Supplemental Digital Content is Available in the Text.
Keywords: Controllability, Pain, Suffering, Assessment, Locus of control
Abstract
Introduction:
Chronic pain and pain-related suffering are major health problems. The lack of controllability of experienced pain seems to greatly contribute to the extent of suffering. This study examined how controllability affects the perception of pain and pain-related suffering, and the modulation of this effect by beliefs and emotions such as locus of control of reinforcement, pain catastrophizing, and fear of pain.
Methods:
Twenty-six healthy subjects received painful electric stimulation in both controllable and uncontrollable conditions. Visual analogue scales and the “Pictorial Representation of Illness and Self Measure” were used to assess pain intensity, unpleasantness, pain-related suffering, and the level of perceived control. We also investigated nonverbal indicators of pain and suffering such as heart rate, skin conductance, and corrugator electromyogram.
Results:
Controllability selectively reduced the experience of pain-related suffering, but did not affect pain intensity or pain unpleasantness. This effect was modulated by chance locus of control but was unrelated to fear of pain or catastrophizing. Physiological responses were not affected by controllability. In a second sample of 25 participants, we varied the instruction. The effect of controllability on pain-related suffering was only present when instructions focused on the person being able to stop the pain.
Discussion:
Our data suggest that the additional measure of pain-related suffering may be important in the assessment of pain and may be more susceptible to the effects of perceived control than pain intensity and unpleasantness. We also show that this effect depends on personal involvement.
1. Introduction
Pain perception is modulated by cognitive and emotional variables such as predictability,12,39 controllability,7,60 attentional focus,1,48 or fear of pain.31,45,46 Studies in healthy volunteers showed that controllable situations reduce pain intensity7,40,60 and unpleasantness7; however, controllability did not always change pain perception.21,28,49 Although actual control was more effective than perceived control alone in reducing pain,40 this dissociation cannot fully explain these ambiguous findings because exerted control did not reduce pain intensity59 and perceived control was found to decrease pain intensity7 in other studies. Anxiety,59 helplessness,60 or pain unpleasantness7 were not assessed consistently across studies, although these variables may modulate the effects of control on pain perception. Carnevale13 suggested that suffering is the most important factor that drives patients to seek medical attention. This is also true for patients with chronic pain.3 Suffering is an overwhelming experience, which is perceived when the intactness of the person is threatened. Distress evoked by pain can induce suffering. However, the meaning assigned to distressing events may vary between persons, based on their personality and personal history. Suffering correlates only moderately with fear and anxiety where avoidance of a feared object prevails, whereas suffering focusses on the self.8,14–16,54 We have demonstrated that pain-related suffering is an additional component of pain that can be assessed independently of pain intensity and unpleasantness.10 The lack of controllability might be especially important for the experience of pain-related suffering in both experimental pain studies and in patients.6,17 Ongoing but unsuccessful efforts to influence the pain make patients especially vulnerable to suffering.56 In addition to observing the effects of control on verbal reports of pain and suffering, a secondary goal of the study was to see if there are also effects on physiological indicators of pain and suffering such as skin conductance responses (SCRs), corrugator electromyogram (EMG), and heart rate (HR).8 This would indicate multilevel effects and would permit bias-free assessments of pain and suffering also in groups that may not easily give verbal reports such as children or incapacitated persons.
The effect of the experimental manipulation of controllability is modulated by individual differences in the perception of control. Wiech et al.59 reported that exerted control over painful stimulation led to reduced pain perception in half of the subjects, whereas the other half showed increased pain intensity ratings. The authors hypothesized that the individuals' locus of control might explain these interindividual variations. We therefore examined locus of control of reinforcement, which is the degree to which people believe that they have control over the outcome of positive or negative events in their lives as opposed to external forces beyond their control.36,36,47 We hypothesized that an internal locus of control would modulate higher effects of uncontrollability on pain than an external or chance locus of control.
The current study examined the influence of controllability on pain intensity, unpleasantness, and pain-related suffering in 2 experiments that differed in the instructions that were used to announce controllability. We hypothesized that control over pain would positively affect all 3 dimensions, with the strongest reduction related to suffering. We expected reductions in SCR and EMG, but not HR. A high chance locus of control, the belief that powerful others control one's life, high catastrophizing, and high fear of pain were assumed to reduce the positive effects of perceived control on suffering.
2. Methods
2.1. Participants
Twenty-six right-handed subjects (13 male) between 18 and 43 years of age (mean: 25.5, SD = 5.81) participated in the study (sample 1). Twenty-five right-handed subjects (8 male) between 20 and 49 years of age (mean: 25.32, SD = 5.79) participated in study 2 (sample 2). Sample sizes were based on power calculations to detect large effects (effect size d > 0.5) in a within-subjects comparison, which have previously been reported in a similar study design.60 The ethics committee of the Medical Faculty Mannheim, University of Heidelberg, Germany, approved the study, and written informed consent was obtained from each participant. Exclusion criteria were cardiovascular or neurological disorders, brain injury, acute or chronic pain, current use of pain medication, pregnancy, lifetime and current substance abuse or dependence, and any other mental disorders. The subjects were screened by a psychologist using the German version of the Structured Clinical Interviews for the Diagnostic and Statistical Manual IV (SCID)61 Axis I to exclude subjects who fulfilled the criteria for a mental disorder.
2.2. Apparatus and application of painful stimuli
Pain processing was investigated in response to a series of painful electrical stimuli applied under conditions of controllability vs uncontrollability. A pair of subcutaneous needle electrodes (20 mm long, 0.35-mm uninsulated tip, 2-mm2 stimulation area, model: 9013R0272, 28G; Alpine Biomed ApS, Skovlunde, Denmark) were placed at the left upper back, at the midtrapezius muscle (1-mm needle separation). The stimulation site was chosen to mimic a clinical condition, like chronic back pain as closely as possible under experimental conditions and to allow similar experiments in chronic back pain patients. Needle electrodes mainly activating Aδ fibers27 were chosen to elicit a rapid and sharp painful sensation. The invasive character of these needles was also expected to result in sufficiently high suffering ratings to avoid floor effects in the rating data. Electric stimuli (2 ms stimulus duration, 400 V, interstimulus interval 500 ms) were applied using a constant current stimulator (model DS7A; Digitimer, Hertfordshire, England). The experiment was performed using Presentation software (Version 14.0, http://www.neurobs.com).
2.3. Psychophysical thresholds and stimulus calibration
The electrical stimulation parameters were determined individually, first by assessing pain-related thresholds by the method of limits. For this purpose, perception threshold, pain threshold, and pain tolerance were assessed during 4 ascending series of electric stimuli. The participants were instructed to press a button when they felt the stimulus for the first time (perception threshold), when the stimulus was painful for the first time (pain threshold), and when they could no longer tolerate the stimulus intensity (pain tolerance). Each threshold was acquired once per ascending series. The first ascending series was discarded as a practice trial, to exclude early fatigue and/or sensitization. Thus, the average of 3 ratings per threshold served as the final parameter. A painful stimulus intensity was preset at 70% of the interval between pain threshold and pain tolerance.
Next, the painful stimulus intensity was adjusted to a perceived pain intensity of 70% on a visual analogue scale (VAS) with the endpoints “no pain” (0) to “extreme pain” (100). Magnitude estimates of pain intensity were assessed during 3 test trials (duration 10 seconds each). Stimulus intensity was adjusted to reach a VAS rating of about 70%. The resulting individual stimulus intensity of each subject was used for all further procedures (sample 1: mean: 8.75, SD = 11.2; sample 2: mean: 10.35, SD = 18.38, values in mA).
2.4. Experimental procedure
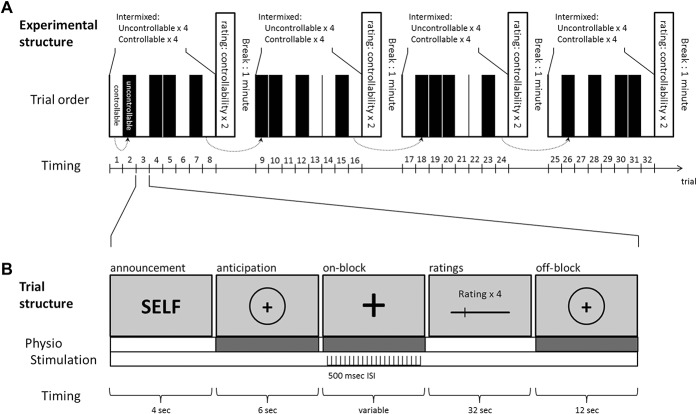
We used a within-subjects design, where each subject received painful electric stimulation in a controllable condition and an uncontrollable condition. The experiment consisted of 4 blocks in which participants received 8 series of painful stimulations (Fig. 1A). Each of the 4 blocks comprised 4 controllable and 4 uncontrollable trials, which were presented in an intermixed order (randomized within each block).
Figure 1.
Structure of the experiment: (A) The experiment consisted of 4 blocks with 8 stimulation trials each. In half of the cases, the stimulation could be stopped by the participant (controllable condition). The remaining trials were stopped by the computer (uncontrollable condition). The duration of the self-controlled trials equaled the duration of the computer-controlled trials in the subsequent block (dotted arrows). The order of the trials was randomized within each block. (B) Each trial was announced by a slide that indicated the type of the trial. Note that in 25 participants, the word “self” was replaced by the word “button press” to announce the controllable trials. The anticipation phase was followed by a varying interval of painful stimulation, ratings of pain intensity, pain unpleasantness, and pain-related suffering. Each trial ended with an off-block lasting 12 seconds. ISI, interstimulus interval.
In the controllable condition, the participants had the possibility to stop the painful electric stimulation through a button press. They were instructed to press the button when the stimulation became intolerable. Painful stimulation continued until it was ended by the participants. In the uncontrollable condition, the participants were informed that the duration of the stimulation was randomly determined by the computer. In reality, we used a yoked control design to match the length of the stimulations in the uncontrollable condition with those in the controllable condition.60 The duration of the 4 uncontrollable trials was predetermined by the duration of the 4 controllable trials of the preceding block. The first block started with a controllable trial. Durations of the uncontrollable trials of the first block were predetermined randomly by one of the preceding controllable trials of the same block. In all other blocks, the order of the trials was randomized. This led to comparable durations of the controllable and uncontrollable trials (sample 1: controllable: 22.8 ± 19.03 seconds, uncontrollable: 22.33 ± 18.33 seconds; sample 2: controllable: 16.9 ± 15.05 seconds, uncontrollable: 18.2 ± 15.28 seconds). The blocks were separated by 1-minute breaks.
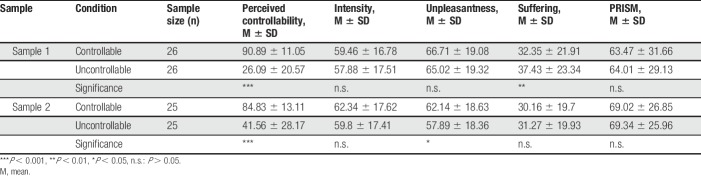
Due to our experimental manipulation, the exerted control had to be accompanied by increased perceptions of control in the controllable trials. We therefore assessed perceived controllability of the pain during the controllability and uncontrollability trials after each block of 8 trials. The same VAS was used for the controllable and uncontrollable trials. The VAS was 800 pixels (23.5 cm) long and ranged from “not at all controllable” to “extremely controllable,” with a visual angle of 16.7°. The pixels were linearly transformed to values of 0 to 100. Perceived controllability differed significantly between the controllable and uncontrollable conditions (sample 1: t(25) = 12.91, P < 0.001, d = 2.53 [CI95: 1.73–3.32]; sample 2: t(24) = 6.96, P < 0.001, d = 1.39 [CI95: 0.83–1.94]). The participants rated perceived controllability higher when they were able to stop the stimulation compared with the uncontrollable condition.
2.5. Instructions and trial structure
In each trial, the condition was announced on the computer screen during a 4-second prestimulus time span. The stimulus was expected during the following 6 seconds of an anticipation phase. Controllable trials were announced during the prestimulus interval with the words: “SELF; please press the button to terminate the stimulation.” Uncontrollable trials were announced, for 4 seconds, with a slide stating: “COMPUTER; stimulation will be terminated by the computer.” The assessment of perceived stimulus intensity, unpleasantness, and related suffering on a VAS, as well as the PRISM task, providing an alternate estimate of suffering, was presented in random order after each trial. The participants had a 32-second time frame to rate all 4 scales, followed by a resting time span (off-block) of 12 seconds.
2.6. Ratings
The assessment of pain intensity, unpleasantness, and suffering was performed on horizontal VASs (800 pixels = 235 mm) with appropriate endpoints (pain intensity: “no pain” and “extreme pain”; pain unpleasantness: “not unpleasant” and “extremely unpleasant”; and suffering: “no suffering” and “extreme suffering”). The scales were presented on a computer screen and comprised an angular view of 16.7°. The VAS ratings were transformed to values ranging from 0% to 100% of the distance between the endpoints of the scale.
In the absence of a gold standard on how to measure suffering,9 we complemented the “suffering” VAS with the suffering scale implemented in the “Pictorial Representation of Illness and Self Measure” (PRISM), because it performed best on quality criteria compared with other instruments for measuring suffering.35
The PRISM task was presented as a computerized version of the original task. The participants viewed a gray screen with a fixed yellow circle in the bottom right corner, representing their self. A moveable red circle was located in the center of the screen, representing the current painful stimulation (corresponding to “illness” in the original PRISM task). The participants were instructed to estimate the importance of the painful stimulation in their life by placing the red circle in an appropriate distance to the yellow circle. According to the PRISM rationale, the amount of suffering is coded by the inverse of the distance of the centers of the red and yellow circles. Thus, the closer the red circle (painful stimulation) is found in relation to the yellow circle (self), the higher is the suffering felt under the aversive stimulation, whereas the further the red circle is away from the yellow circle, the lower is the suffering indicated. The raw PRISM scale values were linearly transformed to values ranging from 0% to 100%, with 0% representing no and 100% representing maximal suffering.
Before the experiment, all participants were asked whether they were able to discriminate between the ratings. Because suffering is a personal and individual experience conveying multiple meanings that we did not want to delimit,14 no definition was given to the participants. If the participants struggled with differentiating between suffering and any of the pain scales, they were asked: “Do you think one can suffer without being in pain—can you give an example?” and “Do you think one can be in pain without suffering—can you give an example?” All participants confirmed those questions and were able to give examples. If participants struggled with the discrimination of pain intensity and pain unpleasantness, it was explained to them by using the radio metaphor by Price et al.43 In this metaphor, experiencing pain is compared with listening to the sound of a radio, where pain intensity refers to the loudness of the music and pain unpleasantness refers to the quality of the music played. The participants had a practice run for each rating before the experiment commenced.
2.7. Adjustment of the experimental procedure
This experiment was initially performed in 26 participants. During the debriefing session after the experiment, 4 participants reported being confused by the overlap between the PRISM rating and the instructions. The controllable trials were announced with the word “self,” whereas during the PRISM task, the participants had to place a token of the painful stimulation in relation to their self (yellow circle), with closer placements representing higher suffering.
The participants stated that in the PRISM task, they were confused between 2 possible meanings of the word “self” on the display and did not know whether they were expected to rate the amount of their own suffering (with ratings closer to the self, indicating higher suffering) or how much they felt being the agent in control of the previous stimulation (with ratings closer to the self, indicating higher agency).
This instruction-related issue was not anticipated and therefore not systematically assessed in all participants. It can hence not be ruled out that other participants were having the same problem without reporting it. This renders a subsample analysis impossible, and PRISM ratings in this sample should therefore be interpreted with caution. To rule out this ambiguity, the experiment was repeated in another 25 participants. For those 25 participants, the general instructions before the experiment were changed and referred to controllable trials as “stoppable by a button press.” Controllable trials were announced with a slide stating: “BUTTON PRESS; please press the button to terminate the stimulation” and uncontrollable trials with the following slide: “COMPUTER; stimulation will be terminated by the computer”. The data of the second experiment (sample 2) were analyzed in the same way as the initial experiment (sample 1).
2.8. Questionnaires
Before the experiment, the participants completed the Locus of Control Scale,34 which assesses beliefs about control of reinforcement with the subscales chance (IPC-C), control by powerful others (IPC-P), and perceived mastery over one's personal life (IPC-I). The scale has good test–retest reliability (IPC-I: r = 0.55, IPC-P: r = 0.66, and IPC-C: r = 0.70), internal consistency (α = 0.91 for IPC-I, α = 0.95 for IPC-P, and α = 0.9 for IPC-C), and validity.33 A general locus of control scale was chosen because we examined healthy individuals, and the painful stimulation was not related to any health problem.57
The participants also completed the Pain-Related Self Statements Scale (PRSS23), which assesses catastrophizing and active coping. The scale is validated in German participants and has excellent reliability (α = 0.92 for catastrophizing and α = 0.88 for active coping) and validity, as shown by significantly higher values for pain catastrophizing and significantly lower values for active coping in pain patients compared with healthy controls, and low to moderate correlations with other pain-related variables such as amount of daily activity, affective distress, or pain severity.
Furthermore, the participants completed the Fear of Pain Questionnaire (FPQ-III). The FPQ-III is a self-report measure designed to evaluate fears about severe, minor, and medical pain with higher scores indicating more fear. The FPQ-III has shown to be a valid and reliable instrument with good test–retest reliability, predictive validity, and internal consistency (for severe pain: α = 0.88, r = 0.69; for minor pain: α = 0.87, r = 0.73; and for medical pain α = 0.87, r = 0.76)38,51 and has been validated in a German population. The PRSS and FPQ-III were chosen to allow for comparison with previous experimental work on pain-related suffering8,10 and because they exist in validated German versions.
2.9. Physiological assessments
Electromyography, SCRs, and electrocardiogram were recorded and amplified using a BrainAmp ExG amplifier (Brain Products GmbH, München, Germany) and registered with a sampling frequency of 500 Hz. The data in one participant had to be discarded due to technical problems.
Electromyographic activity was recorded from the musculus corrugator supercilii using small surface electrodes (1.5 mm Ag/AgCl) that were placed in a bipolar fashion above the left eye, using the placement recommended by Fridlund and Cacioppo.25 The SCRs were recorded from 2 electrodes (5 mm Ag/AgCl), which were placed on the medial phalanges of digits III and IV of the left hand.4 Skin conductance response analysis was performed using the Ledalab V3.4.6c software package for Matlab and followed the guidelines of Fowles et al.24 Electrocardiogram was recorded using two 7-mm Ag/AgCl electrodes (Asmuth GmbH Medizintechnik, Minden, Germany), placed on the subjects' left lateral sternum at the upper and lower edges of the musculus pectoralis major. The ground electrode was placed on the right hip bone. Calculation of interbeat latencies and artefact correction were performed by the KubiosHRV software.53 Details on preprocessing of the physiological data can be found in the supplementary material (available at http://links.lww.com/PR9/A34).
3. Statistical analyses
As explained in the methods section, 2 samples were assessed in 2 separate experimental runs, with differing instructions for the controllability condition. In the original sample (sample 1), the instructions referred to controllable trials as being stoppable by the participant. In the sample of the second experiment (sample 2), the instructions were changed and referred to controllable trials as “stoppable by a button press.” All results are reported for both experimental runs. Statistical analyses were performed using RStudio 1.0.143 (RStudio, Inc., Boston, MA) with R 3.4.0 (The R Foundation for Statistical Computing).
3.1. Controllability and its influence on pain and suffering
To test the specificity of the effect of controllability on suffering compared with pain intensity and pain unpleasantness, we used an analysis of variance (ANOVA) to examine the within-subject effects of controllability (controllable vs uncontrollable) and rating dimension (intensity vs unpleasantness vs suffering VAS vs PRISM). Effect sizes for the ANOVAs are reported as partial η2, including 90% confidence intervals (CIs) of the effect sizes. Confidence interval for partial η2 was chosen following the recommendations by Steiger et al.52 for one-sided tests (CI = 100 [1 − 2α]%). Lower limits of the CIs are reported as 0 in cases where the F test is not statistically significant (α = 0.05). We used pairwise post hoc t tests (false discovery rate, FDR-corrected5) to compare the VAS ratings for perceived controllability, pain intensity, unpleasantness, and suffering as well as PRISM ratings in the controllable and uncontrollable conditions. Effect sizes for the t tests are reported as Cohen's d, including 95% CIs of the effect sizes. Confidence interval for Cohen's d was chosen following the recommendations by Steiger et al.52 for two-sided tests (CI = 100 [1 − α]%). Lower limits of the CIs are reported as <0 and upper limits as >0 in cases where the t test is not statistically significant (α = 0.05).
3.2. Individual differences in the effects of controllability on pain and suffering
To explore the impact of locus of control, fear of pain, and pain catastrophizing on the effect of controllability, the IPC, FPQ-III, and PRSS subscales and the difference in ratings (controllable minus uncontrollable) were correlated for each rating (intensity, unpleasantness, suffering VAS, and PRISM).
3.3. Sample differences in the effects of controllability on pain and suffering
To explore sample differences in the effect of controllability on the ratings, we implemented separate ANOVAs for each rating dimension (pain intensity, unpleasantness, suffering VAS, and PRISM) to test the interaction of sample (sample 1 vs sample 2) and controllability (controllable vs uncontrollable). To examine the association of enduring beliefs and pain controllability, we compared the correlations of the IPC, FPQ-III, and PRSS subscales with differences in controllability (controllable minus uncontrollable) between samples using Fisher's z.
3.4. Physiological assessments
For details on statistical analysis of physiological data, see supplementary material (available at http://links.lww.com/PR9/A34).
4. Results
4.1. Controllability and its influence on pain and suffering
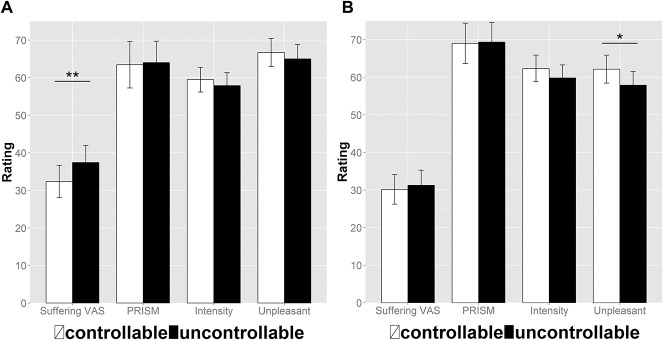
The ANOVAs using controllability (controllable vs uncontrollable) and the rating dimension (intensity vs unpleasantness vs suffering VAS vs PRISM) as within-subject effects were significant for rating dimension (sample 1: F(3,75) = 15.41, P < 0.001, η2 = 0.381 [CI90: 0.220–0.480]; sample 2: F(3,72) = 24.47, P < 0.001, η2 = 0.505 [CI90: 0.349–0.590]) with lower suffering than intensity and unpleasantness ratings. The main effect of controllability was not significant in this analysis (sample 1: F(1,25) = 0.40, P = 0.53, η2 = 0.016 [CI90: 0.000–0.159]; sample 2: F(1,24) = 0.93, P = 0.34, η2 = 0.037 [CI90: 0.000–0.208]); however, we found a significant interaction for controllability X rating dimension (sample 1: F(3,75) = 6.59, P < 0.001, η2 = 0.209 [CI90: 0.066–0.312]; sample 2: F(3,72) = 5.69, P = 0.001, η2 = 0.192 [CI90: 0.051–0.296]). This illustrates a reduction of suffering VAS (t(25) = 3.42, P = 0.008, d = 0.67 [CI95: 0.24–1.09]) in the controllable condition of sample 1, which was not present in the intensity (t(25) = −1.37, P = 0.26, d = 0.27 [CI95: −0.12 to 0.66]), unpleasantness (t(25) = −1.33, P = 0.26, d = 0.26 [CI95: −0.13 to 0.65]), and PRISM (t(25) = 0.31, P = 0.75, d = 0.06 [CI95: −0.32 to 0.45]) ratings (Fig. 2A). For sample 2, it illustrates an increase of unpleasantness ratings in the controllable condition (t(24) = −2.11, P = 0.04, d = 0.42 [CI95: 0.01–0.83]), which was not present in the intensity (t(24) = −1.76, P = 0.09, d = 0.35 [CI95: −0.05 to 0.75]), suffering VAS (t(24) = 0.78, P = 0.44, d = −0.16 [CI95: −0.55 to 0.24]), and PRISM ratings (t(24) = 0.18, P = 0.85, d = 0.04 [CI95: −0.35 to 0.43]) (Fig. 2B and Tables 1 and 2).
Figure 2.
Effect of controllability on ratings in the original sample (A) and the second experiment (B): Bars show mean percentages of the pain intensity, pain unpleasantness, suffering VAS, and PRISM ratings for the controllable condition (white) and the uncontrollable (black) condition, and error bars depict the standard error of the mean. Asterisks show significant repeated-measures t tests (controllable vs uncontrollable) with *P < 0.05 and **P < 0.01. VAS, visual analogue scale.
Table 1.
Pain and suffering scales within experimental conditions: intensity, unpleasantness, suffering, and PRISM ratings and ratings of perceived controllability are shown over all conditions and expressed as percentage values.
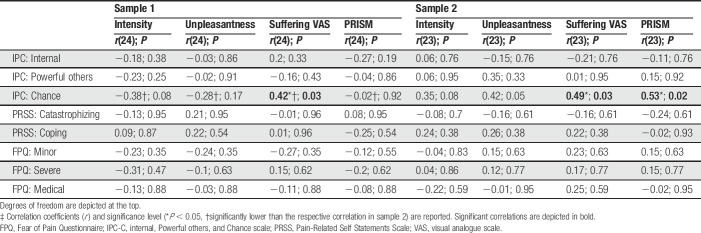
Table 2.
Correlations of attributional style, coping strategies, and fear of pain with differences in ratings (controllable minus uncontrollable) for each rating (intensity, unpleasantness, suffering VAS, and PRISM).‡
4.2. Individual differences in the effects of controllability on pain and suffering
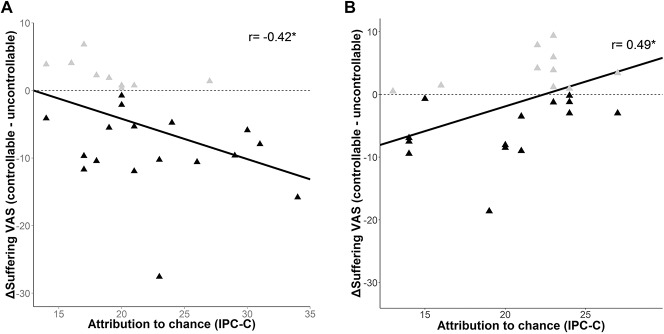
Subjects with a high chance-related locus of control showed significantly more reduction in suffering VAS ratings in sample 1 (rp(24) = −0.42, P = 0.03), but less reduction in suffering VAS (rp(23) = 0.49, P = 0.03) and suffering as assessed with the PRISM (rp(23) = 0.53, P = 0.02) in sample 2 in the controllable vs uncontrollable trials (Fig. 3). There was no significant influence of chance-related locus of control on the effect of experimental control for pain intensity (sample 1: rp(24) = −0.38, P = 0.08; sample 2: rp(23) = 0.35, P = 0.08), unpleasantness (sample 1: rp(24) = −0.28, P = 0.17; sample 2: rp(23) = 0.42, P = 0.05), and also not for PRISM ratings in sample 1 (rp(24) = −0.02, P = 0.92). There was no significant effect of internal locus of control, locus of control directed to powerful others, catastrophizing, active coping, or fear of minor, severe, or medical pain on the difference between controllable and uncontrollable conditions (all r < 0.35, P > 0.18).
Figure 3.
Impact of attributional style on the effects of control in the original sample (A) and the second experiment (B): the x-axis shows the chance subscale of the IPC. The y axis shows the difference (Δ) in the suffering VAS ratings, depicted as percentage values. Ratings in the uncontrollable condition were subtracted from ratings in the controllable condition. The black triangles depict the participants who indicated more suffering when pain could not be controlled. The gray triangles depict participants who indicated more suffering when pain could be controlled. IPC-C, chance subscale of the internal, powerful others, and chance scale; VAS, visual analogue scale. * P < 0.05.
4.3. Sample differences in the effects of controllability on pain and suffering
There was no significant interaction of sample and controllability for pain intensity (F(1,49) = 0.28, P = 0.60, η2 = 0.006 [CI90: 0.000–0.082]), unpleasantness (F(1,49) = 1.18, P = 0.28, η2 = 0.024 [CI90: 0.000–0.128]), suffering VAS (F(1,49) = 3.74, P = 0.06, η2 = 0.071 [CI90: 0.000–0.203]), or PRISM ratings (F(1,49) = 0.01, P = 0.93, η2 < 0.001 [CI90: 0.000–0.018]). The correlation of chance-related locus of control with the difference in controllability ratings was significantly higher in sample 2 than in sample 1 for pain intensity (z = 2.57, P = 0.01), pain unpleasantness (z = 2.47, P = 0.01), suffering VAS (z = 3.30, P = 0.001), and PRISM ratings (z = 2.05, P = 0.04). The samples did not significantly differ in any of the correlations between the rating differences in pain intensity, pain unpleasantness, suffering VAS, or PRISM with the subscales of the FPQ-III (all z < 1.72, P > 0.08), PRSS (all z < 1.27, P > 0.20), or IPC-I and IPC-P subscales (all z < 1.40, P > 0.15).
4.4. Physiological correlates of experimental controllability
We found a significant increase of SCR during painful stimulation (F(2,50) = 27.5, P < 0.001, η2 = 0.524 [CI90: 0.343–0.624]), an HR deceleration during painful stimulation (F(2,50) = 14.01, P < 0.001, η2 = 0.359 [CI90: 0.169–0.484]), and an anticipatory deceleration of corrugator EMG before the onset of painful stimulation (F(2,50) = 9.89, P < 0.001, η2 = 0.283 [CI90: 0.103–0.415]). None of the physiological measures differed significantly between controllable and uncontrollable trials. For further details on physiological data, see supplementary material (available at http://links.lww.com/PR9/A34).
5. Discussion and conclusions
This study shows that control over pain primarily reduces the degree of perceived suffering. This effect was modulated by the subjects' locus of control: The more participants attributed their behavior to chance, the greater was the reduction of suffering when they had control over their pain. Pain intensity and unpleasantness ratings, by contrast, were unaffected by control over pain. This effect was only present in the experiment initiated by an instruction that focused on the person being able to stop the pain. In a second experiment, where the instructions referred to controllable trials as “stoppable by a button press,” suffering was not influenced by controllability. Here, controllability increased unpleasantness ratings, whereas pain intensity and suffering remained unaffected. Interestingly, the modulation by attribution to chance was inverse in this second sample: The more participants attributed their behavior to chance, the smaller was the reduction of suffering when they had control over their pain.
5.1. Controllability and its influence on suffering
The finding that controllability reduces suffering extends the view of the impact of controllability on the pain experience by ascribing a key role to uncontrollability in the manifestation of suffering. According to Thompson,54 the effects of control depend on the meaning the individual ascribes to control, which matches the view that the transition from pain to suffering results when patients feel out of control, and that this transition is influenced by the meaning the individual ascribes to the pain (eg, when chest pain is mistaken as a life-threatening symptom by patients with a panic disorder).15,30 Thus, perceived controllability may act as an assurance that one will not face an event that is beyond the limits of endurance, and suffering can be relieved by changing the meaning of the pain3,14,44 to an experience that one can cope with.
Our results could therefore shed a light on the inconsistencies of previous studies, which examined the relationship of controllability and pain perception by relying exclusively on the pain intensity and unpleasantness dimensions. Because previous studies did not assess pain-related suffering, it is not clear to what extent these measures implicitly related to suffering. Depending on the relevance of suffering for the given experimental setup, pain relief,7,40,60 no changes,21,28,49 or in some cases even increases in perceived pain50,59 may be obtained. The choice of outcome measure is thus important for the detection of the effects of controllability. Suffering has recently been proposed as an outcome measure in patients with chronic pain in addition to pain intensity and unpleasantness measures because it encompasses aspects of helplessness, hopelessness, and the feeling of being overwhelmed.3
5.2. Controllability and its influence on pain unpleasantness
In the second study, the pain unpleasantness ratings in the controllable condition were increased, and controllability was not found to alleviate suffering. Testing for sample interaction effects showed that the effects of controllability on pain and suffering did not differ between the samples (Fig. 2). As reported above, increased pain ratings in response to controllability have been found before.50,59 Our experimental design was similar to the one used in a study on brain mechanisms of pain controllability,60 but did not implement a button press at the end of the uncontrollable trials. This was included in the original study to account for motor responses in the brain. This missing button press at the end of the uncontrollable trials may have induced a different attentional state as compared to the controllable trials. An attentive, but nonreactive awareness was previously shown to reduce pain unpleasantness.42
5.3. Locus of control and suffering
An external locus of control directed towards luck, fate, or chance has been associated with maladaptive pain-coping strategies20 and higher levels of pain despite patient-controlled analgesia.29 Patients with chronic pain who attributed pain to chance experienced pain more frequently and showed high pain intensity ratings.11 Overall, chance locus of control might be associated with less physical activity, more medication abuse, and higher interference of pain in daily life.11 Internal locus of control, by contrast, has been associated with positive outcomes such as lower pain scores, higher satisfaction, and lower disability levels.19,29
In sum, although an internal locus of control seems to be a resilience factor against chronic pain,2,26 it does not seem to be associated with pain perception in experimental pain.60 Rather, attribution to chance seems to lead to worse outcome expectations. Our results suggest that attribution to chance modulates the effects of control over pain on pain-related suffering. Fear of pain or pain catastrophizing did not modulate the effects of control, which implies a specific effect of the attribution to chance.
In study 1, higher attribution to chance led to greater reduction of suffering when pain could be controlled. However, in contrast to these findings, we found the inverse relationship in our second experiment. The circumstances under which controllability reduces suffering therefore remain incompletely understood. The 2 studies differed in the instructions given to the participants. Instructional context and individual control beliefs may modulate the effect; however, we cannot fully explain the exact interaction effects. Future studies should therefore more stringently target the modulatory effects of instructions on the effects of pain controllability on suffering. In addition, cultural factors should be considered because it has been shown that the influence of external locus of control on affective symptoms is weaker for collectivistic societies.18 Moreover, the meaning of suffering differs between cultures and depends not only on the cultural background of the patient but also on the cultural background of the caregiver.22,37,58
5.4. Physiological correlates of experimental controllability
In this study, neither corrugator EMG nor SCR or HR were affected by control over pain. This is in line with a study showing that changes in SCR were not associated with the failure to control pain.28 This study, however, also showed that changes in HR were associated with the failure to control pain. We previously found that SCR and corrugator EMG, but not HR, were associated with suffering.8 Given the explorative nature of our hypothesis on physiological correlates of controllability effects and the sensitivity of those effects to the modulation by individual and contextual factors, there is a need for further research on this issue. This should address the effects of perceived and exerted control on physiological correlates or the modulatory effects of personality.
5.5. Limitations
This study has several limitations. First, the instructions in our experiment were not optimal for the use of the PRISM task. It therefore cannot be ruled out that this ambiguity affected other aspects of the experiment. However, because all reports of ambiguity referred to and were limited to the PRISM rating, it is unlikely that our results were biased by misunderstandings of the instructions. A change of the instructions in a second sample did not yield any changes in the effects of control on the PRISM task. The PRISM task was developed and validated for the application in health issues such as posttraumatic stress disorder,62 lung disease, psoriasis, breast cancer,63 or chronic urticaria.55 The sensitivity of the PRISM task in an experimental setting with healthy individuals has not been tested, and classic VAS measures seem to perform better in this task. A second limitation of our study was the unclear significance of the PRISM instructions for the participants. In both samples, some people may have understood the instructions differently from the others and this may have added additional variance. In future studies, the instructions for the PRISM ratings must be clarified. Third, the suffering VAS scores were rather low compared with clinical settings. The experimental situation does not fully resemble the suffering experienced in a clinical pain condition and does not cover all existential aspects of the suffering experience.32,41 Future studies are needed to evaluate the translational value of our experimental findings in clinical settings. Fourth, the sample effects that we observed need to be addressed more stringently. In study 1, we observed the opposite relationship between attribution to chance and the effect of controllability on suffering compared with study 2. This is a result that we had not predicted a priori and should therefore be viewed as exploratory. A fifth limitation relates to the number of variables tested in this study, which increases the risk of chance findings. We tested the impact of several individual difference variables on the effect of controllability. These results therefore need to be replicated. Finally, we have made an attempt to disentangle the effects of controllability on pain and pain-related suffering. We have, however, not dissociated these effects from the effect of controllability on fear, anxiety, or distress. These are related variables and their modulation by controllability may confound the effect of controllability on suffering.
5.6. Conclusion
This study demonstrates that controllability primarily affects suffering rather than pain severity or unpleasantness. In addition, this result helps to understand the previous inconclusive findings on the effect of controllability on pain. We propose a complex interaction between individual control beliefs and instructional context that influence the experience of suffering. Future studies should take these factors into account when studying the effects of controllability and assessing its significance in a clinical context.
Disclosures
The authors have no conflicts of interest to declare.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to HF (FL 156/34-1) and Frauke Nees and HF (SFB1158/B03) and the Fonds National de la Recherche Luxembourg to FA.
Acknowledgments
Author contributions: M. Löffler, S. Kamping, M. Brunner, and H. Flor designed the study; M. Löffler and M. Brunner collected the data; M. Löffler and D. Kleinböhl analyzed the data; M. Löffler, S. Kamping, M. Brunner, and H. Flor wrote the article; S. Bustan, D. Kleinböhl, and F. Anton commented on the article; F. Anton and H. Flor acquired the funding. All authors contributed to the interpretation of the data, revised the manuscript, and approved the final version of the manuscript.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A34.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Arntz A, De Jong P. Anxiety, attention and pain. J Psychosom Res 1993;37:423–31. [DOI] [PubMed] [Google Scholar]
- [2].Baker TA, Buchanan NT, Corson N. Factors influencing chronic pain intensity in older black women: examining depression, locus of control, and physical health. J Womens Health (Larchmt) 2008;17:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ballantyne JC, Sullivan MD. Intensity of chronic pain—the wrong metric? N Engl J Med 2015;373:2098–9. [DOI] [PubMed] [Google Scholar]
- [4].Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods 2010;190:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Stat Methodol 1995;57:289–300. [Google Scholar]
- [6].Black HK, Rubinstein RL. Themes of suffering in later life. J Gerontol B Psychol Sci Soc Sci 2004;59:S17–24. [DOI] [PubMed] [Google Scholar]
- [7].Borckardt JJ, Reeves ST, Frohman H, Madan A, Jensen MP, Patterson D, Barth K, Smith AR, Gracely R, George MS. Fast left prefrontal rTMS acutely suppresses analgesic effects of perceived controllability on the emotional component of pain experience. PAIN 2011;152:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brunner M, Löffler M, Kamping S, Bustan S, González-Roldán A, Anton F, Flor H. Assessing suffering in experimental pain models. Z für Psychol 2017;225:45–53. [Google Scholar]
- [9].Büchi S, Buddeberg C, Klaghofer R, Russi EW, Brändli O, Schlösser C, Stoll T, Villiger PM, Sensky T. Preliminary validation of PRISM (Pictorial Representation of Illness and Self Measure)–A brief method to assess suffering. Psychother Psychosom 2002;71:333–41. [DOI] [PubMed] [Google Scholar]
- [10].Bustan S, Gonzalez-Roldan A, Kamping S, Brunner M, Löffler M, Flor H, Anton F. Suffering as an independent component of the experience of pain. Eur J Pain 2015;19:1035–48. [DOI] [PubMed] [Google Scholar]
- [11].Cano-García F, Rodriguez-Franco L, López-Jiménez A. Locus of control patterns in headaches and chronic pain. Pain Res Manag 2013;18:e48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carlsson K, Andersson J, Petrovic P, Petersson KM, Öhman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage 2006;32:1804–14. [DOI] [PubMed] [Google Scholar]
- [13].Carnevale FA. A conceptual and moral analysis of suffering. Nurs Ethics 2009;16:173–83. [DOI] [PubMed] [Google Scholar]
- [14].Cassel EJ. The nature of suffering and the goals of medicine. N Engl J Med 1982;306:639–45. [DOI] [PubMed] [Google Scholar]
- [15].Cassell EJ. Diagnosing suffering: a perspective. Ann Intern Med 1999;131:531–4. [DOI] [PubMed] [Google Scholar]
- [16].Chapman C, Gavrin J. Suffering: the contributions of persistent pain. Lancet 1999;353:2233. [DOI] [PubMed] [Google Scholar]
- [17].Charmaz K. Loss of self: a fundamental form of suffering in the chronically ill. Sociol Health Illn 1983;5:168–95. [DOI] [PubMed] [Google Scholar]
- [18].Cheng C, Cheung SF, Chio JHM, Chan MPS. Cultural meaning of perceived control: a meta-analysis of locus of control and psychological symptoms across 18 cultural regions. Psychol Bull 2013;139:152. [DOI] [PubMed] [Google Scholar]
- [19].Cheng SK, Leung F. Catastrophizing, locus of control, pain, and disability in Chinese chronic low back pain patients. Psychol Health 2000;15:721–30. [Google Scholar]
- [20].Crisson JE, Keefe FJ. The relationship of locus of control to pain coping strategies and psychological distress in chronic pain patients. PAIN 1988;35:147–54. [DOI] [PubMed] [Google Scholar]
- [21].Crombez G, Eccleston C, De Vlieger P, Van Damme S, De Clercq A. Is it better to have controlled and lost than never to have controlled at all? An experimental investigation of control over pain. PAIN 2008;137:631–9. [DOI] [PubMed] [Google Scholar]
- [22].Davitz L, Sameshima Y, Davitz J. Suffering as viewed in six different cultures. Am J Nurs 1976;76:1296. [PubMed] [Google Scholar]
- [23].Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther 1993;31:63–73. [DOI] [PubMed] [Google Scholar]
- [24].Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology 1981;18:232–9. [DOI] [PubMed] [Google Scholar]
- [25].Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology 1986;23:567–89. [DOI] [PubMed] [Google Scholar]
- [26].Härkäpää K, Järvikoski A, Mellin G, Hurri H, Luoma J. Health locus of control beliefs and psychological distress as predictors for treatment outcome in low-back pain patients: results of a 3-month follow-up of a controlled intervention study. PAIN 1991;46:35–41. [DOI] [PubMed] [Google Scholar]
- [27].Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Aδ fibers by intra-epidermal needle electrode in humans. PAIN 2002;96:247–52. [DOI] [PubMed] [Google Scholar]
- [28].Janssen SA, Spinhoven P, Arntz A. The effects of failing to control pain: an experimental investigation. PAIN 2004;107:227–33. [DOI] [PubMed] [Google Scholar]
- [29].Johnson LR, Magnani B, Chan V, Ferrante FM. Modifiers of patient-controlled analgesia efficacy. I. Locus of control. PAIN 1989;39:17–22. [DOI] [PubMed] [Google Scholar]
- [30].Kahn DL, Steeves RH. The experience of suffering: conceptual clarification and theoretical definition. J Adv Nurs 1986;11:623–31. [DOI] [PubMed] [Google Scholar]
- [31].Kamping S, Bomba IC, Kanske P, Diesch E, Flor H. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. PAIN 2013;154:1846–55. [DOI] [PubMed] [Google Scholar]
- [32].Kissane D. The relief of existential suffering. Arch Intern Med 2012;172:1501. [DOI] [PubMed] [Google Scholar]
- [33].Krampen G. Differenzierung des Konstruktes der Kontrollüberzeugung. Deutsche Bearbeitung und Anwendung der IPC-Skalen [in German]. Z Exp Angew Psych 1979;26:576–595. [Google Scholar]
- [34].Krampen G. IPC-Fragebogen zu Kontrollüberzeugungen. Hogrefe: Verlag für Psychologie, 1981. [Google Scholar]
- [35].Krikorian A, Limonero JT, Corey MT. Suffering assessment: a review of available instruments for use in palliative care. J Palliat Med 2013;16:130–42. [DOI] [PubMed] [Google Scholar]
- [36].Levenson H. Multidimensional locus of control in psychiatric patients. J Consult Clin Psychol 1973;41:397. [DOI] [PubMed] [Google Scholar]
- [37].Long C. Cultural and spiritual considerations in palliative care. J Pediatr Hematol Oncol 2011;33:S96. [DOI] [PubMed] [Google Scholar]
- [38].McNeil DW, Rainwater AJ. Development of the fear of pain questionnaire-III. J Behav Med 1998;21:389–410. [DOI] [PubMed] [Google Scholar]
- [39].Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un) predictable painful electrocutaneous stimulation: evidence for mediation by fear of pain. PAIN 2012;153:1030–41. [DOI] [PubMed] [Google Scholar]
- [40].Mohr C, Leyendecker S, Petersen D, Helmchen C. Effects of perceived and exerted pain control on neural activity during pain relief in experimental heat hyperalgesia: a fMRI study. Eur J Pain 2012;16:496–508. [DOI] [PubMed] [Google Scholar]
- [41].Mount BM. Existential suffering and the determinants of healing. Eur J Palliat Care 2003;10:40–2. [Google Scholar]
- [42].Perlman D, Salomons T, Davidson R, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion 2010;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. PAIN 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- [44].Rehnsfeldt A, Eriksson K. The progression of suffering implies alleviated suffering. Scand J Caring Sci 2004;18:264–72. [DOI] [PubMed] [Google Scholar]
- [45].Rhudy JL, DelVentura JL, Terry EL, Bartley EJ, Olech E, Palit S, Kerr KL. Emotional modulation of pain and spinal nociception in fibromyalgia. PAIN 2013;154:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rhudy JL, Williams AE, McCabe KM, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 2005;42:579–87. [DOI] [PubMed] [Google Scholar]
- [47].Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr 1966;80:1. [PubMed] [Google Scholar]
- [48].Ryckeghem D, Crombez G, Eccleston C, Legrain V, Damme S. Keeping pain out of your mind: the role of attentional set in pain. Eur J Pain 2013;17:402–11. [DOI] [PubMed] [Google Scholar]
- [49].Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci 2004;24:7199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Salomons TV, Nusslock R, Detloff A, Johnstone T, Davidson RJ. Neural emotion regulation circuitry underlying anxiolytic effects of perceived control over pain. J Cogn Neurosci 2014;27:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sperry-Clark JA, McNeil DW, Ciano-Federoff L. Assessing chronic pain patients: the fear of pain questionnaire-III. In: VandeCreek L, TL J, editors. Innovations in clinical practice: a source book. Sarasota: Professional Resource Press, 1999. pp. 293–305. [Google Scholar]
- [52].Steiger JH. Beyond the F test: effect size confidence intervals and tests of close fit in the analysis of variance and contrast analysis. Psychol Methods 2004;9:164. [DOI] [PubMed] [Google Scholar]
- [53].Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–Heart rate variability analysis software. Comp Methods Programs Biomed 2014;113:210–20. [DOI] [PubMed] [Google Scholar]
- [54].Thompson SC. Will it hurt less if i can control it? A complex answer to a simple question. Psychol Bull 1981;90:89. [PubMed] [Google Scholar]
- [55].Töndury B, Muehleisen B, Ballmer-Weber B, Hofbauer G, Schmid-Grendelmeier P, French L, Büchi S. The Pictorial Representation of Illness and Self Measure (PRISM) instrument reveals a high burden of suffering in patients with chronic urticaria. J Investig Allergol Clin Immunol 2011;21:93. [PubMed] [Google Scholar]
- [56].Turk D, Rudy T. Toward an empirically derived taxonomy of chronic pain patients: integration of psychological assessment data. J Consult Clin Psychol 1988;56:233. [DOI] [PubMed] [Google Scholar]
- [57].Turnipseed DL. Context-specific locus of control scales: poor psychometrics and cluttered theory? Compr Psychol 2014;3:14. [Google Scholar]
- [58].Wein S. Impact of culture on the expression of pain and suffering. J Pediatr Hematol Oncol 2011;33:S105–7. [DOI] [PubMed] [Google Scholar]
- [59].Wiech K, Edwards R, Moseley GL, Berna C, Ploner M, Tracey I. Dissociable neural mechanisms underlying the modulation of pain and anxiety? An FMRI pilot study. PLoS One 2014;9:e110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci 2006;26:11501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wittchen H, Zaudig M, Fydrich T. Strukturiertes klinisches interview für DSM-IV (SKID), achse 1 [structured clinical interview for DSM-IV (SCID), axis 1 disorders]. Göttingen: Hogrefe, 1997. [Google Scholar]
- [62].Wittmann L, Schnyder U, Büchi S. PRISM (Pictorial Representation of Illness and Self Measure): a new method for the assessment of suffering after trauma. J Trauma Stress 2012;25:94–7. [DOI] [PubMed] [Google Scholar]
- [63].Wouters E, Reimus J, van Nunen A, Blokhorst M, Vingerhoets A. Suffering quantified? Feasibility and psychometric characteristics of 2 revised versions of the Pictorial Representation of Illness and Self Measure (PRISM). Behav Med 2008;34:65. [DOI] [PubMed] [Google Scholar]