Abstract
Background:
Molecular targeted therapies were found to be efficacious and safer in the treatment of metastatic renal cell carcinoma (mRCC). Sorafenib is the first target agent (TA) to report a benefit in this disease and has largely established a prominent role in progression-free survival (PFS). However, there have been conflicting results across the trials that evaluated the efficacy of sorafenib.
Objective:
The aim of the study was to perform a meta-analysis to compare the efficacy and safety of sorafenib in first-line treatments of mRCC.
Methods:
We searched online electronic databases: PubMed, Embase, and the Cochrane Library updated on September 2017. Trials on the efficacy of sorafenib in first-line treatments of advanced RCC were included, of which the primary outcomes were objective response rate (ORR), PFS, overall survival (OS), and grade 3/4 adverse events (AEs).
Results:
A total of 5 trials were included in this analysis. The group of AEs showed significantly improved PFS (odds ratio [OR] = 0.78, 95% confidence interval [CI] = 0.70–0.86, P < .001), as well with the ORR (OR = 1.89, 95%CI = 1.38–2.59, P < .0001) compared with sorafenib. However, there was no significant difference in OS (OR = 0.97, 95%CI = 0.78–1.22, P = .82).
Conclusion:
Sorafenib did not achieve efficacy and safety benefit in patients with mRCC compared with those treated with TAs. The role of sorafenib in first-line treatments of mRCC may change in favor of newer drugs. More research is needed to confirm whether these new TAs could replace sorafenib as the gold standard in the future.
Keywords: meta-analysis, metastatic renal cell carcinoma, sorafenib, targeted therapy
1. Introduction
Renal cell carcinoma (RCC) is one of the most progressive urologic cancers with a poor prognosis.[1,2] Approximately 25% to 30% of patients with RCC have overt metastatic or advanced disease, with a dismal 5-year survival rate (only 10–12%).[3] The therapeutic effect of conventional cytokine-based therapies is rather limited because of their high toxicity profile,[4,5] and advanced RCC is highly chemoresistant.[1]
In recent years, there has been growing evidence on the association of molecular mechanisms with metastatic RCC (mRCC). Molecular targeted therapy has recently successfully developed and has shown promising results in first-line treatments of advanced RCC.[6–8] RCC with clear cell histology is linked to the overexpression of vascular endothelial growth factor (VEGF), which is involved in tumor angiogenesis.[9] Currently approved and available VEGF/VEGFR-targeted drugs have been introduced to the clinical armamentarium with a beneficial effect in terms of improving progression-free survival (PFS) and overall survival (OS) rates.[10,11] Tyrosine kinase inhibitors (TKIs) interrupt the intracellular signaling of several pathways involved in the progression of mRCC by targeting growth factors such as VEGF.
Sorafenib was the first plausible TKI alternative to front-line treatment for RCC,[12] which showed antitumor activity in phase III trials based on its ability to improve PFS.[13] Nonetheless, this is challenged by more recent trials including sorafenib as an optional treatment in first-line therapies and as a standard treatment which showed that, compared to other target agents (TAs), sorafenib had no significant benefits in PFS in treating mRCC.[14] Moreover, improvements in OS were not found when using sorafenib as neoadjuvant therapy in patients with mRCC.
To make more rational choice of treatment for patients with mRCC, we performed a meta-analysis of studies to evaluate the therapeutic effect and adverse effects of sorafenib compared to other TAs in the treatment of mRCC.
2. Methods and materials
2.1. Ethical review
Ethics approval was waived because this study did not include any human participants or animals.
2.2. Search strategy
Two investigators independently searched electronic databases: PubMed, Embase, and the Cochrane Library up to September 2017. The process was established to identify all articles with the keywords “renal cell carcinoma” AND “first line”, AND “sorafenib”, and relevant Medical Subject Heading (MeSH) terms were used. The reference lists of all articles that dealt with the topic of interest were also manually checked for additional relevant publications.
2.3. Eligibility criteria
Studies included in the meta-analysis should meet the following criteria: studies were designed as randomized or nonrandomized controlled trials comparing sorafenib with other TA-based chemotherapy as a first-line treatment; studies enrolled patients with mRCC and/or patients with advanced RCC; and the outcomes of interest were efficacy (survival, tumor response) and toxicity (incidence of severe adverse effects [SAEs]), and hazard ratios (HRs) with corresponding 95% CIs were provided. For duplicated or overlapped data in multiple reports, we just included the one with most complete information.
2.4. Quality assessment
Two investigators separately rated the quality of the retrieved studies. We chose the risk of bias items recommended by The Cochrane Handbook for Systematic Reviews of Interventions.
2.5. Data extraction
Two authors independently extracted the relevant data from each trial. Disagreement was revolved by consensus. From each of the eligible studies, the main categories were based on the following: family name of the first author, publication year, study type, trial name, treatment regimen, and endpoint of interests. We extracted the corresponding HRs and risk ratios to describe the strength of the association for OS and PFS and dichotomous (overall response rate (ORR) and SAE rate) data, respectively, with corresponding 95% confidence intervals (CIs).
2.6. Statistical analysis
The endpoints of interest in the pooled analysis were OS, PFS, ORR, and SAE data, and the endpoint outcomes were considered as a weighted average of individual estimate of the HR in every included study, using the inverse variance method. If HRs and corresponding 95% CIs were reported, lnHRs and the corresponding lnLLs and lnULs were used as data points in pooling analysis. Meanwhile, if the study did not provide HRs or 95% CIs, the only available data were in the form of K–M curves. Survival data were extracted from amplified K–M curves, according to the methods described by Tierney et al.[15]
A sensitivity analysis was also performed to examine the impact on the overall results, depending on heterogeneity across the included studies, which was examined the I2 statistic.[16] Studies with an I2 of 25% to 50%, 50% to 75%, or >75% were considered to have low, moderate, or high heterogeneity, respectively.[17] When there was low heterogeneity among studies, the fixed-effects model was used. Otherwise, the random effects model was used. A P value less than .05 was considered statistically significant. Statistical analyses were performed using Review Manager version 5.3 software (Revman; The Cochrane collaboration Oxford, United Kingdom). The findings of our meta-analysis were shown in forest plots. The Begg test and the Egger test were conducted to evaluate publication bias.
3. Results
3.1. Overview of literature search and study characteristics
A total of 364 studies were retrieved initially for evaluation. Based on the criteria described in the methods, 10 publications were evaluated in more detail, but some did not provide enough detail of outcomes of 2 approaches. Therefore, a final total of 5 randomized controlled trials [18–22] addressed the addition of sorafenib to chemotherapy. The search process is described in Figure 1.
Figure 1.
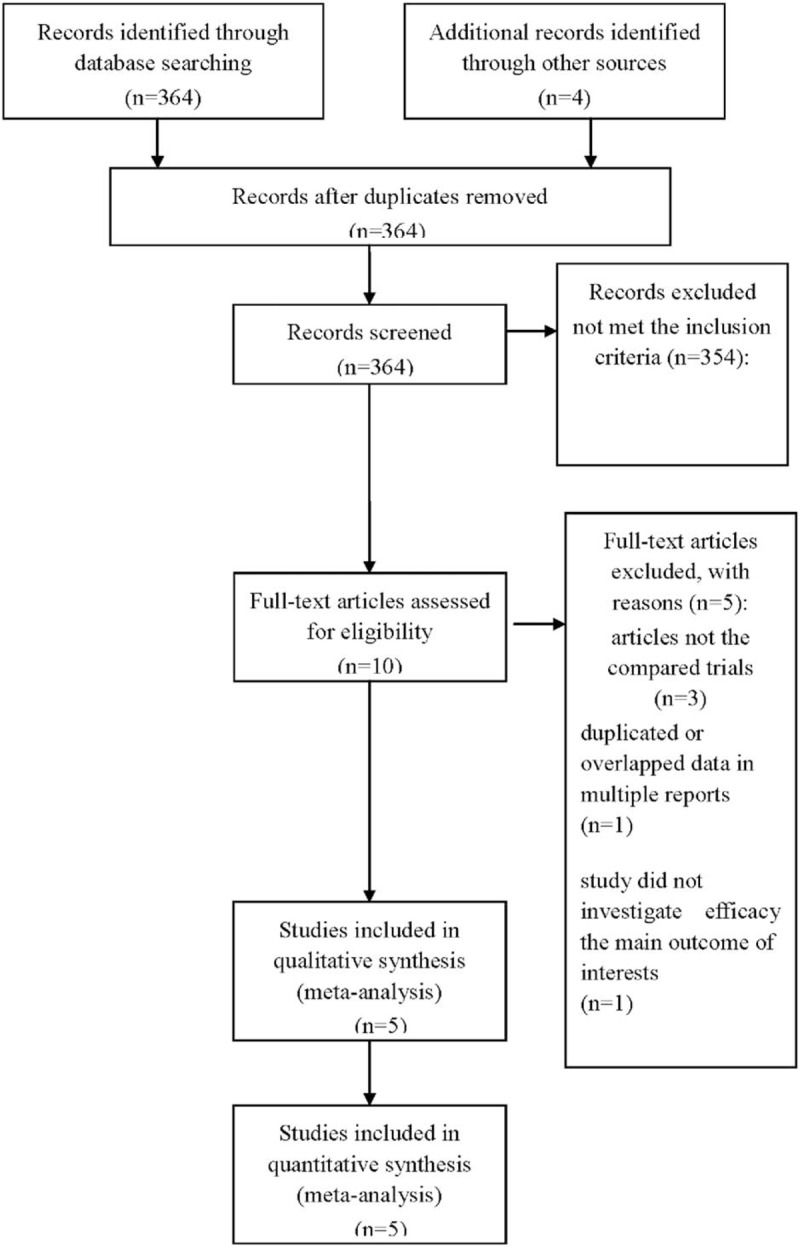
PRISMA flow chart of selection process to identify studies eligible for pooling.
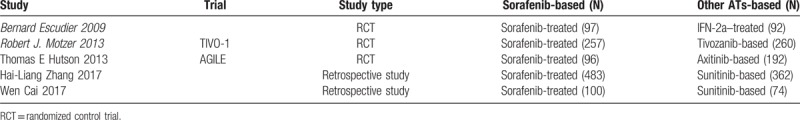
All included studies in this study were based on moderate- to high-quality evidence. Table 1 describes the primary characteristics of the eligible studies in more detail.
Table 1.
The primary characteristics of the eligible studies.
3.2. Clinical and methodological heterogeneity
3.2.1. Pooled analysis of PFS comparing the addition of sorafenib with chemotherapy
Pooled PFS data from all 5 studies[18–22] showed that other TAs prolonged PFS (OR = 0.78, 95%CI = 0.70–0.86, P<.001) compared with the chemotherapy group (Fig. 2).
Figure 2.
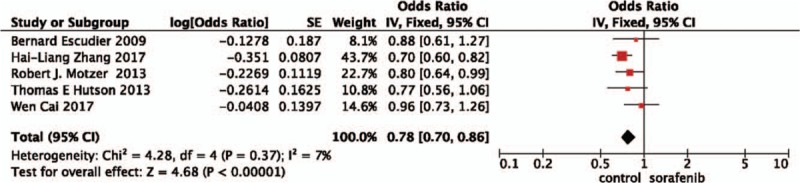
Pooled analysis of progression-free survival comparing the addition of sorafenib with chemotherapy.
3.2.2. Pooled analysis of OS comparing the addition of sorafenib with chemotherapy
A random-effects model was used to pool the OS data,[19–22] since heterogeneity across the 4 studies was high. The pooled data showed that sorafenib plus chemotherapy did not improve OS (OR = 0.97, 95%CI = 0.78–1.22, P = .82) when compared with other TA treatments (Fig. 3).
Figure 3.
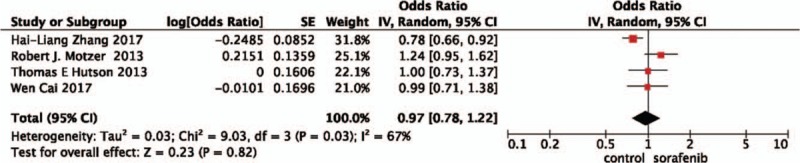
Pooled analysis of overall survival comparing the addition of sorafenib with chemotherapy.
3.2.3. Pooled analysis of ORR comparing the addition of sorafenib with chemotherapy
The pooled ORR data[18–20] did achieve advantage in the other TAs (OR = 1.89, 95%CI = 1.38–2.59, P<.0001). In other words, the addition of sorafenib did not increase the rate of ORR (Fig. 4).
Figure 4.
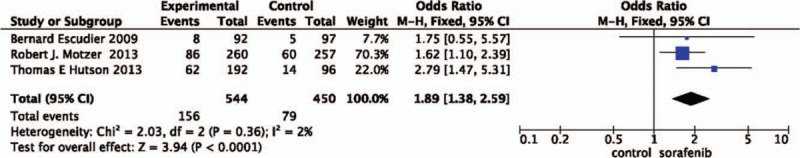
Pooled analysis of objective response rate comparing the addition of sorafenib with chemotherapy.
3.2.4. Pooled analysis of AEs comparing the addition of sorafenib with chemotherapy
Only 2 studies reported available data on AEs,[18,20] so it was not possible to perform meta-analysis. In Escudier et al's study,[18] treatment-emergent AEs of any grade were similar in both arms. Hutson et al [20] showed that SAEs were reported in 64 (34%) of 189 patients receiving axitinib, and 24 (25%) of 96 patients receiving sorafenib.
4. Discussion
VEGF-targeted antiangiogenic agents have proven as the primary mechanism for antitumor effects in RCC,[23–26] and it has been the preferred therapeutic option for patients with mRCC.[4,5,27]
Sorafenib is the first TA and inhibits the VEGFR-2/PDGFR-beta signaling cascade, and is a plausible alternative to front-line immunotherapies for RCC.[13] In a randomized phase II trial of sorafenib as a first-line therapy for mRCC,[18] patients in the sorafenib arm of this study had a longer PFS than anticipated. However, the efficacy of sorafenib used in cancer chemotherapy is often associated with distinctive challenges due to negative first-line data and evidence of the greater activity of sunitinib for mRCC.
At present, sorafenib is included in ESMO's guidelines as first- and second-line therapies for patients with mRCC. However, current clinical trials compare new targeted therapies with sorafenib to achieve benefit in efficacies, and health-related quality of life, while maintaining an acceptable safety profile. Sorafenib appears to be challenged by newer targeted therapies.[28]
In our research, we performed a meta-analysis of all available studies involving sorafenib as a first-line treatment of mRCC. In our study, we demonstrated that combination of sorafenib did not have advantages in ORR compared with TAs. This suggests that the most significant advantage of other TAs compared with sorafenib is their effectiveness in objective response.
Moreover, PFS did not translate into a benefit. Multivariate analysis revealed significant association between PFS and several predictors such as demographic and clinical characteristics, ECOG performance status, MSKCC score for risk, Heng risk criteria, and the number of metastases. In Escudier et al study,[18] the intermediate MSKCC risk group showed a trend toward improved PFS for sorafenib (HR, 1.16).Thus, sorafenib may be beneficial for intermediate-risk patients, who constitute the majority of newly diagnosed patients. Moreover, there was a positive trend of PFS that favored sorafenib in patients who had liver metastases (HR, 1.52) and in patients who had bone metastases (HR, 1.17). Bone metastasis and liver metastasis have been shown as significant predictors for PFS. Moreover, it is intriguing that ECOG performance status seemed to affect PFS of patients with a combined use of axitinib or tivozanib, but not in those receiving sorafenib.[23] However, it is worth noting that ECOG performance status 0 and 1 are broad categories that allow much clinical latitude and remain somewhat subjective. As improved clinical[29] and molecular [30] prognostic categories are developed, older classifications (e.g., ECOG performance status) are likely to be replaced with more objective ones in future clinical trials. Furthermore, the discrepancy of the PFS findings with sorafenib therapy may be related to the diversity of patient populations enrolled in each study differing in many aspects related to prognosis and ethnicity. A Chinese study conducted by Zhang et al[21] showed that superior efficacy of sorafenib in Chinese patients with mRCC yielded PFS of 11.1 months, in line with previous Chinese,[31] Korean [32,33] and Italian [34] studies. However, the results from TIVO-1 trial suggested that treatment with sorafenib as a first-line treatment yielded PFS of 9.1 months.[19] This suggests that patients with different prognostic profiles need different approaches.
For the OS data that were reported, there were issues of confounding due to crossover to differential use of next-line targeted cancer therapies.[26] Both of these factors can underestimate the difference between 2 treatments in a trial as the OS for patients receiving the less efficacious treatment may be improved by the addition of further treatments or there may be some patient populations included in the study too ill to receive treatment. It may be more difficult to show the trend toward longer OS in the sorafenib arm in recent studies due to the increased availability of a number of poststudy treatment options over time. Finally, it has been suggested that crossover treatments should be taken into consideration as prognostic factor for OS.[35,36]
Although the efficacy of targeted therapies in terms of tumor growth control at metastatic sites is definite, their AEs are often major limitations. In the present study, we could have hypothesized that sorafenib may have comparable toxicity with TAs as a first-line therapy for mRCC. Previous studies have shown that TA-related AEs were associated with different ethnicities. Ye and Zhang[37] indicated that patients experienced hand-foot syndrome, and significant difference was found in favor of Chinese patients over Western patients with sorafenib treatment. A Japanese study showed that hand-foot syndrome was the most frequent AE and 10.7% patients had SAEs.[5] The TARGET study indicated that diarrhea, rash, fatigue, and hand-foot syndrome were common AEs in a Western population and hypertension and cardiac ischemia were SAEs in patients using sorafenib.[38] Therefore, a more potent, highly selective inhibitor of TAs may improve tolerability, and thus a comparison of safety in clinical practice would provide important information for patient counseling and option for subsequent lines of treatment.
Our study has several limitations. First and foremost, as this study was a study-level meta-analysis, there is publication bias leading to heterogeneity among included studies. The retrospective studies included had an inherent limitation, and differences in patient comorbidities could not be incorporated in such an analysis. Second, only 2 studies reported available data on AEs, so we did not have access to predict efficacy in AEs.
5. Conclusion
This study demonstrates that other TAs have shown promising response rates and PFS when compared to sorafenib. Sorafenib showed an acceptable safety profile as a first-line therapy for patients with mRCC. Over recent years, as much progress has been achieved in understanding of molecular mechanism of advanced RCC and development of new targeting drugs, the overall efficiency of sorafenib is far from satisfactory. Meanwhile, some studies indicated that sorafenib might retain a role in the treatment of mRCC for some selected patients.
Therefore, the combination of these targeted drugs still requires more studies and trials to identify its application. It must be concluded that more research is needed to confirm whether these new cancer therapies should replace sorafenib as the gold standard in the future. Considering the physical conditions of patients with advanced RCC, appropriate selection of treatments is required to improve their quality of life.
Author contributions
Conceptualization: Hai-Tao Wang.
Writing – original draft: Ming Xia.
Footnotes
Abbreviations: AE = adverse event, CI = confidence interval, mRCC = metastatic renal cell carcinoma, OR = odds ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, TA = targeted agent, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Decastro GJ, McKiernan JM. Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol Clin North Am 2008;35:581–92. vi. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [4].Motzer RJ, Basch E. Targeted drugs for metastatic renal cell carcinoma. Lancet 2007;370:2071–3. [DOI] [PubMed] [Google Scholar]
- [5].Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103–11. [DOI] [PubMed] [Google Scholar]
- [6].Patard JJ, Pignot G, Escudier B, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol 2011;60:684–90. [DOI] [PubMed] [Google Scholar]
- [7].Harshman LC, Xie W, Bjarnason GA, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol 2012;13:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choueiri TK, Duh MS, Clement J, et al. Angiogenesis inhibitor therapies for metastatic renal cell carcinoma: effectiveness, safety and treatment patterns in clinical practice-based on medical chart review. BJU Int 2010;105:1247–54. [DOI] [PubMed] [Google Scholar]
- [9].Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist 2007;12:1404–15. [DOI] [PubMed] [Google Scholar]
- [10].Palazzo A, Iacovelli R, Cortesi E. Past, present and future of targeted therapy in solid tumors. Curr Cancer Drug Targets 2010;10:433–61. [DOI] [PubMed] [Google Scholar]
- [11].Iacovelli R, Sternberg CN, Porta C, et al. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: a systematic review and meta-analysis. Curr Drug Targets 2015;16:164–70. [DOI] [PubMed] [Google Scholar]
- [12].Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:2505–12. [DOI] [PubMed] [Google Scholar]
- [13].Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25Suppl 3: iii49-56. [DOI] [PubMed] [Google Scholar]
- [14].Iacovelli R, Verri E, Cossu Rocca M, et al. Is there still a role for sorafenib in metastatic renal cell carcinoma? A systematic review and meta-analysis of the effectiveness of sorafenib over other targeted agents. Crit Rev Oncol Hematol 2016;99:324–31. [DOI] [PubMed] [Google Scholar]
- [15].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. doi:10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [17].Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280–9. [DOI] [PubMed] [Google Scholar]
- [19].Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287–94. [DOI] [PubMed] [Google Scholar]
- [21].Zhang HL, Sheng XN, Li XS, et al. Sorafenib versus sunitinib as first-line treatment agents in Chinese patients with metastatic renal cell carcinoma: the largest multicenter retrospective analysis of survival and prognostic factors. BMC Cancer 2017;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cai W, Kong W, Dong B, et al. Comparison of efficacy, safety, and quality of life between sorafenib and sunitinib as first-line therapy for Chinese patients with metastatic renal cell carcinoma. Chin J Cancer 2017;36:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- [24].Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. [DOI] [PubMed] [Google Scholar]
- [27].Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Randrup Hansen C, Grimm D, Bauer J, et al. Effects and side effects of using sorafenib and sunitinib in the treatment of metastatic renal cell carcinoma. Int J Mol Sci 2017;18(2.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- [30].Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012;13:827–37. [DOI] [PubMed] [Google Scholar]
- [31].Yu X, Guo G, Li X, et al. Retrospective analysis of the efficacy and safety of sorafenib in Chinese patients with metastatic renal cell carcinoma and prognostic factors related to overall survival. Medicine (Baltimore) 2015;94:e1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Park SJ, Lee JL, Park I, et al. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy 2012;58:468–74. [DOI] [PubMed] [Google Scholar]
- [33].Kim SH, Kim S, Nam BH, et al. Efficacy and safety of sorafenib therapy on metastatic renal cell carcinoma in Korean patients: results from a retrospective multicenter study. PLoS One 2015;10:e0135165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Procopio G, Derosa L, Gernone A, et al. Sorafenib as first- or second-line therapy in patients with metastatic renal cell carcinoma in a community setting. Future Oncol 2014;10:1741–50. [DOI] [PubMed] [Google Scholar]
- [35].Lebwohl D, Kay A, Berg W, et al. Progression-free survival: gaining on overall survival as a gold standard and accelerating drug development. Cancer J 2009;15:386–94. [DOI] [PubMed] [Google Scholar]
- [36].Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ye DW, Zhang HL. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. Onco Targets Ther 2014;7:925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Akaza H, Tsukamoto T, Murai M, et al. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J ClinOncol 2007;37:755–62. [DOI] [PubMed] [Google Scholar]


