Supplemental Digital Content is available in the text
Keywords: breast cancer, DCIS, ductal carcinoma in situ, sentinel lymph node metastasis
Abstract
With the introduction of an organized mammographic screening, the incidence of ductal carcinoma in situ (DCIS) has experienced an important increase. Our experience with sentinel lymph node biopsy (SLNB) among patients with DCIS is reviewed.
We collected retrospective data on patients operated on their breasts for DCIS (pTis), DCIS with microinvasion (DCISM) (pT1mi) and invasive ductal carcinoma (IDC) sized ≤2 cm (pT1) between January 2002 and June 2016, focusing on the result of SLNB.
543 DCIS, 84 DCISM, and 2111 IDC were included. In cases of DCIS and DCISM, SLNB resulted micrometastatic respectively in 1.7% and 6.0% of cases and macrometastatic respectively in 0.9% and 3.6% of cases. 5-year disease-free survival and overall survival in DCISM and IDC were similar, while significantly longer in DCIS. 5-year local recurrence rate of DCIS and DCISM were respectively 2.5% and 7.9%, and their 5-year distant recurrence rate respectively 0% and 4%. IDC, tumor grading ≥2 and lymph node (LN) macrometastasis were significant predictors for decreased overall survival. Significant predictors for distant metastases were DCISM, IDC, macroscopic nodal metastasis, and tumor grading ≥2. Predictors for the microinvasive component in DCIS were tumor multifocality/multicentricity, grading ≥2, ITCs and micrometastases.
Our study suggests that despite its rarity, sentinel node metastasis may also occur in case of DCIS, which in most cases are micrometastases. Even in the absence of an evident invasive component, microinvasion should always be suspected in these cases, and their management should be the same as for IDC.
Key Points
What is this paper adds: ductal carcinoma in situ with microinvasion presented disease free survival and overall survival similar to invasive ductal carcinoma;
Ductal carcinoma in situ with microinvasion presented disease free survival and overall survival shorter than ductal carcinoma in situ;
Ductal carcinoma in situ with microinvasion had shorter disease-free survival mainly because distant metastases occurrence;
Lymph node status could predict tumor microinvasion;
Isolated tumor cells could have a role in tumor microinvasion prediction.
1. Introduction
Although usually in situ cancer should not be able to shed neoplastic cells into the bloodstream or infiltrate the lymphatic net, there is a variable reported percentage of ductal carcinoma in situ (DCIS) which present an axillary nodal involvement, with higher rates noted in the premammographic era.[1–6] Nodal involvement in DCIS likely depends on the misdetection of occult microscopic invasive foci (occult microinvasion) due to technical limitations in specimen pathological assessment.[1–6] Microinvasion is defined as the extension of cancer cells beyond the basal membrane into the adjacent tissues, sized ≤1 mm,[7] but a recent study hypothesized that tumor cell dissemination may also occur before stroma invasion.[8] Some authors have described a prevalence of even 58.3% of occult invasion by histological re-examination of specimens of patients affected by DCIS with nodal metastasis, and thus significantly higher than that of specimens of pTisN0 patients.[9] However, microinvasive foci are shown to be very difficult to detect especially in the case of very wide intraductal carcinoma.[10,11]
Considering the controversy around pathogenesis and management of axillary metastases in DCIS, we reviewed our experience with sentinel lymph node biopsy (SLNB) among patients with DCIS. In particular, in this study, we evaluated the prevalence of sentinel node metastasis and their clinical role by SLNB performed in women affected by DCIS, as well as their influence on patient outcome in terms of overall survival (OS) and disease-free survival (DFS).
2. Materials and methods
For this chart review study, we collected retrospective data about all consecutive women operated on their breast for DCIS or invasive ductal carcinoma (IDC) sized ≤2 cm (pT1) in our Department between January 2002 and June 2016. The study was designed according to the dictates of the general authorization to process personal data for scientific research purposes by the Italian Data Protection Authority. We excluded all histotypes other than ductal carcinoma, male breast cancer, and tumors sized >2 cm by radiological diagnosis or microscopical evaluation.
Collected data included patient characteristics (age at diagnosis, body mass index [BMI], positive family history for breast or ovarian cancer, fertility status, eventual use of estroprogestinic therapies), tumor characteristics (histotype, grading, expression of estrogen receptor (ER), progesterone receptor (PR), HER2/neu expression, and Mib1/Ki-67, multifocality/multicentricity, peritumoral vascular invasion (PVI), peritumoral inflammation, nodal extracapsular invasion or bunched axillary nodes ),[12] surgical and non surgical management.
European guidelines were followed to routinely assess the pathological specimens.[13,14] The samples with a maximum diameter of 30 mm or less were completely sliced and examined, while for larger specimens, the sampling method followed the European guidelines.[13,14] The World Health Organization criteria were used to classify tumor histology[15] and nodal status (tumor, noes, and metastasis [TNM] classification VII ed. American Joint Cancer Committee/Union Internationale Contre le Cancer [AJCC/UICC], 2009), and the recommendations of AFIP (DCIS) and Elston Ellis (IDC) were used to evaluate tumor grade.[16,17] Peritumoral inflammation, PVI, multifocality/multicentricity, and nodal status were defined as previously described.[18,19] PR, ER, Ki-67/Mib-1, and Her-2/Neu expression were evaluated by immunohistochemistry. The authors considered PR or ER receptor positivity as positive in any nuclear staining ≥1%. In addition, Her-2/Neu was considered overexpressed when staining 3+ or 2+ with FISH amplification, negative if value was 0, 1+ or 2+ without FISH amplification. As previously stated, considered molecular subtypes of breast cancer were luminal A, luminal B, luminal Her, Her2-enriched, and basal-like.[18]
The study population was divided into 3 groups, by the ductal in situ component and the ductal invasive component: DCIS (pTis), ductal carcinoma in situ with microinvasion (DCISM) (pT1mi), and IDC sized ≤2 cm (pT1). Data were analyzed using R (version 3.2.3; R Core Team (2016); R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; URL https://www.R-project.org/) and considering as significant P <.05. Univariate analysis was performed by t test in cases of continuous variables, chi-square test or Fisher exact test in cases of categorical variables. We also performed univariate and multivariate survival analyses by Kaplan-Meier curves, Log-rank test, and Cox proportional hazards regression models. OS and DFS were considered to be the main outcomes, and were compared among the 3 groups drawing Kaplan–Meier curves. Moreover, DFS was considered separately for loco-regional and distant recurrences.
Univariate and multivariate Cox proportional hazards regression analysis was performed. In multivariate models included were all factors with less than 20% missing values and that had a P value <.200 in univariate analysis. In addition, in the multivariate model all selected factors and their interactions were accommodated in a single analysis, except when the interaction term was non-significant (in which case we analyzed the no-interaction model). Then a stepwise selection was performed to obtain the final multivariate regression model. For the variables with a P value <.200 and missing values between 20% to 40% of the total subjects a second multivariate Cox proportional hazards regression analysis was performed with random imputation of missing values and with a subsequent stepwise selection to obtain the final multivariate regression model. Univariate and multivariate logistic regression analyses were also performed.
In the multivariate logistic regression model considering only DCIS and DCISM, the following were considered as the dependent variable: DCISM, the presence of nodal metastases, and the presence of ITCs. The possible predictive factors (for DCISM or nodal involvement among DCIS) were considered as independent variables. In the multivariate model, all potentially influencing factors and their interactions were accommodated in a single analysis, except when the interaction term was non-significant (in which case we analyzed the no-interaction model). Furthermore, we included in the initial multivariate model all the factors with less than 20% missing values, that had a P value <.200 in univariate analysis and then we performed a stepwise selection to obtain the final multivariate logistic regression model. For variables with a P value <.200 and missing values between 20% and 40% of the total subjects, a second multivariate logistic regression analysis was performed with random imputation of missing values. Benjamini and Hochberg correction was applied to all multivariate analysis.[20]
3. Results
3.1. Patient characteristics and treatment
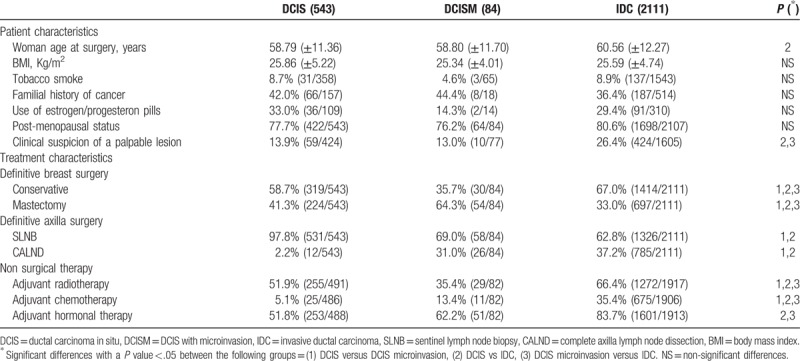
Among 7798 breast cancers operated on in our clinic, 543 DCIS, 84 DCISM, and 2111 IDC pT1 were found. Table 1 shows the population characteristics among the 3 studied groups. Women affected by IDC presented older age than the other groups and breast tumors were more frequently discovered by clinical examination. In addition, complete axillary lymph node dissection (CALND) and adjuvant treatments were more commonly performed in the IDC group (Table 1).
Table 1.
Population characteristics and treatment description. The reported values are means (± standard deviation) or percentages (and absolute values: cases/total available data excluding the unknown). Where appropriate p-values refers to t-test, chi-square test or Fisher exact test.
3.2. Tumor characteristics and staging
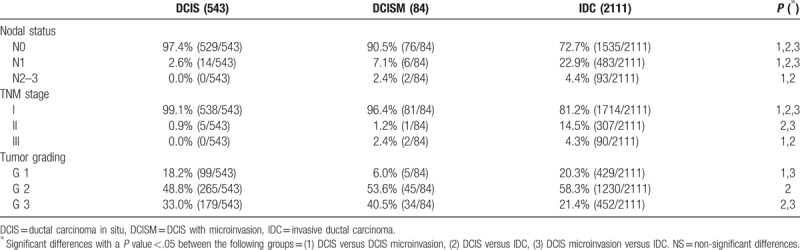
Tumor and axilla characteristics are shown in Tables 2 and 3. Comedo-like necrosis was more frequent in DCISM than in DCIS or IDC and it was more frequent in DCIS than IDC (P <.05) (Table 2). Multifocality/multicentricity was more common in DCISM than in the other 2 groups (P <.05) (Table 2). In cases of DCIS with or without microinvasion, SLNB resulted micrometastatic respectively in 6.0% and 1.7% of cases, and macrometastatic respectively in 3.6% and 0.9% of cases (Table 2). Table 3 shows the tumor staging characteristics. LN status in DCIS has entailed a TNM stage II in 0.9% of cases, while in cases of DCISM the nodal status has entailed a TNM stage II in 1.2% of cases and III in 2.4% of cases (Table 3).
Table 2.
Tumor and axilla characteristics. The reported values are percentages (and absolute values: cases/total available data excluding the unknown). Where appropriate P values refers to chi-square test or Fisher exact test.
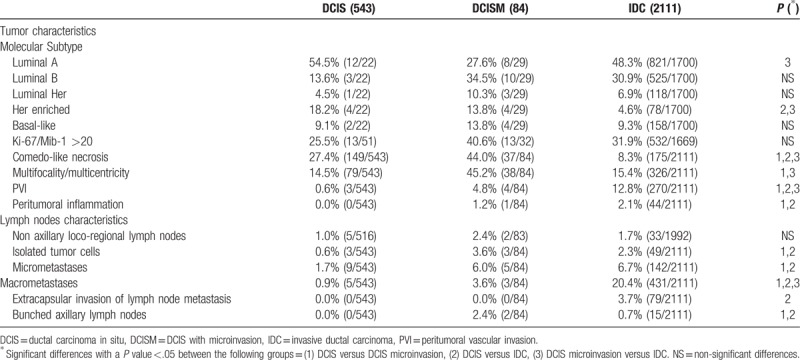
Table 3.
Tumor staging. The reported values are percentages (and absolute values = cases/total available data excluding the unknown). Where appropriate P values refers to chi-square test or Fisher exact test.
3.3. Overall and DFS
Figure 1 shows the Kaplan–Meier analysis. DCIS had better OS and DFS than IDC and DCISM (P <.05) (Fig. 1 A and B). As shown in Figure 1 C and D the most significant difference (DCIS had better DFS than IDC and DCISM) was observed among DFS considering distant metastases. Furthermore, 5-year OS of patients affected by DCIS with and without any microinvasive component resulted respectively 98.8% (95% [confidence interval] CI 96.4%–100.0%) and 99.8% (95% CI 99.4%–100.0%), while for IDC was 98.3% (95% CI 97.7%–98.9%). 5-year local recurrence rate of DCIS and DCISM were respectively 2.5% and 7.9%, and their 5-year distant recurrence rate respectively 0% and 4%.
Figure 1.
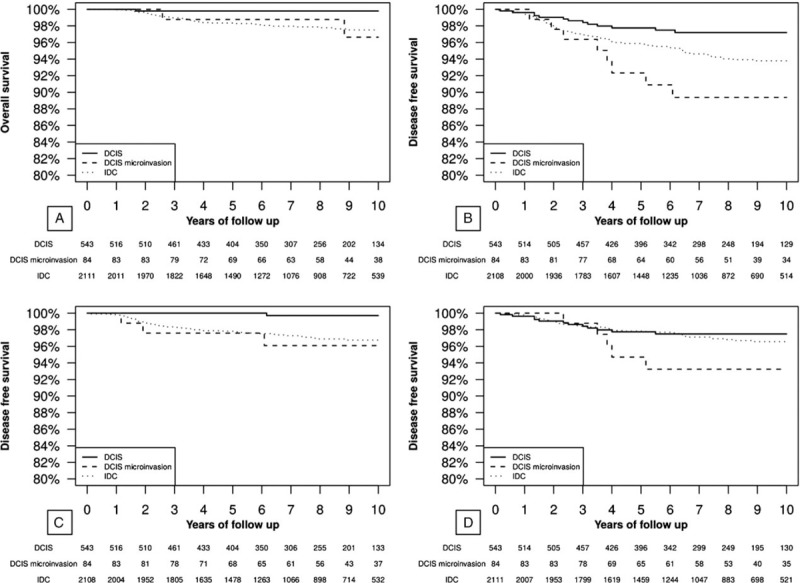
Kaplan–Meier analysis. Panel (A) overall survival in the 3 analyzed groups. At the log-rank test the following differences were significant: DCIS verses DCISM (P <.05) and DCIS versus IDC (P <.05). Panel (B) disease free survival in the 3 analyzed groups. At the log-rank test the following differences were significant: DCIS versus DCISM (P <.05) and DCIS versus IDC (P <.05). Panel (C) disease free survival considering only distant metastasis in the 3 analyzed groups. At the log-rank test the following differences were significant: DCIS versus DCISM (P <.05) and DCIS versus IDC (P <.05). Panel (D) disease free survival considering only loco-regional recurrences (breast and axilla) in the 3 analyzed groups. At the log-rank test the following differences were significant: DCIS versus DCISM (P = .070). DCIS = ductal carcinoma in situ, DCISM = DCIS with microinvasion.
In Table 4A the predictive factors for OS in our population were tested. In multivariate analysis, we found as significant predictive factors: IDC, tumor grading ≥2, and LN macrometastases. Furthermore, the most predictive multivariate model had predictive support from the following included factors: DCISM and peritumoral inflammation. In addition, considering the multivariate model with imputation of the missing values, also basal-like subtype (that was available only in a minority of DCIS or DCISM for missing data) and multifocality/multicentricity were found to be predictive.
Table 4.
Univariate and multivariate Cox proportional hazards regression analysis. The results are reported as hazard ratio (HR) with 95% confidence interval (CI).
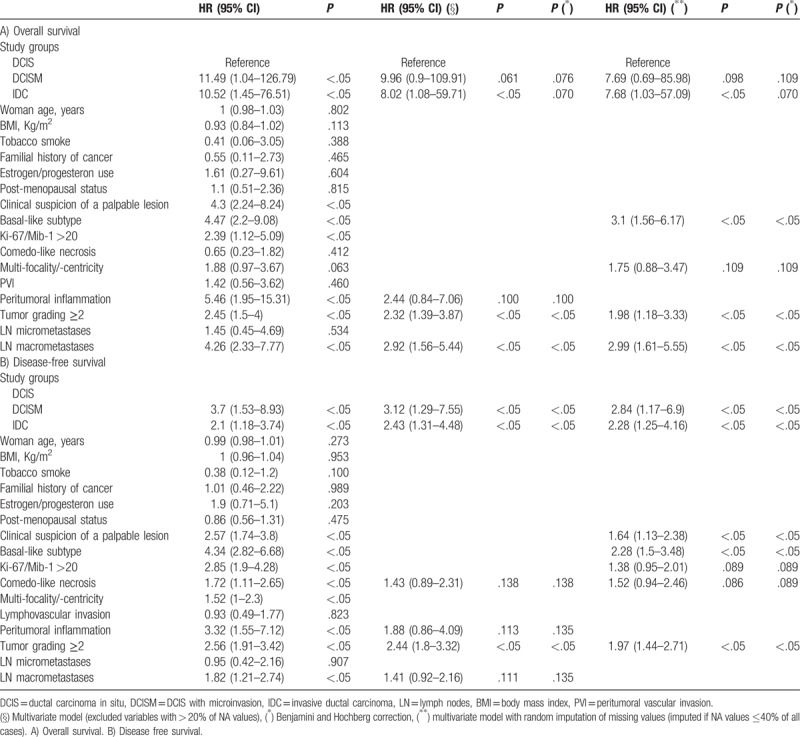
In Table 4B the predictive factors for DFS were assessed. The following factors were found to be significant predictors for reduced DFS: DCISM, IDC, and tumor grading ≥2. Other factors found to be predictive in the multivariate model were comedo-like necrosis and peritumoral inflammation. Moreover, after random imputation of the missing values, the following were also found to be predictive factors for DFS: clinical suspicion of a palpable lesion, basal-like subtype, and Mib1 >20%. Supplemental Tables 1 and 2 show that DCISM and IDC were associated with a reduced DFS in comparison to DCIS mainly due to distant metastases occurrence. In the initial models for prediction of OS and DFS (Table 4 A, 4B, Supplemental Table 1, and 2), we considered the basal information, and not the treatment information such as radiotherapy or chemotherapy, because treatment was standard among the considered patients. However, findings did not change by implementing the models with radiotherapy or chemotherapy. In particular, considering the model with non surgical therapy adjustment (adjuvant chemotherapy, adjuvant radiotherapy, and adjuvant hormonal therapy), as in Table 4B, DFS was significantly influenced by DCISM, clinical suspicion of a palpable lesion, and tumor grading ≥2.
We also assessed the role of lymph node (LN) metastases in DCISM. DCISM without LN metastasis had a 5 year OS of 99.83% (95% CI 99.49%–100%) while DCISM with LN metastasis had a 5 year OS of 85.71% (95% CI 63.34%–100%) (P <.05).
3.4. Predictive factors
Table 5 presents the predictive factors for microinvasion among DCIS. The following were found to be significantly associated with an increased occurrence of microinvasion: tumor multifocality/multicentricity, ITCs, and nodal micrometastases. In the multivariate model, also even tumor grading ≥2, PVI, and comedo-like necrosis were found to be significantly predictive. The area below the curve of the multivariate model was 72.5% (95% CI: 66.7%–78.3%).
Table 5.
Predictive factors for local tumor micronivasion among the 627 cases of DCIS or DCIS with micronivasion. Univariate and multivariate logistic regression analysis.
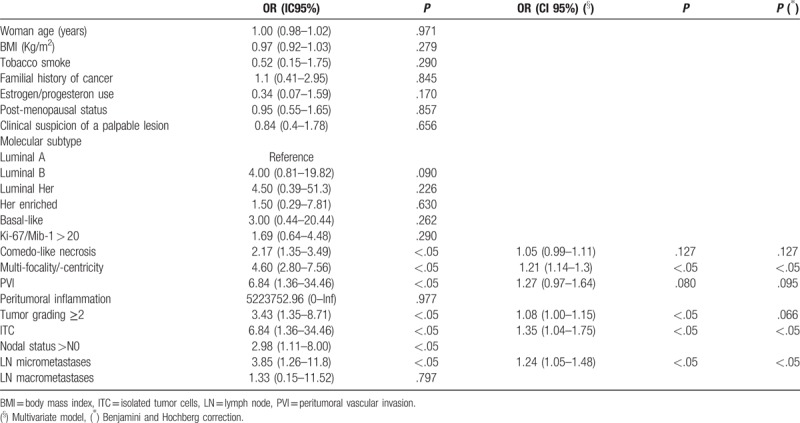
The predictive factors for nodal metastases among DCIS patients were also investigated, and only postmenopausal status was found to be a protective factor (OR 0.96 95% CI 0.93–0.99 P <.05) and DCISM to be a risk factor (OR 1.04 95% CI 1.00–1.09 P <.05). Furthermore, we tested the predictive factors for ITCs and found only older age to be a protective factor (OR 0.99 95% CI 0.90–1.00 P <.05) and DCISM to be a risk factor (OR 1.03 95% CI 1.01–1.05 P <.05).
4. Discussion
In this study, we reviewed our experience with SLNB in patients affected by DCIS and DCISM and compared their clinical outcome with IDC sized up to pT1. Nodal micrometastases resulted 6.0% in DCISM and 1.7% in DCIS while macrometastases were 3.6% in DCISM and 0.9% in DCIS. OS and DFS of DCISM resulted similar to IDC and shorter than DCIS. Significant predictors for OS or DFS, apart from microinvasion in DCIS, were also: IDC, tumor grading ≥2, nodal macrometastases, peritumoral inflammation, basal-like subtype, tumor multifocality/multicentricity, comedo-like necrosis, clinical suspicion of a palpable lesion, and Mib1 >20%. In the multivariate model, among DCIS, resulting predictive factors for microinvasion are: comedo-like necrosis, tumor multifocality/multicentricity, PVI, tumor grading ≥2, ITCs, and nodal micrometastases.
Since the introduction of a mammographic screening in our region, the prevalence of DCIS has increased, thanks to better microcalcifications detection.[21,22] The current literature advises SLNB for DCIS only in the case of very large DCIS with microinvasion suspicion and mastectomy, as the procedure will no longer be reliable.[23–27]
Regarding population characteristics, invasive carcinomas resulted clinically palpable in more than half the patients with DCIS with and without microinvasion. This may be a good indicator of the efficacy of mammographic screening, which is very sensitive for microcalcifications and consequently very accurate in early DCIS detection.
Other interesting data emerge for what concerns breast surgery. In fact, the mastectomy rate resulted surely higher in cases of DCIS and DCISM than IDC within 2 cm of size. In fact, DCIS is frequently multifocal or multicentric and, despite its low aggressivity, can more frequently require complete breast demolition.
While taking into account tumor characteristics, DCISM resulted in a higher prevalence of less favorable prognostic factors, including basal-like subtype (available only in a minority of DCIS or DCISM, due to missing data), high nuclear grading, high proliferation index, comedo-like necrosis, multifocality/multicentricity, and bunched axillary nodes. Most probably also due to these unfavorable characteristics, DCISM OS and DFS resulted more similar to those of IDC than of pure DCIS, which has a very favorable prognosis. However, in the literature, there is still disagreement about the impact of microinvasion on patient prognosis, without any differences between DCISM and DCIS in certain instances.[28–30] Our results confirmed previous studies that showed a reduced OS in DCISM than DCIS.[28,29] In addition, several studies suggested that DCISM survival outcomes were intermediate between DCIS and IDC, while in our study we found it to be very similar to IDC, mainly because of distant metastases occurrence.[30,31]
In the literature, the incidence of positive sentinel nodes in case of DCIS varies between 1.4% and 13%.[32,33] In our population, the prevalence of positive sentinel nodes in cases of DCIS resulted 2.6%. Usually, in these particular cases, surgical specimen was reviewed in order to detect eventual occult microinvasive foci, which in some cases were not found. Some authors described an upstage of DCIS to IDC after definitive surgery of 16.6%, and to microinvasive carcinoma of 16.6%, with a global underestimation rate of invasive disease of even 33%.[34] Therefore, any case of a positive sentinel node in the absence of evident microinvasive foci should be treated as a sort of cancer of unknown primary (CUP) syndrome, and thus therapies have been administered as in cases of IDC.[35] In addition, as emerged from our study, 5 year OS of DCISM with LN metastases was significantly lower than that of DCISM without LN metastases.
Taking into consideration predictive factors for microinvasion in cases of DCIS, in the literature it is associated with comedo-like necrosis, hormone receptor negativity, extensive DCIS or high grading.[28,36] Moreover, some radiological aspects may predict microinvasion by DCIS, including microcalcifications and high-degree of vascularization.[37] Among predictive factors for microinvasion in our population, comedo-like necrosis and PVI may represent an advanced evolutive stage of DCIS, multifocality/multicentricity may correlate with larger lesions, tumor grading ≥2, ITCs or nodal micrometastases may be associated with a more aggressive biological behavior. Moreover, this data suggests the importance of recognizing ITCs and micrometastases in cases of DCIS, as they may be a sign of misdiagnosed microinvasion foci. In fact, it is probably easier to find ITCs in an SLNB than a microinvasion focus in an extended DCIS. Among articles studying the predictive factors for microinvasion in DCIS, postoperative upstaging was mostly correlated with younger age, palpable mass, mammographic or ultrasonographic mass, core-needle biopsy, microinvasion suspicion at biopsy, large DCIS, high grading, comedo-like necrosis, hormonal receptors negativity, Her2/neu overexpression, and periductal inflammation.[36,38–42] In addition, Wang et al and Sakr et al agree with our results that a positive SLNB may be predictive of microinvasion and allow a prompt upstaging of DCIS when required.[29,43]
For what concerns nodal metastases in cases of DCIS, there are still many controversies. In particular, some authors found that an associated intraductal component of carcinoma >2 cm in size is a specific risk factor for ITCs and nodal metastases in cases of microinvasive carcinoma.[44] In our study, we found DCISM to be a risk factor for nodal metastases or ITCs, and older age or postmenopausal status to be protective factors. On the contrary, another study excluded the role of the number and extension of microinvasive foci in the development of nodal metastases.[45] In our opinion, these data from the literature and our results support the hypothesis of a molecular heterogeneity in DCIS,[46] so that a more aggressive DCIS results more likely to develop nodal metastases than a milder one. Supplemental Table 3 shows a review of the current literature on SLNB in cases of DCISM. Among the 32 studies about SLNB in DCISM, 37.50% did not show a clear opinion, 43.75% were in favor of performing SLNB, while only 18.75% were clearly contrary to SLNB procedure. In particular, the studies with wider population size were in favor of performing SLNB in DCISM. Despite the quite low percentage of positive nodes in DCISM in cases of LN positivity, the survival was significantly reduced in our study.
Some recent studies pointed out the negative prognostic value of Her2 in both DCIS and DCISM.[47–49] Unfortunately, this data may be missing in cases of microinvasion if the focus does not provide enough material to be immunohistochemically tested. In fact, in our study, we could not find survival differences in DCIS or DCISM in respect to Her2 positivity, because it was available only in a limited number of cases. Moreover, a possible role of Her2 in predicting in situ local recurrences in DCIS supports the implementation of routine Her2 testing in patients with any type of ductal intraepithelial neoplasia.[50]
The main limitations of this study were the retrospective design and the missing data regarding some biological characteristics of breast cancer, such as Her2 expression, which was not routinely assessed in DCIS, and in many cases of DCISM the invasive component could not be tested because of the paucity of invasive tissue. On the other hand, its points of strength were the wide number of considered DCIS and DCISM, the uniform management due to regular breast meeting discussions in a single center experience, and the median follow up of 95 months (interquartile range (IQR) 61–123) among DCISM and DCIS patients, which represents one of the longest follow-ups in the current literature.[29]
In summary, our study suggests that despite its rarity, nodal metastasis may occur also in cases of DCIS, which in most cases are micrometastasis. Even in the absence of an evident invasive component, microinvasion should always be suspected in these cases, and their management should be the same as for IDC. In our opinion, ITCs in association with nodal micrometastases could be a useful marker for DCISM, and ITCs should be considered in future studies for DCISM prediction.
Acknowledgments
The authors would like to thank the whole staff collaborating in clinical practice and in the study, particularly during data collection, and professor Kelly Wager for her precious help in the linguistic revision.
Author contributions
Substantial contributions to conception and design or acquisition of data or to analysis and interpretation of data (SB, CC, APL, BB, FG, DC, MT, AU, LM, AR). Drafting the article or revising it critically for important intellectual content (SB, CC, APL, BB, FG, DC, MT, AU, LM, AR). All authors have read and approved the final manuscript.
Conceptualization: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Francesco Giacomuzzi, Alessandro Uzzau, Laura Mariuzzi, Andrea Risaliti.
Data curation: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Barbara Baita, Francesco Giacomuzzi, Decio Capobianco, Marta Tortelli.
Formal analysis: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero.
Funding acquisition: Serena Bertozzi, Carla Cedolini.
Investigation: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Francesco Giacomuzzi, Decio Capobianco, Marta Tortelli, Alessandro Uzzau, Andrea Risaliti.
Methodology: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Decio Capobianco, Marta Tortelli, Alessandro Uzzau, Laura Mariuzzi.
Project administration: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Barbara Baita.
Resources: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Francesco Giacomuzzi, Decio Capobianco, Marta Tortelli, Andrea Risaliti.
Software: Ambrogio P Londero.
Supervision: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Laura Mariuzzi, Andrea Risaliti.
Validation: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Andrea Risaliti.
Visualization: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero.
Writing – original draft: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Barbara Baita, Francesco Giacomuzzi, Decio Capobianco, Marta Tortelli, Alessandro Uzzau, Laura Mariuzzi, Andrea Risaliti.
Writing – review & editing: Serena Bertozzi, Carla Cedolini, Ambrogio P Londero, Barbara Baita, Francesco Giacomuzzi, Decio Capobianco, Marta Tortelli, Alessandro Uzzau, Laura Mariuzzi, Andrea Risaliti.
Ambrogio P Londero orcid: 0000-0001-6429-1220.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DCIS = ductal carcinoma in situ, DCISM = ductal carcinoma in situ with microinvasion, DFS = disease-free survival, ER = estrogen receptor, IDC = invasive ductal carcinoma, LN = lymph node, OS = overall survival, PR = progesteron receptor, PVI = peritumoral vascular invasion, SLNB = sentinel lymph node biopsy, TNM = tumor, noes, and metastasis.
SB and CC contributed equally to this work.
The authors declare that they have no potential conflicts of interest relevant to this article. This study has had no financial support.
Supplemental Digital Content is available for this article.
References
- [1].Boler DE, Cabioglu N, Ince U, et al. Sentinel lymph node biopsy in pure DCIS: is it necessary. ISRN Surg 2012;2012:394095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Son BK, Bong JG, Park SH, et al. Ductal carcinoma in situ and sentinel lymph node biopsy. J Breast Cancer 2011;14:301–7. 00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Solin LJ, Kurtz J, Fourquet A, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J Clin Oncol 1996;14:754–63. [DOI] [PubMed] [Google Scholar]
- [4].Silverstein MJ, Gierson ED, Colburn WJ, et al. Can intraductal breast carcinoma be excised completely by local excision? Clinical and pathologic predictors. Cancer 1994;73:2985–9. [DOI] [PubMed] [Google Scholar]
- [5].von Rueden DG, Wilson RE. Intraductal carcinoma of the breast. Surg Gynecol Obstet 1984;158:105–11. [PubMed] [Google Scholar]
- [6].Winchester DP, Menck HR, Osteen RT, et al. Treatment trends for ductal carcinoma in situ of the breast. Ann Surg Oncol 1995;2:207–13. [DOI] [PubMed] [Google Scholar]
- [7].Singletary SE, Allred C, Ashley P, et al. Revision of the American joint committee on cancer staging system for breast cancer. J Clin Oncol 2002;20:3628–36. [DOI] [PubMed] [Google Scholar]
- [8].Banys M, Gruber I, Krawczyk N, et al. Hematogenous and lymphatic tumor cell dissemination may be detected in patients diagnosed with ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2012;131:801–8. [DOI] [PubMed] [Google Scholar]
- [9].Osako T, Iwase T, Kimura K, et al. Detection of occult invasion in ductal carcinoma in situ of the breast with sentinel node metastasis. Cancer Sci 2013;104:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schulz S, Sinn P, Golatta M, et al. Prediction of underestimated invasiveness in patients with ductal carcinoma in situ of the breast on percutaneous biopsy as rationale for recommending concurrent sentinel lymph node biopsy. Breast 2013;22:537–42. [DOI] [PubMed] [Google Scholar]
- [11].Fisher ER. Pathobiological considerations relating to the treatment of intraductal carcinoma (ductal carcinoma in situ) of the breast. CA Cancer J Clin 1997;47:52–64. [DOI] [PubMed] [Google Scholar]
- [12].Arnone P, Zurrida S, Viale G, et al. The TNM classification of breast cancer: need for change. Updates Surg 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
- [13].Perry N. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis-/Ed.: N. Perry et al. Luxembourg: office for official publications of the European Communities. 2006. 00687. [Google Scholar]
- [14].Cedolini C, Bertozzi S, Londero AP, et al. Impact of the presence and quantity of ductal carcinoma in situ component on the outcome of invasive breast cancer. Int J Clin Exp Pathol 2015;8:13304–13. [PMC free article] [PubMed] [Google Scholar]
- [15].Lakhani SR, International Agency for Research on Cancer, World Health Organization, editors. WHO Classification of Tumours of the Breast. Number fourth in World Health Organization classification of tumours, 4th edition. International Agency for Research on Cancer, Lyon. 2012. 00857. [Google Scholar]
- [16].Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- [17].Tavassoli FA, Eusebi V. Tumors of the Mammary Gland. Number fasc. 10 in AFIP atlas of tumor pathology. Fourth series. American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology, Washington, DC: 2009. 00060. [Google Scholar]
- [18].Bernardi S, Bertozzi S, Londero AP, et al. Prevalence and risk factors of intraoperative identification failure of sentinel lymph nodes in patients affected by breast cancer. Nucl Med Commun 2013;34:664–73. [DOI] [PubMed] [Google Scholar]
- [19].Cedolini C, Bertozzi S, Seriau L, et al. Eight-year experience with the intraoperative frozen section examination of sentinel lymph node biopsy for breast cancer in a North-Italian university center. Int J Clin Exp Pathol 2014;7:364–71. [PMC free article] [PubMed] [Google Scholar]
- [20].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Method) 1995;57:289–300. [Google Scholar]
- [21].Driul L, Bernardi S, Bertozzi S, et al. New surgical trends in breast cancer treatment: conservative interventions and oncoplastic breast surgery. Minerva Ginecol 2013;65:289–96. [PubMed] [Google Scholar]
- [22].Cedolini C, Bertozzi S, Londero AP, et al. Type of breast cancer diagnosis, screening, and survival. Clin Breast Cancer 2014;14:235–40. [DOI] [PubMed] [Google Scholar]
- [23].van Roozendaal LM, Goorts B, Klinkert M, et al. Sentinel lymph node biopsy can be omitted in DCIS patients treated with breast conserving therapy. Breast Cancer Res Treat 2016;156:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaufman SA, Harris EER, Bailey L, et al. ACR appropriateness criteria®; ductal carcinoma in situ. Oncology (Williston Park, NY) 2015;29: [PubMed] [Google Scholar]
- [25].Harnett A, Smallwood J, Titshall V, et al. Guideline development group. Diagnosis and treatment of early breast cancer, including locally advanced disease-summary of NICE guidance. BMJ 2009;338:b438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].D’Eredità G, Giardina C, Napoli A, et al. Sentinel lymph node biopsy in patients with pure and high-risk ductal carcinoma in situ of the breast. Tumori 2009;95:706–11. [DOI] [PubMed] [Google Scholar]
- [27].AOIM. Linee guida—Neoplasia della mammella. 2016.00000. [Google Scholar]
- [28].Sue GR, Lannin DR, Killelea B, et al. Predictors of microinvasion and its prognostic role in ductal carcinoma in situ. Am J Surg 2013;206:478–81. [DOI] [PubMed] [Google Scholar]
- [29].Wang W, Zhu W, Du F, et al. Clinicopathological characteristics and cancer-specific outcomes for patients with microinvasive breast cancer: a SEER database analysis. Sci Rep 2017;7:42045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parikh RR, Haffty BG, Lannin D, et al. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys 2012;82:7–13. [DOI] [PubMed] [Google Scholar]
- [31].de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, et al. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer 2002;94:2134–42. [DOI] [PubMed] [Google Scholar]
- [32].Zetterlund L, Stemme S, Arnrup H, et al. Incidence of and risk factors for sentinel lymph node metastasis in patients with a postoperative diagnosis of ductal carcinoma in situ. Br J Surg 2014;101:488–94. [DOI] [PubMed] [Google Scholar]
- [33].Ruvalcaba-Limón E, de Jesús Garduño-Raya M, Bautista-Piña V, et al. Sentinel lymph node metastasis in patients with ductal breast carcinoma in situ. Cir Cir 2014;82:129–41. [PubMed] [Google Scholar]
- [34].Al-Ameer AY, Al Nefaie S, Al Johani B, et al. Sentinel lymph node biopsy in clinically detected ductal carcinoma in situ. World J Clin Oncol 2016;7:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bertozzi S, Londero AP, Petri R, et al. Isolated axillary nodal swelling and cancer of unknown primary. Eur J Gynaecol Oncol 2015;36:131–7. [PubMed] [Google Scholar]
- [36].Ozkan-Gurdal S, Cabioglu N, Ozcinar B, et al. Factors predicting microinvasion in ductal carcinoma in situ. Asian Pac J Cancer Prev 2014;15:55–60. [DOI] [PubMed] [Google Scholar]
- [37].Yao JJ, Zhan WW, Chen M, et al. Sonographic features of ductal carcinoma in situ of the breast with microinvasion: correlation with clinicopathologic findings and biomarkers. J Ultrasound Med 2015;34:1761–8. [DOI] [PubMed] [Google Scholar]
- [38].Yen TWF, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: A guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg 2005;200:516–26. [DOI] [PubMed] [Google Scholar]
- [39].Meijnen P, Oldenburg HSA, Loo CE, et al. Risk of invasion and axillary lymph node metastasis in ductal carcinoma in situ diagnosed by core-needle biopsy. Br J Surg 2007;94:952–6. [DOI] [PubMed] [Google Scholar]
- [40].Sakr R, Bezu C, Raoust I, et al. The sentinel lymph node procedure for patients with preoperative diagnosis of ductal carcinoma in situ: Risk factors for unsuspected invasive disease and for metastatic sentinel lymph nodes. Int J Clin Pract 2008;62:1730–5. [DOI] [PubMed] [Google Scholar]
- [41].Trentin C, Dominelli V, Maisonneuve P, et al. Predictors of invasive breast cancer and lymph node involvement in ductal carcinoma in situ initially diagnosed by vacuum-assisted breast biopsy: Experience of 733 cases. Breast 2012;21:635–40. [DOI] [PubMed] [Google Scholar]
- [42].Lee SK, Yang JH, Woo SY, et al. Nomogram for predicting invasion in patients with a preoperative diagnosis of ductal carcinoma in situ of the breast. Br J Surg 2013;100:1756–63. [DOI] [PubMed] [Google Scholar]
- [43].Sakr R, Antoine M, Barranger E, et al. Value of sentinel lymph node biopsy in breast ductal carcinoma in situ upstaged to invasive carcinoma. Breast J 2008;14:55–60. [DOI] [PubMed] [Google Scholar]
- [44].Orzalesi L, Casella D, Criscenti V, et al. Microinvasive breast cancer: Pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single-institution series. Breast Cancer 2016;23:640–8. [DOI] [PubMed] [Google Scholar]
- [45].Matsen CB, Hirsch A, Eaton A, et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol 2014;21:3330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rohilla M, Bal A, Singh G, et al. Prediction of heterogeneity in breast cancer immunophenotype at ductal carcinoma in situ stage? J Cancer Res Ther 2016;12:1249–56. [DOI] [PubMed] [Google Scholar]
- [47].Fang Y, Wu J, Wang W, et al. Biologic behavior and long-term outcomes of breast ductal carcinoma in situ with microinvasion. Oncotarget 2016;7:64182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Di Cesare P, Pavesi L, Villani L, et al. The Relationships between HER2 Overexpression and DCIS Characteristics. Breast J 2016. [DOI] [PubMed] [Google Scholar]
- [49].Agosto-Arroyo E, Isayeva T, Wei S, et al. Differential gene expression in ductal carcinoma in situ of the breast based on ERBB2 status. Cancer Control 2017;24:102–10. [DOI] [PubMed] [Google Scholar]
- [50].Daoud SA, Ismail WM, Abdelhamid MS, et al. Possible prognostic role of HER2/Neu in ductal carcinoma in situ and atypical ductal proliferative lesions of the breast. Asian Pac J Cancer Prev 2016;17:3733–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


