Supplemental Digital Content is Available in the Text.
Keywords: Neuropathic pain, Gene therapy, Helper-dependent adenoviral vectors, Homing peptide, GAD67, DRG
Abstract
Introduction:
Currently available medications for neuropathic pain are of limited efficacy. Moreover, they are administered systemically and are associated with significant side effects. Ideally, one can circumvent systemic side effects if such treatment can be administered by delivery of the therapeutic agent directly to the diseased neurons. Towards this end, we previously reported the production of a recombinant helper-dependent adenovirus (HDAd) armed with a tissue-specific homing peptide to deliver transgenes targeting sensory neurons with high efficacy.
Objectives:
To develop an effective gene therapy for neuropathic pain by producing a dorsal root ganglion (DRG)-targeted HDAd vector that specifically expresses glutamic acid decarboxylase (GAD) 67 (HDAd-DRG-GAD67).
Methods:
We produced spinal nerve transection (SNT) mice as a neuropathic pain model and delivered HDAd-DRG-GAD67 by injection into spinal nerve or intrathecally to these animals. We evaluated the therapeutic efficacy by measuring ion channel gene expression and quantifying mechanical allodynia, a representative symptom of neuropathic pain, in treated animals.
Results:
Glutamic acid decarboxylase expression by HDAd-DRG-GAD67 reduced allodynia significantly in SNT mice. In addition, HDAd-DRG-GAD67 had a much greater transduction efficacy and expressed the therapeutic gene for a much longer time and at a lower dose of viral particles than wild-type HDAd. We found that SNT induced the upregulation of Cav3.2 mRNA in the DRG and GAD67 overexpression suppressed the elevation. Furthermore, the HDAd-DRG-GAD67–induced allodynia amelioration occurred even when we delayed intrathecal delivery of the therapeutic vector to day 7 after SNT.
Conclusion:
HDAd-mediated DRG-targeted gene therapy delivering GAD67 is an efficacious treatment for neuropathic pain in SNT mice.
1. Introduction
Neuropathic pain is defined as pain caused by a lesion or disease in the somatosensory nervous system.34 Current treatments for the condition remain unsatisfactory.2 Some types of neuropathic pain are characterized by sensory abnormalities that range from unpleasant abnormal sensations (dysesthesia), to an increased response to painful stimuli (hyperalgesia), to pain in response to a stimulus that does not normally provoke pain (allodynia).52 These chronic symptoms adversely impact the daily activities and quality of life of affected patients.
In the pathogenesis of neuropathic pain, several ion channels, eg, voltage-gated sodium channels Nav1.3 and 1.8, and voltage-gated calcium channel Cav3.2, are upregulated in the dorsal root ganglion (DRG), increasing the level of neuronal excitability in the injured nerve.3,31,49,53 Neuropathic pain induced by nerve injury can also result from increased chronic excitability in the spinal dorsal horn,36 whereas the hypofunction of gamma-aminobutyric acid (GABA)ergic neurons is involved in the pathogenesis of allodynia.30 Gamma-aminobutyric acid, synthesized from glutamate by glutamic acid decarboxylase (GAD), is the principal inhibitory neurotransmitter in the nervous system. There are 2 GAD isoforms, GAD65 and GAD67, of which GAD67 is mainly expressed in the cytosol of DRG neurons where it acts as the rate-limiting enzyme of GABA synthesis.6,29 Previous studies showed that GAD knockout mice develop induced pain,54 and that GAD and GABA contribute to ameliorating hyperexcitability to pain induced in neuropathic pain model animals.18,24 Although the mechanism whereby the spinal GABAergic system suppresses neuropathic pain remains poorly understood, a number of studies showed that GAD expression in the DRG/spinal cord effectively relieves neuropathic pain states.7,12,19,20,27,29,50 It is conceivable that synaptic connections between DRG neurons and the spinal dorsal horn modulate excitability of the GABAergic neurons. Thus, the induced activation of GABAergic inhibition in the injured DRG/spinal dorsal horn may constitute a promising treatment strategy.
The stability of neurotrophic polypeptides delivered to the DRG remains challenging because of their susceptibility to proteolytic degeneration, markedly limiting the duration of their therapeutic effects. Although the direct delivery of GABA to DRG was found to reduce neuropathic pain,8,9 its application necessitated additional measures, such as implantation of a pump device for continuous infusion to compensate for the short half-life of exogenous GABA delivered in vivo.8 To circumvent this drawback, we developed an in vivo strategy that enabled the stable induction of GABA expression in DRG using targeted gene therapy. This approach offers the advantage of a reduction in the number of injections needed, and thus the potential systemic side effects associated with the treatment. We believe that the same principle applies to the treatment of neuropathic pain. Recently, we developed DRG-targeted helper-dependent adenoviral vectors (HDAds) that display low toxicity and high transduction efficiency.46 In this study, we tested the efficacy of using such vectors for the treatment of neuropathic pain. Use of fiber-modified HDAds enabling the DRG-specific overexpression of GAD67 was found to be highly effective in the treatment of allodynia in spinal nerve transection (SNT) mice, a widely used neuropathic pain model.17,21
2. Materials and methods
2.1. Construction of the fiber-modified helper-dependent adenoviral vector expressing GAD67 with a dorsal root ganglion homing peptide (HDAd-DRG-GAD67/HDAd-GAD67)
We prepared HDAd vector 293Cre cells and a fiber-modified HDAd vector as described previously.38,39,46 To engineer HDAd-expressing GAD67 with a modified fiber containing the DRG homing peptide sequence (HDAd-DRG-GAD67), we cloned mouse GAD67 cDNA (NM_008077.4) from mouse brain tissue into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) using reverse transcriptase–polymerase chain reaction (PCR) with the following primers: forward, 5′-ATGGCATCTTCCACTCCTTC-3′; reverse, 5′-TTACAGATCCTGACCCAACCT-3′. These cDNAs, which included a Kozak sequence immediately preceding the ATG codon, were subcloned into pBOS vector that contains the elongation factor-1 promoter and rabbit beta-globin polyadenylation signal. The expression cassettes were excised by AscI digestion and subcloned into pΔ28 vectors for producing HDAd vectors (pΔ28-BOS-GAD67-pA). We then used the AdEasy system (Stratagene) to generate fiber-modified helper virus (HV) containing the DRG homing peptide (amino acids sequence: SPGARAF) inserted into the BspEI site at the center of artificial linker (GGSGGG) between amino acid number 542 and 543 in the HI loop of the fiber protein (HV-DRG, in Ref. 46 and Fig. 1A, B). Helper virus or HV-DRG are the first generation adenoviral vectors containing the packaging signal (Φ) flanked by 2 loxP sites, beta-geo gene, and no motif or DRG motif with a deleted E1 site, which are amplified in 293 or 293fiber cells. These HV or HV-DRG provide all the components necessary for replication and packaging of the HDAd genome in trans but cannot be packaged by itself on coinfection with HDAd to 293Cre cells because it lacks an HV packaging signal (Fig. 1B).
Figure 1.
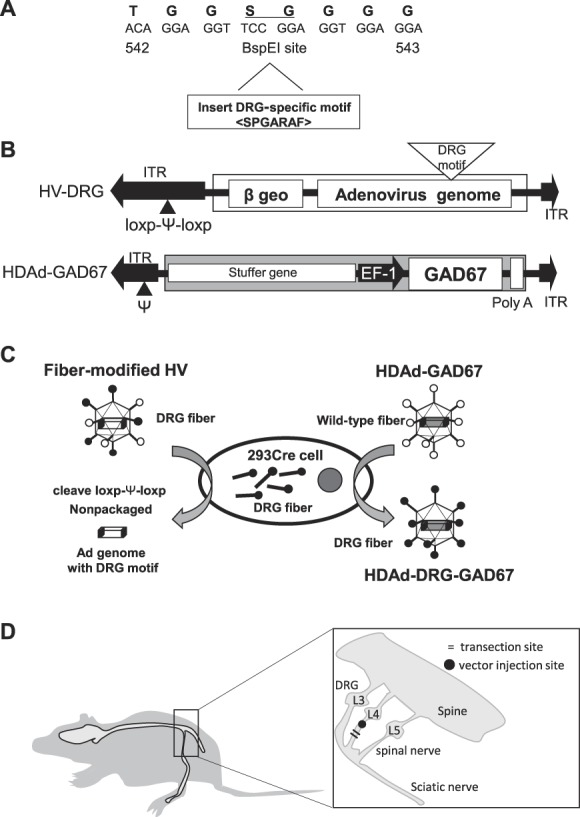
Construction and administration of HDAd containing DRG-specific motif in the fiber. (A) DNA coding for DRG homing peptide (SPGARAF) was cloned into the BspEI site present in the linker peptide (GGSGGG) between amino acid number 542 and 543 in the HI loop of adenoviral fiber protein. DNA sequence coding for the peptide is shown under the amino acid sequence. Underline shows the BspEI site. A, alanine; F, phenylalanine; G, glycine; P, proline; R, arginine; S, serine; T, threonine. (B) Schematic presentation for the construction of helper virus with a chimeric fiber containing DRG homing peptide (HV-DRG) and HDAd expressing the glutamic acid decarboxylase (GAD) 67 gene (HDAd-GAD67). ITR, inverted terminal repeat; Ψ, packaging signal; EF-1, elongation factor 1 promoter. (C) Schematic presentation of the final step in generating HDAd expressing GAD67 with DRG homing peptide (HDAd-DRG-GAD67). HDAd-DRG-GAD67 was constructed in 293Cre cells by coinfecting HDAd-GAD67 and fiber-modified HV. Helper virus genome is not packaged into HDAd-DRG-GAD67 because the packaging signal (Ψ) of HV is excised by Cre in 293Cre cells. Open circle indicates wild-type fiber; black circle indicates DRG fiber. (D) Schematic presentation of neuropathic pain model of mouse induced by spinal nerve transection (SNT). Lumbar (L)4 SNT induces mechanical allodynia in the ipsilateral hind paw. We administrated therapeutic vectors into the L4 spinal nerve immediately after SNT. Right panel shows an enlargement of the transection site. DRG, dorsal root ganglion; HDAd, helper-dependent adenovirus; HV, helper virus.
The therapeutic gene cassette (pΔ28-BOS-GAD67-pA) was transfected into 293Cre cells and followed infection of HV into same cells to generate HDAd-GAD67. To generate HDAd-DRG-GAD67, HDAd-GAD67 and HV-DRG were coinfected into 293Cre cells (Fig. 1C), and the vectors were purified and stored at −80°C until required. The vector titer was determined from a purified DNA concentration using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific K.K., Tokyo, Japan).
2.2. Cell culture
We maintained 293Cre and 293fiber cells in a 5% CO2 incubator at 37°C and grew them in α-MEM medium (Wako Pure Chemical Industries, Ltd, Osaka, Japan) with 10% fetal bovine serum and antibiotics: G418 (0.8 mg/mL) for 293Cre cells and puromycin (1 µg/mL) for 293fiber cells. Infection was performed in serum-free medium (α-MEM; Wako Pure Chemical Industries, Ltd).
2.3. Animal studies
2.3.1. Ethics statement
All animal protocols were approved by the Institutional Animal Care and Use Committee of Shiga University of Medical Science (Approval Number: 2015-12-3). All procedures were performed in accordance with the guidelines of the Research Center for Animal Life Science of Shiga University of Medical Science.
2.3.2. Animals
We used 9- to 10-week-old male C57BL6 mice (Japan CLEA, Osaka, Japan) weighing 19.0 to 22.0 g in this study. The mice were housed in separate cages with free access to food and water under a 12-hour:12-hour light–dark cycle.
2.3.3. Surgical procedures
We generated L4 SNT neuropathic pain model as described previously.21,37,41 Briefly, we anesthetized mice by the intraperitoneal administration of sodium pentobarbital (5 mg/kg). After the midline incision of the mouse back skin, we removed the bilateral L5 transverse processes of the lumbar spine, exposed the L4 spinal nerves bilaterally, which were the largest nerve connected to sciatic nerve, and pinched and transected only the left nerve (Fig. 1D). The right spinal nerve, as a control, was exposed without transection.
2.3.4. Vector administration for the in vivo study
We used 2 different vector injection protocols in this study. In the prevention protocol, we injected 1 µL of treatment vector or vehicle (saline) into the proximal transected site of the left L4 spinal nerve using a Hamilton syringe (8001 1701LT; Hamilton Co., Reno, NV) with a 30-G needle immediately after transecting the left L4 spinal nerve (on day 0 after SNT) (Fig. 1D). Briefly, we gently pinched up the transected spinal nerve and touched the epineurium of L4 spinal nerve with needle and then injected vectors into the subepineurium during the SNT procedure, incurring minimal additional nerve damage. We used 3 different vector concentrations: 107 vp/µL, 108 vp/µL, and 109 vp/µL for this study.
In the treatment study, we prepared SNT mice on day 0 and administrated 3 µL of therapeutic vector vs vehicle control intrathecally using a Hamilton syringe with a 30-G needle 7 days after transecting the left L4 spinal nerve (day 7 after SNT).
2.3.5. Behavioral test
We evaluated the mechanical allodynia before injury (day-2) and at 1, 3, 5, 7, 10, 12, and 14 days after SNT in the prevention study. In the treatment study, we assessed mechanical allodynia on day-2 and at 1, 3, 5, 7, 8, 10, 12, and 14 days after SNT. In both studies, mechanical allodynia was measured as the withdrawal threshold against the response to mechanical stimuli by a dynamic plantar aesthesiometer (Ugo Basil, Varese, Italy) as described previously.37 Briefly, we placed individual mice in polypropylene boxes with a metallic mesh floor and allowed them to acclimatize to the testing environment for at least 1 hour. A stimulating filament probe was then positioned under the hind paw, which was gradually applied until the mouse withdrew its paw. The pressure was increased at approximately 10 g/mm2/s. The test was performed on both the intact right or neuropathic left hind paw. The withdrawal threshold was determined as the mean of 3 trials. For the interval of each stimulus, sufficient time was allowed to pass until mice become stable. The examiner was blinded to the groups during the study period.
And, we also tested withdrawal latency from a radiant heat source, for which no differences were noted (data not shown). Therefore, quantification of mechanical allodynia was used as the indicator of the treatment effects for neuropathic pain.
2.3.6. Reverse transcription–polymerase chain reaction and quantitative reverse transcription polymerase chain reaction analysis
We removed animal tissues under deep anesthesia and froze them immediately in liquid nitrogen. Total RNA was extracted from frozen tissues using the RNeasy mini kit (Qiagen, Valencia, CA) with DNase I (Rnase-free DNase set, Qiagen) treatment. Reverse transcription was performed from 100 ng of total RNA using Prime Script Perfect Real Time (Takara Bio Inc, Kusatsu, Shiga, Japan). Reverse transcription PCR was performed with the following primers: 5′-AACGACCCCTTCATTGAC-3′ and 5′-TCCACGACATACTCAGCAC-3′ for mouse GAPDH, 5′-CACAAACTCAGCGGCATAGA-3′ and 5′-CTGGAAGAGGTAGCCTGCAC-3′ for mouse GAD67, 5′-GCTGTTTGGGAGGCTAGAAT-3′ and 5′-CGAAGGTGACGAAGTAGACG-3′ for mouse Cav3.2, 5′-ACGTGGGGTCTGAGAATGAC-3′ and 5′-TGGCTATGCTCATGGCTCTT-3′ for mouse Nav1.3, and 5′-ATGTGGGTGCAGCGATAGAC-3′ and 5′-CCAAGGCAAAGACACTCAGG-3′ for mouse Nav1.8. For the quantification of each gene, real-time PCR was performed using a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) using the SYBR Green method. Polymerase chain reaction parameters were 95°C for 3 minutes, followed by 50 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. The emitted fluorescence for each reaction was measured 3 times during the annealing extension phase, and amplification plots were analyzed using LightCycler 480 software, version 1.5 (Roche Diagnostics). Potential genomic DNA contamination was controlled by the use of intron-encompassing primers and DNase digestion. Normalization and relative expression analysis of target genes were performed by the 2−ΔΔCt method with GAPDH as a control.
2.3.7. Immunohistochemistry
We prepared tissues for immunohistochemistry with perfusion fixation. Animals were anesthetized by the intraperitoneal administration of sodium pentobarbital (50 mg/kg) and intracardially perfused with 30 mL of phosphate-buffered saline followed by a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer. After perfusion fixation, animal tissues were kept in the same fixative at 4°C overnight and permeated with 15% (wt/vol) sucrose buffer with agitation. The DRG or spinal cord was embedded in Tissue-Tek O.C.T compound (Sakura, Tokyo, Japan), frozen with liquid nitrogen, and cut on a cryostat into 8-μm sections that were collected on Matsunami aggressive silane-coated glass slides. Sections were blocked with 3% normal goat serum in phosphate-buffered saline at room temperature for 15 minutes and processed for immunohistochemistry. The following antibodies were used: anti-GAD65 + GAD67 primary antibody (ab49832, 1:100; Abcam, Cambridge, United Kingdom) and AlexaFluor 594 (1:1000; Abcam) secondary antibody for single staining of GAD expression in the DRG or lumbar spinal cord. For double staining with GAD and Cav3.2 in the DRG, mouse anti-GAD67 monoclonal antibody (MAB5406, 1:100; Millipore, Temecula, CA) and rabbit anti-Cav3.2 antibody (C1868; Sigma, St. Louis, MO) were used as primary antibodies. Then, each section was incubated with species-matched secondary antibodies (AlexaFluor 555 anti-mouse IgG [1:1000; Abcam] and AlexaFluor 647 anti-rabbit IgG [1:1000; Abcam]). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured and analyzed using a Nikon C1si system and EZ-C1 software, version 3.90 (Nikon, Tokyo, Japan). We prepared 3 consecutive sections (each 30-µm interval) from mid of the L4 DRG per mouse and randomly selected over 3 neuron-rich/fiber-poor areas (100 × 100-µm square each), regardless of proximal or distal including over 180 neurons. The fluorescence intensity with anti-GAD antibody-positive stain was determined by ImageJ software (National Institute of Health, Bethesda, MD).43 To evaluate transduction efficacy after administration of the therapeutic vectors, we counted the number of GAD-positive neurons under pretreatment conditions, and on day 7 and 14 after vector injection into the spinal nerve. As with GAD-positive neurons, we measured Cav3.2-positive neurons at pretreatment condition and on day 14 after vector injection. We analyzed approximately 300 neurons in consecutive sections (30-µm intervals) for each left L4 DRG.
2.4. Statistical analysis
All analyses were performed by SPSS software 22.0 (Chicago, IL). For behavioral tests through the entire period, the difference in mechanical allodynia among the groups was examined by 1-way repeated-measures ANOVA with post-hoc comparison using the Tukey method. On each time point of behavioral tests, 1-way ANOVA followed by the Tukey test was conducted. For the analysis of mRNA expression, cell counts, and fluorescence intensity, the difference was determined by 1-way ANOVA followed by the Tukey multiple comparison test among the groups. Data were considered significant at P < 0.05. All results are expressed as means ±SE.
3. Results
3.1. HDAd-mediated GAD67 gene delivery protects against spinal nerve transection–induced neuropathic pain in a dose-dependent manner
To examine whether HDAd-GAD67 could prevent neuropathic pain induced by SNT, we evaluated the antiallodynic effect in SNT mice by measuring the mechanical threshold on day − 2 as a control, and on days 1, 3, 5, 7, 10, 12, and 14 days after SNT as the allodynic state. We evaluated the efficacy of HDAd-GAD67 treatment at 3 concentrations, 107 vp/µL, 108 vp/µL, and 109 vp/µL, and the same volume of vehicle (control group). Each dose of HDAd-GAD67 was administrated once into the proximal site of the injured spinal nerve immediately after SNT. Although the mechanical threshold was significantly reduced in the ipsilateral hind paw in all groups, the threshold was much higher in the 108 vp and 109 vp HDAd-GAD67 groups compared with the 107 vp HDAd-GAD67 and vehicle groups 14 days after SNT (Fig. 2). In the contralateral hind paw, mechanical allodynia was absent in all groups (Fig. 2). In addition, the mechanical threshold in the ipsilateral hind paw was significantly higher in the 109 vp HDAd-GAD67 group compared with the 108 vp HDAd-GAD67 group on days 5 and 7 after SNT (Fig. 2). However, on days 10, 12, and 14 after SNT, the mechanical thresholds were not significantly different between the 108 vp and 109 vp groups, although both these groups had a higher mechanical threshold than the 107 vp and vehicle groups (Fig. 2). These results indicate that HDAd-GAD67 prevented severe mechanical allodynia in a dose-dependent manner. Although HDAd-GAD67 produced a strong therapeutic effect with a high dose of vector, the effect gradually disappeared over the course of 7 to 14 days. The best therapeutic response against neuropathic pain was obtained with a dose of 109 vp of HDAd-GAD67.
Figure 2.
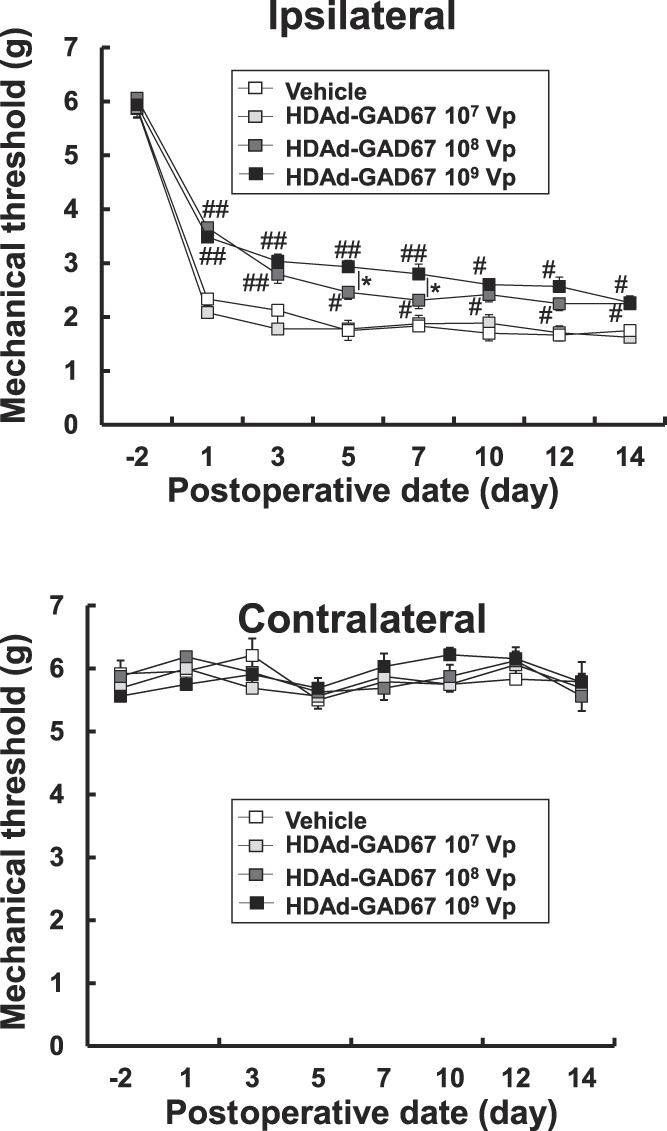
The preventive gene therapy with HDAd-GAD67 for neuropathic pain induced by spinal nerve transection (SNT). The mechanical threshold was measured in the bilateral hind paw 14 days after SNT and treatment with HDAd-GAD67 (n = 8 per each group) or vehicle (control, n = 6). ##P < 0.01, #P < 0.05 compared with HDAd-GAD67 107 Vp or vehicle group, *P < 0.05. Bars indicate mean ±SE. HDAd, helper-dependent adenovirus.
3.2. Dose-dependent dorsal root ganglion–targeted HDAd-mediated GAD67 gene delivery protects against spinal nerve transection–induced neuropathic pain
We next determined whether the DRG-targeted HDAd vector, HDAd-DRG-GAD67, could effectively reduce the neuropathic pain induced by SNT. We measured the mechanical threshold on day-2 as a pre-SNT condition and on days 1, 3, 5, 7, 10, 12, and 14 after SNT as for the HDAd-GAD67 vector, and tested 3 concentrations of HDAd-DRG-GAD67 (107 vp/µL, 108 vp/µL, and 109 vp/µL), using vehicle as a control. The identical protocol was used to deliver HDAd-DRG-GAD67 as for HDAd-GAD67 as described above.
The mechanical threshold was reduced in the ipsilateral hind paw in all groups but was markedly higher in 108 vp and 109 vp HDAd-DRG-GAD67 groups compared with 107 vp HDAd-DRG-GAD67 or with vehicle groups 14 days after SNT (Fig. 3). In the contralateral hind paw, there was no evidence of mechanical allodynia in any of the treatment groups (Fig. 3). Even on days 7 to 14 after SNT, the mechanical threshold in the ipsilateral hind paw was much higher in the 108 vp and 109 vp groups, and therefore, there was no difference in the degree of allodynia between the 108 vp and 109 vp treatment groups (Fig. 3). These results indicate that the administration of 108 vp HDAd-DRG-GAD67 produced an antiallodynic effect that is as good as 109 vp, a response that lasted at least 14 days.
Figure 3.
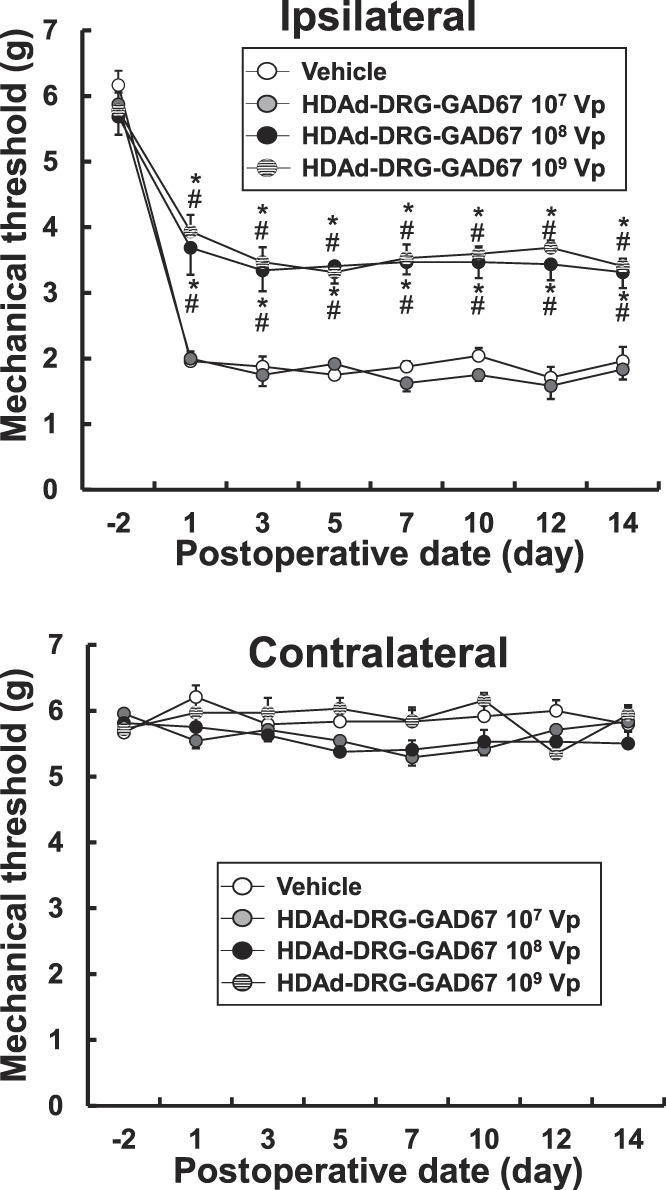
The preventive gene therapy with HDAd-DRG-GAD67 against neuropathic pain induced by spinal nerve transection (SNT). The mechanical threshold was measured in the bilateral hind paw 14 days after SNT and treatment with 107 Vp (n = 6), 108 Vp (n = 8), and 109 Vp (n = 8) HDAd-DRG-GAD67 groups, which were compared with the vehicle group (n = 6). #P < 0.01 compared with the HDAd-DRG-GAD67 107 Vp group and *P < 0.01 compared with the vehicle group. Bars indicate mean ±SE. DRG, dorsal root ganglion; HDAd, helper-dependent adenovirus.
3.3. Efficacy of 109 VP HDAd-GAD67 vs 108 VP HDAd-DRG-GAD67 in preventing spinal nerve transection–induced neuropathic pain
The above experiments indicate that both HDAd-GAD67 and HDAD-DRG-GAD67 were efficacious in preventing SNT-induced neuropathic pain in mice. Furthermore, to be effective, the doses had to be >107 vp. For the HDAd-GAD67, a non–DRG-targeted vector, 109 vp was the optimal dose used. By contrast, for the DRG-targeted vector, HDAd-DRG-GAD67, a dose of 108 vp was as effective as the higher dose of 109 vp. We next compared the efficacy of 109 vp HDAd-GAD67 vs 108 vp HDAd-DRG-GAD67 against SNT-induced allodynia. We administered single injections of HDAd-GAD67 (109 vp) and HDAd-DRG-GAD67 (108 vp) as before and measured mechanical thresholds before and at various times after vector administration. Significant treatment benefits were observed in both treatment groups and continued at a high level for 14 days without diminishing in the HDAd-DRG-GAD67 group, which was markedly, and significantly, better than in the HDAd-GAD67 group, especially on days 5 to 14 after SNT (Fig. 4A). Therefore, use of the DRG-targeted HDAd-DRG-GAD67 was substantially more effective than a 10-fold higher dose of a non–DRG-targeted HDAd-GAD67 in protecting against neuropathic pain in the SNT mouse model.
Figure 4.
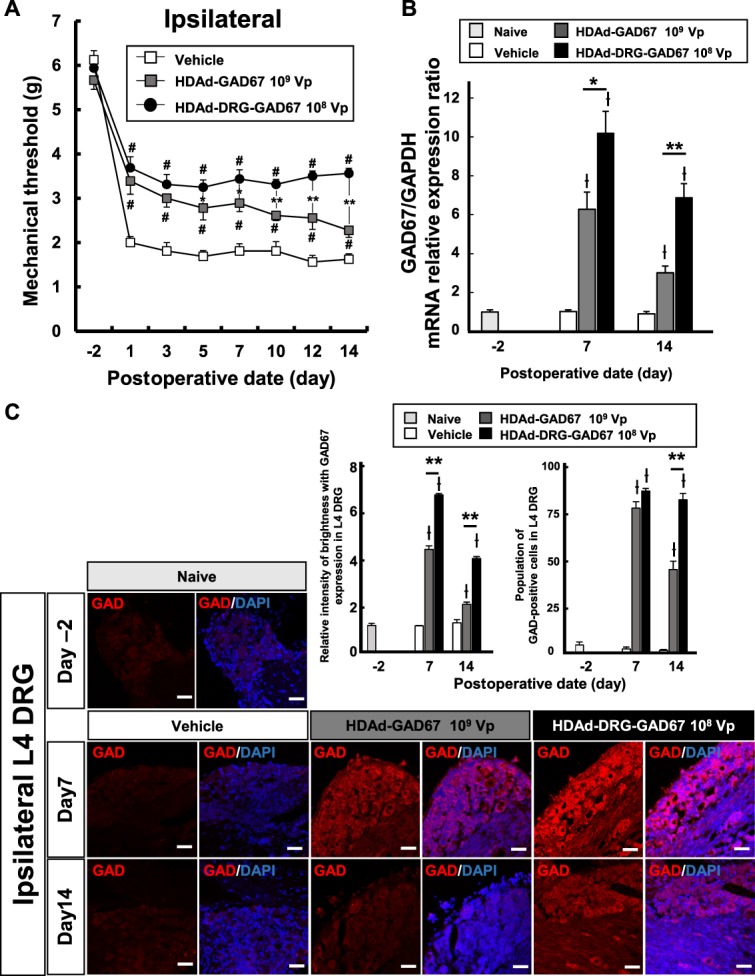
The preventative effect of optimal dose of HDAd-GAD67 or HDAd-DRG-GAD67 against neuropathic pain. (A) The mechanical threshold was measured in 109 Vp HDAd-GAD67 and 108 Vp HDAd-DRG-GAD67 groups, which were compared with the vehicle group for 14 days (n = 8 per group). (B) Relative GAD67 mRNA expression in L4 DRG tissues after SNT and injection of HDAd-GAD67 or HDAd-DRG-GAD67 (n = 6 per group) against the naive group (n = 4). Data were standardized to GAPDH mRNA expression. (C) Immunohistochemistry with anti-GAD antibodies (red) and DAPI (blue) in the ipsilateral L4 DRG on day −2, 7, and 14 after SNT with HDAd-GAD67 or HDAd-DRG-GAD67. Left panels show immunostainings of anti-GAD, and right panels show merged images of GAD and DAPI staining. Bar graphs show relative intensities of GAD staining (n = 5 per each group) in the left side and the percentage of GAD-positive neurons in the L4 DRG (n = 5 per each group) in the right side. #P < 0.01 compared with vehicle, *P < 0.05, **P < 0.01, ƚP < 0.01 compared with the naive group. Bars indicate mean ±SE. Scale bar = 50 μm. DRG, dorsal root ganglion; GAD, glutamic acid decarboxylase; HDAd, helper-dependent adenovirus; SNT, spinal nerve transection.
3.4. Vector-induced glutamic acid decarboxylase expression in L4 dorsal root ganglion tissue after HDAd-GAD67 or HDAd-DRG-GAD67 delivery in the prevention of neuropathic pain
We used quantitative reverse transcription PCR of GAD67 mRNA and GAD protein immunohistochemistry to quantify GAD gene expression in the ipsilateral L4 DRG after SNT. GAD67 mRNA level was unchanged by SNT but was markedly increased after the delivery of the 2 gene vector (Fig. 4B). In addition, the expression of GAD67 mRNA was ∼50% higher on day 7 and ∼100% higher on day 14 after delivery of 108 vp of HDAd-DRG-GAD67 compared with that of 109 vp of HDAd-GAD67 (Fig. 4B).
Similarly, the expression of GAD protein was very low and unchanged by SNT in naive DRG and in the control group on days −2, 7, and 14 after SNT (Fig. 4C). However, GAD protein expression in injured DRG was much higher in both treatment groups than in the naive group (Fig. 4C). Relative GAD protein expression increased significantly on days 7 and 14 after SNT in both HDAd-GAD67 and HDAd-DRG-GAD67 groups compared with the naive group (Fig. 4C). In particular, expression in the HDAd-DRG-GAD67 group was significantly higher than that in the HDAd-GAD67 group on days 7 and 14 (Fig. 4C). In addition, we evaluated the transduction efficacy by counting the GAD-positive neurons. Both treatments induced GAD-positive neurons in the DRG much greater number in DRG tissue on days 7 and 14 after SNT with treatment compared with the naive or vehicle group. Furthermore, the number of such neurons was highest with HDAd-DRG-GAD67 group in all groups on day 14 after SNT (Fig. 4C).
These data suggest that HDAd-DRG-GAD67 induced a continuous high expression of GAD67 mRNA and GAD protein at a lower dose of viral particles compared with the untargeted HDAd-GAD67, consistent with a much higher transduction efficiency for the DRG-targeted vector.
3.5. mRNA expression of ion channels related to neuropathic pain in dorsal root ganglia after HDAd-GAD67 or HDAd-DRG-GAD67 treatment
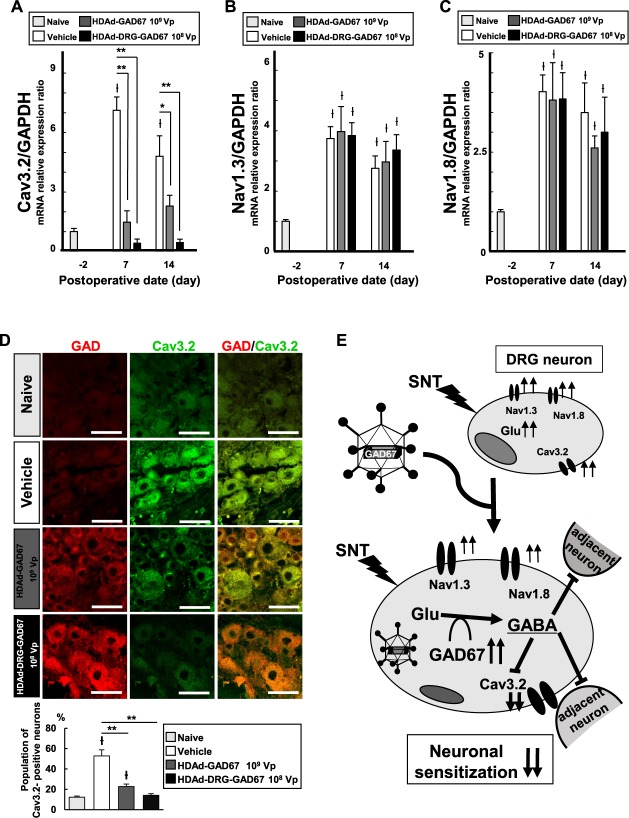
Previous reports suggested that several ion channels are upregulated in DRG in a neuropathic state, and that they individually play an important role in neuropathic pain.5,13 Specifically, Cav3.2,53 Nav1.3,26 and Nav1.823 are upregulated in the DRG neurons of rodent models of neuropathic pain. We therefore analyzed the level of expression of these mRNAs expression in ipsilateral L4 DRG in SNT mice.
We measured the mRNA levels of Cav3.2 (Fig. 5A), Nav1.3 (Fig. 5B), and Nav1.8 (Fig. 5C) by quantitative PCR in DRG tissues on days −2, 7, and 14 after SNT. We found all 3 mRNAs to be significantly upregulated after SNT in ipsilateral L4 DRG tissue; levels tended to be higher on day 7 than on day 14 (Fig. 5A–C). However, the elevation of Cav3.2 mRNA expression was markedly suppressed by the overexpression of GAD67 by HDAd-GAD67 and HDAd-DRG-GAD67 on days 7 and 14 after SNT (Fig. 5A). Cav3.2 mRNA suppression was achieved by a lower dose of HDAd-DRG-GAD67 vector compared with HDAd-GAD67; in fact, HDAd-DRG-GAD67 completely reversed the Cav3.2 mRNA levels down to baseline (Fig. 5A). Nav1.3 and Nav1.8 mRNA expression upregulated by SNT was unchanged in the absence or presence of HDAd-GAD67 or HDAd-DRG-GAD67 treatment (Fig. 5B, C). In immunohistochemical staining with Cav3.2 and GAD67 in DRG, GAD-positive neurons displayed less intense Cav3.2 staining in the HDAd-DRG-GAD67 group (Fig. 5D). In quantitative evaluation of Cav3.2 staining, SNT significantly upregulated the population of Cav3.2-positive neurons in the L4 DRG from the vehicle group (Fig. 5D, bar graph). In both HDAd-GAD67 and HDAd-DRG-GAD67 treatment groups, their population was much lesser than that in the vehicle group. Moreover, the population in the HDAd-DRG-GAD67 group was as low as the naive group (Fig. 5D, bar graph). These data suggest that GAD67 gene transduction by HDAd-DRG-GAD67 ameliorated neuropathic pain behavior at least in part by suppressing Cav3.2 expression (Fig. 5E).
Figure 5.
Relative mRNAs expression of ion channels and immunohistochemistry of GAD67 and Cav3.2 in the injured DRG after SNT and injection of the optimal dose of HDAd-GAD67 or HDAd-DRG-GAD67. (A–C) Cav3.2, Nav1.3, and Nav1.8 mRNA expression ratio in the ipsilateral L4 DRG were measured on day 7 and 14 after SNT and treatment against the mRNA expression at day − 2. Data were standardized to GAPDH mRNA expression (n = 4–6 per each group). (D) Immunohistochemistry with anti-GAD67 and anti-Cav3.2, and the population of Cav3.2-positive neurons in the injured L4 DRG on day 14 after SNT and treatment (n = 4–5 per each group). Left panels show immunostaining of anti-GAD67 (red), middle panels show that of anti-Cav3.2 (Green), and right panels show merged images in each group. Bar graphs show the percentage of the population of Cav3.2-positive neurons in the injured L4 DRG. (E) Schematic molecular mechanism underlying neuropathic pain. HDAd-DRG-GAD67 efficiently induced GAD67 overexpression in targeted DRG. GAD67 promoted the conversion glutamic acid (Glu) to γ-aminobutyric acid (GABA). Gamma-aminobutyric acid decreases Cav3.2 upregulation and acts as an inhibitory neurotransmitter for adjacent neurons. Regular icosahedron with black circle indicates HDAd-DRG-GAD67. *P < 0.05, **P < 0.01, and ƚP < 0.05 compared with the naive group. Bars indicate mean ± SE. Scale bar = 50 μm. DRG, dorsal root ganglion; HDAd, helper-dependent adenovirus; SNT, spinal nerve transection.
3.6. Treatment of neuropathic pain with intrathecal injections of HDAd-GAD67 and HDAd-DRG-GAD67 in the late phase injury after spinal nerve transection
We performed a treatment study by overexpressing GAD67 induced by a single intrathecal injection of HDAd-GAD67 or HDAd-DRG-GAD67 against neuropathic pain induced by SNT (Fig. 6A). We evaluated the treatment effect in SNT mice by measuring the mechanical threshold on day − 2 as a control state of SNT, and on days 1, 3, 5, and 7 after SNT as a pretreatment condition. After measuring the mechanical threshold on day 7, we injected 3 µL of vehicle (as a control), HDAd-DRG-GAD67 108 vp/µL, or HDAd-GAD67 at 108 vp/µL or 109 vp/µL to compare their level of efficacy of treatment. After the injections, mechanical stimuli measurements were continued on days 8, 10, 12, and 14 (as a days 1, 3, 5, and 7 after treatment) (Fig. 6B). In all groups, SNT induced mechanical allodynia in the left hind paw for 14 days, and there was no difference among the groups until day 7 after SNT.
Figure 6.
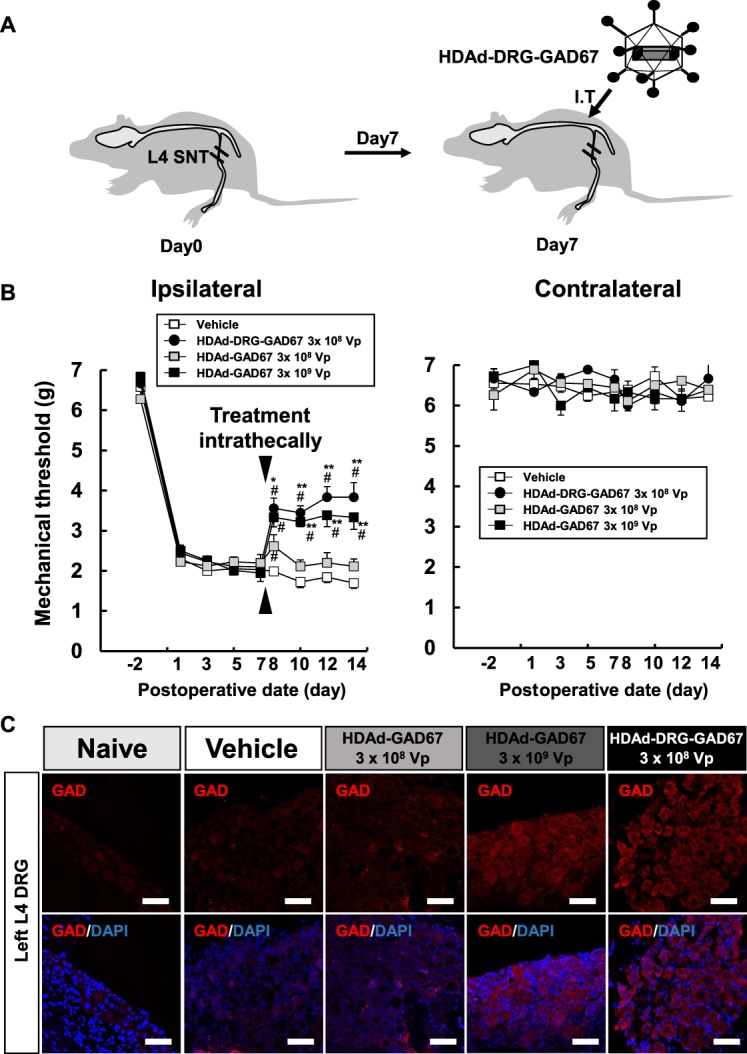
HDAd-DRG-GAD67 gene therapy ameliorates neuropathic pain induced by SNT. (A) Schematic presentation of treatment for SNT-induced neuropathic pain. Spinal nerve transection was performed on day 0 and treatment vectors were administrated intrathecally on day 7. Regular icosahedron indicates HDAd-DRG-GAD67. I.T., intrathecal injection. (B) The mechanical threshold on the bilateral hind paw was measured on days −2, 1, 3, 5, and 7 after SNT as pretreatment condition and days 8, 10, 12, and 14 after SNT as posttreatment condition (n = 5 per vehicle or HDAd-GAD67 3 × 108 Vp group, n = 6 per HDAd-DRG-GAD67 3 × 108 Vp or HDAd-GAD67 3 × 109 Vp group). (C) Immunohistochemistry of GAD in the injured L4 DRG on day 14 after SNT (day 7 after intrathecal treatment) with treatment. Upper panels show immunostainings of anti-GAD (red), and lower panels show merged images with DAPI (blue) staining. #P < 0.01 compared with the vehicle group, **P < 0.01, *P < 0.05 compared with the HDAd-GAD67 3 × 108 Vp group. Bars indicate mean ± SE. Scale bar = 50 μm. DRG, dorsal root ganglion; GAD, glutamic acid decarboxylase; HDAd, helper-dependent adenovirus; SNT, spinal nerve transection.
After treatment on day 7, mechanical allodynia was significantly reduced in the gene therapy groups compared with the control group, with the highest antiallodynic effect observed in the HDAd-DRG-GAD67 group (Fig. 6B). Treatment with HDAd-DRG-GAD67 produced a better improvement at a lower dose than HDAd-GAD67 treatment. Furthermore, the high dose (3 × 109 vp) group of HDAd-GAD67 showed an equivalent or slightly inferior effect than the 3 × 108 vp HDAd-DRG-GAD67 group, but the low-dose (3 × 108 vp) HDAd-GAD67 group achieved a much smaller and shorter antiallodynic effect (Fig. 6B). Spinal nerve transection and vector administration did not induce allodynia in the contralateral hind paw (Fig. 6B). We examined the expression of GAD protein in the target DRG on day 14 after SNT (day 7 after intrathecal injection of treatment vectors). As observed in the prevention study with injection into the spinal nerve, the expression of GAD was as low as in naive DRG and in treatment with the vehicle group and in the treatment group with low dose of HDAd-GAD67 groups on day 14 after SNT (Fig. 6C). However, GAD protein expression in injured DRG was very high in treatment groups with 3.0 × 108 vp of HDAD-DRG-GAD67 and high dose of HDAd-GAD67.
These data suggest that HDAd-DRG-GAD67 induced GAD protein at a lower dose of viral particles compared with HDAd-GAD67 even administrated intrathecally. In addition, we tested whether the GAD expression in the spinal cord was elevated by intrathecal injection of the treatment vector. The mRNA and the protein expression of GAD67 were not elevated by either treatment in spinal dorsal horns at 14 days after SNT (supplemental Figure, available at http://links.lww.com/PR9/A33). These results demonstrate that HDAd-DRG-GAD67 is a powerful gene therapy tool against neuropathic pain in any phase of pain development; as the vector seems to work even after the onset of neuropathic pain in a mouse model, it may be a practical therapeutic approach to this challenging clinical condition.
4. Discussion
In this study, we demonstrated a novel strategy for treating neuropathic pain using fiber-modified HDAd to deliver GAD67 to the DRG. Overexpression of GAD67 in the DRG suppressed the SNT-induced elevation of Cav3.2 mRNA and Cav3.2-positive neuron in the DRG. Our data suggested that GAD67 expression in the DRG induced by DRG-targeted HDAd significantly and more persistently reduced neuropathic pain compared with nontargeted HDAd. In addition, DRG-targeted HDAd had a much greater transduction efficiency and expressed the therapeutic gene at a lower dose and for a much longer time in the DRG compared with treatment using HDAd with wild-type fibers. Furthermore, DRG-targeted HDAd treatment was effective for neuropathic pain both at the early and late phases after nerve injury. Intrathecal administration is routinely used in clinical practice, so our strategy of gene therapy against neuropathic pain could have promising applications in the clinic.
4.1. Benefits of gene therapy with helper-dependent adenoviral vector for neuropathic pain
In the clinical state, neuropathic pain is associated not only with trauma but also systemic diseases such as autoimmune disease (eg, rheumatoid arthritis, vasculitis, or Sjögren syndrome), metabolic disease (diabetes mellitus), and neurological disease of peripheral nerves, including small fiber neuropathy and chemotherapy for cancer or HIV.1,10,22,44 Several medicines such as antiepileptic drugs, antidepressants, and opioids have been used to reduce neuropathic pain based on the pharmacological action of ion channel blocking. However, these agents have limited efficacy and possess considerable unwanted side effects on motor function, the central nervous system, and cardiac function.14 Gene therapy could be a promising approach to reduce systemic side effects; it could effect long-term therapeutic transgene expression with a single administration and could be used to deliver multiple and/or large genes to sensory neurons.16 However, viral vector-mediated gene therapy is associated with a number of problems including limited transduction efficiency, relatively nonspecific targeting, and safety.48 In addition, the clinical application of gene therapy is limited by potential oncogenesis, mutations, host immune response, and transduction efficacy.48 In response to these concerns, HDAd offers the advantages of a high transduction efficacy, the absence of a viral genome, a relatively low immune response and toxicity, and the absence of induced oncogenesis.4,38,39,46 Furthermore, the fiber-modified HDAd developed in our laboratory displays a highly selective tropism for target organs, including neurons, which markedly reduces its toxicity in nontargeted cells and tissues compared with wild-type HDAd.46
In this study, we demonstrate the power of DRG-targeted HDAd in gene therapy for neuropathic pain. We believe that tissue-targeted HDAd has potential applications in clinical trials. Indeed, there are ongoing clinical trials using viral vectors for the treatment of cancer45 and neurodegenerative diseases.32 Here, we achieved a high transduction efficiency and long-term efficacy of GAD67 overexpression by a single injection of tissue-targeted HDAd against neuropathic pain, which could help prepare the way towards clinical trials using this powerful vector.
4.2. Administration method
The safety of the administration method must be taken into account in clinical applications. We demonstrated 2 administration approaches in this study: injection into the spinal nerve and intrathecal injection. In both approaches, HDAd-DRG-GAD67 induced a high transduction efficacy from a single injection, supporting our previous observation of high transduction efficacy after the intrathecal injection of DRG-targeted HDAd.46
After spinal nerve injection, HDAd-DRG-GAD67 could potentially access the injured DRG by direct infiltration and/or axonal transport similar to that reported after injection into the gastrocnemius muscle for motor neuron targeting.35 After intrathecal injection, HDAd-DRG-GAD67 could directly access the DRG through the cerebrospinal fluid. The intrathecal HDAd delivery could deliver the therapeutic gene to multiple DRG by a single intrathecal injection, even if multiple DRG were injured at the same time, not only by trauma but also by systemic disease. In addition, the routinely practiced intrathecal injection of DRG-targeted HDAd offers advantages for clinical applications. Direct injection of therapeutic vectors into the DRG would provide high transduction efficacy.11 However, direct injection is a required advanced technique and radiation facility compared with lumbar puncture because of the pin-point injection to the DRG or spinal nerve. And, the procedure is taken time and effort because the tip of needle for injection should be checked by radiography or fluoroscopy.40 It is noteworthy that DRG-targeted HDAd vectors enable the high-level transduction of therapeutic gene comparable with that of direct injection of nontargeted vectors. In this study, intrathecal injection of HDAd-DRG showed high transduction efficiency and significant relief of neuropathic pain, which suggested that our strategy was superior at feasibility and efficacy.
4.3. Gene therapy for neuropathic pain with GAD67
Several studies demonstrated the potential of alternative treatment for neuropathic pain with the elevation of GAD67 in sensory neurons.7,12,19,20,27,29,50 These studies also reported success of treatment by gene therapeutic methods against the pain behavior induced by spinal cord injury,27,29 streptozotocin-induced diabetes,50 and HIV drugs,19,20 whether GAD67 expression was reduced in sensory neurons under those disease condition or not. In this study, overexpression of GAD67 effectively relieved neuropathic pain, although GAD expression did not change in the untreated DRG after SNT. To express GAD67 in the DRG or spinal dorsal horn, previous studies used recombinant herpes simplex viral (HSV) vector19,20,27,50 or human foamy virus (HFV) vector.29 These studies administrated the respective vectors subcutaneously in the hind paw and achieved gene delivery to DRG at the lumbar level and/or spinal dorsal horn. These vectors are indeed quite promising, especially in the case of HSV-based vector that displays fine tropism for sensory neurons and also has the potential of axonal transport in general. However, wild-type HSV itself causes neuropathic pain and encephalitis, so the safety of recombinant HSV vectors using for neuropathic pain must be thoroughly assess before its clinical use. In addition, the level of HSV-mediated GAD67 expression 2 weeks after administration of vector subcutaneously in hind paw appeared to be relatively modest19; in comparison, the DRG-targeted HDAd vector seemed to achieve substantial overexpression of GAD67 in DRG even 14 days after vector administration. This high level of transduction efficiency could be important for the treatment of chronic pain. In HFV vectors, however, it was not reported the relation to disease in naturally or experimentally infected animals with HFV; further studies would be needed before the vector can be considered for clinical application.28,29,33,42
The administration of treatment vectors subcutaneously was the safety procedure for clinical use. The injection subcutaneously and the method we demonstrated the local injection into injury site of nerve could be promising for delivering therapeutic gene to the single DRG or DRG related to identifiable lesion of injured sensory nerve located in specific anatomical regions. The treatment target tissue against neuropathic pain was the multiple or overall DRG with several degree of injury in systemic disease. In these conditions, taken together, it was not suitable to inject into injury site of the sensory nerve, or subcutaneously, but intrathecal HDAd-DRG-GAD67 could circumvent many of the toxicity of systemic administration.
4.4. Molecular mechanism of neuropathic pain
Recent studies suggested that crosstalk between the immune and nervous systems might underlie neuropathic pain.47 Indeed, nerve injury–induced increases in neuronal excitability resulted in the upregulation of several ion channels in the sensory nervous system, such as some of voltage-gated Na+ and Ca2+ channels in the DRG and spinal dorsal horn.3,31,36,49,53 Here, we found that the suppression of neuronal sensitization after induced GAD67 overexpression in the DRG that increased GABAergic transmission. Our data indicate that GAD67 overexpression suppressed the elevation of Cav3.2 expression in the DRG, but not Na+ channels. We propose that complete remission for neuropathic pain could be achieved by targeting the combination of GABAergic and other neuronal systems that involved in pain regulation.
GABAergic transmission appears to have less effect on the immune system than the nervous system. Proinflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, and IL-6 play a pivotal role in multiple neuropathic pain pathways and are clear treatment targets.25,47,51 In this study, we showed that SNT upregulate Nav1.3 and Nav1.8 mRNA expression, and that GAD67 overexpression does not affect Na+ channel expression. Both Nav1.3 and Nav1.8 had previously been shown to increase the current densities of tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels in DRG neurons by TNF-α.15 In an earlier study, we reported the shRNA silencing of TNF-α in the DRG using a lentiviral vector against neuropathic pain.37 Because the immune system is associated with the initiation and maintenance of neuropathic pain,25,47 a dual treatment approach targeting both the immune and nervous systems might prove effective.
Furthermore, in the pain model induced by HIV chemotherapy, it was reported that downregulation of GAD occurred in the DRG and spine.19 By contrast, our data suggest that SNT was not associated with downregulation of GAD in the DRG. The difference could be related to the use of different animal species or model. It was noted again overexpression of GAD67 in the DRG is effective in alleviating neuropathic pain; it may also suppress neuronal sensitization through downregulation of expression of Cav3.2.
In conclusion, we presented a novel strategy for treating neuropathic pain using DRG-targeted HDAd-expressing GAD67, which suppressed the elevation of Cav3.2 mRNA expression and significantly reduced mechanical allodynia. HDAd-DRG-GAD67 also provided long-term therapeutic effects after a single injection and high transduction efficacies at a lower dose compared with wild-type HDAd. Moreover, this treatment was successful, although it started at a late phase after the onset of injury. Our findings might facilitate the clinical application of gene therapy for neuropathic pain.
Disclosures
The authors have no conflicts of interest to declare.
This study was supported by a Grant-in-Aid (#23790988 to T.T.) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by research funding from The Nakatomi Foundation.
Acknowledgements
The authors thank Fumiko Kimura, Jun Munekata, Astuki Tanaka, and the Central Research Laboratory of Shiga University of Medical Science for excellent technical assistance.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A33.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].André N, Carré M, Brasseur G, Pourroy B, Kovacic H, Briand C, Braguer D. Paclitaxel targets mitochondria upstream of caspase activation in intact human neuroblastoma cells. FEBS Lett 2002;532:256–60. [DOI] [PubMed] [Google Scholar]
- [2].Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol 2018;17:456–66. [DOI] [PubMed] [Google Scholar]
- [3].Bourinet E, Francois A, Laffray S. T-type calcium channels in neuropathic pain. PAIN 2016;157:S15–S22. [DOI] [PubMed] [Google Scholar]
- [4].Brunetti-Pierri N, Ng P. Gene therapy with helper-dependent adenoviral vectors: lessons from studies in large animal models. Virus Genes 2017;53:684–91. [DOI] [PubMed] [Google Scholar]
- [5].Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chattopadhyaya B, Cristo DG, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang J. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 2007;54:889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chattopadhyay M, Mata M, Fink DJ. Vector-mediated release of GABA attenuates pain-related behaviors and reduces Na(V)1.7 in DRG neurons. Eur J Pain 2011;15:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Du X, Hao H, Yang Y, Huang S, Wang C, Gigout S, Ramli R, Li X, Jaworska E, Edwards I, Deuchars J, Yanagawa Y, Qi J, Guan B, Jaffe DB, Zhang H, Gamper N. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 2017;127:1741–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eaton MJ, Martinez MA, Karmally S. A single intrathecal injection of GABA permanently reverses neuropathic pain after nerve injury. Brain Res 1999;835:334–9. [DOI] [PubMed] [Google Scholar]
- [10].Ghosh AK, Jana S, Das T, Sa G, Mandal N, Ray PK. Protection by protein A of apoptotic cell death caused by anti-AIDS drug zidovudine. Biochem Biophys Res Commun 1999;264:601–4. [DOI] [PubMed] [Google Scholar]
- [11].Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Büeler H, Aguzzi A. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A 2000;97:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol 2005;57:914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harriott AM, Gold MS. Contribution of primary afferent channels to neuropathic pain. Curr Pain Headache Rep 2009;13:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haslam C, Nurmikko T. Pharmacological treatment of neuropathic pain in older persons. Clin Interv Aging 2008;3:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, Liu XG. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. PAIN 2010;151:266–79. [DOI] [PubMed] [Google Scholar]
- [16].Huang Y, Liu X, Dong L, Liu Z, He X, Liu W. Development of viral vectors for gene therapy for chronic pain. Pain Res Treat 2011;2011:968218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol 2011;25:1–28. [DOI] [PubMed] [Google Scholar]
- [18].Kami K, Taguchi S, Tajima F, Senba E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol Pain 2016;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kanao M, Kanda H, Huang W, Liu S, Yi H, Candiotti KA, Lubarsky DA, Levitt RC, Hao S. Gene transfer of glutamic acid decarboxylase 67 by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 combined with ddC in rats. Anesth Analg 2015;120:1294–404. [DOI] [PubMed] [Google Scholar]
- [20].Kanda H, Kanao M, Liu S, Yi H, Iida T, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. HSV vector-mediated GAD67 suppresses neuropathic pain induced by perineural HIV gp120 in rats through inhibition of ROS and Wnt5a. Gene Ther 2016;23:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. PAIN 1992;50:355–63. [DOI] [PubMed] [Google Scholar]
- [22].Kuntzer T, Antoine JC, Steck AJ. Clinical features and pathophysiological basis of sensory neuronopathies (ganglionopathies). Muscle Nerve 2004;30:255–68. [DOI] [PubMed] [Google Scholar]
- [23].Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. PAIN 2002;95:143–52. [DOI] [PubMed] [Google Scholar]
- [24].Lee JY, Fink DJ, Mata M. Vector-mediated gene transfer to express inhibitory neurotransmitters in dorsal root ganglion reduces pain in a rodent model of lumbar radiculopathy. Spine (Phila Pa 1976) 2006;31:1555–8. [DOI] [PubMed] [Google Scholar]
- [25].Leung L, Cahill CM. TNF-alpha and neuropathic pain—a review. J Neuroinflamation 2010;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lindia JA, Kohler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1. 3 expression and neuropathic pain behavior in rats. PAIN 2005;117:145–53. [DOI] [PubMed] [Google Scholar]
- [27].Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, Fink DJ. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther 2004;10:57–66. [DOI] [PubMed] [Google Scholar]
- [28].Liu W, He X, Cao Z, Sheng J, Liu H, Li Z, Li W. Efficient therapeutic gene expression in cultured rat hippocampal neurons mediated by human foamy virus vectors: a potential for the treatment of neurological diseases. Intervirology 2005;48:329–35. [DOI] [PubMed] [Google Scholar]
- [29].Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, Xue L, Miao L, He X, Li W. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett 2008;432:13–18. [DOI] [PubMed] [Google Scholar]
- [30].Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology 2002;96:1161–7. [DOI] [PubMed] [Google Scholar]
- [31].Markman JD, Dworkin RH. Ion channel targets and treatment efficacy in neuropathic pain. J Pain 2006;7:S38–S47. [DOI] [PubMed] [Google Scholar]
- [32].Marks WJ, Jr, Baumann TL, Bartus RT. Long-term safety of patients with Parkinson's disease receiving rAAV2-neurturin (CERE-120) gene transfer. Hum Gene Ther 2016;27:522–7. [DOI] [PubMed] [Google Scholar]
- [33].Mergia A, Chari S, Kolson DL, Goodenow MM, Ciccarone T. The efficiency of simian foamy virus vector type-1 (SFV-1) in nondividing cells and in human PBLs. Virology 2001;280:243–52. [DOI] [PubMed] [Google Scholar]
- [34].Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. In: IASP task force on taxonomy. Seattle: IASP Press; 1994. p. 209–14. [Google Scholar]
- [35].Murakami T, Nagano I, Hayashi T, Manabe Y, Shoji M, Setoguchi Y, Abe K. Impaired retrograde axonal transport of adenovirus-mediated E. coli LacZ gene in the mice carrying mutant SOD1 gene. Neurosci Lett 2001;308:149–52. [DOI] [PubMed] [Google Scholar]
- [36].Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol 2012;22:81–91. [DOI] [PubMed] [Google Scholar]
- [37].Ogawa N, Kawai H, Terashima T, Kojima H, Oka K, Chan L, Maegawa H. Gene therapy for neuropathic pain by silencing of TNF-α expression with lentiviral vectors targeting the dorsal root ganglion in mice. PLoS One 2014;9:e92073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oka K, Chan L. Helper-dependent adenoviral vectors. In: Current protocols in molecular biology. Hoboken: John Wiley & Sons Inc., 2005. p. 16.24.11–16.24.23. [DOI] [PubMed] [Google Scholar]
- [39].Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, Beaudet A, Chan L. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation 2001;103:1274–81. [DOI] [PubMed] [Google Scholar]
- [40].Pfirrmann CW, Oberholzer PA, Zanetti M, Boos N, Trudell DJ, Resnick D, Hodler J. Selective nerve root blocks for the treatment of sciatica: evaluation of injection site and effectiveness—a study with patients and cadavers. Radiology 2001;221:704–11. [DOI] [PubMed] [Google Scholar]
- [41].Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. PAIN 2008;136:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Saïb A, Puvion-Dutilleul F, Schmid M, Périès J, de Thé H. The HD: nuclear targeting of incoming human foamy virus Gag proteins involves a centriolar step. J Virol 1997;71:1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine 2018;85:553–9. [DOI] [PubMed] [Google Scholar]
- [45].Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, Gomella LG, Kamat AM, Lotan Y, Svatek RS, Bivalacqua TJ, Grubb RL, III, Krupski TL, Lerner SP, Woods ME, Inman BA, Milowsky MI, Boyd A, Treasure FP, Gregory G, Sawutz DG, Yla-Herttuala S, Parker NR, Dinney CPN. Intravesical rAd-IFNα/Syn3 for patients with high-grade, Bacillus Calmette-Guerin-refractory or relapsed non-muscle-invasive bladder cancer: a phase II randomized study. J Clin Oncol 2017;35:3410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Terashima T, Oka K, Kritz AB, Kojima H, Baker AH, Chan L. DRG-targeted helper-dependent adenoviruses mediate selective gene delivery for therapeutic rescue of sensory neuronopathies in mice. J Clin Invest 2009;119:2100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vallejo R, Tilley D, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 2010;10:167–84. [DOI] [PubMed] [Google Scholar]
- [48].Verma IM. Somia N Gene therapy-promises, problems and prospects. Nature 1997;389:239–42. [DOI] [PubMed] [Google Scholar]
- [49].Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain 2011;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang Y, Nowicki MO, Wang X, Arnold WD, Fernandez SA, Mo X, Wechuk J, Krisky D, Goss J, Wolfe D, Popovich PG, Lawler S, Chiocca EA. Comparative effectiveness of antinociceptive gene therapies in animal models of diabetic neuropathic pain. Gene Ther 2013;20:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wells MR, Racis SP, Jr, Vaidya U. Changes in plasma cytokines associated with peripheral nerve injury. J Neuroimmunol 1992;39:261–8. [DOI] [PubMed] [Google Scholar]
- [52].Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999;353:1959–64. [DOI] [PubMed] [Google Scholar]
- [53].Yue J, Liu L, Liu Z, Shu B, Zhang Y. Upregulation of T-type Ca2+ channels in primary sensory neurons in spinal nerve injury. Spine (Phila Pa 1976) 2013;38:463–70. [DOI] [PubMed] [Google Scholar]
- [54].Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 2011;17:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]