Abstract
Mental and physical stress is thought to play an important causative factor in apical ballooning syndrome (ABS) likely secondary to the vasomotor dysfunction. However, there are currently few data related to the impact of physical stress in this unique cardiomyopathy.
A total 18 patients, including 8 females with history of ABS and 10 post-menopausal controls, underwent physical and mental tests. Assessments included the pain-induced peripheral artery tonometry scores (PIPATs) and mental stress peripheral artery tonometry scores (PATs).
Compared with control group, PIPATs were significantly attenuated in patients with ABS in both baseline and post-mental stress (0.94 ± 0.08 vs1.30 ± 0.54, P <.05 and 0.87 ± 0.19 vs1.24 ± 0.21 P = .01, respectively); mental stress PATs were significantly lower in patients with ABS, both in Stroop test (0.79 ± 0.30 vs 1.24 ± 0.43, P = .01) and arithmetic test (0.91 ± 0.27 vs 1.36 ± 0.57, P = .01). PIPATs correlated significantly with mental stress PATs, both in arithmetic and Stroop test (P <.05).
The PIPATs were attenuated in female with history of ABS and the vascular response to pain may provide a different pathogenesis mechanism on detecting patients with ABS.
Keywords: apical ballooning syndrome, physical stress, vascular response
1. Introduction
Stress—physical, mental, and emotional—is increasingly recognized as an important issue and a potential modifiable risk factor toward the development of cardiovascular diseases.[1] The physiological response to the both physical and mental stress may be reflective of a sympathetic hyper-reactivity,[2] however, mental stress is more commonly associated with silent ischemic episodes and transient ischemic dilation of left ventricle.[3] Nevertheless, the independent or concomitant mechanisms by which physical stress may affect vascular function are relatively understudied.
Apical ballooning syndrome (ABS) had been recognized as an acute cardiomyopathy defined as a transient reduction in left ventricular function in the absence of obstructive coronary artery disease, occurring almost exclusively in post-menopausal women.[4–6] A known trigger is intense emotional or physical stress manifesting as reversible left ventricular dysfunction. Previously we demonstrated a significant reduction in endothelial function at rest as well as in response to mental stress in post-menopausal women diagnosed with ABS compared to women without ABS or women with recent myocardial infarction (MI). However, the vascular response to physical stress in post-menopausal women with ABS is not well defined. In the present study, we first proposed the pain-induced peripheral artery tonometry scores (PIPATs) and hypothesized the novel micro vascular function measurement had association with endothelial response to mental stress in patients with ABS.
2. Methods
2.1. Participants
In accordance with Mayo Clinic Institutional Review Board (IRB) approved protocol, we enrolled 18 female patients who gave written informed consent and including diagnosed of ABS[4,7] (n = 8) and post-menopausal female controls (n = 10). The diagnosis of ABS required fulfillment of the following Mayo diagnostic criteria[8]:
-
1)
transient akinesis, hypo-kinesis, or dys-kinesis of the left ventricular mid segments with or without apical involvement, and regional wall motion abnormalities extending beyond a single epicardial vascular distribution;
-
2)
absence of obstructive coronary disease or angiographic evidence of acute plaque rupture;
-
3)
new electrocardiography abnormalities at the time of the episode (either ST segment elevation and/or T-wave inversion) or elevated cardiac troponin; and
-
4)
absence of pheochromocytoma or myocarditis.
A control group of post-menopausal women with no history of ABS or MI was recruited by advertisements (n = 10). The exclusion criteria for all participants included a history of liver or kidney disease, hormone replacement therapy or significant cardiovascular disease. Written informed consent was obtained from each participant.
2.2. Experimental protocol
Study design and patient progression through the protocol are presented in Figure 1. All studies were performed in a quiet, temperature-controlled room. Participants fasted for 4 hours before the study and abstained from coffee or tobacco use on the day of the examination. None of the participants used vasoactive medications within 24 hours of the study. The study was conducted in the sitting position in a comfortable chair with armrests. A fitted blood pressure cuff on the test arm was inflated to 20 mm Hg above systolic pressure for 5 minutes. The finger cuffs of the Endo-peripheral artery tonometry scores (PAT) 2000 device (Itamar Medical Inc. Ltd., Caesarea, Israel) was placed on the middle finger of each hand.[9] This noninvasive device allows continuous recording of the signal and interpretation of the data is operator-independent as previously described.[10] The finger probes consist of inflatable latex air cuffs connected by pneumatic tubes to an inflating device controlled through a computer algorithm. A pulsatile volume change of the distal digit induces pressure alterations in the finger cuff, which are sensed by pressure transducers and transmitted to and recorded by the Endo-PAT 2000 device. A decrease in the arterial blood volume in the distal finger-tip causes a decrease in pulsatile arterial column changes reflected as a decrease in the measured PAT signal, and vice versa. Participants relaxed for 10 minutes before initiation of the protocol. Baseline blood pressure and heart rate (HR) were obtained via a digital automated blood pressure cuff (Omron Healthcare, Inc., Vernon Hills, Illinois). Double product was calculated as systolic blood pressure (SBP) multiplied by HR.
Figure 1.
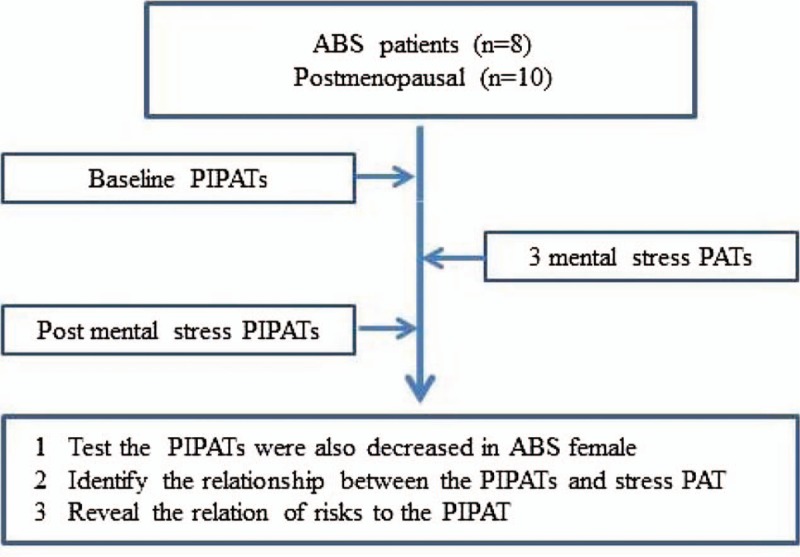
Flow chart.
2.3. Mental stress protocol
The mental stress testing protocol was administered under the supervision of a licensed psychometrist as we previously described.[10] Following the first reactive hyperemia trial and a subsequent rest period the 3, 6-minute mental stress tasks were performed in random order. All tasks were externally paced to accentuate the induced mental stress. Blood pressure and HR measurements were taken 30 seconds before, 2 minutes into, and at the end of each 6-minute mental stress task. Participants relaxed for 5 minutes between each mental stress task. At the end of the third and final mental stress task, the reactive hyperemia protocol was repeated. The tasks consisted of a number-letter recall challenge of increasing length and complexity (spiral omnibus), number subtraction of increasing difficulty (subtracting 1 digit from 2-digit numbers up to subtracting 3 digits from 3-digit numbers), and computerized version of the Stroop word-color conflict.
2.4. PIPATs measurement protocol
Both baseline and post mental stress PIPATs were obtained using the Endo-PAT 2000 device, as described in Figure 2. PIPATs data were analyzed by a computer in an operator-independent manner. PIPATs were calculated as the ratio of the average amplitude of the PAT signal in the control arm when the test arm was occluded divided by the average amplitude of the PAT signal of a 3.5-min time period before cuff inflation.
Figure 2.
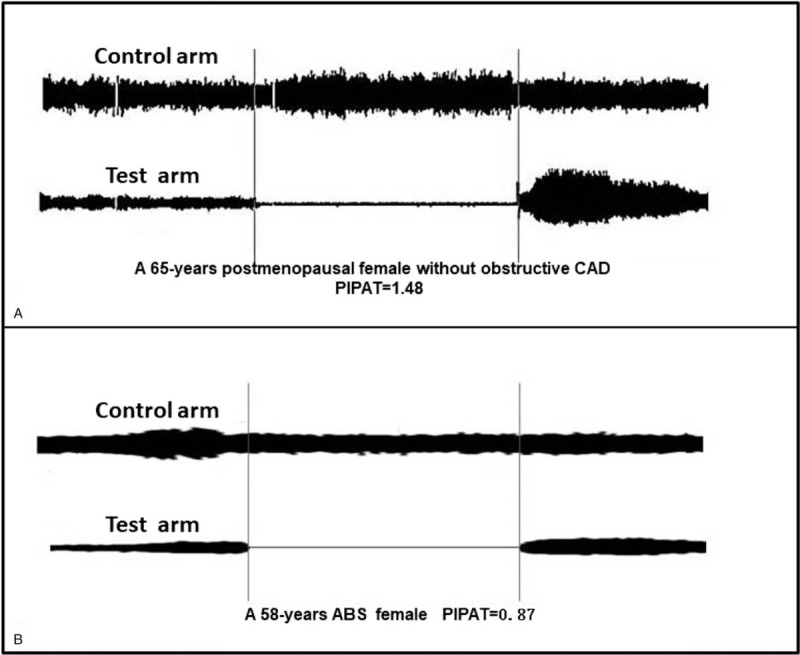
Representative PIPATs tracing in ABS and post-menopausal female. ABS = apical ballooning syndrome, PIPATs = pain-induced peripheral artery tonometry scores.
2.5. Statistical analysis
Continuous variables were expressed as means ± standard deviation. Categorical variables were expressed as percentages and compared by use of the Fisher exact test or χ2 tests as appropriate. Continuous variables were compared by use of unpaired t test or a Wilcoxon rank sum test. Pearson's correlation coefficient was calculated to assess the linear correlation between the vascular responses investigated. Multivariable regression models were constructed to assess how much variation is explained by the covariates in both PIPAT and stress PAT. Stepwise regression was used to identify independent predictors of PIPAT. All statistical tests were 2 sided. A value of P <.05 was set a priori and considered statistically significant. All statistical analyses were performed with the Statistical Package for Social Sciences version 11.5 for Windows (SPSS, Chicago, IL)
3. Results
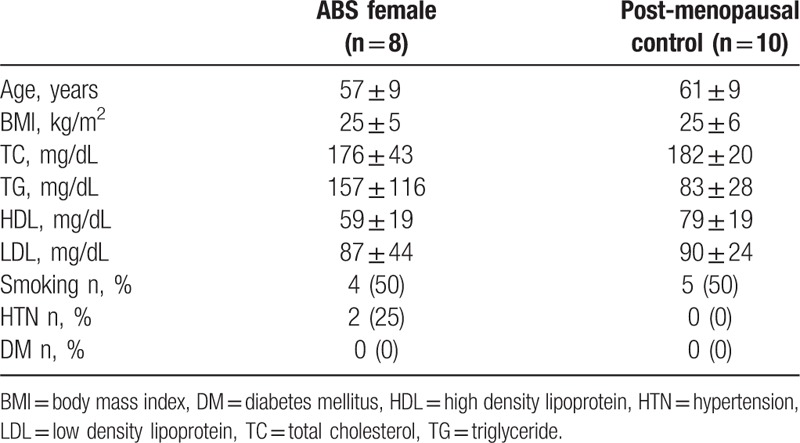
There were no significant differences in baseline characteristics (age, weight, body mass index [BMI] lipid concentration) between patients with ABS (n = 8) and postmenopausal controls (n = 10) (Table 1). Compared with postmenopausal controls, we found no significant difference in the number of nonsmokers, medication adherence, history of diabetes, history of hypertension, or family history of cardiovascular disease in ABS group.
Table 1.
The baseline characteristic of enrolled patients.
As shown in Table 2, there was no difference in the baseline physiologic variables including SBP, HR, or double product between the ABS and postmenopausal groups. The baseline SBPs were 130 ± 18mmHg and 120 ± 13mmHg for patients with ABS and postmenopausal control (P = .19); the baseline HR was 59 ± 9 beats/min and 67 ± 9 beat/min for patients with ABS and postmenopausal controls, respectively (P = .059); the baseline double products were 7866 ± 2120 and 8204 ± 1620 for patients with ABS and postmenopausal controls, respectively (P = .71).
Table 2.
Hemodynamic value of baseline and mental stress tests.
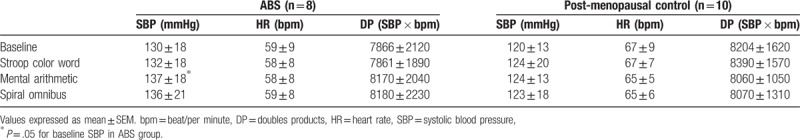
During the Stroop color word test, SBPs increased from baseline to 132 ± 18mmHg and 124 ± 20mmHg for patients with ABS and postmenopausal controls (P = .50 and P = .55, respectively); During the mental arithmetic test, SBPs increased to 137 ± 18 mmHg and 124 ± 13 mmHg for patients with ABS and postmenopausal controls (P = .05 and P = .20, respectively); During the spiral omnibus test, SBPs increased to 136 ± 21 mmHg and 123 ± 18 mmHg for patients with ABS and postmenopausal control (P = .10 and P = .44, respectively).
During the Stroop color-word test, HR was 58 ± 8 beats/min and 67 ± 7 beats/min for patients with ABS and postmenopausal control; during the mental arithmetic test, HRs were 58 ± 8 beats/min and 65 ± 5 beats/min for patients with ABS and postmenopausal controls. During the spiral omnibus test, HR was 59 ± 8 beats/min and 65 ± 6 beats/min for patients with ABS and postmenopausal controls. There was no significantly HR increase above baseline values in mental stress tests, both in patients with ABS and menopausal controls.
During the Stroop color-word test, double products were 7861 ± 1890 and 8390 ± 1570 for patients with ABS and postmenopausal controls; during the mental arithmetic test, double products were 8170 ± 2040 and 8060 ± 1050 for patients with ABS and postmenopausal controls, respectively. During the spiral omnibus test, double products were 8180 ± 2230 and 8070 ± 1310 for patients with ABS and postmenopausal controls, respectively. Compared with baseline values, there were no significantly increases in double products during mental stress tests, both in patients with ABS and menopausal controls (all P = NS).
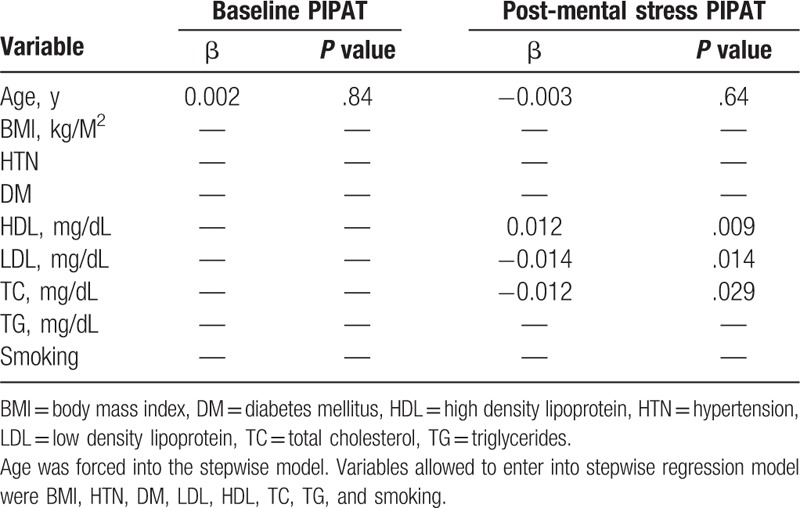
Both baseline and post mental stress PIPATs were significantly lower in patients with ABS, compared with post-menopausal controls (Fig. 3), there was no PIPATs values difference between smokers and non-smokers (not shown). As we previously established that stress PATs (Stroop color-words and mental arithmetic) in patients with ABS were significantly attenuated compared with post-menopausal controls.[10] Baseline PIPATs were significantly correlated with arithmetic PAT and Stroop PAT (r = 0.47, P = .04 and r = 0.45, P = .05, respectively). Post mental stress PIPATs were significantly correlated with arithmetic PAT and Stroop PAT (r = 0.52, P = .03 and r = 0.62, P = .007, respectively). However, the spiral omnibus PATs were not significantly correlated with baseline and post mental stress PIPATs (r = 0.32, P = .20 and r = 0.22, P = .38, respectively) (Fig. 4). In age-adjusted stepwise regression model, lower density lipoprotein (β = −0.014, P = .014), high density lipoprotein (β = 0.012, P = .009) and total cholesterol (TC) (β = −0.012, P = .029) were significantly correlated with post-mental stress PIPAT but not baseline PIPAT (Table 3).
Figure 3.
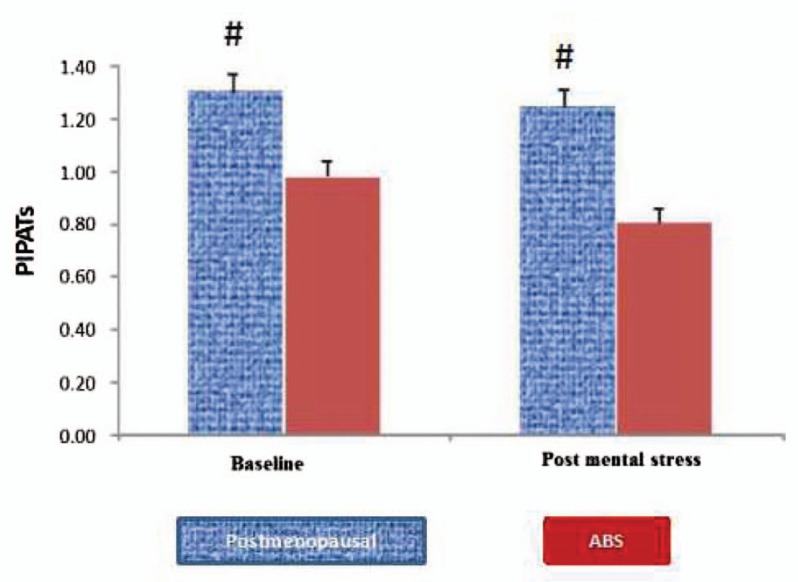
The pain-induced vascular responses to cuff inflation in the control arm were compared between the ABS and post-menopausal female. ABS = apical ballooning syndrome.
Figure 4.
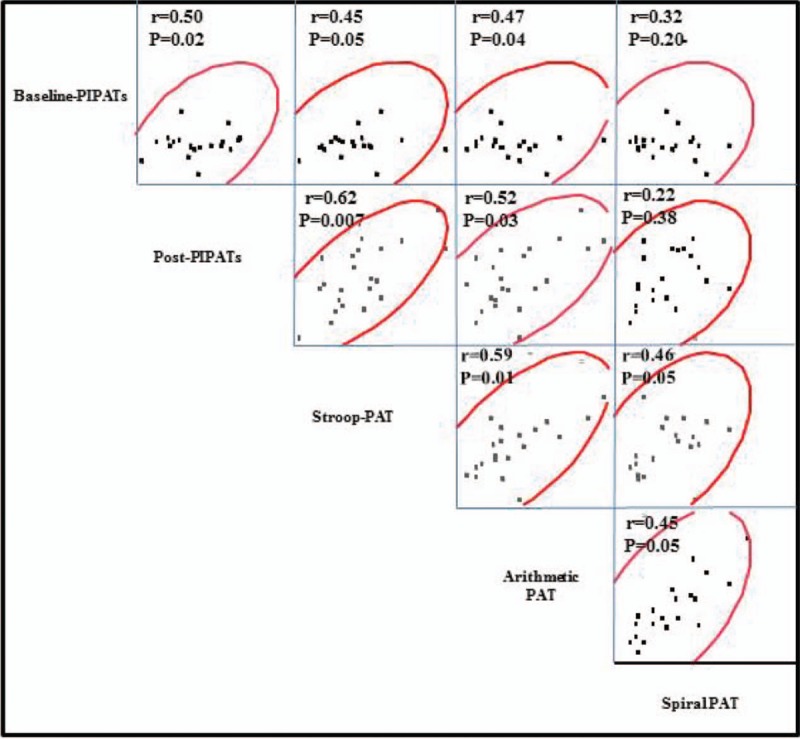
The correlates between the PIPATs and mental stress PATs in the enrolled patients. PATs = artery tonometry scores, PIPATs = pain-induced peripheral artery tonometry scores.
Table 3.
The stepwise linear regression models for the relationship of clinical factors and PAT.
4. Discussion
In this study, we demonstrated for the first time that there is reduced vascular response to blood pressure cuff inflation induced physical pain in patients with ABS. We also provided a direct link between the vascular response to pain and the responses to mental stress. The observation that these vascular responses to pain and mental stress appear to be similar would suggest a common underlying mechanism-potentially autonomic dysfunction previously described.[11] Using the unique PIPATs to understand the ABS mechanisms and detect the potential populations at risk may help avoid catastrophic episodes and provide treatment to those affected.
Numerous studies had established that acute stress has been linked to increased morbidity and mortality in coronary heart disease and progression of arteriosclerosis.[12–14] The phenomena are believed to be secondary to the endothelial dysfunction and micro vascular disease,[15,16] especially in women.[17] In this cohort of ABS patients, the majority provided a history of strong emotional trigger. Martin, et al[10] first observed increased vascular reactivity response to acute mental stress in patients with a prior episode of ABS. Until recently, Park, et al[18] established that sepsis, identified as physical stress and profound inflammatory state, was another sentinel variable associated with the development of ABS. However, until these data, there was no direct evidence to elucidate the cardiovascular responses to the physical stress in ABS.
The physiological response to physical stress is similar to mental stress and involves adaptive change such as mobilization of energy sources and redistribution of blood flow.[1] These stressors may adversely impact vascular response via the actions of corticotrophin-releasing hormone, cortisol, enhanced sympathetic nerve activation[19–23] and pro-inflammatory cytokines.[22,23] In healthy arteries, the activation of the sympathetic system usually leads to release of nitric oxide (NO) and endothelium-derived hyperpolarizing factors via stimulation of endothelial α2-adrenergic receptors and consequently vasodilation,[24] whereas in dysfunctional endothelium α1-adrenergic-mediated constriction of smooth muscle cells will dominate.[25] Furthermore, there are data demonstrating acute stress could increase cortisol and Endothelin-1, which could induce the impairment of endothelial function and lead to the excessive vasoconstriction. The above-mentioned studies indicated that it is likely not sole mechanism was involved in the current response. Future studies will be needed to identify more reasonable mechanistic insight into the observed responses.
In the present study, the PIPATs appeared to provide additional information, complementary to the “traditional” mental stress-induced vascular response, suggesting the notion of possible different mechanisms of ABS. The novel stress model might be useful in future studies of potential predictive methodologies or even the treatment of ABS. Understanding the complexities of PIPATs aids in providing the assessment of response to psychological treatment aimed at improving the responses to stress. Furthermore, it appeared that the metabolic alterations associated with dyslipidemia are key factors in predicting the unique PIPATs in the enrolled women.
A study such as this certainly has limitations related to small sample size, referral bias for a large tertiary care center, and the retrospective nature of the design. In terms of technical difficulties, we estimated the PIPATs using the ratio of control arm PAT signal during cuff inflation compared with baseline amplitude. However, it might provide more information regarding to the PIPATs if we calculated the PIPATs at each 1minute interval during the cuff occlusion. Finally, the small nature of the study did not allow for mechanistic explanation or long-term follow up—both of which are studies ripe for further thought and discussion.
5. Conclusions
In summary, our study demonstrated that PIPATs are attenuated in ABS subjects and significantly correlated with mental stress-induced vascular dysfunction. PIPATs could potentially serve as a novel, rapid vascular response in patients at risk for and who have suffered from ABS.
Acknowledgments
The authors wish to thank Monica L. Olson for their assistance with these studies.
Author contributions
Conceptualization: Lilach O Lerman, Amir Lerman.
Methodology: Robert Jay Widmer.
Software: Ryan J Lennon.
Supervision: Lilach O Lerman, Amir Lerman.
Writing – original draft: Tao Sun, Kyoung H Park.
Writing – review & editing: Yasushi Matsuzawa, Kyoung H Park.
Footnotes
Abbreviations: ABS = apical ballooning syndrome, HR = heart rate, MI = myocardial infarction, PATs = artery tonometry scores, PIPATs = pain-induced peripheral artery tonometry scores, SBP = systolic blood pressure.
National Institute of Health (NIH Grant HL-92954 and AG-31750 to A.L).
The authors have no conflicts of interest to disclose.
References
- [1].Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol 2013;88:124–35. [DOI] [PubMed] [Google Scholar]
- [2].Seliger SL, Katzel LI, Fink JC, et al. Renal function and cardiovascular response to mental stress. Am J Nephrol 2008;28:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kop WJ, Verdino RJ, Gottdiener JS, et al. Changes in heart rate and heart rate variability before ambulatory ischemic events (1). J Am Coll Cardiol 2001;38:742–9. [DOI] [PubMed] [Google Scholar]
- [4].Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 2007;50:448–52. [DOI] [PubMed] [Google Scholar]
- [5].Merli E, Sutcliffe S, Gori M, et al. Tako-tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006;7:53–61. [DOI] [PubMed] [Google Scholar]
- [6].Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472–9. [DOI] [PubMed] [Google Scholar]
- [7].Bybee KA, Murphy J, Prasad A, et al. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol 2006;13:244–50. [DOI] [PubMed] [Google Scholar]
- [8].Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–17. [DOI] [PubMed] [Google Scholar]
- [9].Martin EA, Tan SL, MacBride LR, et al. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res 2008;18:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martin EA, Prasad A, Rihal CS, et al. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010;56:1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Norcliffe-Kaufmann L, Kaufmann H, Martinez J, et al. Autonomic findings in takotsubo cardiomyopathy. Am J Cardiol 2016;117:206–13. [DOI] [PubMed] [Google Scholar]
- [12].Hamer M, Endrighi R, Venuraju SM, et al. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One 2012;7:e31356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Burg MM, Meadows J, Shimbo D, et al. Confluence of depression and acute psychological stress among patients with stable coronary heart disease: effects on myocardial perfusion. J Am Heart Assoc 2014;3:e000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giannoglou GD, Koskinas KC. Mental stress and cardiovascular disease: growing evidence into the complex interrelation between mind and heart. Angiology 2015;66:5–7. [DOI] [PubMed] [Google Scholar]
- [15].Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in kenya. N Engl J Med 2005;352:39–47. [DOI] [PubMed] [Google Scholar]
- [16].Redberg RF, Cannon RO, 3rd, Bairey Merz N, et al. Women's ischemic syndrome evaluation: current status and future research directions: report of the national heart, lung and blood institute workshop: October 2–4, 2002: Section 2: stable ischemia: pathophysiology and gender differences. Circulation 2004;109:e47–9. [DOI] [PubMed] [Google Scholar]
- [17].Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the nhlbi-sponsored women's ischemia syndrome evaluation (wise) study: Part i: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006;47:S4–20. [DOI] [PubMed] [Google Scholar]
- [18].Park JH, Kang SJ, Song JK, et al. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical icu. Chest 2005;128:296–302. [DOI] [PubMed] [Google Scholar]
- [19].Broadley AJ, Korszun A, Abdelaal E, et al. Inhibition of cortisol production with metyrapone prevents mental stress-induced endothelial dysfunction and baroreflex impairment. J Am Coll Cardiol 2005;46:344–50. [DOI] [PubMed] [Google Scholar]
- [20].Gottdiener JS, Kop WJ, Hausner E, et al. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol 2003;92:687–91. [DOI] [PubMed] [Google Scholar]
- [21].Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press 2002;11:22–7. [DOI] [PubMed] [Google Scholar]
- [22].Huang CJ, Stewart JK, Franco RL, et al. Lps-stimulated tumor necrosis factor-alpha and interleukin-6 mrna and cytokine responses following acute psychological stress. Psychoneuroendocrinology 2011;36:1553–61. [DOI] [PubMed] [Google Scholar]
- [23].Aschbacher K, Epel E, Wolkowitz OM, et al. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav Immun 2012;26:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tschudi M, Richard V, Buhler FR, et al. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. Am J Physiol 1991;260:H13–20. [DOI] [PubMed] [Google Scholar]
- [25].Zeiher AM, Drexler H, Wollschlaeger H, et al. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol 1989;14:1181–90. [DOI] [PubMed] [Google Scholar]


