Abstract
De Quervain's disease, carpal tunnel syndrome (CTS), and trigger finger (digit) are three common pathological conditions of the hand. They are considered overuse syndromes and occur predominantly in females. The prevalence rate and cause-specific risks of these three tendinopathies have not yet been clarified. Data from 41,871 cases listed in the Taiwan National Health Insurance Research Database (NHIRD) from 2010 to 2014 were analyzed. The prevalence rate of these 3 conditions by age, sex, and the risk factors of female-dominant diseases (e.g., osteoporosis, rheumatoid arthritis [RA], and tendinopathy), diabetes mellitus, and hormone antagonist treatment was evaluated. We found that 1.59% of the population developed CTS, 0.49% developed de Quervain's, and 1.07% developed trigger finger. Cases were more likely to develop the three hand tendinopathies if they were female, between 50 and 59 years old, and, according to a multivariate analysis, comorbid with RA, diabetes, using hormone antagonists. Our findings should provide an understanding of the risk factors associated with hand tendinopathy.
Keywords: hand tendinopathy, national health insurance research database, risk factors
1. Introduction
De Quervain's disease, carpal tunnel syndrome (CTS), and trigger finger (digit) are three common pathological conditions of the hand. They are considered overuse syndromes and occur predominantly in females. De Quervain's disease is precisely defined as “stenosing tenosynovitis of the first dorsal compartment”.[1] Because the sheath that contains the abductor pollicis longus and extensor pollicis brevis tendons at the radial styloid process thickens and swells, it causes pain on the radial side of the wrist. The patient gradually loses grip strength as the symptoms become more severe. CTS is a “peripheral nerve compression neuropathy”.[2] Its underlying mechanism involves elevated carpal tunnel pressure because of a reduction in carpal tunnel dimension or an increase in the volume of carpal tunnel content. Most CTS cases are idiopathic and are characterized by noninflammatory fibrosis of the subsynovial connective tissue within the carpal tunnel.[3] Trigger finger is generally caused by a size mismatch between the flexor tendon and the first annular (A-1) pulley. All three hand conditions can be conservatively managed using splinting, corticosteroid injections, nonsteroidal anti-inflammatory drugs (NSAIDs), and other adjuvant modalities; surgery is another option.[4–6] Patients are at greater risk for developing postoperative trigger finger after carpal tunnel release.[7] A meta-analysis[8] suggests that both type 1 and type 2 diabetes are risk factors for CTS. The prevalence of trigger finger after carpal tunnel release was higher for patients with diabetes than without it.[9] CTS, trigger finger, and de Quervain's disease are all musculoskeletal disorders caused by repetitive hand posture and motion.[10] These hand tendinopathies appear to be related, but the underlying mechanism is unclear.
Females are more likely to develop autoimmune and inflammatory diseases because of sexual dimorphism.[11,12] De Quervain's disease is found more commonly in women during the later stages of pregnancy and in the early postpartum period.[13] Epidemiologic studies have shown that de Quervain's disease occurs most often in women. The prevalence rate is more than three times higher than in men in the working population.[14] Other studies[15] have reported that CTS is highly associated with postmenopausal women and women who have had an oophorectomy. Similarly, women are more significantly affected by trigger finger than are men, especially women more than 53 years old.[16] Hormonal changes have been suggested to be associated with these diseases.[13,15] However, why this is so is unclear.
Endogenously induced hormonal changes and external mechanical stress might mutually affect each other during the disease pathogenesis. We previously[17] reported that estrogen receptor (ER)-β expression was higher in patients with de Quervain's disease, that its expression level was related to the disease severity, and that disease severity was also associated with tissue inflammation and angiogenesis. Others have reported that some hand abnormalities are related to sex and specific occupations.[10,16,18,19] However, the prevalence rate and cause-specific risks of these three conditions have not yet been clarified. We hypothesize that change in estrogen expression levels affect the soft tissue and result in hand abnormalities. We used the Taiwan National Health Insurance Research Database (NHIRD) (http://nhird.nhri.org.tw/en/Background.html) to assess the musculoskeletal diseases considered sexual dimorphisms. We determined their prevalence rates from 2010 through 2014 by age, sex, chronic diseases (e.g., diabetes mellitus [DM]), and risk factors. This is the first large national database study of the factors associated with these hand tendinopathies.
2. Methods
2.1. Study design and data source
Taiwan implemented a compulsory National Health Insurance (NHI) program in March 1995 that covers 99.6% of its 23 million residents.[20] It records clinical diagnoses and procedures based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.[21] Our study design and study population use retrospective nationwide cohorts based on Taiwan's NHIRD derived from the registration files and original claims data of the NHI program. The NHIRD includes medical information about beneficiary characteristics, insurance information, date of admission, diagnosis at discharge (up to 5 diagnoses), procedures undergone (up to 5), expenditures, registration, ambulatory and inpatient care, examinations, and medication. This study and the related data were gathered from the ambulatory care expenditures by visits (CD), details of ambulatory care orders (OO), inpatient expenditures by admissions (DD), details of inpatient orders (DO), and registry for beneficiaries (ID) from 2010 through 2014. The data used in this study are taken from the NHI's Longitudinal Health Insurance Database (LHID) 2010. The LHID 2010 includes all the medical claims of 2 million individuals who were systematically and randomly sampled and selected from the 2010 Registry of Beneficiaries of the NHIRD. The LHID 2010 data contained 41,871 hand tendinopathy cases and 167,484 age-matched controls (ratio: 4:1). We defined de Quervain's disease (ICD-9-CM codes 727.9), CTS (ICD-9-CM codes 354.0), and trigger finger (ICD-9-CM codes 727.03) cases in Taiwan in the NHIRD using ICD-9 codes. We used SAS 9.4 (SAS Institute Inc., Cary, NC) for related variable analyses: age (age groups: ≤ 29 years, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80); joint disease (ICD-9-CM codes 726.0, 726.1, 715.3, or 716.3); osteoporosis (ICD-9-CM codes 733.0 or 733.1); rheumatoid arthritis (RA) (ICD-9-CM codes 719.3, 714.0, 714.2, or 714); diabetes (ICD-9-CM codes 250.0 or 250.5 or 250.7 or 250.8 or 250.9); marital status (“unmarried” or “married, divorced, widowed”); education level (“high school and below” or “university and above”); low income (“yes” or “no”); and whether the patient had been treated with a hormone antagonist which was used as an antiestrogen to treat breast or ovary cancers within the previous 3 years if diagnosed with hand syndromes (“yes” or “no”).
2.2. Ethics statement
The Institutional Review Board of the Jianan Psychiatric Center, a Taiwan Ministry of Health and Welfare hospital approved the study (IRB: 15–010). Informed consent was waived because patient names and incidence densities (IDs) in the NHIRD were de-identified before being released for research.
2.3. Statistical analysis
We cleaned, merged, and analyzed the data using SAS 9.4. IDs of all-cause and cause-specific hand tendinopathy were calculated by dividing the number of people who sought medical care for hand tendinopathy by the total of person-years determined. Descriptive statistics were analyzed for all variables using counts and percentages. All analyses were 2-tailed, and significance was set at P <.05. To reduce potential confounders, a multivariate logistic regression analysis controlled for age, marital status, educational level, low income, joint disease, osteoporosis, RA, diabetes, and hormone antagonist treatment in the three years before hand tendinopathy was used to determine the factors that affected De Quervain's disease, CTS, and trigger finger. The effects of outcome variables were measured using an adjusted odds ratio (AOR) with a 95% confidence interval (CI). All data were retrieved and processed within the Taiwan Data Science Center of the Ministry of Health and Welfare.
3. Results
From 2010 to 2014, the total of the prevalence rates of these 3 hand tendinopathies was 3.16% (Table 1). Their individual prevalence rates were 1.59% for CTS, 0.49% for de Quervain's disease, and 1.07% for trigger finger. Prevalence rates were twice as high for women (66.27%) than for men (Table 1). The most female-dominant condition was de Quervain's disease (72.74%). More ≥80-year-old men than women had trigger finger. De Quervain's disease was most prevalent in 30- to 39-year-old men; the highest prevalence of most other cases of hand tendinopathy was in 50- to 59-year-old men and women.
Table 1.
The number of cases of hand tendinopathy during 2010-2014.
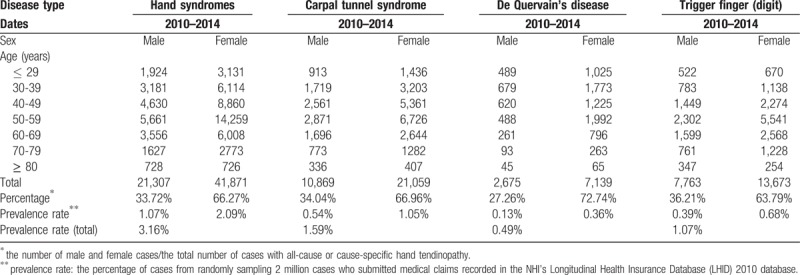
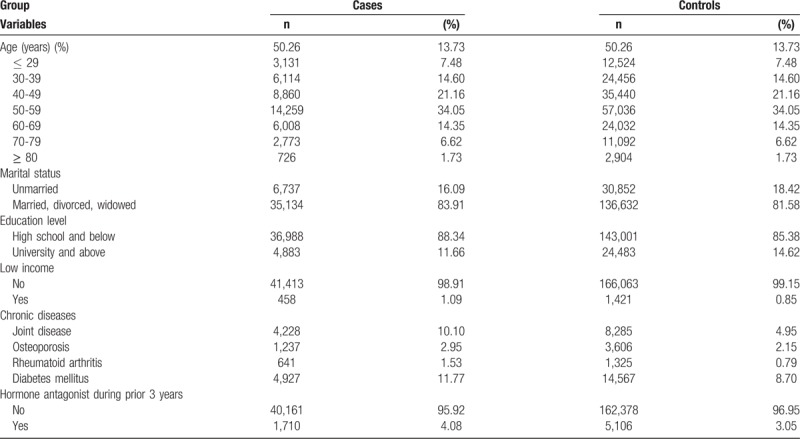
In female patients, most cases of de Quervain's disease, CTS, and trigger finger were in the 50- to 59-year-olds (34.05%) and in the 40- to 49-year-olds (21.16%) (Table 2). Most (83.91%) case group members were married, divorced, or widowed. Fewer case group members had a high educational level (11.66% vs 14.62%), but more had a low income (1.09% vs 0.85%), chronic comorbid joint disease (10.10% vs 4.95%), and diabetes (11.77% vs 8.70%) than did the control group. Most (95.92%) case group members had not taken a hormone antagonist within 3 years before having one of these three tendinopathies.
Table 2.
Demographics of female cases and controls.
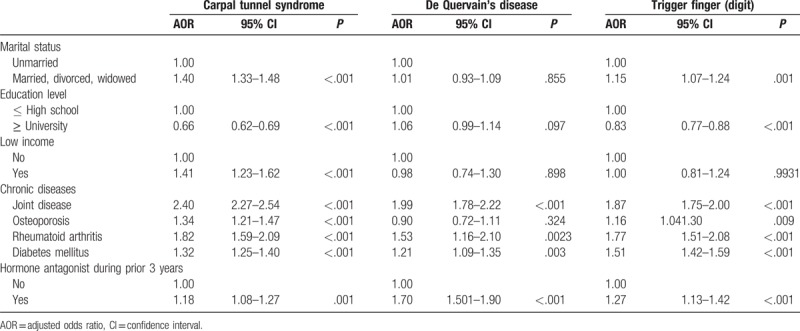
Multivariate logistic regression analyses adjusted for marital status, education level, income, chronic diseases, and taking hormone antagonists in the previous three years (after a diagnosis for hand tendinopathy) indicated that marital status and education level were significantly related to CTS and trigger finger: married, divorced, and widowed case-group members were 1.40 times and 1.15 times more likely to have CTS and trigger finger than were unmarried case-group members (95% CI = 1.33–1.48, P <.001; 95% CI = 1.07–1.24, P =.001, respectively). The case s had an educational level of university and above were 0.66 times and 0.83 times more likely to have CTS and trigger finger as compared with case s had an educational level of high school and below (95% CI = 0.62–0.69, P <.001; 95% CI = 0.77–0.88, P <.001). The case s with low income were 1.41 times more likely to have CTS (95% CI = 1.23–1.62, P <.001). The case s had joint disease were 2.40 times, 1.99 times and 1.87 times more likely to have CTS, de Quervain's disease, and trigger finger (95% CI = 2.27–2.54, P <.0001; 95% CI = 1.78–2.22, P <.001; 95% CI = 1.75–2.00, P <.001). The cases had osteoporosis were 1.34 times and 1.16 times more likely to have CTS and trigger finger (95% CI = 1.21–1.47, P <.001; 95% CI = 1.04–1.30, P =.009). The case s had RA were 1.82 times, 1.53 times, and 1.77 times more likely to have CTS, de Quervain's disease, and trigger finger (95% CI = 1.59–2.09, P <.001; 95% CI = 1.16–2.01, P = .003; 95% CI = 1.51–2.09, P <.001). The cases had DM were 1.32 times, 1.21 times and 1.51 times more likely to have CTS, de Quervain's disease, and trigger finger (95% CI = 1.25–1.40, P <.001; 95% CI = 1.09–1.35, P =.003; 95% CI = 1.42–1.59, P <.001). Taking hormone antagonists in the previous 3 years after case had hand tendinopathies were 1.18 times, 1.69 times and 1.27 times more likely to have CTS, de Quervain's disease, and trigger finger (95% CI = 1.08–1.274, P = .001; 95% CI = 1.51–1.90, P <.001; 95% CI = 1.13–1.42, P <.001) (Table 3).
Table 3.
Multivariate logistic regression analysis of factors associated with hand tendinopathy in female patients.
4. Discussion
We found that, during the five years from 2010 to 2014, CTS was the most prevalent type of hand tendinopathy, trigger finger was second, and de Quervain's disease was third. This was consistent with a study[10] that evaluated 867 hand tendinopathy patients from 2009 to 2010. The gender ratio (female to male) of morbidity rate of 3 conditions is 1.94; the highest rate is 2.70: de Quervain's disease. The peak age of disease prevalence is 50 to 59 years old. Joint disease and RA were the most frequent comorbid chronic diseases.
Our findings support previous epidemiologic studies[11–16] which reported that females were more likely to develop hand diseases than were males. Hormonal changes might account for the prevalence of hand tendinopathy. The median age at menopause of Asian women is 51.1 years old; it ranges between 51.4 and 54 years old in North American and European women.[22] In Taiwanese women, the peak age for our target tendinopathies was between 50 and 59 years old, which includes the average age at onset of menopause and, therefore, suggests the involvement of hormones in the pathogenesis of these diseases. Postmenopausal women have a significantly higher prevalence of musculoskeletal symptoms than do premenopausal women. Abrupt changes of estrogen expression during menopause exacerbate osteoarthritis (OA), RA, and osteoporosis. Estrogen might have intrinsic immune-regulatory properties that upregulate immunoglobulin G (IgG) sialylation in RA and in chondrogenesis in OA.[23,24] Estrogen deficiency also upregulates T cell-mediated osteoclast activation and leads to osteoporosis.[25] Hormone regulates the immune response might occur during the pathogenesis of these three hand tendinopathies. In addition, men ≥ 80 years were more likely to have trigger finger than were age-matched women. Taiwan's Bureau of Statistics reported that 54.02% to 56.82% of the country's total labor force was between 25 and 44 years old from 2010 through 2014 (https://eng.stat.gov.tw/mp.asp?mp = 5) but that the number of women ≥ 65 years old in the labor force dropped quickly. The peak age of de Quervain's disease in men was 30 to 39 years old, which was also at the peak age of male laborers. Mechanical loading seems to be a significant factor in the pathogenesis of de Quervain's disease. Nevertheless, a large population study[26] reported that women had four times more CTS than did men. The causes of diseases are most likely more complicated than simple sex differences, the combined effects of hormonal changes, and mechanical loading. The type and duration of labor performed and the laborer's age group might also affect the type of tendinopathy developed. However, this hypothesis requires further investigation.
Hormonal changes are associated with musculoskeletal diseases.[27,28] A direct effect of estrogen antagonist (aromatase-inhibitor [AI]) therapy was reported[26] to induce a musculoskeletal syndrome characterized by tenosynovial change and post-treatment intra-articular fluid.[29] AI treatment is associated with a higher prevalence of CTS and trigger finger.[30] One of our novel findings is that patients treated with AIs had higher AORs for de Quervain's disease (1.69, 95% CI = 1.51–1.90, P <.0001), trigger finger (1.27, 95% CI = 1.13–1.42, P <.0001), and CTS (1.18, 95% CI = 1.08–1.27, P <.0001). Our study is the first to report that in a large group of AI patients, AI therapy is a cause-specific factor for common hand tendinopathy. Patients with cancer should be informed when they are scheduled for AI therapy. There is a wealth of data on the effects of hormones on inflammatory and degenerative arthritis like RA and OA.[31] RA affects about 1% of the worldwide population (6:1 [female:male] in young adults).[32] The Women's Health Initiative (https://www.whi.org/SitePages/WHI%20Home.aspx) said[33] that 44% of the participating postmenopausal women reported OA. A systematic review and meta-analysis[34] suggest that both RA and OA increase the risk of CTS. They evaluated 23 studies for the meta-analysis, and the pooled confounder-adjusted AORs were 1.96 for RA and 1.87 for OA. We found that the AORs for CTS of case-group members were 2.40 for joint diseases and 1.82 for RA. We included all joint diseases in all joints. This might be why our study had higher AORs for CTS. Moreover, we found that RA and OA also increased the risk of de Quervain's disease and trigger finger. Our study had, for patients with de Quervain's disease, higher AORs for patients comorbid with joint diseases (1.99, 95% CI = 1.78–2.22, P <.0001) than for patients comorbid with RA (1.53, 95% CI = 1.16–2.01, P = .0027). The AORs of patients with trigger finger had a higher AOR for patients comorbid with joint diseases (1.87, 95% CI = 1.75–2.00, P <.0001) than for patients comorbid with RA (1.77, 95% CI = 1.51–2.08, P <.0001), but they were not significantly different; this suggested that de Quervain's disease might be more associated with joint diseases. It is not clear whether autoimmune disease or degenerative inflammatory disease had stronger effects on the pathogenesis of the three targeted tendinopathies. Nevertheless, they must be differentially diagnosed from RA-induced phalangeal joint pain to avoid overestimating the occurrence of this RA-related symptom. Trigger finger and CTS are common clinical problems for patients with DM. Other studies[35–38] report that trigger finger and CTS are more common in patients with than without DM. Because DM causes peripheral neuropathy, also a symptom of CTS, a differential diagnosis is necessary. Osteoporosis is considered a common postmenopausal disease.[39] Surprisingly, however, the association of this disease with musculoskeletal symptoms is not widely studied. CTS is more associated with osteoporosis than with trigger finger and de Quervain's disease, which suggests that CTS and osteoporosis share some common pathogenic causes. However, this hypothesis requires further investigation.
4.1. Limitations
Our study has some limitations. Because we relied on insurance claims data instead of actual medical records, the disease prevalence might be underestimated, and there might be a misclassification bias. Second, the availability of other socioeconomic factors (occupation and financial status) is limited. We found that a lower educational level and lower income were significantly associated with hand tendinopathy, especially with CTS. Case-group members’ job types and work behaviors were not provided in these data; thus, we were unable to associate these tendinopathies with socioeconomic factors. However, we currently use an in vitro cyclic stretching culture system (ATMS Boxer TM, TAIHOYA Corporation, Kaohsiung, Taiwan) to study the effects of mechanical stress on tissue. It partly answers the question. Third, most of the AI-treated patients had cancer. AI therapy usually lasts from 6 months to 3 years; therefore, we controlled for that. However, cancer might be a potential confounder; we did not rule that out in this study.
5. Conclusion
In conclusion, we surveyed the epidemiology of three common hand tendinopathies in Taiwan between 2010 and 2014. We found that they are all female-dominant conditions. We hypothesize that the combined effects of hormonal changes and mechanical loading lead to different types and severities of hand tendinopathy. The risk factors of these tendinopathies are joint disease, RA, DM, and hormonal antagonist therapy. A differential diagnosis is required between these three hand tendinopathies and RA- and DM-induced symptoms. Patients should be informed at the start of AI treatment of their risk of developing one or more of these tendinopathies and of other risks. Our findings should provide a greater understanding of the risk factors associated with hand tendinopathy and should be a valuable reference for clinicians.
Author contributions
Conceptualization: Po-Chuan Shen, I-Ming Jou, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Data curation: Po-Chuan Shen, I-Ming Jou, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Formal analysis: Po-Chuan Shen, Po-Chun Chang, I-Ming Jou, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Funding acquisition: Jeng-Long Hsieh, Po-Chun Chang.
Investigation: Po-Chun Chang, Fang-Hsin Lee, Jeng-Long Hsieh.
Methodology: Po-Chuan Shen, Po-Chun Chang, I-Ming Jou, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Project administration: Po-Chuan Shen, I-Ming Jou, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Resources: Fang-Hsin Lee, Jeng-Long Hsieh.
Software: Po-Chun Chang, Chung-Hwan Chen, Fang-Hsin Lee, Jeng-Long Hsieh.
Supervision: Fang-Hsin Lee, Jeng-Long Hsieh.
Validation: Fang-Hsin Lee, Jeng-Long Hsieh.
Visualization: Fang-Hsin Lee, Jeng-Long Hsieh.
Writing – original draft: Po-Chuan Shen, Fang-Hsin Lee, Jeng-Long Hsieh.
Writing – review & editing: Jeng-Long Hsieh.
Footnotes
Abbreviations: AI = aromatase-inhibitor, AOR = adjusted odds ratio, CI = confidence interval, CTS = carpal tunnel syndrome, DM = diabetes mellitus, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IDs = incidence densities, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = Taiwan National Health Insurance Research Database, RA = rheumatoid arthritis.
This study is based in part on data from the NHIRD provided by the Taiwan National Health Insurance Administration, Ministry of Health and Welfare, and managed by the National Health Research Institutes. Our interpretations and conclusions do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes. This work was supported by a grant from the Taiwan National Science Council, Ministry of Science and Technology (MOST 105–2314-B-273–001-MY3) and An Nan hospital, China Medical University (ANHRF107–08).
The authors have no conflicts of interest to disclose.
References
- [1].de Quervain F. On a form of chronic tendovaginitis by Dr. Fritz de Quervain in la Chaux-de-Fonds. 1895. Am J Orthop 2005;26:641–4. [PubMed] [Google Scholar]
- [2].Bland JD. Carpal tunnel syndrome. Curr Opin Neuro 2005;18:581–5. [DOI] [PubMed] [Google Scholar]
- [3].Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am 2004;86:1458–66. [DOI] [PubMed] [Google Scholar]
- [4].Kim JK, Hann HJ, Kim MJ, et al. The expression of estrogen receptors in the tenosynovium of postmenopausal women with idiopathic carpal tunnel syndrome. J Orthop Res 2010;28:1469–74. [DOI] [PubMed] [Google Scholar]
- [5].Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am 2006;31:135–46. [DOI] [PubMed] [Google Scholar]
- [6].Di Sante L, Martino M, Manganiello I, et al. Ultrasound-guided corticosteroid injection for the treatment of de Quervain's tenosynovitis. Am J Phys Med Rehabil 2013;92:637–8. [DOI] [PubMed] [Google Scholar]
- [7].Hayashi M, Uchiyama S, Toriumi H, et al. Carpal tunnel syndrome and development of trigger digit. J Clin Neurosci 2005;12:39–41. [DOI] [PubMed] [Google Scholar]
- [8].Pourmemari MH, Shiri R. Diabetes as a risk factor for carpal tunnel syndrome: a systematic review and meta-analysis. Diabet Med 2016;33:10–6. [DOI] [PubMed] [Google Scholar]
- [9].Grandizio LC, Beck JD, Rutter MR, et al. The incidence of trigger digit after carpal tunnel release in diabetic and nondiabetic patients. J Hand Surg Am 2014;39:280–5. [DOI] [PubMed] [Google Scholar]
- [10].Laoopugsin N, Laoopugsin S. The study of work behaviours and risks for occupational overuse syndrome. Hand Surg 2012;17:205–12. [DOI] [PubMed] [Google Scholar]
- [11].Bearoff F, Case LK, Krementsov DN, et al. Identification of genetic determinants of the sexual dimorphism in CNS autoimmunity. PLoS One 2015;10:e0117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kovacs WJ, Olsen NJ. Sexual dimorphism of RA manifestations: genes, hormones and behavior. Nat Rev Rheumatol 2011;7:307–10. [DOI] [PubMed] [Google Scholar]
- [13].Tendon trouble in the hands: de Quervain's tenosynovitis and trigger finger. Women are more likely than men to develop these painful conditions. Harv Womens Health Watch 2010;17:4–5. [PubMed] [Google Scholar]
- [14].Petit Le Manac’h A, Roquelaure Y, Ha C, et al. Risk factors for de Quervain's disease in a French working population. Scand J Work Environ Health 2011;37:394–401. [DOI] [PubMed] [Google Scholar]
- [15].Pascual E, Giner V, Aróstegui A, et al. Higher incidence of carpal tunnel syndrome in oophorectomized women. Br J Rheumatol 1991;30:60–2. [DOI] [PubMed] [Google Scholar]
- [16].De la Parra-Márquez ML, Tamez-Cavazos R, Zertuche-Cedillo L, et al. Risk factors associated with trigger finger. Case-control study. Cir Cir 2008;76:323–7. [PubMed] [Google Scholar]
- [17].Shen PC, Wang PH, Wu PT, et al. The estrogen receptor-( expression in de Quervain's disease. Int J Mol Sci 2015;16:26452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dale AM, Zeringue A, Harris-Adamson C, et al. General population job exposure matrix applied to a pooled study of prevalent carpal tunnel syndrome. Am J Epidemiol 2015;181:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heilskov-Hansen T, Mikkelsen S, Svendsen SW, et al. Exposure-response relationships between movements and postures of the wrist and carpal tunnel syndrome among male and female house painters: a retrospective cohort study. Occup Environ Med 2016;73:401–8. [DOI] [PubMed] [Google Scholar]
- [20].National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. A new era of National Health Insurance—towards the universal coverage of health care. Available at: https://ww.w.nhi.gov.tw/Resource/webdata/13767_1_National%20Health%20Insurance%20in%20Taiwan%202016-2017(bilingual).pdf Accessed July 9, 2018. [Google Scholar]
- [21].Chang GH, Tsai MS, Liu CY, et al. End-stage renal disease: a risk factor of deep neck infection-nationwide follow-up study in Taiwan. BMC Infect Dis 2017;17:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Palacios S, Henderson VW, Siseles N, et al. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 2010;13:419–28. [DOI] [PubMed] [Google Scholar]
- [23].Engdahl C, Bondt A, Harre U, et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther 2018;20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koelling S, Miosge N. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum 2010;62:1077–87. [DOI] [PubMed] [Google Scholar]
- [25].Zhao R, Wang X, Feng F. Upregulated cellular expression of IL-17 by CD4+ T-cells in osteoporotic postmenopausal women. Ann Nutr Metab 2016;68:113–8. [DOI] [PubMed] [Google Scholar]
- [26].Farioli A, Curti S, Bonfiglioli R, et al. Observed differences between males and females in surgically treated carpal tunnel syndrome among non-manual workers: a sensitivity analysis of findings from a large population study. Ann Work Expo Health 2018;62:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon JL, Girdler SS. Hormone replacement therapy in the treatment of perimenopausal depression. Curr Psychiatry Rep 2014;16:517. [DOI] [PubMed] [Google Scholar]
- [28].Gao HL, Lin SQ, Wei Y, et al. The effect of age and menopausal status on musculoskeletal symptoms in Chinese women aged 35-64 years. Climacteric 2013;16:639–45. [DOI] [PubMed] [Google Scholar]
- [29].Lintermans A, Laenen A, Van Calster B, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Ann Oncol 2013;24:350–5. [DOI] [PubMed] [Google Scholar]
- [30].Spagnolo F, Sestak I, Howell A, et al. Anastrozole-induced carpal tunnel syndrome: results from the International Breast Cancer Intervention Study II Prevention Trial. J Clin Oncol 2016;34:139–43. [DOI] [PubMed] [Google Scholar]
- [31].Talsania M, Scofield RH. Menopause and rheumatic disease. Rheum Dis Clin North Am 2017;43:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 2001;27:269–81. [DOI] [PubMed] [Google Scholar]
- [33].Women's Health Initiative. Welcome to the Women's Health Initiative. Available at: https://www.whi.org/SitePages/WHI%20Home.aspx Accessed November 9, 2018. [Google Scholar]
- [34].Shiri R. Arthritis as a risk factor for carpal tunnel syndrome: a meta-analysis. Scand J Rheumatol 2016;45:339–46. [DOI] [PubMed] [Google Scholar]
- [35].Yosipovitch G, Yosipovitch Z, Karp M, et al. Trigger finger in young patients with insulin dependent diabetes. J Rheumatol 1990;17:951–2. [PubMed] [Google Scholar]
- [36].Chammas M, Bousquet P, Renard E, et al. Dupuytren's disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am 1995;20:109–14. [DOI] [PubMed] [Google Scholar]
- [37].Gamstedt A, Holm-Glad J, Ohlson CG, et al. Hand abnormalities are strongly associated with the duration of diabetes mellitus. J Intern Med 1993;234:189–93. [DOI] [PubMed] [Google Scholar]
- [38].Chen LH, Li CY, Kuo LC, et al. Risk of hand syndromes in patients with diabetes mellitus: a population-based cohort study in Taiwan. Medicine (Baltimore) 2015;94:e1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sözen T, Özişik L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol 2017;4:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


