Abstract
Sustained biomaterial thromboresistance has long been a goal and challenge in blood-contacting device design. Endothelialization is one of the most successful strategies to achieve long-term thromboresistance of blood-contacting devices, with the endothelial cell layer providing dynamic hemostatic regulation. It is well established that endothelial cell behavior is influenced by interactions with the underlying extracellular matrix (ECM). Numerous researchers have sought to exploit these interactions to generate improved blood-contacting devices by investigating the expression of hemostatic regulators in endothelial cells on various ECM coatings. The ability to select substrates that promote endothelial cell-mediated thromboresistance is crucial to advancing material design strategies to improve cardiovascular device outcomes. This review provides an overview of endothelial cell regulation of hemostasis, the major components found within the cardiovascular basal lamina, and the interactions of endothelial cells with prominent ECM components of the basement membrane. A summary of ECM-mimetic strategies used in cardiovascular devices is provided with a focus on the effects of key adhesion modalities on endothelial cell regulators of hemostasis.
Keywords: Coagulation, integrin, cardiovascular devices, endothelial cells, hemostatic regulation
1. Introduction
A critical limitation of early blood-contacting medical devices was their propensity to fail due to thrombus formation from poor biomaterial hemocompatibility. Aggregation of activated platelets can occlude blood vessels and lead to downstream morbidity due to emboli that travel to the patient’s lungs or brain. These early failures led to a critical investigation of methods to prevent coagulation, namely the generation of anti-thrombotic coatings and hemocompatible biomaterials for medical devices. Current research aims to understand and recapitulate the body’s anti-thrombotic surfaces to improve the patency of blood-contacting medical devices. The endothelium provides dynamic hemostatic regulation of all blood-contacting surfaces in the body. Thus, promoting endothelialization of cardiovascular devices is a popular strategy for generating long-term thromboresistance and controlling coagulation without the need for systemic anti-platelet therapies. Endothelial cells prevent platelet activation, provide a protective and selective barrier to underlying tissues, respond to injury, and activate clotting when necessary.110, 123, 129, 155 Importantly, the endothelial cell layer is a dynamic system that accomplishes all of these tasks by responding to cues not only from circulating blood but also from the underlying extracellular matrix (ECM).138 These cues can induce changes in endothelial cell phenotype with a resulting change in hemostatic regulation through the expression and release of anti- or pro-thrombotic constituents.4, 5, 13, 47, 97, 104, 108, 118, 122, 127, 132, 141, 153, 154 Although initial efforts were focused primarily on creating substrates that support endothelial cell adhesion and migration, it is also important to understand the influence of these substrates on the endothelial cell phenotype in regards to hemostatic regulation. The ability to select particular interactions between the endothelium and substrates that limit platelet aggregation and thrombosis will enable advancements in thromboresistant biomaterial design.
The compositional make up and material properties of the ECM vary throughout the body to provide the appropriate cell-material interactions depending on the function of the local tissue. The basement membrane is the ECM that supports endothelial cells in the cardiovascular system with major components consisting of collagen, laminin, nidogen, proteoglycans, and glycosaminoglycans.72, 73, 156 Endothelial cells bind uniquely to these components using different transmembrane proteins, such as integrins and syndecans,.72, 74, 75 Integrin and syndecan binding to ligands on the basement membrane initiates intracellular signaling cascades that affect many cell behaviors including migration, proliferation, apoptosis, and hemostatic regulation.62, 74, 89, 101, 103, 109–111 However, the limited understanding of the individual effects of these relationships has limited thromboresistant biomaterial design. Elucidating key relationships between integrin binding, signaling cascades, and the corollary changes in a cellular hemostatic regulators has become an area of interest for researchers. Elucidation of the key mediators of anti-thrombotic cell behavior can provide improved material design of thromboresistant coatings for blood-contacting devices.
This review will first provide an overview of endothelial cell regulation of hemostasis as it relates to preventing platelet activation and coagulation. A summary of the individual components of the basement membrane and basal lamina and the modalities used by cells to attach to the ECM will then be identified as key regulators of coagulation. Finally, ECM-mimetic strategies used in cardiovascular devices will be reviewed with a focus on the effects of these adhesion modalities (e.g. integrin, syndecan attachment) on endothelial cell behavior.
2. Endothelial Cell Regulation of Hemostasis
Endothelial cells have several mechanisms for regulating coagulation and inflammation.147, 167 In addition to providing a physical barrier to the pro-thrombotic ECM, endothelial cells are responsible for the initiation or direct regulation of coagulation, platelet function, and fibrinolysis to minimize adverse consequences of vascular injury, as well as maximize vascular repair capabilities.119, 123, 145 Disruption of these regulatory functions can lead to cardiovascular disease and eventually death, highlighting the importance of the endothelial layer in cardiovascular systems.2, 11, 26, 36, 39, 95, 110, 123, 155 For example, in Marfan’s Syndrome, mutations in fibrillin-1 result in endothelial dysregulation characterized by low nitric oxide (NO) production that leads to numerous complications in the disease state.24 Therefore, in order to understand the effects of various influencers on endothelial cell hemostatic regulation, it is important to describe this functionality in full.
In controlling coagulation, the endothelial cells bind antithrombin III that is responsible for inactivation of thrombin, Factor Xa, and Factor IXa in the coagulation pathway, slowing coagulation.123 The ECs also express thrombomodulin, which in turn promotes the activation of protein C in concert with endothelial protein C receptor.43, 56 Activated protein C is an anticoagulant that limits the conversion of Factor VIII to Factor VIIIa and prevents the conversion of Factor V to Factor Va.43, 56 Endothelial cells also prevent coagulation by expressing tissue factor pathway inhibitor (TFPI) which inhibits the conversion of Factor VII to Factor VIIa.162 Without these controls, the coagulation pathway would proceed unchecked and clotting would be prolific in the body leading to increased rates of stroke, embolisms, and heart attacks.110, 123
Platelets play a critical role in coagulation with fibrin-stabilized platelet aggregates able to rapidly form hemostatic plugs upon vessel damage.120 Endothelial cells can rapidly promote platelet adhesion and activation by producing and releasing von Willebrand factor (vWF), a blood glycoprotein that binds to Factor VIII as a stabilizing agent against protein C, platelet surface glycoproteins, and constituents of the ECM. vWF exists in two compartments in endothelial cells: constitutively secreted pathway where dimerized vWF is exported to the plasma and subendothelial matrix, and residing in a granular store containing very highly multimerized vWF that can be mobilized rapidly in response to agonists such as thrombin.34 Endothelial cells also produce ADAMTS13, which cleaves the ultra-long vWF strings (ULVWF) that form to capture platelets.15, 45, 141, 151, 170 Endothelial cells also reduce platelet activation by producing prostaglandin I2 (PGI2) and endothelial nitric oxide synthase (eNOS).161 Synthesis of these molecules is triggered by increases of intracellular calcium ion concentrations in endothelial cells.163 PGI2 and nitric oxide (NO) are both potent vasodilators and inhibit platelet activation.78 The powerful anti-aggregatory and vasodilator properties of PGI2 and nitric oxide make them critical regulators of coagulation.
Endothelial cells also play an important role in fibrinolysis, or the enzymatic breakdown of blood clots, an important consideration for medical devices by enabling endothelial cells to help eliminate small clots and prevent large thrombi formation. To this end, endothelial cells synthesize and acutely release tissue plasminogen activator (t-PA), a protein that is involved in the dissolution of blood clots by converting plasminogen to active plasmin. tPA is constitutively released from small granular stores that are separate from vWF stores.12 Endothelial cells release plasminogen activator inhibitor PAI-1 (the main t-PA inhibitor) in activated conditions to prevent excessive fibrinolysis by blocking the action of t-PA.12 The regulation of t-PA and PAI-1 is vital to healthy vasculature because an imbalance of either of these factors leads to hemorrhagic disease or hypercoagulable states.
Endothelial cells are responsible for releasing a number of products that trigger signaling cascades that carefully balance the cell response to maintain hemostasis. A summary of the prothrombotic and antithrombotic agents regulated by endothelial cells is provided in Table 1. When disease states are induced in endothelial cells, either from extracellular or intracellular cues, this balance is perturbed. Therefore, it is critical to maintain endothelial cell health when attempting to recapitulate the endothelial cell environment for cardiovascular applications.
Table 1:
Common hemostatic regulators synthesized by endothelial cells in response to environmental cues.
| Protein | Function | |
|---|---|---|
| Prothrombotic Proteins | Von Willebrand Factor (vWF) | Large multimeric glycoprotein that binds to platelets and proteins during thrombus formation34 |
| Tissue Factor (TF) | Surface protein expressed by activated endothelial cells to initiate coagulation cascade162 | |
| Plasminogen activator inhibitor (PAI-1) | Inhibitor of tPA12 | |
| Antithrombotic Proteins | A disintegrin and metalloproteinase with a thrombospondin type I motif, member 10 (ADAMTS-13) | Enzyme that cleaves vWF141 |
| Tissue factor pathway inhibitor (TFPI) | Major inhibitor of TF, Factor Xa, and thrombin162 | |
| Tissue plasminogen activator (tPA) | Regulator of fibrinolysis12 | |
| Endothelial nitric oxide synthase (eNOS) | enzyme that catalyzes the production of nitric oxide which inhibits platelet aggregation161 | |
| Activated protein C (APC) | Glycoprotein that proteolytically inactivates factor Va and VIIIa to reduce thrombin formation56 |
3. The Cardiovascular Basement Membrane and Basal Lamina
Most vasculature consists of a tri-layer structure, with the tunica adventitia as the outer layer, the tunica media as the middle layer, and the tunica intima as the inner-most layer (Figure 1). Each layer is separated by a fibrous elastin layer. The intimal layer is of particular interest, as it consists of the basement membrane as the innermost layer and the basal lamina supporting the basement membrane directly beneath it. The basement membrane is crucial for the maintenance of a confluent and functional endothelial cell monolayer that provides the dynamic hemostatic regulation described above.26, 72, 75 It is typically 20–120 nm thick and can prevent the movement of cells from one layer to the next while selectively filtering molecules that are transported across it.14, 110 The basement membrane and the basal lamina are composed of many components that work synergistically together to not only promote cell adhesion but also influence cell phenotype and genotype.116, 126, 174 The basement membrane is composed of collagen, laminin, nidogen, glycosaminoglycans, and proteoglycans.65, 106, 171 The basal lamina directly beneath it is composed of collagen, fibronectin, laminin, glycosaminoglycans, and proteoglycans in varying concentrations depending on the location of the tissue in the cardiovascular system.73, 155, 156 Varying these components can change the cell response based on ligand type and availability.68 Although it is the combined presentation of the individual components that drives cellular functions, understanding how each component contributes to the mechanical and biochemical characteristics of the basement membrane will allow for improved constituent selection when creating substrates for endothelial cell growth, whether for antithrombotic coatings or for investigating cell behavior. There are well described differences between endothelial cells of arteries, veins, and capillaries, and researchers have suggested these differences could be associated with basement membrane compositional differences.23, 90 However, these differences have yet to be described. There is strong evidence, particularly in the recent comparisons of organ specific venous and arterial endothelial cells, that the basement membrane composition may differ and affect endothelial cell survival and phenotype.91 There is further evidence that atherosclerotic plaques may be limited by culturing endothelial cells on collagen as compared to fibronectin and fibrinogen.117 This highlights an important future direction of research that could enhance development of vascular coatings and cardiovascular tissue engineering. Until these differences in basement membrane composition are fully understood, we must rely on the current understanding of individual components to guide cell behavior. A summary of the key components of the basal lamina is provided in Table 2 and detailed in the sections below.
Figure 1:
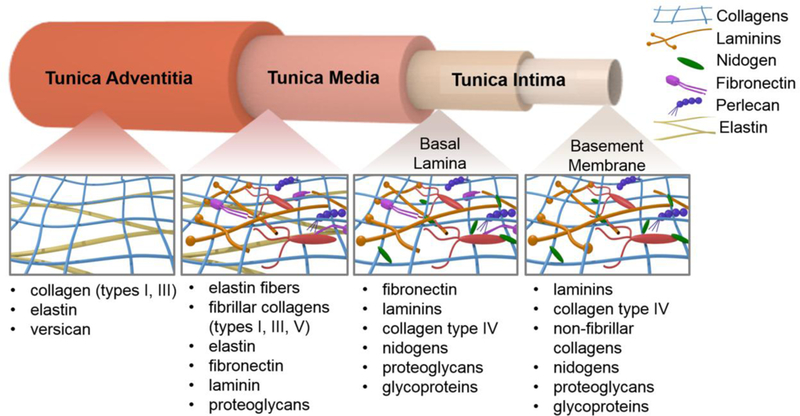
The vascular layers and their associated extracellular matrix components.
Table 2:
Summary of the main ECM components found in the vascular wall, their roles, and respective locations.
| ECM Component | Role | Location in the Vascular Wall |
|---|---|---|
| Collagen | Cell adhesion and signaling; tensile strength | tunica intima (type IV and non-fibrillar), tunica media (types I, III, IV), high relative content in tunica adventitia (types I, III)160 |
| Laminin | Cell adhesion and signaling; ECM structural organization | basement membrane149 |
| Fibronectin | Cell adhesion and signaling | basement membrane 160 |
| Nidogen | Crosslinking of other ECM components | basement membrane, basal lamina148 |
| Glycosaminoglycans, proteoglycans | Cell signaling; hydration | basal lamina, basement membrane115, 136 |
| Elastin | Tissue recoil and elasticity | tunica media50, tunica adventitia80 |
3.1. Collagen
Collagen is the most prominent constituent of the basement membrane and basal lamina and is responsible for tensile strength and cellular adhesion. Although 28 types of collagen have been identified, collagen type I and IV are the most prevalent types found in the cardiovascular system.128 Other non-fibrillar collagens, such as type VIII (formerly known as endothelial collagen), are also important in ECM signaling to endothelial cells.79 Collagens have a hallmark triple helical structure and are typically involved in forming fibrillar networks in the ECM that impart strength and structure to the basement membrane.73 Collagen’s rope-like structure provides resistance to tensile forces by carrying stress.64 Furthermore, collagen is one of the main ECM components responsible for imparting cellular adhesive properties through several receptors including binding sites for the α1β1, α2β1, α10β1, and α11β1 integrins, known as the collagen receptor subfamily of integrins.67 Attachment to these integrins is promoted via the GFOGER peptide sequence on collagen.85, 128, 168 In addition to binding via integrins, cells also bind to collagen through syndecan-1.159 Therefore, collagen is vital to basal lamina due to providing significant mechanical strength and biochemical cues that enable cell adhesion and migration.
3.2. Laminin
Laminin is a key organizer of the basement membrane and basal lamina’s structure as it can self-assemble into sheets, bringing together the other ECM components through crosslinking.172 Laminin is composed of three long polypeptide chains (an α, a β, and a γ chain) held together by disulfide bonds.149 18 laminin trimers have been investigated and described, with laminin-1 as the most prominent in the basal lamina. In addition to being an important ECM crosslinker, laminin enables ECM interactions with many different cell types through its diverse binding sites for cellular surface receptors. For example, laminin has binding sites for integrins α3β1, α6β1, α7β1, and α6β4 as well as binding sites for syndecans 1, 2, and 4, creating a diverse array of cellular responses and interactions.21, 28, 30, 40, 152, 164
3.3. Fibronectin
Fibronectin, a glycoprotein, is another major constituent of the basal lamina and is formed by two nearly identical polypeptide chains attached via disulfide bonds to form a dimer structure.42, 121, 137 Fibronectin exists in both soluble and insoluble forms.121,59 Soluble fibronectin circulates in the blood and other body fluids, and insoluble fibronectin is found within the ECM.144 Although transcribed from a single gene, fibronectin within the ECM has multiple forms as a result of alternative splicing that can generate up to 20 variants.121 These fibronectin variants promote specific cellular and ECM interactions by generating different adhesive ligands. For instance, fibronectin facilitates cellular attachment of endothelial cells to the ECM via integrin binding sites, primarily integrin α5β1, but also αvβ3, α4β1, α4β7, and α9β1 with RGD, PHSRN, LDV, and REDV binding sites.58, 92, 121, 125, 176 Additionally, fibronectin not only binds to cells but also promotes adhesion to other ECM components such as collagen, primarily in denatured regions of collagen triple helices through functional and structural domains, as well as heparin and fibrin through specific binding domains.121 Overall, fibronectin offers complex interactions of the basal lamina with ECs and their environment.
3.4. Nidogen
Nidogen is a small glycoprotein that makes up about 2–3% of the basement membrane.148 Nodogen plays a critical role in ECM organization in the basement membrane as it is responsible for cross-linking collagen, laminin, perlecan, fibrinogen, and fibronectin.1, 25, 41 There are two types, nidogen1 and nidogen2, that are very similar structurally, but have varying relative abundances in different basement membranes.83 Nidogen2 appears to be the most prevalent type in the vascular basement membrane.
3.5. Glycosaminoglycans and Proteoglycans
Glycosaminoglycans (GAGs) are unbranched polysaccharide chains composed of repeating disaccharide units and are essential to the formation of the ECM as hydrophilic space-fillers in both the basement membrane and the basal lamina. GAGs form gels at low concentrations and allow the ECM to resist compressive forces by hydrating and filling most of the extracellular space.18, 52, 64 GAGs are considered the “most anionic molecules produced by animal cells” and hydrate the ECM by attracting water molecules due to their high negative charge.64 GAG chains can be covalently linked to a core protein, forming a proteoglycan.63 Proteoglycans are abundant in the ECM and can regulate the activities of secreted ECM proteins by binding to them. They play a major role in chemical signaling between cells by changing conformations or blocking of binding sites.61, 64 Although many proteoglycans are secreted, some remain as trans-membrane proteins, known as syndecans, and act as receptors for ECM proteins.32 The diverse family of GAGs and proteoglycans are responsible for not only hydration but also ECM-endothelial cell interactions that regulate cellular behavior.
3.6. Elastin
Although it is not a component of the intimal layer that directly affects endothelial cell behavior via binding events, elastin is another major component of other layers of vasculature and provides elastic recovery after stretch or deformation of the tissue that can affect endothelial cell behavior via mechanical cues. Elastin is made from the soluble precursor molecule tropoelastin that generate a highly insoluble crosslinked network. 64, 133, 146 The lysine amino acids of tropoelastin are extensively crosslinked immediately after release from the cell by oxidative deamination of the lysine side chains via the enzyme lysil oxidase with subsequent condensation linking two, three, or four side chains.29, 64 The elasticity of the resulting elastin network is attributed to the loose, random coil conformation of the resulting polypeptide chain. The stretching of elastin is limited by interwoven stiff collagen fibers, and the overall stress response of ECM is dictated by the interplay and concentrations of collagen to elastin. Elastin has not shown cell adhesive properties and the function appears to be limited to providing important mechanical recoil to tissues.64
In summary, the individual components of the ECM work in concert to define the biochemical and mechanical landscape of the basement membrane and basal lamina. Interactions of endothelial cells with these individual components then initiate signaling cascades to affect cytoskeletal organization and gene expression. Understanding the individual qualities and combined synergistic effects of the basement membrane and basal lamina constituents allows for tailoring of substrates for desired cell growth and behavior.
4. Endothelial Cell Interactions with the Extracellular Matrix
Integrins and syndecans are transmembrane receptors that facilitate ECM-endothelial cell adhesion. These transmembrane proteins uniquely interact with ECM ligands and provide a method for signal transduction from the exterior of the cell to the interior. Understanding which integrins and syndecans are responsible for attachment to individual ECM components in the basal lamina will elucidate the signaling cascades leading to changes in endothelial cell gene expression, Figure 2.
Figure 2:
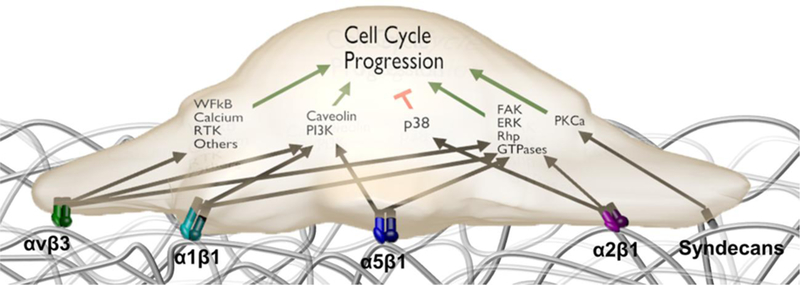
Common integrin and syndecan signaling pathways in endothelial cells.
4.1. Integrin Expression in Endothelial Cells
Integrins are critical for not only anchoring cells to the ECM and mediating migration but also important transducers of intracellular signaling that influence cell phenotype.140,51 Integrins are a large family of transmembrane proteins that exist as heterodimers, with 18 unique α and β subunits that combine to form 24 distinct dimers that bind to specific amino acid sequences within ECM proteins.74 A list of common integrins and their respective ligands are listed in Table 3. Endothelial cells express α1β1 that binds to collagen, α2β1 that binds to collagen and laminin, α3β1, α6β1, and α6β4 that bind to laminin, α4β1 and α5β1 that find to fibronectin, and αvβ3 and αvβ5 that selectively bind to vitronectin.87, 103, 142 The extracellular portion of these transmembrane proteins links to ligands on the ECM, and the intracellular part of integrins associate with actin binding proteins, including vinculin, α-actinin, paxillin, talin, zyxin, tensin, and filamin.93 Signaling pathways are then activated by the actin binding proteins which may lead to downstream changes in the chemical or mechanical composition of the ECM, or affect cell behavior such as proliferation, migration, and differentiation.77, 113 One of the key regulators of integrin-matrix signaling includes the focal adhesion kinase, or FAK, and can play a role in inflammation and hemostasis.98 Focal adhesion complexes also recruit intracellular proteins such as focal adhesion kinase (FAK), a cytoplasmic tyrosine kinase that plays an important role in cell survival by activating essential signaling pathways critical for the prevention of apoptosis.27, 69, 74,130, 135 For example, one of the major signaling pathways initiated by the Src-FAK complex is the Ras-MEK-MAPK pathway that affects the transcription of genes important to cell cycle progression.93
Table 3:
Summary of integrins, cell expression, and attachment proteins.
| Integrin | Proteins with attachment ligands |
|---|---|
| α1β1 | Collagen IV, Colllagen I, Laminin30, 82, 124 |
| α2β1 | Collagen 1, Collagen IV, Laminin143, 152 |
| α5β1 | Gelatin, Fibronectin, Fibrillin-135, 57, 8, 125 |
| αvβ1 | Laminin, Fibronectin, Osteopontin, Vitronectin28, 71, 105, 176 |
| αvβ3 | Gelatin, Fibrinogen, Vitronectin, Thrombospondin, Osteopontin, Fibronectin, VEFG-A, Fibrillin-1, vWF35, 57, 66, 71, 92, 131 |
| α4β1 | Fibronectin58 |
| α6β4 | Laminin164 |
| α6β1 | Laminin21 |
| αvβ5 | Osteopontin, Fibrinogen, Vitronectin, Fibronectin, Thrombospondin71, 76, 157 |
Not only does the type of integrin affect cell adhesion and signaling, but the location of the integrins and their abundance affect the strength of cellular adhesions and response of the cell. For example, focal adhesion complexes are strong, stable adhesions formed when integrins are clustered on the cell surface, allowing for many cytoskeletal filaments to attach at the resulting plaque.48 Endothelial cells are dependent on these attachments for cell survival, as detachment from their substrate leads to upregulation of apoptotic signaling.
There are a multitude of other focal adhesion proteins involved in establishing and maintaining cytoskeletal linkages: integrin-bound proteins that directly bind actin, such as talin, α-actinin, and filamin; integrin-bound proteins that indirectly associate with and regulate the cytoskeleton such as kindling, integrin-linked kinase (ILK), paxillin, and FAK; non-integrin-bound actin-binding proteins, such as vinculin; and adaptor and signaling molecules that regulate the interactions of the proteins of the afore-mentioned groups.93 These molecules then go on to affect many common cellular pathways such as the Akt, ERK, JNK, RhoA, Rac1, and Cdc42 pathways. Each of these pathways then uniquely affects cell survival, proliferation, differentiation, migration, adhesion, and polarity by modulating gene expression, cell cycle regulation, focal adhesion turnover, and actin dynamics. A review by Legate et al. provides detailed information about the individual pathways and their effects.93 For example, β1 integrins are involved in a signaling pathway with RACK that results in increased cell migration toward insulin-like growth factor 1, or IGF-1. Binding of the αvβ3 integrin has been shown to induce a β3-SHP-2 interaction that sequesters phosphatase and prolongs IGF1R signaling, that plays an important role in growth and development.93, 101, 173, 177
Overall, integrins provide complex and wide-ranging modes of attachment and signaling in endothelial cells. The endothelial cell presentation and available ECM binding sites dictate the resulting cell behavior. However, it remains challenging to isolate the role of one integrin from another in order to discern the individual and synergistic contributions of these interactions. Elucidation of these roles would provide greater understanding of how cells regulate hemostasis and how specific binding events can be incorporated into material design to facilitate that process.
4.2. Syndecans
The evolving roles of syndecans, type I membrane glycoproteins composed of GAG chains covalently linked to a core protein, is becoming increasingly important in understanding ECM -endothelial cell interactions.10 Syndecans often act as co-receptors to ligands such as vascular endothelial growth factor (VEGF) and fibronectin that have interactions of particular interest in the ECM and are relevant for tissue engineering.6 The syndecan family is organized into four group members: syndecans-1, −2, −3, and −4, each with distinct functions as shown in Table 4. For example, syndecan-1 regulates cell-interstitial collagen adhesion and binds to fibronectin, as well as growth factors via heparin sulfate chains.32 Syndecan-1 is down regulated in endothelial cells and can promote differentiation in vascular smooth muscle cells.22 Syndecan-2 is also found in endothelial cells and binds to ECM components such as fibronectin and growth factors.32, 44, 166 However, further research is required to understand the specific roles of syndecan-2 in cell adhesion. Syndecan-3 is predominantly found in muscle cells and neuroblastoma cells within the nervous system and binds to certain growth factors.166 However, it has low affinity for fibronectin, collagen I, III, and laminin and therefore plays a limited role in cell adhesion to ECM.46, 84 Therefore, syndecan-3 is of limited interest in hemostatic regulation. Syndecan-4 is found to be ubiquitously expressed in all cell types, making it a more widespread component than the other syndecans and is known to be involved in focal cell adhesion, a tight interaction between the cell and ECM, ensuring intracellular signaling.111 Syndecans influence cell interactions significantly in their roles in cell adhesion and binding to ligands in the ECM.10 Although the importance of syndecans is established, there is still much to learn about the nuances of their function.3, 32, 111, 159, 166
Table 4:
Summary of the syndecan family and their roles as cell attachment mediators.
| Syndecan | Cell type | Syndecan receptors |
|---|---|---|
| Syndecan-1 | Vascular endothelial cells, human umbilical vein endothelial cells, microvascular endothelial cells | Fibronectin, collagen, growth factors9, 70 |
| Syndecan-2 | Human umbilical vein endothelial cells, microvascular endothelial cells | Fibronectin, laminin, collagen, growth factors37, 60, 114 |
| Syndecan-3 | Human coronary artery endothelial cells, brain endothelial cells | Matrix molecules*, growth factors20, 38 |
| Syndecan-4 | Human umbilical vein endothelial cells | Fibronectin, laminin, collagen, growth factors54, 158 |
Matrix molecule not specified
5. Influence of ECM on Endothelial Cell Hemostasis
In an effort to recapitulate the basal lamina, many ECM-mimetic platforms have been used to culture and examine the behavior of endothelial cells.17, 19 As discussed earlier, endothelial cells bind to ECM proteins via integrins and syndecans, which then initiates intracellular signaling that modulates cell behavior. Although many of the intricacies of the cell-ECM binding are not explicitly discussed in many of the reviewed studies, comparisons of behavior between various ECM components can be used to identify roles of different integrin or syndecan binding and corollary effects on coagulation.
5.1. Collagen-initiated Hemostatic Regulation
Collagen is one of the most prevalent adhesive proteins found in the body and is a commonly selected protein to promote enhanced cellular adhesion to biomaterials. Collagen studies are typically performed using mammalian-derived collagen type I or IV either as a coating or as a crosslinked gel.49, 53, 94, 96, 165 Endothelial cell adhesion to collagen is mediated by integrins α1β1, α2β1, α10β1, and α11β1 as well as syndecans 1 and 4.82, 124, 142, 143, 152 These integrins can bind to collagen peptide sequences GFOGER, GLOGERGRO, and GFOGERGVQ.85, 168 Studies have also been performed on collagen mimics such as these peptide sequences that have more tailored integrin interactions.49, 112, 139 These typically require coating studies or chemical crosslinking into another network, as they do not form networks on their own.107
Collagen coat studies have demonstrated a difference in not only cell behaviors such as migration and proliferation as compared to TCPS but also differences in hemostatic regulation.53, 96, 112, 165 For example, endothelial cells cultured on collagen-coated ePTFE demonstrated lower levels of PGI2 and tPA as compared to endothelial cells on an un-coated ePTFE control.100 This would indicated a less thromboresistant phenotype, but this study did not analyze the production of complementary pro-thrombotic factors in the endothelial cells cultured on these substrates.100 Another collagen coat study noted an increase in NO production with endothelial cells on the collagen coat as compared to endothelial cells on TCPS, indicating a more thromboresistant phenotype.49 However, like the previous study, the authors did not discuss the production of pro-thrombotic factors and how the substrate difference would affect their expression. Studies on collagen gels have shown that endothelial cells have decreased expression of PGI2 and vWF, indicating the signaling from collagen attachment is important for endothelial cell regulation of hemostasis. 165
Studies on collagen mimics that allow for more specific integrin binding are utilized to further elucidate the interactions between endothelial cells and collagen. Streptococcal collagen-like protein, or Scl proteins, are a triple helical protein that are recombinantly expressed in E. coli and have no natural adhesion sites. These proteins have tunable bioactivity as peptide sequences can be inserted via site directed mutagenesis in order to target specific binding proteins.169 For example, these studies have incorporated the GFPGER sequence targeting integrins α1β1 and α2β1 that are expressed by endothelial cells to bind to collagen and laminin, creating the Scl2 proteins. Endothelial cells showed increased adhesion on PEG hydrogels containing the Scl2 proteins compared to PEG gels alone, as well as comparable adhesion to PEG hydrogels containing collagen.16, 31 Endothelial cells seeded on these scaffolds demonstrated a decrease in NOS3 and TM gene expression, and e-selectin gene expression increased compared to collagen gels. This suggests that integrins α1β1 and α2β1 binding are responsible for these gene expression changes.112
Based on the evidence in the studies described above and shown in Table 5, the binding of these integrins and syndecans to collagen are responsible for the observed changes in the endothelial cell expression of hemostatic regulators. These integrins could be used as targets to increase the thromboresistance such as through increased NO production. However, parsing out the specific integrin interactions is needed to identify specific integrin binding targets for tissue engineering and cardiovascular device coatings.
Table 5:
Summary of the effects of endothelial cell attachment to collagen on hemostatic regulator molecule expression.
| Substrate Application | Cell type | Molecules tested | Results |
|---|---|---|---|
| Collagen-derived peptide coat | Human umbilical vein endothelial cell; human aortic endothelial cell | PGI2, vWF; PECAM-1, VE-Cadherin, NOS3, TM, E-selectin; | Increase in NOS3 compared to TCPS; No significant change in PGI2, vWF; PECAM-1, VE-Cadherin, TM, E-selectin49 |
| Crosslinked collagen coat | Human umbilical vein endothelial cell; human saphenous vein | PGI2, vWF, tPA, PAI-1 | Decrease in PGI2 and vWF secretion; No difference in basal levels of PGI2, tPA increase compared to bare53, 165 |
| ePTFE with collagen-1 coat | Human saphenous vein; human umbilical vein | tPA, PGI2, PAI-1 | Decrease in PGI2 and tPA; no difference in tPA secretion; PAI increased; Different levels of PGI2, PAI-1 and tPA between unmodified versus modified PTFE53, 96, 175 |
| Collagen derived peptide hydrogel (PEG-Scl2) | Human aortic endothelial cell | PECAM-1, VE-Cadherin, NOS3, TM, E-selectin | Decrease in NOS3 and TM on PEG-Scl2 and E-selectin increased compared to collagen; no significant change in PECAM-1112 |
5.2. Fibronectin and Gelatin-initiated Hemostatic Regulation
Endothelial cells bind to fibronectin via integrin α5β1, but also α4β1, α4β7, and α9β1 as well as syndecan 4.58, 92, 121, 176 When endothelial cells are seeded on fibronectin coats, a measured increase in PGI2 as well as tPA was observed as compared to uncoated ePTFE and TCPS.53, 96, 100 Endothelial cells bind to gelatin also through integrin α5β1 as well as αvβ3, binding specifically to the RGD binding sequence that becomes accessible on denaturation of the collagen triple helix.35, 57 When gelatin is coated on ePTFE, there are increased levels of PGI2 (anti-coagulant), PAI-1 (pro-coagulant), and tPa (anti-coagulant) compared to the unmodified ePTFE, suggesting a more thromboresistant phenotype.175 Studies are commonly performed using RGD, as it is a readily available peptide sequence. When endothelial cells are cultured on RGD that is incorporated into hydrogels, ADAMTS-13 (anti-coagulant), TFPI (anti-coagulant), tPA (anti-coagulant), vWF (pro-coagulant), TF (pro-coagulant), P-selectin (pro-coagulant) all increased compared to TCPS.6 With these increases and results summarized in Table 6, endothelial cells appear to be much more activated on RGD peptides that attach to the integrins α5β1 and αvβ3. Again, these changes highlight specific integrin and syndecan targeting are influencing the endothelial cell’s ability to promote coagulation.
Table 6:
Summary of the effects of endothelial cell attachment to fibronectin and gelatin on hemostatic regulator molecule expression.
| ECM Protein | Substrate Application | Cell type | Molecules tested | Results |
|---|---|---|---|---|
| Gelatin | Gelatin coat on TCPS | Bovine aortic endothelial cell; human saphenous vein endothelial cell | PGI2, PAI, tPA | Increase in NO and PAI; Decrease in PGI2 compared to TCPS7, 53 |
| ePTFE with gelatin coat | Human umbilical vein endothelial cell | PGI2, PAI-1, tPA | Increase in PGI2, PAI-1 and t-PA compared to bare100 | |
| PEG-RGD hydrogel | Porcine aortic valvular endothelial cell | ADAMTS-13, TFPI, tPA, vWF, TF, P-selectin | ADAMTS-13, TFPI, tPA, tPA, vWF, TF, P-selectin all increased compared to TCPS6 | |
| Fibronectin | Fibronectin coat on TCPS | Bovine aortic; human umbilical vein; human saphenous vein | PGI2, vWF, tPA, PAI-1 | Increase in PGI2; decreased vWF compared to TCPS; no significant change in tPA7, 53, 165 |
| ePTFE with Fn coat | Human saphenous vein endothelial cell; human umbilical vein endothelial cell | tPA, PGI2, PAI-1 | Increase in PGI2 and tPA compared to bare ePTFE; no significant change in PAI-196, 175 |
5.3. Laminin-initiated Hemostatic Regulation
Endothelial cells bind to laminin via integrins α3β1, α6β1, α7β1, and α6β4 as well as syndecan 2.21, 28, 30, 142, 164 These integrins commonly bind to laminin peptide sequences that contain YIGSR.55 Studies on laminin coats have demonstrated an increase in PGI2 expression, indicating a more thromboresistant phenotype of the endothelial cells.7 Studies have also been performed on laminin-mimetic hydrogels. A laminin peptide sequence targeting syndecan binding was covalently linked to the surface of poly(ethylene glycol) diacrylate hydrogels. Cells grown on these constructs were then compared to hydrogels with RGD binding sequences linked to the surface of the gels, which interact with integrins α5β1 and αvβ3.6 The endothelial cells grown on the laminin-mimetic gels showed ADAMTS-13, TFPI, tPA, vWF, TF, and P-selectin all increased, with NO increased compared to plate grown cells.6 The change of gene expression of hemostatic regulators in targeting syndecan binding indicates that syndecans also play an important role in endothelial cell hemostatic regulation. The effects of laminin on endothelial cell hemostasis are shown in Table 7.
Table 7:
Summary of the effects of endothelial cell attachment to laminin on hemostatic regulator molecule expression.
| Substrate Application | Cell Type | Molecule Tested | Results |
|---|---|---|---|
| Laminin coat on TCPS | Bovine aortic endothelial cell | PGI2 | Increase in PGI2 compared to TCPS7 |
| PEG-laminin peptide hydrogel | Porcine aortic valvular endothelial cell; human aortic endothelial cell | ADAMTS-13, TFPI, tPA, vWF, TF, P-selectin; NO | ADAMTS-13, TFPI, tPA, tPA, vWF, TF, P-selectin all increased; NO increased compared to TCPS6, 49 |
6. Summary and Future Directions
This review provides a summary of key interactions of endothelial cells with prominent ECM components of the basal lamina and how these interactions affect endothelial cell hemostatic regulation. Understanding the effects of ECM-endothelial cell interactions provides the potential to design improved blood-contacting materials with long-term thromboresistance. The molecules discussed in this review are focused on the major cellular interactions found between endothelial cells and ECM components of the basal lamina, with more minor and possibly uncharacterized interactions assumed to also be occurring. Further investigation in the area of hemostatic regulation is important because it plays a direct role in thrombotic and embolic complications of cardiovascular devices. It is expected that insight into endothelial cell hemostatic regulation will drive biomedical device design for the reduction of coagulation and increased blood compatibility.
In order to develop an endothelial layer coating with the appropriate hemostatic regulating behavior, the effect of individual and combined integrin interactions should be further elucidated. The studies reviewed here have demonstrated the importance of the substrate on endothelial cell hemostatic regulation, but few have investigated specific binding interactions and the correlated changes in endothelial cell gene expression and thromboresistance. Future investigation would need to isolate integrin interactions on well controlled surfaces for the culture of endothelia cells. Using these platforms it would then be possible to analyze the changes in gene expression as well as the functional changes in endothelial cell hemostatic regulation. Once specific interactions have been correlated to changes in hemostatic regulation, mechanisms and signaling pathways can be elucidated. Understanding signaling pathways and mechanisms could lead to further tailoring of coating designs for endothelialization of biomedical devices. By elucidating the specific effects of integrin attachment on hemostatic regulation, it would then be possible to target specific endothelial cell binding mechanisms to promote a thromboresistant phenotype in an endothelial cell monolayer.
References:
- 1.Aeschlimann D and Paulsson M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. Journal of Biological Chemistry 266: 15308–15317, 1991. [PubMed] [Google Scholar]
- 2.Aird WC Endothelial cell heterogeneity. Cold Spring Harbor perspectives in medicine 2: a006429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexopoulou AN, Multhaupt HA and Couchman JR. Syndecans in wound healing, inflammation and vascular biology. The international journal of biochemistry & cell biology 39: 505–528, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bae J-S, Yang L and Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proceedings of the National Academy of Sciences 104: 2867–2872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajzar L, Morser J and Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. Journal of Biological Chemistry 271: 16603–16608, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Balaoing LR, Post AD, Lin AY, Tseng H, Moake JL and Grande-Allen KJ. Laminin peptide-immobilized hydrogels modulate valve endothelial cell hemostatic regulation. PloS one 10: e0130749, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balcells M and Edelman ER. Effect of pre‐adsorbed proteins on attachment, proliferation, and function of endothelial cells. Journal of cellular physiology 191: 155–161, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ and Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by α5β1 and αvβ3 integrins. Journal of Biological Chemistry 278: 34605–34616, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Beauvais DM, Ell BJ, McWhorter AR and Rapraeger AC. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. Journal of Experimental Medicine 206: 691–705, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernfield M, Kokenyesi R, Kato M, Hinkes M, Spring J, Gallo R and Lose E. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annual review of cell biology 8: 365–393, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Bonetti PO, Lerman LO and Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology 23: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Booyse FM, Aikens ML and Grenett HE. Endothelial Cell Fibrinolysis: Transcriptional Regulation of Fibrinolytic Protein Gene Expression (t‐PA, u‐PA, and PAI‐1) by Low Alcohol. Alcoholism: clinical and experimental research 23: 1119–1124, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Bos GW, Poot AA, Beugeling T, van Aken WG and Feijen J. Small-Diameter Vascular Graft Prostheses: Current Status. Archives of Physiology and Biochemistry 106: 100–115, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Bouïs D, Hospers GA, Meijer C, Molema G and Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4: 91–102, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Broberg M and Nygren H. Von Willebrand factor, a key protein in the exposure of CD62P on platelets. Biomaterials 22: 2403–2409, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Browning MB, Guiza V, Russell B, Rivera J, Cereceres S, Höök M, Hahn MS and Cosgriff-Hernandez EM. Endothelial cell response to chemical, biological, and physical cues in bioactive hydrogels. Tissue Engineering Part A 20: 3130–3141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan MR and Shah MM. Translating biomaterial properties to intracellular signaling. Cell biochemistry and biophysics 54: 1–10, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Cardin AD and Weintraub H. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis: An Official Journal of the American Heart Association, Inc 9: 21–32, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Carey DJ Control of growth and differentiation of vascular cells by extracellular matrix proteins. Annual review of physiology 53: 161–177, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Carey DJ Syndecans: multifunctional cell-surface co-receptors. Biochemical Journal 327: 1–16, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chartier NT, Lainé M, Gout S, Pawlak G, Marie CA, Matos P, Block MR and Jacquier-Sarlin MR. Laminin-5-integrin interaction signals through PI 3-kinase and Rac1b to promote assembly of adherens junctions in HT-29 cells. Journal of cell science 119: 31–46, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chaterji S, Lam CH, Ho DS, Proske DC and Baker AB. Syndecan-1 regulates vascular smooth muscle cell phenotype. PloS one 9: e89824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi J-T, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D and Brown PO. Endothelial cell diversity revealed by global expression profiling. Proceedings of the National Academy of Sciences 100: 10623–10628, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung A, Yeung KA, Cortes S, Sandor G, Judge D, Dietz H and Van Breemen C. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. British journal of pharmacology 150: 1075–1083, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung AE, Dong L-J, Wu C and Durkin ME. Biological functions of entactin. Kidney international 43: 13–19, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt A-M and Stern DM. Endothelial Cells in Physiology and in the Pathophysiology of Vascular Disorders. Blood 91: 3527–3561, 1998. [PubMed] [Google Scholar]
- 27.Clark EA and Brugge JS. Integrins and signal transduction pathways: the road taken. Science 268: 233, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Clark P, Coles D and Peckham M. Preferential adhesion to and survival on patterned laminin organizes myogenesisin vitro. Experimental Cell Research 230: 275–283, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Szot S, Williams MA and Kagan HM. Oxidation of lysine side-chains of elastin by the myeloperoxidase system and by stimulated human neutrophils. Biochemical and biophysical research communications 135: 451–457, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Colognato H, MacCarrick M, Julian J and Yurchenco PD. The laminin α2-chain short arm mediates cell adhesion through both the α1β1 and α2β1 integrins. Journal of Biological Chemistry 272: 29330–29336, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Cosgriff-Hernandez E, Hahn MS, Russell B, Wilems T, Munoz-Pinto D, Browning MB, Rivera J and Höök M. Bioactive hydrogels based on designer collagens. Acta Biomaterialia 6: 3969–3977, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Couchman JR, Chen L and Woods A. Syndecans and cell adhesion. International review of cytology 207: 113–150, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Crawford DC, Chobanian A and Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circulation research 74: 727–739, 1994. [DOI] [PubMed] [Google Scholar]
- 34.da Silva ML and Cutler DF. von Willebrand factor multimerization and the polarity of secretory pathways in endothelial cells. Blood 128: 277–285, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis GE Affinity of integrins for damaged extracellular matrix: αvβ3 binds to denatured collagen type I through RGD sites. Biochemical and biophysical research communications 182: 1025–1031, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Davis GE and Senger DR. Endothelial extracellular matrix biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation research 97: 1093–1107, 2005. [DOI] [PubMed] [Google Scholar]
- 37.De Rossi G, Evans AR, Kay E, Woodfin A, McKay TR, Nourshargh S and Whiteford JR. Shed syndecan-2 inhibits angiogenesis. J Cell Sci 127: 4788–4799, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rossi G and Whiteford JR. A novel role for syndecan-3 in angiogenesis. F1000Research 2: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deanfield JE, Halcox JP and Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation 115: 1285–1295, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Laminins Durbeej M.. Cell and Tissue Research 339: 259–268, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Erickson AC and Couchman JR. Still more complexity in mammalian basement membranes. Journal of Histochemistry & Cytochemistry 48: 1291–1306, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Erickson HP, Carrell N and McDONAGH J. Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol 91: 673–678, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esmon CT Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Critical care medicine 29: S48–S51, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Fears CY, Gladson CL and Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. Journal of Biological Chemistry 281: 14533–14536, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Feys H, Anderson P, Vanhoorelbeke K, Majerus E and Sadler J. Multi‐step binding of ADAMTS‐13 to von Willebrand factor. Journal of Thrombosis and Haemostasis 7: 2088–2095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuentealba L, Carey DJ and Brandan E. Antisense inhibition of syndecan-3 expression during skeletal muscle differentiation accelerates myogenesis through a basic fibroblast growth factor-dependent mechanism. Journal of Biological Chemistry 274: 37876–37884, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Fukudome K, Kurosawa S, Stearns-Kurosawa DJ, He X, Rezaie AR and Esmon CT. The endothelial cell protein C receptor cell surface expression and direct ligand binding by the soluble receptor. Journal of Biological Chemistry 271: 17491–17498, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Geiger B, Spatz JP and Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10: 21–33, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Genové E, Shen C, Zhang S and Semino CE. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 26: 3341–3351, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Gerrity R and Cliff W. The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Laboratory investigation; a journal of technical methods and pathology 32: 585–600, 1975. [PubMed] [Google Scholar]
- 51.Giancotti FG Complexity and specificity of integrin signalling. Nature Cell Biology 2: E13–E14, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Gill VL, Aich U, Rao S, Pohl C and Zaia J. Disaccharide analysis of glycosaminoglycans using hydrophilic interaction chromatography and mass spectrometry. Analytical chemistry 85: 1138–1145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillis C, Bengtsson L and Wiman B. Secretion of prostacyclin, tissue plasminogen activator and its inhibitor by cultured adult human endothelial cells grown on different matrices. European Journal of Vascular and Endovascular Surgery 11: 127–133, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A and Couchman JR. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. Journal of Biological Chemistry jbc M109. 056945, 2010. [DOI] [PMC free article] [PubMed]
- 55.Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, Robey FA and Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell 48: 989–996, 1987. [DOI] [PubMed] [Google Scholar]
- 56.Griffin J, Fernandez J, Gale A and Mosnier L. Activated protein C. Journal of Thrombosis and Haemostasis 5: 73–80, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Grover CN, Gwynne JH, Pugh N, Hamaia S, Farndale RW, Best SM and Cameron RE. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomaterialia 8: 3080–3090, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan J-L and Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell 60: 53–61, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Hahn A, Regenass S, Kern F, Buhler F and Resink T. Expression of soluble and insoluble fibronectin in rat aorta: effects of angiotensin II and endothelin-1. Biochemical and biophysical research communications 192: 189–197, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Halden Y, Angelika R, Atzenhofer W, Szilak L, Wabnig A and Andreas J. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochemical Journal 377: 533–538, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardingham T and Fosang A. Proteoglycans: many forms and many functions. The FASEB Journal 6: 861–870, 1992. [PubMed] [Google Scholar]
- 62.Hasan SS and Siekmann AF. The same but different: signaling pathways in control of endothelial cell migration. Current opinion in cell biology 36: 86–92, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Hassell JR, Kimura JH and Hascall VC. Proteoglycan core protein families. Annual review of biochemistry 55: 539–567, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Hay ED Cell Biology of Extracellular Matrix Springer US, 1991, p. 468. [Google Scholar]
- 65.Hayashi K, Madri JA and Yurchenco PD. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. The Journal of Cell Biology 119: 945–959, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Healy JM, Murayama O, Maeda T, Yoshino K, Sekiguchi K and Kikuchi M. Peptide Ligands for Integrin. alpha. v. beta. 3 Selected from Random Phage Display Libraries. Biochemistry 34: 3948–3955, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Heino J The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biology 19: 319–323, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Herbst TJ, McCarthy JB, Tsilibary EC and Furcht LT. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. The Journal of Cell Biology 106: 1365–1373, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodivala-Dilke KM, Reynolds AR and Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell and Tissue Research 314: 131–144, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Hozumi K, Suzuki N, Nielsen PK, Nomizu M and Yamada Y. Laminin α1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin α2β1. Journal of Biological Chemistry 2006. [DOI] [PubMed]
- 71.Hu DD, Lin EC, Kovach NL, Hoyer JR and Smith JW. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. Journal of Biological Chemistry 270: 26232–26238, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Hynes R Cell–matrix adhesion in vascular development. Journal of Thrombosis and Haemostasis 5: 32–40, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Hynes RO The Extracellular Matrix: Not Just Pretty Fibrils. Science 326: 1216–1219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hynes RO Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25. [DOI] [PubMed] [Google Scholar]
- 75.Iivanainen E, Kähäri VM, Heino J and Elenius K. Endothelial cell–matrix interactions. Microscopy research and technique 60: 13–22, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Isik FF, Gibran NS, Jang YC, Sandell L and Schwartz SM. Vitronectin decreases microvascular endothelial cell apoptosis. Journal of cellular physiology 175: 149–155, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Ivaska J and Heino J *. Adhesion receptors and cell invasion: mechanisms of integrin-guided degradation of extracellular matrix. Cellular and Molecular Life Sciences CMLS 57: 16–24, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kadowitz P, Chapnick B, Feigen L, Hyman A, Nelson P and Spannhake E. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. Journal of Applied Physiology 45: 408–413, 1978. [DOI] [PubMed] [Google Scholar]
- 79.Kalluri R Angiogenesis: Basement membranes: structure, assembly and role in tumour angiogenesis. Nature Reviews Cancer 3: 422, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Karrer H An electron microscope study of the aorta in young and in aging mice. Journal of ultrastructure research 5: 1–27, 1961. [DOI] [PubMed] [Google Scholar]
- 81.Kato H, Suzuki H, Tajima S, Ogata Y, Tominaga T, Sato A and Saruta T. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. Journal of hypertension 9: 17–22, 1991. [PubMed] [Google Scholar]
- 82.Kern A and Marcantonio EE. Role of the I‐domain in collagen binding specificity and activation of the integrins α1β1 and α2β1. Journal of cellular physiology 176: 634–641, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Kimura N, Toyoshima T, Kojima T and Shimane M. Entactin-2: a new member of basement membrane protein with high homology to entactin/nidogen. Experimental Cell Research 241: 36–45, 1998. [DOI] [PubMed] [Google Scholar]
- 84.Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB and Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. Journal of Biological Chemistry 273: 10702–10708, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW and Barnes MJ. The Collagen-binding A-domains of Integrins α1β1 and α2β1recognize the same specific amino acid sequence, GFOGER, in native (Triple-helical) collagens. Journal of Biological Chemistry 275: 35–40, 2000. [DOI] [PubMed] [Google Scholar]
- 86.Kramer R, Bensch K, Davison P and Karasek M. Basal lamina formation by cultured microvascular endothelial cells. The Journal of Cell Biology 99: 692–698, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kreidberg JA Functions of α3β1 integrin. Current opinion in cell biology 12: 548–553, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Kusuma S, Zhao S and Gerecht S. The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells. The FASEB Journal 26: 4925–4936, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamalice L, Le Boeuf F and Huot J. Endothelial Cell Migration During Angiogenesis. Circulation research 100: 782–794, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Lang I, Pabst MA, Hiden U and Blaschitz A. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. European journal of cell biology 82: 163, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Lassance L, Miedl H, Konya V, Heinemann A, Ebner B, Hackl H, Desoye G and Hiden U. Differential response of arterial and venous endothelial cells to extracellular matrix is modulated by oxygen. Histochemistry and cell biology 137: 641–655, 2012. [DOI] [PubMed] [Google Scholar]
- 92.Leahy DJ, Hendrickson WA, Aukhil I and Erickson HP. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science 258: 987–991, 1992. [DOI] [PubMed] [Google Scholar]
- 93.Legate KR, Wickström SA and Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes & Development 23: 397–418, 2009. [DOI] [PubMed] [Google Scholar]
- 94.Leitinger B and Hohenester E. Mammalian collagen receptors. Matrix Biology 26: 146–155, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Lerman A and Zeiher AM. Endothelial function cardiac events. Circulation 111: 363–368, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Li J. m., Menconi MJ, Wheeler HB, Rohrer MJ, Klassen VA, Ansell JE and Appel MC. Precoating expanded polytetrafluoroethylene grafts alters production of endothelial cell—derived thrombomodulators. Journal of Vascular Surgery 15: 1010–1017, 1992. [PubMed] [Google Scholar]
- 97.Li S, Huang NF and Hsu S. Mechanotransduction in endothelial cell migration. Journal of cellular biochemistry 96: 1110–1126, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Lim S-T, Miller NLG, Chen XL, Tancioni I, Walsh CT, Lawson C, Uryu S, Weis SM, Cheresh DA and Schlaepfer DD. Nuclear-localized focal adhesion kinase regulates inflammatory VCAM-1 expression. The Journal of Cell Biology 197: 907–919, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X, Wu H, Byrne M, Krane S and Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proceedings of the National Academy of Sciences 94: 1852–1856, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu A and Sipehia R. Antithrombotic and fibrinolytic system of human endothelial cells seeded on PTFE: the effects of surface modification of PTFE by ammonia plasma treatment and ECM protein coatings. Biomaterials 22: 1439–1446, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Lu Q and Rounds S. Focal Adhesion Kinase and Endothelial Cell Apoptosis. Microvascular research 83: 56–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madri JA and Williams SK. Capillary endothelial cell cultures: phenotypic modulation by matrix components. The Journal of Cell Biology 97: 153–165, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahabeleshwar GH, Chen J, Feng W, Somanath PR, Razorenova OV and Byzova TV. Integrin affinity modulation in angiogenesis. Cell Cycle 7: 335–347, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Majerus EM, Zheng X, Tuley EA and Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. Journal of Biological Chemistry 278: 46643–46648, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall JF, Rutherford DC, McCartney A, Mitjans F, Goodman SL and Hart IR. Alpha v beta 1 is a receptor for vitronectin and fibrinogen, and acts with alpha 5 beta 1 to mediate spreading on fibronectin. Journal of cell science 108: 1227–1238, 1995. [DOI] [PubMed] [Google Scholar]
- 106.Martin GR and Timpl R. Laminin and other basement membrane components. Annual review of cell biology 3: 57–85, 1987. [DOI] [PubMed] [Google Scholar]
- 107.McGuigan AP and Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 28: 2547–2571, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fibrinolysis Medcalf R., inflammation, and regulation of the plasminogen activating system. Journal of Thrombosis and Haemostasis 5: 132–142, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Mehta D and Malik AB. Signaling Mechanisms Regulating Endothelial Permeability. Physiological Reviews 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Michiels C Endothelial cell functions. Journal of cellular physiology 196: 430–443, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Morgan MR, Humphries MJ and Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nature Reviews Molecular Cell Biology 8: 957–969, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Munoz-Pinto DJ, Guiza-Arguello VR, Becerra-Bayona SM, Erndt-Marino J, Samavedi S, Malmut S, Russell B, Höök M and Hahn MS. Collagen-mimetic hydrogels promote human endothelial cell adhesion, migration and phenotypic maturation. Journal of Materials Chemistry B 3: 7912–7919, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niu G and Chen X. Why integrin as a primary target for imaging and therapy. Theranostics 1: 30–47, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noguer O, Villena J, Lorita J, Vilaró S and Reina M. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Experimental Cell Research 315: 795–808, 2009. [DOI] [PubMed] [Google Scholar]
- 115.O’shea K and Dixit V. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. The Journal of Cell Biology 107: 2737–2748, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olivero DK and Furcht LT. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Investigative Ophthalmology & Visual Science 34: 2825–2834, 1993. [PubMed] [Google Scholar]
- 117.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ and Schwartz MA. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J Cell Biol 169: 191–202, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osterud B, Bajaj M and Bajaj S. Sites of tissue factor pathway inhibitor (TFPI) and tissue factor expression under physiologic and pathologic conditions. On behalf of the Subcommittee on Tissue factor Pathway Inhibitor (TFPI) of the Scientific and Standardization Committee of the ISTH. Thrombosis and haemostasis 73: 873–875, 1995. [PubMed] [Google Scholar]
- 119.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD and Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nature Reviews Cardiology 9: 439–453, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Packham MA Role of platelets in thrombosis and hemostasis. Canadian journal of physiology and pharmacology 72: 278–284, 1994. [DOI] [PubMed] [Google Scholar]
- 121.Pankov R and Yamada KM. Fibronectin at a glance. Journal of cell science 115: 3861–3863, 2002. [DOI] [PubMed] [Google Scholar]
- 122.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK and Gimbrone MA Jr. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. The Journal of Clinical Investigation 116: 49–58, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pearson JD Endothelial cell function and thrombosis. Best Practice & Research Clinical Haematology 12: 329–341, 1999. [DOI] [PubMed] [Google Scholar]
- 124.Perret S, Eble JA, Siljander PR-M, Merle C, Farndale RW, Theisen M and Ruggiero F. Prolyl-hydroxylation of collagen type I is required for efficient binding to integrin α1β1 and platelet GPVI but not to α2β1. Journal of Biological Chemistry 2003. [DOI] [PubMed]
- 125.Pytela R, Pierschbacher MD and Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proceedings of the National Academy of Sciences 82: 5766–5770, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raines EW The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. International journal of experimental pathology 81: 173–182, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG and Thorpe PE. Antiphospholipid antibodies promote leukocyte–endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. The Journal of Clinical Investigation 121: 120–131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ricard-Blum S The Collagen Family. Cold Spring Harbor Perspectives in Biology 3: a004978, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodgers GM Hemostatic properties of normal and perturbed vascular cells. The FASEB Journal 2: 116–123, 1988. [DOI] [PubMed] [Google Scholar]
- 130.Rowe SL and Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules 7: 2942–2948, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rüegg C and Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cellular and Molecular Life Sciences CMLS 60: 1135–1157, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sakata Y, Curriden S, Lawrence D, Griffin JH and Loskutoff DJ. Activated protein C stimulates the fibrinolytic activity of cultured endothelial cells and decreases antiactivator activity. Proceedings of the National Academy of Sciences 82: 1121–1125, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sandberg LB, Weissman N and Gray WR. Structural features of tropoelastin related to the sites of cross-links in aortic elastin. Biochemistry 10: 52–56, 1971. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt A, Brixius K and Bloch W. Endothelial precursor cell migration during vasculogenesis. Circulation research 101: 125–136, 2007. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz MA Integrin signaling revisited. Trends in Cell Biology 11: 466–470, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Schwarzbauer J Basement membrane: Putting up the barriers. Current Biology 9: R242–R244, 1999. [DOI] [PubMed] [Google Scholar]
- 137.Schwarzbauer JE, Spencer CS and Wilson CL. Selective secretion of alternatively spliced fibronectin variants. The Journal of Cell Biology 109: 3445–3453, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Semenza GL Vascular responses to hypoxia and ischemia. Arteriosclerosis, Thrombosis, and Vascular Biology 30: 648–652, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR and Detmar M. The α1β1 and α2β1 Integrins Provide Critical Support for Vascular Endothelial Growth Factor Signaling, Endothelial Cell Migration, and Tumor Angiogenesis. The American Journal of Pathology 160: 195–204, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shattil SJ and Ginsberg MH. Perspectives series: cell adhesion in vascular biology. Integrin signaling in vascular biology. Journal of Clinical Investigation 100: 1–5, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shim K, Anderson PJ, Tuley EA, Wiswall E and Sadler JE. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood 111: 651–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Short SM, Talbott GA and Juliano RL. Integrin-mediated signaling events in human endothelial cells. Molecular Biology of the Cell 9: 1969–1980, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Staatz W, Fok K, Zutter M, Adams S, Rodriguez B and Santoro S. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. Journal of Biological Chemistry 266: 7363–7367, 1991. [PubMed] [Google Scholar]
- 144.Stenman S and Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. Journal of Experimental Medicine 147: 1054–1064, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sumpio BE, Timothy Riley J and Dardik A. Cells in focus: endothelial cell. The international journal of biochemistry & cell biology 34: 1508–1512, 2002. [DOI] [PubMed] [Google Scholar]
- 146.Swee MH, Parks WC and Pierce RA. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. Journal of Biological Chemistry 270: 14899–14906, 1995. [DOI] [PubMed] [Google Scholar]
- 147.Szmitko PE, Wang C-H, Weisel RD, de Almeida JR, Anderson TJ and Verma S. New markers of inflammation and endothelial cell activation part I. Circulation 108: 1917–1923, 2003. [DOI] [PubMed] [Google Scholar]
- 148.TIMPL R, DZIADEK M, FUJIWARA S, NOWACK H and WICK G. Nidogen: A new, self‐aggregating basement membrane protein. European journal of biochemistry 137: 455–465, 1983. [DOI] [PubMed] [Google Scholar]
- 149.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart J-M and Martin GR. Laminin--a glycoprotein from basement membranes. Journal of Biological Chemistry 254: 9933–9937, 1979. [PubMed] [Google Scholar]
- 150.Tsamis A, Krawiec JT and Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. Journal of the Royal Society Interface 10: 20121004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Turner NA, Nolasco L, Ruggeri ZM and Moake JL. Endothelial cell ADAMTS-13 and VWF: production, release, and VWF string cleavage. Blood 114: 5102–5111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Underwood PA, Bennett FA, Kirkpatrick A, Bean PA and Moss BA. Evidence for the location of a binding sequence for the α2β1 integrin of endothelial cells, in the β1 subunit of laminin. Biochemical Journal 309: 765–771, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valentijn KM, van Driel LF, Mourik MJ, Hendriks G-J, Arends TJ, Koster AJ and Valentijn JA. Multigranular exocytosis of Weibel-Palade bodies in vascular endothelial cells. Blood 116: 1807–1816, 2010. [DOI] [PubMed] [Google Scholar]
- 154.Van Hinsbergh V, Bertina R, Van Wijngaarden A, Van Tilburg N, Emeis J and Haverkate F. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood 65: 444–451, 1985. [PubMed] [Google Scholar]
- 155.van Hinsbergh VW Endothelium—role in regulation of coagulation and inflammation. In: Seminars in Immunopathology Springer, 2012, p. 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van Hinsbergh VW The endothelium: vascular control of haemostasis. European Journal of Obstetrics & Gynecology and Reproductive Biology 95: 198–201, 2001. [DOI] [PubMed] [Google Scholar]
- 157.Vogel BE, Lee S-J, Hildebrand A, Craig W, Pierschbacher MD, Wong-Staal F and Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. The Journal of Cell Biology 121: 461–468, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vuong TT, Reine TM, Sudworth A, Jenssen TG and Kolset SO. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. Journal of Histochemistry & Cytochemistry 63: 280–292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vuoriluoto K, Jokinen J, Kallio K, Salmivirta M, Heino J and Ivaska J. Syndecan-1 supports integrin α2β1-mediated adhesion to collagen. Experimental Cell Research 314: 3369–3381, 2008. [DOI] [PubMed] [Google Scholar]
- 160.Wagenseil JE and Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiological Reviews 89: 957–989, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Warner TD, Armstrong PC, Chan MV and Knowles RB. The importance of endothelium-derived mediators to the efficacy of dual anti-platelet therapy Taylor & Francis, 2016. [DOI] [PubMed] [Google Scholar]
- 162.White TA, Johnson T, Zarzhevsky N, Tom C, Delacroix S, Holroyd EW, Maroney SA, Singh R, Pan S and Fay WP. Endothelial-derived tissue factor pathway inhibitor regulates arterial thrombosis but is not required for development or hemostasis. Blood 116: 1787–1794, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whorton A, Willis C, Kent R and Young S. The role of calcium in the regulation of prostacyclin synthesis by porcine aortic endothelial cells. Lipids 19: 17–24, 1984. [DOI] [PubMed] [Google Scholar]
- 164.Winterwood NE, Varzavand A, Meland MN, Ashman LK and Stipp CS. A critical role for tetraspanin CD151 in α3β1 and α6β4 integrin–dependent tumor cell functions on laminin-5. Molecular Biology of the Cell 17: 2707–2721, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wissink M, Beernink R, Poot A, Engbers G, Beugeling T, van Aken W and Feijen J. Relation between cell density and the secretion of von Willebrand factor and prostacyclin by human umbilical vein endothelial cells. Biomaterials 22: 2283–2290, 2001. [DOI] [PubMed] [Google Scholar]
- 166.Woods A Syndecans: transmembrane modulators of adhesion and matrix assembly. The Journal of Clinical Investigation 107: 935–941, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu MDKK and Thiagarajan MDP. ROLE OF ENDOTHELIUM IN THROMBOSIS AND HEMOSTASIS. Annual Review of Medicine 47: 315–331, 1996. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Höök A and Höök M. Multiple binding sites in collagen type I for the integrins α1β1 and α2β1. Journal of Biological Chemistry 275: 38981–38989, 2000. [DOI] [PubMed] [Google Scholar]
- 169.Xu Y, Keene DR, Bujnicki JM, Höök M and Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. Journal of Biological Chemistry 277: 27312–27318, 2002. [DOI] [PubMed] [Google Scholar]
- 170.YEH HC, Zhou Z, Choi H, Tekeoglu S, May W, Wang C, Turner N, Scheiflinger F, Moake JL and DONG JF. Disulfide bond reduction of von Willebrand factor by ADAMTS‐13. Journal of Thrombosis and Haemostasis 8: 2778–2788, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yurchenco PD and Schittny JC. Molecular architecture of basement membranes. The FASEB Journal 4: 1577–1590, 1990. [DOI] [PubMed] [Google Scholar]
- 172.Yurchenco PD, Tsilibary E, Charonis A and Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. Journal of Biological Chemistry 260: 7636–7644, 1985. [PubMed] [Google Scholar]
- 173.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R and Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol 9: 858–867, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zamir E and Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. Journal of cell science 114: 3583–3590, 2001. [DOI] [PubMed] [Google Scholar]
- 175.Zhang JC, Wojta J and Binder BR. Growth and fibrinolytic parameters of human umbilical vein endothelial cells seeded onto cardiovascular grafts. The Journal of thoracic and cardiovascular surgery 109: 1059–1065, 1995. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano R and Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. The Journal of Cell Biology 122: 235–242, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao X and Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Advanced Drug Delivery Reviews 63: 610–615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


