Abstract
Objectives:
There is little consensus regarding the prognostic value of symptom duration in predicting clinical disease severity or quality-of-life (QOL) outcomes in patients with chronic rhinosinusitis (CRS). Our objectives were to: 1) determine if patients with longer symptom duration have worse preoperative disease severity and/or QOL, and 2) determine if delayed surgical intervention influences outcomes of endoscopic sinus surgery (ESS).
Methods:
Patients diagnosed with CRS were prospectively enrolled into a multi-center cohort study and observed 14.7 [±4.8] months on average following primary ESS. Preoperative symptom duration was stratified into short-term (<12 months), middle-term (12–60 months), and long-term (>60 months). Disease severity was assessed using endoscopy and computed tomography. Disease-specific QOL was measured with the Sinonasal Outcome Test-22 (SNOT-22) and Rhinosinusitis Disability Index (RSDI). Adjusted bivariate and multivariate associations between symptom duration, disease severity, and QOL scores were evaluated.
Results:
113 patients met inclusion criteria with 35 patients lost to postoperative follow-up. No significant differences in preoperative disease severity or QOL scores were reported between symptom duration subgroups. Participants in the long-term symptom subgroup reported significantly greater mean postoperative improvement on SNOT-22 total scores (n=28; −36.3[SD±22.2]) compared to both short-term (n=27; −23.4[SD±11.3]; p=0.039) and middle-term (n=23; −23.5[SD±20.1]; p=0.050) subgroups. Postoperative QOL improvements in the long-term symptom subgroup remained significantly greater (p≤0.036) after multivariate adjustment.
Conclusion:
Symptom duration was not associated with mean preoperative disease severity or QOL. Patients with long-term symptom duration reported the greatest mean postoperative QOL improvement, suggesting that delayed surgical intervention may not reduce QOL improvements following ESS.
Keywords: Quality of Life, Sinusitis, Patient Reported Outcome Measures, Chronic Disease, Symptom Assessment
INTRODUCTION
Current consensus guidelines recommend endoscopic sinus surgery (ESS) as a treatment option for patients with CRS who have persistent symptomatic burden and objective evidence of disease despite receiving appropriate medical management.1,2 In this paradigm, ESS remains an elective procedure and the choice to pursue surgery is typically based on shared decision-making between the physician and patient which takes into consideration individual symptom burden and personal preferences while balancing risks and patient expectations. Patients with worse sinus-specific quality-of-life (QOL) impairment are more likely to pursue ESS while those with less symptomatic burden are more likely to continue medical therapy alone.3 Although patients who elect continued medical therapy usually report less improvement over time, compared to those undergoing ESS, the choice to delay ESS has, until recently, never been considered inherently harmful.
Recent studies have questioned whether the duration of persistent, symptomatic CRS impacts long-term outcomes. Hopkins et al. utilized the National Comparative Audit of Surgery for Nasal Polyposis and Chronic Rhinosinusitis (NCASNPCR) and found that delayed surgical intervention, relative to symptom onset, was associated with less postoperative improvement in QOL.4 Additionally, using a large secondary database from the United States (U.S.), Benninger et al. reported association between the duration of CRS symptoms and development of comorbid asthma, as well as association with increased long-term sinus-related healthcare utilization.5 The authors of these studies were careful to draw tentative conclusions, however the implications of these findings are potentially paradigm shifting. In fact, if delaying primary ESS predisposes a patient to less improvement or worse long-term outcomes, then patients may elect to pursue ESS with more urgency in attempt to avoid those outcomes.
With these issues in mind, our objective was to further investigate the relationship between symptom duration, clinical measures, and outcomes in patients with CRS. We hypothesized that patients with longer symptom duration would present with worse preoperative disease-severity and QOL. Furthermore, we hypothesized that delayed surgical intervention would be associated with less postoperative improvement in both clinical and patient-reported outcomes following primary ESS.
MATERIALS and METHODS
Study Design and Setting
Study participants were prospectively enrolled from academic, tertiary medical centers between July, 2012 and January, 2016. Participating enrollment sites included Departments/Divisions of Otolaryngology-Head and Neck Surgery within North America including: Oregon Health & Science University (OHSU, Portland, OR), the University of Utah (Salt Lake City, UT), the Medical University of South Carolina (Charleston, SC), Stanford University (Palo Alto, CA), and the University of Calgary (Calgary, AB, Canada). Additional findings from this cohort have been described in the literature.6–8 The Institutional Review Board at each site provided annual review, authorized consent guidance, and data safety monitoring.
Study Participants – Eligibility Criteria
Diagnoses of CRS were confirmed by fellowship trained Rhinologists using current criteria outlined in the Adult Sinusitis Guidelines provided by the American Academy of Otolaryngology.1,9 Adult study participants (≥18 years of age) were asked to provide extensive medical and social history to verify recent therapeutic management including: at least one course (≥14 days) of empiric or culture-directed antibiotics, either corticosteroid nasal spray (≥21 days) or oral corticosteroid therapy (≥5 days), and nasal saline irrigations as needed (~240ml. PRN). Participants voluntarily elected primary ESS following patient counseling. Surgical approach was determined by each enrolling physician using both radiographic imaging and endoscopic examination findings.
Primary ESS was completed under general anesthesia and consisted of: unilateral or bilateral maxillary antrostomy, partial/total ethmoidectomy, sphenoidotomy, and/or frontal sinusotomy, incorporating either inferior turbinate reduction and/or septoplasty if indicated. Postoperative management included nasal saline irrigations and topical corticosteroid sprays/rinses to facilitate optimal recovery if warranted. Study participants were observed up to 18 months postoperatively. Follow-up evaluations occurred during routine clinical appointments or via mailed response surveys.
Exclusion Criteria
Study participants were excluded from analyses if they presented with comorbid conditions which typically impact global health including ciliary dyskinesia/cystic fibrosis and corticosteroid dependency. Additional exclusion consisted of any patients with a history of previous ESS due to the confounding nature of ESS on the primary exposure variables of interest to this study.
Primary Exposure Measurement – Duration of Disease
The main exposure of interest was defined as the date (month / year) in which study participants started experiencing persistent symptoms of CRS based on patient recall. Similar to Hopkins, et al., symptomatic duration was calculated between reported symptom on-set date and the date of primary ESS, then categorized into a ‘short-term’ (<12 months); ‘middle-term’ (12–60 months), or a ‘long-term’ (>60 months) subgroup.4
Data Sources - Clinical Measures of Disease Severity
Preoperative high resolution computed tomography (CT) of the bilateral sinuses was obtained, without contrast, to assess disease severity and quantified by each enrolling physician in accordance with Lund-Mackay staging (range: 0–24).10 Patients were also evaluated using preoperative and postoperative bilateral sinus endoscopy and quantified by each enrolling physician using Lund-Kennedy staging (range: 0–20).11 Higher total scores on both staging systems represent worse overall disease severity.
Olfactory function was measured using the Brief Smell Identification Test (BSIT, Sensonics, Inc., Haddon Heights, NJ). The BSIT is a validated, 12-item diagnostic tool of olfactory fucntion.12 Study participants are directed to identify the correct odorants from 4 options in a forced choice, “scratch-and-sniff” response format. Higher total scores reflect superior olfactory function (range: 0–12).11 A minimal clinically important difference (MCID) reflecting difference within-subject improvement of at least 1.0 point on BSIT scores has been previously described.13
Data Sources - Patient-Reported Outcome Measures (PROMs)
Study participants were asked to complete two PROMs to quantify symptom severity and QOL impairment. Subjects were asked to complete PROMs during initial enrollment meetings and postoperative follow-up. The SinoNasal Outcome Test (SNOT-22) is a 22-item validated survey developed to quantify sinonasal symptom severity (©2006, Washington University, St. Louis, MO) using Likert score responses (range: 0–5), where higher scores reflect worse symptom severity.14 Higher total scores (range: 0–110) reflect worse overall symptom severity and disease impairment. The SNOT-22 items have been previously factored into 5 distinct symptom domains including the: rhinologic symptoms (range: 0–30), extra-nasal rhinologic symptoms (range: 0–15), ear/facial symptoms (range: 0–25), psychological dysfunction (range: 0–35), and sleep dysfunction (range: 0–25) with minimal item cross-loading.8 A MCID value for SNOT-22 total scores has also been previously defined as at least 8.9 points in a cohort with medically refractory CRS.14
Additionally, the 30-item RhinoSinusitis Disability Index (RSDI) was also administered to measure complimentary aspects of CRS disease severity. The RSDI consists of 3 domains which evaluate the impact of CRS on a respondent’s physical (range: 0–44), functional (range: 0–36), and emotional (range: 0–40) domains. Individual item scores are measured using Likert scale responses (range: 0–4) where higher scores indicate worse symptom severity. Higher summarized total scores reflect worse overall symptom severity (range: 0–120).15 A MCID for RSDI total scores has been defined by determining the mean preoperative group score and calculating one-half of the associated standard deviation.16,17
Statistical Methods
Statistical analyses were completed using SPSS software (ver. 24.0; IBM Corp, Armonk, NY). Descriptive patient data were reported and distributions of scaled data were assessed for assumptions of linearity and/or normality. Global comparisons between symptom duration subgroups was completed using analysis of variance (ANOVA), chi-square (χ2) testing, or Kruskall-Wallis (KW) test statistics, where appropriate. Adjustments for multiple bivariate comparisons were completed using two-sided independent t-testing, Mann-Whitney-U (MWU), or χ2 testing when omnibus statistics indicated significant between-group differences. Within-subject improvement was determined using matched pairs t-testing or Wilcoxon signed rank testing.
Primary predictors of interest were symptom duration subgroup variables while the primary outcome of interest was the postoperative change (last available postoperative score – preoperative score) in PROMs. Simple, stepwise, linear regression modeling was used to identify significant risk factors associated with postoperative improvements in PROM score differences. Covariates listed in Table 1 were screened for univariate significance at the 0.200 α-level for preliminary model inclusion. Final models were constructed using manual, forward selection (p<0.100) and backwards elimination (p<0.050). Covariate risk factors, including measures of comorbidity, were included into each bivariate model to evaluate potential effect estimate confounding (≥10% difference in effect estimation for symptom duration subgroup variables). Goodness-of-model-fit was evaluated using coefficients of multiple determination (R2) to determine the total explained model variation (%). Unadjusted and adjusted regression effect estimates (β) associated with symptom duration subgroups, standard errors (SE), 95% confidence intervals, and type-1 error probability (p-values) are reported. To account for postoperative outcome variation due to preoperative PROM scores, individual relative mean improvement (RMI) was calculated using the formula: [(last available postoperative score-preoperative score) / preoperative score] × 100, and then average for each symptom duration subgroup.
Table 1:
Omnibus comparisons between symptom duration subgroups across demographics, comorbidity, clinical measures of disease severity, and patient-reported outcome measure scores at enrollment (n=113)
| Preoperative Cofactors: | ‘Short’ Cohort (< 12 months) n=32 | ‘Middle’ Cohort (12–60 months) n=39 | ‘Long’ Cohort (>60 months) n=42 | Omnibus test statistic | p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 53.1±17.7 | 47.0±15.6 | 45.9±16.5 | F(2)=1.90 | 0.155 | |
| Male | 16 (50%) | 22 (56%) | 23 (55%) | χ2=0.31 | 0.858 | |
| White / caucasian | 30 (94%) | 34 (87%) | 36 (86%) | χ2=1.25 | 0.534 | |
| African American | 1 (3%) | 1 (3%) | 0 (0%) | χ2=1.24 | 0.539 | |
| Asian | 0 (0%) | 2 (5%) | 5 (12%) | χ2=4.55 | 0.103 | |
| Hispanic/Latino | 0 (0%) | 1 (3%) | 3 (7%) | χ2=2.88 | 0.237 | |
| Education (years) | 14.6±2.3 | 15.5±2.5 | 14.3±3.6 | F(2)=1.62 | 0.204 | |
| Household Income: | ---- | ---- | ---- | ---- | ---- | |
| $0-$25,000 | 1 (3%) | 4 (10%) | 5 (12%) | χ2=2.05 | 0.358 | |
| $26,000-$50,000 | 7 (22%) | 5 (13%) | 8 (19%) | χ2=1.27 | 0.530 | |
| $51,000-$75,000 | 9 (28%) | 5 (13%) | 6 (14%) | χ2=3.32 | 0.190 | |
| $76,000-$100,000 | 3 (9%) | 10 (26%) | 9 (21%) | χ2=3.21 | 0.210 | |
| $100,000+ | 9 (28%) | 12 (31%) | 8 (19%) | χ2=1.19 | 0.551 | |
| Medical insurance type: | ---- | ---- | ---- | ---- | ---- | |
| Employer provided | 16 (50%) | 28 (72%) | 25 (60%) | χ2=3.58 | 0.167 | |
| Medicare | 10 (31%) | 4 (10%) | 5 (12%) | χ2=6.69 | 0.035 | |
| Medicaid | 0 (0%) | 0 (0%) | 3 (7%) | χ2=5.21 | 0.074 | |
| State Assisted | 0 (0%) | 1 (3%) | 1 (2%) | χ2=0.81 | 0.668 | |
| Private | 6 (19%) | 1 (3%) | 5 (12%) | χ2=4.97 | 0.083 | |
| Asthma | 13 (41%) | 13 (33%) | 15 (36%) | χ2=0.41 | 0.813 | |
| Nasal Polyposis | 10 (31%) | 16 (41%) | 17 (41%) | χ2=0.88 | 0.644 | |
| Septal deviation | 19 (59%) | 20 (51%) | 18 (43%) | χ2=2.00 | 0.368 | |
| Allergies (tested) | 15 (47%) | 16 (41%) | 19 (45%) | χ2=0.27 | 0.874 | |
| ASA intolerance | 0 (0%) | 2 (5%) | 2 (5%) | χ2=1.65 | 0.439 | |
| COPD | 1 (3%) | 0 (0%) | 1 (2%) | χ2=1.13 | 0.568 | |
| Current smoker | 2 (6%) | 3 (8%) | 2 (5%) | χ2=0.30 | 0.861 | |
| Alcohol use | 10 (31%) | 20 (51%) | 12 (29%) | χ2=5.14 | 0.077 | |
| Depression (self-report) | 3 (9%) | 2 (5%) | 8 (19%) | χ2=4.05 | 0.132 | |
| CT score | 12.0±6.2 | 12.6±5.8 | 13.0±6.4 | F(2)=0.24 | 0.784 | |
| Endoscopy score | 5.0±3.2 | 5.7±3.8 | 5.0±3.2 | F(2)=0.46 | 0.631 | |
| BSIT score | 8.5±3.0 | 9.6±2.2 | 7.5±3.3 | KW(2)=6.48 | 0.039 | |
| Normal olfaction | 18 (64%) | 16 (70%) | 15 (50%) | χ2=2.34 | 0.310 | |
| PROM scores: | ---- | ---- | ---- | ---- | ---- | |
| SNOT-22 total score | 49.0±18.2 | 48.9±18.9 | 55.8±19.2 | F(2)=1.71 | 0.185 | |
| Rhinologic domain | 15.7±7.0 | 16.0±5.8 | 17.5±6.4 | F(2)=0.90 | 0.408 | |
| Extra-nasal rhinologic domain | 8.2±3.7 | 7.5±4.3 | 7.8±3.6 | F(2)=0.29 | 0.752 | |
| Ear / facial domain | 8.5±4.8 | 8.9±5.3 | 9.6±5.2 | F(2)=0.44 | 0.647 | |
| Psychological dysfunction | 14.1±7.8 | 14.2±8.2 | 17.2±8.1 | F(2)=1.84 | 0.163 | |
| Sleep dysfunction | 12.6±6.8 | 12.3±7.1 | 14.7±6.7 | F(2)=1.41 | 0.249 | |
| RSDI total score | 43.6±21.8 | 37.9±24.7 | 45.6±21.3 | F(2)=1.20 | 0.307 | |
| Physical domain | 17.5±8.3 | 17.5±10.4 | 19.8±8.9 | F(2)=0.79 | 0.455 | |
| Functional domain | 14.3±8.2 | 11.4±8.1 | 13.5±6.8 | F(2)=1.33 | 0.269 | |
| Emotional domain | 11.8±7.8 | 9.0±8.1 | 12.3±7.8 | F(2)=1.91 | 0.153 | |
N, sample size; SD, standard deviation; VA, Veterans Affairs; ASA, acetylsalicylic acid (aspirin); COPD, chronic obstructive pulmonary disease; CT, computed tomography, BSIT, Brief Smell Identification Test. Disparities in reported samples sizes for household income and BSIT scores are derived from incomplete data capture/missing values – valid percentages are reported. Test statistics were elected based on evidence of normal distribution of scaled values. F(2), f-test statistic with 2 degrees of freedom, KW, Kruskall-Wallis test statistic, χ2, two-sided Pearson’s chi-squared test statistic. RSDI, Rhinosinusitis Disability Index; SNOT-22, 22-item SinoNasal Outcome Test, PROM, patient-reported outcome measure.
RESULTS
Final Study Population
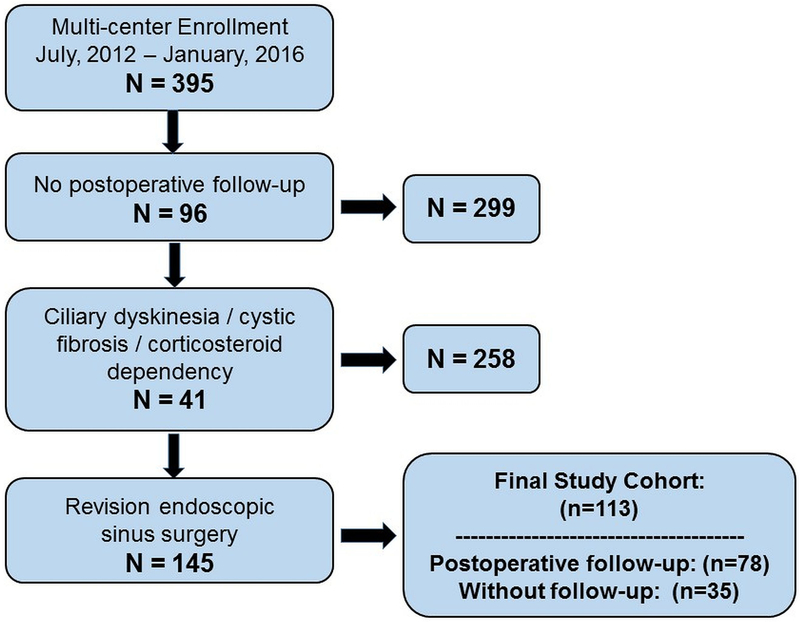
One hundred and thirteen patients met all inclusion criteria (Figure 1) with an average surgical wait time of 4.8 [SD±8.0] weeks. The overall average duration of disease was 91.3 [±133.5] months while 63% of participants reported seeking primary ESS within the first 60 months of symptom on-set. The final study cohort was re-categorized into symptom duration subgroups approximating equal sample size and consisting of ‘short-term’ (<12 months; n=32; 28%), ‘middle-term’ (12–60 months; n=39, 35%), and ‘long-term’ (>60 months; n=42; 37%) subgroups. Demographic factors, comorbid conditions, clinical measures of disease severity, and all preoperative PROM scores are described and compared in Table 1 while the prevalence of surgical procedures is presented in Table 2.
Figure 1:
Flow diagram for study inclusion.
Table 2:
Prevalence of surgical procedures for independent symptom duration subgroups (N=113)
| Procedure type: | ‘Short’ Cohort n=32 | ‘Middle’ Cohort n=39 | ‘Late’ Cohort n=42 | |||
|---|---|---|---|---|---|---|
| Right side n (%) | Left side n (%) | Right side n (%) | Left side n (%) | Right side n (%) | Left side n (%) | |
| Maxillary antrostomy | 28 (88%) | 29 (91%) | 34 (88%) | 36 (92%) | 37 (88%) | 37 (88%) |
| Partial ethmoidectomy | 2 (6%) | 4 (13%) | 8 (21%) | 10 (26%) | 5 (12%) | 6 (14%) |
| Total ethmoidectomy | 28 (88%) | 27 (84%) | 30 (77%) | 29 (74%) | 32 (76%) | 31 (74%) |
| Sphenoidotomy | 28 (88%) | 25 (78%) | 23 (59%) | 26 (67%) | 30 (71%) | 30 (71%) |
| Middle turbinate resection | 3 (9%) | 2 (6%) | 8 (21%) | 7 (18%) | 9 (21%) | 8 (19%) |
| Inferior turbinate reduction | 7 (22%) | 7 (22%) | 19 (49%) | 18 (46%) | 16 (38%) | 16 (38%) |
| Frontal sinustomy (Draf I) | 5 (16%) | 6 (19%) | 5 (13%) | 5 (13%) | 5 (12%) | 5 (12%) |
| Frontal sinusotomy (Draf IIa) | 17 (53%) | 16 (50%) | 19 (49%) | 18 (46%) | 16 (38%) | 17 (41%) |
| Frontal sinusotomy (Draf IIb) | 0 (0%) | 0 (0%) | 2 (5%) | 1 (3%) | 3 (7%) | 2 (5%) |
| Frontal sinusotomy (Draf III) | 1 (3%) | 1 (3%) | 0 (0%) | |||
| Septoplasty | 20 (63%) | 25 (64%) | 23 (55%) | |||
| Image guidance | 20 (63%) | 25 (64%) | 16 (38%) | |||
CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyposis; n, sample size.
Preoperative cofactors were statistically comparable between symptom duration subgroups with a few notable exceptions. After adjustment for multiple comparisons, average preoperative BSIT scores were significantly worse in the long-term subgroup, compared to those in the middle-term subgroup (p=0.035). Participants in the short-term subgroup reported a significantly higher prevalence of Medicare coverage compared to both middle-term and late-term groups (p<0.050), indicating a higher likelihood to pursue earlier surgical intervention. These trends were reversed for patients with Medicaid and state assisted medical coverage, without significant difference likely due to limited sample size. No significant differences between any two symptom duration subgroups were found for any preoperative PROM mean score (Table 1).
Postoperative Improvements in PROMs and Disease Severity Measures
All participants were followed for an average of 14.7 [±4.8] months after primary sinus surgery. Postoperative follow-up was available for 78 (69%) of the total study cohort, consisting of 27/32 (84%) study participants with short-term symptoms, 23/39 (59%) with middle-term symptoms, and 28/42 (67%) with long-term symptom duration. No significant differences between study participants with (n=78) and without (n=35) postoperative follow-up were found across demographic, comorbidity, clinical measures of disease severity, or mean patient-reported outcome measure scores with the exception of age at enrollment. Participants providing postoperative follow-up were significantly older (51.6 [±16.7] vs. 41.1 [±14.3] years) on average (p=0.002). Comparisons in mean postoperative improvements across clinical measures of disease severity and PROMs, between all three subgroups, are described in Table 3. Within-subject mean improvements were highly significant for all three subgroups for all PROMs over time (p<0.050) except for the SNOT-22 rhinologic, extra-nasal rhinologic and sleep domains, and the RSDI emotional domain. Within-subject BSIT scores significantly improved postoperatively in only the long-term cohort (p=0.007). The prevalence of patients reporting at least one postoperative MCID value for BSIT, SNOT-22, and RSDI total scores are described for each symptom duration subgroup in Table 4.
Table 3:
Omnibus comparisons between symptom duration subgroups across average postoperative improvements in patient-reported outcome measure and disease severity scores at last available follow-up (n=78)
| PROM scores: Mean±SD |
‘Short’ Cohort (< 12 months) n=27 | ‘Middle’ Cohort (12–60 months) n=23 | ‘Late’ Cohort (>60 months) n=28 | Omnibus test statistic | p-value |
|---|---|---|---|---|---|
| SNOT-22 total score | −23.4±11.3* | −23.5±20.1* | −36.3±22.2* | F(2)=4.23 | 0.018 |
| Rhinologic domain | −6.7±6.5* | −8.3±7.6* | −11.3±7.0* | F(2)=2.86 | 0.064 |
| Extra-nasal rhinologic domain | −3.8±3.7* | −3.2±4.2* | −4.8±4.6* | F(2)=0.95 | 0.391 |
| Ear / facial domain | −5.2±3.7* | −4.0±4.3* | −7.5±5.3* | F(2)=4.14 | 0.020 |
| Psychological dysfunction | −6.2±6.0* | −6.6±8.3* | −11.3±8.3* | F(2)=3.68 | 0.030 |
| Sleep dysfunction | −5.8±5.1* | −6.2±6.4* | −8.6±6.7* | F(2)=1.65 | 0.199 |
| RSDI total score | −26.6±17.0* | −17.3±24.1* | −34.3±21.5* | F(2)=3.97 | 0.023 |
| Physical domain | −10.3±6.6* | −9.4±8.9* | −14.8±8.4* | F(2)=3.40 | 0.039 |
| Functional domain | −10.2±8.1* | −5.2±8.1* | −11.2±7.7* | F(2)=3.79 | 0.027 |
| Emotional domain | −6.2±5.59* | −2.6±9.1 | −8.2±7.5* | F(2)=3.33 | 0.042 |
| Endoscopy score | −2.1±2.6* | −3.0±3.2* | −2.2±4.2* | F(2)=0.38 | 0.681 |
| BSIT score | 0.4±1.9 | 0.1±2.2 | 2.3±5.3* | KW(2)=8.67 | 0.013 |
SD, standard deviation; RSDI, Rhinosinusitis Disability Index; SNOT-22, 22-item SinoNasal Outcome Test; F(2), f-test statistic with 2 degrees of freedom; KW=Kruskall-Wallis test statistic.
indicates significant bivariate within-subject (group) improvement over time (p<0.050). negative values reflect mean score improvements over time. PROM, patient-reported outcome measure.
Table 4:
Comparison of the prevalence of study participants reporting at least one MCID value following endoscopic sinus surgery between symptom duration subgroups.
| PROM scores: | ‘Short’ Cohort (%) | ‘Middle’ Cohort (%) | ‘Late’ Cohort (%) | χ2 test statistic | p-value |
|---|---|---|---|---|---|
| SNOT-22 total score | 92% | 83% | 93% | 1.65 | 0.438 |
| RSDI total score | 78% | 59% | 85% | 4.32 | 0.115 |
| BSIT score | 50% | 25% | 76% | 9.63 | 0.008 |
PROM, patient-reported outcome measure; SNOT-22, 22-item SinoNasal Outcome Test; RSDI, Rhinosinusitis Disability Index; BSIT, Brief Smell Identification Test; X2, chi-square test statistic. MCID, minimal clinically important difference.
After adjustment for multiple, bivariate comparisons, participants in the long-term cohort reported significantly greater improvement across disease-specific PROMs. Patients in the long-term cohort improved significantly more on SNOT-22 total scores compared to both the short-term cohort (p=0.039) and middle-term cohort (p=0.050). Similarly, patients in the long-term cohort improved significantly more on SNOT-22 ear/ facial scores than study participants in the middle-term cohort (p=0.020) and to a greater magnitude on SNOT-22 psychological dysfunction scores than patients in the short-term cohort (p=0.050). Patients in the long-term cohort also reported significantly better mean, adjusted, postoperative improvement on RSDI total scores compared to those in the middle-term cohort (p=0.019), largely contributable to differences between those groups within physical scores (p=0.062), functional scores (p=0.033), and emotional scores (p=0.038). Differences in mean improvement scores between the short- and middle-term cohorts were not significantly different for any PROM scores (all p≥0.106), except for the RSDI functional domain (p=0.022).
Linear Regression Modeling – Effect estimations for Length of Disease
As indicated in Table 3, bivariate comparisons between short-term and middle-term subgroups revealed no significant differences in mean postoperative improvement for most PROMs or clinical measures of disease severity. Preliminary linear regression modeling adopted a re-categorized primary predictor of interest into two symptom duration groups including: 1) ≤60 symptom months and 2) >60 symptom months, using the former as modeling referent. Unadjusted, univariate modeling revealed that >60 months of previous symptom duration was consistently associated with greater postoperative improvement on all SNOT-22 and RSDI scores, as well as greater improvement on BSIT olfactory scores (Table 5).
Table 5:
Unadjusted average effect estimates (β) associated with symptom duration subgroup (> 60 months) for postoperative improvements in PROMs and clinical measures of disease severity
| Outcome measures: | Unadjusted β | SE | 95% CI | p-value | R2 |
|---|---|---|---|---|---|
| SNOT-22 total score | −12.9 | 4.4 | −21.7, −4.1 | 0.005 | 0.104 |
| Rhinologic domain | −3.8 | 1.7 | −7.2, −0.5 | 0.026 | 0.065 |
| Extra-nasal rhinologic domain | −1.3 | 1.0 | −3.3, 0.7 | 0.200 | 0.022 |
| Ear / facial domain | −2.9 | 1.1 | −5.1, −0.8 | 0.008 | 0.091 |
| Psychological dysfunction | −4.9 | 1.8 | −8.6, −1.3 | 0.008 | 0.091 |
| Sleep dysfunction | −2.6 | 1.5 | −5.5, 0.3 | 0.074 | 0.043 |
| RSDI total score | −11.8 | 5.1 | −22.0, −1.7 | 0.023 | 0.069 |
| Physical domain | −5.0 | 1.9 | −8.8, −1.2 | 0.011 | 0.084 |
| Functional domain | −3.3 | 2.0 | −7.2, 0.7 | 0.102 | 0.036 |
| Emotional domain | −3.6 | 1.8 | −7.3, 0.1 | 0.054 | 0.050 |
| Endoscopy score | 0.3 | 0.9 | −1.6, 2.2 | 0.727 | 0.002 |
| BSIT score | 2.0 | 0.9 | 0.1, 3.9 | 0.039 | 0.073 |
PROMs, patient reported outcome measures; β, effect estimate for the predictor of interest; SE, standard error, CI, confidence interval; R2, coefficient of multiple determination (explained percent variance); SNOT-22, 22-item SinoNasal Outcome Test; RSDI, Rhinosinusitis Disability Index; BSIT, Brief Smell Identification Test.
After covariate screening, additional multivariate modeling was completed for all unadjusted models with significant associations between >60 month symptom duration and individual postoperative improvement measures, with manual adjustment for enrollment site variation (Table 6). Screened covariates, including enrollment location and comorbidity, were not independently associated with postoperative improvement measures (p>0.200) or identified as confounding factors in the association between symptom duration and postoperative differences. After adjustment for significant cofactors, symptom duration >60 months was still significantly associated with greater average postoperative improvement following sinus surgery. Baseline PROM scores were highly significantly associated with all postoperative change scores (p<0.001) in bivariate models, however were not included in final models to avoid potential effect estimate bias in multivariate models of change over time.18
Table 6:
Adjusted average effect estimates (β) associated with symptom duration subgroup (>60 months) for postoperative improvements in PROMs and clinical measures of disease severity
| PROM scores: | Adjusted β | SE | 95% CI | p-value | R2 |
|---|---|---|---|---|---|
| SNOT-22 total score1 | −14.3 | 4.2 | −22.6, −5.9 | 0.001 | 0.366 |
| Rhinologic domain2 | −3.8 | 1.7 | −7.1, −0.5 | 0.023 | 0.122 |
| Ear / facial domain3 | −2.9 | 1.1 | −5.1, −0.8 | 0.009 | 0.091 |
| Psychological dysfunction4 | −5.1 | 1.7 | −8.5, −1.7 | 0.004 | 0.412 |
| RSDI total score5 | −10.9 | 5.1 | −21.0, −0.7 | 0.036 | 0.269 |
| Physical domain6 | −4.5 | 1.9 | −8.3, −0.7 | 0.020 | 0.137 |
| BSIT score7 | 1.9 | 0.9 | −0.2, 3.5 | 0.072 | 0.184 |
PROMs, patient reported outcome measures; β, effect estimate for the predictor of interest; SE, standard error, CI, confidence interval; R2, coefficient of multiple determination (explained percent variance); SNOT-22, 22-item SinoNasal Outcome Test; RSDI, Rhinosinusitis Disability Index; BSIT, Brief Smell Identification Test.
Final model adjusted for covariates including: enrollment site (p=0.236), employer provided insurance (p=0.001), and $0-$25,000 income level (p=0.005).
Final model adjusted for covariates including: enrollment site (p=0.390), employer provided insurance (p=0.045).
Final model adjusted for covariates including: enrollment site (p=0.964).
Final model adjusted for covariates including: enrollment site (p=0.021), employer provided insurance (p=0.002), $0-$25,000 income level (p<0.001), and preoperative CT score (p=0.033).
Final model adjusted for covariates including: enrollment site (p=0.633), $0-$25,000 income level (p=0.017), and Medicare insurance (p=0.008).
Final model adjusted for covariates including: enrollment site (p=0.972) and White/Caucasian race (p=0.042).
Final model adjusted for covariates including: enrollment site (p=0.186) and nasal polyposis (p=0.046).
Relative Mean Improvements
To further account for postoperative score variation due to preoperative PROM status, RMI values were compared across re-categorized symptom duration subgroups (Table 7). Higher mean RMI values were reported from patients with >60 months of symptoms associated with CRS across all outcome measures, except for SNOT-22 extra-nasal rhinologic scores and endoscopy scores. Between-group differences in mean RMI scores were not statistically significant except for postoperative differences in BSIT scores although the RMI of nasal endscopy scores was almost three times that in those patients with symptom duration ≤ 60 months compared to patients with more than 60 months.
Table 7:
Comparison of relative mean improvement as a percentage of baseline score between symptom duration subgroups (n=78)
| PROM scores: | ≤ 60 symptom months (n=50) | > 60 symptom months (n=28) | Test Statistic | p-value |
|---|---|---|---|---|
| RMI (%) | RMI (%) | |||
| SNOT-22 total score | 50.2% | 53.2% | MWU=540 | 0.185 |
| Rhinologic domain | 45.5% | 64.4% | MWU=514 | 0.213 |
| Extra-nasal rhinologic domain | 45.5% | 44.4% | MWU=513 | 0.334 |
| Ear / facial domain | 55.0% | 72.5% | MWU=499 | 0.193 |
| Psychological dysfunction | 39.8% | 68.8% | MWU=459 | 0.098 |
| Sleep dysfunction | 46.1% | 60.4% | MWU=515 | 0.461 |
| RSDI total score | 56.7% | 76.1% | MWU=519 | 0.282 |
| Physical domain | 48.8% | 74.7% | MWU=530 | 0.205 |
| Functional domain | 60.9% | 83.2% | MWU=429 | 0.065 |
| Emotional domain | 34.7% | 73.7% | MWU=367 | 0.166 |
| Endoscopy score | 46.4% | 14.8% | MWU=387 | 0.600 |
| BSIT score | 10.6% | 56.0% | MWU=573 | 0.006 |
PROM, patient reported outcome measure; RMI, relative mean improvement, SNOT-22, 22-item SinoNasal Outcome Test; RSDI, Rhinosinusitis Disability Index; BSIT, Brief Smell Identification Test. MWU, Mann Whitney U test statistic.
DISCUSSION
Key results
A robust body of literature exists outside otolaryngology which has linked chronic inflammation to reduced patient-reported QOL,19 suggesting that chronic disease duration may impact QOL.20,21 This has led to increased interest in understanding whether earlier intervention for CRS, such as ESS, might improve long-term outcomes. Initial investigations have suggested that delayed surgical intervention for CRS may, in fact, adversely impact outcomes,4 with increased risk of developing asthma5, irreversible upper-airway remodeling, and recalcitrant disease.22 This emerging evidence implies that early intervention in CRS might circumvent irreversible changes and improve long-term outcomes. In contrast to these early investigations, we found that patients reporting long-term persistent symptoms experienced better outcomes after primary ESS. In fact, patients with longer symptom duration reported greater mean QOL improvements compared to those patients with shorter symptom duration.
Interpretation
Prevailing literature supports that ESS significantly improves QOL in patients with CRS, although it is unclear if delayed primary surgical intervention for those with long-term (> 60 months) symptom duration is associated with reduced QOL improvements. Additionally, definitions in the literature for what constitutes ‘delayed surgical intervention’ are quite heterogeneous. The seminal manuscript by Hopkins et al. examined the NCASNPCR and noted that the time between nose/sinus symptom on-set to surgery was highly variable but concluded that delayed surgical intervention adversely impacted outcomes after ESS in a large patient cohort.4 Another retrospective analysis of the MarketScan Commercial Claims and Encounter database in the U.S. evaluated the time between CRS diagnosis and primary ESS and found association with the development of comorbid asthma, as well as increased sinus-related long-term healthcare utilization with delayed surgical intervention.5 Investigation of a Canadian surgical registry of 150 patients from the Vancouver Coast Health Authority, Newton et al. reported that delayed ESS, operationalized using surgical wait times of 32.4 weeks (~8 months) on average, was not a significant prognosticator of postoperative SNOT-22 score improvements.23 This study may offer slight contrast to the investigations by Hopkins, et al. and Sahlstrand-Johnson, et al., both whom reported that patients with less symptomatic disease experienced the largest postoperative improvements in SNOT-22 scores on average, however surgical wait time measures an inherently different component of ‘delayed surgical intervention’ than that of symptomatic disease duration.4,24
Our multi-center data found significant, within-subject improvement in mean QOL regardless of symptom duration following ESS (Table 3). Furthermore, the majority of patients in each symptom duration subgroup reported at least one MCID in both the SNOT-22 and RSDI total scores following ESS (Table 4). Interestingly, subjects with long-term symptom duration demonstrated significantly greater QOL improvement compared to those with short-term and middle-term symptom duration after covariate adjustment. Following multivariate adjustment, patients with more than 60 months of symptom duration were still found to report significantly greater postoperative improvement in most SNOT-22 and RSDI scores on average (Table 6).
Additionally, although no significant differences in preoperative PROMs were found between symptom duration subgroups, patientswith longer symptomatic disease did report overall worse mean SNOT-22 scores. To better account for variation in postoperative improvement percentages due to preoperative symptom severity, RMI was also compared across symptom duration subgroups. No significant difference in unadjusted mean relative improvement percentages was found when participant subgroups were recategorized between those with symptom duration ≤ 60 months and those with more than 60 months (Table 7).
The overall extent of surgical intervention also varied between the NHS database and our North American patient cohort with much less extensive surgery reported in the NCASNPCR database compared to our multi-centered patient group (Table 2).25 It is clear that notable differences in both defined predictive variables and outcome measures, relative to symptom duration, as well as sample sizes and the extent of overall intervention exists across these current investigations of English, Canadian, and North American patient databases. This uncertainty suggests that current, available data lacks consistency and external validity and may not be adequate to warrant substantial alterations to the current surgical treatment paradigm for CRS at this time.
Limitations
This current investigation is strengthened through a prospective, multi-center design; however, several limitations should be considered when evaluating these data. First, the referral pattern of this patient population to academic, tertiary care practices in North America may bias towards more severe sinonasal disease. Secondly, there is potential for differential misclassification and/or recall bias when requesting patients provide the approximate date of symptom onset for a chronic disease process. In 2015, Hopkins, et al. also used an alternative approach by defining symptomatic disease on-set using retrospective chart review.26 Although, this removes some potential for recall bias the authors are unable to comment on the timeline of the disease prior to chart diagnosis. Third, predetermined symptom duration subgroup designations may be considered arbitrary and have not been clearly demonstrated to represent differentiations of preoperative disease presentation. Fourth, unmeasured confounding factors such as barriers to care, mucosal remodeling and others not considered during this investigation may be responsible for observed associations between symptomatic symptom duration and PROM scores. Fifth, sample size limitations should be considered when interpreting these findings as sample size can restrict an ability to make a clear generalized statements outside the context of this patient cohort. While investigations with larger sample sizes are likely to provide better reflections of true average population metrics, we were still able to identify significant differences in mean PROMs between symptom duration subgroups due to magnitudes of difference reported by study participants. Lastly, postoperative follow-up was available for 78 /113 (69%) of study subjects and it remains unclear how incomplete follow-up (selection) bias may impact internal study validity for observational clinical research of this patient population.
CONCLUSION
In this study, symptom duration did not associate with preoperative disease severity or QOL. Patients with long-term symptom duration reported the greatest postoperative QOL improvement, suggesting that delayed surgical intervention may not reduce QOL improvements following ESS.These findings challenge previously reported work which suggest earlier surgical intervention may provide greater QOL benefit following ESS. Further investigation is warranted to better define CRS symptom duration prospectively and to identify the optimal timing of surgical intervention for CRS.
Acknowledgments
Funding / Financial Disclosures: Timothy L. Smith, Jeremiah A. Alt, Zachary M. Soler, and Jess C. Mace were supported by a grant for this investigation from a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD; R01 DC005805). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT02720653. The NIDCD did not contribute to the design or conduct of this study; preparation, review, approval or decision to submit this manuscript for publication. Jeremiah A. Alt, is also supported by grants from the University of Utah Program in Personalized Health and the National Center for Advancing Translational Sciences of the National Institute of Health (KL2TR001065) and from the National Institute of Allergy and Infectious Diseases (1R43 AI126987). Richard R. Orlandi is a consultant for BioInspire, and 480 Biomedical. Jeremiah A. Alt and Richard R. Orlandi are consultants for Medtronic, Inc. Jeremiah A. Alt is a consultant for GlycoMira Therapeutics Inc. and Spirox. Zachary M. Soler is a consultant for Olympus, 480 Biomedical and Regeneron. None of these are affiliated with this research.
Footnotes
Conflicts of Interest: None to report
REFERENCES
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007; 137(3 Suppl):S1–31. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg. 2015; 152(4):598–609. [DOI] [PubMed] [Google Scholar]
- 3.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013; 123(10):2341–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins C, Rimmer J, Lund VJ. Does time to endoscopic sinus surgery impact outcomes in Chronic Rhinosinusitis? Prospective findings from the National Comparative Audit of Surgery for Nasal Polyposis and Chronic Rhinosinusitis. Rhinology. 2015; 53(1):10–17. [DOI] [PubMed] [Google Scholar]
- 5.Benninger MS, Sindwani R, Holy CE, Hopkins C. Early versus delayed endoscopic sinus surgery in patients with chronic rhinosinusitis: impact on health care utilization. Otolaryngol Head Neck Surg. 2015; 152(3):546–552. [DOI] [PubMed] [Google Scholar]
- 6.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014; 4(9):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeConde AS, Mace JC, Alt JA, Rudmik L, Soler ZM, Smith TL. Longitudinal improvement and stability of the SNOT-22 survey in the evaluation of surgical management for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015; 5(3): 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014; 140(8):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015; 152(2 Suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 10.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 11.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997; 117(3 Pt 2):S35–40. [DOI] [PubMed] [Google Scholar]
- 12.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996; 106(3 Pt 1):353–356. [DOI] [PubMed] [Google Scholar]
- 13.Levy JM, Mace JC, Bodner TE, Alt JA, Smith TL. Defining the minimal clinically important difference for olfactory outcomes in the surgical treatment of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(8):821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;3 4(5): 447–454. [DOI] [PubMed] [Google Scholar]
- 15.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997; 123(11):1175–1179. [DOI] [PubMed] [Google Scholar]
- 16.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003; 41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 17.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010; 142(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005; 162(3):267–278. [DOI] [PubMed] [Google Scholar]
- 19.Nowakowski AC, Graves KY, Sumerau JE. Mediation analysis of relationships between chronic inflammation and quality of life in older adults. Health Qual Life Outcomes. 2016; 14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalafateli M, Triantos C, Theocharis G, et al. Health-related quality of life in patients with inflammatory bowel disease: a single-center experience. Ann Gastroenterol. 2013; 26(3):243–248. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuriyama M, Kato J, Kuwaki K, et al. Clinical factors that impair health-related quality of life in ulcerative colitis patients vary with the disease duration. Eur J Gastroenterol Hepatol. 2008;20(7):634–641. [DOI] [PubMed] [Google Scholar]
- 22.Do TQ, Barham HP, Earls P, et al. Clinical implications of mucosal remodeling from chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6(8):835–840. [DOI] [PubMed] [Google Scholar]
- 23.Newton E, Janjua A, Lai E, Liu G, Crump T, Sutherland JM. The impact of surgical wait time on patient reported outcomes in sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(12):1156–1161. [DOI] [PubMed] [Google Scholar]
- 24.Sahlstrand-Johnson P, Hopkins C, Ohlsson B, Ahlner-Elmqvist M. The effect of endoscopic sinus surgery on quality of life and absenteeism in patients with chronic rhinosinuitis - a multi-centre study. Rhinology. 2017; 55(3):251–261. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins C, Browne JP, Slack R, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol. 2006; 31(5):390–398. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins C, Andrews P, Holy CE. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Retrospective analysis using the UK clinical practice research data. Rhinology. 2015; 53(1):18–24. [DOI] [PubMed] [Google Scholar]