Abstract
Background:
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors (palbociclib and abemaciclib) and mammalian target of rapamycin (mTOR) inhibitors (everolimus) are effective agents for restoring endocrine sensitivity in patients with advanced breast cancer progression on prior aromatase inhibitors. We conducted a network meta-analysis to compare these treatments in terms of progression-free survival (PFS), objective response rate (ORR), and clinical benefit rate (CBR).
Methods:
The PubMed and Embase databases were searched for relevant publications between January 2000 and June 2018. Treatments were ranked based on a network meta-analysis. Ranking was determined by P-score. A random-effect model was used when heterogeneity was detected; otherwise, a fixed-effect model was used.
Results:
Six trials comprising 4063 patients formed the comparison network. Compared with everolimus plus exemestane, the combinations of palbociclib or abemaciclib with fulvestrant showed similar efficacies in PFS and no differences in ORR. For the CBR, palbociclib demonstrated improvement, while abemaciclib did not. Incidences of severe adverse events did not significantly differ. A total of 29%, 15.9%, and 4% of patients discontinued everolimus, abemaciclib, and palbociclib, respectively, due to toxicity.
Conclusion:
These results suggest similar efficacies between CDK4/6 inhibition and mTOR blockade; however, CDK4/6 inhibitors were associated with favorable toxicity profiles.
Keywords: breast cancer, CDK4/6, endocrine therapy, meta-analysis, mTOR
1. Introduction
Approximately 70% of patients diagnosed with breast cancer are hormone receptor-positive and HER2-negative, which is characterized by expression of the estrogen receptor (ER) and/or progesterone receptor (PR) but without HER2 amplification.[1] For the initial treatment, endocrine therapy was the standard of care for these patients without visceral crisis, due to its efficacy and favorable toxicity profile.[2] Aromatase inhibitors (AI) have shown superiority over tamoxifen in terms of tumor response and progression-free survival (PFS) and were therefore considered the first-line choice.[3]
However, nearly all patients inevitably developed AI resistance. As a second-line treatment, fulvestrant (administered as 500 mg) and exemestane were associated with a moderate PFS of approximately 6 months.[4] Understanding the mechanisms of endocrine resistance has evolved over the past decade. Most importantly, the phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) pathway is likely involved.[5] Additionally, cyclin-dependent kinase 4/6 (CDK4/6) promotes proliferation in hormone receptor-positive breast cancer.[6]
Relevant clinical studies have yielded promising results in patients who have progressed on AI.[7–9] In the BOLERO-2 trial, the mTOR inhibitor, everolimus, plus exemestane significantly prolonged PFS in AI-resistant postmenopausal patients compared with exemestane alone.[7] Two recent studies investigating CDK4/6 inhibitors (PALOMA-3 and MONARCH-2) found that adding palbociclib or abemaciclib to fulvestrant significantly improved PFS in second-line settings.[8,9] However, this combination therapy was associated with increased adverse events.
The mTOR and CDK4/6 inhibitors added to the armory of second-line options in patients who developed resistance to initial endocrine therapy, but it challenged the optimal management regarding treatment sequence. Direct comparisons between these novel combinations are lacking. Therefore, we conducted a network meta-analysis to indirectly compare the efficacy and toxicity of CDK4/6 inhibitors plus fulvestrant versus everolimus plus exemestane.
2. Methods
2.1. Study design and trial inclusion criteria
The PubMed and Embase databases were searched using the terms “breast cancer”, “metastatic”, “advanced”, “hormone receptor-positive”, “endocrine therapy”, and “randomized trial”. The search was limited to articles published between January 2000 and June 2018. Abstracts presented at the annual meetings of the European Society of Medical Oncology and the American Society of Clinical Oncology between 2000 and 2017 were also screened. The study adhered to the recommendations of the PRISMA protocol. The study used data from publications for analysis, and ethical issues were not involved. Therefore, ethical approval was waived.
We included trials that compared endocrine-based therapy with different treatment strategies. Trials were required to be prospective phase II or III randomized controlled trials that reported the numbers of patients showing a stable disease and hazard ratio (HR) with 95% confidence interval (CI) of PFS or time to treatment progression or presented sufficient data to calculate the HRs with 95% CIs. Trials without randomization or control arms were excluded.
2.2. Data extraction and quality assessment
Data were extracted and quality was assessed by two independent reviewers. Disagreement between reviewers was resolved by discussion or a third reviewer. Data extracted from the trials included the trial name, first author, publication year, trial phase, sample size, treatments, adverse events, response rates, clinical benefit rates (CBRs), and PFS. We used the quantitative Jadad scale to assess study quality.[10]
3. Statistical analysis
We analyzed PFS, response rates, CBRs, and rates of severe adverse events. The P value, HRs and their 95% CIs were directly extracted.
For ease of computation and programming, we used a frequentist method to perform the analysis rather than Bayesian modeling.[11] Notably, both approaches were considered to have similar results and rankings in the network analysis.[12] A random-effect model was used when heterogeneity was detected; otherwise, a fixed-effect model was used. Treatments were ranked based on a network meta-analysis. Ranking was determined by P-score through a net rank function in the R package, with higher scores indicating a higher probability of being the best treatment. A sensitivity analysis was also planned and performed in an alternative network.
Statistical tests with P <.05 were considered significant. The results are depicted in all figures as forest plots, where HR <1 corresponds to a lower event rate in the treatment arm. Network meta-analysis was performed using R software, version i386 3.3.2, with the netmeta package.
4. Results
After the screening, 41 studies were identified for further evaluation (Fig. 1). Twenty-one studies that focused on first-line endocrine therapy were excluded. Eight publications were chosen from the remaining 20 trials.[7–9,13–29] Of these 8 publications, 6 were finally identified after excluding 2 duplicate reports.[16,17] The quality was high in all included trials (Jadad score ≥3).
Figure 1.
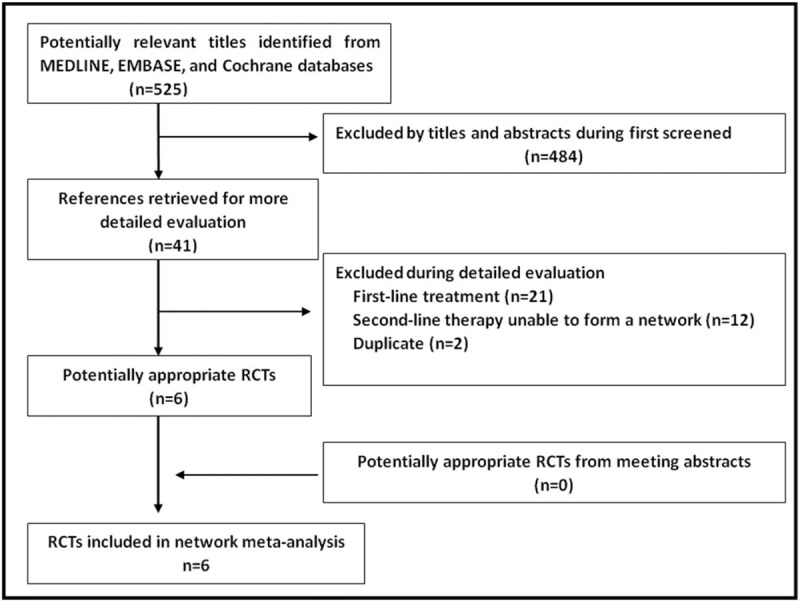
Search strategy results.
The 6 trials (EFECT, SoFEA, CONFIRM, BOLERO-2, PALOMA-2, and MONARCH-2) included 4063 patients.[7–9,13–15] SoFEA was a 3-arm study,[13] but 1 arm (fulvestrant plus anastrozole) was unnecessary in creating the network and was therefore excluded. The other 2 arms (fulvestrant and exemestane) in the SoFEA trial were applied in the sensitivity analysis.
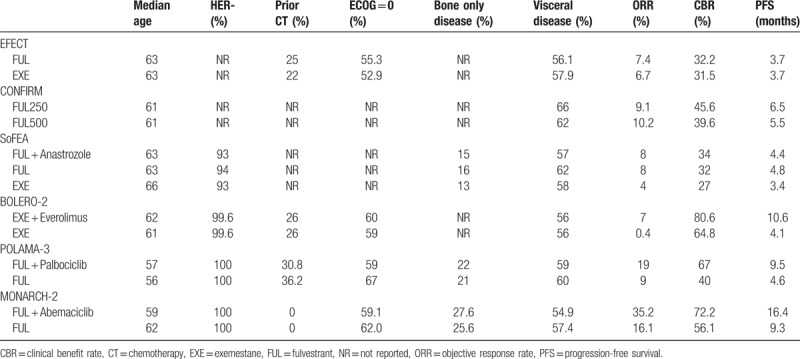
As shown in Figure 2, a network was formed with the 5 comparisons to allow indirectly comparing the combination of palbociclib or abemaciclib plus fulvestrant and the combination of everolimus plus exemestane. Details of the included studies, patient characteristics, and main study outcomes are summarized in Tables 1 and 2.
Figure 2.
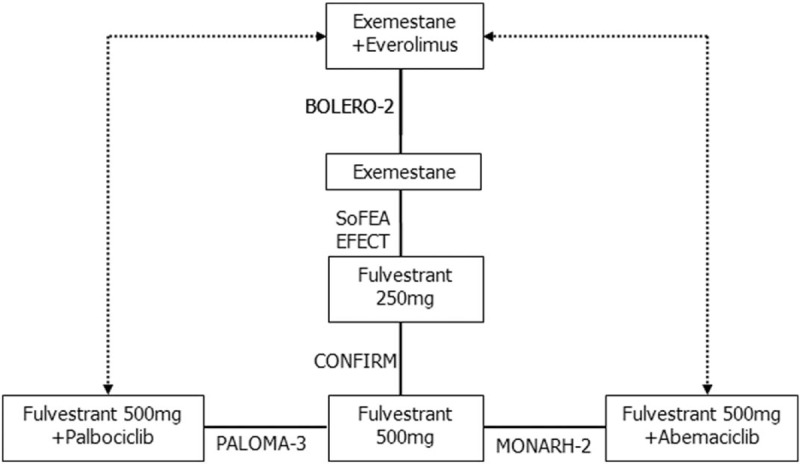
Network of the trials included in the analysis. The boxes denote therapies. Solid lines indicate direct comparisons, and dashed lines indicate indirect comparisons.
Table 1.
Details of trials included in the network analysis.
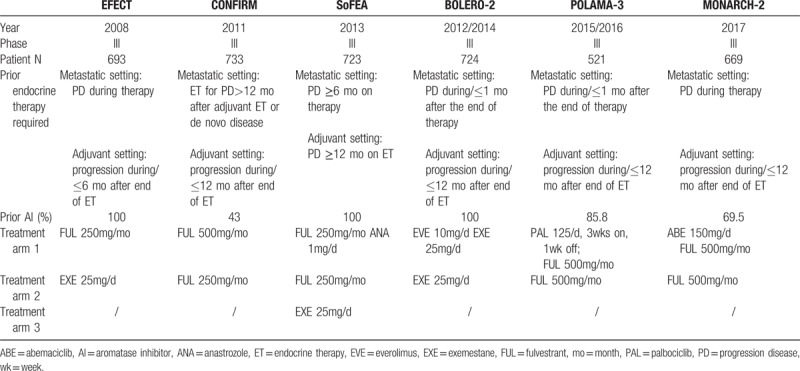
Table 2.
Patient characteristics and main study outcomes.
No significant heterogeneity or inconsistencies were found for the whole network (Q = 0.01, P = .92); therefore, a fixed-effect method was used for the meta-analysis. Network meta-analysis results for the PFS and response rates are summarized in Figures 3 and 4, respectively. The P-scores for each treatment are presented in Table 3.
Figure 3.

Pooled hazard ratios for disease progression. Treatments in the columns are compared with those in the rows.
Figure 4.
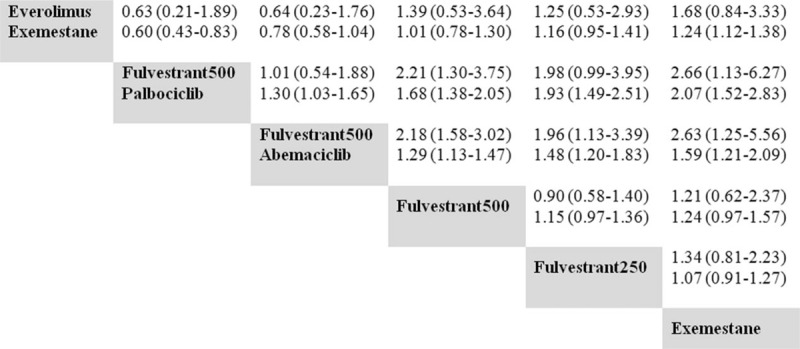
Pooled ORs for response. Treatments in the columns are compared with those in the rows. The first line shows the ORs for overall response rate, and the second line shows the ORs for clinical benefit rates. ORs = odds ratios.
Table 3.
P-scores of treatments in the network meta-analysis.
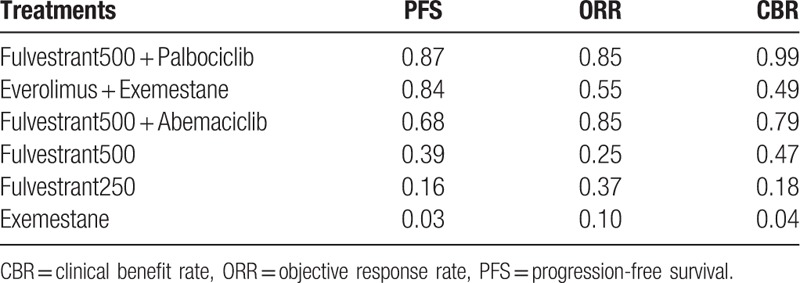
Regarding PFS, the 2 CDK4/6-based combinations showed similar efficacies compared with everolimus plus exemestane. The corresponding P-scores were .87, .84, and .68 for palbociclib plus fulvestrant, abemaciclib plus fulvestrant, and everolimus plus exemestane, respectively. No differences were found in objective response rate (ORR) among the 2 CDK4/6-based combinations and everolimus plus exemestane. For CBR, only palbociclib plus fulvestrant showed improvement compared with everolimus plus exemestane. When excluding either the SoFEA or EFECT studies to form the alternative network, the sensitivity analysis results were generally consistent with those of the original network.
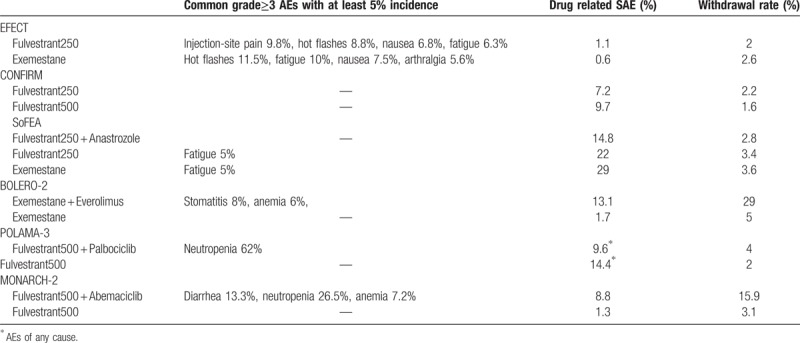
The most common grade 3 or 4 adverse events from the treatments in each trial as well as withdrawal due to toxicity are summarized in Table 4. Regarding severe adverse events, compared with everolimus plus exemestane in the network, both CDK4/6-based combinations showed a nonsignificant increasing trend. The ORs were 1.57 (95% CI, 0.57–4.34) and 1.59 (95% CI, 0.53–4.77) for palbociclib plus fulvestrant and abemaciclib plus fulvestrant, respectively.
Table 4.
Toxicity profile of treatments in each included trial.
5. Discussion
Endocrine therapy is the standard of care for patients with hormone receptor-positive and HER2-negative advanced or metastatic breast cancer. After progressing on the first-line treatment, maintaining blockage of the ER pathway was preferable to starting chemotherapy.[30] Over the past 5 years, marked progress has been made to better understand the mechanisms of endocrine resistance, which has finally led to improved treatment outcomes.[31] Exemestane plus everolimus, palbociclib plus fulvestrant, and abemaciclib plus fulvestrant have all shown remarkably improved PFS in randomized phase III trials.[7–9] Directly comparing these regimens in a head-to-head trial was not viable. However, indirectly comparing their efficacy and toxicity may lead to better-informed treatment decisions.
A recent network analysis investigated the role of CDK4/6 inhibitors in this field,[32] but it involved patients without previous endocrine therapy and did not compare abemaciclib plus fulvestrant with exemestane plus everolimus. In the present study, we employed a network analysis for indirect comparison between CDK4/6 inhibitors plus fulvestrant and everolimus plus exemestane. The 3-combination therapy had a similar effect on PFS, although the combination of abemaciclib with fulvestrant in the MONARCH study was associated with the greatest absolute benefit in PFS (7.1 months).[9]
These 2 strategies represent the latest advances in overcoming endocrine resistance. Preclinical studies showed that mTOR activation played a key role in endocrine resistance and in the close interaction between the mTOR and ER pathways.[33] CDK in the cell-cycle facilitated cancer cell progression from the G0 phase to the G1 phase when bound with D-type cyclins. This governed oncogenic growth and was strongly indicated to be involved in endocrine resistance mechanisms.[34]
Concerns regarding toxicity are always important to consider before starting a treatment. Palbociclib with fulvestrant had a lower rate of discontinuation due to toxicity.[8] Stomatitis was the most noted adverse event that led to dose reductions or withdrawals for patients receiving everolimus plus exemestane. A recent study showed that prophylactic use of dexamethasone oral solution markedly reduced the incidence and severity of stomatitis,[35] which may make this regimen more acceptable.
Our study results provided information on treatment decisions. The best choice should always be guided by a specific biomarker. Exploratory analysis of biomarkers was retrospectively performed.[36,37] In the BOLERO-2 study, the benefit from everolimus was independent of the status of specific genes such as PIK3CA, FGFR1, or CCND1.[36] Similarly, the benefit from palbociclib in the PALOMA-3 study was independent of ESR1 status, the mutation of which was identified as a possible mechanism of AI resistance.[37] Therefore, no biomarkers have yet been identified to optimize treatment.
Overall survival benefit was the most essential factor in treatment selection. In the BOLERO-2 trial, everolimus prolonged overall survival by 4.4 months, though without statistical significance.[16] At the time of this report, overall survival analysis for the PALOMA-3 trial was immature. However, in the phase 2 PALOMA-1 trial, where palbociclib with letrozole was given in a first-line setting, it did not statistically significantly improve overall survival.[38]
More studies are being conducted to combat endocrine resistance. Active PIK3CA mutations were found in approximately 30% of patients resistant to AI.[31] Buparlisib was one of the pan-PI3K inhibitors, and its addition to fulvestrant significantly improved PFS in patients harboring PIK3CA mutations in their plasma DNA.[21] However, exposure to this combination was compromised by its toxicity. Other isoform-specific PI3K inhibitors have shown reduced toxicity and are being investigated in large trials. Recently, the PreECOG 0102 study showed that adding everolimus to fulvestrant improved PFS by 5.3 months [39]. These combinations may provide future treatment options and challenge treatment decisions.
The present study had several limitations. First, patient characteristics varied across the studies included in our analysis. Percentages of patients exposed to prior AI and chemotherapy differed among trials. Some trials involved patients who were HER2-positive, although this number was small. Second, our analysis was not based on individual patient data, which could provide more accurate and convincing results for indirect comparisons. Third, the relatively small number of the included trials precluded further analysis for heterogeneity due to the nature of the indirect comparison.
Based on this network analysis, the combination of palbociclib and fulvestrant seemed to be a better treatment option than everolimus plus exemestane considering their efficacy and toxicity profiles.
Author contributions
Conceptualization: Jia-Zhou Lin.
Data curation: Hong-Wei Huang, Qi-Ni Xu, Hong-Biao Wang.
Formal analysis: Hong-Wei Huang, Qi-Ni Xu, Hong-Biao Wang.
Methodology: Li-Sheng Huang, Jia-Zhou Lin.
Software: Li-Sheng Huang, Qi-Ni Xu.
Writing – original draft: Xu-Yuan Li, Jia-Zhou Lin.
Writing – review & editing: Xu-Yuan Li, Jia-Zhou Lin.
Footnotes
Abbreviations: CBR = clinical benefit rate, CDK4/6 = cyclin-dependent kinase 4/6, CI = confidence interval, ER = estrogen receptor, HR = hazard ratio, mTOR = mammalian target of rapamycin, ORR = objective response rate, PFS = progression-free survival, PI3K = phosphatidylinositol 3-kinase.
The authors have no conflicts of interest to disclose.
References
- [1].Tryfonidis K, Zardavas D, Katzenellenbogen BS, et al. Endocrine treatment in breast cancer: cure, resistance and beyond. Cancer Treat Rev 2016;50:68–81. [DOI] [PubMed] [Google Scholar]
- [2].Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol 2016;34:3069–103. [DOI] [PubMed] [Google Scholar]
- [3].Cardoso F, Bischoff J, Brain E, et al. A review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal women. Cancer Treat Rev 2013;39:457–65. [DOI] [PubMed] [Google Scholar]
- [4].Cope S, Ouwens MJ, Jansen JP, et al. Progression-free survival with fulvestrant 500 mg and alternative endocrine therapies as second-line treatment for advanced breast cancer: a network meta-analysis with parametric survival models. Value Health 2013;16:403–17. [DOI] [PubMed] [Google Scholar]
- [5].Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 2015;12:342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417–30. [DOI] [PubMed] [Google Scholar]
- [7].Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015;373:209–19. [DOI] [PubMed] [Google Scholar]
- [9].Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–84. [DOI] [PubMed] [Google Scholar]
- [10].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [11].Gibas C, Neupane B, Richer D, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One 2014;9:e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 2017;35:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnston SRD, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013;14:989–98. [DOI] [PubMed] [Google Scholar]
- [14].Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive, advanced breast cancer: results from EFECT. J Clin Oncol 2008;26:1664–70. [DOI] [PubMed] [Google Scholar]
- [15].Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor–positive advanced breast cancer. J Clin Oncol 2010;28:4594–600. [DOI] [PubMed] [Google Scholar]
- [16].Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2? Ann Oncol 2014;25:2357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leo AD, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2013;106:djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burstein HJ, Cirrincione CT, Barry WT, et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor–positive advanced breast cancer—CALGB 40302 (Alliance). J Clin Oncol 2014;32:3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baselga J, Im S-A, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tryfonidis K, Basaran G, Bogaerts J, et al. A European organisation for research and treatment of cancer randomized, double-blind, placebo-controlled, multicentre phase II trial of anastrozole in combination with gefitinib or placebo in hormone receptor-positive advanced breast cancer (NCT00066378). Eur J Cancer 2016;53:144–54. [DOI] [PubMed] [Google Scholar]
- [23].Zaman K, Winterhalder R, Mamot C, et al. Fulvestrant with or without selumetinib, a MEK 1/2 inhibitor, in breast cancer progressing after aromatase inhibitor therapy: a multicentre randomised placebo-controlled double-blind phase II trial, SAKK 21/08. Eur J Cancer 2015;51:1212–20. [DOI] [PubMed] [Google Scholar]
- [24].Robertson JFR, Ferrero J-M, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 2013;14:228–35. [DOI] [PubMed] [Google Scholar]
- [25].Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012;30:2718–24. [DOI] [PubMed] [Google Scholar]
- [26].Carlson RW, O’Neill A, Vidaurre T, et al. A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat 2012;133:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu B, Jiang Z, Shao Z, et al. Fulvestrant 250?mg versus anastrozole for Chinese patients with advanced breast cancer: results of a multicentre, double-blind, randomised phase III trial. Cancer Chemother Pharmacol 2010;67:223–30. [DOI] [PubMed] [Google Scholar]
- [28].Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res Treat 2010;123:453–61. [DOI] [PubMed] [Google Scholar]
- [29].Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 2002;20:3386–95. [DOI] [PubMed] [Google Scholar]
- [30].Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Turner NC, Neven P, Loibl S, et al. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 2017;389:2403–14. [DOI] [PubMed] [Google Scholar]
- [32].Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 2017;18:654–62. [DOI] [PubMed] [Google Scholar]
- [33].Ballinger TJ, Meier JB, Jansen VM. Current landscape of targeted therapies for hormone-receptor positive, HER2 negative metastatic breast cancer. Front Oncol 2018;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sherr CJ. A new cell-cycle target in cancer-inhibiting cyclin D-dependent kinases 4 and 6. N Engl J Med 2016;375:1920–3. [DOI] [PubMed] [Google Scholar]
- [35].Hortobagyi GN, Chen D, Piccart M, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 2016;34:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fribbens C, O’Leary B, Kilburn L, et al. PlasmaESR1mutations and the treatment of estrogen receptor–positive advanced breast cancer. J Clin Oncol 2016;34:2961–8. [DOI] [PubMed] [Google Scholar]
- [37].Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- [38].Kornblum NS, Manola J, Klein P, Ramaswamy B, Brufsky A, Stella PJ, et al. PrECOG 0102: a randomized, double-blind, phase II trial of fulvestrant plus everolimus or placebo in post-menopausal women with hormone receptor (HR)-positive, HER2-negative metastatic breast cancer (MBC) resistant to aromatase inhibitor (AI) therapy. San Antonio Breast Cancer Symposium. 2016: abstract S1-02. [Google Scholar]


