Abstract
Background and Aims
Selection exerted by pollinators on flowers is predicted to occur along two distinct axes. While pollinator attraction to flowers is governed by pollinator preferences, pollen transfer efficiency is mediated by the mechanical fit of pollinators to flower morphology. Although pollinator attraction in sexually deceptive orchids is typically underpinned by floral odour, morphological traits are expected to play a vital role in mechanical fit during floral contact with pollinators.
Methods
Here we utilize a comprehensive and novel procedure to test for pollinator-mediated selection through mechanical fit with the flower labellum in the orchid Chiloglottis trapeziformis. This approach combines detailed pollinator observations related to plant reproductive fitness with complementary experimental manipulation and phenotypic selection analysis.
Key Results
Experiments with virgin flowers revealed that pollen removal occurs only during vigorous pseudocopulation. This behaviour involves male wasps that grasp the insectiform callus structure on the labellum while probing the labellum tip in a forward orientation. Both orientation and duration of pseudocopulation were significant predictors of pollen removal, confirming a direct relationship between pollinator behaviour and plant fitness. Controlled floral manipulation that either shortened or elongated the distance between the callus and the labellum tip detected no change in pollinator attraction. The duration of pseudocopulation, however, was significantly reduced on flowers with shortened or elongated callus–tip distances, consistent with stabilizing selection. Phenotypic selection analysis confirmed this prediction in natural populations by uncovering evidence for stabilizing selection on the distance between the callus and the labellum tip.
Conclusions
Our experimental manipulations and selection analysis in natural populations thus demonstrate stabilizing selection on the distance from the callus to the labellum tip, and illustrate the utility of employing multiple approaches to confirm selection exerted by pollinators on floral form.
Keywords: Chiloglottis trapeziformis, floral manipulation, insect behaviour, mechanical fit, mimicry, pollination, pollinator-mediated selection, pseudocopulation, sexual deception, stabilizing selection
INTRODUCTION
Pollinators play a crucial role in floral evolution and diversification (Grimaldi, 1999). Evidence for pollinator-mediated selection on floral form has been reported across the angiosperms (Harder and Johnson, 2009), and can be broadly characterized into two main types. Firstly, selection on attractive floral traits can be exerted via the sensory system of pollinators in terms of their ability to detect and prefer a given floral colour (Schemske and Bradshaw, 1999; Newman et al., 2012), floral scent (Shuttleworth and Johnson, 2010; van der Niet et al., 2011) and floral size (Galen, 1989; Schemske and Agren, 1995). Secondly, pollinator morphology can exert strong selection on floral structures via mechanical fit, as reported for flower tubes (Anderson and Johnson, 2008; Pauw et al., 2009) and flower throat length (Cosacov et al., 2014). Although not expected to affect pollinator attraction, mechanical fit between pollinator and floral morphology promotes plant fitness by enhancing the frequency and efficiency of pollen transfer (Newman et al., 2015), making it a regular target of pollinator-mediated selection in specialized plants (Rosas-Guerrero et al., 2011).
Some of the most compelling evidence for the importance of pollinator preferences comes from highly specialized, rewardless plants that mimic sympatric flowers. In these deceptive mimics, flower colour has repeatedly been shown to be a key trait under strong selection by pollinators (Jersáková et al., 2012; Newman et al., 2012). Extensive support for pollinator-mediated selection on floral scent comes from another group of deceptive plants that exclusively attract male pollinators by mimicking female insects. These sexually deceptive (SD) plants are predominantly found in the Orchidaceae (Bohman et al., 2016). Chemical investigations in the orchid genera Ophrys (Schiestl et al., 1999), Chiloglottis (Schiestl et al., 2003; Peakall et al., 2010), Drakaea (Bohman et al., 2014) and Caladenia (Bohman et al., 2017; Xu et al., 2017) confirm that pollinator attraction is achieved with the same floral compound(s) as found in the sex pheromone of the pollinator’s female.
Although floral scent mimicry is a key factor underpinning pollinator attraction and speciation in SD orchids (Peakall et al., 2010; Ayasse et al., 2011; Peakall and Whitehead, 2014; Bohman et al., 2016), visual and morphological cues are likely also important in sexual mimicry (Kullenberg, 1961; Peakall, 1990; Gaskett, 2011). Comparable experimental evidence for this hypothesis, however, is largely lacking. For example, a phenotypic selection analysis on the South American orchid Geoblasta pennicillata found no evidence of increased fitness for flowers with labella that more accurately mimic the shape of its pollinator’s female (Benitez-Vieyra et al., 2009). Similarly, an analysis of flower shape across four pollinator-sharing Cryptostylis species in Australia (Gaskett, 2012) failed to match orchid shapes with the female insects they putatively mimic. Size and colour matches between female insects and mimicking floral structures, however, have been found in closely related Chiloglottis orchids (de Jager and Peakall, 2016).
The paucity of experimental evidence for morphological mimicry in SD orchids may suggest limited pollinator-mediated selection via pollinator preference on floral traits. In contrast, pollinator-mediated selection via mechanical fit to pollinator morphology is expected in these systems, because SD orchids are typically pollinated by a single pollinator species (Peakall et al., 2010; Bohman et al., 2016; Phillips and Peakall, 2017). The evolution of a tight match between an SD orchid’s flower morphology and the size and shape of its male pollinator, which governs pollen transfer, is thus expected. Surprisingly few studies on SD orchids have investigated mechanical fit with male pollinators. A correlation between labellum length and male pollinator body length in Ophrys has previously been reported (Paulus, 2006) for species where males orientate in the forward position (head pollination), as well as the reverse position (abdominal pollination). A recent experimental study in Ophrys leochroma also documented a reduction in the rate of attempted copulation (pseudocopulation) by pollinators when floral parts that males usually grip during visits are removed (Rakosy et al., 2017), indicating that mechanical fit with the labellum is likely important in governing pollinator behaviour in SD orchids.
Sexually deceptive Chiloglottis in Australia, which are pollinated by male thynnine wasps, are prime candidates for experimental investigations of pollinator-mediated selection. Pollinators in this system readily attempt to mate with featureless, plastic beads spiked with synthetic copies of the relevant flower’s mimetic attractive compounds (Schiestl et al., 2003; Peakall et al., 2010; Peakall and Whitehead, 2014). Because pollinators are sufficiently deceived by olfactory traits, we predict that variation in flower morphology within and among Chiloglottis species is under stronger pollinator-mediated selection for mechanical fit than for female mimicry. Nonetheless, experimental evidence for mimicry has recently been found in two morphologically distinct Chiloglottis species that employ the same attractive mimetic compound (chiloglottone 1) to attract their primary wasp pollinators (de Jager and Peakall, 2016). These pollinators exhibit contrasting orientations on the flowers (forward versus reverse) during pseudocopulation that match the contrasting floral morphologies of the two orchids. This allowed a priori predictions of trait matches between female insects and the floral traits that mimic them, which were confirmed in both species, but more pronounced in Chiloglottis trapeziformis (de Jager and Peakall, 2016).
Chiloglottis trapeziformis exhibits a small diamond-shaped labellum with a dark insectiform callus structure formed by the fusion of calli that the pollinator grips during pseudocopulation (Fig. 1). This process involves abdominal probing of the distal labellum edge, resulting in the deposition of pollinia on the dorsal thorax while the pollinator is in the forward orientation (Peakall et al., 2010). When the labellum is experimentally shortened, the duration of pseudocopulation is significantly reduced (de Jager and Peakall, 2016). This reduction will likely decrease pollinia removal and deposition, which is typically low in this and many other SD orchids (Weinstein et al., 2016). These findings indicate the potential for strong pollinator-mediated selection in C. trapeziformis. In this paper, we comprehensively investigate the hypothesis that pollinators exert selection on C. trapeziformis via mechanical fit with its labellum by combining novel pollinator observations, experimental floral manipulation and phenotypic selection analysis in natural populations.
Fig. 1.
The male pollinator, Neozeleboria cryptoides, exhibiting forward orientation during pseudocopulation on the labellum of Chiloglottis trapeziformis. (A) Pollinators typically first contact the stigma, indicated by the white arrow, before (B) dislodging a pollinium. Scale bar = 10 mm. Photographs: Rod Peakall.
MATERIALS AND METHODS
For all experiments, fresh flowers were collected from Black Mountain Creek (35°16′37.6″ S, 149°6′6.9″ E), Mulloon Farm (35°14′23.9″ S, 149°36′35.0″ E) and the Australian Botanical Gardens (35°16′36.14″ S, 149°6′35.67″ E).
Linking pollinator behaviour and plant fitness
To confirm that pseudocopulation is a robust proxy for pollination success, we secured virgin Chiloglottis trapeziformis flowers to wooden skewers 15 cm above the ground, before exposing them to the pollinator community at Molonglo Gorge (35°16′30.12″ S, 149°5′19.08″ E) during 2015. Chiloglottis trapeziformis does not occur at this site, ensuring sufficient pollinator activity for experimentation, since areas with resident C. trapeziformis often have low visitation due to learned avoidance by pollinators (Whitehead and Peakall, 2013). We observed the behaviour of all visitors in the field, and recorded the orientation and duration of mating attempts by pseudocopulating Neozeleboria cryptoides male wasps on C. trapeziformis flowers with a stopwatch. Orientation was scored as the position the pollinator was in when the pollinium became attached to its body, because males exhibiting pseudocopulation for very long periods may move around on the labellum. For every visit, we also recorded whether a pollinium was removed, noting the precise placement of the pollinium on the pollinator’s body.
Although it would be ideal to record the above data for pollen deposition as well, this is extremely difficult under experimental and natural conditions for two reasons: the rapid decline in response to experimentally presented flowers at a given location (Whitehead and Peakall, 2013) and the extremely low incidence of resident males carrying Chiloglottis pollinia (Bower, 1996; M. L. de Jager, pers. obs.). Nevertheless, pollinator visits resulting in pollinia removal are also expected to contribute to pollen deposition, if the visiting male is carrying Chiloglottis pollinia. This is because in C. trapeziformis the stigma is located directly below the anther (Fig. 1) and contact with the stigma normally occurs before contact with the anther during pseudocopulation. Indeed, as in other Australian orchids whose pollinia lack a connecting stalk and viscidium, such as the sexually deceptive Leporella fimbriata, pollinator contact with the stigma results in the transfer of stigmatic secretions to the insect’s thorax that act as a glue to assist pollinia removal and attachment (Peakall et al., 1987, 1990). Therefore, we treat pollinia removal as a reasonable proxy for pollen deposition. We employed a generalized linear model with a binomial distribution and logit link function to model the effects of orientation (forward versus side), duration of pseudocopulation, and their interaction on pollinia removal.
Linking floral manipulation and pollinator-mediated selection
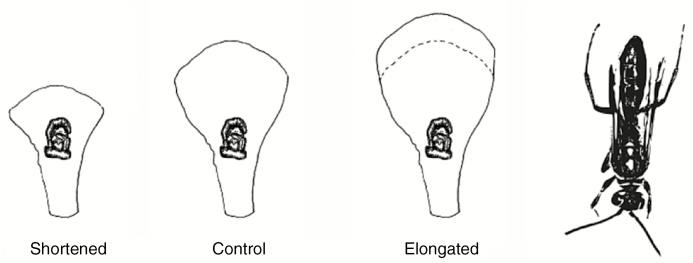
A key trait proven to be mechanically important during the interaction between C. trapeziformis and its pollinator is the distance between the callus and labellum tip (de Jager and Peakall, 2016). Directly manipulating this distance by moving the callus is not possible, because the callus is the site where the pollinator-attracting olfactory compound chiloglottone 1 is produced in C. trapeziformis (Falara et al., 2013). Instead, we can manipulate the labellum length, which automatically shortens or elongates the callus–tip distance. These two traits are strongly correlated in natural populations (Supplementary Data Table S1). To explore the effect of shortening the labellum on pollinator behaviour, de Jager and Peakall (2016) cut off a distal portion of the labellum just posterior to the callus, which yielded manipulated flowers with a labellum length of 5.0–5.5 mm (Fig. 2).
Fig. 2.
Labellum manipulations in C. trapeziformis to produce shortened and elongated floral morphologies (see Materials and methods for protocol). The pollinator N. cryptoides is illustrated for relative scale.
To elongate the labellum of C. trapeziformis beyond its natural range, we attached the distal portion of a donor flower’s labellum to the abaxial side of the recipient flower’s labellum with a thin layer of odourless instant-drying superglue. Each donor labellum was selected to match the recipient labellum in colour, as this trait can vary among individuals within populations. Any experimentally elongated flowers that were not properly secured or aligned were discarded. This procedure produced manipulated flowers with a labellum length of 10.0–10.5 mm. In contrast, the mean labellum length of natural C. trapeziformis flowers is 7.3 ± 0.5 mm (n = 16). The mean length of its pollinator, N. cryptoides, by comparison is 9.7 ± 0.9 mm (n = 16). Each shortened or elongated flower was paired with a control flower separated by 4 cm and presented 15 cm above the ground in water-filled vials. To restrict the potential effects of volatile release due to flower cutting, control flowers in the shortened experiment had a similar-sized portion of their dorsal sepal removed. Similarly, control flowers in the elongation experiment received an equivalent band of superglue on the abaxial side of its labellum to control for any potential scent effects of glue application.
Because C. trapeziformis flowers quickly wilt once pollinated, all control and manipulated flowers were emasculated. Experimental flowers were used within 2 h of manipulation to avoid possible wilting due to cutting or glue application. Manipulation experiments were conducted during 2013 (shortened; de Jager and Peakall, 2016) and 2015 (elongated; this study) at a site on Black Mountain where no C. trapeziformis occurs. Responding N. cryptoides male wasps were scored as approaching, landing or pseudocopulating. For pseudocopulating males, we also recorded the orientation and duration of pseudocopulation. For each manipulation, we conducted ten replicate experiments. Each experiment comprised four trials of 3 min each. We did not repeat trials at the same location on a given day, and considered pollinator visits independent due to the very low probability of resampling N. cryptoides individuals on the same day (Whitehead and Peakall, 2013).
To test whether elongating or shortening the labellum, and consequently the callus–tip distance, impacts pollinator behaviour, we first compared the number of approaches, landings and pseudocopulations between control and manipulated flowers with χ2 tests. These mutually exclusive behavioural categories were analysed separately for the elongation and shortening experiments. To test whether the duration of pseudocopulation differed between control and manipulated flowers, we used Mann–Whitney U tests. If the callus–tip distance is under stabilizing selection, we expect manipulated flowers to receive fewer or shorter pseudocopulations relative to control flowers.
Pollinator-mediated selection analysis
Flowers from Black Mountain Creek (n = 71) and Mulloon Farm (n = 50) were collected during the 2013–15 flowering seasons to assess the extent of pollinator-mediated selection in C. trapeziformis. We randomly picked flowers at least 5 m apart over multiple visits, as most clones in Chiloglottis tend to be smaller than 5 m in size (Whitehead et al., 2015). As these are both large populations (>200 m2, containing many hundreds of flowers), the likelihood of pseudoreplicating clones is low. We never picked recently opened flowers (identified by their vivid, bright yellow pollinia and light green flower colour) to ensure opportunity for pollinator visitation, and only selected flowers with receptive, wet stigmas to ensure that pollinia removal and deposition was possible. We picked flowers on or after the second day of good weather, because the pollinator, N. cryptoides, is mainly active on warm days (>18 °C) without strong wind. Floral traits were measured with digital callipers on the day of picking, and we gave flowers a binary score of 0 (no evidence of either pollinia removal or deposition) or 1 (evidence of pollinia removal or deposition). We selected this approach to explore the floral traits affecting potential pollination (male and/or female fitness), as the rates of pollen deposition were too low for independent statistical analyses. Given the proximity of anthers to the stigma in C. trapeziformis and the fact that males typically contact both reproductive parts during pseudocopulation, we deemed this approach justified.
Seven standardized floral traits (mean 0, s.d. 1) relating to labellum and callus morphology (callus–tip distance, callus length, labellum length, callus width, labellum width, callus height and callus–pollinia distance) were used as predictor variables in multivariate regression (Parachnowitsch and Kessler, 2010). This method employs partial regression coefficients to identify the strength of directional selection (β) on a given trait, independently of variation in other traits (Lande and Arnold, 1983). We also investigated stabilizing and disruptive selection by estimating quadratic (γ) coefficients with multiple linear regression. To estimate parameters, we multiplied the quadratic coefficients by 2, because linear models underestimate stabilizing/disruptive selection gradients by half (Stinchcombe et al., 2008). We calculated linear selection gradients from models including only linear terms, but quadratic gradients from models including both linear and quadratic terms. We did not estimate correlational selection gradients, because models including interactions between all floral traits contained too many parameters for algorithms to converge. Significance was assessed with generalized linear models with a binomial distribution and logit link, as binary response variables tend to have non-normally distributed errors that may violate parametric tests, despite these tests yielding reliable estimates (Lande and Arnold, 1983; Brodie and Janzen, 1996). We estimated the effects of site and year with an analysis of deviance approach by comparing models with and without the effect of interest. All statistics were conducted in R 3.3 (R Development Core Team, 2008).
RESULTS
Linking pollinator behaviour and plant fitness
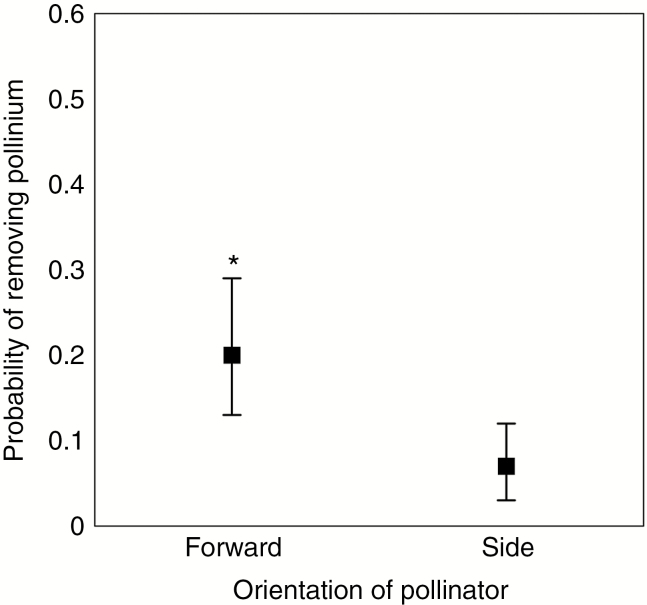
Pseudocopulation occurred in all cases of pollinia removal from virgin flowers, thus confirming its use as a reliable proxy for pollination success in C. trapeziformis. Furthermore, both the orientation of pseudocopulating pollinators and the duration of pseudocopulation were important factors predicting the probability of pollinia removal (Table 1). Pseudocopulating males were more likely to remove pollinia while in the forward orientation (Fig. 3). Longer pseudocopulation also increased the probability of pollinia removal. We detected no interaction between the orientation of pollinators and the duration of pseudocopulation. All removed pollinia attached to the dorsal thorax of male pollinators, as previously reported for Chiloglottis species (Peakall et al., 2010). It is important to note that out of 250 pseudocopulating males, only 32 removed pollinia, indicating that pollination events in C. trapeziformis are rare (12.8 %) even when male visitors are sexually stimulated.
Table 1.
Logistic regression on the effects of pollinator orientation and duration of pseudocopulation on pollinia removal in unmanipulated C. trapeziformis flowers (n = 250)
| B | Wald χ2 | d.f. | P | |
|---|---|---|---|---|
| Orientation | 1.742 | 8.519 | 1 | 0.004 |
| Duration | 0.650 | 12.695 | 1 | <0.001 |
| Orientation × duration | −0.400 | 2.575 | 1 | 0.109 |
Fig. 3.
Probability of male pollinators removing a pollinium from an unmanipulated C. trapeziformis flower while pseudocopulating in the forward and side orientations. *P < 0.005.
Linking floral manipulation and pollinator-mediated selection
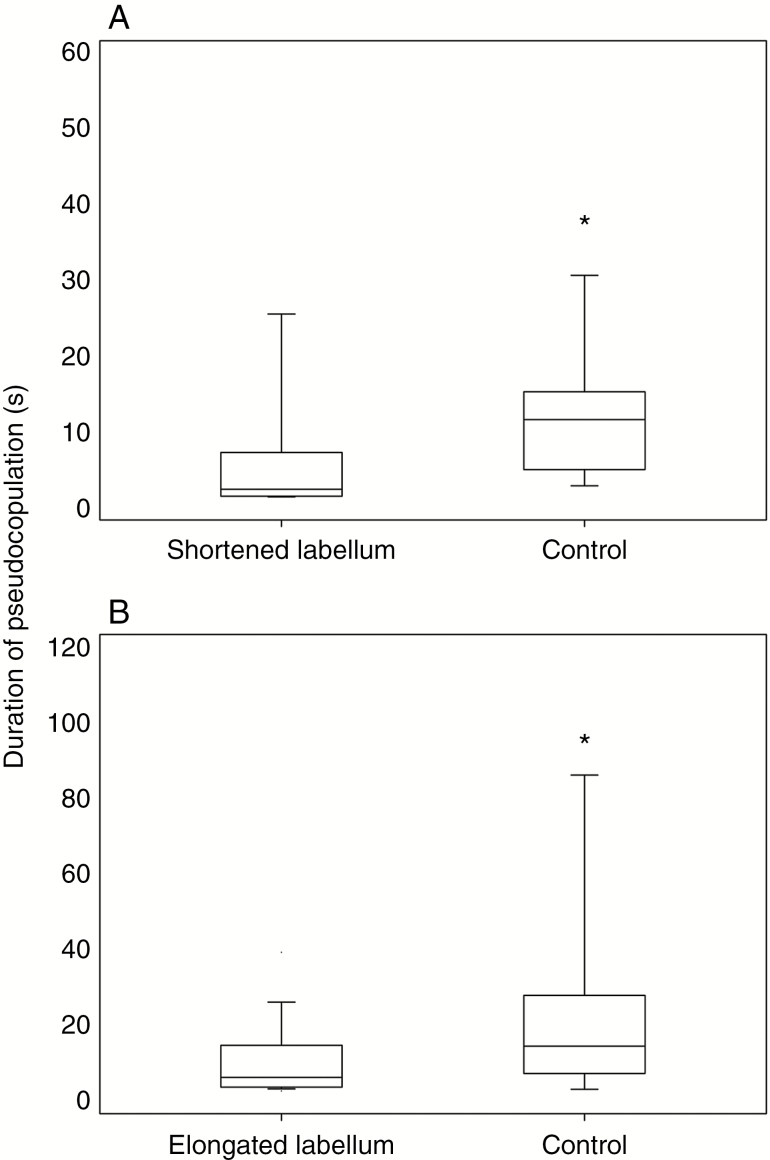
There was no difference in the number of mutually exclusive approach, land or pseudocopulate responses by pollinators at experimental and control flowers (Table 2), indicating that floral manipulation did not alter the attractiveness of C. trapeziformis. The usual forward orientation exhibited by N. cryptoides males during pseudocopulation was strongly reduced on flowers with shortened labella, because males were unable to probe the distal labellum edge on shortened flowers. Pseudocopulating males on flowers with elongated labella exhibited no change in the number of forward orientations, because they could still touch the elongated labellum tip with their abdomen while holding on to the callus. The duration of pseudocopulation was significantly reduced on manipulated flowers with both elongated and shortened labella compared with control flowers (Fig. 4), suggesting that stabilizing selection is likely operating on the callus–tip distance.
Table 2.
Differences in the number of mutually exclusive behaviours exhibited by male pollinators on manipulated C. trapeziformis relative to control flowers
| Shortening experiment | Elongation experiment | |||
|---|---|---|---|---|
| Behaviour | Control | Manipulated | Control | Manipulated |
| Approach | 84 | 85 | 173 | 210 |
| Land | 61 | 49 | 44 | 63 |
| Pseudocopulate | 23 | 21 | 24 | 32 |
| Forward orientation | 14 | 2* | 14 | 20 |
Forward orientation refers to the number of pseudocopulating males that exhibited the forward orientation.
Data were analysed with χ2 tests under an expectation of equal responses between control and manipulated treatments.
*P < 0.005.
Fig. 4.
Duration of pseudocopulation by male N. cryptoides on manipulated flowers of C. trapeziformis with (A) shortened and (A) elongated labella. Medians and 5th and 95th percentiles are shown. *P < 0.05.
Pollinator-mediated selection analysis
As there was no effect of year, we combined the data across years. Site was a significant factor (χ2 = 4.777, P = 0.029) in explaining pollination potential (evidence of either pollinia removal or deposition), driven by the lower rates of pollination at Mulloon Farm (pollinia removal = 16.0 %, pollen deposition = 4.0 %) than at Black Mountain (pollinia removal = 33.8 %, pollen deposition = 12.7 %). Although we detected no evidence of directional selection on any floral traits in C. trapeziformis (Table 3), we documented a significant negative quadratic coefficient, indicating stabilizing selection on the distance between the callus and the tip of the labellum (callus–tip).
Table 3.
Selection coefficients ± s.e. for directional (β) and stabilizing (γ) selection on the floral traits of C. trapeziformis (n = 121 flowers)
| Potential fitness (pollinia removal and deposition) | ||
|---|---|---|
| Trait | β coefficient | γ coefficient |
| Callus–tip distance | 0.007 ± 0.063 | −0.191 ± 0.075* |
| Callus length | 0.064 ± 0.048 | 0.049 ± 0.070 |
| Labellum length | 0.023 ± 0.072 | 0.094 ± 0.089 |
| Callus width | 0.078 ± 0.046 | −0.058 ± 0.066 |
| Labellum width | 0.043 ± 0.059 | 0.001 ± 0.058 |
| Callus height | −0.046 ± 0.049 | −0.015 ± 0.069 |
| Callus–pollinium distance | −0.004 ± 0.044 | 0.056 ± 0.063 |
*P < 0.05.
DISCUSSION
Our study investigated the effect of variation in flower morphology on pollinator behaviour in C. trapeziformis. While sexually deceived pollinators vary in their behaviour at flowers (Peakall, 1990), only pseudocopulatory behaviour is predicted to promote pollination in C. trapeziformis (de Jager and Peakall, 2016), as this process brings the male into contact with the reproductive column (Peakall et al., 2010; Peakall and Whitehead, 2014). Our first experiment validates this prediction, by revealing that pollinia removal only occurs during pseudocopulation with the labellum. However, pollinia removal occurred in only a fraction (13%) of these pseudocopulation events. Consistent with expectations from our earlier work (de Jager and Peakall, 2016), pollinia removal in C. trapeziformis mainly occurs when the pollinator is positioned in the appropriate forward orientation (Fig. 3).
We also provide the first experimental evidence that the probability of pollinia removal is correlated with the duration of pseudocopulation. A recent study on sexually deceptive O. leochroma offers complementary evidence for the importance of pseudocopulation, by revealing an increase in the deposition of massulae with increasing pseudocopulation duration (Rakosy et al., 2017). The frequency and duration of pseudocopulation as a proxy for plant reproductive fitness likely applies broadly across sexually deceptive plants (Ellis and Johnson, 2010; Gaskett, 2011). However, an association between pseudocopulation and pollination is not necessarily expected in all cases (Weinstein et al., 2016). In Drakaea orchids, for instance, only the pre-copulatory pollinator behaviour of attempted pick-up of the pseudo-female labellum is essential for pollination, because it brings the pollinator into contact with the column, although pseudocopulation is often also associated with this event (Peakall, 1990; Phillips et al., 2013; Bohman et al., 2014).
Although olfactory cues are crucial for securing pollinator pseudocopulation in C. trapeziformis (Schiestl et al., 2003), there is convincing evidence that morphology does matter. Artificially shortening the distance between the callus and the labellum tip, for instance, reduces the duration of pseudocopulation (de Jager and Peakall, 2016). In an extension of that earlier work, here we experimentally tested the effect of extending the callus–tip distance on pollinator behaviour. Our floral manipulations, which purposefully did not alter the callus tissue, where sexual attractants are produced, revealed no change in the response or behavioural repertoire of male visitors, indicating that manipulated flowers had similar long- and short-range attractiveness to control flowers. This suggests a lack of strong pollinator preference for a specific floral shape and size in driving pollinator behaviour in Chiloglottis, and corroborates previous results in other sexually deceptive orchids (Benitez-Vieyra et al., 2009; Gaskett, 2012).
In contrast to orchids, studies of sexual deception in the daisy Gorteria diffusa reveal that pollinator preference is of prime importance to its floral evolution (Ellis and Johnson, 2010; de Jager and Ellis, 2013). Sexual deception in this daisy differs markedly from sexual deception in orchids, because the mimicry is predominantly visual rather than olfactory (de Jager and Ellis, 2012), and the pollinator is a small dipteran bee fly (Ellis and Johnson, 2009). Beyond the biological idiosyncrasies of these systems, the contrasting findings between sexually deceptive orchids and daisies likely reflect different analytical approaches in studying floral traits that pollinators do not directly interact with (i.e. floral outlines; Gaskett, 2012) versus floral traits clearly associated with pollinator copulatory behaviour (i.e. insectiform structures on the flower; de Jager and Ellis, 2013).
Although we detected no effect of floral manipulation on attractiveness, our experiments revealed the significant impact that manipulation had on the duration of pseudocopulation. Sexually stimulated males that initiated pseudocopulation on flowers with either shortened or elongated labella terminated this behaviour after only a few seconds. Male N. cryptoides wasps have slightly longer abdomens than the labella of C. trapeziformis (de Jager and Peakall, 2016), and typically arch their abdomens to reach its distal edge during pseudocopulation (Fig. 1), which is difficult, or impossible on manipulated flowers. This suggests that the observed reduction in the duration of pseudocopulation is driven by a lack of mechanical fit with the labellum on manipulated flowers. In O. leochroma, a decrease in pseudocopulation duration occurs when the floral components that pollinators functionally interact with during pseudocopulation are removed (Rakosy et al., 2017). In contrast, the removal of floral components with which males infrequently interact during pseudocopulation had little to no effect, leading the authors to class these traits as either mechanically ‘active or inactive’.
Considering the evidence for a link between pseudocopulation duration and pollinia removal in C. trapeziformis, and independent evidence of a decrease in the duration of pseudocopulation on manipulated flowers, we expect pollinators to exert stabilizing selection on the distance from the callus to the labellum tip. To detect pollinator-mediated selection, limitations on male and female pollination success are required. Across the Orchidaceae this is generally the case (Tremblay et al., 2004), and sexually deceptive orchids are no exception (Weinstein et al., 2016). Indeed, in our study, male and female fitness was low in C. trapeziformis, indicating strong potential for pollinator-mediated selection on floral phenotypes that can capitalize on sporadic pollinator visits.
In a final test of pollinator-driven selection on floral traits via mechanical fit, we employed selection analysis. Although phenotypic selection analyses have been applied across a wide range of plants (Harder and Johnson, 2009), this approach has rarely been employed in sexually deceptive species, possibly as a result of a strong research focus on chemistry, which is crucial for securing pollinator attraction. In the only such study to date, Benitez-Vieyra et al. (2009) found no evidence for phenotypic selection on floral shape in the sexually deceptive orchid G. pennicillata. By contrast, we detected a significant negative quadratic coefficient on the distance from the callus to the labellum tip, indicative of stabilizing selection. This independent result, based on a set of flowers different from that used in our behavioural experiments, corroborates our experimental finding of reduced pseudocopulation duration when this distance is either shortened or elongated. The distance from the callus structure, which pollinators grip during pseudocopulation, to the distal labellum edge, where they probe with the tip of their abdomen, is thus strongly indicated to be under selection mediated by pollinators.
Conclusions
Our results demonstrate that, when combined with a focus on relevant pollinator behaviours, and experimental manipulation of floral form, selection analysis can be a powerful tool in the study of sexually deceptive species. However, without these additional behavioural and experimental components, selection analysis alone cannot provide direct evidence that pollinators are the agents of selection, or identify the mechanism of selection. This is a widespread problem in studies investigating pollinator-mediated selection, which tend to explore either phenotypic selection in natural populations or experimental manipulation of flowers (Harder and Johnson, 2009), but not both. Comparing selection gradients of open-pollinated plants with those of hand-pollinated plants can help identify the component of selection exerted by pollinators (Sandring and Ågren, 2009; Sletvold et al., 2010), although including pollinator observations and floral manipulations offers a more comprehensive analysis of pollinator-mediated selection. In sexually deceptive orchids, this may be especially pertinent due to other potential factors influencing the strength of selection observed in phenotypic selection studies, such as learned avoidance of flower patches by experienced pollinators, low sample size of independent plants due to clonality, and variation in the presence, density and sex ratios of pollinators between periods of study.
Recognizing which floral components influence pollen transfer and plant fitness can help elucidate the evolutionary pathways of different floral traits. This rationale has been successfully applied to studies of floral scent, by identifying behaviourally active and inactive chemical compounds to which pollinators either respond or do not respond (Schiestl et al., 1999; Ayasse et al., 2000; Mant et al., 2005). The analogous extension by Rakosy et al. (2017) in identifying active and non-active mechanical traits in sexually deceptive Ophrys provides another exemplar. Similarly, studies on deceptive plants that rely on visual mimicry, have uncovered that suites of floral traits involved in female mimicry are under stronger pollinator-mediated selection than suites of traits not involved in mimicry (Ellis et al., 2014). Considering floral structures from this perspective may be instrumental in explaining why floral traits differ in the amount of variation they exhibit (Cresswell, 1998), and can help reveal which traits produce functional divergence and reproductive isolation (Gögler et al., 2015). We suggest that careful consideration of floral traits, in combination with a multi-pronged experimental approach, promises to shed new light on plant–pollinator interactions and the evolution of floral form in deceptive and non-deceptive systems.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: correlation coefficients between the floral traits of Chiloglottis trapeziformis investigated in this study.
ACKNOWLEDGEMENTS
We would like to thank Ryan Phillips, two anonymous reviewers and James Thomson for useful comments. The ACT government supplied research permits to conduct this work (permit numbers LT2013704 and LT2015836). M.L.D.J. was funded by the National Research Foundation of South Africa (SFP12083011650) and R.P. by the Australian Research Council (DP1094453).
LITERATURE CITED
- Anderson B, Johnson SD. 2008. The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 62: 220–225. [DOI] [PubMed] [Google Scholar]
- Ayasse M, Schiestl F, Paulus H, et al. . 2000. Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: how does flower-specific variation of odor signals influence reproductive success?Evolution 54: 1995–2006. [DOI] [PubMed] [Google Scholar]
- Ayasse M, Stökl J, Francke W. 2011. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry 72: 1667–1677. [DOI] [PubMed] [Google Scholar]
- Benitez-Vieyra S, Medina AM, Cocucci A. 2009. Variable selection patterns on the labellum shape of Geoblasta pennicillata, a sexually deceptive orchid. Journal of Evolutionary Biology 22: 2354–2362. [DOI] [PubMed] [Google Scholar]
- Bohman B, Phillips RD, Menz MHM, et al. . 2014. Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: implications for the evolution of sexual deception. New Phytologist 203: 939–952. [DOI] [PubMed] [Google Scholar]
- Bohman B, Flematti GR, Barrow RA, Pichersky E, Peakall R. 2016. Pollination by sexual deception – it takes chemistry to work. Current Opinion in Plant Biology 32: 37–46. [DOI] [PubMed] [Google Scholar]
- Bohman B, Phillips RD, Flematti GR, Barrow RA, Peakall R. 2017. The spider orchid Caladenia crebra produces sulfurous pheromone mimics to attract its male wasp pollinator. Angewandte Chemie International Edition 56: 8455–8458. [DOI] [PubMed] [Google Scholar]
- Bower C. 1996. Demonstration of pollinator-mediated reproductive isolation in sexually deceptive species of Chiloglottis (Orchidaceae: Caladeniinae). Australian Journal of Botany 44: 15–33. [Google Scholar]
- Brodie ED, Janzen FJ. 1996. On the assignment of fitness values in statistical analyses of selection. Evolution 50: 437–442. [DOI] [PubMed] [Google Scholar]
- Cosacov A, Cocucci AA, Sérsic AN. 2014. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza do pollinators matter?Annals of Botany 113: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell J. 1998. Stabilizing selection and the structural variability of flowers within species. Annals of Botany 81: 463–473. [Google Scholar]
- Ellis AG, Johnson SD. 2009. The evolution of floral variation without pollinator shifts in Gorteria diffusa (Asteraceae). American Journal of Botany 96: 793–801. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Johnson SD. 2010. Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the Orchidaceae. American Naturalist 176: E143–E151. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Brockington SF, de Jager ML, Mellers G, Walker RH, Glover BJ. 2014. Floral trait variation and integration as a function of sexual deception in Gorteria diffusa. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 369: 1471–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falara V, Amarasinghe R, Poldy J, Pichersky E, Barrow RA, Peakall R. 2013. The production of a key floral volatile is dependent on UV light in a sexually deceptive orchid. Annals of Botany 111: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. 1989. Measuring pollinator-mediated selection on morphometric floral traits: bumblebees and the alpine sky pilot, Polemonium viscosum. Evolution 43: 882–890. [DOI] [PubMed] [Google Scholar]
- Gaskett AC. 2011. Orchid pollination by sexual deception: pollinator perspectives. Biological Reviews 86: 33–75. [DOI] [PubMed] [Google Scholar]
- Gaskett AC. 2012. Floral shape mimicry and variation in sexually deceptive orchids with a shared pollinator. Biological Journal of the Linnean Society 106: 469–481. [Google Scholar]
- Gögler J, Stökl J, Cortis P, et al. . 2015. Increased divergence in floral morphology strongly reduces gene flow in sympatric sexually deceptive orchids with the same pollinator. Evolutionary Ecology 29: 703–717. [Google Scholar]
- Grimaldi D. 1999. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden 86: 373–406. [Google Scholar]
- Harder LD, Johnson SD. 2009. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist 183: 530–545. [DOI] [PubMed] [Google Scholar]
- de Jager ML, Ellis AG. 2012. Gender-specific pollinator preference for floral traits. Functional Ecology 26: 1197–1204. [Google Scholar]
- de Jager ML, Ellis AG. 2013. The influence of pollinator phylogeography and mate preference on floral divergence in a sexually deceptive daisy. Evolution 67: 1706–1714. [DOI] [PubMed] [Google Scholar]
- de Jager ML, Peakall R. 2016. Does morphology matter? An explicit assessment of floral morphology in sexual deception. Functional Ecology 30: 537–546. [Google Scholar]
- Jersáková J, Jürgens A, Šmilauer P, Johnson SD. 2012. The evolution of floral mimicry: identifying traits that visually attract pollinators. Functional Ecology 26: 1381–1389. [Google Scholar]
- Kullenberg B. 1961. Studies in Ophrys pollination. Zoologiska Bidrag fran Uppsala 34: 1–340. [Google Scholar]
- Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- Mant J, Peakall R, Schiestl FP. 2005. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys?Evolution 59: 1449–1463. [PubMed] [Google Scholar]
- Newman E, Anderson B, Johnson SD. 2012. Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society B 279: 2309–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. 2015. Local adaptation: mechanical fit between floral ecotypes of Nerine humilis (Amaryllidaceae) and pollinator communities. Evolution 69: 2262–2275. [DOI] [PubMed] [Google Scholar]
- van der Niet T, Hansen DM, Johnson SD. 2011. Carrion mimicry in a South African orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses. Annals of Botany 107: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachnowitsch AL, Kessler A. 2010. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytologist 188: 393–402. [DOI] [PubMed] [Google Scholar]
- Paulus HF. 2006. Deceived males – pollination biology of the Mediterranean orchid genus Ophrys (Orchidaceae). Journal Europäischer Orchideen 38: 303–351. [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. 2009. Flies and flowers in Darwin’s race. Evolution 63: 268–279. [DOI] [PubMed] [Google Scholar]
- Peakall R. 1990. Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology 4: 159–167. [Google Scholar]
- Peakall R, Whitehead MR. 2014. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany 113: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Beattie AJ, James SH. 1987. Pseudocopulation of an orchid by male ants: a test of two hypotheses accounting for the rarity of ant pollination. Oecologia 73: 522–524. [DOI] [PubMed] [Google Scholar]
- Peakall R, Angus CJ, Beattie AJ. 1990. The significance of ant and plant traits for ant pollination in Leporella fimbriata.Oecologia 84: 457–460. [DOI] [PubMed] [Google Scholar]
- Peakall R, Ebert D, Poldy J, et al. . 2010. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator-driven speciation. New Phytologist 188: 437–450. [DOI] [PubMed] [Google Scholar]
- Phillips RD, Peakall R. 2017. Evolutionary relationships among pollinators and repeated pollinator sharing in sexually deceptive orchids. Journal of Evolutionary Biology 30: 1674–1691. [DOI] [PubMed] [Google Scholar]
- Phillips RD, Xu T, Hutchinson MF, Dixon KW, Peakall R. 2013. Convergent specialization – the sharing of pollinators by sympatric genera of sexually deceptive orchids. Journal of Ecology 101: 826–835. [Google Scholar]
- R Development Core Team 2008. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rakosy D, Cuervo M, Paulus HF, Ayasse M. 2017. Looks matter: changes in flower form affect pollination effectiveness in a sexually deceptive orchid. Journal of Evolutionary Biology 30: 1978–1993. [DOI] [PubMed] [Google Scholar]
- Rosas-Guerrero V, Quesada M, Armbruster WS, Pérez-Barrales R, Smith SD. 2011. Influence of pollination specialization and breeding system on floral integration and phenotypic variation in Ipomoea. Evolution 65: 350–364. [DOI] [PubMed] [Google Scholar]
- Sandring S, Ågren J. 2009. Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution 63: 1292–1300. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Agren J. 1995. Deceit pollination and selection on female flower size in Begonia involucrata: an experimental approach. Evolution 49: 207–214. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. 1999. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proceedings of the National Academy of Sciences of the USA 96: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. . 1999. Orchid pollination by sexual swindle. Nature 399: 421–423. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. . 2003. The chemistry of sexual deception in an orchid-wasp pollination system. Science 302: 437–438. [DOI] [PubMed] [Google Scholar]
- Shuttleworth A, Johnson SD. 2010. The missing stink: sulphur compounds can mediate a shift between fly and wasp pollination systems. Proceedings of the Royal Society B 277: 2811–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletvold N, Grindeland JM, Ågren J. 2010. Pollinator-mediated selection on floral display, spur length and flowering phenology in the deceptive orchid Dactylorhiza lapponica. New Phytologist 188: 385–392. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. 2008. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing?Evolution 62: 2435–2440. [DOI] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2004. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society 84: 1–54. [Google Scholar]
- Weinstein A, Davis B, Menz M, Dixon K, Phillips R. 2016. Behaviour of sexually deceived ichneumonid wasps and its implications for pollination in Cryptostylis (Orchidaceae). Biological Journal of the Linnean Society 119: 283–298. [Google Scholar]
- Whitehead MR, Peakall R. 2013. Short-term but not long-term patch avoidance in an orchid-pollinating solitary wasp. Behavioral Ecology 24: 162–168. [Google Scholar]
- Whitehead MR, Linde CC, Peakall R. 2015. Pollination by sexual deception promotes outcrossing and mate diversity in self-compatible clonal orchids. Journal of Evolutionary Biology 28: 1526–1541. [DOI] [PubMed] [Google Scholar]
- Xu H, Bohman B, Wong D, et al. . 2017. Complex sexual deception in an orchid is achieved by co-opting two independent biosynthetic pathways for pollinator attraction. Current Biology 27: 1867–1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.