Abstract
Background and Aims
Enlarged sterile flowers on the periphery of inflorescences increase the attractiveness of floral displays, and previous studies have generally demonstrated that these have positive effects on insect visitation and/or reproductive success. However, experiments have not specifically been designed to examine the benefits of sterile flowers under conditions that reflect the early stages in their evolution, i.e. when plants that produce sterile flowers are at low frequency.
Methods
Over three years, three experiments were performed in natural populations of Viburnum lantanoides, which produces sterile marginal flowers (SMFs). The first experiment established that fruit production in V. lantanoides increases with the receipt of outcross pollen. The second tested the role of SMFs under extant conditions, comparing fruit production in two populations composed entirely of intact plants or entirely of plants with the SMFs removed. The third was designed to mimic the presumed context in which SMFs first evolved; here, SMFs were removed from all but a few plants in a population, and rates of insect visitation and fruit set were compared between plants with intact and denuded SMFs.
Key Results
In comparing whole populations, the presence of SMFs nearly doubled fruit set. Under simulated ‘ancestral’ conditions within a population, plants with intact SMFs received double the insect visits and produced significantly more fruits than denuded plants. There was no significant effect of the number of inflorescences or fertile flowers on insect visitation or fruit set, indicating that the presence of SMFs accounted for these differences.
Conclusions
The presence of SMFs significantly increased pollinator attraction and female reproductive success both in contemporary and simulated ancestral contexts, indicating that stabilizing selection is responsible for their maintenance, and directional selection likely drove their evolution when they first appeared. This study demonstrates a novel approach to incorporating historically relevant scenarios into experimental studies of floral evolution.
Keywords: Viburnum, sterile marginal flowers, pollination, natural selection
INTRODUCTION
Reproductive success in animal-pollinated plants depends on a plant’s ability to attract pollinators to its flowers. Consequently, flowering plants have evolved numerous strategies to increase the attractiveness of their floral displays. One of these strategies is the production of large displays with many flowers (reviewed by Ohashi and Yahara, 2001). Large displays may be more attractive to pollinators, but they may require a major investment and also increase the probability of geitonogamous mating, reducing the number of pollen grains and ovules available for outcrossing (Klinkhamer and de Jong, 1993; Harder and Barrett, 1995, 1996; Barrett and Harder, 1996; Ohashi and Yahara, 2001; Mitchell et al., 2004; Karron et al., 2004; Karron and Mitchell, 2012).
Many species with large floral displays have evolved showy accessory structures that effectively increase the size of the display without increasing the number of flowers (reviewed by Harder and Barrett, 1996). These showy accessory structures can be classified into two categories depending on the organs that endow showiness (Classen-Bockhoff, 1990). The first involves producing large, showy bracts, as in the aroids (Araceae), spurges (Euphorbiaceae) and dogwoods (Cornaceae). The second involves the production of enlarged flowers by expanding either petals or sepals. Such enlarged flowers are often borne on the periphery of the inflorescence and they are often sterile or have greatly reduced sexual function. Such sterile flowers have evolved independently numerous times, and are especially well known in the inflorescences of sunflowers (Asteraceae), hydrangeas (Hydrangeaceae) and viburnums (Adoxaceae).
Darwin (1877) hypothesized that sterile flowers may promote outcrossing by increasing pollinator visitation. Many of the studies testing this hypothesis have been conducted in Asteraceae, at the intra-population scale (i.e. where there is natural or experimentally generated variation in the expression of sterile flowers within the same population). Here the presence of enlarged ray flowers has been shown to have a positive effect on insect visitation and/or reproductive success (Stuessy et al., 1986; Abbott and Irwin, 1988; Andersson, 1991, 1996, 2008; Nielsen et al., 2002). A study performed in Leopoldia comosa (Asparagaceae) provided similar insights; here the interactive effects of display size (i.e. fertile flower number) and sterile flowers increased pollinator attraction, pollen export and pollen receipt, especially in habitats where pollinators were limiting (Morales et al., 2013). Taken together, such studies provide broad support for Darwin’s (1877) hypothesis and imply that sterile flowers evolved via natural selection in the ancestors of these species or clades.
Similar studies conducted in Viburnum have yielded conflicting results, mainly related to the level at which sterile marginal flowers (SMFs) have a measurable effect on pollinator behaviour and/or reproductive success. An early study in V. lantanoides (the hobblebush) compared fruit set between inflorescences with and without SMFs in the same individual plant, and found that intact inflorescences produced significantly more fruit than denuded ones, suggesting that pollinators preferentially visited inflorescences with intact SMFs (Bell, 1985). However, considering that the expression of SMFs is fixed within individual plants in Viburnum, manipulations at the intra-plant scale provide only limited insight into the selective benefits of SMFs. Puzzlingly, subsequent studies in both V. opulus and V. lantanoides, which compared inflorescences and whole plants having intact SMFs with plants that had had the SMFs removed, failed to detected a significant effect of SMFs on fruit set (Krannitz and Maun, 1991; Englund, 1994; P. Wilson and M.J. Donoghue, unpubl. res.). These studies did, however, identify other important aspects of reproductive success in SMF-producing Viburnum species. In V. opulus planted in Ontario, Canada, fruit production was sometimes significantly affected by the number of neighbouring plants (i.e. more fruits were initiated in larger groups of plants; Krannitz and Maun, 1991). In V. lantanoides, fruit production was found to differ significantly among populations of differing density and at different elevations in Massachusetts, New Hampshire and Vermont (P. Wilson and M.J. Donoghue, unpubl. res.). The inability to demonstrate a consistent positive effect of SMFs at the within-population scale calls into question the role of natural selection in driving the evolution of these specialized flowers. However, a study of V. macrocephalum f. keteleeri found that the benefits of SMFs with respect to pollinator visitation and fruit set only become apparent at the whole-population level, i.e. in comparing individuals in populations composed entirely of intact plants or of plants from which SMFs were removed (Jin et al., 2010). In agreement with at least some of the findings of Krannitz and Maun (1991), the findings of Jin et al. (2010) imply that the benefits of SMFs accrue when they are present in a population of neighbouring plants.
In this study, we conducted a series of experiments over a 3-year period in natural populations of V. lantanoides to determine the selective benefits of SMFs in Viburnum at multiple scales. The first experiment was necessary to establish the effects of selfing versus outcrossing on fruit production in these plants, as this would greatly influence our interpretations. The second experiment was designed to test the effects of SMFs on reproductive success in this species. This focused on mean fruit set per inflorescence under contemporary conditions, comparing individuals in whole populations composed entirely of intact plants versus plants from which SMFs were removed. In a third experiment we measured the effects of SMFs on rates of visitation and fruit set in the presumed context under which SMFs initially evolved, namely when ‘mutant’ SMF-producing individuals occurred at low frequency within a population otherwise composed of plants lacking SMFs.
MATERIALS AND METHODS
Study species and sites
Viburnum lantanoides is one of the nine (out of a total of ~165) Viburnum species that produce SMFs. It is a member of the Pseudotinus subclade (Clement et al., 2014; Spriggs et al., 2015), which, like its closest relatives in China (V. sympodiale) and Japan (V. furcatum), is an understorey shrub that inhabits cool, mesic habitats in mixed hardwood forests. The sister group of this clade of three species is V. nervosum, which occupies cool forests extending from the eastern to the western Himalayas. Viburnum nervosum lacks SMFs but is highly unusual in Viburnum in producing fertile flowers that are larger than those in any other Viburnum species.
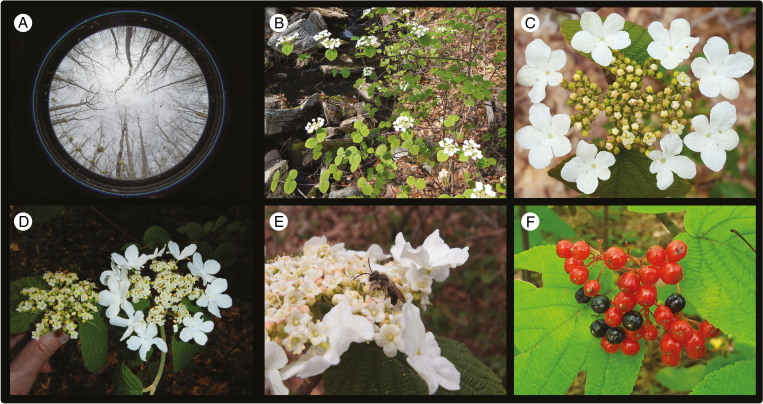
Viburnum lantanoides has an expansive distribution in North America, spanning from the Smoky Mountains in the south to Quebec and Cape Breton Island in the north. It is one of the first woody species to flower in early spring (Fig. 1A), and a single genet can be composed of many ramets, each potentially producing >100 inflorescences a year (Fig. 1B). The flowers are produced in terminal sessile compound umbel-like inflorescences, with generally five branches (rays) (Fig. 1C). Based on our measurements, each inflorescence bears on average 168 fertile flowers (95 % CI 166–70, n = 536 inflorescences) and eight SMFs (range 7–22, n = 48 inflorescences), typically two on the periphery of each of the four lateral rays. The SMFs greatly increase the width of an inflorescence (Fig. 1D; diameter with SMFs 104 mm, 95 % CI 101–107 mm; diameter without SMFs 72.1 mm, 95 % CI 69.4–74.7 mm) and increase its surface area by a factor of 2 (mean ± s.e. 2.16 ± 0.062, n = 48 inflorescences). The SMFs generally begin to open ~7 d before the fertile flowers in the centre of the inflorescence. The fertile flowers typically shed and receive pollen for approximately four to six consecutive days, remaining open day and night. The inflorescences are visited by a variety of insects (e.g. early-emerging andrenid bees, syrphid and muscid flies and elaterid beetles), but at our site the most consistent (and presumably consequential) visitation was by andrenid bees, which were found to actively visit and collect pollen from V. lantanoides. We note that the fertile flowers of V. lantanoides produce a faint sweet odour and a tiny amount of nectar at the base of the short style, near the juncture of the rotate corolla tube. The SMFs, in contrast, produce no nectar, nor do they possess nectar guides. The fruits are drupes that take between 2 and 3 months to reach full maturity. The ovaries start out green in colour, turn red for a prolonged period, and finally mature one at a time to dark purple or nearly black (Fig. 1F). Fruit dispersal has not been studied in detail in this species, but the fruits are primarily dispersed by frugivorous birds and occasionally also by small mammals. However, many fruits appear to fall off the maternal plant before being eaten (Gould, 1966).
Fig. 1.
Viburnum lantanoides. (A) Plants flower for 5–7 d in early spring prior to canopy closure, as shown by a fisheye photograph taken from above one of our focal plants. (B) Genets can be composed of multiple flowering ramets; larger plants can produce >100 inflorescences. (C) Inflorescences bear ~165 fertile flowers on five inflorescence rays, and eight SMFs (two on each of the four lateral rays). (D) The presence of SMFs increases the overall width of an inflorescence by a factor of 2. (E) Flowers are visited by a variety of insects but are most effectively pollinated by early-emerging Andrena bees. (F) Drupe fruits mature to the red-coloured phase ~2 months after flowering and then asynchronously turn black at maturity.
We conducted our experiments in Beartown State Forest (42°12′03.4″ N, 73°16′52.9″ W) in the Berkshire Mountains of western Massachusetts. The site is characteristic of the northern hardwood forests of New England, with sugar maple (Acer saccharum), red oak (Quercus rubra), yellow birch (Betula alleghaniensis) and American beech (Fagus grandifolia) forming the canopy, with a scattering of white pine (Pinus strobus) and hemlock (Tsuga canadensis). Common understorey associates of V. lantanoides at this site included the striped maple (Acer pensylvanicum), witch hazel (Hamamelis virginiana), alternate-leaved dogwood (Cornus alternifolia), mountain laurel (Kalmia latifolia) and wintergreen (Gaultheria procumbens). Two other Viburnum species (V. acerifolium and V. cassinoides) were also present, but these flower several weeks after V. lantanoides. As described below, we conducted experiments in four populations of V. lantanoides within the park.
Conditions during the flowering period varied considerably among the three years that we conducted our experiments (Supplementary Data Table S1). In 2015, flowering occurred over four full days from 7 to 10 May. Unusually warm temperatures during this time (~ 25–30 °C) caused trees to leaf out and typically later-flowering species to flower during this time. In 2016, our observations of flowering individuals were made over four full days from 9 to 12 May, with clear skies and daytime temperatures around 15 °C. In 2017, a warm spell caused flowering to begin in late April. However, cool, rainy conditions in early May extended the flowering season, and we conducted our observations on seven days between 29 April and 11 May.
Experiment 1: breeding system
We performed a crossing experiment to determine the breeding system in V. lantanoides. Each year, 1 week prior to anthesis, we selected six to ten plants and bagged all inflorescences with nylon mesh. After all flowers had opened, we applied the following pollination treatments: (1) unpollinated – to determine rates of autonomous fruit set; (2) self-pollinated – hand pollination with pollen from another inflorescence on the same plant, to determine rates of fruit production through selfing; (3) cross-pollinated – hand pollination with a mixture of pollen from several plants distributed >10 m away from the focal plant, to determine rates of fruit production with outcross pollen; and (4) cross-pollinated with SMFs removed – to determine the effect of SMF removal on fruit production. Pollination treatments were carried out by collecting donor pollen onto a plastic Petri dish and applying pollen onto individual stigmas with a fine-tipped paint brush. Two months following these treatments, we counted the number of fruits produced in each bagged inflorescence. We also counted the fruits produced in an additional (untreated) inflorescence on each experimental plant and in a random selection of unbagged and naturally pollinated plants to determine background rates of fruit set. We averaged across inflorescences when the same treatment was applied to multiple inflorescences on a given plant.
Experiment 2: reproductive success at the whole-population level
We performed an experiment to determine the effect of SMFs on reproductive success under contemporary conditions, in which SMFs are fixed within large populations. To this end, we replicated the experimental design of Jin et al. (2010), comparing mean fruit set per inflorescence between individuals in two populations, one consisting entirely of plants with intact inflorescences and another consisting entirely of plants with the SMFs removed.
For this experiment, we located two populations (populations A and B) of similar spatial extent that were separated by ~100 m, each one consisting of ~100 flowering plants. One week prior to anthesis, we removed SMFs from all plants in population A (the denuded treatment) while leaving them intact in population B (the intact treatment). We then tagged six to ten plants of similar height and inflorescence number in each population to assay fruit set. Ten weeks after the flowering period, we counted fruits in five to ten inflorescences per tagged plant in both populations. We repeated this experiment over three years, reversing the population that was subject to artificial SMF removal (e.g. the intact population in 2015 became the denuded population in 2016), and tagging new plants each year. For analysis, we averaged fruit set per inflorescence per plant.
Experiment 3: pollination and reproductive success under ‘ancestral’ conditions
From 2015 to 2017 we conducted an experiment to determine the effects of SMFs on insect visitation and fruit set under conditions designed to reflect the incipient stages of SMF evolution, i.e. when SMF-producing ‘mutants’ occur in low frequency in a population otherwise composed entirely of ‘ancestral’ non-SMF producing plants.
For this experiment, we located a large population with clearly defined boundaries (~150 m × 75 m), which contained ~250 flowering ramets. We identified eight to 15 natural clusters within this population (with patch identities depending on the number and distribution of flowering ramets in a given year) and tagged two focal plants in each. One week prior to anthesis, SMFs were removed from all plants in the population with the exception of one of the focal plants in every patch. Thus, each patch contained many plants with SMFs removed and two focal plants; one ‘intact’ plant with SMFs and one ‘denuded’ plant for comparison. This yielded a total of eight to 15 intact and denuded plants for analysis. Upon flowering, we conducted pollinator observations on sunny days from 0900 to 1500 h. Observations were conducted in 10-min blocks, during which observers noted the number of visits, their duration, and the identity (to major insect clade) of visitors to each focal plant. Observers moved between plants at the end of each observation block, and three to five observations were performed on each focal plant per day of observation. The total number of insect visits per plant was divided by the number of 10-min observation blocks to yield an average rate of insect visitation over the study period. At the end of the flowering period, we tagged five to ten randomly selected inflorescences for each focal plant to estimate the mean number of fertile flowers per inflorescence and the mean number of fruits produced. Fruits were counted every 10–20 d for 10 weeks.
Data analyses
For experiment 1 we used one-way analysis of variance to test for differences among the different pollination treatments. As some pollination treatments were not replicated across all years of the study, data were pooled across years. Significant differences between pollination treatments were identified with a post hoc Tukey’s honestly significant difference (HSD) test. For experiment 2 we found that fruit-set data were normally distributed, so we used a linear mixed effects model (LMM; Bolker et al., 2009) with SMF treatment and population as fixed effects and year as a random effect. For experiment 3, we square-root-transformed mean visits per 10-min observation period to reach normality and analysed these data with a LMM, with SMF treatment, log-mean inflorescence number per plant and mean fertile flower number per inflorescence as fixed effects and year as a random effect. We found that mean fruit set per inflorescence was normally distributed, so again we used a LMM with SMF treatment, log-mean inflorescence number per plant and mean fertile flower number per inflorescence as fixed effects and year as a random effect. For both analyses for experiment 3, we used the Bayesian information criterion (BIC) to compare simple models without effect interactions with more complex models that included interactions between SMF treatment and log-mean inflorescence number per plant, mean fertile flower number per inflorescence, and year. All analyses were conducted in R v. 3.4.3 (R Core Team, 2017) and models were fitted under restricted maximum likelihood (REML) in the R package lme4 (Bates et al., 2015). F-statistics were estimated with the R package lmerTest (Kuznetsova et al., 2017). All means are presented with standard errors.
RESULTS
Experiment 1: breeding system
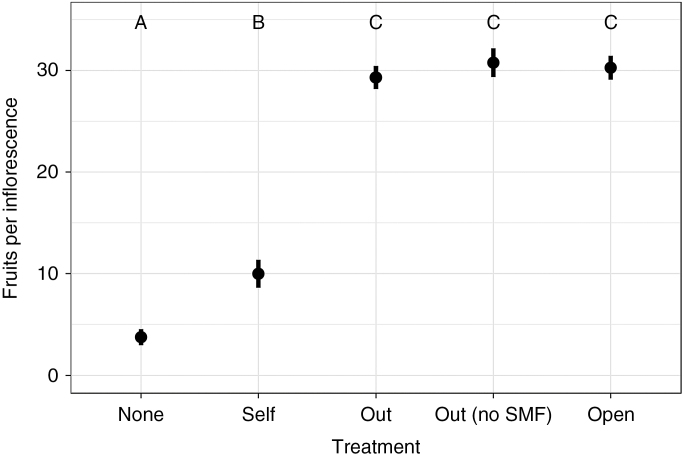
Fruit production varied significantly with pollination treatment (Fig. 2; F4,114 = 112, P < 0.0001, n = 115 inflorescences). Experimentally outcrossed inflorescences with and without intact SMFs and unbagged, open-pollinated inflorescences produced similar numbers of fruits: outcrossed with SMFs, 9.3 ± 1.08 fruits, n = 29; outcrossed without SMFs, 30.8 ± 1.62 fruits, n = 13; unbagged and open, 30.2 ± 1.24 fruits, n = 22. In contrast, bagged, unpollinated inflorescences and experimentally self-pollinated inflorescences produced significantly fewer fruits than did outcrossed and open-pollinated inflorescences (Tukey HSD, q = 2.89, α = 0.05): unpollinated inflorescences produced 3.77 ± 1.22 fruits (n = 23) and self-pollinated inflorescences produced 9.98 ± 1.1 fruits (n = 28). The low rates of fruit production from the autonomous and selfing treatments in contrast to both the cross-pollinated and the naturally pollinated treatments suggest that V. lantanoides possesses a ‘leaky’ self-incompatibility system, in which very few fruits are produced through selfing and fruit production is increased by outcrossing. Furthermore, a similar level of fruit set for artificially outcrossed and open-pollinated inflorescences suggests that fruit production is not pollinator-limited at the study site. Finally, our finding that outcrossing yields a similar number of fruits whether SMFs are left intact or removed indicates that the removal of SMFs does not in itself reduce fruit set.
Fig. 2.
Mean ± s.e. fruit set per inflorescence following experimental pollination treatments: bagged and unpollinated (‘None’); hand self-pollinated (‘Self’); hand-pollinated with outcross pollen (‘Out’); hand-pollinated with outcross pollen but with SMFs removed (‘Out (no SMF)’); unbagged, open-pollinated (‘Open’). Letters A, B and C denote statistically significant differences among treatments (P < 0.05).
Experiment 2: reproductive success at the whole-population level
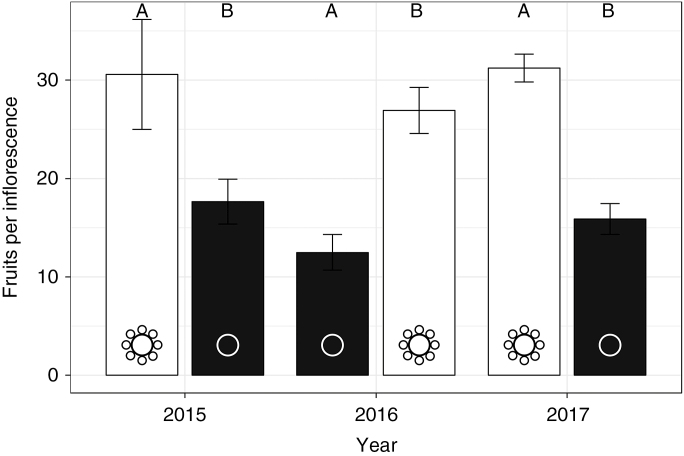
There was a significant negative effect of SMF removal on fruit set per inflorescence across all three years of the study (Fig. 3; F1,70 = 48.6, P < 0.0001). On average, intact plants produced roughly double the number of fruit as did denuded plants (in 2015, 30.5 ± 5.59 versus 17.7 ± 2.28; in 2016, 26.9 ± 2.34 versus 12.5 ± 1.81; in 2017, 31.2 ± 1.41 versus 15.9 ± 1.57). We found no significant effect of population on fruit set (F1,70 = 2.8, P = 0.839), indicating that the presence or absence of SMFs accounted for the differences in fruit set as opposed to any environmental differences between the two sites.
Fig. 3.
Effects of SMFs on fruit set between populations. Mean ± s.e. fruit set per inflorescence per plant in populations composed entirely of individuals with intact SMFs (white bars) or with denuded inflorescences (black bars). Letters A and B denote population identity.
Experiment 3: pollination and reproductive success under ‘ancestral’ conditions
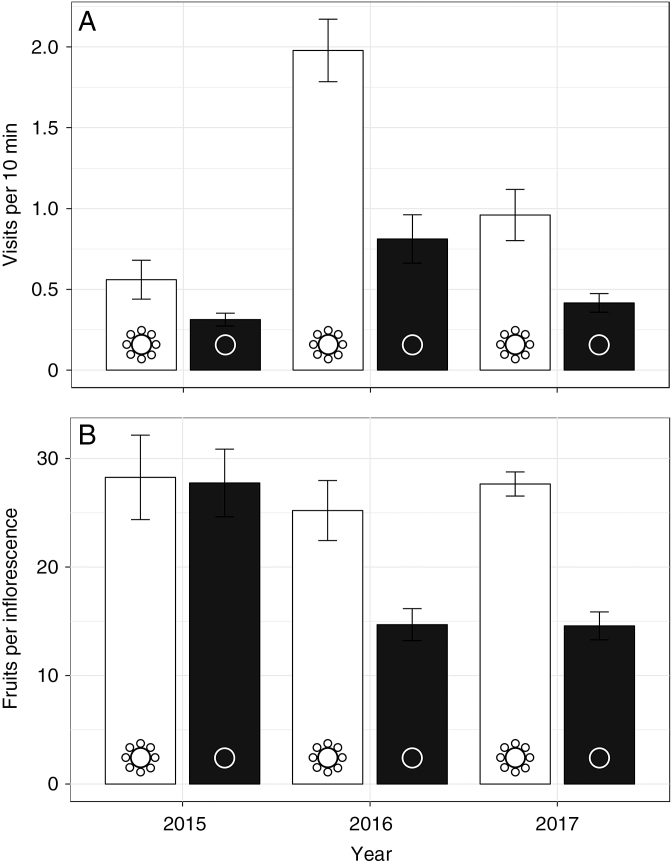
Despite significant climatic variability (see above), intact plants (with SMFs) experienced significantly greater rates of insect visitation than did denuded plants across all three years of the study (Fig. 4A; F1,59 = 29.3, P < 0.0001). The difference in visitation rates (mean ± s.e. visits per 10 min) between intact and denuded plants was greatest in 2016 (1.98 ± 0.612 versus 0.812 ± 0.474), followed by 2017 (0.961 ± 0.614 versus 0.416 ± 0.474), and it was lowest in 2015 (0.56 ± 0.341 versus 0.313 ± 0.112). Most visits were from muscid flies and andrenid bees, the latter likely being the most effective pollinators as they were observed to actively collect pollen and move vigorously between plants. European honey bees (Apis mellifera) were not observed visiting our experimental plants except in 2015, where they accounted for <5 % of total visits (data not shown). We found no significant effect of other aspects of the floral display on fruit set (log-inflorescence number, F1,59 = 3.12, P = 0.139; fertile flower number per inflorescence, F1,59 = 1.37, P = 0.242). More complex models that included interactions between SMF treatment and floral display characters (log-inflorescence number and fertile flower number per inflorescence) and year were not favoured over the simple model according to the BIC.
Fig. 4.
Effects of SMFs on insect visitation and fruit set under ‘ancestral’ conditions (i.e. when plants with SMFs were at low frequency). (A) Mean ± s.e. insect visits per 10-min observation block between plants with intact SMFs (white bars) and plants with SMFs removed (black bars). (B) Mean ± s.e. fruits per inflorescence per plant in intact and denuded plants.
Intact plants (with SMFs) also produced significantly more fruits per inflorescence than did denuded ones (Fig. 4B; F1,59 = 26, P < 0.0001). The difference in mean fruit set (mean ± s.e. fruits per inflorescence) between treatments was greatest in 2017, when intact plants produced nearly double the number of fruits as denuded plants (27.6 ± 1.11 versus 14.6 ± 1.28). In 2016, intact plants produced closer to one-third more fruits than denuded plants (25.2 ± 2.77 versus 14.7 ± 1.48). In 2015 we found almost no difference in fruit set between intact and denuded plants (28.3 ± 3.89 versus 27.7 ± 3.11). We did not find a significant effect of log-inflorescence number on fruit set (F1,59 = 0.481, P = 0.264). There was a slight but significant effect of mean fertile flower number on fruit set (F1,59 = 4.46, P = 0.0347), but no significant difference in mean flower number per inflorescence between treatments (one-tailed t-test: t61.8 = 4.46, P = 0.64). The BIC favoured a more complex model that included the interaction between treatment and year (∆BIC = 16.9) over a simple model without effect interactions. Results from the complex model recapitulated the simple model, with the addition of a significant effect of treatment × year on fruit set (F2,57 = 6.87, P = 0.002). This makes sense considering that SMF treatment did not uniformly affect fruit set across all years (Fig. 4). Taken as a whole, these results imply that the presence or absence of SMFs primarily accounted for the observed differences in insect visitation and fruit set between intact and denuded plants when intact plants were present at low frequency.
DISCUSSION
Our results demonstrate a clear positive effect of SMFs on pollinator attraction and female reproductive success at the whole-population level and even when SMFs are at low frequency within a population. Using experimental hand-pollinations, we found that fruit production is greatly enhanced by outcrossing (Fig. 2), which supports a role for SMFs in promoting insect visitation and fruit set. We tested this hypothesis first by comparing mean fruit set per inflorescence between individuals in a pair of populations composed entirely of intact or of denuded plants. We found that the presence of SMFs roughly doubled fruit production in this experiment (Fig. 3). This same basic result was obtained in an experiment designed to mimic an earlier stage in the evolution of SMFs, where plants with SMFs were present at low frequency in a population of plants that otherwise lacked SMFs. In this experiment the presence of SMFs more than doubled the rate of insect visitation (Fig. 4A), and this usually resulted in increased fruit production (Fig. 4B). Together, our findings provide evidence for natural selection in the maintenance of SMFs under current conditions, as well as in the initial stages of the evolution of SMFs. Overall, these experiments provide new insights into the ecological circumstances that favoured the evolution of this innovation.
The benefits of SMFs under contemporary conditions
Under current conditions, our comparisons at the whole-population level show that plants with intact SMFs produce nearly double the number of fruits per inflorescence as compared with denuded plants (Fig. 3). Our results align well with other studies on the selective benefits of accessory structures and enlarged sterile flowers in a variety of species at similar scales, i.e. when intact and denuded plants occur in roughly equal frequency within or between populations (Lack, 1982; Marshall and Abbott, 1984; Stuessy et al., 1986; Abbott and Irwin, 1988; Sun and Ganders, 1990; Andersson, 1991, 1996, 2008; Nielsen et al., 2002; Morales et al., 2013). Taken together, these studies support the view that SMFs function under present circumstances in attracting insects to patches of plants from a distance (Jin et al., 2010), enabling them to more effectively compete with non-SMF species for pollinators in a community context. In the case of V. lantanoides, which is one of the first understorey woody plants to flower in the spring in north-eastern forests, we suspect that SMFs serve to better attract the generally very limited number of pollinators that are active at that time. We have noted that the numbers of relevant pollinators (e.g. andrenid bees) appear to vary greatly from year to year as a function of weather conditions at the time that V. lantanoides flowers. We speculate that SMFs provide a particularly significant advantage under these highly variable early spring circumstances. It is noteworthy that the SMF-bearing close relatives of V. lantanoides in eastern Asia (V. furcatum and V. sympodiale) also occupy cool–temperate forests and flower early in the spring, and it is likely that this early-flowering strategy was a prelude to the evolution of SMFs in the ancestor of the Pseudotinus clade to which these species belong (B. Park and M.J. Donoghue, unpubl. res.). It seems likely that the relative scarcity and unpredictability of pollinator services in such forests at the time of flowering may have promoted the evolution of SMFs.
Given our findings, it is likely that stabilizing selection is maintaining the production of SMFs under contemporary conditions. However, an alternative explanation is difficult to reject. To our knowledge, there are no reports of non-SMF-bearing individuals within any of the nine Viburnum species that produce SMFs (V. lantanoides, V. sympodiale and V. furcatum in the Pseudotinus clade, V. plicatum and V. hanceanum in the Lutescentia clade, V. opulus, V. sargentii and V. trilobum in the Opulus clade, and V. macrocephalum in the Euviburnum clade). We have personally examined many thousands of plants of these species in the wild and in herbaria, and have never observed an individual lacking SMFs. One explanation for this observation is that such mutants are immediately and strongly selected against. Another explanation is that such mutants appear so rarely that there is virtually no relevant genetic variation within populations upon which selection could act (e.g. Bridgham et al., 2009). However, in Asteraceae, where the production of ray flowers evolved early in the clade, discoid inflorescences (i.e. without ray flowers) have evolved numerous times both across (Bremer and Humphries, 1993) and within (e.g. Bello et al., 2013) species. This provides evidence that reversals are certainly possible in other SMF-producing lineages. It is noteworthy that we do observe variation in the number of SMFs within inflorescences. Although eight is the most common number (two SMFs produced by each of the four lateral inflorescence rays), we observed variation from seven to 20. This variation could provide the basis for selection to higher and lower numbers (and ultimately perhaps to complete loss), but we note that this variation largely occurs idiosyncratically from one inflorescence to the next within individual plants, and we have not observed entire plants that consistently produce more or fewer SMFs per inflorescence. We also note that phylogenetic analyses of Viburnum support four independent origins of SMFs but no instances of the subsequent loss of SMFs (Clement et al., 2014; Spriggs et al., 2015; Eaton et al., 2017). Although we cannot entirely rule out non-adaptive explanations for the maintenance of SMFs in Viburnum without more detailed knowledge of the developmental and genetic underpinnings of the trait (but see Li et al., 2017; Lu et al., 2017), our data do offer positive evidence for the benefit of producing SMFs in modern populations of the hobblebush.
The benefits of SMFs under simulated ‘ancestral’ conditions
When they are present at low frequency within populations, V. lantanoides plants with intact SMFs consistently experienced more than double the rate of insect visitation than denuded plants (Fig. 4A). We found no effect of inflorescence number per plant, or of the mean number of fertile flowers per inflorescence, on rates of insect visitation, indicating that SMFs account for the observed differences. Similarly, in two out of three years of our study, intact plants produced almost double the number of fruits per inflorescence than did denuded plants (Fig. 4B). Again, there was no effect of inflorescence number, and only a slight effect of fertile flower number, on fruit set. As we found no consistent difference in mean fertile flower number between intact and denuded plants, differences in fruit production are unlikely to be the result of differences in fertile flower number. Taken together, our results indicate that insects preferred to visit isolated plants with SMFs, and that this usually resulted in increased fruit set, even when intact plants were present at very low frequency within a population.
These findings support the view that directional selection was likely responsible for increasing the frequency of SMFs when these initially arose within an ancestral population. Ultimately such selection may have resulted in the fixation of SMFs in the ancestors of the SMF-producing Viburnum species, though this could also have been promoted by fluctuations in population size related to past climate change (B. Park and M.J. Donoghue, unpubl. res.). Although we have documented increases in fruit set, our experiments do not allow us to assess the relative importance of male fitness gain through increased pollen export versus female fitness gain through increased seed production. Theory predicts that floral traits that enhance showiness (i.e. flower number and flower size) evolve to increase pollen export (reviewed by Burd and Callahan, 2000), and that showiness may evolve to increase both male and female fitness when fruit set is pollen-limited (reviewed by Burd, 1994; Knight et al., 2005). But the benefits of showiness are likely to be context-dependent (reviewed by Wilson et al., 1994; Ashman and Morgan, 2004), and the selective advantage of showiness for male and female function may vary over time. Here we have shown that SMFs increase fruit set, and we strongly suspect that they enhance male reproductive success as well. However, a critical evaluation of male reproductive success will require paternity analyses, which are especially difficult to carry out in this particular system. Even if plants could be properly genotyped (B. Park and M.J. Donoghue, unpubl. res.), the seeds of V. lantanoides, like those of many Viburnum species, are notoriously hard to germinate (Knowles and Zalik, 1958; Hidayati et al., 2005; Karlsson et al., 2005; Chien et al., 2011; Phartyal et al., 2014).
In the meantime, our observations on temporal heterogeneity in this system bear on the factors that likely promoted the evolution of SMFs. Viburnum lantanoides generally flowers before the trees leaf out in early spring (in late April to early May in southern New England, depending on weather conditions), and it is one of the few resources available to pollinating insects during this time. Though early flowering may be favoured by strong competition for pollinators later in the spring, a shift to earlier flowering can also be risky, rendering species more vulnerable to idiosyncratic weather conditions (e.g. rainy versus sunny days during the short flowering period) as well as to phenological mismatches between flowering and insect activity (Kudo and Ida, 2013). This may have been the case in 2015, when a long winter followed by the sudden onset of unseasonably warm temperatures caused V. lantanoides to flower at a time when other floral resources were becoming more abundant and trees were beginning to leaf out at the study site. Overall rates of visitation to V. lantanoides were low in 2015, which may reflect competition for pollinators with a greater number of other species. Furthermore, the rapid closure of the canopy may have diminished the conspicuousness of the SMFs, resulting in the observed smaller difference in visitation rates between intact and denuded plants in that year. We suspect that lower overall rates of insect visitation during 2015 may have led to fewer high-quality pollination events, which may have contributed to the lack of a significant difference in fruit set between intact and denuded plants in that year. It is interesting to note, however, that although conditions in 2015 were not ideal for pollinator activity, they may have been more favourable for fruit maturation as compared with other years (Fig. 4B). It is difficult to identify a single, overarching factor driving this result, but we suspect that the warmer than average conditions during both the flowering and the fruiting period may have influenced fruit maturation (e.g. frugivore density, resource availability) In contrast, in 2016 and 2017, V. lantanoides flowered under cooler temperatures, conditions that appear to be more typical during its flowering period. With fewer resources available, insects may have focused their foraging efforts on V. lantanoides, as evidenced by greater overall rates of visitation in these years (Fig. 4A). The open light environment may also have enabled insects to more easily distinguish intact plants from denuded ones, leading to more pronounced differences in visitation rate (Fig. 4A). Under these conditions, increased visitation to intact plants may have allowed higher-quality pollination and, in turn, significant differences in fruit set. A better understanding of such year-to-year differences will require additional seasons of experimentation, and will also need to consider a variety of other factors that could influence fruit set at later stages of development.
Overall, our findings are consistent with the hypothesis that SMFs evolved to increase reproductive success in the highly variable early springtime conditions that V. lantanoides and its relatives in the Pseudotinus clade are exposed to. Furthermore, our results suggest that the presence of SMFs led to appreciable differences in fruit set, suggesting that selection on female function alone may be sufficient in promoting SMF evolution, though they likely also impact male reproductive success. In this context, SMFs represent just one of a suite of traits that may have evolved in the Pseudotinus clade to accomplish this end. Like all Pseudotinus species, V. lantanoides can and does reproduce vegetatively; they root opportunistically when their pendulous branches touch the ground, and they spread via sucker shoots (Gould, 1966). Considering that rates of insect visitation and fruit set can vary greatly from year to year (this study) and among populations in V. lantanoides (P. Wilson and M.J. Donoghue, unpubl. res.), and in plant species in general (Herrera, 1988; Horvitz and Schemske, 1990; Campbell, 1991; Maad, 2000; Price et al., 2005; Jacquemyn and Brys, 2010), vegetative reproduction may be critical for the long-term persistence of populations when rates of reproductive success are low, or in marginal habitats where pollinators are in low abundance. Together, these traits work in concert to accommodate spatio-temporal heterogeneity in pollination services.
The importance of ‘origination’ experiments
Experiments are typically performed in species in which the trait of interest has long since arisen and been fixed. These studies may be especially useful in testing the current function and selective maintenance of a trait in a population (e.g. Andersson, 1991; Jin et al., 2010; Morales et al., 2013). In this study we adopted this paradigm and carried out one experiment that tested the difference between a population with the trait of interest present and a population where the trait was absent. But we have also designed an experiment to mimic the circumstances that may have been present when the trait first appeard in a population. The idea is to ask not only how the trait functions under current circumstances (e.g. where it is fixed in a population) but also how it may have functioned in a hypothesized earlier setting when it was very rare in a population and the role of different evolutionary forces in explaining their fixation. Experiments of this type, which explore the role of selection during the early evolution of a trait (Losos, 2011), would clearly benefit from knowledge of the trait’s genetic underpinnings. In this case our knowledge is limited, but we have supposed that SMFs originated via a mutation that caused an individual plant to produce inflorescences with fully formed SMFs. This is not the only possible scenario, of course, but it is the one that we have tested here, and it is useful to have discovered that SMFs can increase fitness even in this particular context.
We note that such ‘origination’ experiments could also be informed by phylogenetic inference of the time and place that a trait evolved, and the environmental conditions that might have existed at that point (Weber and Agrawal, 2012). These circumstances may be more difficult to mimic experimentally, particularly when a trait evolved very early in a clade’s history and the context in which it evolved is poorly understood (e.g. the origin of the flower itself). However, it may well be possible in other cases, and Viburnum may be especially well suited as we now have considerable knowledge of its phylogeny, biogeography, functional traits and ecology (e.g. Chatelet et al., 2013; Clement et al., 2014; Spriggs et al., 2015; Scoffoni et al., 2016; Eaton et al., 2017; Edwards et al., 2017). Viburnum lantanoides is nested within the Pseudotinus clade, and it would be especially instructive, therefore, to conduct a similar set of pollination experiments in populations of its two Asian relatives with SMFs – V. furcatum and V. sympodiale – and to compare the results with V. nervosum, the closest relative that lacks SMFs. Based on our phylogenetic and ecological knowledge of these species we infer that the ancestor of this small clade originated in cool temperate forests in eastern Asia, probably >10 million years ago (B. Park and M.J. Donoghue, unpubl. res.). For the reasons that we have outlined, this environmental setting may have set the stage for the evolution of a more showy floral display. From this perspective, the enlarged fertile flowers of V. nervosum and the SMFs of the lantanoides–furcatum–sympodiale lineage can be viewed as alternative mechanisms to increase showiness coinciding with a shift to flowering early in the short growing season. We note that a similar sequence is also observed in the circumboreal Opulus clade, where the adaptation of the ancestor of this clade to cold climates at high latitudes appears to have served as a precursor to the evolution of large fertile flowers in V. edule (North America) and V. koreanum (North-East Asia), and to SMFs in the ancestor of V. opulus (Europe), V. sargentii (North-East Asia) and V. trilobum (North America) (B. Park and M.J. Donoghue, unpubl. res.).
The phylogenetically correlated evolution of a unique branching pattern in the Pseudotinus clade (the ‘furcatum’ growth pattern of Donoghue, 1981; Edwards et al., 2014) can also be interpreted as a response to these environmental circumstances. This plant architecture is characterized by the production of sympodial plagiotropic axes that are predisposed to touching the ground and rooting at the nodes. This is one important way that these plants spread vegetatively. Such branches also have the effect of positioning inflorescences, often in pairs, in a distinct upright plane along the lateral axes. We hypothesize that this arrangement of inflorescences might have boosted the collective attractiveness of SMFs (Kunin, 1993; Ghazoul, 2005) and that this too may have been a factor favouring the evolution of SMFs in this particular lineage. The interaction between growth architecture and SMFs is also apparent in SMF-producing species in the Lutescentia clade (V. plicatum and V. hanceanum), where long monopodial plagiotropic axes and the production of inflorescences on short lateral shoots along these branches yield the highly conspicuous ‘doublefile’ display that characterizes these plants. This phylogenetically informed outlook helps to identify additional attributes of potential significance and sequences of evolutionary events, and this knowledge of organismal integration can, in turn, help us to design the most relevant manipulative experiments.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: weather conditions during pollinator observations from 2015 to 2017. Data were collected from a weather station in Pittsfield, MA (station KPSF) and were downloaded from Weather Underground (http://www.wunderground.com/).
ACKNOWLEDGEMENTS
We would like to thank Nancy Putnam, Adam Morris and the staff at the Massachusetts Department of Conservation and Recreation for granting us permission to conduct our experiments in the Beartown State Forest. We also thank Justine Cefalu, Malini Gandhi, Jessica Glass, Daemin Kim, Daniel Macuigan, Angus Mossman, Jaeeun Sohng, Sheena Thalma and Pamela Torola for their assistance in the field. This work was funded by a Yale Institute for Biospheric Studies Doctoral Dissertation Improvement Grant awarded to B.P. As members of B.P.’s doctoral dissertation committee, Spencer Barrett and Vivian Irish provided highly useful advice on the design of the study. Our work was stimulated by an earlier effort led by Paul Wilson, to whom we are very grateful for ideas and inspiration.
LITERATURE CITED
- Abbott RJ, Irwin JA. 1988. Pollinator movements and the polymorphism for outcrossing rate at the ray floret locus in groundsel, Senecio vulgaris L. Heredity 60: 295–298. [Google Scholar]
- Andersson S. 1991. Floral display and pollination success in Achillea ptarmica (Asteraceae). Ecography 14: 186–191. [Google Scholar]
- Andersson S. 1996. Floral display and pollination success in Senecio jacobaea (Asteraceae): interactive effects of head and corymb size. American Journal of Botany 83: 71–75. [Google Scholar]
- Andersson S. 2008. Pollinator and nonpollinator selection on ray morphology in Leucanthemum vulgare (oxeye daisy, Asteraceae). American Journal of Botany 95: 1072–1078. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Morgan MT. 2004. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context?Proceedings of the Royal Society B Biological Sciences 271: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. 1996. Ecology and evolution of plant mating. Trends in Ecology and Evolution 11: 73–79. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bell G. 1985. On the function of flowers. Proceedings of the Royal Society B Biological Sciences 224: 223–265. [Google Scholar]
- Bello MA, Álvarez I, Torices R, Fuertes-Aguilar J. 2013. Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae). Annals of Botany 112: 1597–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, et al. . 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Bremer K, Humphries CJ. 1993. Generic monograph of the Asteraceae-Anthemideae. Bulletin of the Natural History Museum of London (Botany) 23: 71–177. [Google Scholar]
- Bridgham JT, Ortlund EA, Thornton JW. 2009. An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M. 1994. Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review 60: 83–139. [Google Scholar]
- Burd M, Callahan HS. 2000. What does the male function hypothesis claim?Journal of Evolutionary Biology 13: 735–742. [Google Scholar]
- Campbell DR. 1991. Effects of floral traits on sequential components of fitness in Ipomopsis aggregata. American Naturalist 137: 713–737. [Google Scholar]
- Chatelet DS, Clement WL, Sack L, Donoghue MJ, Edwards EJ. 2013. The evolution of photosynthetic anatomy in Viburnum. International Journal of Plant Sciences 174: 1277–1291. [Google Scholar]
- Chien C-T, Chen S-Y, Tsai C-C, Baskin JM, Baskin CC, Kuo-Huang L-L. 2011. Deep simple epicotyl morphophysiological dormancy in seeds of two Viburnum species, with special reference to shoot growth and development inside the seed. Annals of Botany 108: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen-Bockhoff R. 1990. Pattern analysis in pseudanthia. Plant Systematics and Evolution 171: 57–88. [Google Scholar]
- Clement WL, Arakaki M, Sweeney PW, Edwards EJ, Donoghue MJ. 2014. A chloroplast tree for Viburnum (Adoxaceae) and its implications for phylogenetic classification and character evolution. American Journal of Botany 101: 1029–1049. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1877. The different forms of flowers on plants of the same species. London: J. Murray. [Google Scholar]
- Donoghue MJ. 1981. Growth patterns in woody plants with examples from the genus Viburnum. Arnoldia 41: 2–23. [Google Scholar]
- Eaton DAR, Spriggs EL, Park B, Donoghue MJ. 2017. Misconceptions on missing data in RAD-seq phylogenetics with a deep-scale example from flowering plants. Systematic Biology 66: 399–412. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Chatelet DS, Sack L, Donoghue MJ. 2014. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. Journal of Ecology 102: 328–336. [Google Scholar]
- Edwards EJ, Chatelet DS, Chen B-C, et al. . 2017. Convergence, consilience, and the evolution of temperate deciduous forests. American Naturalist 190: S87–S104. [DOI] [PubMed] [Google Scholar]
- Englund R. 1994. Male and female reproductive success in the hermaphroditic shrub Viburnum opulus (Caprifoliaceae). PhD Thesis, Uppsala University, Sweden. [Google Scholar]
- Ghazoul J. 2005. Pollen and seed dispersal among dispersed plants. Biological Reviews 80: 413–443. [DOI] [PubMed] [Google Scholar]
- Gould WP. 1966. The ecology of Viburnum alnifolium Marsh. PhD Thesis, State University College of Forestry at Syracuse University, USA. [Google Scholar]
- Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Harder LD, Barrett SCH. 1996. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies of floral evolution in animal-pollinated plants. New York: Chapman & Hall, 140–190. [Google Scholar]
- Herrera CM. 1988. Variation in mutualisms: the spatiotemporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35: 95–125. [Google Scholar]
- Hidayati SN, Baskin JM, Baskin CC. 2005. Epicotyl dormancy in Viburnum acerifolium (Caprifoliaceae). American Midland Naturalist 153: 232–244. [Google Scholar]
- Horvitz CC, Schemske DW. 1990. Spatiotemporal variation in insect mutualists of a neotropical herb. Ecology 71: 1085–1097. [Google Scholar]
- Jacquemyn H, Brys R. 2010. Temporal and spatial variation in flower and fruit production in a food-deceptive orchid: a five-year study. Plant Biology 12: 145–153. [DOI] [PubMed] [Google Scholar]
- Jin B, Wang L, Wang J, et al. . 2010. The structure and roles of sterile flowers in Viburnum macrocephalum f. keteleeri (Adoxaceae). Plant Biology 12: 853–862. [DOI] [PubMed] [Google Scholar]
- Karlsson LM, Hidayati SN, Walck JL, Milberg P. 2005. Complex combination of seed dormancy and seedling development determine emergence of Viburnum tinus (Caprifoliaceae). Annals of Botany 95: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ. 2012. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany 109: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. 2004. The influence of floral display size on selfing rates in Mimulus ringens.Heredity 92: 242–248. [DOI] [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong TJ. 1993. Attractiveness to pollinators: a plant’s dilemma. Oikos 66: 180–184. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. . 2005. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution, and Systematics 36: 467–497. [Google Scholar]
- Knowles RH, Zalik S. 1958. Effects of temperature treatment and of a native inhibitor on seed dormancy and of cotyledon removal on epicotyl growth in Viburnum trilobum Marsh. Canadian Journal of Botany 36: 561–566. [Google Scholar]
- Krannitz PG, Maun MA. 1991. An experimental study of floral display size and reproductive success in Viburnum opulus: importance of grouping. Canadian Journal of Botany 69: 394–399. [Google Scholar]
- Kudo G, Ida TY. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94: 2311–2320. [DOI] [PubMed] [Google Scholar]
- Kunin WE. 1993. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology 74: 2145–2160. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software 82: 1–26. [Google Scholar]
- Lack AJ. 1982. Competition for pollinators in the ecology of Centaurea scabiosa L. and Centurea nigra L. III. Insect visits and the number of successful pollinations. New Phytologist 91: 321–339. [Google Scholar]
- Li W, He Z, Zhang L, et al. . 2017. miRNAs involved in the development and differentiation of fertile and sterile flowers in Viburnum macrocephalum f. keteleeri. BMC Genomics 18: 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB. 2011. Convergence, adaptation, and constraint. Evolution 65: 1827–1840. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu J, Li W, et al. . 2017. Transcriptomic analysis reveals mechanisms of sterile and fertile flower differentiation and development in Viburnum macrocephalum f. keteleeri. Frontiers in Plant Science 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maad J. 2000. Phenotypic selection in hawkmoth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution 54: 112–123. [DOI] [PubMed] [Google Scholar]
- Marshall DF, Abbott RJ. 1984. Polymorphism for outcrossing frequency at the ray floret locus in Senecio vulgaris L. II. Confirmation. Heredity 52: 331–336. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. 2004. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology 18: 116–124. [Google Scholar]
- Morales CL, Traveset A, Harder LD. 2013. Sterile flowers increase pollinator attraction and promote female success in the Mediterranean herb Leopoldia comosa. Annals of Botany 111: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LR, Philipp M, Siegismund HR. 2002. Selective advantage of ray florets in Scalesia affinis and S. pedunculata (Asteraceae), two endemic species from the Galápagos. Evolutionary Ecology 16: 139–153. [Google Scholar]
- Ohashi K, Yahara T. 2001. Behavioural responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In: Chittka L, Thomson JD, eds. Cognitive ecology of pollination. Cambridge: Cambridge University Press, 274–296. [Google Scholar]
- Phartyal SS, Kondo T, Fuji A, Hidayati SN, Walck JL. 2014. A comprehensive view of epicotyl dormancy in Viburnum furcatum: combining field studies with laboratory studies using temperature sequences. Seed Science Research 24: 281–292. [Google Scholar]
- Price MV, Waser NM, Irwin RE, Campbell DR, Brody AK. 2005. Temporal and spatial variation in pollination of a montane herb: a seven-year study. Ecology 86: 2106–2116. [Google Scholar]
- R Core Team 2017. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-Kok J, et al. . 2016. Hydraulic basis for the evolution of photosynthetic productivity. Nature Plants 2: 16072. [DOI] [PubMed] [Google Scholar]
- Spriggs EL, Clement WL, Sweeney PW, Madriñán S, Edwards EJ, Donoghue MJ. 2015. Temperate radiations and dying embers of a tropical past: the diversification of Viburnum. New Phytologist 207: 340–354. [DOI] [PubMed] [Google Scholar]
- Stuessy TF, Spooner DM, Evans KA. 1986. Adaptive significance of ray corollas in Helianthus grosseserratus (Compositae). American Midland Naturalist 115: 191–197. [Google Scholar]
- Sun M, Ganders FR. 1990. Outcrossing rates and allozyme variation in rayed and rayless morphs of Bidens pilosa. Heredity 64: 139–143. [Google Scholar]
- Weber MG, Agrawal AA. 2012. Phylogeny, ecology, and the coupling of comparative and experimental approaches. Trends in Ecology and Evolution 27: 394–403. [DOI] [PubMed] [Google Scholar]
- Wilson P, Thomson JD, Stanton ML, Rigney LP. 1994. Beyond floral Batemania: gender biases in selection for pollination success. American Naturalist 143: 283–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.