Abstract
Background and Aims
Intraspecific trait variation (ITV) is an important dimension of plant ecological diversity, particularly in agroecosystems, where phenotypic ITV (within crop genotypes) is an important correlate of key agroecosystem processes including yield. There are few studies that have evaluated whether plants of the same genotype vary along well-defined axes of biological variation, such as the leaf economics spectrum (LES). There is even less information disentangling environmental and ontogenetic determinants of crop ITV along an intraspecific LES, and whether or not a plant’s position along an intraspecific LES is correlated with reproductive output.
Methods
We sought to capture the extent of phenotypic ITV within a single cultivar of soy (Glycine max) – the world’s most commonly cultivated legume – using a data set of nine leaf traits measured on 402 leaves, sampled from 134 plants in both agroforestry and monoculture management systems, across three distinct whole-plant ontogenetic stages (while holding leaf age and canopy position stable).
Key Results
Leaf traits covaried strongly along an intraspecific LES, in patterns that were largely statistically indistinguishable from the ‘universal LES’ observed across non-domesticated plants. Whole-plant ontogenetic stage explained the highest proportion of phenotypic ITV in LES traits, with plants progressively expressing more ‘resource-conservative’ LES syndromes throughout development. Within ontogenetic stages, leaf traits differed systematically across management systems, with plants growing in monoculture expressing more ‘resource-conservative’ trait syndromes: trends largely owing to an approximately ≥50% increases in leaf mass per area (LMA) in high-light monoculture vs. shaded agroforestry systems. Certain traits, particularly LMA, leaf area and maximum photosynthetic rates, correlated closely with plant-level reproductive output.
Conclusions
Phenotypic ITV in soy is governed by constraints in trait trade-offs along an intraspecific LES, which in turn (1) underpins plant responses to managed environmental gradients, and (2) reflects shifts in plant functional biology and resource allocation that occur throughout whole-plant ontogeny.
Keywords: Agroecology, functional traits, Glycine max, intraspecific trait variation, leaf economics spectrum, leaf mass per area, leaf trait, photosynthesis
INTRODUCTION
Understanding differences in ecological strategies among plant species has emerged as a critical means by which ecologists test hypotheses on mechanisms of species coexistence (e.g. Kraft et al., 2015), and understand the mechanistic linkages and feedbacks between biodiversity and ecosystem functioning (Lavorel and Garnier, 2002; Cadotte et al., 2011). Considerable evidence now exists indicating that certain suites of functional traits can be used to directly describe ecological variation among plants (e.g. Reich et al., 1997; Westoby, 1998; Westoby et al., 2002; Wright et al., 2004; Diaz et al., 2016). Arguably the most commonly evaluated plant functional traits are those forming the ‘leaf economics spectrum’ (LES): a suite of covarying leaf functional characteristics that cumulatively differentiate plants along a continuous axis of ecological differences, from short-lived resource-acquiring plants on one end with low leaf mass per area (LMA), high photosynthetic assimilation rates (A) and high leaf nitrogen (N) concentrations, to long-lived resource-conserving species with the opposite suite of traits on the other (Wright et al., 2004). Even when considering a broader suite of traits, species differences along the LES remain as one of the primary defining axes of ecological variation among plants worldwide (Diaz et al., 2016).
While the vast majority of research on leaf traits focuses on differences among species, there has been increasing interest in understanding patterns and consequences of intraspecific trait variation (ITV) (Albert et al., 2010a, b, 2011; Messier et al., 2010; Siefert et al., 2015). Ecological research broadly categorizes ITV as differences in trait expression within a species (among either conspecific individuals or populations), which may be owing to genotypic variability among plants, or phenotypic responses to local environmental conditions (e.g. Moran et al., 2016). There is now increasing evidence to indicate that individual plants vary considerably along an ‘intraspecific LES’ due to phenotypic and/or genotypic variation (Blonder et al., 2013; Hu et al., 2015; Niinemets, 2015; Martin et al., 2017), and this ITV has important consequences for ecosystem functioning including plant decomposition, reproduction and plant responses to above- and below-ground environmental change (e.g. Lecerf and Chauvet, 2008; Jung et al., 2014; Gagliardi et al., 2015; Isaac et al., 2017).
Exploring patterns of intraspecific variation in the LES traits of crops has recently emerged as a critical application of trait-based ecology (Martin and Isaac, 2015, 2018; Milla et al., 2015). From an agroecosystem functioning perspective, intraspecific variation in crop LES traits, either within or across cultivars, (1) modulates plant responses to above- and below-ground environmental gradients (Isaac et al., 2017); (2) is empirically linked with crop yield (Gagliardi et al., 2015); (3) is a key input into process-based models of agricultural yield (Bouman and van Laar, 2006; Martin et al., 2018); and (4) in certain systems forms the basis of local knowledge used to inform management decisions (Isaac et al., 2018). Moreover, variation along an intraspecific LES in crops has provided evidence on the ecological consequences of plant breeding (Milla et al., 2015; Martin et al., 2017).
Nevertheless there is still a paucity of research assessing patterns of intragenotype LES trait variation within the world’s most common crops (Martin and Isaac, 2015). For example, N2 fixation is a key attribute in many (but not all) of the world’s most widespread leguminous crops, yet there are no studies evaluating how this characteristic might influence patterns of intragenotype LES variation. Research on non-domesticated plant species indicates that legumes may deviate from a wider LES, and, in particular, express weaker relationships between certain traits such as A and leaf N concentrations (Adams et al., 2016). However, to our knowledge, no studies have assessed patterns or consequences of LES traits in the world’s most common leguminous crops. In particular, soybean [Glycine max (L.) Merr.] – the focus of this present study – currently covers >100 Mha of agricultural land globally, making it the world’s fourth most widespread crop, and the world’s most abundant domesticated N2-fixing species (Martin and Isaac, 2015). Although many independent studies have evaluated ITV for one or a few individual traits, there are no studies to date evaluating if and why soy varies along a prospective within-genotype intraspecific LES.
Decades of intensive selection would suggest that soy has been shifted towards the extreme ‘resource-acquiring’ end of the LES, probably expressing among the highest values of A and leaf N, and low LMA observed across a global species pool (Milla et al., 2015). However, while this might be the case broadly, ITV along the LES within a given genotype is also likely to be governed by key managed environmental gradients such as light and temperature, which may lead to soy covering a broad spectrum of the LES (e.g. Campbell et al., 1990). Spatial or temporal heterogeneity in soil nutrients is also expected to drive intraspecific variation in crop LES traits within genotypes, yet these relationships may be more variable in soy due to differences in rates of N2 fixation across managed environmental gradients (Isaac et al., 2014; Nasielski et al., 2015).
It is also likely that the position of soy leaves along the LES will shift during the course of leaf and whole-plant development. As leaves age, reductions in photosynthetic rate are often associated with changes in LMA and especially leaf N; such patterns have been observed in multiple plant species, including soy (Reich et al., 1986, 1991). Furthermore, although much less studied, as plants enter into reproductive stages later in the growing season, greater resource allocation to N-intensive reproductive structures (including pods and seeds) may contribute to a plant progressively expressing more resource-conservative LES traits, notably reductions in leaf N and in turn A. Such ‘whole-plant ontogenetic effects’ associated with reproductive allocation (i.e. changes in traits that are detected when controlling for leaf age and canopy position) have been observed in some domesticated species and trees (Steppe et al., 2011; Mason et al., 2013; Martin and Thomas, 2013; Sendall and Reich, 2013), but to our knowledge this has not been evaluated in any of the world’s most common crops.
Our study seeks to address some of these expectations by evaluating phenotypic dimensions of ITV in soy within a single genotype, in order to address the following research questions. (1) Do soy plants of the same genotype vary along an intraspecific LES? If so, then (2) do soy LES traits covary with one another in patterns that are similar to the LES observed across non-domesticated plant species? (3) Is the position of soy plants along an intraspecific LES predominantly explained by managed environmental gradients, whole-plant ontogenetic stage and/or N2 fixation? Finally, we ask (4) is ITV in soy along the LES correlated with plant-level yield?
MATERIALS AND METHODS
Experimental site
Our study was based at the University of Guelph Agroforestry research site located in Guelph, Canada (43°32ʹ28ʺN, 80°12ʹ32ʺW, 334 m a.s.l.), a 30 ha experimental farm established in 1987. Soils at the site are described as Grey Brown Luvisol with a sandy loam texture (but see Oelbermann and Voroney, 2007 for detailed soil analyses). The site experiences average weekly minimum and maximum temperatures of 6.0 and 27.3 °C, respectively, and mean monthly rainfall of 78.3 mm (Furze et al., 2017).
At the site, three different major crop species including soybean (G. max), corn (Zea mays L.) and winter wheat (Triticum aestivum L.) are planted on annual rotations in both monoculture and agroforestry management systems. The agroforestry management treatments entail intercropping one of the aforementioned crops with varying combinations of ten deciduous and coniferous tree species, with trees planted 3–6 m apart within 800 m long rows and with 12.5–15 m spacing between rows (Thevathasan and Gordon, 2004; Reynolds et al., 2007). The monoculture site is located immediately adjacent (within 300 m) to the east of the agroforestry site, and receives the same no-till cultivation, zero fertilization, rain-fed irrigation and crop rotation regimes as the agroforestry systems (Furze et al., 2017). In May 2017, G. max (variety: Pioneer P90Y90) was planted in both management systems in crop rows spaced 18 cm apart, and at densities of approx. 450 000 seeds ha–1 (Furze et al., 2017).
Plant and leaf trait sampling
Individual soy plants were sampled for leaf traits in a total of three primary management systems (two of which are further sub-divided below): (1) monoculture; (2) agroforestry bordered with hybrid poplar (Populus tremuloides Michx. × P. nigra L. clone DNN177); and (3) agroforestry bordered with spruce [Picea abies (L.) H. Karst.]. In the monoculture site, we established three 3 m2 sample ‘blocks’, which were spaced 25 m apart to ensure spatial interspersion of samples. Within each of the agroforestry management systems, six 3 m2 sample blocks were established at the same spacing, three of which were located at 2 m from the treeline (hereafter, ‘treeline’ management conditions), while the remaining three blocks were paired with treeline blocks, but situated 5–6 m into the crop row (hereafter, ‘crop row’ management conditions). Pairing treeline and crop row blocks within the agroforestry system was done in order to capture the effects of tree competition on soybean leaf functional traits (Reynolds et al., 2007).
Within each block, three individual soybean plants were identified that were free of major damage, vigorous and healthy in appearance. For each selected plant, traits were sampled on three leaves (detailed below). This sampling schedule was replicated at three different soybean plant developmental stages: (1) late vegetative growth (V6, defined as soy plants with six nodes on the main stem with fully developed leaves; 10–13 July 2017); (2) pod production (R3–R4, defined as soy plants bearing pods at one of the four uppermost nodes, which are 0.5–2cm in length; 31 July–4 August 2017); and (3) late pod filling (R6, defined by soy plants with a fully developed leaf at one of the four uppermost nodes which bears a leaf pod containing a green seed that fills the pod cavity; 21–24 August 2017) (Fehr et al., 1971). These stages were chosen to coincide approximately with key ontogenetic factors that might influence leaf traits including the amount of N2 fixation and reproductive onset. In sum, our study design resulted in traits being measured according to the following nested design: traits from n = 402 leaves measured on n = 134 soy plants, situated in n = 15 different sampling blocks located within n = 5 management conditions, which were then sampled at n = 3 different plant developmental stages.
Soybean leaf functional traits, nodules and reproduction
Leaves were selected in order to best control for the potentially confounding influence of individual leaf age on trait values (Mason et al., 2013). Therefore all soy leaf traits were measured on recently matured, fully expanded, terminal trifoliate leaflets that were sampled from the upper 25 % of individual plant canopies (Cornelissen et al., 2003; Perez-Harguindeguy et al., 2013). For each leaf, we first measured the maximum photosynthetic rate on an area basis (Aarea, μmol CO2 m–2 s–1) in the field using a Li-Cor XT 6400 CO2 portable gas analyser (Li-Cor Biosciences, Lincoln, NE, USA) that was equipped with a red/blue light source (6000-02B Red-Blue SI-0951). All Aarea measurements were taken between 8.00 and 14.00 h, at saturating photosynthetic photon flux densities of 1500 μmol m–2 s–1, a CO2 concentration of 400 ppm, intercellular CO2 partial pressures >200 μmol CO2 mol–1 air, relative humidity between 50 and 80 %, and leaf temperatures of 25–30 °C. After leaf fluxes were allowed to stabilize to these conditions, Aarea measurements were taken as the mean value of three replicate measurements at 20 s intervals. We used these same gas flux measurements also to calculate stomatal conductance (gs, mol H2O m–2 s–1) and instantaneous water use efficiency (WUE, mmol CO2 mol H2O–1).
Following gas exchange, we then collected each leaf, excavated each whole plant by hand to the most distal fine roots possible and transported all plant material to the University of Toronto Scarborough, Canada for analysis of morphological and chemical traits, as well as root nodule and reproduction analyses. First, leaf area was calculated by scanning each leaf and analysing images using ImageJ software (Abramoff et al., 2004). Fresh leaves were then dried at 65 °C for 48 h and weighed, and LMA (g m–2) was calculated as leaf dry mass divided by leaf area; LMA was then used to derive mass-based photosynthetic rates (Amass, mmol CO2 g–1 s–1). Dried leaves were ground into a homogeneous powder using a Retsch ball Mill (Retsch Ltd, Haan, Germany), and 0.01–0.1 g of leaf tissue was weighed and analysed for leaf C and N concentrations (% mass basis) using a CN 628 elemental analyzer (LECO Instruments, Ontario, Canada).
Following leaf trait analyses, we then washed and rinsed the excavated root masses, and counted and weighed all nodules on each plant. Evaluating relationships between soy reproduction and leaf traits is challenging, since the complete maturation of pods and seeds occurs after (at least partial) leaf senescence (Fehr et al., 1971). Therefore, we focused our analysis of trait–reproduction relationships at the late pod filling stage where initial seed formation, but not complete pod filling, is accomplished (Fehr et al., 1971). For each plant sampled at our third time period, the total number of pods and seeds were counted on each excavated plant.
Environmental data
Upon harvesting of each soy plant, approx. 20 g of soil was collected from the entire area immediately surrounding the plant roots (to the depth of the deepest roots) using a trowel, placed in polyethylene bags, frozen and transported to the University of Toronto Scarborough where they were thawed and measured for gravimetric soil moisture content, as well as plant-available inorganic N. All soil measurements were performed in the same facility, and following the same methodologies, as those employed by Gagliardi et al. (2015). Light environment was quantified as canopy openness at the block level, using digital hemispherical photography with a Nikon Coolpix 950 digital camera affixed with a FC-E8 fisheye lens (Nikon, Tokyo, Japan). All images were taken between 08.00 and 10.00 h under overcast conditions to avoid light scatter, and images were analysed for the percentage canopy openness using Gap Light Analyzer v. 2.0 (Frazer et al., 1999).
Statistical analysis
Describing ITV.
All data analysis was conducted using R v. 3.3.2 (R Foundations for Statistical Computing, Vienna, Austria). To describe overall ITV in soybean LES traits (as well as nodule and reproduction data), we used the ‘fitdist’ R package (Delignette-Muller and Dutang, 2015) to fit, compare and describe ITV as either normal or log-normal distributions based on log-likelihood scores. If traits were better described by log-normal distributions, we employed median and median absolute deviation (mad) values to describe central tendency and variation about the mean, respectively, and calculated quartile-based coefficients of variation (CVs) for these traits as mad/median; otherwise, standard parametric descriptive statistics were calculated. Based on this procedure, log-transformed values of LMA, leaf area, WUE and gs were used for all subsequent analyses. This same procedure was then performed on environmental variables.
An intraspecific LES.
We used the ‘smatr’ R package (Warton et al., 2012) to perform a series of standardized major axis (SMA) regression analyses among all pairwise leaf trait combinations. To evaluate how patterns of LES trait covariation in soy compare with the same relationships observed among wild species, we also focused in more detail on pairwise relationships that are key in the LES, namely those among Amass, LMA and leaf N. These analyses then entailed comparing the slope of the SMA relationship observed between these traits in soy with the same relationships observed in the GLOPNET data set populated by ‘wild species’ trait values, that was used to derive the initial LES hypothesis (Table 1 in Wright et al., 2004). For these comparisons of slopes, we used log-transformed values of all traits in both the soy and GLOPNET data sets.
Table 1.
Intraspecific variation in leaf traits of Glycine max
| Trait | n | Log-likelihood values | Descriptive statistics | ||||
|---|---|---|---|---|---|---|---|
| Normal | Log-normal | Range | Mean/median | S.d./mad | CV | ||
| Leaf area (cm2) | 402 | –1505.9 | –1492.4 | 12.1–61.5 | 29.5 | 10.1 | 34.3 |
| LMA (g m–2) | 402 | –1514.9 | –1500.2 | 23.5–74.9 | 41.2 | 11.1 | 27.1 |
| A area (μmol m–2 s–1) | 402 | –1223.0 | –1272.8 | 3.96–32.39 | 20.84 | 5.08 | 24.4 |
| A mass (μmol g–1 s–1) | 402 | 212.2 | 175.8 | 0.08–1.05 | 0.50 | 0.14 | 28.5 |
| Leaf C (% mass) | 402 | –737.6 | –740.3 | 38.2–45.7 | 42.8 | 1.5 | 3.6 |
| Leaf N (% mass) | 402 | –460.5 | –463.7 | 2.4–6.2 | 4.1 | 0.8 | 18.7 |
| Leaf C:N | 402 | –815.0 | –798.7 | 7.1–16.9 | 10.5 | 1.9 | 17.6 |
| WUE (mmol CO2 mol H2O–1) | 399 | –873.9 | –692.7 | 1.37–18.98 | 3.35 | 1.05 | 31.3 |
| g s (mol H2O m–2 s–1) | 399 | –27.0 | –138.7 | 0.019–1.338 | 0.57 | 0.259 | 45.5 |
| Nodules per plant | 134 | –686.2 | –660.0 | 4–230 | 54 | 31.1 | 65.0 |
| Nodule mass | 134 | –20.4 | 24.9 | 0.01–1.5 | 0.22 | 0.2 | 85.0 |
| Pods per plant | 45 | –188.0 | –165.3 | 4–82 | 14 | 8.9 | 87.1 |
| Seeds per plant | 45 | –225.3 | –202.4 | 11–195 | 32 | 17.8 | 86.3 |
For each trait, the best distribution fit models based on log-likelihood values are highlighted in bold. Where traits were best described by a normal distribution, descriptive statistics include mean, standard deviation (s.d.) and coefficient of variation (CV). For traits best described by a log-normal distribution, descriptive statistic include median and median absolute deviation (mad); CVs were quartile based (calculated as mad/median).
We also evaluated multivariate patterns in trait covariation using a principal components analysis (PCA) that included all traits except Aarea since it was highly correlated with Amass. We then coupled the PCA with a permutational multivariate analysis of variance (PerMANOVA) to evaluate if traits varied in multivariate space as a function of plant developmental stage, management and a plant stage × management interaction term (as informed by the variance decomposition procedure detailed below). Both the PCA and PerMANOVA were implemented using the ‘vegan’ R package (Oksanen et al., 2016); more specifically, the PerMANOVA was based on Euclidean distances among data points with 10 000 permutations used.
Predictors of ITV along the LES.
We followed the approach of Messier et al. (2010) to determine how our four nested levels of organization explained variation in soy leaf traits (as well as PCA axis 1 and 2 scores). This was done using the ‘nlme’ R package (Pinheiro et al., 2016) to fit first a linear mixed effects model for each trait individually, where all four levels were included as nested random effects (i.e. plants within blocks within management within plant developmental stage), and the overall intercept is the only fixed effect. The ‘varcomp’ function in the ‘ape’ R package (Paradis et al., 2004) was then used in order to partition the variation in soybean LES traits among these nested levels. This analysis was also done to evaluate ITV in nodule and reproduction data.
This variance partitioning technique informed our next analysis, which was designed to evaluate differences in leaf traits (as well as nodule and reproduction data) across plant developmental stages and management conditions. To account for non-independence of samples, this was done using a linear mixed-effects model where variation in traits was assessed as a function of plant developmental stage, management conditions and developmental stage × management interaction term as fixed effects, and plant identity and block as random effects. Mixed-effects models were fit using the ‘nlme’ R package (Pinheiro et al., 2016), and least square mean trait values and associated 95 % confidence intervals were calculated using the ‘lsmeans’ R package (Lenth, 2016).
Leaf traits and root nodules.
Relationships between leaf traits and nodules were determined at the plant level, such that plant-level leaf trait values were calculated as the mean of three observations for each trait per plant. We then used simple linear regression analysis to test if the number of nodules per plant predicted leaf trait values (see the Supplementary statistical methods).
However, since inferences regarding the relationships between traits and nodules are likely to be confounded by autocorrelation among data within plant developmental stages and management conditions, we also analysed trait–nodule relationships using a linear mixed-effects model. This model predicted traits as a function of nodules, while accounting for plants within blocks within management treatments within developmental stages, as four nested random effects (see the Supplementary statistical methods). We then used the ‘sem.model.fits’ function in the ‘piecewiseSEM’ R package (Lecerf and Chauvet, 2008) to calculate the marginal and conditional r2 values for these mixed models. Marginal r2 values correspond to the proportion of variation in traits explained by nodules per plant (while assuming the nested structure of data); conditional r2 values correspond to the proportion of variation in traits explained by nodule count as well as the nested random effects.
Leaf traits and reproduction.
We used a simple linear regression that included a second-order polynomial term to evaluate the relationship between traits and reproductive output (measured and analysed as both pod and seed count separately; Supplementary statistical methods). We also used linear mixed-effects models to evaluate relationships between pod and seed counts, and leaf traits while accounting for our nested random effects (i.e. plants within blocks within management treatments; see the Supplementary statistical methods). For all trait–reproduction models we then calculated marginal and conditional r2 values.
Leaf traits and environmental variables.
We used a linear mixed-effects model to evaluate how continuous environmental variables influence leaf trait expression. Within these models, traits were predicted as a function of five environmental variables incorporated as fixed effects: log-transformed canopy openness (%), soil moisture (% mass), log-transformed total soil C (% mass), total soil N (% mass) and plant-available N (mg kg–1; see Supplementary Data Table S5). These models also accounted for nested random effects of plants within blocks within management treatments within developmental stages (see the Supplementary statistical methods).
RESULTS
Intraspecific leaf trait variation
The largest ITV in G. max leaf traits was observed in traits associated with water fluxes including gs and WUE, which varied over one and two orders of magnitude, respectively, with CVs of 31.3 and 45.5 %, respectively (Table 1). In comparison, ITV in Aarea and Amass was intermediate, with CV values of 24.4 and 28.5 %, respectively, but still varied widely over approximately one order of magnitude (Table 1). Similarly, leaf morphological traits, namely LMA and leaf area, also expressed intermediate values of ITV (CV = 27.1 and 34.3 %, respectively), ranging 5- and 3-fold across the entire data set, respectively (Table 1). Leaf chemical traits expressed the lowest degree of ITV, with CV values of 3.6, 18.7 and 17.6 % for leaf C, leaf N and leaf C:N, respectively (Table 1).
An intraspecific LES in soy
In a bivariate framework, nearly all traits were statistically significantly correlated with one another, with the exception of leaf chemical traits and those associated with leaf hydraulic function (Table 2). Specifically, leaf N and C:N ratios did not covary with either WUE or gs, leaf C did not significantly covary with WUE, leaf area was unrelated to gs and Aarea, and LMA was unrelated to gs. Otherwise all leaf traits significantly covaried (Table 2).
Table 2.
Bivariate correlations among nine leaf functional traits in G. max
| A mass | A area | Log LMA | Leaf N | Leaf C | Log leaf CN | Log leaf area | Log WUE | g s | |
|---|---|---|---|---|---|---|---|---|---|
| A mass | – | 35.5 (32.8, 38.5) | –1.7 (–1.8, –1.6) | 5.3 (4.9, 5.8) | 10.6 (9.7, 11.6) | –1.2 (–1.3, –1.1) | –0.4 (–0.5, –0.4) | –2.8 (–3.1, –2.6) | 1.8 (1.7, 2.0) |
| Aarea | 0.348 (<0.001) | – | 0.1 (0.04, 0.1) | 0.2 (0.1, 0.2) | 0.3 (0.3, 0.3) | 0.03 (–0.04, –0.03) | 14.7 (13.3, 16.2) | –0.1 (–0.1, –0.1) | 0.1 (0.1, 0.1) |
| Log LMA | 0.337 (<0.001) | 0.083 (<0.001) | – | –3.2 (–3.5, –2.9) | –6.3 (–6.9, –5.8) | 0.7 (0.6, 0.8) | 0.7 (0.7, 0.8) | 1.7 (1.5, 1.8) | 1.1 (1.0, 1.2) |
| Leaf N | 0.286 (<0.001) | 0.034 (<0.001) | 0.222 (<0.001) | – | 2.0 (1.9, 2.1) | –0.2 (–0.2, –0.2) | 2.2 (2.0, 2.4) | –0.5 (–0.6, –0.5) | –0.3 (–0.4, –0.3) |
| Leaf C | 0.200 (<0.001) | 0.004 (0.102) | 0.233 (<0.001) | 0.596 (<0.001) | – | –0.1 (–0.1, –0.1) | 4.4 (4.0, 4.8) | –0.3 (–0.3, –0.2) | –0.2 (–0.2, –0.2) |
| Log leaf C:N | 0.286 (<0.001) | 0.056 (<0.001) | 0.181 (<0.001) | 0.97 (<0.001) | 0.466 (<0.001) | – | –0.5 (–0.5, –0.4) | 2.4 (2.2, 2.7) | –1.6 (–1.7, –1.4) |
| Log leaf area | 0.0002 (0.767) | 0.019 (0.006) | 0.029 (0.001) | 0.076 (<0.001) | 0.166 (<0.001) | 0.051 (<0.001) | – | 1.2 (1.1, 1.3) | –0.8 (–0.8, –0.7) |
| Log WUE | 0.186 (<0.001) | 0.022 (0.002) | 0.171 (<0.001) | 0.0002 (0.403) | 0.001 (0.29) | 0.00003 (0.456) | 0.08 (<0.001) | – | –0.7 (–0.7, –0.6) |
| g s |
0.144
(<0.001) |
0.295 (<0.001) | 0.005 (0.079) | 0.001 (0.291) | 0.025 (0.001) | 0.0001 (0.435) | 0.003 (0.269) | 0.14 (<0.001) | – |
Values in the table above the diagonal correspond to the slope of the relationship (with 95 % confidence limits in parentheses), which are based on a standardized major axis (SMA) regression model. Values below the diagonal correspond to the r2 (and P-values in parentheses) associated with each SMA model, with significant relationships highlighted in bold.
Samples sizes for SMA models are n = 402 in all cases, except for correlations involving log water use efficiency (WUE) and stomatal conductance (gs) here n = 309.
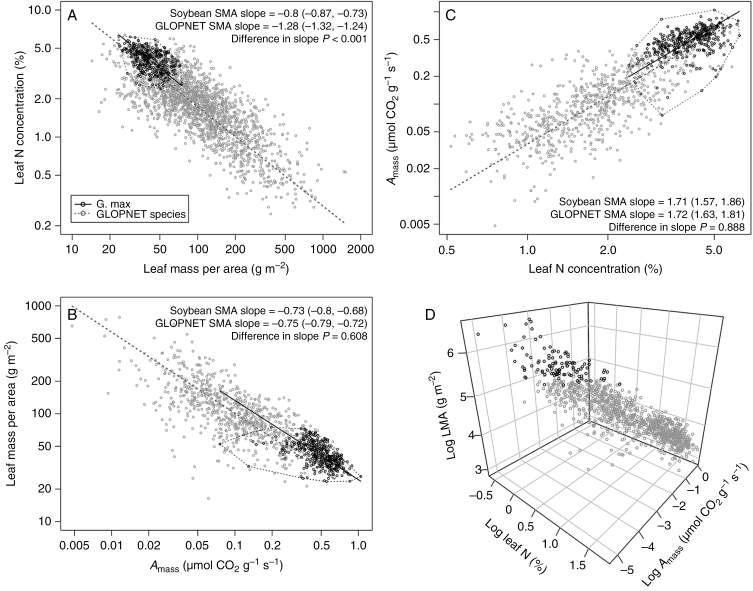
Bivariate LES trait relationships in soy were statistically indistinguishable from those observed across plants in the GLOPNET database (Fig. 1). Specifically, as in the LES there was a significant positive relationship between log leaf N and log Amass (r2 = 0.263, P < 0.001), with the slope of the G. max relationship (1.71) being statistically indistinguishable from that of the GLOPNET data set (slope = 1.72, slope test P-value = 0.888; Fig. 1). Similarly, log Amass and log LMA in soy were significantly negatively related (r2 = 0.28, P < 0.001) with a slope that was statistically indistinguishable from that in the GLOPNET data set (–0.73 vs. –0.75, respectively; Fig. 1). As in the LES, log LMA and log leaf N were significantly negatively related in soy (r2 = 0.209, P < 0.001); although the slope of this relationship was qualitatively similar to that observed in GLOPNET species (–0.8 vs. –1.28, respectively), these relationships were statistically distinguishable from one another (Fig. 1).
Fig. 1.
Relationships among three leaf economic spectrum (LES) traits in Glycine max compared with global patterns among species in the GLOPNET database. Open black circles represent observations of LES traits in G. max. In (A–C), solid black trend lines correspond to standardized major axis (SMA) regression models fit to log-transformed G. max data, and dashed black lines represent convex hull models surrounding the trait space occupied by G. max; in all cases n = 402 G. max observations. Grey circles correspond to trait values for species represented in the GLOPNET data set of Wright et al. (2004), and the dashed grey line corresponds to SMA models fit to these data sets, where n = 1958, 746, 712 and 706 for relationships presented in (A), (B), (C) and (D), respectively. Also shown in (A–C) are the results of an SMA slope test where the slope of the G. max SMA is compared with that of the GLOPNET data set; in these analyses, P-values ≥0.05 indicate that slopes of bivariate trait relationships in soy do not differ significantly from those observed across GLOPNET species.
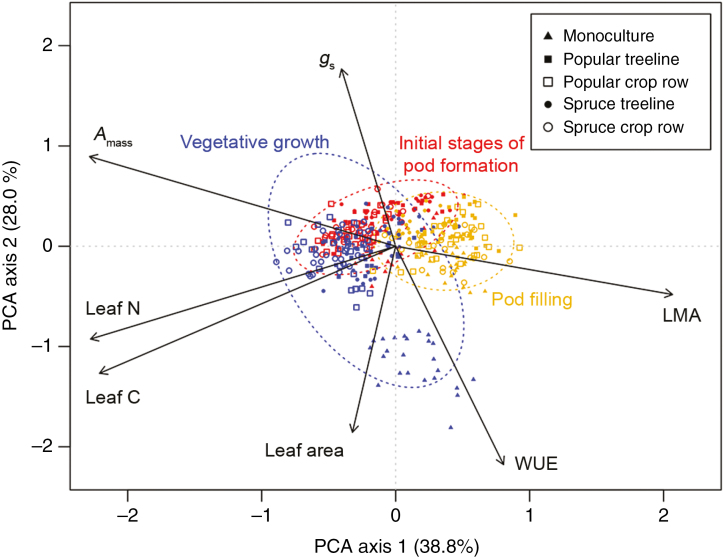
In a multivariate framework, soy leaf traits covaried across two primary axes that cumulatively explained 66.8 % of the variation across the entire data set (Fig. 2). The first PCA axis explained 38.8 % of the variation, and was consistent with representing an intraspecific LES, being positively associated with LMA and negatively associated with Amass, leaf N and leaf C (Fig. 2). The second PCA axis explained an additional 28.0 % of the variation in soy traits and was positively associated with gs, and negatively associated with WUE and leaf area (Fig. 2). PerMANOVA indicated that the position of soy leaves in multivariate trait space differed significantly as a function of both plant developmental stage (r2 = 0.232, P < 0.001) and management system (r2 = 0.346, P < 0.001; Fig. 2). Over the course of the growing season, soy plants tended to move towards the resource-conserving end of the LES along PCA axis 1 (Fig. 2). Differences in multivariate soy traits among management systems were largely associated with pronounced differences in soy leaf water use traits of plants sampled in the monoculture at the first sampling period (Fig. 2).
Fig. 2.
Principal components analysis (PCA) representing multivariate relationships among seven leaf functional traits in Glycine max. Sample size for PCA was n = 399 leaves; different symbols correspond to the five different management conditions, and three colours correspond to three different leaf trait sampling periods. Coloured dashed lines correspond to 95 % confidence ellipses surrounding leaves measured within the same plant developmental stage. Plant developmental stage explained the largest proportion of variation (55.1 %) in PCA axis 1 scores, while management conditions explained the largest proportion of variation (55.7 %) of the variation in PCA axis 2 scores (see Fig. 3; Supplementary Data Table S1).
Variance partitioning
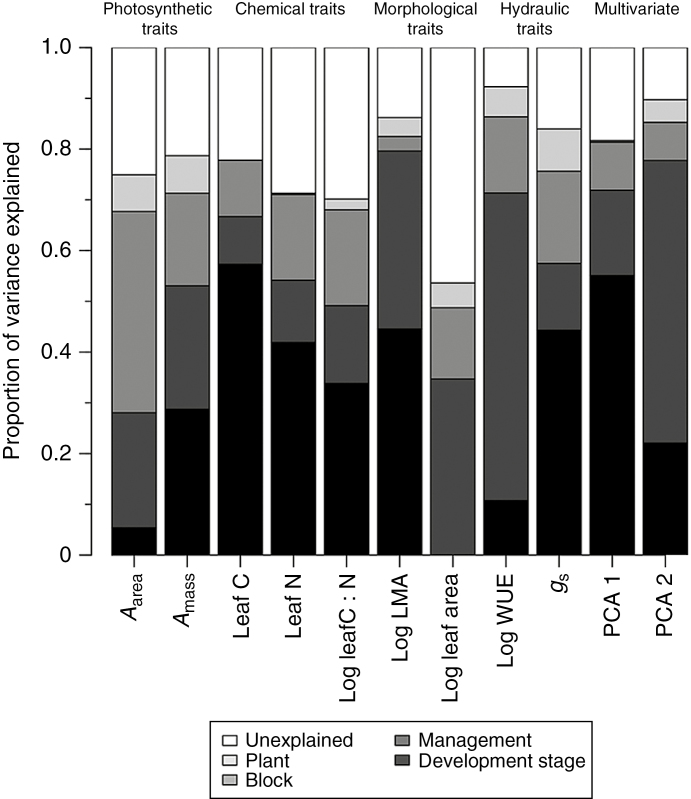
Plant developmental stage accounted for the largest portion of variation in six of the nine traits evaluated here, explaining between 28.7 and 57.3 % of the variation in Amass, leaf C, leaf N, leaf C:N, LMA and gs (Fig. 3; Supplementary Data Table S1). The exceptions to this were Aarea, leaf area and WUE, where developmental stage accounted for <10.7 % of ITV (Fig. 3; Supplementary Data Table S1). Management system accounted for the highest proportion of variation in leaf area and WUE (34.7 and 60.6 %, respectively). Management system also accounted for 22.7 % of the variation in Aarea, while block accounted for the largest proportion of intraspecific variation in this trait (Fig. 3; Supplementary Data Table S1). Individual plant identity explained <9 % of overall variation across all traits, while 7.7–46.4 % of trait variation was unexplained by the nested levels evaluated here (Fig. 3; Supplementary Data Table S1). Consistent with the results of our PerMANOVA (Fig. 2), 55.1 % of the variation in PCA axis 1 scores was explained by plant developmental stage, while 55.7 % of the variation in PCA axis 2 scores was explained by management (Fig. 3; Supplementary Data Table S1).
Fig. 3.
Variance partitioning of nine leaf functional traits and two multivariate indicators of leaf trait syndromes of Glycine max, across four nested levels of plant developmental stage, management, block and plant. Sample sizes for all physiological, chemical and morphological traits are n = 402, while sample sizes for hydraulic traits and multivariate indices are n = 399. Specific values for each trait × nested level combination are presented in Supplementary Data Table S1.
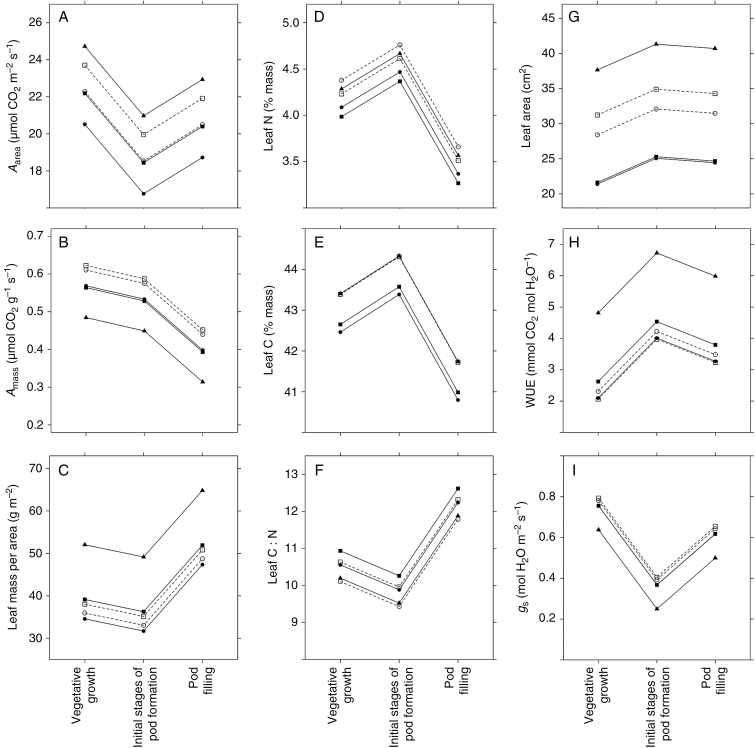
ITV across plant developmental stages and management
All traits varied significantly as a function of plant developmental stage, management conditions and a developmental stage × management interaction term (Fig. 4; Supplementary Data Tables S2 and S3), although the largest differences in LES traits occurred between the first two stages (vegetative growth/initial stages of pod formation) and the pod filling stage (Fig. 4). These two time periods mark a transition of soy from expressing resource-acquiring trait values – higher Amass and leaf N, and lower LMA – towards more resource-conserving trait values – lower Amass and leaf N, and higher LMA (Fig. 4,; Supplementary Data Tables S2 and S3). The primary ontogenetic differences in leaf area, WUE and gs were associated with changes from vegetative growth to the initial stages of pod formation (Fig. 4G–I).
Fig. 4.
Intraspecific variation in nine leaf functional traits in Glycine max across three plant developmental stages and five management conditions. Points correspond to least square means for traits at each developmental stage × management combination, based on a linear mixed -effects model (see Supplementary Data Table S3 for values and confidence intervals). Based on the same models, all traits varied significantly across developmental stages, management conditions and as a function of a developmental stage × management interaction term (P ≤ 0.001 in all cases; Supplementary Data Table S2). Sample sizes for all traits are n = 402, expect for water use efficiency (WUE) and stomatal conductance (gs) where n = 399.
Among management conditions, plants in the monoculture expressed the highest Aarea values coupled with the highest LMA values, and therefore also expressed the lowest Amass (Fig. 4; Supplementary Data Tables S2 and S3). Within agroforestry conditions, proximity to shade trees did not have a systematic impact on traits, except for leaf area which was larger in the crop rows than in the treeline in both poplar and spruce management (Fig. 4). Leaf WUE was highest, and gs the lowest, in plants growing in monoculture (Fig. 4H, I; Supplementary Data Tables S2 and S3).
ITV root nodules
Simple linear regression indicated that key LES traits including Amass, LMA and leaf N all covaried as a function of root nodule mass (P < 0.001, r2 = 0.153–0.421; Table 3; Supplementary Data Fig. S1). However, mixed-effects models indicated that within any given plant developmental stage × management condition combination, nodule mass was largely a weak predictor of functional traits, explaining only 0.02–8.9 % of ITV (Table 3).
Table 3.
Relationships between root nodule mass and leaf functional traits in Glycine max
| Trait | Simple linear models | Mixed model diagnostics | ||||
|---|---|---|---|---|---|---|
| Intercept | Nodule mass | Model P | r 2 | Marginal r2 | Conditional r2 | |
| A mass | 0.4 | –0.2 | <0.001 | 0.275 | 0.024 | 0.81 |
| A area | 21.2 | 0.5 | 0.636 | 0.002 | 0.002 | 0.813 |
| Log LMA | 1.7 | 0.2 | <0.001 | 0.421 | 0.019 | 0.902 |
| Leaf N | 3.6 | –0.7 | <0.001 | 0.153 | 0.002 | 0.885 |
| Leaf C | 42.0 | –1.4 | <0.001 | 0.138 | 0.011 | 0.928 |
| Log leaf C:N | 1.1 | 0.1 | <0.001 | 0.136 | 0.001 | 0.853 |
| Log WUE | 0.6 | 0.2 | <0.001 | 0.122 | 0.009 | 0.908 |
| g s | 0.5 | –0.1 | 0.265 | 0.01 | 0.0002 | 0.847 |
| Log leaf area | 1.6 | 0.1 | <0.001 | 0.156 | 0.089 | 0.736 |
Simple linear regression models were used to assess relationships across the entire data set, while linear mixed-effects models were employed to describe the relationship between traits and nodule mass while accounting for the nested structure of data. Marginal r2 values therefore correspond to the variance in traits explained by nodule mass alone, while the conditional r2 value corresponds to the variance in traits explained by both nodule mass and the nested random effects (namely sample blocks nested within management conditions nested within plant developmental stages).
Sample size for all models was n = 134 plants, except for WUE and gs analyses where n = 133 plants. Nodule mass data were log transformed prior to analyses.
ITV and soy reproduction
When evaluated individually, polynomial regression models indicated that certain leaf function traits, particularly Aarea, Amass, leaf N, LMA and leaf area, were all statistically significant correlates of both pod and seed counts (r2 = 0.110–0.685; Table 4). When accounting for differences in reproduction across blocks nested within management conditions, LMA explained more than half of the variation in pod and seed counts (64.3 and 54.5 %, respectively; Table 4). Similarly, Aarea and leaf area explained between 19.9 and 34.0 % of the variation in pod and seed counts when accounting for random effects (Table 4). Leaf C concentrations as well as WUE and gs were generally the weakest predictors of reproductive output, explaining only between 0.8 and 6.7 % of the variation in pod and seed counts (Table 4).
Table 4.
Relationships between leaf functional traits and reproduction in Glycine max
| Variable | Trait | Simple linear models | Mixed model diagnostics | |||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Trait | Trait2 | Model P | r 2 | Marginal r2 | Conditional r2 | ||
| Pod count | A mass | –1.2 | 11.8 | –14.1 | 0.087 | 0.11 | 0.071 | 0.757 |
| A area | 1.1 | –0.1 | 0.002 | <0.001 | 0.466 | 0.204 | 0.751 | |
| Log LMA | 60.9 | –73.2 | 22.3 | <0.001 | 0.685 | 0.643 | 0.75 | |
| Leaf N | –2.3 | 1.8 | –0.2 | 0.084 | 0.112 | 0.127 | 0.79 | |
| Leaf C | –16.2 | 0.64 | –0.01 | 0.003 | 0.239 | 0.043 | 0.715 | |
| Log leaf C:N | –14.7 | 30.5 | –14.6 | 0.164 | 0.083 | 0.122 | 0.799 | |
| Log WUE | 1.5 | –0.4 | –0.4 | 0.232 | 0.067 | 0.019 | 0.743 | |
| g s | 1.7 | –1.4 | 0.7 | 0.12 | 0.096 | 0.022 | 0.766 | |
| Log leaf area | –11.0 | 14.3 | –4.1 | <0.001 | 0.442 | 0.282 | 0.792 | |
| Seed count | A mass | –1.0 | 12.5 | –14.9 | 0.048 | 0.135 | 0.088 | 0.772 |
| A area | 1.4 | –0.03 | 0.002 | <0.001 | 0.453 | 0.199 | 0.77 | |
| Log LMA | 71.0 | –84.0 | 25.4 | <0.001 | 0.625 | 0.545 | 0.714 | |
| Leaf N | –2.5 | 2.2 | –0.3 | 0.03 | 0.153 | 0.128 | 0.766 | |
| Leaf C | –40.1 | 1.8 | –0.02 | 0.001 | 0.294 | 0.067 | 0.717 | |
| Log Leaf C:N | –18.2 | 37.8 | –18.0 | 0.068 | 0.12 | 0.119 | 0.774 | |
| Log WUE | 2.3 | –1.8 | 0.8 | 0.291 | 0.057 | 0.023 | 0.782 | |
| g s | 2.1 | –1.4 | 0.8 | 0.11 | 0.1 | 0.008 | 0.76 | |
| Log leaf area | –8.7 | 11.6 | –3.2 | <0.001 | 0.478 | 0.34 | 0.839 | |
Simple linear regression models including a second-order polynomial term were used to assess relationships across the entire data set, whereas linear mixed-effects models were employed to describe the relationship between traits and nodules while accounting for the nested structure of data. Marginal r2 values therefore correspond to the variance in pod and seed counts explained by traits alone, while the conditional r2 value corresponds to the variance in reproduction explained by traits as well as the nested random effects (namely sample blocks nested within management conditions).
Sample sizes for all models are n = 134 plants, except for WUE and gs analyses where n = 133 plants. Pod and seed count data were log transformed prior to analyses.
ITV and environmental variables
Continuous environmental variables including canopy openness, soil moisture, soil C and N, and plant-available N all differed significantly across management treatments, and – with the exception of canopy openness and soil C – also among sampling times (Supplementary Data Table S4). The environmental variables measured here were most closely linked with ITV in LMA, leaf area and WUE [variance explained (i.e. marginal r2 = 0.206–0.287)], and to a lesser degree Aarea and gs (marginal r2 = 0.136 and 0.123, respectively; Table 5). Continuous environmental variables explained 3.3–6.7 % of ITV in leaf chemical traits (Table 5). Of all environmental variables, canopy openness was arguably the most important predictor of ITV, being significantly positively associated with LMA, leaf area and WUE, and negatively associated with gs. With the exception of a positive association between plant-available N and Aarea, soil N variables were unrelated to ITV in LES traits (Table 5).
Table 5.
Results of the linear mixed-effects model evaluating relationships between nine leaf functional traits in Glycine max and five environmental variables
| Trait | n | Fixed effects | Explained variance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Log canopy openness (%) | Soil moisture (%) | Log soil C (%) | Soil N (%) | Log plant-available N (mg kg–1) | Marginal r2 | Conditional r2 | ||
| A area | 134 | –16.99 | 3.98 | 0.08 | 6.07 | 0.48 | 1.19 | 0.136 | 0.875 |
| A mass | 134 | 0.43 | –0.1 | 0.002 | 0.11 | 0.02 | 0.02 | 0.079 | 0.857 |
| Leaf C | 134 | 35.92 | 0.49 | 0.09 | 1.32 | –0.17 | 0.01 | 0.033 | 0.932 |
| Leaf N | 134 | 0.73 | 0.09 | 0.07 | 0.72 | –0.15 | 0.03 | 0.056 | 0.924 |
| Log leaf C:N | 134 | 3.23 | –0.02 | –0.02 | –0.19 | 0.02 | –0.01 | 0.067 | 0.906 |
| Log LMA | 134 | 2.4 | 0.39 | –0.01 | 0.004 | –0.04 | 0.02 | 0.287 | 0.92 |
| Log leaf area | 134 | 0.21 | 0.37 | 0.03 | 0.38 | 0.18 | 0.05 | 0.28 | 0.818 |
| Log WUE | 133 | –1.04 | 0.53 | –0.004 | 0.07 | 0.08 | 0.04 | 0.206 | 0.92 |
| Log gs | 133 | –0.32 | –0.63 | 0.04 | 0.43 | 0.05 | –0.001 | 0.123 | 0.933 |
Numbers shown are the parameter estimates for each variable, with parameters that differ significantly from 0 (P ≤ 0.001) highlighted in bold. Each model was fit assuming non-independence of errors such that observations in blocks were nested within management nested within time. Marginal r2 values therefore correspond to the variance in pod and seed counts explained by traits alone, while the conditional r2 value corresponds to the variance in reproduction explained by traits as well as the nested random effects (namely sample blocks nested with management conditions).
Sample sizes for all models were n = 134 plants, except for WUE and gs analyses where n =133 plants.
Mean values, s.e. and confidence limits surrounding environmental data are presented in Supplementary Data Table S4.
DISCUSSION
Intraspecific variation in LES traits of the world’s most common leguminous crop is broad (Table 1) and falls along a defined intraspecific LES, the shape of which is remarkably similar to, and largely statistically indistinguishable from, the LES observed across ‘wild’ plants globally (Figs 1 and 2; Table 2). Our study is similar to others (Li et al., 2015; Martin et al., 2017) in showing that, within crop genotypes, phenotypic ITV in leaf hydraulic properties – namely instantaneous WUE and gs – is also considerable, but forms an axis of intraspecific biological variation that is distinct from an intraspecific LES (Fig. 2). Our results (Figs 3 and 4; Supplementary Data Tables S1–S3) provide additional evidence suggesting that a considerable proportion of phenotypic ITV within crop genotypes can be captured by sampling leaf traits across different whole-plant developmental stages, particularly those related to reproductive onset (cf. Thomas, 2010; Martin and Thomas, 2013; Mason et al., 2013), and across management systems (Gagliardi et al., 2015; Martin et al., 2017). Accounting for these dimensions of ITV is critical when designing trait sampling strategies (Carmona et al., 2015), compiling crop trait databases (Martin and Isaac, 2015) and, ultimately, when evaluating relationships between functional traits and ecosystem functioning such as plant-level reproductive output (Table 4).
Patterns of LES in soy vs. other plants
The relationships between LES traits in soy (Fig. 1) were nearly identical to the interspecific patterns observed among wild plant species in the GLOPNET data set (Wright et al., 2004). This finding differs from studies that have detected a decoupling of key LES traits – namely Aarea and leaf N on an area basis – in N2-fixing plants in non-agricultural systems (Adams et al., 2016). However, finding LES trait relationships that did not differ in soy vs. GLOPNET species is similar to other studies that have observed that patterns of trait covariation along an intraspecific LES are similar to global LES patterns (Blonder et al., 2013); other studies also allude to similarities between an intraspecific and a global interspecific LES, but do not include a formal statistical evaluation (Hu et al., 2015; Niinemets, 2015). Although our soy data indicate a universal LES that describes both inter- and intraspecific trait covariation within a single genotype, this finding is in contrast to other studies which have found that plants within a crop genotype express significantly different patterns of trait covariation along an intraspecific LES (Martin et al., 2017). Specifically, relationships between leaf N and Amass in coffee (Coffea arabica) – also evaluated across high (monoculture) and low light (agroforestry) management systems – were less tightly coupled compared with GLOPNET species, which was speculated to be related to artificial selection for N-based compounds (namely caffeine) that are unrelated to C assimilation (fig. 2 in Martin et al., 2017). In soy, this is not the case (Fig. 2), indicating that certain aspects of the domestication syndrome of a crop – such as selection for N-based secondary compounds as in coffee – may influence the shape of trait covariation along an intraspecific LES.
Recent work has shown that crops tend to devote a large fractional allocation of leaf N to C assimilation, as compared with non-domesticated plants (Ghimire et al., 2017), and that overall artificial selection has, either directly or indirectly, selected for traits that confer high rates of resource acquisition (Milla et al., 2014, 2015; Roucou et al., 2018). Taken together with our results here (Fig. 1), these lines of reasoning suggest that some of the world’s most common crops, particularly annual herbaceous and graminoid crops such as wheat, maize and soy, now occupy the most extreme resource-acquiring end of the LES. Defining the dominant positions of crops along the LES, as well as the intraspecific trait space occupied by the world’s most common domesticated crops (as in Fig. 1), is a key step in further refining and understanding the role that crop expansion or diversity plays in driving global models of net primary productivity (Martin and Isaac, 2015).
Causes of intraspecific trait variation
In standardizing our analysis by leaf age and canopy position, our data and analysis suggest that phenotypic ITV within a single soy genotype across the LES is largely explained by changes in traits observed over the course of whole-plant development, and to a lesser degree across management systems that differ in broad environmental conditions (Fig. 4; Table 5). There was significant ITV in all traits across all soy life stages evaluated here, with the largest differences in suites of LES traits occurring between the stages of initial pod formation and pod filling (Fig. 4). These ontogenetic shifts are consistent with expectations that resource allocation to reproduction influences ITV in LES traits, particularly in terms of declines in Amass and leaf N, and increases in LMA, that occur following reproductive onset (Thomas, 2010, 2011; Martin and Thomas, 2013; Mason et al., 2013).
Various authors have noted that quantifying the continuous ontogenetic variation in leaf traits of perennial plant species occurring throughout growing seasons (McKown et al., 2013; Fajardo and Siefert, 2016) or across plant size continua (Thomas, 2010; Martin and Thomas, 2013; Sendall and Reich, 2013) is critical for understanding the structure, function and dynamics of terrestrial ecosystems (e.g. Herault et al., 2011). However, considering constraints on how in-depth ITV sampling protocols may feasibly be (Carmona et al., 2015), our results (Fig. 2; Supplementary Data Table S1) and those of others (Mason et al., 2013) indicate that sampling traits at distinct crop developmental stages is critical and feasible for capturing a significant proportion of trait variation within genotypes in agricultural systems.
Soy traits also expressed within-genotype phenotypic ITV among management systems, but the species identity of nearby trees within the agroforestry systems did not have a large effect on leaf traits (Figs 3 and 4). Instead, ITV associated with management in our data set was due to major differences in trait values observed between plants in the monoculture as compared with all agroforestry management conditions (Fig. 3). Most notably, monoculture plants expressed extremely high values of WUE, LMA and Aarea along with the lowest rates of Amass and gs (Fig. 4H). These patterns – in particular high values of LMA and Aarea in monoculture – are consistent with studies evaluating responses of shade-intolerant species (such as soy) to high light (Table 5; Valladares and Niinemets, 2008; Lusk et al., 2008; Poorter et al., 2009). Selection for rapid growth under high light has probably resulted in soy plants expressing greater symplastic cell components (i.e. mesophyll and/or parenchyma per unit area) under high irradiance (Bunce et al., 1977). These light responses should lead to higher LMA and Aarea and lower Amass for soy plants in high light monoculture (Lusk et al., 2008; Poorter et al., 2009). Our data are therefore consistent with expected crop trait acclimation to above-ground environmental gradients, as compared with a relatively small role for below-ground resource availability in driving ITV in LES traits of crops (Table 5; Gagliardi et al., 2015; Martin et al., 2017).
Intraspecific trait variation and agroecosystem function
Leaf economics traits are largely hypothesized to underpin individual plant performance, with implications for ecosystem-scale functioning (Reich et al., 1992; Reich, 2012; Violle et al., 2007). In agroecology, this general ecological hypothesis is explicitly incorporated into many of the most prominent simulation models that employ leaf economics traits, notably Aarea and specific leaf area (the inverse of LMA), as key data inputs when predicting crop yield under climate change scenarios (e.g. Jones et al., 2003; Martin et al., 2018). In addition to leaf area, LMA and Aarea covaried most strongly with individual reproductive output in soy, explaining 45.3–68.5 % of the variation in individual seed or pod output (Supplementary Data Fig. S2). Beyond the individual scale, observational studies also indicate that ITV in LES traits scales up to influence reproduction rates at the plot or farm scale, across management gradients (Reynolds et al., 2007). From a modelling perspective then, accounting for phenotypic ITV in certain traits – particularly leaf area, Aarea and LMA which are all explicitly incorporated into crop models – is key for refining predictions and projections of soy yield into the future.
CONCLUSIONS
In addition to an extensive literature on the benefits of ITV for agroecological processes (reviewed by Brooker et al., 2015), recent analyses indicate that phenotypic ITV among cultivars of the same crop species contributes significantly to the maintenance of agroecological functions (Reiss and Drinkwater, 2018). Our findings show that phenotypic ITV within genotypes across ontogenetic stages and managed environmental gradients also influences rates of agroecological functioning. When taken with other research (Milla et al., 2014, 2015; Martin et al., 2017), our results also give insights into the constraints that might predict or limit ITV in crops, and inform an understanding of the ecological consequences of artificial selection. Research that explores patterns of ITV across a greater range of key plant life history traits, within and across a greater number of crops, presents a major stepping stone towards applying the principles of trait-based ecology into agricultural management.
SUPPLEMENTARY DATA
Supplementary data for this article are available online at https://academic.oup.com/aob and consist of the following. Supplementary information on statistical methods. Table S1: variance partitioning of nine leaf functional traits and two multivariate indicators of leaf trait syndromes of Glycine max, across four nested levels, namely plant developmental stage, management, block and plant. Table S2: results of ANOVA evaluating intraspecific variation in nine leaf functional traits, two nodule traits and two reproduction traits in Glycine max across three plant developmental stages and five different management conditions. Table S3: leaf functional traits, root nodule counts and mass, and pod and seed counts in Glycine max across three plant developmental stages and five management conditions. Table S4: environmental variables across three plant developmental stages and five management conditions in Guelph Agroforestry Research Station, Ontario, Canada. Table S5: variation in five environmental variables across three plant developmental stages and five different management conditions at Guelph Agroforestry Research Station, Ontario, Canada. Figure S1: intraspecific variation in root nodule mass in Glycine max across three plant developmental stages and five management conditions. Figure S2: intraspecific variation in pod count and seed count in Glycine max across five management conditions.
ACKNOWLEDGEMENTS
We thank Jacky Tong, Mahendra Doraisami, Victor Truong, Ravin Dyal, Sylwia Pucek, Arika Hisatsune, Senping Zhang, Jason Ngo, Tom Man Hui, Motaseem Jamal, Manisha Mistry, Anisha Prasad, Luke Greco, Julia Romano, Maimuna Hafiz and Maathura Perapakaran for their assistance in the lab. We also thank Marney Isaac and Roberta Fulthorpe for helpful comments on earlier versions of this manuscript, as well as two anonymous reviewers for comments that improved the manuscript. This research was supported by the Canada Research Chairs program and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to Marney E. Isaac, as well as a graduate research assistantship granted to F.H. courtesy of the Department of Physical and Environmental Sciences, University of Toronto Scarborough.
LITERATURE CITED
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42. [Google Scholar]
- Adams MA, Turnbull TL, Sprent JI, Buchmann N. 2016. Legumes are different: leaf nitrogen, photosynthesis, and water use efficiency. Proceedings of the National Academy of Sciences, USA 113: 4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S. 2010a. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Functional Ecology 24: 1192–1201. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, et al. . 2010b. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology 98: 604–613. [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. 2011. When and how should intraspecific variability be considered in trait-based plant ecology?Perspectives in Plant Ecology, Evolution and Systematics 13: 217–225. [Google Scholar]
- Blonder B, Violle C, Enquist BJ. 2013. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. Journal of Ecology 101: 981–989. [Google Scholar]
- Bouman BAM, van Laar HH. 2006. Description and evaluation of the rice growth model ORYZA2000 under nitrogen-limited conditions. Agricultural Systems 87: 249–273. [Google Scholar]
- Brooker RW, Bennett AE, Cong WF, et al. . 2015. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytologist 206: 107–117. [DOI] [PubMed] [Google Scholar]
- Bunce JA, Patterson DT, Peet MM, Alberte RS. 1977. Light acclimation during and after leaf expansion in soybean. Plant Physiology 60: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte MW, Carscadden K, Mirotchnick N. 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48: 1079–1087. [Google Scholar]
- Campbell WJ, Allen LH, Bowes G. 1990. Response of soybean canopy photosynthesis to CO2 concentration, light, and temperature. Journal of Experimental Botany 41: 427–433. [Google Scholar]
- Carmona CP, Rota C, Azcarate FM, Peco B. 2015. More for less: sampling strategies of plant functional traits across local environmental gradients. Functional Ecology 29: 579–588. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. . 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- Delignette-Muller ML, Dutang C. 2015. fitdistrplus: an R package for fitting distributions. Journal of Statistical Software 64: 1–34. [Google Scholar]
- Diaz S, Kattge J, Cornelissen JHC, et al. . 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Fajardo A, Siefert A. 2016. Phenological variation of leaf functional traits within species. Oecologia 180: 951–959. [DOI] [PubMed] [Google Scholar]
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. 1971. Stage of development descriptions for soybeans, Glycine max (L) Merrill. Crop Science 11: 929–931. [Google Scholar]
- Frazer GW, Canham CD, Lertzmen KP. 1999. Gap Light Analyzer (GLA) Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs. Simon Fraser University, Burnaby, BC and the Institute of Ecosystem Studies, Millbrook, NY. [Google Scholar]
- Furze JR, Martin AR, Nasielski J, Thevathasan NV, Gordon AM, Isaac ME. 2017. Resistance and resilience of root fungal communities to water limitation in a temperate agroecosystem. Ecology and Evolution 7: 3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi S, Martin AR, Virginio ED, Rapidel B, Isaac ME. 2015. Intraspecific leaf economic trait variation partially explains coffee performance across agroforestry management regimes. Agriculture Ecosystems & Environment 200: 151–160. [Google Scholar]
- Ghimire B, Riley WJ, Koven CD, et al. . 2017. A global trait-based approach to estimate leaf nitrogen functional allocation from observations. Ecological Applications 27: 1421–1434. [DOI] [PubMed] [Google Scholar]
- Herault B, Bachelot B, Poorter L, et al. . 2011. Functional traits shape ontogenetic growth trajectories of rain forest tree species. Journal of Ecology 99: 1431–1440. [Google Scholar]
- Hu YK, Pan X, Liu GF, et al. . 2015. Novel evidence for within-species leaf economics spectrum at multiple spatial scales. Frontiers in Plant Science 6: 901. doi: 10.3389/fpls.2015.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac ME, Carlsson G, Ghoulam C, Makhani M, Thevathasan NV, Gordon AM. 2014. Legume performance and nitrogen acquisition strategies in a tree-based agroecosystem. Agroecology and Sustainable Food Systems 38: 686–703. [Google Scholar]
- Isaac ME, Martin AR, Virginio ED, Rapidel B, Roupsard O, Van den Meersche K. 2017. Intraspecific trait variation and coordination: root and leaf economics spectra in coffee across environmental gradients. Frontiers in Plant Science 8: 1196. doi: 10.3389/fpls.2017.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac ME, Cerda R, Rapidel B, Martin AR, Dickinson AK, Sibelet N. 2018. Farmer perception and utilization of leaf functional traits in managing agroecosystems. Journal of Applied Ecology 55: 69–80. [Google Scholar]
- Jones JW, Hoogenboom G, Porter CH, et al. . 2003. The DSSAT cropping system model. European Journal of Agronomy 18: 235–265. [Google Scholar]
- Jung V, Albert CH, Violle C, Kunstler G, Loucougaray G, Spiegelberger T. 2014. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. Journal of Ecology 102: 45–53. [Google Scholar]
- Kraft NJB, Godoy O, Levine JM. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proceedings of the National Academy of Sciences, USA 112: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16: 545–556. [Google Scholar]
- Lecerf A, Chauvet E. 2008. Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic and Applied Ecology 9: 598–605. [Google Scholar]
- Lenth RV. 2016. Least-squares means: the R package lsmeans. Journal of Statistical Software 69: 1–33. [Google Scholar]
- Li L, McCormack ML, Ma CG, et al. . 2015. Leaf economics and hydraulic traits are decoupled in five species-rich tropical–subtropical forests. Ecology Letters 18: 899–906. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Reich PB, Montgomery RA, Ackerly DD, Cavender-Bares J. 2008. Why are evergreen leaves so contrary about shade?Trends in Ecology & Evolution 23: 299–303. [DOI] [PubMed] [Google Scholar]
- Martin AR, Isaac ME. 2015. Plant functional traits in agroecosystems: a blueprint for research. Journal of Applied Ecology 52: 1425–1435. [Google Scholar]
- Martin AR, Isaac ME. 2018. Functional traits in agroecology: advancing description and prediction in agroecosystems. Journal of Applied Ecology 55: 5–11. [Google Scholar]
- Martin AR, Thomas SC. 2013. Size-dependent changes in leaf and wood chemical traits in two Caribbean rainforest trees. Tree Physiology 33: 1338–1353. [DOI] [PubMed] [Google Scholar]
- Martin AR, Rapidel B, Roupsard O, et al. . 2017. Intraspecific trait variation across multiple scales: the Leaf Economics Spectrum in coffee. Functional Ecology 31: 604–612. [Google Scholar]
- Martin AR, Hale CE, Cerabolini BEL, et al. . 2018. Inter- and intraspecific variation in leaf economics traits in wheat and maize. AoB Plants 10: ply006. doi: 10.1093/aobpla/ply006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CM, McGaughey SE, Donovan LA. 2013. Ontogeny strongly and differentially alters leaf economic and other key traits in three diverse Helianthus species. Journal of Experimental Botany 64: 4089–4099. [DOI] [PubMed] [Google Scholar]
- McKown AD, Guy RD, Azam MS, Drewes EC, Quamme LK. 2013. Seasonality and phenology alter functional leaf traits. Oecologia 172: 653–665. [DOI] [PubMed] [Google Scholar]
- Messier J, McGill BJ, Lechowicz MJ. 2010. How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters 13: 838–848. [DOI] [PubMed] [Google Scholar]
- Milla R, Morente-Lopez J, Alonso-Rodrigo JM, Martin-Robles N, Chapin FS. 2014. Shifts and disruptions in resource-use trait syndromes during the evolution of herbaceous crops. Proceedings of the Royal Society B: Biological Sciences 281: pii: 20141429. doi: 10.1098/rspb.2014.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla R, Osborne CP, Turcotte MM, Violle C. 2015. Plant domestication through an ecological lens. Trends in Ecology & Evolution 30: 463–469. [DOI] [PubMed] [Google Scholar]
- Moran EV, Hartig F, Bell DM. 2016. Intraspecific trait variation across scales: implications for understanding global change responses. Global Change Biology 22: 137–150. [DOI] [PubMed] [Google Scholar]
- Nasielski J, Furze JR, Tan J, Bargaz A, Thevathasan NV, Isaac ME. 2015. Agroforestry promotes soybean yield stability and N2-fixation under water stress. Agronomy for Sustainable Development 35: 1541–1549. [Google Scholar]
- Niinemets U. 2015. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytologist 205: 79–96. [DOI] [PubMed] [Google Scholar]
- Oelbermann M, Voroney RP. 2007. Carbon and nitrogen in a temperate agroforestry system: using stable isotopes as a tool to understand soil dynamics. Ecological Engineering 29: 342–349. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, et al. . 2016. vegan: community ecology package in R version 2.3–5. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Perez-Harguindeguy N, Diaz S, Garnier E, et al. . 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2016. nlme: linear and nonlinear mixed effects models. R package version 3.1–127. [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 183: 1222–1222. [DOI] [PubMed] [Google Scholar]
- Reich PB. 2012. Key canopy traits drive forest productivity. Proceedings of the Royal Society B: Biological Sciences 279: 2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Schoettle AW, Raba RM, Amundson RG. 1986. Response of soybean to low concentrations of ozone. 1. Reductions in leaf and whole plant net photosynthesis and leaf chlorophyll content. Journal of Environmental Quality 15: 31–36. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1991. Leaf age and season influence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant, Cell and Environment 14: 251–259. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1992. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs 62: 365–392. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences, USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss ER, Drinkwater LE. 2018. Cultivar mixtures: a meta-analysis of the effect of intraspecific diversity on crop yield. Ecological Applications 28: 62–77. [DOI] [PubMed] [Google Scholar]
- Reynolds PE, Simpson JA, Thevathasan NV, Gordon AM. 2007. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecological Engineering 29: 362–371. [Google Scholar]
- Roucou A, Violle C, Fort F, Roumet P, Ecarnot M, Vile D. 2018. Shifts in plant functional strategies over the course of wheat domestication. Journal of Applied Ecology 55: 25–37. [Google Scholar]
- Sendall KM, Reich PB. 2013. Variation in leaf and twig CO2 flux as a function of plant size: a comparison of seedlings, saplings and trees. Tree Physiology 33: 713–729. [DOI] [PubMed] [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, et al. . 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18: 1406–1419. [DOI] [PubMed] [Google Scholar]
- Steppe K, Niinemets Ü, Teskey RO. 2011. Tree size- and age-related changes in leaf physiology and their influence on carbon gain. In: Meinzer F, Lachenbruch B, Dawson T, eds. Size- and age-related changes in tree structure and function. Dordrecht: Springer, 235–253. [Google Scholar]
- Thevathasan NV, Gordon AM. 2004. Ecology of tree intercropping systems in the North temperate region: experiences from southern Ontario, Canada. Agroforestry Systems 61–62: 257–268. [Google Scholar]
- Thomas SC. 2010. Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiology 30: 555–573. [DOI] [PubMed] [Google Scholar]
- Thomas SC. 2011. Age-related changes in tree growth and functional biology: the role of reproduction. In: Meinzer FC, Dawson T, Lachenbruch BJ, eds. Size- and age-related changes in tree structure and function. Dordrecht: Springer, 33–64. [Google Scholar]
- Valladares F, Niinemets U. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology Evolution and Systematics 39: 237–257. [Google Scholar]
- Violle C, Navas ML, Vile D, et al. . 2007. Let the concept of trait be functional!Oikos 116: 882–892. [Google Scholar]
- Warton DI, Duursma RA, Falster DS, Taskinen S. 2012. smatr 3 – an R package for estimation and inference about allometric lines. Methods in Ecology and Evolution 3: 257–259. [Google Scholar]
- Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil 199: 213–227. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.