those described for the teratological seed cones above and illustrated in Figs 3–5. However, the free lobes of the aril were not the only fleshy structure within these seed cones; several of the lower (formerly scale-like) leaves inserted on the seed-cone stalk also became strongly swollen and fleshy, except at their hyaline margins, which remained membranaceous. In these teratological seed cones, the fleshy lobes of the aril alternated perfectly with the lowermost anomalously swollen pair of leaves (Fig. 6D–F).
Taxus baccata: wild-type seed cones (Fig. 7)
The position of seed cones within the branching pattern is similar to Pseudotaxus (Figs 1A and 2B). At pollination time, the seed cones and their pollination drops are oriented in a downward position (Fig. 7A). There are seven or eight pairs of decussate scale leaves inserted on the stalk of the seed cone (Fig. 7A, B). At pollination time, the scale leaves almost entirely surround the ovule, except the micropylar region (Fig. 7A). The aril develops relatively late, and is not visible externally at pollination time (Fig. 7A). It is initialized as two small lateral primordia directly below the ovule, which are located in the transverse plane, in an alternating arrangement with the lowermost pair of scale leaves (Fig. 7B, C). Subsequently the broad bases of the two primordia become extended, fuse to each other and form a ring primordium (Fig. 7D, E), which is characterized by apical growth. The ovule is supplied by two collateral vascular bundle strands, whereas the aril lacks vasculature entirely. The initial ring primordium is slow-growing and becomes visible externally ~2–3 months after pollination, when the aril starts to exceed the surrounding scale leaves in length (Fig. 7D). At this ontogenetic stage, the aril is still green and not fleshy (Fig. 7F). After the aril has exceeded the scale leaves in length, further development is rapid (Fig. 7E). Mature seeds are surrounded entirely or even exceeded by the aril, which becomes red and fleshy (Fig. 7F). The mature aril is a cup-like structure that remains unfused to the seed (Fig. 7F). All other features are similar to Pseudotaxus (Figs 1 and 2).
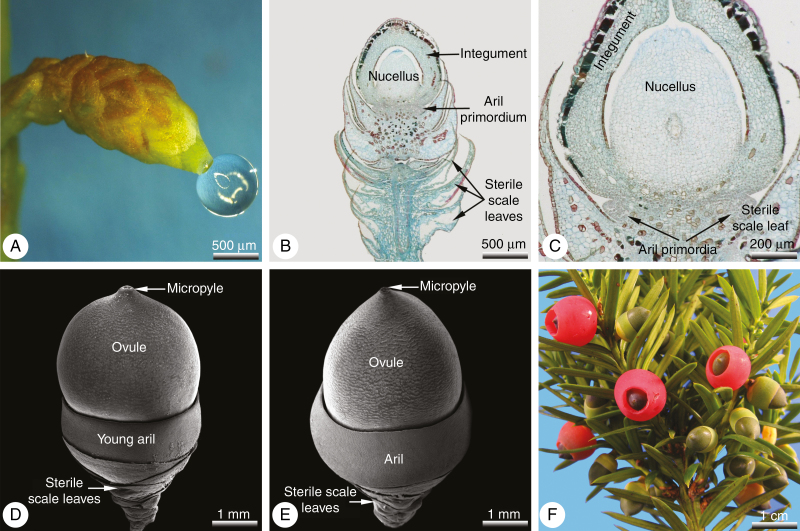
Fig. 7.
Taxus baccata, aril development. (A–C) Seed cones at pollination time. (A) Seed cone with pollination drop; no aril structures visible externally. (B) Longitudinal section of the seed cone illustrated in (A); the aril primordia located below the ovule develop at a late ontogenetic stage. (C) Detail of (B); ovule base showing aril primordia in the transverse plane. (D, E) After pollination the aril starts enclosing the seed; in wild-type seed cones it forms a cup-like structure with apical growth. (F) Lateral branch with seed cones in different ontogenetic stages: immature ones with a green aril, mature ones with a fleshy, red aril.
DISCUSSION
Comparison with other Taxaceae
Our data show that the wild-type aril of Pseudotaxus closely resembles that of Taxus in both structure and development. In both genera, the aril is green before turning white (in Pseudotaxus) or red (in Taxus). In both genera, aril initiation commences with formation of two lateral primordia in a transverse plane. The initials are located directly below the ovules and their tips are free at early stages, but later they form a ring primordium and ultimately a cup-shaped fleshy structure that is not fused to the ovule/seed (Mundry, 2000). Thus, in both Taxus and Pseudotaxus the aril could be interpreted as two fused, strongly swollen and fleshy scale leaves rather than as a modified integument, as other authors have also suggested for Taxus (e.g. Van Tieghem, 1869; early literature summarized by Dupler, 1920). Mature aril structure in the closely related genus Austrotaxus also resembles that of Taxus and Pseudotaxus (e.g. Florin, 1948a, b; Krüssmann, 1983; Bobrov et al., 2004), though detailed comparative ontogenetic studies are lacking. Based on comparative morphology, Florin (1948b) described the free upper collar in arils of Austrotaxus, Taxus and Pseudotaxus (treated as Nothotaxus) as their main similarity with the arils of Torreya and Amentotaxus, suggesting that the first three genera represent the more ‘primitive’ condition in Taxaceae.
In Torreya, aril development does not commence until the integument has already formed the micropyle. As in Taxus and Pseudotaxus, the aril is initiated by two lateral opposite primordia in the transverse plane, but the primordia become strongly extended into a ring primordium very early in ontogeny. Mundry (2000) demonstrated a clear correlation between the arrangement of the aril initials and the bract that bears the seed cone; the initials always develop in a transverse plane and the transition zone between the transverse primordia is located in the median plane. Thus, the juvenile Torreya aril is strongly bilobed; at its earliest ontogenetic stages it resembles Taxus and Pseudotaxus but differs considerably from them at later stages. Soon after pollination, the two free aril lobes exceed the distal micropyle and are pushed upwards to envelop the entire ovule. Subsequently, the aril becomes basally fused to the ovule and develops further by intercalary basal growth, so that at maturity the seed and aril are almost entirely fused to each other, only the micropylar parts remaining free. The outer surface of the mature aril is also characterized by elongated cells arranged in axial rows (Mundry, 2000).
In Cephalotaxus, aril development begins significantly later than in Taxus, Pseudotaxus, Austrotaxus and Torreya, so that the aril is invisible before fertilization. It is initiated as a ring primordium and is almost entirely congenitally fused to the ovuliferous integument; hence some authors have interpreted this structure as a fleshy seed coat (sarcotesta), comparable with the outer fleshy structure in seeds of cycads and Ginkgo (e.g. Vierhapper, 1910; Page, 1990; Contreras et al., 2017). However, in cycads and Ginkgo the maturing integuments differentiate into three layers (endotesta, sclerotesta and sarcotesta), which are initiated regularly over the entire integument (e.g. Coulter and Chamberlain, 1917; Obermann, 2003). Thus, the term ‘sarcotesta’ should be avoided in describing the fleshy outer layer of the Cephalotaxus seed, which is best interpreted as an aril. The ring primordium of Cephalotaxus shows distinct intercalary growth, so that the aril envelops the entire seed except for the distal micropylar region (Mundry, 2000). Cephalotaxus lacks the free distal tips as developed in Torreya, but the outer surface of the mature aril resembles that of Torreya (Mundry, 2000).
Keng (1969) described aril development in Amentotaxus as closely similar to that of Cephalotaxus; the ovule is embedded in the aril at an early stage. However, the earliest cones examined were already 3 mm long, so it remains undetermined whether the Amentotaxus aril is initiated from a ring primordium (as in Cephalotaxus) or two lateral primordia in the transverse plane that later form a ring primordium, as in Taxus, Pseudotaxus and Torreya. Subsequent aril development in Amentotaxus is characterized by fusion of the aril to the integument and intercalary growth (Ghimire and Heo, 2014). Ultimately, the aril is entirely fused to the seed except at the micropylar region (Krüssmann 1955, 1983; Keng 1969; Ghimire and Heo, 2014; Eckenwalder, 2009; Farjon, 2010), as in Cephalotaxus. However, vasculature is reduced within the seed cone and the ovule is supplied by a single bundle strand (Keng, 1969); in all other Taxaceae the ovule is supplied by two vascular bundles.
Thus, among extant Taxaceae, two generic groupings exist with respect to the structure and ontogeny of the fleshy structure (aril): (1) Austrotaxus–Pseudotaxus–Taxus (the APT group) and (2) Amentotaxus–Cephalotaxus–Torreya (the ACT group). The most striking differences between the two groups relate to fusion and growth (Table 1). The aril is strongly fused to the ovule in the ACT group, compared with a free cup-like structure in the APT group. Aril growth is intercalary in the ACT group compared with apical (distal) growth in the APT group. In the ACT group, the aril has several vertical intercellular spaces and a non-papillate epidermis, compared with a strongly papillate epidermis and internal tissues lacking intercellular spaces in the APT group. The strong initial distal growth of the free lobes in Torreya represents a marked difference not only with Amentotaxus and Cephalotaxus but also with all other Taxaceae.
Table 1.
Comparison of fleshy ovule/seed structures in genera of Taxaceae
| Fleshy structure (aril) | Austrotaxus, Pseudotaxus, Taxus (APT group) | Amentotaxus, Cephalotaxus, Torreya (ACT group) |
|---|---|---|
| Attachment | Aril free, cup-like (not strongly fused to the ovule) | Aril strongly fused to the ovule |
| Early development and growth | Development initiated by two lateral primordia with mostly apical growth; subsequently a ring primordium is formed (Austrotaxus unknown) | In Amentotaxus and Cephalotaxus, growth commences with a ring primordium, and is intercalary (though there are few data on Amentotaxus). In Torreya, an initial distal growth phase forms two free distal lobes; subsequently a ring primordium is formed, with an intercalary growth phase. |
| Epidermis | Papillate | Non-papillate |
| Anatomy | Internal tissues lacking large intercellular spaces | Internal tissues with several large, vertical intercellular spaces |
| Vasculature | Absent | In Torreya, two vascular strands are present; in Amentotaxus absent; in Cephalotaxus they are probably absent (based on images in Mundry, 2000) |
Conversely, the genera share some morpho-anatomical similarities. In five genera (Amentotaxus, Austrotaxus, Cephalotaxus, Pseudotaxus, Taxus) the micropyle remains visible externally even at maturity, but in Torreya it is soon enveloped by the two free distal lobes of the aril. In three genera (Pseudotaxus, Taxus, Torreya), aril development is initiated by two lateral primordia in the transverse plane before later forming a ring primordium, but in Cephalotaxus and Amentotaxus such distinct transverse initial lobes are not visible and aril development commences with a ring primordium (Table 1). No comparative data exist for Austrotaxus for this character, and further studies are desirable for aril initiation and ontogeny in Austrotaxus and early ontogeny in Amentotaxus.
Interpretation of the Pseudotaxus aril
The wild-type aril of Pseudotaxus originates at a relatively late stage from an abaxial ring primordium adjacent to the ovule, making it difficult to resolve its homologies based on typical seed cones. Thus, the teratological seed cones investigated here provide significant relevant data. In the numerous teratological seed cones examined, the aril was bilobed or entirely divided into two free lobes. In both wild-type and teratological seed cones, the aril initiates with two opposite primordia in the transverse plane, but in wild-type seed cones the two primordia rapidly fuse at their margins to form a ring primordium, even at early developmental stages. In contrast, in the teratological seed cones the two lateral primordia remain free throughout the entire maturation of the seed cone. In both wild-type and teratological seed cones, the two transverse aril primordia alternate with the distalmost pair of scale-like leaves inserted on the seed-cone stalk, and this alternation is maintained to maturity in mature teratological seed cones.
Thus, the two lobes of the aril could readily be interpreted as two opposite leaves that become strongly swollen and fleshy during development. The following data support this hypothesis: (1) the seeds and fleshy aril lobes are not fused to each other; (2) the two aril lobes are inserted below the ovule in the same plane; (3) the two fleshy aril lobes precisely alternate with the distalmost pair of scale-like leaves on the seed-cone stalk and therefore form part of the same organ series; (4) juvenile lobes are green and chlorophyll-rich before ultimately turning white. Finally, further convincing evidence (5) was provided by a teratological seed cone that formed additional fleshy structures below it, apart from the distal fleshy aril lobes (Fig. 6); our study shows that these additional fleshy structures are transformed leaves of the seed-cone stalk that have become unusually swollen, fleshy and white.
All these features combined support the hypothesis that the typical, cup-like aril of wild-type Pseudotaxus could have originated as two fused and strongly swollen leaves. Thus, an origin of the aril as an outgrowth of the seed scale, shoot axis, funiculus or integument is unlikely. These teratological data are congruent with the hypothesis of some previous authors (e.g. Taylor et al., 2009) that the taxaceous aril is derived from modified sterile scales at the base of the ovule-carrying short shoot. A remarkably similar condition occurs among other gymnosperms, in some species of the gnetalean genus Ephedra, in which the seeds are surrounded by several decussate fleshy and intensely coloured bracts (e.g. Stützel and Mundry, 2001; Huang et al., 2005; Rydin et al., 2010; Yang and Wang, 2013). These fleshy bracts are attractive to animals that disperse the seeds, such as frugivorous birds or seed-caching rodents (Ickert-Bond and Wojciechowski, 2004; Hollander and Vander Wall, 2009, 2010). Each pair of fleshy bracts is connate to a degree that is species-specific, with fusion of one- to two-thirds of their length (Huang et al., 2005), comparable with the bilobed arils in the teratological seed cones of Pseudotaxus investigated here (Figs 3–6).
Conversely, other factors could contradict this interpretation, including spatial constraints, the relatively late development of the aril, and the absence of vasculature. Spatial constraints during early aril development could result in the formation of two apparently distinct primordia. Initiation and subsequent development of the aril represent the final steps in seed-cone formation in this species, after all other structures are formed. If the aril represents a fusion product of the modified distal pair of scale leaves, as suggested here, then much earlier initiation would be expected, directly subsequent to the formation of the distal pair of scale leaves and not after a break of several weeks. One possibility is that aril initiation does takes place earlier, but long before the first initials become visible externally. Furthermore, while each scale leaf of the stalk is supplied with a single collateral vascular strand, the Pseudotaxus aril lacks vasculature entirely, as also in Taxus (Dupler, 1920; Mundry, 2000). However, the absence of aril vasculature in Pseudotaxus does not exclude derivation of the aril from fused scale leaves. In contrast, within Torreya, the aril is supplied with two vascular bundle strands, one at each lateral side (Vierhapper, 1910; Herzfeld, 1914), perhaps due to the relatively large size of the mature Torreya seeds compared with the small mature seeds of Taxus and Pseudotaxus.
Conclusions
Fleshy structures that promote biotic dispersal by ingestion (either as fleshy seeds or fleshy fruits) have evolved many times in seed plants. Examples include Ginkgo, cycads, Ephedra (Gnetales), some conifers (e.g. Juniperus, Podocarpus, Taxaceae) and angiosperms (e.g. fleshy-seeded Magnolia: Lovisetto et al., 2015) and some extinct seed plants such as some Cordaitales (e.g. Arber, 1914). The ‘fleshiness’ syndrome, in which the relevant structure becomes not only enlarged, soft and sweet-tasting but also colourful and attractive, encompasses a complex suite of changes in tissue texture and physiology and pigment synthesis. A suite of MADS-box genes is implicated in the development of fleshy fruits and seeds in both angiosperms and gymnosperms (e.g. Prasad et al., 2010; Lovisetto et al., 2012, 2013, 2015). Lovisetto et al. (2012) found that although AGAMOUS (AG) is widely expressed in both male and female structures in Taxus, AG expression is particularly localized to the young developing aril during later stages of seed development, and that other (AGL6 and TM8-like) MADS-box genes were also apparently involved. They hypothesized that this suite of genes represents a basic genetic toolkit that is common to all fleshy structures, regardless of their morpho-anatomical origin (Lovisetto et al., 2015).
The hypothesis that iterative gene co-option results in iterative evolution of anatomically similar but morphologically non-homologous structures accords with the structural diversity of the fleshy seed structures within conifer families such as Taxaceae and Podocarpaceae, in which the fleshy region is variously interpreted as an aril, epimatium or sarcotesta. Our investigations of the cup-like aril of Pseudotaxus demonstrate a similarity not only with other Taxaceae but also with some other extant conifers, such as Phyllocladus (Podocarpaceae s.l.), in which development of the fleshy structure associated with the seed shares several similarities with Taxus and Pseudotaxus, despite their relatively distant relationship. As in some Taxaceae, the aril of Phyllocladus is free and not fused to the seed. As in Pseudotaxus, aril development is relatively late in Phyllocladus glaucus, initially as an outgrowth at the ovule base on its lateral margins, but rapidly forming a ring primordium, leading to a closed collar and finally a cup-like aril with the two lateral initials visible as distinct lobes on the distal collar (Tomlinson and Takaso, 1989). Vascularization of the ovule in Phyllocladus also resembles most Taxaceae (except Amentotaxus), with two vascular strands. Thus, fleshy structures in gymnosperms could well be highly homoplastic. Recent studies on gene expression in conifers and other living gymnosperms are contributing to the debate on their homologies, but more comparative data are needed to untangle the diverse range of structures and development in these taxa. Our comparative investigation of teratological seed cones in Pseudotaxus strongly indicates that the typical aril of Taxaceae could be interpreted not as a modified integument but as a fused pair of strongly swollen leaves.
ACKNOWLEDGEMENTS
We thank Michael Laumann and Paavo Bergmann (Electron Microscopy Center, Department of Biology, University of Konstanz, Germany) for technical support (SEM and paraffin technique).
LITERATURE CITED
- Arber EAN. 1914. A revision of the seed impressions of the British coal measures. Annals of Botany 28: 81–108. [Google Scholar]
- Bobrov AVFCH, Melikan AP, Romanov MS, Sorokin AN. 2004. Seed morphology and anatomy of Austrotaxus spicata (Taxaceae) and its systematic position. Botanical Journal of the Linnean Society 145: 437–443. [Google Scholar]
- Buchholz JT, Gray NE. 1948. A taxonomic revision of Podocarpus: I. The sections of the genus and their subdivisions with special reference to leaf anatomy. Journal of the Arnold Arboretum 29: 49–63. [Google Scholar]
- Burleigh JG, Mathews S. 2004. Phylogenetic signal in nucleotide data from seed plants: implications for resolving the seed plant tree of life. American Journal of Botany 91: 1599–1613. [DOI] [PubMed] [Google Scholar]
- Chaw SM, Parkinson CL, Cheng Y, Vincent TM, Palmer JD. 2000. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proceedings of the National Academy of Sciences of the USA 97: 4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Nicolson RG, Tripp K, Chaw SM. 2000. Phylogeny of Taxaceae and Cephalotaxaceae genera inferred from chloroplast matK gene and nuclear rDNA ITS region. Molecular Phylogenetics and Evolution 14: 353–365. [DOI] [PubMed] [Google Scholar]
- Contreras DL, Duijnstee IAP, Ranks S, Marshall CR, Looy CV. 2017. Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Perspectives in Plant Ecology, Evolution and Systematics 74: 93–117. [Google Scholar]
- Coulter JM, Chamberlain CJ. 1917. Morphology of gymnosperms, 2nd edn. Chicago: University of Chicago Press; (reprinted 1982). [Google Scholar]
- Dörken VM, Hetzel I. 2017. Taxus baccata – Europäische Eibe (Taxaceae). Jahrbuch Bochumer Bototanischer Verein 8: 298–305. [Google Scholar]
- Dörken VM, Zhang ZX, Mundry IB, Stützel T. 2011. Morphology and anatomy of male reproductive structures in Pseudotaxus chienii (W.C. Cheng) W.C. Cheng (Taxaceae). Flora 206: 444–450. [Google Scholar]
- Dupler AW. 1920. Ovuliferous structures of Taxus canadensis. Botanical Gazette 69: 492–520 [Google Scholar]
- Eckenwalder JE. 2009. Conifers of the world. Portland: Timber Press. [Google Scholar]
- Englund M, Carlsbecker A, Engstrom P, Vergara-Silva F. 2011. Morphological “primary homology” and expression of AG-subfamily MADS-box genes in pines, podocarps, and yews. Evolution and Development 13: 171–181. [DOI] [PubMed] [Google Scholar]
- Farjon A. 2007. A natural history of conifers. Portland: Timber Press. [Google Scholar]
- Farjon A. 2010. A handbook of the world’s conifers, Vol. 1 Leiden: Brill. [Google Scholar]
- Farjon A, Ortiz-Garcia S. 2003. Cone and ovule development in Cunninghamia and Taiwania (Cupressaceae sensu lato) and its significance for conifer evolution. American Journal of Botany 90: 8–16. [DOI] [PubMed] [Google Scholar]
- Florin R. 1948a On Nothotaxus, a new genus of the Taxaceae, from Eastern China. Acta Horti Bergiani 110: 385–395. [Google Scholar]
- Florin R. 1948b On the morphology and relationships of the Taxaceae. Botanical Gazette 14: 31–39. [Google Scholar]
- Florin R. 1951. Evolution in cordaites and conifers. Acta Horti Bergiani 15: 285–388. [Google Scholar]
- Florin R. 1954. The female reproductive organs of conifers and taxads. Biological Reviews 29: 367–389. [Google Scholar]
- Gerlach D. 1984. Botanische Mikrotomtechnik, eine Einführung, 2nd edn. Stuttgart: Thieme. [Google Scholar]
- Gerstberger P, Leins P. 1978. Rasterelektronenmikroskopische Untersuchungen an Blütenknospen von Physalis philadelphia (Solanaceae). Berichte der Deutschen Botanischen Gesellschaft 91: 381–387. [Google Scholar]
- Ghimire B, Heo K. 2014. Cladistic analysis of Taxaceae s.l. Plant Systematics and Evolution 300: 217–223. [Google Scholar]
- Ghimire B, Lee C, Heo K. 2014. Leaf anatomy and its implication for phylogenetic relationships in Taxaceae s.l. Journal of Plant Research 127: 373–388. [DOI] [PubMed] [Google Scholar]
- Harris TM. 1976. The Mesozoic gymnosperms. Review of Palaeobotany and Palynology 21: 119–134. [Google Scholar]
- Herzfeld S. 1914. Die weibliche Koniferenblüte. Österreichische Botanische Zeitschrift 64: 321–358. [Google Scholar]
- Hollander JL, Vander Wall S. 2009. Dispersal syndromes in North American Ephedra. International Journal of Plant Science 170: 323–330. [Google Scholar]
- Hollander JL, Vander Wall S. 2010. Evolution of dispersal in North American Ephedra. Evolutionary Ecology 24: 333–345. [Google Scholar]
- Huang J, Giannasi DE, Price RA. 2005. Phylogenetic relationships in Ephedra (Ephedraceae) inferred from chloroplast and nuclear DNA sequences. Molecular Phylogenetics and Evolution 35: 48–59. [DOI] [PubMed] [Google Scholar]
- Ickert-Bond SM, Wojciechowski MF. 2004. Phylogenetic relationships in Ephedra (Gnetales): evidence from nuclear and chloroplast DNA sequence data. Systematic Botany 29: 834–849. [Google Scholar]
- Jagel A, Dörken VM. 2014. Morphology and morphogenesis of the seed cones of the Cupressaceae, I: Cunninghamioideae, Athrotaxioideae, Taiwanioideae, Sequoioideae, Taxodioideae. Bulletin of the Cupressus Conservation Project 3: 99–121. [Google Scholar]
- Jagel A, Dörken VM. 2015a Morphology and morphogenesis of the seed cones of the Cupressaceae, II: Cupressoideae. Bulletin of the Cupressus Conservation Project 4: 51–78. [Google Scholar]
- Jagel A, Dörken VM. 2015b Morphology and morphogenesis of the seed cones of the Cupressaceae, III: Callitroideae. Bulletin of the Cupressus Conservation Project 4: 91–108. [Google Scholar]
- Keng H. 1969. Aspects of morphology of Amentotaxus formosana with a note on the taxonomic position of the genus. Journal of the Arnold Arboretum 50: 432–448. [Google Scholar]
- Krüssmann G. 1955. Handbuch der Nadelgehölze, 1st edn. Hamburg: Parey. [Google Scholar]
- Krüssmann G. 1983. Handbuch der Nadelgehölze, 2nd edn. Hamburg: Parey. [Google Scholar]
- Lang XD, Su JR, Lu SG, Zhang ZJ. 2013. A taxonomic revision of the genus Cephalotaxus (Taxaceae). Phytotaxa 84: 1–24. [Google Scholar]
- Lovisetto A, Guzzo F, Tadiello A, Toffali K, Favretto A, Casadoro G. 2012. Molecular analyses of MADS-box genes trace back to gymnosperms the invention of fleshy fruits. Molecular Biology and Evolution 29: 409–419. [DOI] [PubMed] [Google Scholar]
- Lovisetto A, Guzzo F, Busatto N, Casadoro G. 2013. Annals of Botany 112: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovisetto A, Masiero S, Rahim MA, Mendes MAM, Casadoro G. 2015. Fleshy seeds form in the basal angiosperm Magnolia grandiflora and several MADS-box genes are expressed as fleshy seed tissues develop. Evolution and Development 17: 82–91. [DOI] [PubMed] [Google Scholar]
- Meyen SV. 1987. Fundamentals of palaeobotany. London: Chapman and Hall. [Google Scholar]
- Mundry I. 2000. Morphologische und morphogenetische Untersuchungen zur Evolution der Gymnospermen. Bibliotheca Botanica 152. [Google Scholar]
- Mundry I, Mundry M. 2001. Male cones in Taxaceae s.l. - an example of Wettstein’s pseudanthium concept. Plant Biology 3: 405–416. [Google Scholar]
- Obermann J. 2003. Vergleichende Untersuchungen an den Samenanlagen spermatozoidbefruchteter Gymnospermen mit REM und Anatomie unter Berücksichtigung fossiler Strukturen. Diploma Thesis, Ruhr University Bochum, Germany. [Google Scholar]
- Page CN. 1990. Gymnosperms. In: Kubitzki K. ed. The families and genera of vascular plants, Vol. 1 Berlin: Springer. [Google Scholar]
- Prasad K, Zhang X, Tobon E, Ambrose BA. 2010. The Arabidopsis B-sister MADS-box protein, GORDITA, represses fruit growth and contributes to integument development. Plant Journal 62: 203–214 [DOI] [PubMed] [Google Scholar]
- Quinn CJ, Price RA, Gadek PA. 2002. Familial concept and relationships in the conifers based on rbcL and matK sequence comparisons. Kew Bulletin 57: 513–531. [Google Scholar]
- Rydin C, Khodabandeh A, Endress PK. 2010. The female reproductive unit of Ephedra (Gnetales): comparative morphology and evolutionary perspectives. Botanical Journal of the Linnean Society 163: 387–430. [DOI] [PubMed] [Google Scholar]
- Stevens PF. 2001. Angiosperm Phylogeny Website. Version 14, 2017. http://www.mobot.org/MOBOT/research/APweb. Accessed 3 January 2018. [Google Scholar]
- Stützel T, Mundry I. 2001. Gnetidae: Ihre Blüten und die Verwandtschaft zu den Blütenpflanzen. Palmengarten 64: 109–116. [Google Scholar]
- Stützel T, Röwekamp I. 1999. Female reproductive structures in Taxales. Flora 194: 145–157. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. 2009. Palaeobotany: the biology and evolution of fossil plants, 2nd edn. Burlington, MA: Academic Press, Elsevier. [Google Scholar]
- Tomlinson PB. 1992. Aspects of cone morphology and development in Podocarpaceae (Coniferales). International Journal of Plant Sciences 153: 572–588. [Google Scholar]
- Tomlinson PB, Takaso T. 1989. Cone and ovule ontogeny in Phyllocladus (Podocarpaceae). Botanical Journal of the Linnean Society 99: 209–221. [Google Scholar]
- Tomlinson PB, Takaso T. 2002. Seed cone structure in conifers in relation to development and pollination: a biological approach. Canadian Journal of Botany 80: 1250–1273. [Google Scholar]
- Van Tieghem PH. 1869. Anatomie comparee de la fleur femelle et du fruit des cycadées, des conifères, et des gnetacées. Annales des sciences naturelles, Botanique, series V 10: 269–304. [Google Scholar]
- Vázquez-Lobo A, Carlsbecker A, Vergara-Silva F, Alvarez-Buylla ER, Piñero D, Engström P. 2007. Characterization of the expression patterns of LEAFY/FLORICAULA and NEEDLY orthologs in female and male cones of the conifer genera Picea, Podocarpus, and Taxus: implications for current evo-devo hypotheses for gymnosperms. Evolution & Development 9: 446–459. [DOI] [PubMed] [Google Scholar]
- Vierhapper F. 1910. Entwurf eines neuen Systems der Coniferen. Abhandlungen der Kaiserlich-Königlichen Zoologisch-botanischen Gesellschaft in Wien 5: 1–52. [Google Scholar]
- Wilde MH. 1944. A new interpretation of coniferous cones: I. Podocarpaceae (Podocarpus). Annals of Botany 8: 1–41. [Google Scholar]
- Wilde MH. 1975. A new interpretation of microsporangiate cones in Cephalotaxaceae and Taxaceae. Phytomorphology 25: 434–450. [Google Scholar]
- Yang Y, Wang Q. 2013. The earliest fleshy cone of Ephedra from the Early Cretaceous Yixian Formation of Northeast China. PLoS One 8: e53652. [DOI] [PMC free article] [PubMed] [Google Scholar]