Abstract
Background
Flower coloration is a key enabler for pollinator attraction. Floral visual signals comprise several components that are generated by specific anatomical structures and pigmentation, and often have different functions in pollinator attraction. Anatomical studies have advanced our understanding of the optical properties of flowers, and evidence from behavioural experiments has elucidated the biological relevance of different components of floral visual signals, but these two lines of research are often considered independently.
Scope
Here, we review current knowledge about different aspects of the floral visual signals, their anatomical and optical properties, and their functional significance in plant–pollinator visual signalling. We discuss common aspects, such as chromatic and achromatic contrast, hue, saturation and brightness, as well as less common types of visual signals, including gloss, fluorescence, polarization and iridescence in the context of salience of floral colour signals and their evolution, and highlight promising avenues for future research.
Keywords: Absorbance, colour preference, colour vision, evolution, flower colour, pigment, pollination, reflectance, structure, iridescence, gloss
INTRODUCTION
Many plants attract pollinators by displaying coloured flowers. The diversity of floral colours is wide, which can partly be explained by the fact that featuring distinct colours increases dissimilarity of flowers from their neighbourhood (Kevan, 1972; Levin, 1985; McEwen and Vamosi, 2010; Hopkins and Rausher, 2012). Flower colour is often described by the key elemental factors that influence human perception (Wyszecki and Stiles, 1982; Kelber and Osorio, 2010), i.e. hue (the spectral descriptor of colour), saturation (the purity of a colour) and brightness (the intensity of a signal). In addition, colour contrast and green contrast (the contrast between a stimulus and its background mediated solely by the green photoreceptor) are also considered to be important factors enabling the visual perception of flowers by pollinators (Table 1).
Table 1.
Glossary of terms for different aspects of the visual signals of flowers
| Visual signal | Perceptual counterpart | Measurement | Optical characteristics | Current evidence for functional significance for visual signalling in nature |
|---|---|---|---|---|
| Dominant wavelength | Hue | Reflectance spectrum measured with bifurcated probe or integrating sphere and a colour space model. | Wavelength-selective absorption by pigments. | Important; many pollinators have innate and learned preferences for specific hues. Effects of dominant wavelength cannot always be disentangled from colour contrast. |
| Spectral purity | Saturation | Reflectance spectrum measured with bifurcated probe or integrating sphere and a colour space model. | Colourfulness of the stimulus as opposed to greyness; characterized by the slope and amplitude at the inflection points of the reflectance curve. | Has been shown to be important for a few bee species. Effects of spectral purity cannot always be disentangled from colour contrast and dominant wavelength. |
| Intensity | Brightness/luminance | Integrating sphere or other technique that allows measuring the absolute amount of reflectance. | Amplitude of the reflectance curve. | Not known to be important for diurnal pollinators. |
| Colour contrast | Perceptual contrast between two colours as detected by all photoreceptors. Depending on the species, this may be affected by dominant wavelength and/or spectral purity. | Reflectance spectrum and colour vision model. | Difference in the amplitude of the stimuli’s reflectance spectra in the visible range of wavelengths. | Important – many species use colour contrast to distinguish and/or detect flowers from close and large distances. Increased colour contrast has been shown in several bee species to increase the probability of correct target flower identification. The relative importance of hue versus saturation can be difficult to discern. |
| Green contrast | Perceptual contrast between two colours as detected by the green photoreceptor. | Reflectance spectrum measured with bifurcated probe or integrating sphere and excitation values of green photoreceptor for background versus stimulus. | Difference in the amplitude of the stimulus’ and background’s spectra in the green wavelength range. | In honey bees important for long-distance detection. In many insects vital for motion processing, and the processing of complex patterns, shapes or sizes of objects such as flowers. |
| Specular reflection | Gloss | Angle-dependent reflectance measurements | Specular (mirror-like) reflection. Gloss requires very smooth and flat flower surfaces (Fig. 4). | Unclear. No evidence supporting or rejecting gloss as a signalling function. |
| Iridescence | Angle-dependent coloration | Angle-dependent reflectance measurements | Physical interactions of light waves with periodically ordered nanostructures that differ in refractive index. | Very little or no functional significance in natural conditions. |
| Polarization | Gloss | Angle-dependent reflectance measurements or high-quality photo camera with polarization-sensitive filter. | Geometrical orientation of the oscillation of light waves. Polarization effects generally occur at smooth and flat surfaces and often co-occur with gloss (Fig. 4). | Very little or no functional significance in natural conditions. |
| Fluorescence | Fluorescence | Fluorescence microscopy. | Emission of light by pigments. | Very little or no functional significance in natural conditions. |
For each aspect, we list the perceptual and physical counterpart, how it is measured, its optical characteristics and a conclusion as to its currently believed importance for visual signalling to pollinators in natural conditions. For details and calculations see Supplementary Data File S1.
A challenge for modern studies of plant–pollinator interactions is understanding which perceptual factors are biologically relevant for flower evolution, and how such factors interact with other types of visual signals, such as gloss, fluorescence or iridescence effects (Table 1). Different components of the flower’s visual signals are attributable to specific structural features and/or pigmentation. By collecting high-quality data on the physical properties of flowers it may be possible to untangle major traits that influenced evolutionary processes.
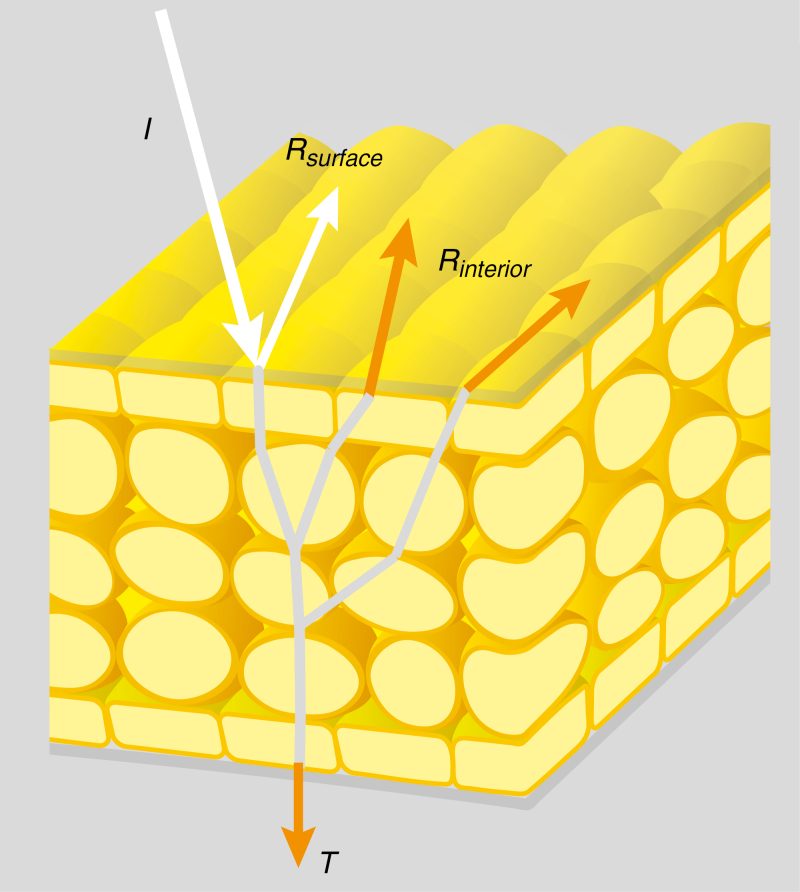
The coloration of flowers is typically due to the combined effect of light scattering by floral structures and wavelength-selective absorption by pigments (Kay et al., 1981; Kevan and Backhaus, 1998; van der Kooi et al., 2016a). Backscattering of light occurs at boundaries of media with different refractive indices, such as air/cell wall or water/cell wall interfaces and cellular inhomogeneities such as (pigment) granules (Fig. 1) (van der Kooi et al., 2016a). Flower interiors are commonly stratified, with different layers having specific scattering and pigmentation properties. For example, floral interior layers can vary in shape, size and type (e.g. mesophyll or starch cells), and pigments can be distributed throughout the flower or in specific layers (e.g. Kay et al., 1981; Koes et al., 1994; Kevan and Backhaus, 1998; Vignolini et al., 2012; van der Kooi et al., 2016a). Light that is not backscattered by the flower’s interior or surface is transmitted through the flower (Fig. 1); transmitted light may under specific circumstances contribute to the visual signal (van der Kooi et al., 2016a).
Fig. 1.
Diagram of light reflection in a flower. Part of the incident light (I) is reflected by the surface (Rsurface) or by the interior (Rinterior), part of the light is transmitted (T) through the flower, and light of a specific wavelength range is absorbed by pigments. The light that is reflected by the surface is largely unmodulated by pigments, whereas light that is reflected by the petal interior or transmitted through the flower will be modulated by pigments inside the flower (for visualization purposes the light rays inside the flower are shown in grey).
Floral pigments selectively filter the backscattered and transmitted light. For example, flowers with blue–green-absorbing anthocyanins are purple, and flowers with blue-absorbing carotenoids are yellow (reviewed by Mol et al., 1998; Grotewold, 2006). For some pigments, in particular anthocyanins, the absorption of light depends on the vacuolar pH (Mol et al., 1998). The degree of filtering by pigments strongly determines the strength of a flower’s visual signal (Figs 2 and 3) (van der Kooi et al., 2016a) and whether a pollinator may view such information as salient or cryptic (Dyer et al., 2007).
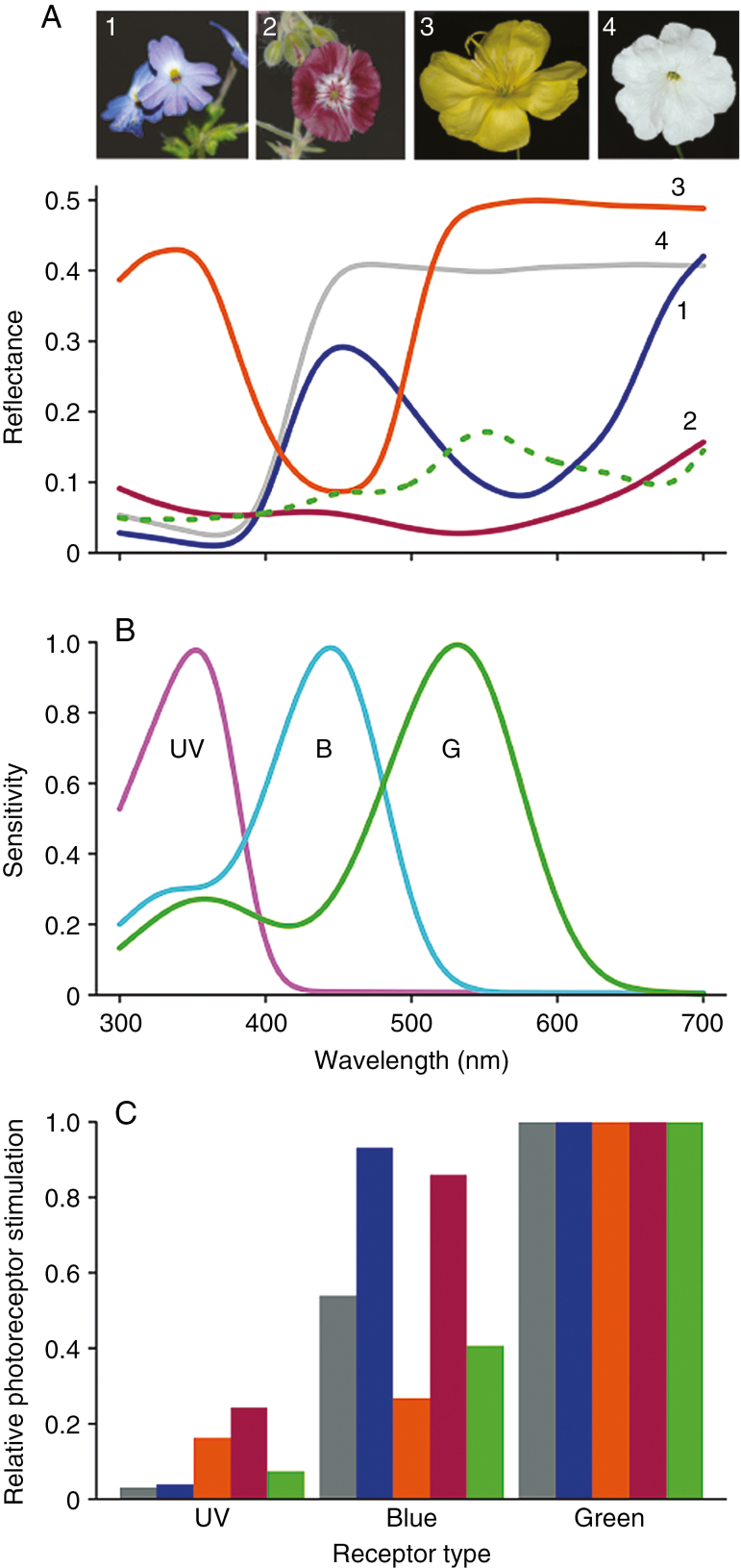
Fig. 2.
Reflectance spectra and their visual signals for four example flowers. (A) Reflectance spectra of four differently coloured flowers and an average green background spectrum: 1, Browallia americana; 2, Geranium phaeum; 3, Oenothera glazioviana; 4, Petunia nyctaginiflora; dashed curve, average green background. (B) Honey bee photoreceptor spectral sensitivities (after Peitsch et al., 1992). Relative photon stimulation for the different reflectance spectra of panel A for the different photoreceptors, using the photoreceptor spectral sensitivity shown in panel B. See Supplementary Data Table S1 for specific aspects of the spectra and interpretation with vision models.
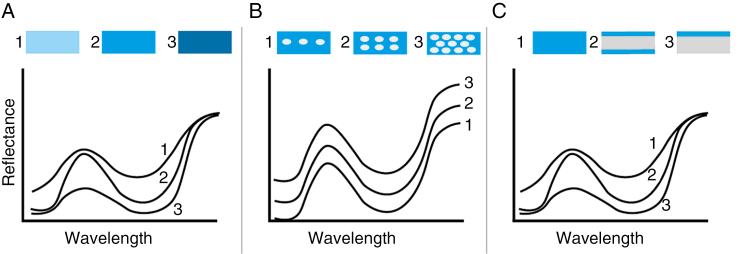
Fig. 3.
Diagram showing how different optical properties determine a flower’s reflectance spectrum. In each panel only one optical property is varied to illustrate the different mechanisms; at the top of each panel three model petals are shown (illumination and observation are from above). (A) Changes in the amount of pigment will alter the modulation of the reflected light; little pigment yields a pale colour, and very much pigment yields a dull colour. (B) The intensity of the reflected light increases with the number of backscattering structures, which are illustrated by white ovals. When the reflectance increases, the transmittance will decrease. For the sake of simplicity, the relative modulation of the spectra was kept constant, although in real flowers the filtering by pigments will change with floral anatomy. (C) When the total amount of pigment remains constant but its localization changes, this will alter the modulation of the reflectance spectrum. Pigment deposition at the side of viewing yields the strongest modulation. For details, see van der Kooi et al. (2016a) and Stavenga and van der Kooi (2016).
Detailed measurements suggest a wide variance in floral reflectance spectra between species, although in pollinator visual space this diversity can be different (e.g. Daumer, 1958; Kevan, 1972; Menzel and Shmida, 1993; Chittka et al., 1994). A large variety of animals, particularly numerous insect species, with often very different visual capabilities, is involved in plant–pollinator interactions. It is nevertheless convenient to consider some key pollinators such as bees for appropriate ways to investigate how flower colour may be perceived. Behavioural assays with pollinators using tailored stimuli can help to elucidate the relative importance of different aspects of floral signals. Data from behavioural assays can subsequently be compared with modelling results that enable interpretation of a flower’s reflectance spectrum with a ‘pollinator-subjective view’. Bees have a phylogenetically conserved trichromatic visual system with peak photoreceptor sensitivities at about 350, 440 and 540 nm (Fig. 2) (Peitsch et al., 1992; Briscoe and Chittka, 2001), and by using colour vision models such as the colour hexagon (Chittka, 1992) or the receptor noise-limited model (Vorobyev and Osorio, 1998) perceptual contrast values can be calculated. Hence, data from behavioural and modelling studies provide insight into which spectral receptors govern the decisions of pollinators and whether the spectral characteristics of the flowers and the pollinators’ vision are tuned.
Recent flower studies using anatomy and spectroscopy have elucidated the anatomical features that can cause various optical effects, and behavioural experiments with insect pollinators have tested the relative importance of different types of visual signals. However, knowledge on the behaviour and visual systems of pollinators is often considered independently from current knowledge on the optical properties of flowers.
In this review, we aim to link the studies on the optical properties of flowers with experimental studies on pollinator visual systems and behaviour. We discuss how the flower’s anatomy and pigments generate different components of the visual signal, and we discuss the current scientific evidence supporting each type of visual signal. We examine what is currently known about the achromatic aspects of bee pollinator perception, i.e. green contrast and brightness, as well as the chromatic aspects of floral colours, i.e. hue and saturation. Finally, we discuss several types of more uncommon visual cues that are due to specific anatomical properties, namely fluorescence, iridescence, gloss and polarization effects. For each visual cue we first consider the optical mechanism and subsequently its contribution to the overall visual signal of flowers and the current knowledge on its biological significance in natural conditions. Throughout the text we highlight promising avenues for future research. We will not discuss the principles of different vision models, as this subject has recently been reviewed elsewhere (Kemp et al., 2015; Renoult et al., 2017).
ACHROMATIC ASPECTS OF FLOWER COLORATION
Brightness
Brightness refers to the perceived intensity of a stimulus, independent of hue and saturation. The perceived brightness depends on adaptation processes in the visual system, and it is often considered to be a relative percept, because a stimulus is judged relative to its surroundings. Hence, a piece of white paper is perceived as white in both open daylight and indoors, even though there may be a couple of orders of intensity differences reflected from that same page in the different environments. It is therefore important to consider the perception of a pollinator when considering the light intensity reflected by a flower as its visual signal.
Brightness is a product of information processing by the brain, whereas intensity is a physical property of a stimulus (Table 1). In humans, brightness is a dimension of our colour sense because the primate brain has multiple pathways for chromatic and intensity signals, and the brain combines such signals to enable a combined precept (Clery et al., 2013). However, given the complex nature of visual information processing, we may not readily assume that insect pollinators such as bees also perceive brightness in the context of colour signalling. Stimulus brightness as potentially perceived by bees has been modelled as the sum of all photoreceptor excitations (Spaethe et al., 2001). The brightness contrast between a stimulus and its background is the difference between stimulus brightness and background brightness (Hempel de Ibarra et al., 2014). Brightness contrast can be positive or negative, i.e. a flower can have a higher or lower brightness than its background.
A flower’s reflectance (the fraction of incident light that is reflected) is determined by the type and amount of structures inside the petals. The absolute amount of backscattering by a flower can be measured with an integrating sphere (Vukusic and Stavenga, 2009; van der Kooi et al., 2016a). Apart from pigments that selectively absorb light at specific wavelength ranges, three main characteristics determine a flower’s reflectance: the refractive index difference between floral structures, the flower’s interior inhomogeneity and the flower’s thickness. Firstly, the refractive index difference between two structures determines the reflectance at their boundaries; an increase in refractive index difference causes a higher reflectance. In contrast to animals, where the tissue’s refractive indices have been studied for various species, such as bird feathers and butterfly wing scales (Leertouwer et al., 2011), there have been virtually no studies on the refractive indices of floral tissue. Although the refractive indices of media commonly found in flowers, e.g. water and cell walls (Stavenga and van der Kooi, 2016), are expected to be similar between species, we are not aware of any comparative studies on this subject. Secondly, a flower’s reflectance will increase when the amount of scattering structures per unit thickness increases (Fig. 3B). For example, flower areas with relatively uncompressed cells scatter less light than veined areas, where many cells are packed together (Kevan and Backhaus, 1998; Stavenga and van der Kooi, 2016). Thirdly, when the flower’s total thickness increases, the amount of scattering structures a propagating light wave encounters also increases, resulting in higher reflectance.
A comparative study covering 39 species from 23 plant families showed that the interior inhomogeneity and thickness of flowers varies greatly, but that the overall reflectance is rather similar among species (van der Kooi et al., 2016a). In the long-wavelength range, i.e. where modulation by pigments is negligible, the reflectance value – and thus the amount of backscattering – can be estimated. Flowers reflect between 20 % and 50 % of the incident light, which suggests that a reflectance in this range is sufficient for strong visual signalling to pollinators (van der Kooi et al., 2016a). In other words, a 20 % light reflectance allows for a sufficient overall contrast with the average leaf background. Given the great variation in interior inhomogeneity and thickness of flowers, it seems unlikely that physiological restrictions would thwart reflectance values above 50 %; rather, this suggests that further increases in the amount of light reflected will not significantly enhance the flower’s visibility for pollinators (van der Kooi et al., 2016a).
Evidence suggests that overall floral brightness is not likely to be a major cue for detection of colour signals from flowers by insect pollinators. Multiple behavioural experiments with bees, flies and wasps showed that these insects do not use brightness for object detection, at least in the presence of colour signals (Daumer, 1956; Backhaus et al., 1987; Chittka et al., 1992; Spaethe et al., 2001; Reser et al., 2012; Papiorek et al., 2013; Rohde et al., 2013). Early as well as more recent studies suggest that bees ignore intensity differences when foraging (Daumer, 1956; Backhaus et al., 1987; Chittka et al., 1992; reviewed by Kevan et al., 1996). Indeed, bees have great difficulty in detecting and learning to recognize targets that differ from their background only in brightness (Ng et al., 2018), and also flies and the diurnal hummingbird hawkmoth, Macroglossum stellatarum, (largely) ignore intensity differences and are more triggered by specific hues and/or overall chromatic contrast (Goyret and Kelber, 2012; Woodcock et al., 2014). To date, the number of species studied in this respect is fairly small, however, so this claim cannot be simply extrapolated to all insect pollinators.
Brightness can generally be considered an unlikely trait that drove flower signal evolution in habitats where bees are the main pollinators. Previously reported effects of pollinators selecting for flower brightness in the cornflower Centauria cyanus (Renoult et al., 2013) and the deceptive orchid Anacamptis morio (Sletvold et al., 2016) may be a result of the fact that the spectral signal intensity is often confounded with other components of the visual signal. Brightness differences are often difficult to disentangle from differences in green contrast, because flowers with a high overall reflectance reflect more light at long wavelength ranges, and may thus exhibit a higher green contrast to the background. Additional evidence suggesting brightness is not very important comes from the fact that intensity differences may be unreliable in nature. Brightness per se can be considered as an unreliable signal (Kelber et al., 2003), because it is determined both by the object and by illumination conditions, such as shadows, weather and time of day, and greatly differs between and during days.
Although brightness may not be very important for the detection of visual signals in most insect pollinators, at least some species can learn to use it as a stimulus. Behavioural experiments have shown that two diurnal Lepidoptera species are able to distinguish stimuli solely based on brightness contrast with the background, namely the hummingbird hawkmoth (Kelber, 2005) and a swallowtail butterfly (Kinoshita et al., 2012). In addition, the nocturnal moth Deilephila elpenor can more successfully distinguish bright than non-bright stimuli at low illumination conditions (Kelber et al., 2002), suggesting that for nocturnal insects brightness may be important. For nocturnally pollinated flowers, strong brightness between the flower and background may enhance conspicuousness, as colour vision becomes less reliable when illumination intensities are low (Endler, 1993; Cronin et al., 2014). In line with this hypothesis is the observation that many nocturnally pollinated flowers seem to be bright to the human eye (White et al., 1994), although this observation would benefit from further studies. Hence, under nocturnal conditions, plants with brighter flowers could be more conspicuous than their co-flowering conspecific neighbours.
In summary, evidence suggests that brightness is not a major visual signal for detection by diurnal pollinators, especially bees, but this may differ between species and habitats. We emphasize that there is a need for systematic comparative studies on the functional significance of brightness perception.
Green contrast
In addition to brightness contrast, many insects detect flowers from large distances by means of their green-receptor contrast. Green contrast is the contrast between a stimulus and its background mediated solely by the green photoreceptor (reviewed by Osorio and Vorobyev, 2005). Green contrast can be positive or negative, i.e. a flower can have a higher or a lower green reflectance than the background vegetation. For many insects, the sensitivity of the long-wavelength photoreceptor extends from the ultraviolet to long wavelengths (Fig. 2). The bimodal nature of the bees’ long-wavelength photoreceptor is due to the secondary sensitivity in the ultraviolet of nearly all photoreceptors (Fig. 2B) (Peitsch et al., 1992). The green photoreceptor provides a high signal-to-noise ratio compared to the other photoreceptors, so this receptor may have a particular role in signal detection (Vasas et al., 2017). Green contrast is an achromatic signal and thus partly similar to brightness contrast (i.e. high reflectance difference between flower and background yields a strong contrast), but for green contrast, modulation by floral pigments is important.
Green contrast can vary over a wide range between wild flowers (Papiorek et al., 2014; Vasas et al., 2017), but whether the variation is linked to the pollination system or plant life-history traits is unclear. Two life-history traits have been suggested to influence green contrast of flowers: floral display size and the plant’s habitat. Spaethe et al. (2001) showed that bumble bees rely more on green contrast when searching for small flowers, but more on colour contrast when searching for large flowers. This suggests that plants with small floral displays need to compensate for their reduced conspicuousness via stronger green contrast. Indeed, a comparative analysis of floral patterns and green contrast by Hempel de Ibarra and Vorobyev (2009) suggested that smaller flowers exhibit stronger green contrast. However, given these preliminary results are based on photographed flowers and not on spectral and anatomical measurements, this hypothesis needs further testing. In a comparative study on Israeli flora, it was found that desert plants exhibited weaker green contrast than plants growing in the Mediterranean region (Menzel et al., 1997). Interestingly, this was not due to a change in the desert flowers’ visual signal, but due to a relatively strongly coloured background of desert plants. Whether floral green contrast is less important for desert plants than for Mediterranean plants remains unknown. Finally, Ohashi et al. (2015) found that flowers that undergo a colour change (e.g. following pollination) generally retain their (high) green contrast. This presumably increases long-distance detection of the floral display as a whole, so as to increase visitation of flowers that have not yet lost their colour and require pollination.
Green contrast of flowers seems mostly important for long-distance detection by pollinators, and its relative importance may depend on the type of pollinator and habitat. Bees have difficulty in detecting flowers based solely on green contrast. In experiments with honey bees and bumble bees, green contrast as a sole factor results in rather poor flower detection, but combined green and chromatic contrast leads to very reliable flower detection, especially at greater distances (Giurfa et al., 1996; Dyer et al., 2008). There is a clear need for large-scale comparative studies on the degree of variation of green contrast between species, and to what extent this is determined by pollinator-mediated selection, phylogenetic ancestry or habitat.
CHROMATIC ASPECTS OF FLOWER COLORATION
Colour Contrast
Whereas green contrast involves only one photoreceptor class, colour contrast is the overall contrast between two visual stimuli mediated by all photoreceptor types involved in colour vision. Colour contrast and detection thresholds are often inferred using vision models for bee colour perception (Chittka, 1992; Vorobyev and Osorio, 1998). Colour contrast can represent the perceptual difference between the flower and background, between flowers or within flowers in the case of colour patterns. Differences in either hue or saturation can contribute to the colour contrast between two stimuli, but such factors may also be considered separately for what are the main driving factors for the evolution of specific flower spectral properties (see section on Hue and saturation). Floral pigments play a key role in the colour contrast of flowers, but because of the complex interplay of pigments and light-scattering structures, understanding the relative importance of the key elements may be difficult.
Colour contrast has been proven to be important at the plant community level, between flowers and their background as well as between different areas within single flowers. Several hypotheses predict the evolution of flower colours at the plant community level, with different predictions depending on the hypothesis. First, floral signals may have converged to some degree, for example when they are serviced by the same (generalist) pollinator (Schiestl and Johnson, 2013). This was found in an island community for plants pollinated by flies (Shrestha et al., 2016), and on the European mainland for plants that were rare in their community (Gumbert et al., 1999). Second, plants may maximize floral colour contrast in respect to other species blooming at the same time, to distinguish themselves from their co-flowering neighbours (Levin, 1985; McEwen and Vamosi, 2010; van der Kooi et al., 2016b). This was convincingly shown in Phlox drummondii that produces light blue flowers in most of its range, but in areas where it co-occurs with the light blue-flowered P. cuspidata it produces dark-red flowers (Levin, 1985), in order to reduce interspecies hybridization (Hopkins and Rausher, 2012).
In addition to contrasting with neighbouring flowers, colour contrast to the background and within-flower contrast improves detection and navigation by pollinators. Colour patterns are generally due to differences in pigmentation between flower areas, although in some cases the anatomy of differently coloured flower areas varies (Kay et al., 1981; van der Kooi et al., 2017). Behavioural choice tests confirmed the importance of colour contrast against the background for visual detection by bees, for both artificial stimuli (Giurfa et al., 1996; Spaethe et al., 2001; Morawetz et al., 2013) and real flowers (Dyer et al., 2007). A bumble bee’s decision to visit a flower depends both on the colour contrast between the central and peripheral part of the colour pattern, and on the direction of the colour contrast, i.e. on the colour of the centre versus that of the periphery. Many flowers have ultraviolet-absorbing centres and ultraviolet-reflecting peripheries (sometimes referred to as floral ‘bulls-eyes’). Such a pattern increases both the contrast with the background and that within the flower (Kevan and Backhaus, 1998). Ultraviolet-reflecting petal tips have high contrast with the green background (which has low ultraviolet reflectance; Fig. 2A), and at short range the bee will perceive a strong contrast between the ultraviolet-absorbing centre and the ultraviolet-reflecting periphery. In addition to providing high contrast, such colour patterns – in both the ultraviolet and the visible wavelength ranges – can constitute a gradient of increasing spectral purity towards the centre, which bumble bees prefer (Lunau, 1992). Nonetheless, as discussed above, green contrast also appears to be important for flower detection at a distance, and there seems to be a complex way in which these visual descriptors interact to influence bee target detection (Giurfa et al., 1996; Dyer et al., 2016b).
We conclude that colour contrast is important at different levels and in different contexts. For honey bees green contrast of the flower against the background is important for detection from longer distances and colour contrast is important from shorter distances, but other important pollinators such as bumble bees and stingless bees use colour and green contrast at similar distances (Table 2; Dyer et al., 2008; Wertlen et al., 2008). It will thus be of high value to evaluate the importance of chromatic and achromatic contrast in other important pollinators. We will now discuss the different aspects of colour contrast.
Table 2.
Behavioural studies with free flying bee species on the relative importance of hue and saturation, in conjunction with other aspects of the visual signal
| Species | Tested parameters | Stimuli | Main finding | Reference |
|---|---|---|---|---|
| Apis mellifera | Dominant wavelength | Interference filters | Strong preference for dominant wavelength parameter of stimuli | (Menzel, 1967) |
| Apis mellifera | Dominant wavelength, colour contrast, green contrast | Artificial coloured paper stimuli | Strong preference for dominant wavelength parameter of stimuli | (Giurfa et al., 1995) |
| Apis mellifera | Dominant wavelength, spectral purity, intensity, green contrast, colour contrast | Artificial printed colours with very small perceptual differences | Bees approached stimuli according to spectral purity difference as compared to the background | (Rohde et al., 2013) |
| Bombus terrestris | Dominant wavelength, spectral purity, intensity, colour contrast | Artificial stimuli were presented simultaneously and the choices by bees were recorded | Bees approached stimuli according to spectral purity difference as compared to the background | (Lunau, 1990) |
| Bombus terrestris | Dominant wavelength, spectral purity, intensity, green contrast, colour contrast | Artificial coloured paper stimuli | Choices by bees were principally mediated by hue | (Gumbert, 2000) |
| Bombus terrestris | Hue | Artificial colour stimuli and field- based testing with real flowers | Preference for hue observed in lab-based studies results in improved foraging efficiency on flowers at field sites | (Raine and Chittka, 2007) |
| Bombus terrestris | Dominant wavelength, spectral purity, intensity, green contrast, colour contrast | Artificial coloured paper with very small perceptual differences | Bees approached stimuli according to spectral purity difference as compared to the background | (Rohde et al., 2013) |
| Bombus terrestris | Hue, saturation, intensity, colour contrast, green contrast | Snapdragon (Antirrhinum majus) flowers with genetic modification of colour and wild type | Bees ignored intensity cues and preferred flowers of higher chromatic contrast | (Dyer et al., 2007) |
| Melipona mondury | Dominant wavelength, spectral purity, intensity, green contrast | Artificial mixtures of pigments | Strong preference for dominant wavelength parameter of stimuli | (Koethe et al., 2016) |
| Melipona quadrifasciata | Dominant wavelength, spectral purity, intensity, green contrast | Artificial mixtures of pigments | Strong preference for dominant wavelength parameter of stimuli | (Koethe et al., 2016) |
| Tetragonala carbonaria | Dominant wavelength, spectral purity, intensity, green contrast, colour contrast | Artificial coloured papers | Preference for spectral purity, but only when in interaction with green contrast. Preference also observed for dominant wavelength | (Dyer et al., 2016a) |
Hue and saturation
Colour contrast is determined by differences in hue and saturation between a stimulus and the background. Hue is generally described as a categorization of what humans consider ‘colour’, e.g. blue, yellow or red (Wyszecki and Stiles, 1982); however, for pollinators hue is typically quantified as an angle in a colour space, expressed in dominant wavelength (Chittka, 1992) (Table 1, Supplementary Data File S1). The saturation of a colour refers to its ‘pureness’, e.g. for human perception red is more saturated than pink. The saturation of a colour is determined by the slope of the reflectance curve at the inflection point(s), i.e. the wavelength range where the slope is maximal (Fig. 2). Very steep changes in the reflectance curve yield visual signals of high saturation. A decrease in slope yields a more uniform reflectance at different wavelengths, resulting in a greyer visual signal, i.e. a visual signal of lower saturation. These quantitative parameters can be determined using a colour space (Menzel and Shmida, 1993; Chittka et al., 1994; Vorobyev and Brandt, 1997; Kevan and Backhaus, 1998) (see File S1).
Floral pigments determine a flower’s colour and thus its hue and saturation. The type of pigment determines the hue, and the degree of filtering by pigments determines the flower’s saturation. The degree of absorption by pigments is due to both the amount and localization of pigment. Generally, increases in pigment concentration will lead to stronger spectral modulation of the reflected light (Fig. 3A). Deposition of pigment in specific floral layers can also dramatically change the absorption by pigments (Fig. 3C). When pigments occur in the outer (epidermal) layers of the flower, the light that is backscattered by the interior traverses the pigmented layer twice and thus is more modulated than when pigments are located throughout the flower (Stavenga and van der Kooi, 2016; van der Kooi et al., 2016a). Pigment deposition is probably phylogenetically constrained; anthocyanins generally occur in epidermal layers, whereas carotenoids are often more evenly distributed through the flower (reviewed by Mol et al., 1998; Grotewold, 2006).
Evidence from studies on the optical properties of flowers and behaviour of bees suggests that intermediate amounts of floral pigments yield the highest salience to pollinators. For flowers with a low amount of pigment, an increase in pigment concentration will increase the flower’s saturation. However, extreme increases in pigment concentration will yield a dull appearance and reduce the flower’s conspicuousness, because floral pigments absorb in a broad wavelength range (see figure 3 in van der Kooi et al., 2016a). This is in accordance with behavioural experiments with bees, which showed that stimuli with intermediate amounts of pigment yielded the strongest visual signal and were most easily detected by bees (Papiorek et al., 2013). In other words, there will be a certain amount of pigment necessary to have a sufficiently strong visual signal, but having more pigments is not always better.
Hue may be important because many insect species from different orders have innate or learned preferences for specific hues. Menzel (1967) showed that honey bees exhibit innate preferences for colours of short wavelengths, which was also found in Australian native stingless bees (Dyer et al., 2016a). It has been suggested that in honey bees, innate preferences for particular hues are more important than flower brightness, colour contrast and green contrast (Giurfa et al., 1995), although this probably depends on the experimental setup. Innate preferences for specific hues were also documented for flower-visiting flies, beetles and butterflies (Lunau and Maier, 1995; Johnson and Midgley, 2001; Woodcock et al., 2014; Kinoshita et al., 2017). For example, the diurnal hummingbird hawkmoth M. stellatarum has increased spectral sensitivity and innate preferences for blue light (Telles et al., 2014), and various species of hoverflies, including frequent flower-visitors such as Eristalis tenax and Episyrphus balteatus, have innate preferences for yellow (reviewed by Lunau, 2014). In an island environment where flies were the sole pollinators their visual systems appeared to impose strong selection on flower colours (Shrestha et al., 2016). In contrast to the wide-held belief that birds innately prefer red hues, experimental and field studies have found no results supporting that idea; preferential visits of birds to red flowers are probably the result of learning behaviour (reviewed by Lunau and Maier, 1995). At least bees and various species of Lepidoptera can learn to associate specific colours with a reward and so overcome their innate preferences (Lunau and Maier, 1995; Gumbert, 2000; Chittka and Raine, 2006; Goyret et al., 2008; Rohde et al., 2013). Flies are less likely to overcome their innate colour preferences due to limited learning capability. Whether a species strictly follows its innate colour preference during foraging can be relevant when certain plant species are rewarding at a specific moment and the pollinator can learn to associate a specific hue with a reward.
The importance of hue versus saturation differs strongly between pollinators. For five flower-visiting insect species (the Western honey bee, one bumble bee and three stingless bee species) the relative importance of hue versus saturation – sometimes in combination with other aspects of the visual signal – has been experimentally investigated in laboratory and field settings (Table 2). In these experiments, bees were presented with artificially coloured stimuli that systematically differed in one or a few aspects of the visual stimuli. By combining different types of hues with various degrees of saturation, the relative importance of both could be determined. Whereas for honey bees and bumble bees saturation seems (slightly) more important than hue, for two stingless bee species, Melipona mondura and M. quadrifasciata, innate preferences for a particular hue are more important than high saturation (Table 2). In these four species, the (short-distance) detection was unrelated to the degree of green contrast. Interestingly, Tetragonala carbonaria stingless bee individuals preferred increased saturation, but only when in combination with high green contrast (Dyer et al., 2016a). These bees also demonstrated a preference for hue (Table 2). Depending on the stimuli used, different aspects of a visual signal can be correlated. For example, saturation and colour contrast may be correlated (Gumbert, 2000), rendering it difficult to test the relevance of these different aspects in isolation. In addition, a pollinator’s perceptions and preferences may be driven by multiple factors to enable a decision, meaning that testing potential signals in isolation could yield incomplete answers. For example, the blue hue preference of honey bees can mask other information present in complex colour signals (Morawetz et al., 2013).
We conclude that chromatic contrast is important for plant–pollinator signalling, especially for short-distance discrimination. Hue and saturation are both potentially very important, but their relative importance seems to be species-specific. The great variation in preference for hue versus saturation between flower-visiting species opens perspectives for comparative studies between species. It would be particularly interesting to investigate whether the variation in hue and saturation found in natural populations of flowering plants can be linked to preferences by local pollinators.
OTHER TYPES OF VISUAL PHENOMENA SUGGESTED TO PLAY A ROLE IN VISUAL SIGNALLING
The surface properties of flowers can contribute to the flower’s reflection and/or modify the light that is backscattered by the interior. The shape of both the epidermal surface and the cuticle may contribute to the visual signal. For example, very flat epidermal surfaces can give flowers a glossy appearance (Parkin, 1928; Vignolini et al., 2012; Papiorek et al., 2014; van der Kooi et al., 2017). In order for surface reflections to be visible to pollinators under normal (daylight) conditions, the salience of the visual signal needs to be comparable to that of the visual signal generated by the petal interior. Comparing the relative conspicuousness of particular visual signals to the overall visual signal requires detailed optical and behavioural studies.
Structural coloration and iridescence
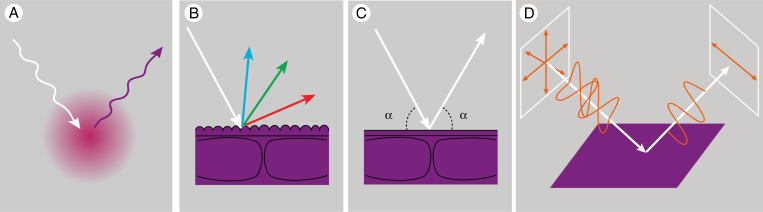
Structural colours arise in regularly ordered, nano-sized structures composed of materials with different refractive indices (Srinivasarao, 1999; Kinoshita, 2008), and are fairly common among animals (reviewed by Vukusic and Sambles, 2003). Typical for structural coloration is that it is highly directional and angle-dependent (i.e. iridescent); thus, the hue changes with angle of observation or illumination (Fig. 4).
Fig. 4.
Various types of visual signals are not due to backscattering by the flower’s interior. (A) Fluorescence is the emission of light by pigments that absorbed light, for example absorption of ultraviolet light leads to emission of long-wavelength light such as blue. (B) Periodically structured striations of the flower’s cuticle may cause incident light to be diffracted, yielding a visual signal that is dependent on the angle of illumination and observation, a phenomenon known as iridescence. (C) A very flat and smooth flower surface may cause specular reflection when the angle of illumination is identical to the angle of observation (denoted by α). Light that is reflected by the surface will not be modified by floral pigments inside the flower, so the gloss will generally be perceived as (achromatic) white. (D) Unpolarized light illuminating the flower becomes linearly horizontally polarized when it is reflected by the smooth surface of a flower; the degree of polarization depends on the angle of incidence. In glossy areas and under large angles the ratio of surface versus subsurface reflection – and thus the polarization effect – will be maximal.
Some plant species have petals with (quasi-regular) cuticular striations (Kay et al., 1981) that could potentially serve as a diffractive grating. Although several studies on communities of plants suggested the absence of any iridescence effects (Kay et al., 1981; Menzel and Shmida, 1993; Lee, 2007), more recently several species have been suggested to be iridescent (Whitney et al., 2009). However, detailed optical analyses narrowed the possible incidence of floral iridescence in wild species to only one variety of the flower-of-an-hour, Hibiscus trionum (Vignolini et al., 2015; van der Kooi et al., 2014, 2015). The virtual absence of iridescent wild flowers is due to the highly irregular periodicity of the petal’s striations. In many species the irregularity of the striation’s periodicity, combined with light backscattering by irregularities inside the petal, prevent iridescence effects being visible under natural conditions (van der Kooi et al., 2014).
Despite advances in our understanding of its mechanistic basis, it remains unknown whether iridescence plays a role in plant–pollinator signalling. The contribution of surface structures to the flower’s visual signal under natural conditions is virtually always very low, thereby limiting a role as signal to pollinators (van der Kooi et al., 2014, 2015; Lunau, 2016). Laboratory experiments showed that bees could be trained to associate highly iridescent artificial objects with a reward (Whitney et al., 2009, 2016; Moyroud et al., 2017); however, it remains unclear whether the bees were attracted by the changes in spectral purity (Lunau, 2016), by the change in hue (i.e. iridescence) or by just one hue, such as blue, green or yellow (Morehouse and Rutowski, 2009; van der Kooi et al., 2015; de Premorel et al., 2017). Moreover, the fact that bees can be trained to detect a particular stimulus should not be regarded as evidence for its biological relevance; bees can be trained to a suite of ecologically irrelevant stimuli, for example explosives and dynamite or different artistic painting styles (Rodacy et al., 2002; Wu et al., 2013; Avarguès-Weber and Giurfa, 2014; van der Kooi et al., 2015). Finally, iridescent stimuli have dynamically changing visual signals, which may hamper detection and the learning process (Menzel and Shmida, 1993; Pike, 2015; Kjernsmo et al., 2018). Thus, although very profound in the animal kingdom, iridescence is unlikely to play a major role in plant–pollinator signalling.
Gloss
Gloss (or specularity) is directional reflection of light at a surface. Unlike iridescence, gloss is wavelength-independent and reflection most strongly occurs under mirroring angles (Fig. 4). If the angle of observation or illumination changes to a non-mirroring angle, the observer will only perceive light scattered by the flower interior. In waxy flowers or flowers with very flat epidermises, the surface can act as a mirror, yielding a glossy appearance (Parkin, 1928; Galsterer et al., 1999; van der Kooi et al., 2014). Buttercups and related species (Ranunculus and Ficaria spp.) are plants with exceptionally glossy flowers (Parkin, 1928). Buttercup flowers have a very flat and smooth epidermal surface, and due to a thin air layer immediately below the epidermis (Vignolini et al., 2012), the epidermis acts as an optical thin film – similar to an oil layer on water or a soap bubble – causing a very glossy appearance (van der Kooi et al., 2017). Some other plant groups feature glossy flowers (e.g. Adonis as well as many succulent plant species), although in many species (e.g. Tulipa) this is due to non-ordered epidermal striations that yield an overall glossy appearance (see above, section on structural colour and iridescence). It remains unclear how widespread glossiness is throughout the plant kingdom.
To our knowledge, there is neither evidence supporting nor evidence rejecting the hypothesis that glossiness is a signal for pollinators. On the one hand, insects that approach the flower under an angle mirroring the sun may perceive the gloss as a bright flash, increasing the flower’s conspicuousness. On the other hand, the spectrum of the directionally reflected light may be colourless to pollinators, because the reflected light is not (or only weakly) modulated by pigments (Galsterer et al., 1999; van der Kooi et al., 2017). In buttercups, the gloss may enhance the long-distance visual signal, but for short distances, the overall yellow petal colour is probably important. Finally, flower surface properties may play a role in several non-visual functions, such as temperature regulation of floral organs, wettability of the flowers, and tactile cues or grip to pollinators (Kevan and Lane, 1985; Whitney et al., 2011; van der Kooi, 2016; van der Kooi et al., 2017).
In almost all plant species, for near perpendicular illumination the surface reflectance is very small (<5 %) and the visual signal thus is largely due to diffuse light reflection by the scattering structures inside the flower (Kay et al., 1981; Kevan and Backhaus, 1998; Lee, 2007; van der Kooi et al., 2014; Stavenga and van der Kooi, 2016). Many flower surfaces have cuticular microstructures and/or conical epidermal cells (Kay et al., 1981; Lee, 2007; Costa et al., 2017), prohibiting specular reflections (van der Kooi et al., 2014). In Antirrhinum snapdragons, these conical epidermal cells were suggested to focus incident light on vacuoles containing (anthocyanin) pigments, so as to increase the saturation of the purple coloration and hence the visibility to pollinators (e.g. Gorton and Vogelmann, 1996). However, the presence of cone-shaped epidermal cells does not change the visitation rate by bees (Dyer et al., 2007) or the spectral properties of the visual signal as perceived by bees (Papiorek et al., 2014). Given the highly irregular shape and orientation of the cones, an alternative, possibly more important role of the cones may be to scatter incident light to many angles, providing a matt visual signal that is visible from many directions (Wehner and Bernard, 1993; Lee, 2007; van der Kooi et al., 2014). Nonetheless, further studies examining the functional significance of floral epidermal cones would be very valuable, especially as many species of flower have cone-shaped epidermal cells (Kay et al., 1981; van der Kooi et al., 2014).
Polarization
Light is polarized when the light wave vibrations occur in a single plane. Sunlight is unpolarized and remains unpolarized when it is diffusely reflected by a rough surface, such as an irregularly shaped petal’s surface. However, oblique illumination of a flat and smooth petal surface can result in strongly polarized reflected light (Fig. 4). The degree to which surface-reflected polarized light can be observed depends on its intensity relative to that of the (unpolarized) light backscattered by the petal interior. If the surface reflectance dominates, as in specular reflection of a glossy flower, polarized light can provide a visual signal to pollinators.
Although polarization vision is a common ability in many insects (reviewed by Cronin et al., 2014), its significance in floral signalling may be expected to be small. A well-known case where polarization is used is that of bees that use skylight polarization as a navigational cue (Cronin et al., 2014). To detect polarized light independent of the intensity of the signal, bees use specialized photoreceptors, which are located in the dorsal part of the eye (Wehner et al., 1975). Polarized light reflected by flowers in front or below the foraging bee will not be perceived, as the distal and ventral parts of the bee’s eyes have photoreceptors that effectively abolish polarization effects (Wehner et al., 1975), so as to enhance chromatic detectability (Wehner and Bernard, 1993). In a laboratory experiment by Foster et al. (2014), honey bees could be trained to detect downward-facing stimuli with a strong polarization stimulus. The stimulus could only be detected when the bees approached it from below, i.e. when the dorsal part of the eyes faced the stimulus. The significance of polarization patterns in flowers under natural conditions is small, given the widespread occurrence of petal microstructures, such as striations and cones, which prevent a polarization pattern from occurring in virtually all species (Horváth et al., 2002). In addition, the variable approach angle of a foraging bee to different flowers in natural conditions would probably yield polarization as an unreliable cue for identifying conspecific flowers, and thus would not serve as useful information that was evolved for plant–pollinator signalling.
Fluorescence
Fluorescence is the property of materials to absorb light at a particular wavelength and to subsequently emit light of longer wavelengths (Fig. 4). When the emitted light is in the visible wavelength range, it can potentially yield a striking visual appearance. The occurrence of fluorescence is widespread, but the quantum efficiency (the ratio of photons absorbed to the number of photons emitted) of most natural pigments is so low that fluorescence as a visual signal is rare (reviewed by Marshall and Johnsen, 2017). For flowers, fluorescence signalling was suggested to occur in nectar (Thorp et al., 1975), petals (Gandía-Herrero et al., 2005a, b) and pollen (Mori et al., 2018). There is, however, no clear experimental evidence showing that plant fluorescence signalling increases the visibility to pollinators. As with most animals, fluorescence quantum efficiency of floral pigments is very low (~1 %), meaning that under natural conditions a fluorescence effect will be swamped by petal reflections (Kevan, 1976; Iriel and Lagorio, 2010a, b). Hence, fluorescence can currently be regarded as unimportant for visual signalling for pollinators.
CONCLUSIONS AND OUTLOOK
The plant kingdom offers a bewildering array of flower colours. Floral visual signals have different components (Table 1), each with different relevancy that may vary between environmental conditions and pollinator species. When discussing the importance of floral visual signals in the context of pollinator attraction, it is important to consider that a flower visit by a pollinator is a series of behavioural reactions that might be triggered by different signals. Some visual signals may be more important to detect flowers from long distances, whereas others are more important for reliable recognition at a close range. Navigation and foraging behaviour within the flower may subsequently be modulated by within-flower colour patterns (Kevan et al., 2001; Koski and Ashman, 2014).
Different aspects of the flower’s visual signals have different importance for detecting pollinators. Evidence suggests that signal intensity, perceived as brightness, plays no major role in plant–pollinator signalling, but this may differ between habitats and pollinator species. Green contrast perceived by the long-wavelength photoreceptor may play a role, especially in long-distance signalling for honey bees, and at shorter ranges chromatic contrast seems more important (Giurfa et al., 1996; Hempel de Ibarra et al., 2014). Chromatic contrast is determined by both the flower’s hue and spectral purity. The relative importance of hue and spectral purity – which has been tested for only a few bee species – remains largely unknown and is likely to be very species- and context-specific. From an evolutionary point of view, changes in the amount of pigment produced (and thus spectral purity) may evolve more rapidly than changes in the type of pigment produced (and thus hue), as it requires only quantitative changes in an already existing pigment synthesis pathway. We note that whereas molecular techniques have been used successfully to study the genetic basis of floral pigment synthesis (Rausher, 2008; Hopkins and Rausher, 2011; Bombarely et al., 2016; Sheehan et al., 2016; Du et al., 2018), we are much further behind in our knowledge on the genetic basis of other aspects of the visual signal (e.g. green contrast or gloss).
There are various other types of potential visual cues caused by the flower’s surface (gloss, iridescence and polarization effects) or interior (fluorescence), but these cues are in most cases not of biological significance. This is because such effects are only very locally visible and will thus be swamped by the petal’s backscattering. Moreover, visualizing these optical effects generally requires artificial (lighting) conditions, which are so specific that they are unlikely be found in the complex visual environment where pollinators forage. This means that this is not biologically meaningful in a way that would lead to evolution of a signal. Petal gloss may be an exception in this context because it is visible under natural conditions, although there are currently no behavioural experiments that have tested whether pollinators detect and respond to gloss. The important point from an evolutionary perspective is to understand which of these potential traits are evolved signals and which are incidental as being just part of the physical properties of the floral structure. The colours of rocks, for example, can be amazing to humans, but are clearly not evolved signals for any visual system.
Flower detection by pollinators does not depend solely on chromatic and achromatic properties, but also on the flower’s background (Kevan, 1972; Forrest and Thomson, 2009; Bukovac et al., 2017). It is known that colour discrimination and preference is strongly dependent on the background colour in at least bumble bees (Lunau et al., 1996), stingless bees (Koethe et al., 2016), honey bees (Hempel de Ibarra et al., 2000) and diurnal hawk moths (Kelber, 1997). Because in natural settings there is a wide range of backgrounds on which flowers may appear, it is likely that background coloration plays a role in the evolution of floral visual signals.
The anatomy of flowers and how this shapes the visual signal is relatively little studied, leaving many questions on the evolution of the optical properties of flowers unanswered. Formation of the visual signal of a flower is always an interaction of different aspects, including scattering structures, stratification, pigment localization and pigment concentration, which each have their own level of (dis)order (Figs 1 and 3). Recent studies have started to explore how these properties contribute to the visual signal (Stavenga and van der Kooi, 2016; van der Kooi et al., 2016a), but whether there are systematic differences between pollination guilds or taxonomic groups and phylogenetic constraints is virtually unstudied. By the same token, little is known about the degree of intraspecific variation in flower colour and its effect on visitation by pollinators in natural populations. Many studies on flower colour variability focused on discrete colour morphs, whilst in many species there is continuous variability in flower colour, with small but to pollinators probably visible changes in floral visual signals (e.g. Chittka et al., 1994; Frey, 2004; Rakosy et al., 2012; Narbona et al., 2018). Experiments with pollinators in response to real (Frey, 2004; Rakosy et al., 2012) and artificial flowers (Papiorek et al., 2013) suggest that pollinators respond to such (small) differences. Hence, it remains largely unknown to what extent small changes in hue, saturation or brightness correspond to changes in reproductive success, which is pivotal in understanding the effects on plant fitness.
When one studies the visual signals of flowers, one must always bear in mind the visual perception of the pollinators (Kelber and Osorio, 2010; Kemp et al., 2015). At present, for model pollinator bee species there is sufficient information on colour processing, including the spectral sensitivity of photoreceptors, opponent channels and psychophysics, to build sophisticated (vision) models; however, for the vast majority of pollinators, including birds, bats and flies, a lot more work needs to be done on if and how particular species process colour. In addition, we emphasize that there is a need for studies that bring laboratory-based knowledge to field experiments. An elegant example are the studies on sexually deceptive Ophrys orchids that used standardized field experiments with real pollinators in the plants’ natural habitat, to study the (natural variability of) floral visual signals and (its) their importance for pollination (Streinzer et al., 2009; Rakosy et al., 2012). Nonetheless we emphasize that detailed optical investigations of flowers are often best carried out in laboratory environments, with controlled (illumination) conditions and high standards, to meet the needs of reproducible colour science (sensu White et al., 2015). Indeed, whereas portable equipment such as a bifurcated probe may provide a reasonable overall impression of the reflectance spectrum, high-level precision on the absolute amount of backscattering (and thereby brightness) of flowers requires measurements with an integrating sphere (Vukusic and Stavenga, 2009; van der Kooi et al., 2016a).
We conclude that the field of flower coloration is rapidly expanding, aided by developments in different scientific domains. In this review we have considered visual signals of flowers mostly as advertising signals to pollinators; however, these signals can also function to deter antagonists, such as florivores or nectar robbers (Strauss and Whittall, 2006; Renoult et al., 2014; Papiorek et al., 2016). In addition, abiotic effects may impose selective pressures on visual effects of flowers and the underlying optical properties. For example, floral pigments can protect against (harmful) ultraviolet light (Koes et al., 1994) meaning that they may occur in certain floral layers for non-visual signalling purposes. We welcome further multi-disciplinary studies that combine physics, chemistry and evolutionary theory to understand the multiple functions and evolution of floral colours.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. File S1: Calculation of colour parameters. Table S1: Characteristics of the spectra shown in Fig. 2A using vision models.
ACKNOWLEDGEMENTS
CJvdK was financially supported by a VENI grant (number 016.Veni.181.025) financed by the Netherlands Organisation for Scientific Research (NWO). AGD acknowledges The Australian Research Council Grant DP160100161. Doekele Stavenga is gratefully acknowledged for comments on the manuscript. CJvdK conceived the idea, designed the figures and drafted the manuscript together with AGD and KL. All authors provided input for the manuscript and approved the final version.
LITERATURE CITED
- Avarguès-Weber A, Giurfa M. 2014. Cognitive components of color vision in honey bees: how conditioning variables modulate color learning and discrimination. Journal of Comparative Physiology A 200: 449–461. [DOI] [PubMed] [Google Scholar]
- Backhaus W, Menzel R, Kreißl S. 1987. Multidimensional scaling of color similarity in bees. Biological Cybernetics 56: 293–304. [Google Scholar]
- Bombarely A, Moser M, Amrad A, et al. 2016. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nature Plants 2: 16074. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annual Review of Entomology 46: 471–510. [DOI] [PubMed] [Google Scholar]
- Bukovac Z, Shrestha M, Garcia JE, Burd M, Dorin A, Dyer AG. 2017. Why background colour matters to bees and flowers. Journal of Comparative Physiology A 203: 369–380. [DOI] [PubMed] [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170: 533–543. [Google Scholar]
- Chittka L, Raine NE. 2006. Recognition of flowers by pollinators. Current Opinion in Plant Biology 9: 428–435. [DOI] [PubMed] [Google Scholar]
- Chittka L, Beier W, Hertel H, Steinmann E, Menzel R. 1992. Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in Hymenoptera. Journal of Comparative Physiology A 170: 545–563. [DOI] [PubMed] [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. 1994. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vision Research 34: 1489–1508. [DOI] [PubMed] [Google Scholar]
- Clery S, Bloj M, Harris JM. 2013. Interactions between luminance and color signals: effects on shape. Journal of Vision 13: 16–16. [DOI] [PubMed] [Google Scholar]
- Costa V, Pimentel R, Chagas M, Alves G, Castro C. 2017. Petal micromorphology and its relationship to pollination. Plant Biology 19: 115–122. [DOI] [PubMed] [Google Scholar]
- Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton: Princeton University Press. [Google Scholar]
- Daumer K. 1956. Reizmetrische Untersuchung des Farbensehens der Bienen. Zeitschrift für vergleichende Physiologie 38: 413–478. [Google Scholar]
- Daumer K. 1958. Blumenfarben, wie sie die Bienen sehen. Zeitschrift für vergleichende Physiologie 41: 49–110. [Google Scholar]
- de Premorel G, Giurfa M, Andraud C, Gomez D. 2017. Higher iridescent-to-pigment optical effect in flowers facilitates learning, memory and generalization in foraging bumblebees. Proceedings of the Royal Society B: Biological Sciences 284: 20171097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Lai L, Wang F, et al. 2018. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biology 20: 121–129. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Whitney HM, Arnold SE, Glover BJ, Chittka L. 2007. Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod-Plant Interactions 1: 45–55. [Google Scholar]
- Dyer AG, Spaethe J, Prack S. 2008. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. Journal of Comparative Physiology A 194: 617–627. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Boyd-Gerny S, Shrestha M, et al. 2016. a Innate colour preferences of the Australian native stingless bee Tetragonula carbonaria Sm. Journal of Comparative Physiology A 202: 603–613. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Streinzer M, Garcia J. 2016. b Flower detection and acuity of the Australian native stingless bee Tetragonula carbonaria Sm. Journal of Comparative Physiology A 202: 629–639. [DOI] [PubMed] [Google Scholar]
- Endler JA. 1993. The color of light in forests and its implications. Ecological Monographs 63: 2–27. [Google Scholar]
- Forrest J, Thomson JD. 2009. Background complexity affects colour preference in bumblebees. Naturwissenschaften 96: 921–925. [DOI] [PubMed] [Google Scholar]
- Foster JJ, Sharkey CR, Gaworska AV, Roberts NW, Whitney HM, Partridge JC. 2014. Bumblebees learn polarization patterns. Current Biology 24: 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey FM. 2004. Opposing natural selection from herbivores and pathogens may maintain floral color variation in Claytonia virginica (Portulacaceae). Evolution 58: 2426–2437. [DOI] [PubMed] [Google Scholar]
- Galsterer S, Musso M, Asenbaum A, Fürnkranz D. 1999. Reflectance measurements of glossy petals of Ranunculus lingua (Ranunculaceae) and of non-glossy petals of Heliopsis helianthoides (Asteraceae). Plant Biology 1: 670–678. [Google Scholar]
- Gandía-Herrero F, Escribano J, García-Carmona F. 2005. a Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 222: 586–593. [DOI] [PubMed] [Google Scholar]
- Gandía-Herrero F, García-Carmona F, Escribano J. 2005. b Botany: floral fluorescence effect. Nature 437: 334–334. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Nunez J, Chittka L, Menzel R. 1995. Colour preferences of flower-naive honeybees. Journal of Comparative Physiology A 177: 247–259. [Google Scholar]
- Giurfa M, Vorobyev M, Kevan P, Menzel R. 1996. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. Journal of Comparative Physiology A 178: 699–709. [Google Scholar]
- Gorton HL, Vogelmann TC. 1996. Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiology 112: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyret J, Kelber A. 2012. Chromatic signals control proboscis movements during hovering flight in the hummingbird hawkmoth Macroglossum stellatarum. PloS ONE 7: e34629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyret J, Pfaff M, Raguso RA, Kelber A. 2008. Why do Manduca sexta feed from white flowers? Innate and learnt colour preferences in a hawkmoth. Naturwissenschaften 95: 569–576. [DOI] [PubMed] [Google Scholar]
- Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology 57: 761–780. [DOI] [PubMed] [Google Scholar]
- Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology 48: 36–43. [Google Scholar]
- Gumbert A, Kunze J, Chittka L. 1999. Floral colour diversity in plant communities, bee colour space and a null model. Proceedings of the Royal Society B: Biological Sciences 266: 1711–1716. [Google Scholar]
- Hempel de Ibarra N, Vorobyev M. 2009. Flower patterns are adapted for detection by bees. Journal of Comparative Physiology A 195: 319–323. [DOI] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M, Brandt R, Giurfa M. 2000. Detection of bright and dim colours by honeybees. Journal of Experimental Biology 203: 3289–3298. [DOI] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M, Menzel R. 2014. Mechanisms, functions and ecology of colour vision in the honeybee. Journal of Comparative Physiology A 200: 411–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R, Rausher MD. 2011. Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii. Nature 469: 411–414. [DOI] [PubMed] [Google Scholar]
- Hopkins R, Rausher MD. 2012. Pollinator-mediated selection on flower color allele drives reinforcement. Science 335: 1090–1092. [DOI] [PubMed] [Google Scholar]
- Horváth G, Gál J, Labhart T, Wehner R. 2002. Does reflection polarization by plants influence colour perception in insects? Polarimetric measurements applied to a polarization-sensitive model retina of Papilio butterflies. Journal of Experimental Biology 205: 3281–3298. [DOI] [PubMed] [Google Scholar]
- Iriel A, Lagorio MG. 2010. a Implications of reflectance and fluorescence of Rhododendron indicum flowers in biosignaling. Photochemical & Photobiological Sciences 9: 342–348. [DOI] [PubMed] [Google Scholar]
- Iriel A, Lagorio MG. 2010. b Is the flower fluorescence relevant in biocommunication?Naturwissenschaften 97: 915–924. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Midgley JJ. 2001. Pollination by monkey beetles (Scarabaeidae: Hopliini): do color and dark centers of flowers influence alighting behavior?Environmental Entomology 30: 861–868. [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. 1981. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society 83: 57–83. [Google Scholar]
- Kelber A. 1997. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. Journal of Experimental Biology 200: 827–836. [DOI] [PubMed] [Google Scholar]
- Kelber A. 2005. Alternative use of chromatic and achromatic cues in a hawkmoth. Proceedings of the Royal Society B: Biological Sciences 272: 2143–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A, Osorio D. 2010. From spectral information to animal colour vision: experiments and concepts. Proceedings of the Royal Society B: Biological Sciences 277: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A, Balkenius A, Warrant EJ. 2002. Scotopic colour vision in nocturnal hawkmoths. Nature 419: 922–925. [DOI] [PubMed] [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. 2003. Animal colour vision–behavioural tests and physiological concepts. Biological Reviews 78: 81–118. [DOI] [PubMed] [Google Scholar]
- Kemp DJ, Herberstein ME, Fleishman LJ, et al. 2015. An integrative framework for the appraisal of coloration in nature. American Naturalist 185: 705–724. [DOI] [PubMed] [Google Scholar]
- Kevan PG. 1972. Floral colors in the high arctic with reference to insect–flower relations and pollination. Canadian Journal of Botany 50: 2289–2316. [Google Scholar]
- Kevan PG. 1976. Fluorescent nectar. Science 194: 341–342. [DOI] [PubMed] [Google Scholar]
- Kevan PG, Backhaus WGK. 1998. Color vision: ecology and evolution in making the best of the photic environment. In: Backhaus WGK, Kliegl R, Werner JS, eds. Color vision: perspectives from different disciplines. Berlin: De Gruyter. [Google Scholar]
- Kevan PG, Lane MA. 1985. Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Sciences USA 82: 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevan P, Giurfa M, Chittka L. 1996. Why are there so many and so few white flowers?Trends in Plant Science 1: 252–527. [Google Scholar]
- Kevan PG, Chittka L, Dyer AG. 2001. Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. Journal of Experimental Biology 204: 2571–2580. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Takahashi Y, Arikawa K. 2012. Simultaneous brightness contrast of foraging Papilio butterflies. Proceedings of the Royal Society of London B: Biological Sciences 279: 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Stewart FJ, Ômura H. 2017. Multisensory integration in Lepidoptera: insights into flower–visitor interactions. BioEssays 39. [DOI] [PubMed] [Google Scholar]
- Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific. [Google Scholar]
- Kjernsmo K, Hall JR, Doyle C, Khuzayim N, Cuthill IC, Scott-Samuel NE, Whitney HM. 2018. Iridescence impairs object recognition in bumblebees. Scientific Reports 8: 8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koes RE, Quattrocchio F, Mol JN. 1994. The flavonoid biosynthetic pathway in plants: function and evolution. BioEssays 16: 123–132. [Google Scholar]
- Koethe S, Bossems J, Dyer AG, Lunau K. 2016. Colour is more than hue: preferences for compiled colour traits in the stingless bees Melipona mondury and M. quadrifasciata. Journal of Comparative Physiology A 202: 615–627. [DOI] [PubMed] [Google Scholar]
- Koski MH, Ashman TL. 2014. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Functional Ecology 28: 868–877. [Google Scholar]
- Lee DW. 2007. Nature’s palette. The science of plant color. Chicago: University of Chicago Press. [Google Scholar]
- Leertouwer HL, Wilts BD, Stavenga DG. 2011. Refractive index and dispersion of butterfly chitin and bird keratin measured by polarizing interference microscopy. Optics Express 19: 24061–24066. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1985. Reproductive character displacement in Phlox. Evolution 39: 1275–1281. [DOI] [PubMed] [Google Scholar]
- Lunau K. 1990. Colour saturation triggers innate reactions to flower signals: flower dummy experiments with bumblebees. Journal of Comparative Physiology A 166: 827–834. [Google Scholar]
- Lunau K. 1992. A new interpretation of flower guide colouration: absorption of ultraviolet light enhances colour saturation. Plant Systematics and Evolution 183: 51–65. [Google Scholar]
- Lunau K. 2014. Visual ecology of flies with particular reference to colour vision and colour preferences. Journal of Comparative Physiology A 200: 497–512. [DOI] [PubMed] [Google Scholar]
- Lunau K. 2016. Flower color: How bumblebees handle colours with perceptually changing hues. Current Biology 26: R229–R231. [DOI] [PubMed] [Google Scholar]
- Lunau K, Maier E. 1995. Innate colour preferences of flower visitors. Journal of Comparative Physiology A 177: 1–19. [Google Scholar]
- Lunau K, Wacht S, Chittka L. 1996. Colour choices of naive bumble bees and their implications for colour perception. Journal of Comparative Physiology A 178: 477–489. [Google Scholar]
- Marshall J, Johnsen S. 2017. Fluorescence as a means of colour signal enhancement. Philosophical Transactions of the Royal Society B 372: 20160335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen JR, Vamosi JC. 2010. Floral colour versus phylogeny in structuring subalpine flowering communities. Proceedings of the Royal Society B: Biological Sciences 277: 2957–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. 1967. Untersuchungen zum Erlernen von Spektralfarben durch die Honigbiene (Apis mellifica). Journal of Comparative Physiology A 56: 22–62. [Google Scholar]
- Menzel R, Shmida A. 1993. The ecology of flower colours and the natural colour vision of insect pollinators: the Israeli flora as a study case. Biological Reviews 68: 81–120. [Google Scholar]
- Menzel R, Gumbert A, Kunze J, Shmida A, Vorobyev M. 1997. Pollinators’ strategies in finding flowers. Israel Journal of Plant Sciences 45: 141–156. [Google Scholar]
- Mol J, Grotewold E, Koes R. 1998. How genes paint flowers and seeds. Trends in Plant Science 3: 212–217. [Google Scholar]
- Morawetz L, Svoboda A, Spaethe J, Dyer AG. 2013. Blue colour preference in honeybees distracts visual attention for learning closed shapes. Journal of Comparative Physiology A 199: 817–827. [DOI] [PubMed] [Google Scholar]
- Morehouse NI, Rutowski RL. 2009. Comment on “Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators”. Science 325: 1072. [DOI] [PubMed] [Google Scholar]
- Mori S, Fukui H, Oishi M, et al. 2018. Biocommunication between plants and pollinating insects through fluorescence of pollen and anthers. Journal of Chemical Ecology 44: 591–600. [DOI] [PubMed] [Google Scholar]
- Moyroud E, Wenzel T, Middleton R, et al. 2017. Disorder in convergent floral nanostructures enhances signalling to bees. Nature 550: 469–474. [DOI] [PubMed] [Google Scholar]
- Narbona E, Wang H, Ortiz P, Arista M, Imbert E. 2018. Flower colour polymorphism in the Mediterranean Basin: occurrence, maintenance and implications for speciation. Plant Biology 20: 8–20. [DOI] [PubMed] [Google Scholar]
- Ng L, Garcia JE, Dyer AG. 2018. Why colour is complex: evidence that bees perceive neither brightness nor green contrast in colour signal processing. Facets. In Press. doi:10.1139/facets-2017-0116. [Google Scholar]
- Ohashi K, Makino TT, Arikawa K. 2015. Floral colour change in the eyes of pollinators: testing possible constraints and correlated evolution. Functional Ecology 29: 1144–1155. [Google Scholar]
- Osorio D, Vorobyev M. 2005. Photoreceptor sectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proceedings of the Royal Society of London B: Biological Sciences 272: 1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiorek S, Rohde K, Lunau K. 2013. Bees’ subtle colour preferences: how bees respond to small changes in pigment concentration. Naturwissenschaften 100: 633–643. [DOI] [PubMed] [Google Scholar]
- Papiorek S, Junker RR, Lunau K. 2014. Gloss, colour and grip: multifunctional epidermal cell shapes in bee-and bird-pollinated flowers. PloS ONE 9: e112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiorek S, Junker RR, Alves‐dos‐Santos I, et al. 2016. Bees, birds and yellow flowers: pollinator‐dependent convergent evolution of UV patterns. Plant Biology 18: 46–55. [DOI] [PubMed] [Google Scholar]
- Parkin J. 1928. The glossy petals of Ranunculus. Annals of Botany 42: 739–755. [Google Scholar]
- Peitsch D, Fietz A, Hertel H, Desouza J, Ventura DF, Menzel R. 1992. The spectral input systems of hymenopteran insects and their receptor-based color-vision. Journal of Comparative Physiology A 170: 23–40. [DOI] [PubMed] [Google Scholar]
- Pike TW. 2015. Interference coloration as an anti-predator defence. Biology Letters 11: 20150159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine NE, Chittka L. 2007. The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. PLoS ONE 2: e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakosy D, Streinzer M, Paulus HF, Spaethe J. 2012. Floral visual signal increases reproductive success in a sexually deceptive orchid. Arthropod–Plant Interactions 6: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD. 2008. Evolutionary transitions in floral color. International Journal of Plant Sciences 169: 7–21. [Google Scholar]
- Renoult JP, Thomann M, Schaefer HM, Cheptou PO. 2013. Selection on quantitative colour variation in Centaurea cyanus: the role of the pollinator’s visual system. Journal of Evolutionary Biology 26: 2415–2427. [DOI] [PubMed] [Google Scholar]
- Renoult JP, Valido A, Jordano P, Schaefer HM. 2014. Adaptation of flower and fruit colours to multiple, distinct mutualists. New Phytologist 201: 678–686. [DOI] [PubMed] [Google Scholar]
- Renoult JP, Kelber A, Schaefer HM. 2017. Colour spaces in ecology and evolutionary biology. Biological Reviews 92: 292–315. [DOI] [PubMed] [Google Scholar]
- Reser DH, Witharanage RW, Rosa MG, Dyer AG. 2012. Honeybees (Apis mellifera) learn color discriminations via differential conditioning independent of long wavelength (green) photoreceptor modulation. PloS ONE 7: e48577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacy PJ, Bender S, Bromenshenk J, Henderson C, Bender G. 2002. Training and deployment of honeybees to detect explosives and other agents of harm. Proceedings SPIE 4742: 474–482. [Google Scholar]
- Rohde K, Papiorek S, Lunau K. 2013. Bumblebees (Bombus terrestris) and honeybees (Apis mellifera) prefer similar colours of higher spectral purity over trained colours. Journal of Comparative Physiology A 199: 197–210. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology and Evolution 28: 307–315. [DOI] [PubMed] [Google Scholar]
- Sheehan H, Moser M, Klahre U, et al. 2016. MYB-FL controls gain and loss of floral UV absorbance, a key trait affecting pollinator preference and reproductive isolation. Nature Genetics 48: 159. [DOI] [PubMed] [Google Scholar]
- Shrestha M, Lunau K, Dorin A, et al. 2016. Floral colours in a world without birds and bees: the plants of Macquarie Island. Plant Biology 18: 842–850. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Trunschke J, Smit M, Verbeek J, Ågren J. 2016. Strong pollinator‐mediated selection for increased flower brightness and contrast in a deceptive orchid. Evolution 70: 716–724. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proceedings of the National Academy of Sciences USA 98: 3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasarao M. 1999. Nano-optics in the biological world: beetles, butterflies, birds, and moths. Chemical Reviews 99: 1935–1962. [DOI] [PubMed] [Google Scholar]
- Stavenga DG, van der Kooi CJ. 2016. Coloration of the Chilean Bellflower, Nolana paradoxa, interpreted with a scattering and absorbing layer stack model. Planta 243: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SY, Whittall JB. 2006. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press. [Google Scholar]
- Streinzer M, Paulus HF, Spaethe J. 2009. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. Journal of Experimental Biology 212: 1365–1370. [DOI] [PubMed] [Google Scholar]
- Telles FJ, Lind O, Henze MJ, Rodríguez-Gironés MA, Goyret J, Kelber A. 2014. Out of the blue: the spectral sensitivity of hummingbird hawkmoths. Journal of Comparative Physiology A 200: 537–546. [DOI] [PubMed] [Google Scholar]
- Thorp RW, Briggs DL, Estes JR, Erickson EH. 1975. Nectar fluorescence under ultraviolet irradiation. Science 189: 476–478. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ. 2016. Plant biology: flower orientation, temperature regulation and pollinator attraction. Current Biology 26: R1143–R1145. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Wilts BD, Leertouwer HL, Staal M, Elzenga JTM, Stavenga DG. 2014. Iridescent flowers? Contribution of surface structures to optical signaling. New Phytologist 203: 667–673. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Dyer AG, Stavenga DG. 2015. Is floral iridescence a biologically relevant cue in plant-pollinator signaling?New Phytologist 205: 18–20. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Staal M, Stavenga DG. 2016. a How to colour a flower: on the optical principles of flower coloration. Proceedings of the Royal Society B: Biological Sciences 283: 20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Pen I, Staal M, Stavenga DG, Elzenga JTM. 2016. b Competition for pollinators and intra‐communal spectral dissimilarity of flowers. Plant Biology 18: 56–62. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Dijksterhuis J, Stavenga DG. 2017. Functional optics of glossy buttercup flowers. Journal of the Royal Society Interface 17: 20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasas V, Hanley D, Kevan PG, Chittka L. 2017. Multispectral images of flowers reveal the adaptive significance of using long-wavelength-sensitive receptors for edge detection in bees. Journal of Comparative Physiology A 203: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignolini S, Thomas MM, Kolle M, et al. 2012. Directional scattering from the glossy flower of Ranunculus: how the buttercup lights up your chin. Journal of the Royal Society, Interface 9: 1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignolini S, Moyroud E, Hingant T, et al. 2015. The flower of Hibiscus trionum is both visibly and measurably iridescent. New Phytologist 205: 97–101. [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Brandt R. 1997. How do insect pollinators discriminate colors?Israel Journal of Plant Sciences 45: 103–113. [Google Scholar]
- Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proceedings of the Royal Society of London B 265: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424: 852–855. [DOI] [PubMed] [Google Scholar]
- Vukusic P, Stavenga DG. 2009. Physical methods for investigating structural colours in biological systems. Journal of the Royal Society, Interface 6: S133–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R, Bernard GD. 1993. Photoreceptor twist: a solution to the false-color problem. Proceedings of the National Academy of Sciences USA 90: 4132–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R, Bernard GD, Geiger E. 1975. Twisted and non-twisted rhabdoms and their significance for polarization detection in the bee. Journal of Comparative Physiology A 104: 225–245. [Google Scholar]
- Wertlen AM, Niggebrügge C, Vorobyev M, de Ibarra NH. 2008. Detection of patches of coloured discs by bees. Journal of Experimental Biology 211: 2101–2104. [DOI] [PubMed] [Google Scholar]
- White RH, Stevenson RD, Bennett RR, Cutler DE, Haber WA. 1994. Wavelength discrimination and the role of ultraviolet vision in the feeding behavior of hawkmoths. Biotropica 26: 427–435. [Google Scholar]
- White TE, Dalrymple RL, Noble DW, Zurek DB, Umbers KD. 2015. Reproducible research in the study of biological coloration. Animal Behaviour 106: 1e57. [Google Scholar]
- Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. 2009. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323: 130–133. [DOI] [PubMed] [Google Scholar]
- Whitney HM, Bennett KMV, Dorling M, et al. 2011. Why do so many petals have conical epidermal cells?Annals of Botany 108: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney HM, Reed A, Rands SA, Chittka L, Glover BJ. 2016. Flower iridescence increases object detection in the insect visual system without compromising object identity. Current Biology 26: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock TS, Larson BM, Kevan PG, Inouye DW, Lunau K. 2014. Flies and flowers II: Floral attractants and rewards. Journal of Pollination Ecology 12: 63–94. [Google Scholar]
- Wu W, Moreno AM, Tangen JM, Reinhard J. 2013. Honeybees can discriminate between Monet and Picasso paintings. Journal of Comparative Physiology A 199: 45–55. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. 1982. Color science. New York: Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.