Abstract
Spondyloepiphyseal dysplasia congenita (SEDC) is an autosomal dominant disorder, characterized by disproportionate dwarfism with short spine, short neck associated with variable degrees of coxa vara. Cervical cord compression is the most hazardous skeletal deformity in patients with SEDC which requires special attention and management.
Ten patients with the clinical and the radiographic phenotypes of spondyloepiphyseal dysplasia congenita have been recognized and the genotype was compatible with single base substitutions, deletions or duplication of part of the COL2A1 gene (6 patients out of ten have been sequenced). Cervical spine radiographs showed apparent atlantoaxial instability in correlation with odontoid hypoplasia or os-odontoideum.
Instability of 8 mm or more and or the presence of symptoms of myelopathy were the main indications for surgery. Posterior cervical fusion from the occiput or C1–3, decompression of C1–2 and application of autorib transfer followed by halo vest immobilization have been applied accordingly.
Orthopedic management of children with spondyloepiphyseal dysplasia congenita (SEDC) should begin with the cervical spine to avoid serious neurological deficits and or mortality.
Keywords: cranio-vertebral junction, management, spondyloepiphyseal dysplasia congenita
1. Introduction
Spondyloepiphyseal dysplasia congenita (SEDC) is a rare autosomal dominant inherited chondrodysplasia. It was first described by Spranger and Wiedemann in 1966.[1] Children with spondyloepiphyseal dysplasia congenita (SEDC) do present with a wide spectrum of orthopedic abnormalities. Angular deformity of the lower limbs, particularly genu valgum. Lumbar lordosis is an apparent abnormality which in fact mostly related to hip flexion contractures. Coxa vara leads to waddling gait. The latter has been confused by some physicians with myopathy or mucopolysaccharidosis.[2,3]
Prenatal ultrasound showed shortening of long bones (arms, legs) < the 5th percentile, flat facies, and delayed ossification of spine and knee.[4] Onset is at birth, but severe short stature may not be obvious until 2 to 3 years. In infancy the vertebral bodies are ovoid or pear-shaped but later platyspondyly with irregular endplates develops. Bone age is markedly delayed and the epiphyses are flattened and fragmented. The capital femoral epiphysis is severely affected. The femoral heads might be absent. Delay in ossification of the pubic rami is characteristic.[5]
SEDC is a heritable bone dysplasia which mostly results from random mutations sparsely distributed in the 54 exons of the COL2A1 gene.[6] The genetic defect is in the COL2A1 gene located on chromosome 12q13.1-q13.2, and it results in defective procollagen type 2 subunits.[7] To date, more than 500 different mutations have been identified globally and listed in the Human Gene Mutation Database.
Congenital hypothyroidism should be ruled out as occasionally this can mimic spondyloepiphyseal dysplasia.[8,9]
Many patients with SEDC, presented with C1–2 instability which might progress to subluxation/ dislocation and subsequently lead to cervical myelopathy and quadriparesis with potentially lethal outcome. The incidence of myelopathy reaches 35% and above.[10] Spinal cord injury may lead to quadriplegia and sudden death due to respiratory failure. These outcomes have led several authors to recommend fusion of the upper cervical spine once instability is demonstrated.[11,12] The instability can occur due to odontoid hypoplasia, os odontoideum (OsO) and/or ligamentous laxity.[12] It was noted that C1 inner diameter is smaller than in healthy people, which further compromised the space available for the cord (SAC) at this level in cases of instability.[13,14] Traditionally, C0-C2 relationship has been assessed by measuring of anterior atlanto-dental interval (ADI), posterior atlanto-dental interval (PADI), vertical atlanto-axial index (VAAI), basion-axial interval (BAI) and basion-dens interval (BDI).[15–18]
The literature review was carried out in German, English and Russian in PubMed (NCBI), eLibrary, and Google scholar databases. Search queries were: spondylo-epiphyseal dysplasia congenita (SEDC), atlantoaxial dislocation in skeletal dysplasia, atlantoaxial subluxation in skeletal dysplasia, atlantoaxial instability in skeletal dysplasia, os odontoideum, odontoid hypoplasia and odontoid aplasia, cervical spine in skeletal dysplasia, cervical spine in genetic syndromes, cervical fusion in skeletal dysplasia.
This paper attempts to show that clinical picture of SEDC may obscure signs of cervical myelopathy and such patients need to examine cervical spine annually. Another aim of this study was to compose diagnostic and treatment algorithm for SEDC patients with cervical spine pathology.
2. Materials and methods
We evaluated 10 patients with SEDC and abnormalities of cervical spine. They have been seen and treated in: Orthopaedic Hospital of Speising, Vienna, Austria, Pediatric Orthopedic Institute n.a. H. Turner, Saint-Petersburg, Russia. Ilizarov Center, Kurgan, Russia and department of Pediatric orthopedic surgery, children Hospital, Tunis, Tunisia. The study protocol was approved by the Medical Committee (Ethics Committee of the Turner Scientific Research Institute, No. 3/2016, Saint-Petersburg, Russia) of the Paediatric Orthopedic Institute n.a. H. Turner, Department of Foot and Ankle Surgery. Signed consents were obtained from the guardians.
Inclusion criteria:
-
1)
presence of SEDC (genetically confirmed),
-
2)
presence of cervical spine abnormalities,
-
3)
presence of cervical instability, cervical stenosis or/and cervical deformity and 4) surgical treatment.
C0-C2 relationship has been assessed by measuring of anterior atlanto-dental interval (ADI), posterior atlanto-dental interval (PADI), vertical atlanto-axial index (VAAI), basion-axial interval (BAI) and basion-dens interval (BDI). In the subaxial cervical spine, we measured: value of cervical kyphosis/lordosis on sagittal radiograph, cervical scoliosis on anteroposterior radiograph, SAC C3-C7.
For this study, following clinical tests were used: modified Japanese Orthopedic Association (mJOA) scale (Benzel modification), Functional Independent Measure (FIM) scale and Visual Analog Scale for pain (VAS). All patients underwent X-ray of spine and pelvis, cervical spine computerized tomography (CT) and magnetic resonance imaging (MRI). The following parameters were evaluated by radiographs, computerized tomography and MRI: type of anomaly, SAC (C1), sagittal atlantal diameter (SAD) and foramen magnum (SDFM), ADI, the presence of atlanto-occipital dissociation (AOD), according to BAI and BDI measurements, the value of pathologic kyphosis, lordosis or scoliosis. In cases of odontoid abnormalities we measured ADI between the inferior rim of the C1 anterior arch and the line made between of C2 anterior-superior edge and remaining attached part of the odontoid.
3. Results
The clinical phenotypic characterizations: Table 1 and Figure 1 demonstrates typical clinical changes in SEDC patients: wide frontal area, wide set eyes and full cheeks, short neck, barrel-shaped chest, angular deformities of lower limb, lumbar hyper lordosis, protuberant abdomen, coxa vara with subsequent development of waddling gait was additional feature. All presented with short stature (-7SD). The subsequent developmental motor history in all children showed delayed walking (the average age of walking unsupported was 14 months, albeit with difficulty).
Table 1.
Clinical symptoms in 10 patients with spondyloepiphyseal dysplasia congenita.
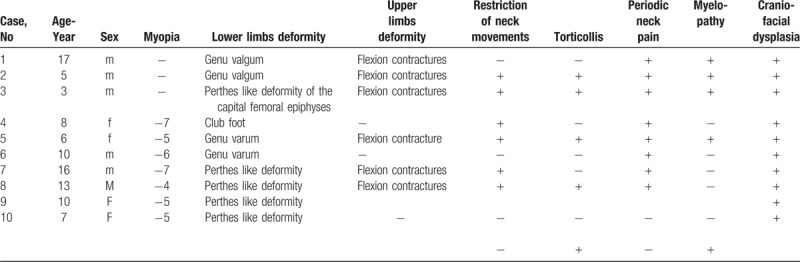
Figure 1.
(A) A-13-months-old-girl-presented with delayed walking, short stature of (-4SD), barrel-shaped chest, protuberant abdomen, and exaggerated lumbar lordosis. A-5-years-old-girl presented with severe short stature (-4SD), exaggerated lumbar lordosis and myopia of -6 diopters (B). A 17-years-old-male patient presented with severe short stature, large head in comparison to small body, flexion contractures of the upper limbs, scoliosis associated with exaggerated lumbar lordosis and genu valgum (C).
Clinical picture of myelopathy has been encountered in 4 patients out of 10. Flexion contractures of the upper limbs have been encountered in 3 patients.
From Table 1 we can see that symptoms of myelopathy prevailed, also 60% of patients had periodic mild neck pain. Only 2 patients had local symptoms: torticollis and restriction of neck movement.
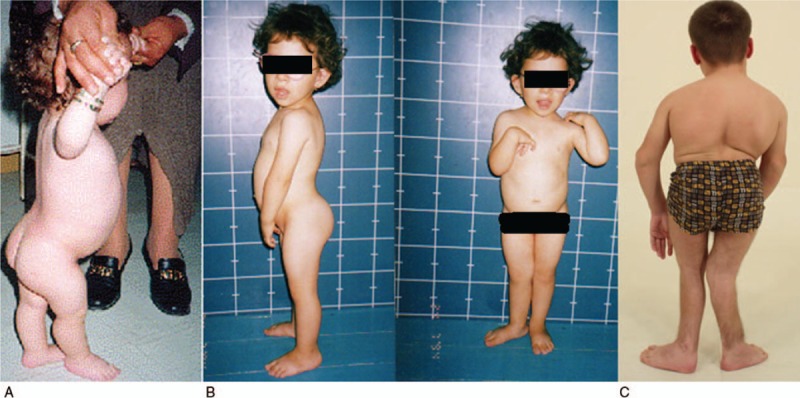
Skeletal survey showed marked delay in bone age, angular deformities of the lower limbs (Fig. 2a). Radiographs of the pelvis in some patients demonstrated such typical changes as flattened and irregular epiphyses, small iliac wings and horizontal acetabulae (Fig. 2b).
Figure 2.
(A) Anteroposterior radiograph of the lower extremities showed a 3-year-old-boy presented with valgus knee and pes valgus. Anteroposterior radiograph of the pelvis in a-5-year-old-girl showed rudimentary and somehow flattened capital femoral epiphysis, horizontal acetabulae, the iliac wings are small and coxa vara associated with metaphyseal irregularities (B).
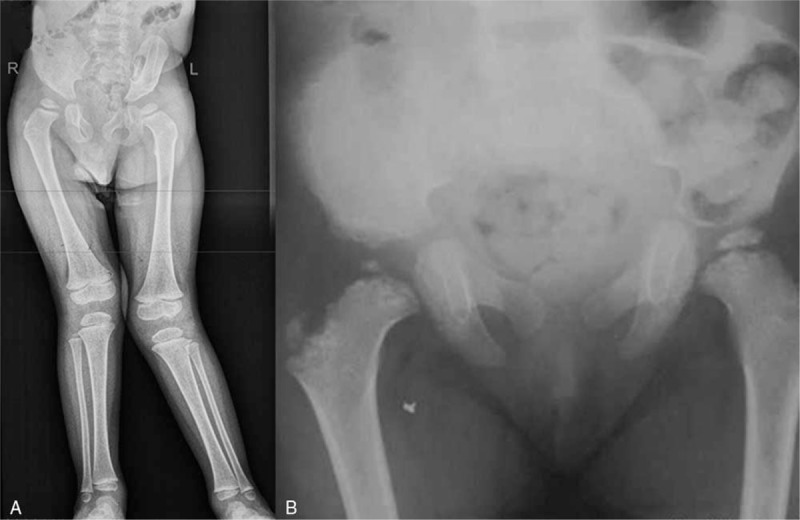
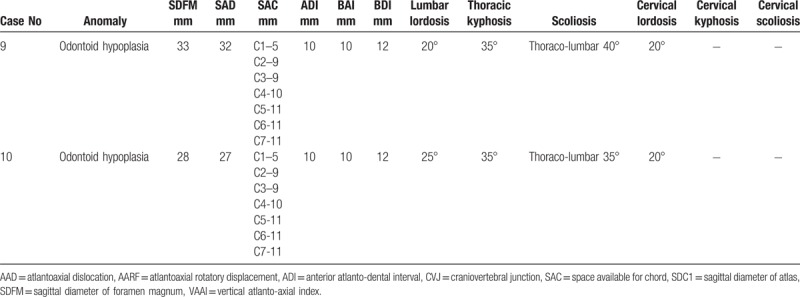
Spine radiographs showed oval shaped vertebrae or flattening of the vertebral bodies with irregular endplates (Fig. 3). All patients had CVJ instability (Table 2 ), C0-C1 stenosis and 80% had AOD. Diameter of anatomical holes was relatively less than in the general population. Odontoid hypoplasia was presented in 2 patients (Fig. 3).
Figure 3.
A. Lateral spine radiograph in a-6-years-old- boy showed platyspondyly with oval shaped vertebral bodies associated with exaggerated lumbar lordosis. Lateral and antero-posterior spine X-rays of 3-years-old boy demonstrated flattening of the vertebral bodies with irregular endplates, with Th12-L2 hypoplasia and kyphosis (B). Lateral and antero-posterior spine X-rays of 3-years-old boy demonstrated ovoid lumbar spine bodies and hypoplasia and flattening of the thoracic vertebral bodies with irregular endplates (C).
Table 2.
Radiographic features in 10 patients with spondyloepiphisal dysplasia congenita.
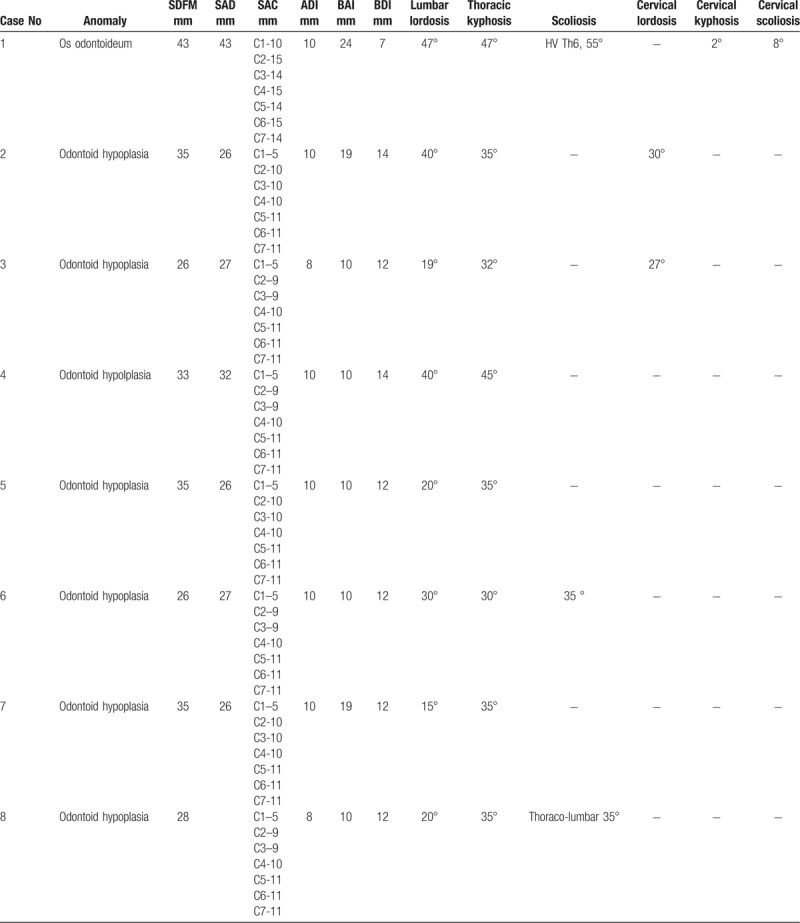
Table 2 (Continued).
Radiographic features in 10 patients with spondyloepiphisal dysplasia congenita.
3.1. Molecular genetics
SEDC is basically an autosomal dominant disorder caused by single allele mutations. In the present study, 6 unrelated patients were screened for putative mutation(s) in the COL2A1 gene (MIM: 120140; NM_001844.4) and 2 heterozygous missense mutations were identified. The c.1916G>A (p.Gly639Asp) mutation in exon 29 was detected in 2 patients and the c.2965C>T (p.Arg989Cys) mutation in exon 43 was identified in 4 patients. The rest of our patients were diagnosed in accordance with clinical and radiographic phenotypic characterizations.
3.2. Surgical interventions
Preoperative indications for spinal fusion included:
-
(1)
clinical signs of cervical myelopathy;
-
(2)
cervical spine instability in flexion-extension surveys and
-
(3)
cervical spine stenosis.
Surgery was performed under neurophysiologic control: transcranial somatosensory-evoked potential and transcranial motor-evoked potentials with total intravenous anesthesia. Instrumentation included posterior wiring techniques with insertion of transarticular screws, titanium cables, and rods with polyaxial screws.
Group of male patients of 17, 5, and 3-years-old respectively, presented with chronic progressive tetraparesis. Radiological assessment showed odontoid hypoplasia, vertebral hypoplasia, pronounced decrease of SAC C1, increase of ADI and AOD. They underwent occipito-spondylodesis with C1 posterior laminectomy. During follow-up period neurological status improved and fusion was achieved (Figs. 4–6).
Figure 4.
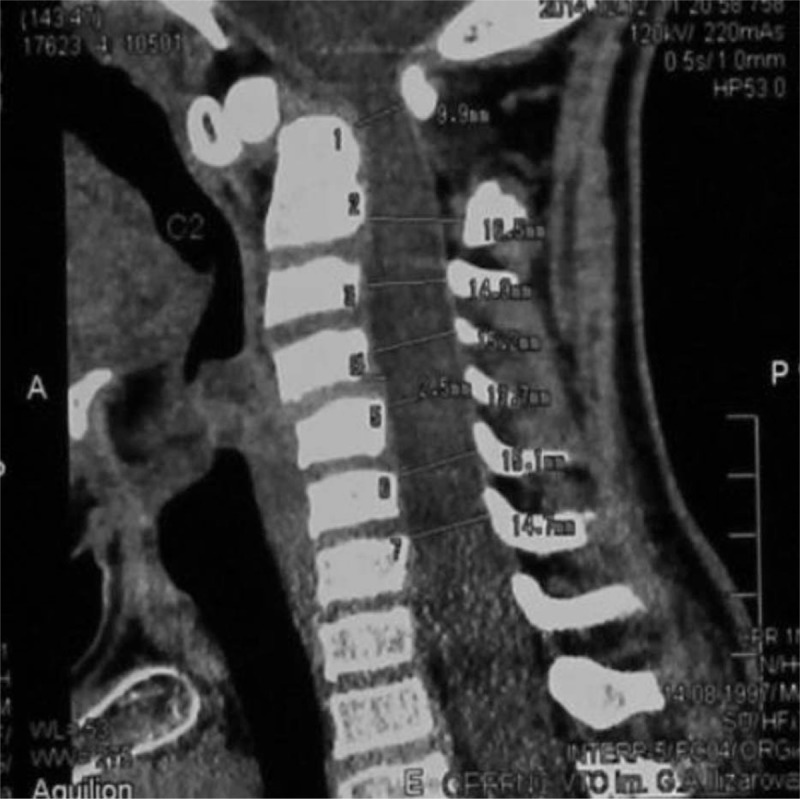
Sagittal 3DCT scna of the cervical spine of a-17-years-old male patient showed C1–2 instability (A), os-odontoidium associated with flattened cervical vertebrae (B).
Figure 6.
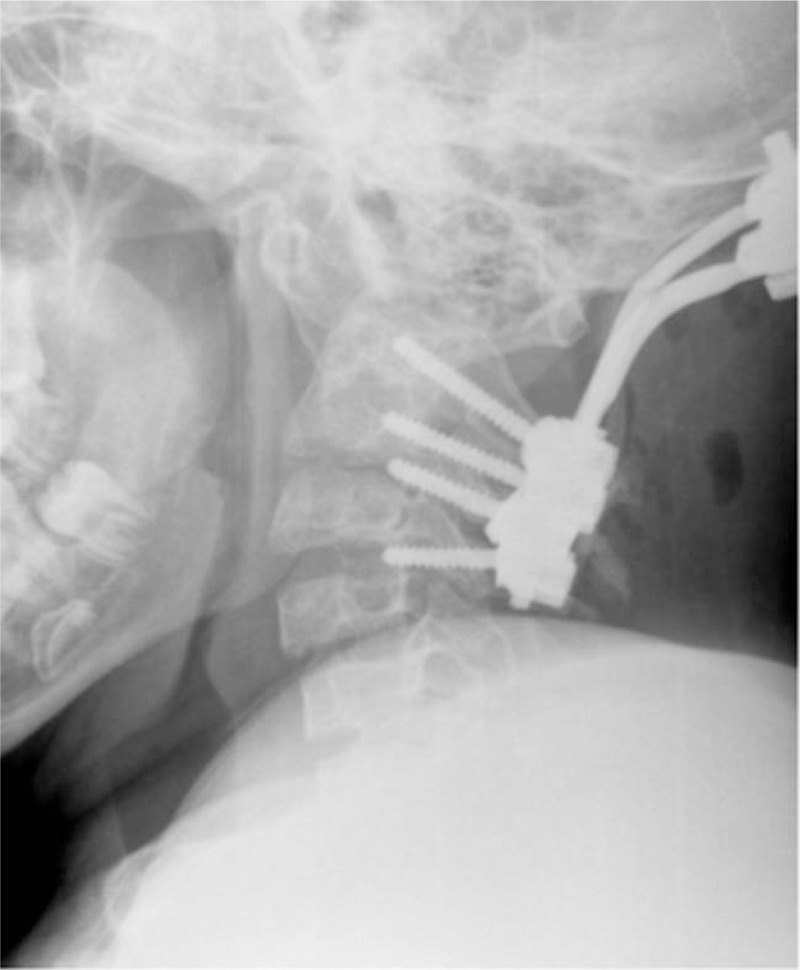
Lateral cervical spine radiograph of a-17-years-old-male patient with SEDC underwent posterior occipito-fusion C0-C3. SEDC = Spondyloepiphyseal dysplasia congenita.
Figure 5.
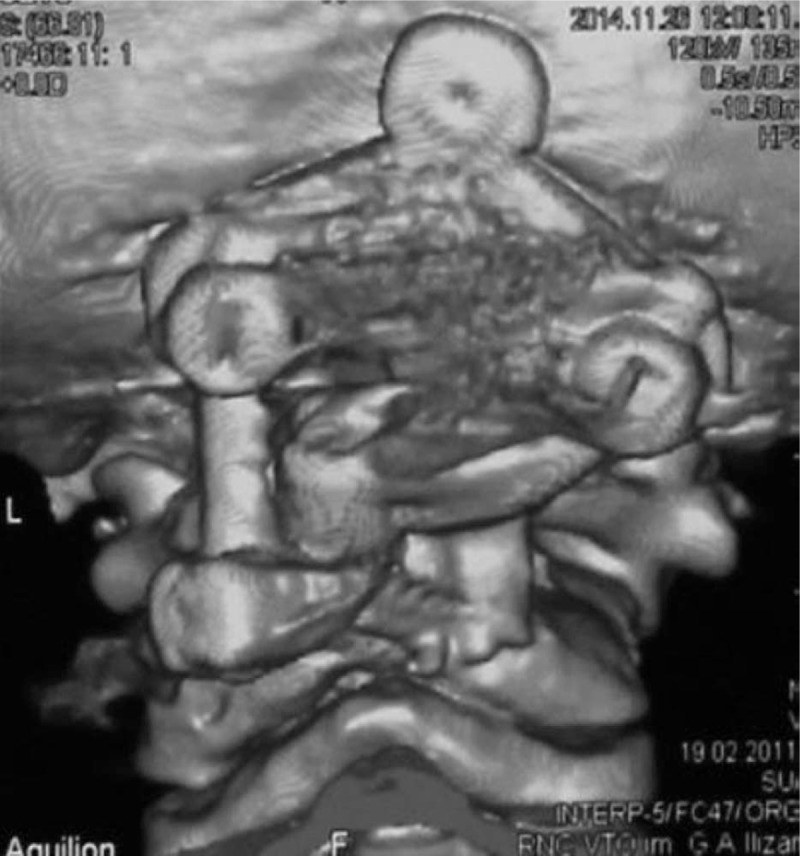
3D reconstruction CT scan of a-3-years-old-boy with SED underwent posterior occipito-fusion with C0-C2 and by decompression of C1/2.
4. Discussion
Spondyloepiphyseal dysplasia congenita (SEDC) is an autosomal dominant disorder linked to mutation in COL2A1. The disease is characterized by small stature of pre and postnatal onset. Disproportionate short stature with short trunk, flat face, hypertelorism, cleft palate, myopia, vitreoretinal degeneration and retinal detachment at time of growth spurt. Short neck, cervical myopathy (C1–2 instability), barrel-shaped thorax and increased thoracic kyphosis and marked lumbar lordosis with short spine are evident features. The limbs are short, the musculo skeletal phenotype is characterized by hypotonia and waddling gait.[4] In neonatal and infancy the radiographic manifestations are ovoid or pear-shaped vertebrae. Generalized shortness of the long bones with normal modeling is evident. The pubic and the ischium are hypoplastic. Ossification defects along the epiphyses at hips, knees and no talus or calcaneal ossification. In childhood and later in life, platyspondyly, odontoid hypoplasia and C1–2 instability are characteristic features. The epiphyses are characterized by delay in maturation and irregularity. Restrictive lung disease, laryngeal hypoplasia are additional pathologies.[10,20,21]
Radiographic findings include the delayed appearance of the epiphysis. Femoral heads are not apparent on radiographs until the patient is approximately 5 years of age.[22,23]
The initial aim of the paper was to compose diagnostic and treatment algorithm for SEDC patients with cervical spine pathology.
This study confirms that incomplete ossification, coexisting osseous anomalies and angular deformity in SEDC patients may have been an important factors in the high frequency of atlantoaxial dislocation and myelopathy occurrence.[10,14,19,24–28] Atlanto-occipital dissociation was often presented in patients with SEDC. So, occipitospondylodesis could be the best treatment strategy for fusion in such cases.
Significant instability has been described in infants with SEDC younger than 1 year of age. For this reason, flexion and extension cervical radiographs should be obtained before the administration of any anesthetic procedures in children with SEDC[20] In children with odontoid hypoplasia, instability may be present with extension because the odointoid is not sufficiently large to prevent posterior migration of C1. When odontoid hypoplasia produces atlantoaxial instability, posterior decompression and fusion is the surgical procedure of choice.[10,21] Orthopedic treatment begins with the cervical spine. Signs and symptoms of cervical instability include hypotonia, sleep apnea, respiratory insufficiency, and myelopathy. Respiratory insufficiency has been seen in infants with SEDC secondary to thoracic dysplasia and cervical cord compression: Thereby, children with pulmonary problems must be carefully assessed for cervical instability.[29,30]
Nakamura et al evaluated 16 patients with SEDC (aged 3–37 years). Six patients out of 16 manifested atalnto-axial instability and 5 of them presented with clinical picture suggestive of myelopathy. They concluded that the risk factors for cord compression included growth deficiency (-7SD) and severe coxa vara.[13]
In a study on congenital osseous anomalies of the cervical spine, 31% of pediatric patients presented with neurological symptoms, and the incidence of myelopathy was estimated to increase on follow-ups. The authors recommend periodic follow-ups for all patients, with dynamic lateral radiographs to identify segmental instability. In our series, we chose to follow-up the patients regularly and opted for surgery when instability was evident or when progressive neurological findings necessitated intervention.[31]
Atlantoaxial dislocation was initially classified by Greenberg into 2 subcategories—reducible and irreducible. Greenberg further devised a treatment strategy based on this system. For irreducible atlantoaxial dislocation, Greenberg specifically stated that the treatment must be aimed at immediate decompression and achieving stabilization
Greenberg work has been considered a landmark publication and is considered by many to be the gold standard for atlantoaxial dislocation treatment in the subsequent literature.[32] Fielding and Hawkins subsequently developed a new classification system according to the direction of dislocation—anterior, posterior, lateral, and rotational.[33] Wang has recently proposed a novel classification system that aims to standardize atlantoaxial dislocation classification and treatment strategy. Referred to as the “Wang classification system” in this article, it is derived from Greenberg classification and is primarily based on classifying dislocations as reducible or irreducible atlantoaxial dislocation.[34] The certain diagnosis of skeletal dysplasias is a challenge. Ultrasound has been considered as a fundamental screening exploration for fetal assessment in non-lethal osteochondrodysplasias. The full prenatal assessment of a fetus with a generic diagnosis of skeletal dysplasia is very difficult. The heterogeneity of ultrasound signs, the syndromic polymorphism, the highly diversified phenotypic expression, the very variable perinatal evolution and prognosis, make this group of conditions very difficult to assess prenatally in an accurate way. Fetal MRI has become a useful examination in maternal-fetal medicine, with very extensive indications and encouraging results in skeletal dysplasias. Fetal MRI is always complementary to the US. The details brought by the fetal MRI are useful, and the exploration is harmless for the fetus and the mother. The fetal imagining through conventional ultrasound or advanced techniques, alternatively completed with a fetal MRI must underlie the prenatal diagnosis of fetal osteochondrodysplasias. A certain diagnosis cannot be accurate and complete without the contribution of genetics, maternal and fetal medicine, obstetrics or radiology.[4]
5. Conclusions
This study shows that SEDC patients with CVJ anomalies often had anterior and central dislocation and myelopathy, atlanto-occipital dissociation due to incomplete ossification, coexisting osseous anomalies and angular deformity. These patients require early aggressive surgical treatment. Finally, a number of important limitations need to be considered.
Acknowledgments
We wish to thank Mr. Hamza Al Kaissi, student at the Bratislava Medical University for his help in collecting / translating German literature.
Author contributions
Conceptualization: Ali Al Kaissi, Sergey Ryabykh, Olga M Pavlova, Farid Ben Chehida, Franz Grill, Susanne Gerit Kircher.
Data curation: Ali Al Kaissi, Sergey Ryabykh, Polina Ochirova, Vladimir Kenis, Rudolf Ganger.
Formal analysis: Sergey Ryabykh, Vladimir Kenis, Franz Grill.
Investigation: Farid Ben Chehida, Susanne Gerit Kircher.
Methodology: Ali Al Kaissi, Sergey Ryabykh.
Resources: Franz Grill.
Supervision: Franz Grill, Susanne Gerit Kircher.
Validation: Susanne Gerit Kircher.
Writing – original draft: Ali Al Kaissi.
Writing – review & editing: Ali Al Kaissi.
Footnotes
Abbreviations: ADI = anterior atlanto-dental interval, AOD = atlanto-occipital dissociation, BAI = basion-axial interval, BDI = basion-dens interval, C0-C2 = Occiput/Atlanto-Axial, COL2A1 = Collagen 2 Alpha 1, CVJ = Cranio vertebral junction, FIM = Functional Independent Measure, mJOA = modified Japanese Orthopedic Association, PAD1 = posterior atlanto-dental interval, SAC = space available for cord, SAD = sagittal atlantal diameter, SDFM = Space diameter for foramen magnum, SEDC = Spondyloepiphyseal dysplasia congenita, VAAI = vertical atlanto-axial index.
Given that all investigations and interventions in this report were performed as part of the regular health care whose informed consent was obtained on admission, an ethical approval was obtained.
The authors have no conflicts of interest to disclose.
References
- [1].Spranger J, Wiedemann H-R. Dysplasia spondyloepiphysaria congenita. Lancet 1966;288:642. [Google Scholar]
- [2].Deng H, Huang X, Yuan L. Molecular genetics of the COL2A1-related disorders. Mutat Res Rev Mutat Res 2016;768:1–3. [DOI] [PubMed] [Google Scholar]
- [3].Al Kaissi A, Ryabykh S, Ochirova P, et al. Muscle weakness: a misleading presentation in children with distinctive syndromic entities (Clinical case reports). J Investig Med High Impact Case Rep 2017;January–March:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berceanu C, Gheonea AI, Vlădăreanu S, et al. Ultrasound and MRI comprehensive approach in prenatal diagnosis of fetal osteochondrodysplasias. Cases series. Med Ultrason 2017;19:66–72. [DOI] [PubMed] [Google Scholar]
- [5].Jung SC, Mathew S, Li QW, et al. Spondyloepiphyseal dysplasia congenita with absent femoral head. J Pediatr Orthop B 2004;13:63–9. [DOI] [PubMed] [Google Scholar]
- [6].Xiong Q, Liu Y, Xue Y, et al. A novel de novo mutation in COL2A1 leading to spondyloepiphyseal dysplasia congenita in a Chinese family. Hum Genome Var 2018;5:17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tiller GE, Weis AM, Polumbo AP, et al. An RNA-splicing mutation (G+5IVS20) in the type II collagen gene (COL2A1) in a family with spondyloepiphyseal dysplasia congenita. Am J Hum Genet 1995;56:388–95. [PMC free article] [PubMed] [Google Scholar]
- [8].Wiedemann HR. Oligosymptomatic hypothyroidism presenting as apparent spondyloepiphyseal dysplasia (Letter). Am J Med Genet 1994;50:385. [DOI] [PubMed] [Google Scholar]
- [9].Anderson IJ, Goldberg RB, Marion RW, et al. Spondyloepiphyseal dysplasia congenita: genetic linkage to type II collagen (COL2AI). Am J Hum Genet 1990;46:896–901. [PMC free article] [PubMed] [Google Scholar]
- [10].Miyoshi K, Nakamura K, Haga N, et al. Surgical treatment for atlantoaxial subluxation with myelopathy in spondyloepiphyseal dysplasia congenita. Spine (Phila Pa 1976) 2004;29:E488–91. [DOI] [PubMed] [Google Scholar]
- [11].Serhan Er M, Abousamra O, Rogers K, et al. Upper cervical fusion in children with spondyloepiphyseal dysplasia congenita. J Pediatr Orthop 2017;37:466–72. [DOI] [PubMed] [Google Scholar]
- [12].Svensson O, Aaro S. Cervical instability in skeletal dysplasia report of 6 surgically fused cases. Acta Orthop Scand 1988;59:66–70. [DOI] [PubMed] [Google Scholar]
- [13].Nakamura K, Miyoshi K, Haga N, et al. Risk factors of myelopathy at the atlantoaxial level in spondyloepiphyseal dysplasia congenita. Arch Orthop Trauma Surg 1998;117:468–70. [DOI] [PubMed] [Google Scholar]
- [14].McKay SD, Al-Omari A, Tomlinson LA, et al. Review of cervical spine anomalies in genetic syndromes. Spine (Phila Pa 1976) 2012;37:E269–77. [DOI] [PubMed] [Google Scholar]
- [15].Liu K, Xie F, Wang D, et al. Reference ranges for atlantodental interval in adults and its variation with age and gender in a large series of subjects on multidetector computed tomography. Acta Radiol 2015;56:465–70. [DOI] [PubMed] [Google Scholar]
- [16].Locke GR, Gardner JI, Epps Van EF. Atlas-dens interval (ADI) in children: a survey based on 200 normal cervical spines. Am J Roentgenol Radium Ther Nucl Med 1966;97:135–40. [DOI] [PubMed] [Google Scholar]
- [17].Kulkarni AG, Goel AH. Vertical atlantoaxial index: a new craniovertebral radiographic index. J Spinal Disord Tech 2008;21:4–10. [DOI] [PubMed] [Google Scholar]
- [18].Madadin M, Samaranayake PR, O’Donnell C, et al. Post-mortem CT evaluation of atlanto-occipital dissociation. J Forensic Leg Med 2017;46:16–9. [DOI] [PubMed] [Google Scholar]
- [19].Benzel EC, Lancon J, Kesterson L, et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord 1991;4:286–95. [DOI] [PubMed] [Google Scholar]
- [20].Roberts W, Henson LC. Anesthesia for scoliosis: dwarfism and congenitally absent odontoid process. AANA J 1995;63:332–7. [PubMed] [Google Scholar]
- [21].LeDoux MS, Naftalis RC, Aronin PA. Stabilization of the cervical spine in spondyloepiphyseal dysplasia congenita. Neurosurgery 1991;28:580–3. [DOI] [PubMed] [Google Scholar]
- [22].Ikegawa S, Iwaya T, Taniguchi K, et al. Retinal detachment in spondyloepiphyseal dysplasia congenita. J Pediatr Orthop 1993;13:791–2. [DOI] [PubMed] [Google Scholar]
- [23].Crossan J, Wynne-Davies R, Fulford G. lateral failure of the capital femoral epiphysis: bilateral Perthes disease, multiple epiphyseal dysplasia, pseudoachondroplasia, and spondyloepiphyseal dysplasia congenita and tarda. J Pediatr Orthop 1983;3:297–301. [DOI] [PubMed] [Google Scholar]
- [24].Benglis DM, Sandberg DI. Acute neurological deficit after minor trauma in an infant with achondroplasia and cervicomedullary compression. Case report and review of the literature. J Neurosurg 2007;107(2 Suppl):152–5. [DOI] [PubMed] [Google Scholar]
- [25].Benson DR, Newman DC. The spine and surgical treatment in osteogenesis imperfecta. Clin Orthop Relat Res 1981;159:147–53. [PubMed] [Google Scholar]
- [26].Klimo P, Jr, Kan P, Rao G, et al. Os odontoideum: presentation, diagnosis, and treatment in a series of 78 patients. J Neurosurg Spine 2008;9:332–42. [DOI] [PubMed] [Google Scholar]
- [27].Mohindra S, Tripathi M, Arora S. Atlanto-axial instability in achondroplastic dwarfs: a report of two cases and literature review. Pediatr Neurosurg 2011;47:284–7. [DOI] [PubMed] [Google Scholar]
- [28].Zhao D, Wang S, Passias PG, et al. Craniocervical instability in the setting of os odontoideum: assessment of cause, presentation, and surgical outcomes in a series of 279 cases. Neurosurgery 2015;76:514–21. [DOI] [PubMed] [Google Scholar]
- [29].Harding CO, Green CG, Perloff WH, et al. Respiratory complications in children with spondyloepiphyseal dysplasia congenita. Pediatr Pulmonol 1990;9:49–54. [DOI] [PubMed] [Google Scholar]
- [30].Naumoff P. Thoracic dysplasia in spondyloepiphyseal dysplasia congenita. Am J Dis Child 1977;131:653–4. [DOI] [PubMed] [Google Scholar]
- [31].Hosalkar HS, Sankar WN, Wills BP, et al. Congenital osseous anomalies of the upper cervical spine. JBJS Am 2008;90:337–48. [DOI] [PubMed] [Google Scholar]
- [32].Greenberg AD. Atlanto-axial dislocations. Brain 1968;91:655–84. [DOI] [PubMed] [Google Scholar]
- [33].Fielding JW, Hawkins R. Atlanto-axial rotatory fixation. (Fixed rotatory subluxation of the atlanto-axial joint). J Bone Joint Surg Am (Am Vol) 1977;59:37–44. [PubMed] [Google Scholar]
- [34].Wang S, Wang C, Yan M, et al. Novel surgical classification and treatment strategy for atlantoaxial dislocations. Spine 2013;38:E1348–56. [DOI] [PubMed] [Google Scholar]


