Abstract
Background:
Among groups of persons living with HIV (PLWH), high-risk drinking trajectories are associated with HIV severity. Whether changes in individuals’ alcohol use are associated with changes in HIV severity over the same period is unknown.
Methods:
Veterans Aging Cohort Study (VACS) data from VA’s EHR (2/1/2008–9/30/16) identified AUDITC screens for all PLWH. Pairs of AUDIT-C screens within 9–15 months were included if CD4 and/or viral load (VL) was measured within 9 months after baseline and follow-up AUDIT-Cs. Linear regression assessed change in HIV severity (CD4 and logVL) associated with AUDIT-C change adjusted for confounders. Mean changes in HIV severity were estimated for each AUDIT-C change value. For all measures of change, positive values indicate improvements (lower drinking and improved HIV severity).
Results:
Among PLWH, 21,999 and 22,143 were eligible for CD4 and VL analyses, respectively. Most had non- or low-level drinking and stable consumption over time (mean AUDIT-C change=.08, SD=1.91). HIV severity improved over time [mean CD4 change=20.5 (SD 180.8); mean logVL change=0.12 (SD 0.71)]. AUDIT-C changes were associated non-linearly with changes in CD4 (p=0.03) and logVL (p<0.001). Improvement in HIV severity was greatest among those with stable AUDIT-C scores over time; those with greater AUDIT-C increases fared worse than those with smaller increases in or stable AUDIT-Cs.
Conclusions:
Improvement in HIV severity was greatest among PLWH with relatively stable drinking, most of whom initially did not drink or drank at low levels. Those with large changes (especially increases) in drinking appear at greatest risk for poor HIV control.
Keywords: HIV, CD4, viral load, alcohol use, alcohol use disorders, veterans
1. INTRODUCTION.
Over half of people living with HIV (PLWH) in the U.S. report past-year alcohol use (Williams et al., 2016b), and ~25% report unhealthy use (Saitz, 2005; Williams et al., 2016b). Alcohol use is a health concern for PLWH because it is associated with increased risk of HIV transmission (Scott-Sheldon et al., 2016), poorer HIV disease management (Braithwaite et al., 2005; Hendershot et al., 2009; Vagenas et al., 2015), and HIV disease progression (Hahn and Samet, 2010; Justice et al., 2016; Williams et al., 2016a).
While a large literature has reported associations between alcohol use and HIV-relevant outcomes (Williams et al., 2016a), including HIV disease severity (Baum et al., 2010; Deiss et al., 2016; Samet et al., 2007), longitudinal studies are rare. Further longitudinal work is needed to understand which specific patterns of alcohol use increase risks and how changes in alcohol use may alter the course of HIV-related outcomes over time (Williams et al., 2016a). One previous study conducted in the Veterans Aging Cohort Study (VACS) survey sample (3,539 PLWH recruited from 8 urban HIV clinics) used group-based trajectory analysis to understand associations between alcohol use trajectory groups and HIV disease severity trajectory groups based on a composite index of HIV disease severity (Justice et al., 2012; Justice et al., 2013). This study identified the greatest likelihood of belonging to an extreme HIV severity risk group among those with high likelihood of consistent heavy alcohol use over time (Marshall et al., 2017). However, further longitudinal investigations of the influence of alcohol use on markers of HIV disease severity are needed. Longitudinal studies are needed to evaluate markers of HIV disease severity used clinically, such as CD4 and HIV viral load, and to address questions related to “counterfactual causality” (Hill, 1965, 2015)—specifically, whether HIV disease severity in an individual is altered by a change in their alcohol use over time.
Understanding whether and in what subgroups individual-level changes in alcohol use correspond to concomitant changes in markers of HIV disease used for routine viral monitoring among PLWH could help further elucidate these issues. Therefore, in the VACS national sample of PLWH receiving care in the Veterans Health Administration (VA)—we evaluated whether individual-level changes in alcohol use were associated with concomitant changes in two routine laboratory measures of HIV disease severity—CD4 cells/μl and HIVRNA viral load.
2. METHODS.
2.1. Data Source and Study Samples
Using national VACS data from VA electronic health records (Fultz et al., 2006; Justice et al., 2006) 2/1/2008 to 4/30/2016, we identified all PLWH receiving VA care nationally who had documented alcohol screening with the Alcohol Use Disorders Identification Test Consumption (AUDIT-C) (Bradley et al., 2006). To allow for sufficient follow-up time, participants needed to have at least one initial AUDIT-C (the “baseline” time point) prior to 5/1/2014.
Within this “Total Study Sample” (see Supplemental File 1), we identified PLWH with a follow-up AUDITC screen recorded 9–15 months after a prior baseline screen (up to 07/31/2015). To maximize power, patients could contribute multiple pairs of alcohol screens during the study as long as there were at least 9 months between each baseline date; no maximum number of months between baseline screens was specified. For each baseline screen, the follow-up screen closest to 12 months was selected. Among those with at least 1 pair of AUDIT-C screens, we identified two analytic samples based on availability of CD4 and HIV-RNA viral load (VL) data documented following each AUDIT-C measurement. The “CD4 Sample” and the “VL Sample” included those with CD4 cells/μl and HIV-RNA VL measured within 9 months following both baseline and follow-up AUDIT-C screening, respectively. Although we assessed the feasibility of evaluating CD4 and VL outcomes within 3- and 6-month windows following AUDIT-C measures, the 9-month time window was selected to maximize sample size.
2.2. Measures.
2.2.1. Exposure Measure.
Changes in alcohol use over time were measured using the AUDIT-C, a validated screen for unhealthy use (Bradley et al., 2003; Bush et al., 1998). AUDIT-C scores range from 0 to 12; higher scores reflect higher levels of consumption and greater severity of unhealthy use (Rubinsky et al., 2013; Rubinsky et al., 2010). The primary exposure measure was AUDIT-C change score, calculated as baseline AUDIT-C minus follow-up AUDIT-C score. Possible change scores ranged from −12 to 12, with negative values reflecting increased drinking, positive values reflecting decreased drinking, and 0 values reflecting stable drinking over time. AUDIT-C change scores were also categorized −6 to −12, −3 to −5, −2, −1, 0, 1, 2, 3 to 5, and 6 to 12 for descriptive purposes.
2.2.2. Outcome Measures.
Changes in HIV disease severity were measured using two routine clinical markers of HIV: 1) change in CD4 cells/μl and 2) change in log base 10 HIV-RNA copies/ml (logVL) from baseline to follow-up. Measures were created so that negative values indicate lower HIV control; positive values indicate improved HIV control; and 0 indicates no change in HIV control over time.
2.2.3. Other Measures.
Demographic characteristics included age at baseline AUDIT-C (<50, 50–65, >65), gender (male/female), and race/ethnicity (black, Hispanic, white, and other/unknown). International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes documented in the year prior to baseline AUDIT-C were used to measure depression, anxiety, serious mental illness, stimulant use, opioid use, other drug use, alcohol use disorder, and any alcohol-specific medical condition (e.g., alcoholic cirrhosis). Three baseline HIV clinical measures were derived: 1) on ART in year prior to baseline AUDIT-C defined as one or more prescriptions filled; 2) mean adherence to ART defined as the proportion of medication doses prescribed in year prior to baseline that were filled; and 3) detectable viral load at baseline (HIV-RNA ≥500 copies/ml). A cut point of ≥500 copies/ml was used to categorize viral load detection because, though tests have had increased sensitivity at lower cut-points over time (Dombrowski et al., 2013; North American AIDS Cohort, 2017), this cut-point is relevant for all years of the study. Baseline AUDIT-C scores were categorized into alcohol use risk groups: 0 for non-drinking, 1–3 (1–2 for women) for low-level, 4–5 (3–5 for women) for mild, 6–7 for moderate, and 8–12 for severe alcohol use (Justice et al., 2016; Kinder et al., 2009). Because HIV control improves over time among PLWH in treatment, number of days between baseline and follow-up CD4 and VL measures was also calculated.
2.3. Analyses.
2.3.1. Patient-level Descriptive Analyses.
At the patient level, based on the first AUDIT-C pair contributed, characteristics of CD4 and VL analytic samples were described and compared to characteristics in the Total Study Sample to assess the generalizability of the samples with complete data to the larger sample of individuals with at least one AUDIT-C screen. For both analytic samples, we also summarized patient-level mean initial values of CD4 and logVL, as well as the proportion with detectable VL, across baseline AUDIT-C scores.
2.3.2. Observation-level Descriptive and Regression Analyses.
At the observation level (pair of screens), we described the count of observations by AUDIT-C change categories and baseline AUDIT-C. We then used linear regression models to assess change in CD4 and change in logVL (outcome measures) associated with change in AUDIT-C (independent variable). To allow for a non-linear association, we modeled this association flexibly using restricted cubic splines, a function formed by connecting segments (thus allowing non-linear combinations of estimates) (Khamis and Kepler, 2002). Spline knots (i.e., points where segments connect) were set at −3, −1, 0, 1, and 3 based on examination of the unadjusted association using different knot placements and identification of knots that approximated the fully nonparametric association (i.e., the model that allowed for a different effect at each value of AUDIT-C change) (Royston, 2000). For both outcomes, three separate models were run: unadjusted (Block 1); adjusted for demographics (age, race, and gender), initial CD4 (or logVL), and days between CD4 (or VL) measures (Block 2; primary model); and additionally adjusted for baseline depression and anxiety diagnoses (Block 3). To account for potential dependence due to multiple observations per patient, we estimated standard errors using the robust sandwich estimator (Liang and Zeger, 1986). Finally, we applied inverse probability weighting (Little and Rubin, 2002) to weight samples back to the Total Study Sample. Inverse probability weights were estimated in the Total Study Sample using logistic regression in which an indicator for being in the analytic sample was regressed on demographic and clinical characteristics.
For all outcome regression models, the association between AUDIT-C change score and change in CD4 or logVL was assessed by testing the significance of the spline terms using an overall Wald test. To visualize the exposure-response relationship of AUDIT-C change with change in CD4 and logVL, we plotted mean change in CD4 and logVL for each possible AUDIT-C change score (−12 to +12) using the spline model. For adjusted models, predicted mean changes in CD4 and logVL were obtained using fixed covariate values; specifically: mean age, male gender, black race, mean values of initial CD4 or logVL, mean days between CD4 or VL measures, and absence of baseline depression and anxiety disorder diagnoses.
Based on possible social desirability bias in reporting reduced drinking, and because previous research among general outpatients demonstrated that decreases in alcohol use were not consistently associated with improvements in medical outcomes in the following year while increases were associated with increased risk of diverse medical outcomes (Bradley et al., 2016), we hypothesized that increases in AUDIT-C scores would be associated with decreases in HIV control, whereas decreases in AUDIT-C score may not be reflected in outcomes. To test these hypotheses, we tested a priori-specified contrasts to assess for differences in the mean change in CD4 and mean change in logVL associated with an increase in AUDIT-C by 2 points (AUDIT-C change score −2) vs. remaining stable (AUDIT-C change score 0); we similarly compared an increase in AUDIT-C by 5 points (AUDIT-C change −5) to remaining stable (AUDIT-C change 0) and an increase in AUDIT-C by 5 points (AUDIT-C change −5) to increased AUDIT-C by 8 points (AUDIT-C change −8).
2.3.3. Secondary Analyses.
Four sets of secondary analyses were conducted for each outcome. First, because initial level of drinking could result in ceiling effects, and because a previous study found that associations between changes in alcohol use and changes in medical outcomes depended on baseline level of drinking (Bradley et al., 2016), we repeated all regression analyses including the baseline AUDIT-C category as well as interaction terms between the baseline AUDIT-C category and the spline terms of AUDIT-C change. We conducted overall tests of interaction terms using Wald tests and then plotted the exposure-response function for each baseline AUDIT-C category. Second, because studies suggest associations between alcohol use and CD4 and VL may primarily operate indirectly through alcohol’s influence on ART adherence and other self-care behaviors (Azar et al., 2010; Hahn and Samet, 2010; Williams et al., 2016a), we repeated primary models (Block 2 models) in each sample stratified by detectable VL (500+ copies/ml) vs. suppressed VL (<500 copies/ml), using viral suppression as a proxy for ART adherence and self-care given the strong correlation with these constructs. Third, because the past-year timeframe elicited by the AUDIT-C does not enable distinction between lifetime abstinence and being a “sick-quitter” (Shaper et al., 1988), we repeated analyses among only patients who reported past-year drinking (AUDIT-C>0) at baseline and follow-up. Finally, though we selected to measure CD4 and VL within 9 months of AUDIT-C screens to maximize sample size and generalizability of results, we re-ran primary analyses in subsamples of patients with CD4 and VL measured within 6 months to decrease time between AUDIT-C and HIV severity measures.
All analyses were conducted using Stata v14 (StataCorp., 2014).
3. RESULTS.
3.1. Patient-Level Descriptive Results
Among 33,224 PLWH in the Total Study Sample, 21,999 patients (64,679 observations) met eligibility criteria for the CD4 sample and 22,143 (66,166 observations) met criteria for the VL sample (see Supplemental File 1). Overlap between the two was substantial: of the CD4 sample (n=21,999), 99% (n=21,734) were also in the VL sample; of the VL sample (n=22,143), 98% (n=21,734) were also in the CD4 sample. The median number of observations (pair of screens) each patient contributed was 3 (range 1–8) for both samples. The number of days between baseline and follow-up AUDIT-C screens ranged from 270 to 450 with a median of 364 and an inter-quartile range (IQR) of 333 – 394. For both CD4 and VL, the number of days between baseline and follow-up measures ranged from 271 to 711 with a median of 371 and an IQR of 329 – 421.
Patients were similar across the two samples (Table 1) and largely male (97%) and 50 years or older (65%); nearly half were black race/ethnicity, and mental health diagnoses were common, with nearly one-third of patients having depression, and nearly one-tenth having anxiety or serious mental illness. Approximately 14% had documented alcohol use disorders, and the prevalence of other substance use diagnoses ranged from 1.5% to 7.5%. Three-fourths of each sample was on ART at baseline, mean adherence was ~82%, and nearly three-fourths were virally suppressed. Characteristics of patients in the analytic sample were overall quite similar to those in the Total Study Sample though statistically significant differences were observed for all characteristics except serious mental illness (Table 1). Based on initial values in each analytic sample, mean CD4 decreased as AUDIT-C increased, the proportion with detectable VL increased as AUDIT-C increased and mean logVL increased slightly (all tests for linear trend p<0.001, Table 2).
Table 1.
Characteristics of VA Patients Living With HIV: CD4 and Viral Load Samples with Comparison to Total Study Sample*
| CD4 Sample N= 21,999 | Viral Load Sample N=22,143 | Total Study Sample* N = 33,224 | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | p-value** | N | (%) | p-value** | N | (%) |
| Demographics | ||||||||
| Gender (Female) | 606 | (2.8) | 0.011 | 613 | (2.8) | 0.02 | 971 | (2.9) |
| Age | <0.001 | <0.001 | ||||||
| <50 | 7,763 | (35.3) | 7,837 | (35.4) | 11,661 | (35.1) | ||
| 50—64 | 12,071 | (54.9) | 12,136 | (54.8) | 17,963 | (54.1) | ||
| ≥65 | 2,165 | (9.8) | 2,170 | (9.8) | 3,600 | (10.8) | ||
| Race/Ethnicity | <0.001 | <0.001 | ||||||
| Black | 10,676 | (48.5) | 10,741 | (48.5) | 16,028 | (48.2) | ||
| Hispanic | 1,870 | (8.5) | 1,880 | (8.5) | 2,691 | (8.1) | ||
| White | 8,864 | (40.3) | 8,916 | (40.3) | 13,346 | (40.2) | ||
| Other/Unknown | 589 | (2.7) | 606 | (2.7) | 1,159 | (3.5) | ||
| MH and SUD | ||||||||
| Depressive Disorder | 6,639 | (30.2) | <0.001 | 6,658 | (30.1) | <0.001 | 9,502 | (28.6) |
| Anxiety Disorder | 1,951 | (8.9) | 0.005 | 1,952 | (8.8) | 0.02 | 2,845 | (8.6) |
| Serious Mental Illness | 2,127 | (9.7) | 0.36 | 2,125 | (9.6) | 0.80 | 3,177 | (9.6) |
| Stimulant Use Disorder | 332 | (1.5) | 0.008 | 332 | (1.5) | 0.003 | 461 | (1.4) |
| Opioid Use Disorder | 807 | (3.7) | <0.001 | 811 | (3.7) | <0.001 | 1,325 | (4.0) |
| Other Drug Use Disorder | 1,654 | (7.5) | 0.04 | 1,653 | (7.5) | 0.01 | 2,570 | (7.7) |
| HIV Clinical Measure | ||||||||
| On antiretroviral therapy (ART) at Baseline | 16,690 | (75.9) | <0.001 | 16,761 | (75.7) | <0.001 | 21,080 | (63.5) |
| Mean ART Adherence (SD) | 81.2 | (24.4) | <.001 | 82.7 | (23.4) | <.001 | 79.1# | (26.2) |
| Detectable Viral Load | 4,581 | (23.5) | <.001 | 4,624 | (23.6) | <.001 | 6,650 | (26.2) |
| Alcohol Use Severity | ||||||||
| Alcohol Use Disorder | 3,061 | (13.9) | 0.009 | 3,053 | (13.8) | <0.001 | 4,742 | (14.3) |
| Alcohol-Specific Condition | 102 | (0.5) | <.001 | 104 | (0.5) | <.001 | 213 | (0.6) |
| Baseline AUDIT-C Category | <0.001 | <0.001 | ||||||
| 0 | 10,372 | (47.2) | 10,434 | (47.1) | 15,563 | (46.8) | ||
| 1–3 (1–2 for women) | 8,760 | (39.8) | 8,828 | (39.9) | 12,988 | (39.1) | ||
| 4–5 (3–5 for women) | 1,676 | (7.6) | 1,705 | (7.7) | 2,612 | (7.9) | ||
| 6–7 | 512 | (2.3) | 502 | (2.3) | 854 | (2.6) | ||
| 8–12 | 679 | (3.1) | 674 | (3.0) | 1,207 | (3.6) | ||
Total Study Sample includes all in the eligible HIV+ sample who had at least one AUDIT-C screen
all p-values reflect comparison between those who did and did not meet criteria for the analytic samples out of those in the Total Study Sample (e.g., comparison of those 21,999 in the CD4 analytic sample to the 11,225 not in CD4 analytic sample)
N=21,080 patients prescribed ART
Table 2.
Mean Baseline Values of HIV Disease Severity Across Baseline AUDIT-C Score Categories for Both Analytic Samples*
| CD4 Sample (n= 21,999) | Test for Trend p-value | ||
|---|---|---|---|
| Baseline AUDIT-C Score | N | Mean (SD) Baseline CD4 | <0.001 |
| 0 | 10372 | 511.3 (303.8) | |
| 1–3 (1–2 for women) | 8760 | 516.7 (293.6) | |
| 4–5 (3–5 for women) | 1676 | 506.0 (284.2) | |
| 6–7 | 512 | 504.2 (313.5) | |
| 8–12 | 679 | 434.7 (278.0) | |
| Viral Load Sample (n=22,143) | |||
| Baseline AUDIT-C Score | N | Mean (SD) Baseline logVL** | <0.001 |
| 0 | 10434 | 2.97 (0.68) | |
| 1–3 (1–2 for women) | 8828 | 3.03 (0.74) | |
| 4–5 (3–5 for women) | 1705 | 3.06 (0.74) | |
| 6–7 | 502 | 3.11 (0.80) | |
| 8–12 | 674 | 3.26 (0.88) | |
| N (%) with Detectable VL | <0.001 | ||
| 0 | 10434 | 1,979 (19.0) | |
| 1–3 (1–2 for women) | 8828 | 2,011 (22.8) | |
| 4–5 (3–5 for women) | 1705 | 447 (26.2) | |
| 6–7 | 502 | 149 (29.7) | |
| 8–12 | 674 | 246 (36.5) | |
Analyses were conducted at the patient (as opposed to the observation) level
Estimates for baseline logVL are high relative to current targets for suppression (<1.7 logs / < 50); viral suppression in the present study was at <2.7 logs / <500 copies/ml.
3.2. Observation-Level Descriptive Results
AUDIT-C scores generally stayed stable over time [mean change in AUDIT-C=0.08 (SD=1.9); median=0.00 (IQR=0.0–1.0)]. Overall, CD4 and VL improved over time [mean CD4 change=20.5 (SD 180.8); median=20.0 (IQR= −66.0–109.0); mean logVL change=0.12 (SD 0.71); median=0.0, IQR=0.0–0.0)]. The distribution of observations across AUDIT-C change score and baseline AUDIT-C categories are presented in Supplemental File 2. Approximately half of observations had AUDIT-C scores of 0 at baseline, and more than 40% had AUDIT-C scores of 0 at both baseline and follow-up. Among those with AUDIT-C>0, most were low-level drinkers at baseline and had little change in AUDIT-C. For a patient to have a large decrease in AUDIT-C, it would have been necessary to start with a very high score (e.g., a patient would have had an initial AUDIT-C of at least 6 to have a decrease in AUDIT-C of 6). In both samples, only ~10% of observations had a baseline AUDIT-C of 4 or more, with declining proportions at higher scores.
3.3. Observation-Level Regression Results
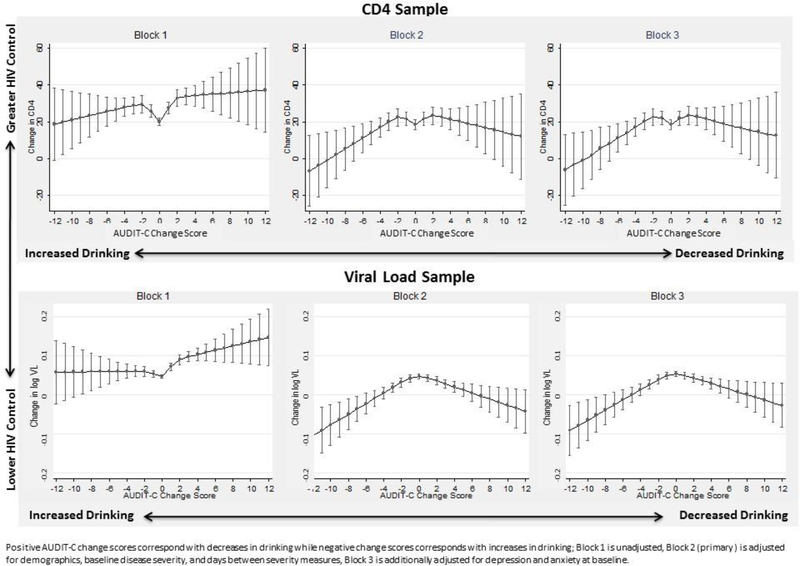
AUDIT-C change scores were associated non-linearly with changes in CD4 (Block 1 p<0.001, Block 2 p=0.03, Block 3 p=0.03) and changes in logVL (all Blocks p<0.001). In unadjusted analyses (Block 1), compared to observations with stable drinking (AUDIT-C change score 0) observations with increases and especially decreases in drinking tended to have greater improvements in CD4 compared to those with stable drinking (Figure 1). After covariate adjustment (Blocks 2 and 3), estimated improvement appeared highest among those with stable or small changes in AUDIT-C and lowest among those with the largest increases in drinking (Figure 1). For instance, among those with relatively stable AUDIT-C scores, estimated CD4 improvements ranged from 18.5 (95% CI 15.9–21.2) cells/μl for stable AUDIT-C to 23.3 (18.6–28.1) cells/μl for those with a 2-point AUDIT-C increase. In general, though estimates of the change in HIV disease severity were less precise among those with large changes in drinking due to fewer observations, large increases in drinking over time (negative AUDIT-C change score) were associated with less improvement in CD4 and logVL than commensurately large reductions in drinking (positive AUDIT-C change score). For instance, mean CD4 change for those whose AUDIT-C increased 6 points (AUDIT-C change score −6) was 11.2 cells/μl (95% CI 3.4–19.0) whereas the mean CD4 change for those whose AUDIT-C decreased 6 points (AUDIT-C change +6) was 19.1 cells/μl (95% CI 9.7–28.4). Results were similar when models were further adjusted for mental health diagnoses (Figure 1, Block 3).
Figure 1.
Unadjusted and adjusted association between AUDIT-C change scores and change in measures of HIV disease severity over one year among VA patients with HIV
In tests of pre-specified contrasts in the primary model (Block 2), mean CD4 change did not significantly differ between those whose AUDIT-C increased by either 2 or 5 points (AUDIT-C change scores −2 and −5, respectively), as compared to those whose AUDIT-C did not change. However, mean CD4 change was significantly lower for those whose AUDIT-C increased by 8 points [AUDIT-C change score −8; 5.2 cells/μl (95% CI −6.1–16.6)] compared to those whose AUDIT-C increased by 5 points [AUDIT-C change score −5; 14.1 cells/μl (95% CI 7.8–20.4)] (p=0.005; Table 3). For logVL, even incremental increases in drinking were associated with less improvement in logVL compared with individuals with stable drinking. Increasing drinking 2 and 5 points (AUDIT-C change scores −2 and −5, respectively) were both significantly associated with less improvement in logVL relative to no AUDIT-C change, as were AUDIT-C increases of 8 relative to 5 points (Table 3).
Table 3.
Association between AUDIT-C change scores and change in CD4 and log VL: Results of contrast tests* for comparison of mean changes in CD4 and log Viral Load between a priori- and post hoc- specified change scores.
| AUDIT-C change score | Predicted change in HIV-related Outcome | Difference in change in HIV-related outcome (95% CI) | p-value* | ||
|---|---|---|---|---|---|
| ΔA | ΔA' | ΔY (95% CI) | ΔY' (95% CI) | ΔY' − ΔY (95% CI) | |
|
CD4 Sample | |||||
| A priori contrasts to test hypothesis | |||||
| 0 | −2 | 18.5 (15.9, 21.2) | 22.5 (17.8, 27.1) | 4.0 (−1.1, 9.0) | 0.12 |
| 0 | −5 | 18.5 (15.9, 21.2) | 14.1 (7.8, 20.4) | −4.2 (−10.7, 2.2) | 0.20 |
| −5 | −8 | 14.1 (7.8, 20.4) | 5.2 (−6.1, 16.6) | −8.8 (−15.0, −2.8) | 0.005 |
|
Post-hoc contrast tests to assess effects of decreases in drinking | |||||
| 0 | +2 | 18.5 (15.9 – 21.2) | 23.3 (18.6, 28.1) | −4.9 (−10.1, 0.4) | 0.07 |
| 0 | +5 | 18.5 (15.9 – 21.2) | 20.2 (12.9, 27.6) | −1.7 (−9.3, 5.8) | 0.66 |
| +5 | +8 | 20.2 (12.9 – 27.6) | 16.7 (3.0, 30.5) | 3.5 (−3.8, 10.8) | 0.35 |
| +8 | −8 | 16.7 (3.0, 30.5) | 5.2 (−6.1, 16.6) | 11.5 (−6.5, 29.5) | 0.21 |
|
Viral Load Sample | |||||
| A priori contrasts to test hypothesis | |||||
| 0 | −2 | 0.05 (0.04, 0.05) | 0.03 (0.02, 0.04) | −0.02 (−0.03, −0.00) | 0.01 |
| 0 | −5 | 0.05 (0.04, 0.05) | −0.01 (−0.03, 0.01) | −0.06 (−0.08, 0.04) | <0.0001 |
| −5 | −8 | −0.01 (−0.03, 0.01) | −0.05 (−0.09, −0.01) | −0.04 (−0.06, −0.02) | 0.0001 |
|
Post-hoc contrast tests to assess effects of decreases in drinking | |||||
| 0 | +2 | 0.05 (0.04, 0.05) | 0.04 (0.02, 0.5) | −0.01 (−0.02, 0.00) | 0.07 |
| 0 | +5 | 0.05 (0.04, 0.05) | 0.01 (−0.00, 0.03) | −0.03 (−0.5, −0.02) | 0.0002 |
| +5 | +8 | 0.01 (−0.00, 0.03) | −0.01 (−0.04, 0.02) | −0.02 (−0.04, −0.01) | 0.009 |
| +8 | −8 | −0.01 (−0.04, 0.02) | −0.05 (−0.09, −0.01) | −0.04 (10.09, −0.01) | 0.13 |
KEY: Above, mean changes in the outcomes (ΔY) associated with an AUDIT-C change score (ΔA) are compared to mean changes in the outcomes ( ΔY') associated with another AUDIT-C change score (ΔA'); negative AUDIT-C change score indicates increased AUDIT-C; positive AUDIT-C change score indicates decreased AUDIT-C
p-value for contrast, tested using primary model (Block 2) adjusted for demographics, disease severity at baseline, and days between severity measures.
Although we hypothesized a priori that decreases in drinking may not be reflected in measures of HIV severity, plots of associations (Figure 1) suggest that changes in HIV severity may be sensitive to decreases in drinking. Thus, we conducted post-hoc contrast tests to understand whether decreases in AUDIT-C of 2 and 5 points (AUDIT-C change score +2 and +5, respectively) differed from no change, whether decreases of 8 points differed from decreases of 5 points (AUDIT-C change score +8 and +5, respectively), and whether decreases of 8 points (AUDIT-C change score +8) differed from increases of 8 points (AUDIT-C change score −8). For changes in CD4, there were no statistically significant differences for any tested contrast (Table 3). For changes in VL, there was no difference between decreases in AUDIT-C scores of 2 points and no change or between decreases of 8 points and increases of 8 points (Table 3), though sample sizes for tests were small (see Supplemental File 2). However, there was less improvement in VL associated with decreases in AUDIT-C scores of 5 points relative to no change and with decreases in AUDIT-C scores of 8 relative to 5 points.
3.4. Secondary Observational-Level Regression Results
No significant interactions between baseline AUDIT-C category and AUDIT-C change score were observed in associations with either change in CD4 (Block 1 p=0.83, Block 2 p=0.75, Block3 p=0.75) or logVL (Block 1 p=0.67, Block 2 p=0.28, Block 3 p=0.28). Plots of mean changes in outcomes across AUDIT-C changes scores were similar across baseline AUDIT-C strata (see Supplemental File 3).
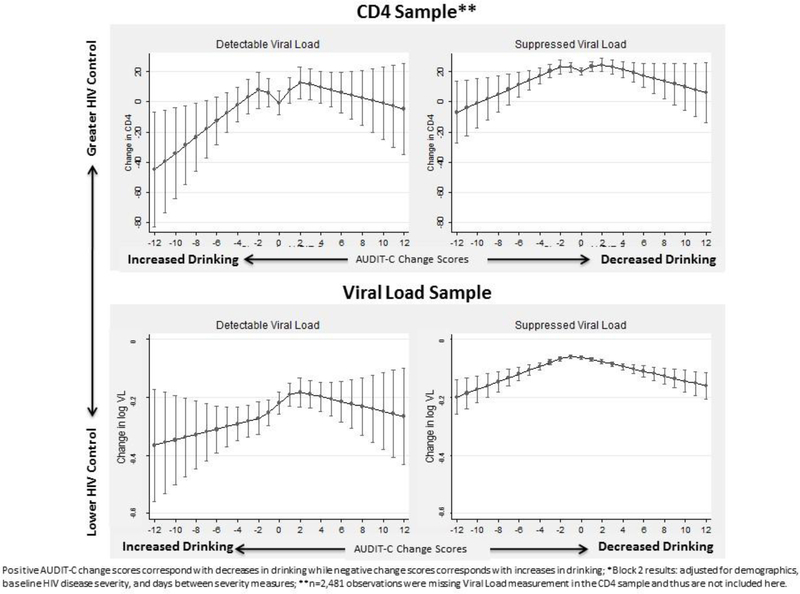
In analyses stratified by detectable versus suppressed viral load, a significant association between AUDIT-C change score and change in both CD4 and logVL was identified for each strata (p-values all <0.05). Patterns of associations were similar in those with detectable and suppressed viral loads (Figure 2). Because three-fourths of patients had suppressed viral loads, estimates were more precise among those with suppressed viral loads. Results were also similar when non-drinkers (those with AUDIT-C=0) at both time points were omitted and when a 6-month timeframe was used (instead of 9 months) for outcomes assessment (data not shown).
Figure 2.
Association* between AUDIT-C change scores and change in measures of HIV disease severity over one year: Stratified by HIV viral suppression
4. DISCUSSION
In this national sample of PLWH, most patients reported non- or low-level drinking at baseline, and greater alcohol use corresponded to greater HIV disease severity measured by CD4 and VL. Over time, drinking remained relatively stable, and HIV disease severity generally improved. Changes in alcohol use were associated with changes in HIV disease severity; PLWH with relatively stable drinking over time had the greatest improvements in both measures of HIV disease severity, while those whose drinking increased over time had the smallest improvements. This study is the first to our knowledge to assess whether individual-level (i.e., within person) changes in patient-reported alcohol use are associated with concomitant changes in markers of HIV disease severity over time, and thus the first to take a “counterfactual causality” (Hill, 2015) approach to assessing the influence of alcohol use on HIV disease severity. This is also the first study to evaluate the association between changes in alcohol use and HIV severity based on direct laboratory measures commonly used in HIV care to monitor disease progression.
Findings from this national study are consistent with prior studies that have found that alcohol use is associated with HIV control (Justice et al., 2016; Williams et al., 2016a) and with a previous study in general outpatients that found increases in drinking over time were associated with higher probability of subsequent gastrointestinal bleeding and trauma (Bradley et al., 2016). Findings from this study also complement those from the previous longitudinal study that assessed a scaled composite measure of HIV disease severity (Justice et al., 2012; Justice et al., 2013) using group-based trajectory analyses and identified the greatest likelihood of extreme HIV disease severity over time among a group of PLWH with consistent heavy alcohol use (Marshall et al., 2017).
In the present study, patients with relatively stable drinking fared best in terms of improvement in HIV severity. In secondary analyses plots stratified by baseline drinking revealed that, compared to relatively stable drinking, increases were generally associated with less improvement and decreases were not associated with additional improvement in CD4 or VL for any baseline AUDIT-C group except baseline 6–7 (the smallest group, for which both increases and decreases were associated with more improvement than relatively stable drinking). Many persons who drink at high levels and/or have alcohol use disorders have more dynamic drinking patterns than those who drink at low levels. Findings from this study suggest the possibility that patients with unstable drinking, especially those who increase drinking greatly over time, may be at greatest risk for poor HIV disease management. Further research is needed to investigate the influence of stable high-level drinking on HIV disease severity.
Though we hypothesized reductions in drinking may not be reflected in measures of HIV severity, patients who substantially decreased drinking at subsequent screening surprisingly had greater increases in VL compared to those with stable and smaller decreases in drinking. These findings may reflect uncontrolled alcohol use disorders, which commonly relapse and remit; decreases in drinking among patients with alcohol use disorders can be short-lived due to difficulty controlling drinking and may not result in immediate biological or behavioral changes. Alternatively, reductions in drinking may have been catalyzed by declining health (Shaper et al., 1988), thus associations observed may reflect reverse causality. These findings could also relate to unreliable reports of decreases over time. Known limitations to the quality of clinical alcohol screening in VA, which result in under-detection of drinking (Bradley et al., 2011; McGinnis et al., 2016; Williams et al., 2015), may have resulted in misclassification. Finally, it is possible that we did not have adequate power to understand associations between changes in drinking and changes in HIV disease severity for those with large changes due to increasingly small sample sizes.
Notably, associations between changes in drinking and changes in HIV severity measures did not differ substantially when stratified by detectable versus suppressed VL, though precision was limited among the former. Findings from these secondary analyses suggest the possibility that changes in alcohol use may influence changes in HIV disease severity regardless of baseline viral suppression. Further work is needed to investigate the potential moderating role of adherence and other HIV self-care behaviors.
This study has several limitations. First, while minority racial/ethnic groups are well-represented among PLWH receiving VA care, the VA population is largely older and male; thus, findings may not generalize to younger persons and/or women. Moreover, the study was conducted among patients receiving healthcare with documentation of key measures. Although analyses used inverse probability weighting to account for biases arising from differential loss to follow-up, results may not be generalizable to PLWH not engaged in healthcare and/or not screened for unhealthy alcohol use. Second, alcohol use measurement may have been influenced by both patient and provider-level factors (Bradley et al., 2011; Lapham et al., 2013; McGinnis et al., 2016; Williams et al., 2015) which may have resulted in inaccurate reflections of changes—particularly decreases—in drinking. Further research should be conducted using gold-standard assessments of alcohol use, (e.g., timeline follow-back methods) and/or biomarkers (Williams et al., 2016a). Third, though we adjusted for measured factors that could confound associations, residual confounding by unmeasured factors is possible. Finally, while a 9-month time window for identifying outcomes data was chosen to maximize sample size and findings were unchanged when a 6-month window was applied, sensitivity of outcomes to the alcohol use data may be limited.
5. CONCLUSIONS
Despite these limitations, this is the first large study to our knowledge to evaluate whether person-level changes in alcohol use were associated with concomitant changes in measures of HIV disease severity used clinically. Findings from this individual-level evaluation of the responsiveness of HIV disease severity to changes in drinking provide further support for the adverse influence of alcohol use on the health of PLWH and can therefore be useful for clinicians counseling patients about alcohol-related risks. These findings complement previous VACS research in a smaller, recruited sample, which assessed a scaled measure of risk for HIV disease severity and demonstrated that groups of PLWH with consistent heavy drinking trajectories have greater likelihood of trajectories of extreme HIV disease severity compared to PLWH with stable low drinking trajectories (Marshall et al., 2017). Future work is needed to evaluate the independent and combined influences of varying patterns of alcohol use (e.g., relative importance of stable versus dynamic compared to high versus low levels drinking) over time on HIV-relevant outcomes.
Supplementary Material
HIGHLIGHTS.
In PLWH receiving care, baseline alcohol use corresponds with HIV disease severity
Changes in alcohol use over time corresponded with changes in HIV disease severity
PLWH with stable alcohol use had the greatest improvement in HIV disease severity.
PLWH whose alcohol use increased over time had the smallest improvements.
ACKNOWLEDGMENTS
Views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the United States Government, the University of Washington, the University of California or the Group Health Research Institute.
ROLE OF FUNDING
This research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA022866-01; Williams/Bradley PIs) and COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795,U01-AA020799; U10 AA013566). Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12-276), and Dr. Bradley is supported by a mid-career mentorship award from NIAAA (K24-AA022128). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
CONFLICTS OF INTEREST
Dr. Bradley owns stock in Pfizer Pharmaceuticals. All other authors declare no potential conflicts of interest. Preliminary findings of this research study were presented at the Research Society on Alcoholism (RSA) Conference in Denver, CO in June 2017
AVAILABILITY OF DATA
The data that support the findings of this study are available from the Veterans Health Administration (VA) but restrictions apply to the availability of these data, due to patient confidentiality, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of VA, in compliance with current VA data management guidelines.
REFERENCES
- Azar MM, Springer SA, Meyer JP, Altice FL, 2010. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 112, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A, 2010. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 26, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, Maynard C, Burman ML, Kivlahan DR, 2003. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med 163, 821829. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, Kivlahan DR, 2011. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med 26, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Rubinsky AD, Lapham GT, Berger D, Bryson C, Achtmeyer C, Hawkins EJ, Chavez LJ, Williams EC, Kivlahan DR, 2016. Predictive Validity of Clinical AUDIT-C Alcohol Screening Scores and Changes in Scores for Three Objective Alcohol-related Outcomes in a Veterans Affairs (VA) Population. Addiction. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR, 2006. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care 12, 597–606. [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF, Justice AC, 2005. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res 29, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 158, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Deiss RG, Mesner O, Agan BK, Ganesan A, Okulicz JF, Bavaro M, Lalani T, O’Bryan TA, Bebu I, Macalino GE, 2016. Characterizing the Association Between Alcohol and HIV Virologic Failure in a Military Cohort on Antiretroviral Therapy. Alcohol Clin Exp Res 40, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski JC, Kitahata MM, Van Rompaey SE, Crane HM, Mugavero MJ, Eron JJ, Boswell SL, Rodriguez B, Mathews WC, Martin JN, Moore RD, Golden MR, 2013. High levels of antiretroviral use and viral suppression among persons in HIV care in the United States, 2010. J Acquir Immune Defic Syndr 63, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC, 2006. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 44, S25–30. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Samet JH, 2010. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep 7, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM, 2009. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr 52, 180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB, 1965. The Environment and Disease: Association or Causation? Proc R Soc Med 58, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB, 2015. The environment and disease: association or causation? 1965. J R Soc Med 108, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K, 2006. Veterans Aging Cohort Study (VACS): Overview and description. Med Care 44, S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K, 2012. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 54, 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, Edelman EJ, Fiellin LE, Freiberg MS, Gordon AJ, Kraemer KL, Marshall BD, Williams EC, Fiellin DA, 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 161, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, Kitahata MM, Horberg MA, Brooks JT, Buchacz K, Rourke SB, Rachlis A, Napravnik S, Eron J, Willig JH, Moore R, Kirk GD, Bosch R, Rodriguez B, Hogg RS, Thorne J, Goedert JJ, Klein M, Gill J, Deeks S, Sterling TR, Anastos K, Gange SJ, 2013. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 62, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis H, Kepler M, 2002. Multivariate cubic spline smoothing in multiple prediction. Comput Methods Programs Biomed 67, 131–136. [DOI] [PubMed] [Google Scholar]
- Kinder LS, Bryson CL, Sun H, Williams EC, Bradley KA, 2009. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs 70, 253–260. [DOI] [PubMed] [Google Scholar]
- Lapham GT, Rubinsky AD, Heagerty P, Williams EC, Hawkins EJ, Maynard C, Kivlahan DR, Bradley KA, 2013. Annual rescreening for alcohol misuse: diminishing returns for some patient subgroups. Med Care Epub 2013/08/21. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL, 1986. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22. [Google Scholar]
- Little RJA, Rubin DB, 2002. Complete-Case and Available-Case Analysis, Including Weighting Methods Statistical Analysis with Missing Data, Second Edition. John Wiley & Sons, Inc., [Google Scholar]
- Hoboken NJ. Marshall BDL, Tate JP, McGinnis KA, Bryant KJ, Cook RL, Edelman EJ, Gaither JR, Kahler CW, Operario D, Fiellin DA, Justice AC, 2017. Long-term alcohol use patterns and HIV disease severity. AIDS 31, 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, Kraemer KL, Maisto SA, Crystal S, Fiellin DA, Justice AC, 2016. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend 168, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American AIDS Cohort, 2017. North American AIDS Cohort collaboration on research and design https://statepiaps7.jhsph.edu/naaccord/.
- Royston P, 2000. Choice of scale for cubic smoothing spline models in medical applications. Stat Med 19, 1191–1205. [DOI] [PubMed] [Google Scholar]
- Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA, 2013. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res 37, 1380–1390. [DOI] [PubMed] [Google Scholar]
- Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA, 2010. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend 108, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, 2005. Alcohol dependence: chronic care for a chronic disease J Bras Psiquiatr 54, 268–269. [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R, 2007. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr 46, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Carey KB, Cunningham K, Johnson BT, Carey MP, 2016. Alcohol use predicts sexual decision-making: A systematic review and meta-analysis of the experimental literature. AIDS Behav 20 Suppl 1, 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, Walker M, 1988. Alcohol and mortality in British men: explaining the Ushaped curve. Lancet 2, 1267–1273. [DOI] [PubMed] [Google Scholar]
- StataCorp., 2014. Stata Statistical Software: Release 14. StataCorp LP, College Station, TX. [Google Scholar]
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL, 2015. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review : alcohol and the HIV continuum of care. Curr HIV/AIDS Rep 12, 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, Bradley KA, 2015. Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study. J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH, 2016a. Alcohol use and Human Immunodeficiency Virus (HIV) Infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res 4 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Joo YS, Lipira L, Glass JE, 2016b. Psychosocial stressors and alcohol use, severity, and treatment receipt across human immunodeficiency virus (HIV) status in a nationally representative sample of US residents. Subst Abus, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.