Abstract
Evidence demonstrated that glutamatergic system is implicated in mediating relapse to several drugs of abuse, including methamphetamine (METH). Glutamate homeostasis is maintained by a number of glutamate transporters, such as glutamate transporter type 1 (GLT-1), cystine/glutamate transporter (xCT), and glutamate aspartate transporter (GLAST). In addition, group II metabotropic glutamate receptors (mGluR2/3) were found to be implicated in relapse-seeking behavior. Ample evidence showed that β-lactam antibiotics are effective in upregulating GLT-1 and xCT expression, thus improving glutamate homeostasis and attenuating relapse to drugs of abuse. In this study, we investigated the reinstatement of METH using conditioned place preference (CPP) in male alcohol-preferring (P) rats exposed to home-cage free choice ethanol drinking. Here, we tested the effect of clavulanic acid (CA), a β-lactam, on the reinstatement of METH-seeking and ethanol drinking. In addition, we examined the expression of GLT-1, xCT and GLAST as well as metabotropic glutamate receptor (mGluR2/3) in the nucleus accumbens (NAc) shell, NAc core and dorsomedial prefrontal cortex (dmPFC). A priming i.p. injection of METH reinstated preference in METH-paired chamber following extinction. Chronic exposure to ethanol decreased the expression of GLT-1 and xCT in the NAc shell, but not in the NAc core or dmPFC. CA treatment blocked the reinstatement of METH-seeking, decreased ethanol intake and restored the expression of GLT-1 and xCT in the NAc shell. In addition, the expression of mGluR2/3 was increased by CA treatment in the NAc shell and dmPFC. These findings suggest that these glutamate transporters and mGluR2/3 might be potential therapeutic targets for the attenuation of reinstatement to METH-seeking.
Keywords: Methamphetamine, glutamatergic transporters, conditioned place preference, mGluR2/3, clavulanic acid
INTRODUCTION
Ample evidence suggests that ethanol use disorder is exceptionally high in amphetamine-dependent subjects (Stinson et al., 2005). Methamphetamine (METH) users frequently use ethanol (Bujarski et al., 2014; Furr et al., 2000), which may result in several deteriorating effects [For review see ref. (Althobaiti and Sari, 2016b)]. Despite the evidence of the high rate of the co-abuse of METH and ethanol, little is known about the effects of co-exposure of these drugs or METH on ethanol drinking in rodent models. However, a recent study showed that METH self-administration decreased ethanol intake (Winkler et al., 2016). In this study, we examined the effects of METH co-exposure for seven consecutive days on home-cage voluntary ethanol intake in alcohol-preferring (P) rats and the role of the glutamatergic system in this drug co-exposure animal model.
One of the main brain regions involved in the rewarding and reinforcing effects of drugs of abuse is the nucleus accumbens (NAc) (Bardo, 1998a; Wise and Rompré, 1989). Glutamatergic neurotransmission in the NAc plays a critical role in the relapse behavior to different drugs of abuse. Importantly, deficits in glutamate clearance in the NAc have been found to be associated with chronic drug use and drug-seeking behavior (Das et al., 2015b; Fujio et al., 2005; Knackstedt et al., 2010; Melendez et al., 2005). The NAc receives glutamatergic inputs from the prefrontal cortex (PFC) as well as other brain regions (Kelley et al., 1981; Phillipson and Griffiths, 1985). Dorsomedial PFC (dmPFC), in particular, has been implicated in mediating the reinstatement of cocaine-seeking (Berglind et al., 2009; McLaughlin and See, 2003). Glutamate release from dorsal PFC projections to the NAc core has been found to mediate the reinstatement of cocaine-seeking (McFarland et al., 2003; McFarland et al., 2004). Of note, studies demonstrated that blockade of metabotropic glutamate receptor 1 (mGluR1) and mGluR5 as well as activation of mGluR2/3 in central reward brain regions can decrease the reinstatement to several drugs of abuse (Bossert et al., 2006; Cornish and Kalivas, 2000; Dravolina et al., 2007; Lee et al., 2005; McFarland et al., 2004; Tessari et al., 2004). Therefore, modulating glutamatergic system at the synaptic and extra-synaptic receptors might be an effective strategy for attenuating reinstatement of drug-seeking.
Glutamate homeostasis is maintained by several glutamate transporters, including glutamate transporter type 1 (GLT-1, its human homolog excitatory amino acid 2, EAAT2), cystine/glutamate transporter (xCT) and glutamate-aspartate transporter (GLAST). GLT-1 clears about 90% of extracellular glutamate and is frequently reported to be downregulated in the NAc following exposure to drugs of abuse (Fischer et al., 2013b; Knackstedt et al., 2009; Rothstein et al., 1995; Sari and Sreemantula, 2012; Shen et al., 2014). Furthermore, xCT was also downregulated in the NAc following cocaine withdrawal (Baker et al., 2003). In fact, decrease in the xCT expression in the NAc, which has a significant role in regulating glutamate homeostasis (Alhaddad et al., 2014; Baker et al., 2002), is associated with decrease in basal glutamate concentrations and subsequent impairment of the activation of mGluR2/3. Decrease in glutamatergic tone on mGluR2/3 can lead to an increase in glutamate release in the NAc during reinstatement (Dietrich et al., 2002; LaLumiere and Kalivas, 2008; Madayag et al., 2007; Moran et al., 2005).
Importantly, treatment with ceftriaxone (CEF), a known GLT-1 upregulator, has been reported to prevent the reinstatement of METH, cocaine, ethanol, nicotine, and heroin seeking (Abulseoud et al., 2012; Alajaji et al., 2013; Knackstedt et al., 2010; Qrunfleh et al., 2013; Sari et al., 2009; Shen et al., 2014). Moreover, our lab recently showed that METH exposure could decrease the expression of GLT-1 in the NAc. In addition, chronic ethanol drinking was shown to decrease GLT-1 and xCT expression in the NAc (Alhaddad et al., 2014; Das et al., 2015b). Therefore, CEF treatment decreased ethanol intake and upregulated GLT-1 in the NAc and PFC (Alhaddad et al., 2014; Althobaiti et al., 2016b; Sari et al., 2011; Sari et al., 2013). CEF’s upregulatory effect on GLT-1 expression is suggested to be mainly attributed to the β-lactam ring found in this drug and other β-lactam antibiotics (Rothstein et al., 2005). Thus, clavulanic acid (CA), which is a non-antibiotic β-lactam compound with desirable therapeutic profile relative to CEF, could be a better alternative. In fact, CA is orally available, relatively safe, and can cross the blood-brain barrier (Münch et al., 1981; Nakagawa et al., 1994b). Importantly, CA (1 mg/kg and 10 mg/kg, i.p) was shown to decrease cocaine self-administration in part through upregulation of GLT-1 in the NAc (Kim et al., 2016). Also, CA ( 5 mg/kg) was reported to decrease chronic ethanol drinking, which was associated with an increase in GLT-1 and xCT expression in the NAc and PFC in P rats (Hakami and Sari, 2017).
Therefore, we examined the CA effect on the reinstatement to METH in conditioned place preference (CPP) paradigm in P rats that had free access to ethanol. P rats were used in this study as an established animal model of alcoholism since these rats can drink 5–8 g ethanol/kg/day voluntarily (Li et al., 1993). Moreover, according to previous reports, the reinforcing effects of stimulants, such as cocaine, were more pronounced in P rats as compared to Wistar rats (Katner et al., 2011). P rats have also been reported to be less sensitive to the sedating effects of ethanol [for a review, see ref. (McBride and Li, 1998)]. This tolerance to the sedating effects of ethanol makes P rats a good animal model for testing ethanol and METH co-abuse using the CPP procedure. Of note, the CPP has been an important alternative procedure to self-administration in investigating reward and relapse of several drugs of abuse [for review, see ref. (Tzschentke, 1998)]. In this paradigm, the drug of abuse is associated with specific environmental cues. As a result, when the drug is rewarding, animals prefer to be in contact with the drug-paired environment [for review, see ref. (Carr et al., 1989)]. Importantly, CPP has been used for all drugs of abuse, including ethanol, opiates (such as heroin, morphine, and methadone), and stimulants (such as cocaine and METH) (Ashby et al., 2003; Mueller and Stewart, 2000; Reid et al., 1985; Steinpreis et al., 1996; Tzschentke and Schmidt, 1995; Zakharova et al., 2009).
Also, we investigated the effect of METH on ethanol drinking and the effect of co-exposure of these drugs on locomotor activity in P rat model. We also tested the effects of CA on ethanol intake in P rats pre-exposed to METH for seven consecutive days. Finally, to explore the possible role of the glutamatergic system, we investigated the expression of different glutamate transporters (GLT-1, xCT and GLAST) and mGluR2/3 in the NAc shell and core, as well as the dmPFC.
MATERIALS AND METHODS
Animals
Male P rats, at the age of 21–30 days, were obtained from the Indiana University Medical Center (Indianapolis, IN) Indiana Alcohol Research Center breeding colonies. Animals were single-housed in standard plastic cages with a controlled temperature (21°C) and humidity (30%) on 12:12 light-dark cycle and had free access to food, and a three-bottle drinking paradigm [water and two ethanol concentrations (15% and 30% v/v)]. Rats had ad libitum food, water, and ethanol throughout the experimental procedure, except for the control (water group) which did not have access to ethanol. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Drugs
(+)-METH hydrochloride and CA were purchased from Sigma-Aldrich (St. Louis, MO). Saline solution (0.9% NaCl) was used to dissolve both drugs. Ethanol (95%; Decon Labs, Inc.) was diluted in water.
Ethanol drinking paradigm
Rats had free access to a three-bottle drinking paradigm [two ethanol concentrations (15% and 30% v/v) and water] and food, except for the control (water group) which did not have access to ethanol, for five weeks before CPP testing. Ethanol intake, water intake, and body weight were measured three times a week during Week 4 and 5, and the average values of these two weeks were considered as the baseline. The measurements of fluid intake and body weight were then recorded daily throughout the experiment.
Experimental Design
Experimental groups and schedule are illustrated in Table 1. A separate group of rats was utilized to investigate whether CA has any effect on CPP. These rats were treated with CA (5 mg/kg, i.p.) and underwent the same procedure as described below in the conditioning phase. After completion of the seven conditioning days, rats were tested for preference. During Week 6, the CPP experiment was initiated, in which each group received either saline or METH (2.5 mg/ kg, i.p.) injections for seven days (conditioning phase), followed by seven days treatment with either saline or CA (5 mg/kg, i.p.) (extinction phase). Finally, rats were tested for reinstatement produced by an i.p. injection of METH (2.5 mg/kg). A one water control group and three ethanol exposed groups have been used in this study as follows: a) Control (water group) group had free access to water and food throughout the whole experiment and received saline i.p. injections in conditioning, extinction and reinstatement phases; b) Saline-Saline group had free access to ethanol (15% and 30% v/v), as well as ad libitum food and water throughout the experiment. Rats received saline i.p. injections in conditioning, extinction and reinstatement phases; c) METH-Saline group had free access to ethanol (15% and 30% v/v), as well as ad libitum food and water throughout the experiment. Rats received METH i.p. injections in conditioning, saline in extinction and METH in the reinstatement phase; and d) METH-CA group had free access to ethanol (15% and 30% v/v), as well as ad libitum food and water throughout the experiment. Rats received METH in conditioning, CA in extinction and METH in the reinstatement phase.
Table 1.
| Group | Post conditioning | Extinction | Reinstatement | |
| Control (water group) | Saline | Saline | Saline | |
| Ethanol-Exposed Groups | CA group | CA or Saline | - | - |
| Saline-Saline | Saline | Saline | Saline | |
| METH-Saline | METH or Saline | Saline | METH | |
| METH-CA | METH or Saline | CA or Saline | METH | |
Conditioned place preference paradigm
Apparatus
A three-chamber CPP apparatus made of Plexiglas was used in this study. Two equal-sized chambers were distinguished by both visual and tactile cues (40 cm × 40 cm × 40 cm), and one smaller middle chamber (30 cm × 40 cm × 40 cm). The inner walls of the first chamber are white with horizontal black stripes and textured black floor (chamber 1). The inner walls of the chamber 2 are black with vertical white stripes and smooth white floor. The middle chamber is featureless and separated from the two chambers by two guillotine doors. The CPP experiment was conducted according to previous studies in four distinct phases (Abulseoud et al., 2012; Arezoomandan et al., 2016).
The habituation phase
The first day of habituation was considered as Day 1. On Days 1–3, each rat was placed in the middle chamber with both doors closed for three minutes. Both doors were then opened, and the rats had free access to explore the entire apparatus for 20 minutes. On Day 3, the test was recorded by a digital camera fixed above the apparatus (CPP Test). The time spent in each chamber was measured by an observer who was blinded to the experimental design. The locomotor activity was analyzed using ANY-maze video tracking system. Rats were excluded from the study when they showed strong initial preference to any chamber for more than 67% of the total time as conducted previously (Alshehri et al., 2018; Fujio et al., 2005; Hammad et al., 2017a).
The conditioning phase
The conditioning phase (Days 4–10) consisted of fourteen conditioning sessions conducted on seven consecutive days (morning and afternoon sessions). Each rat received i.p. injections of either 2.5 mg/kg METH or saline, and was placed in the corresponding chamber with the door closed for 20 minutes in the morning session. In the afternoon session (~5 hours after the morning session), each rat received saline or 2.5 mg/kg METH i.p. and was placed in the opposite chamber with the door closed for 20 minutes. Since initial time spent in Chamber 1, Chamber 2, and the middle chamber was not significantly different; an unbiased design was utilized. Thus, random assignment of rats was utilized in which half of the animals received METH in Chamber 1, and the other half received the drug in Chamber 2. The control group received saline in both morning and afternoon sessions, and placed in either chambers (i.e., in chamber 1 in the morning and chamber 2 in the afternoon session) with doors closed for 20 minutes. The order of METH and saline administration was counterbalanced. METH was administered in the morning for half of the group of rats and saline for the other group of rats in the afternoon. On the following day, saline was administered to the same group of rats in the morning and METH was given in the afternoon, and the pattern was reversed for the other half of group of rats. Similarly, the orientation of the chambers was alternated as described in a previous study (Cunningham et al., 2006). On Day 11, the CPP test was performed again as described previously. Animals were conditioned twice a day based on a previous report (Abulseoud et al., 2012).
The extinction phase
This phase is similar to the conditioning phase except for the rats that received METH in the conditioning phase were randomly assigned to receive either CA (5 mg/kg, i.p.) or saline (1 ml/kg) instead of 2.5 mg/kg METH (Day 12–18). The dose of CA (5 mg/kg, i.p.) was chosen based on previous studies (Kim et al., 2016; Schroeder et al., 2014). The control group received saline in morning and afternoon sessions as described in the conditioning phase. On Day 19, another CPP test was performed. Rats must show 25% reduction in time spent in the Meth-paired chamber following the extinction training to be considered extinguished as described in previous work (Abulseoud et al., 2012).
Reinstatement phase
On the morning of Day 20, each rat was administered a single dose of either METH (2.5 mg/kg) or saline and placed in the corresponding chamber with the door closed for 20 minutes. In the afternoon, saline or METH was administered (i.p.) and the rat was placed in the opposite chamber. On Day 21, each rat was tested for CPP as described above.
Brain tissue harvesting
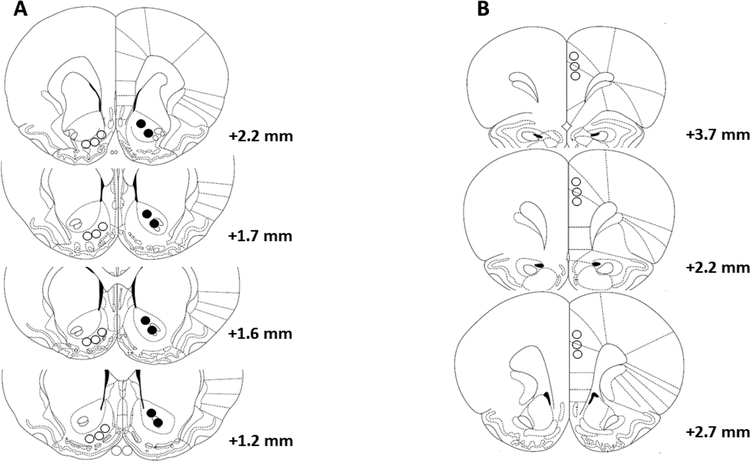
Rats were rapidly euthanized following reinstatement using CPP test by CO2 inhalation and immediately decapitated. Brains were then removed and rapidly frozen on dry ice and stored at −80°C. Brain sectioning and micro-punch procedure were performed as described previously (Sari and Sreemantula, 2012). The NAc shell and core, and dmPFC were identified using stereotaxic according to The Rat Brain Atlas as shown in Fig. 1 (Paxinos and Watson, 2007).
Figure 1.
Representative micro-punch of brain regions: NAc shell (open circles) and core (closed circles) (A), and dmPFC (B). Numbers indicate the anteroposterior distance from bregma according to The Rat Brain Atlas (Paxinos and Watson 2007).
Western blot
Western blot procedure was performed as previously described (Sari et al., 2009). Briefly, brain tissue was lysed in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1% Triton, 0.1% SDS) containing protease inhibitor cocktail. Extracted proteins were loaded onto 10–20% Tris-glycine gel. After separation, proteins were transferred from the gel onto PVDF membrane. The membrane was then blocked using 3% milk in Tris-buffered saline Tween 20 for 30 minutes. Guinea pig anti-GLT1 (Millipore Bioscience Research Reagents; 1:5000 dilution), rabbit anti-xCT antibody (Abcam; 1:1000 dilution), rabbit anti-mGluR2/3 antibody (Upstate Biotechnology; 1:1000 dilution), rabbit anti-GLAST (Abcam; 1:5000 dilution), or mouse anti β-tubulin antibody (Covance; 1:5000 dilution) was then added to the blocking buffer, and the membrane was incubated overnight at 4°C. The membrane was then washed and incubated with horseradish peroxidase-labeled (HRP) anti-Guinea pig, anti-rabbit, or anti-mouse secondary antibody (1:5000). Chemiluminescent kit (SuperSignal West Pico) was used to incubate membrane for protein detection. The membrane was exposed to Kodak BioMax MR films (Thermo Fisher Scientific). Films were then developed using an SRX-101A machine. Blots for each detected protein were digitized and quantified using an MCID system. Data calculated as ratios of GLT-1/β-tubulin, xCT/β-tubulin, GLAST/β-tubulin, and mGluR2/3/β-tubulin relative to control group (100% control-value). In each gel run, control (water group) was set as 100% and the expression of protein of interest for the other ethanol-exposed groups was computed relative to it as described in previous studies (Hammad et al., 2017b; Li et al., 2003; Miller et al., 2008; Raval et al., 2003; Zhang and Tan, 2011).
Statistical analysis
Time spent in conditioning chambers (preference) and locomotor activity were analyzed using two-way repeated measures ANOVA, followed by Newman-Keuls as post hoc test. In case of locomotion, distance travelled in the whole apparatus was measured at two different time-points (baseline, and postconditioning) in response to different treatments (control, ethanol-saline and ethanol-METH). In case of preference, chambers represent the place that specific treatment was given (saline or METH) and time spent in each chamber was tested at different time-points (preconditioning, postconditioning, extinction and reinstatement), and the statistical analysis was conducted in METH-Saline group as well as METH-CA group.
Average ethanol intake, ethanol preference, water intake, and body weight were analyzed using two-way repeated measures ANOVA, followed by Bonferroni’s multiple comparisons post hoc test. Immunoblot data were analyzed using one-way ANOVA, followed by Newman-Keuls as a post hoc test. GraphPad Prism was used to analyze all data. The p values were set at <0.05 level of significance.
RESULTS
Ethanol drinking and preference as well as water intake and body weight
Effect of METH during the conditioning phase
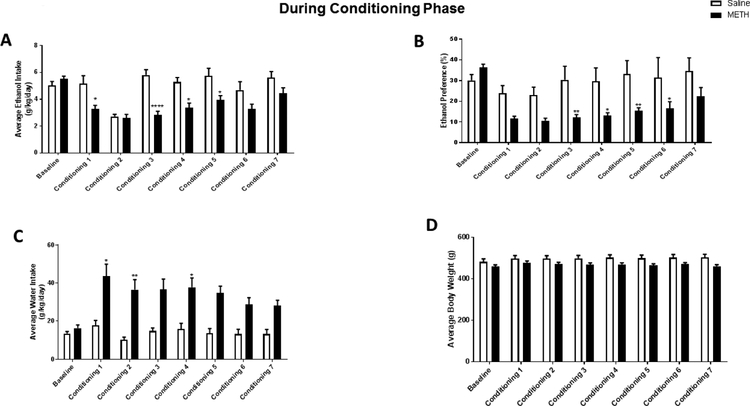
We tested the effect of METH during the conditioning phase (conditioning 1–7) on ethanol intake and preference, water intake, and body weight. Statistical analysis using two-way repeated measures ANOVA revealed a significant effect of time [F (7, 182) = 11.43, p < 0.0001], treatment [F (1, 26) = 11.01, p = 0.0027], and a significant interaction between time and treatment [F (7, 182) = 5.372, p < 0.0001]. Bonferroni’s multiple comparisons revealed that METH significantly decreased the average ethanol intake during Day 1, 3, 4, and 5 of conditioning as compared to the saline treatment group (p < 0.05, 0.0001, 0.05, and 0.05, respectively; Fig. 2A). No significant change in ethanol intake between METH and saline treatment groups was found during Day 2, 6, and 7 of conditioning phase.
Figure 2.
Effect of METH on ethanol intake and preference during the conditioning phase. Rats had free access to three bottles containing water, 15% ethanol, and 30% ethanol for five weeks. Ethanol intake and preference were measured during the last two weeks and served as a baseline. Rats were then administered METH (2.5 mg/kg/day) or saline for seven days during the conditioning phase, and ethanol intake (A), preference (B), water intake (C), and body weight (D) were assessed daily. METH significantly decreased ethanol intake as compared to the saline group during Day 1, 3–5 of conditioning. Ethanol preference was decreased following METH in Day 3–6 of conditioning. Water intake was significantly increased in METH treated rats as compared to salin- treated rats during Day 1, 2 and 4 of conditioning. Body weight was not significantly changed following METH during the conditioning phase *p<0.05, **p<0.01, and ****p<0.0001. (n = 7–21).
We further investigated the effect of METH on ethanol preference. Two-way repeated measures ANOVA revealed a significant effect of time [F (7, 182) = 7.597, p < 0.0001], treatment [F (1, 26) = 11.29, p = 0.0024], and a significant interaction between time and treatment [F (7, 182) = 3.956, p < 0.0005]. Bonferroni’s multiple comparisons revealed that METH significantly decreased ethanol preference during Day 3 (p < 0.01), Day 4 (p < 0.05), Day 5 (p < 0.01), and Day 6 (p < 0.05) of conditioning as compared to saline treatment (Fig. 2B). No significant change was found in ethanol preference during Day 1, 2, and 7 of the conditioning phase between METH and saline treatment groups.
Two-way repeated measures ANOVA were conducted on water intake, and revealed a significant effect of time [F (7, 182) = 2.171, p = 0.0387], treatment [F (1, 26) = 13.34, p = 0.0011], and no significant interaction between time and treatment [F (7, 182) = 1.416, p = 0.2014]. Bonferroni’s multiple comparisons revealed that METH significantly increased the average water intake in Day 1 (p < 0.05), Day 2 (p < 0.01), and Day 4 (p < 0.05) of conditioning as compared to saline group (Fig. 2C). No significant change was found in ethanol intake during Day 3, 5, 6, and 7 of the conditioning phase between METH and saline treatment groups. A significant effect of time [F (7, 182) = 24.27, p < 0.0001], no significant effect of treatment [F (1, 26) = 3.676, p = 0.0662], and a significant interaction between time and treatment [F (7, 182) = 10.89, p < 0.0001] were revealed on body weight using two-way repeated measures ANOVA. Bonferroni’s multiple comparisons test did not reveal any significant change in body weight following METH treatment in all conditioning days as compared to saline group (p > 0.05; Fig. 2D).
Effect of CA during the extinction phase
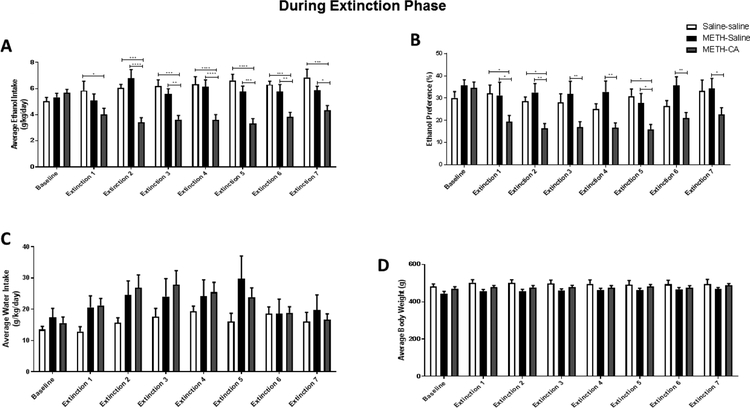
We tested the effect of CA during the extinction phase (extinction 1–7) on ethanol intake and preference as well as water intake and body weight. Statistical analysis using two-way repeated measures ANOVA revealed a no significant effect of time [F (7, 175) = 1.027, p = 0.4141], a significant effect of treatment [F (2, 25) = 18.14, p < 0.0001], and a significant interaction between time and treatment [F (14, 175) = 4.333, p < 0.0001]. Bonferroni’s multiple comparisons test revealed that average ethanol intake was significantly decreased in the METHCA group during Day 1 of extinction as compared to the Saline-Saline group (p < 0.05; Fig. 3A); and decreased ethanol intake was observed as compared to METH-Saline and Saline-Saline groups during Day 2 through 7 of the extinction phase. No significant change in ethanol intake was observed between METH-Saline and Saline-Saline groups in all days of extinction.
Figure 3.
Effect of CA on ethanol intake and preference during the extinction phase. Rats continued to have free access to three bottles containing water, 15% ethanol, and 30% ethanol. Rats received either CA (5 mg/kg/day) or saline for seven days during the extinction phase. Ethanol intake (A), preference (B), water intake (C), and body weight (D) were measured daily during the extinction phase. CA significantly decreased ethanol intake and preference during Day 1–7 of the extinction phase. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. (n = 7–12)
We further investigated the effect of CA on ethanol preference. Two-way repeated measures ANOVA revealed a significant effect of time [F (7, 175) = 2.832, p = 0.0081], treatment [F (2, 25) = 10.64, p = 0.0005], and a no significant interaction between time and treatment [F (14, 175) = 1.476, p = 0.1244]. Bonferroni’s multiple comparisons revealed that ethanol preference was significantly decreased in the METH-CA group as compared to METH-Saline group during Day 1 through 7 of extinction (p < 0.01– 0.05; Fig. 3B). Statistical analysis revealed that ethanol preference was significantly decreased in the METH-CA group as compared to Saline-Saline group during Day 1, 2 and 5 of extinction (p < 0.05; Fig. 3B). No significant change in ethanol preference was observed between the METH-Saline and Saline-Saline groups in all days of extinction. A significant effect of time [F (7, 175) = 4.13, p = 0.0003], no significant effect of treatment [F (2, 25) = 1.255, p = 0.3025], and no significant interaction between time and treatment [F (14, 175) = 1.066, p = 0.3920] were revealed using two-way repeated measures ANOVA conducted on water intake. Bonferroni’s multiple comparisons test did not reveal any significant change in water intake among all groups during the extinction phase (p > 0.05; Fig. 3C). Two-way repeated measures ANOVA conducted on body weight revealed a significant effect of time [F (7, 175) = 5.486, p < 0.0001], no significant effect of treatment [F (2, 25) = 1.722, p = 0.1992], and no significant interaction between time and treatment [F (14, 175) = 1.352, p = 0.1815]. Bonferroni’s multiple comparisons did not reveal any significant change in body weight among any groups during the extinction phase (p > 0.05; Fig. 3D).
Conditioned place preference
Effect of CA on CPP
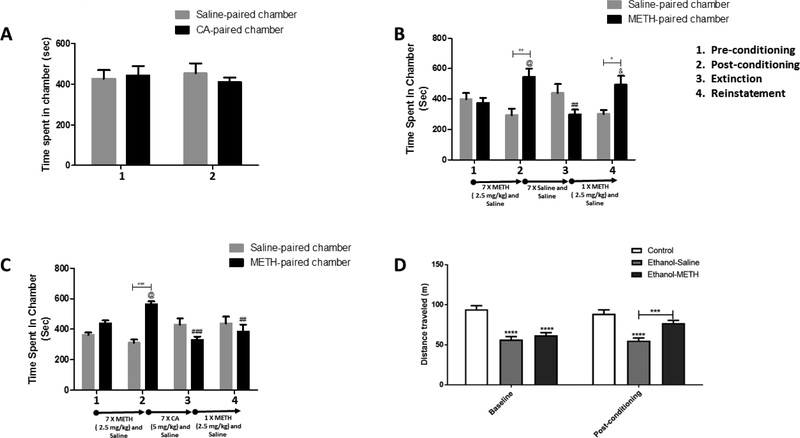
The effects of CA were tested in control animals using the CPP paradigm. Two-way repeated measures ANOVA revealed a no significant effect of time [F (1, 6) = 0.02707, p = 0.8747], no significant effect of chamber [F (1, 6) = 0.04274, p = 0.8431], and no significant interaction between time and chamber [F (1, 6) = 1.397, p = 0.2820] (Fig. 4A).
Figure 4.
Time spent and locomotor activity. Time spent in each conditioning chamber during different CPP phases in CA administered alone (A), METH-Saline group (B) and METH-CA group (C). Time spent in the CA-paired chamber was not significantly changed following seven days treatment with CA (A). Time spent in the METH-paired chamber was significantly increased in reinstatement as compared to extinction training in METH-Saline treated rats (B). CA blocked the reinstatement effect of METH (C). Distance traveled for rats during the baseline and after the conditioning with METH (D). Locomotor activity was lower in ethanol groups during the baseline as compared to the control group. Following the conditioning with METH, the locomotor activity was increased as compared to the saline group. No significant difference in distance traveled between the control group and Ethanol-METH group following conditioning (n = 9–12 for each group). Values shown as means ± S.E.M. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. [(@p<0.05) as compared to pre-conditioning, (#p<0.05, ##p<0.01 and ###p<0.001) as compared to post-conditioning, while (&p<0.05) as compared to extinction phase].
Effect of CA on METH-induced reinstatement using CPP paradigm
METH-Saline treated group.
Two-way repeated measures ANOVA revealed no significant effect of time [F (3, 24) = 1.005, p = 0.4077], no significant effect of chamber [F (1, 8) = 1.514, p = 0.2534], and significant interaction between time and chamber [F (3, 24) = 10.39, p = 0.0001]. Newman-Keuls, multiple comparisons test, showed a significant increase in time spent following conditioning in the METH-paired chamber as compared to pre-conditioning (p < 0.05) and saline-paired chamber in post-conditioning test (p < 0.01; Fig. 4B). A significant decrease in time spent in the METH-paired chamber was revealed following extinction training as compared to post-conditioning (p < 0.01; Fig. 4B). METH increased time spent in the METH-paired chamber as compared to the extinction phase (p < 0.05). In addition, METH increased time spent in the METH-paired chamber as compared to saline-paired chamber (p < 0.05) in the reinstatement phase.
METH-CA treated group.
Two-way repeated measures ANOVA revealed a significant effect of time [F (3, 33) = 2.066, p = 0.1236], no significant effect of chamber [F (1, 11) = 2.401, p = 0.1495], and significant interaction between time and chamber [F (3, 33) = 10.56, p < 0.0001]. Newman-Keuls multiple comparisons showed a significant increase in time spent following conditioning training in the METH-paired chamber as compared to pre-conditioning (p < 0.05) and saline-paired chamber in post-conditioning (p < 0.001; Fig. 4C). A significant decrease in time spent in the METH-paired chamber was revealed following extinction training and reinstatement as compared to post-conditioning (p < 0.001 and p < 0.01, respectively; Fig. 4C). Time spent in the METH-paired chamber was not significantly changed following reinstatement as compared to extinction or saline-paired chamber (p > 0.05).
Locomotor activity
Two-way repeated measures ANOVA revealed no significant effect of time [F (1, 32) = 1.223, p = 0.2771], significant effect of treatment [F (2, 32) = 15.73, p < 0.0001], and significant interaction between time and treatment [F (2, 32) = 8.657, p = 0.0010]. A significant decrease in locomotor activity was revealed at baseline in Ethanol-Saline and Ethanol-METH groups as compared to the control group (p < 0.0001; Fig. 4D). Newman-Keuls multiple comparisons showed a significant increase in locomotor activity following seven days conditioning treatment with METH as compared to saline treatment in ethanol-exposed rats (p < 0.001; Fig. 4D). A significant decrease in locomotor activity was revealed between the Ethanol-Saline group as compared to Water control group (p < 0.0001; Fig. 4D). No significant change in locomotor activity was revealed between the METH-Saline group and control group following conditioning. Importantly, CA did not change locomotor activity following seven days treatment with this drug; mean distance traveled ± SEM in post-conditioning = 56.16 ± 4.57 m as compared to baseline = 50.46 ± 5.39 m [Paired t-test, t(6) = 1.662, p = 0.1477].
Determination of the expression of GLT-1, xCT, GLAST, mGluR2/3 in the NAc shell and core as well as the dmPFC
GLT-1 expression.
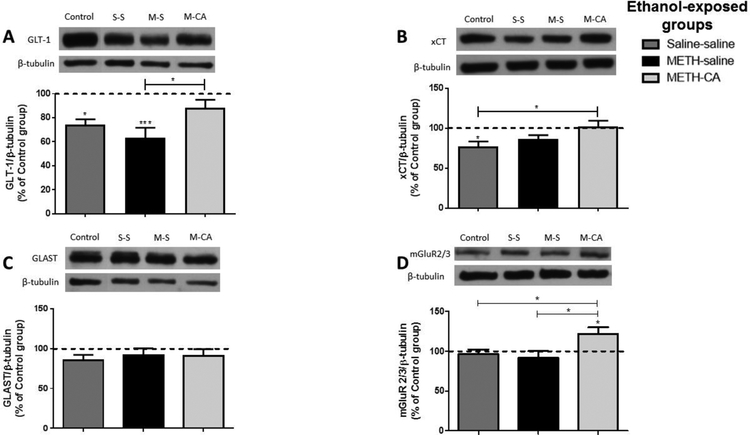
We investigated the effect of ethanol, METH and CA on GLT-1 expression in the dmPFC and NAc shell and core following the reinstatement test. One-way ANOVA revealed a significant main effect of treatment in the NAc shell [F (3, 24) = 7.866, p = 0.0008; Fig. 5A], but not in the core [F (3, 24) = 0.5286, p < 0.6669; Fig. 6A] or dmPFC [F (3, 24) = 0.8643, p = 0.4731; Fig. 7A]. Newman-Keuls multiple comparisons showed a significant increase in the expression of GLT-1 in METH-CA treated rats compared to METH-Saline treated rats in the NAc shell (p < 0.05; Fig. 5A). However, statistical analyses showed significant downregulation in GLT-1 expression in the Saline-Saline and METH-Saline groups compared to the control group in the NAc shell (p < 0.05 and p < 0.001, respectively; Fig. 5A). No significant difference in GLT-1 expression was revealed between the METH-CA group and the control group.
Figure 5.
Effects of ethanol, METH (2.5 mg/kg) and CA (5 mg/kg) on the expression of GLT-1 (A), xCT (B), GLAST (C), and mGluR2/3 (D) in the NAc shell. Quantitative analysis revealed a significant increase in the ratio of GLT-1/β-tubulin in METH-CA treated rats compared to the METH-Saline treated rats. A significant downregulation of GLT-1 expression was revealed in the METH-Saline and Saline-Saline (exposed to ethanol without METH) groups compared to the control water group. A significant increase in the ratio of xCT/β-tubulin was revealed in the METH-CA group compared to the Saline-Saline (exposed to ethanol without METH) group. Significant downregulation of xCT expression was revealed in Saline-Saline treated group compared to the control water group. A significant increase in the ratio of mGluR2/3/β-tubulin was revealed in METH-CA group compared to METH-Saline, Saline-Saline and control groups. No significant difference in GLAST expression was revealed among all tested groups in the NAc shell. * p<0.05. Values are shown as means ± S.E.M. n = 6–7 for each group.
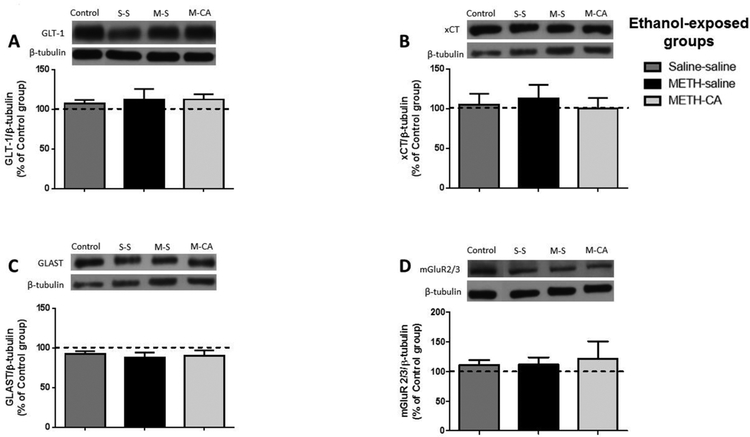
Figure 6.
Effects of METH (2.5 mg/kg) and CA (5 mg/kg) on the expression of GLT-1 (A), xCT (B), GLAST (C), and mGluR2/3 (D) in the NAc core. The quantitative analysis did not reveal any significant change in the expression of GLT-1, xCT, GLAST, and mGluR2/3 among all tested groups in the NAc core. Values are shown as means ± S.E.M. n = 6–7 for each group.
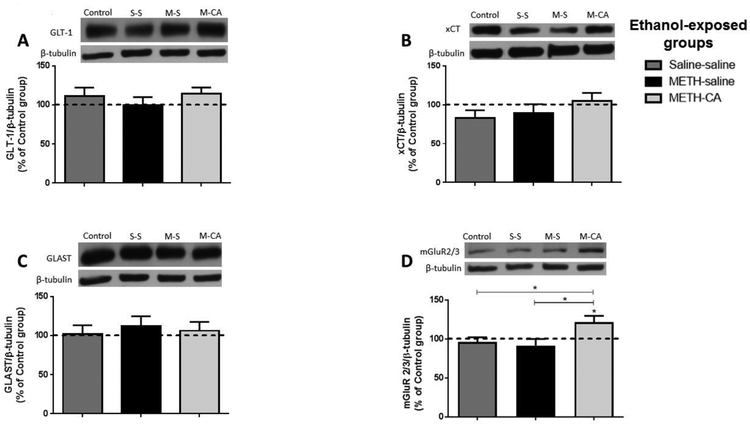
Figure 7.
Effects of METH (2.5 mg/kg) and CA (5 mg/kg) on the expression of GLT-1 (A), xCT (B), GLAST (C) and mGluR2/3 (D) in dmPFC. Quantitative analysis revealed a significant increase in the ratio of mGluR2/3/β-tubulin in the METH-CA group compared to the control group. No significant difference in GLT-1, xCT or GLAST expression was revealed among all tested groups in dmPFC. * p<0.05. Values are shown as means ± S.E.M. n = 6–7 for each group.
xCT expression.
We investigated xCT expression in the NAc shell and core, as well as dmPFC, following the reinstatement test. One-way ANOVA revealed a significant main effect in the NAc shell [F (3, 20) = 4.831, p = 0.0109, Fig. 5B], but not in the NAc core [F (3, 24) = 0.2322, p < 0.8730; Fig. 6B] or dmPFC [F (3, 24) = 1.15, p = 0.3491; Fig. 7B]. Newman-Keuls multiple comparisons showed a significant increase in the expression of xCT in METH-CA treated rats compared to the Saline-Saline treated rats in the NAc shell (p < 0.05; Fig. 5B). However, statistical analyses showed significant downregulation in xCT expression in the Saline-Saline group compared to the control group in the NAc shell (p < 0.05; Fig. 5B). No significant difference in xCT expression was revealed between the METH-CA and METH-Saline groups as compared to control group.
GLAST expression.
We further tested the effect of ethanol, METH and CA on GLAST expression in the NAc shell and core, and dmPFC following reinstatement test. One-way ANOVA revealed a non-significant main effect of treatment in the NAc shell [F (3, 24) = 0.7276, p = 0.5456; Fig. 5C], NAc core [F (3, 20) = 1.096, p = 0.3740; Fig. 6C] and dmPFC [F (3, 20) = 0.2637, p = 0.8507; Fig. 7C].
mGluR2/3 expression.
We investigated the effect of ethanol, METH and CA on mGluR2/3 expression in the dmPFC, and NAc shell and core following reinstatement test. One-way ANOVA revealed a significant main effect in the NAc shell [F (3, 24) = 3.996, p = 0.0193; Fig. 5D], dmPFC [F (3, 20) = 3.116, p = 0.0492; Fig. 8D], but not in NAc core [F (3, 24) = 0.294, p = 0.8293; Fig. 6D]. Newman-Keuls multiple comparisons showed a significant increase in the expression of mGluR2/3 in the METH-CA treated rats compared to control group in the NAc shell (p < 0.05; Fig. 6D), dmPFC (p < 0.05; Fig. 7D). Moreover, statistical analyses showed a significant increase in mGluR2/3 expression in the METH-CA group compared to Saline-Saline and METH-Saline group in the NAc shell (p < 0.05; Fig. 5D), but not in dmPFC (p > 0.05; Fig. 7D).
DISCUSSION
In this study, we showed that chronic ethanol exposure caused significant downregulation in GLT-1 and xCT expression in the NAc shell. Of note, GLT-1 and xCT are responsible for clearing and maintaining extracellular glutamate to be at physiological levels in several brain regions implicated in drug dependence (Baker et al., 2002; Danbolt, 2001; Rothstein et al., 1995). In line with previous reports, GLT-1 and xCT were downregulated in the NAc following exposure to various drugs of abuse, including ethanol and METH (Alhaddad et al., 2014; Althobaiti et al., 2016a; Das et al., 2015a; Fischer et al., 2013a; Goodwani et al., 2015; He et al., 2014; Knackstedt et al., 2009; Sari and Sreemantula, 2012; Sari et al., 2013). Importantly, NAc is a well- known brain region involved in the rewarding and reinforcing effects of drugs of abuse (Bardo, 1998b; Koob and Bloom, 1988; Wise and Rompré, 1989). Deficits in glutamate clearance in the NAc have been found to be associated with chronic drug use and drug seeking behavior (Das et al., 2015a; Fujio et al., 2005; Knackstedt et al., 2010; Melendez et al., 2005). Importantly, studies showed that chronic ethanol drinking decreased the expression of GLT-1 and xCT in the NAc and other brain regions, and restoring the expression of these proteins was associated with reduction in ethanol drinking (Aal‐Aaboda et al., 2015; Alhaddad et al., 2014; Das et al., 2015b; Sari and Sreemantula, 2012). In accordance with the present study, CA treatment restored GLT-1 and xCT expression levels in the NAc shell and decreased ethanol intake. This is also in accordance with our previous reports that demonstrated decrease in ethanol intake following CEF treatment, which was associated with upregulation of GLT-1 in the NAc (Alhaddad et al., 2014; Sari et al., 2011; Sari et al., 2013). Importantly, CA has some advantages over CEF such as lack of antibiotic activity, oral availability, relative safety, and the ability to cross blood brain barrier (Münch et al., 1981; Nakagawa et al., 1994a). These advantages suggest that CA might be a potential compound for the management of drug dependence.
In this study, we revealed that the reduction in the expression of GLT-1 and xCT following chronic ethanol exposure is specific to the shell rather than the core subregion of the NAc. This suggests the important role of NAc shell in mediating the rewarding and reinforcing effects of ethanol. Indeed, ethanol exposure has been shown to increase the release of dopamine in the NAc shell of rats selectively bred to prefer ethanol more than rats bred to avoid ethanol (Bustamante et al., 2008). Moreover, a recent report showed that blocking dopamine D1 receptor in the NAc shell but not core decreased ethanol-seeking behavior in P rats (Hauser et al., 2015). Moreover, the reinforcing effect of ethanol has been shown to be mediated through the activation of dopaminergic receptors in the NAc shell, but not NAc core (Ding et al., 2015). However, we have previously shown that ethanol drinking for six weeks caused downregulation of GLT-1 expression in both the NAc shell and core (Sari et al., 2013). In this study, rats were exposed to chronic ethanol drinking for a more extended period (eight weeks). This might indicate possible neuroadaptation that might have occurred in the core, but not NAc shell, to the effect of ethanol on GLT-1 expression. We suggest that GLT-1 is more sensitive to the effect of ethanol in the shell subregion of the NAc than the core. However, further studies are still needed to examine the subregional differences of the NAc as well as other brain regions. On the other hand, the expression of GLAST was not altered in any brain regions tested, which is in line with previous reports (Alhaddad et al., 2014; Hakami et al., 2016). Studies suggested that GLAST is highly expressed in cerebellum and retina, while GLT-1 and xCT predominantly regulate glutamate homeostasis as compared to GLAST in forebrain regions, including the NAc (Baker et al., 2002; Danbolt, 2001; Lehre et al., 1997; Rothstein et al., 1994; Rothstein et al., 1995). This might be a key factor in mediating the effects of ethanol on the expression of these transporters.
Moreover, chronic ethanol exposure caused reduction in the locomotor activity of rats as compared to control group, which is consistent with the sedative effect of ethanol. Interestingly, METH reversed the negative effect of ethanol drinking on locomotor activity, which corroborates with a recent clinical study (Kirkpatrick et al., 2012). METH stimulant effect resulted in an improvement of the detrimental effect of ethanol on locomotor activity. This might indicate that the high prevalence of METH and ethanol co-abuse found in clinical studies may involve some degree of compensation or self-medication [For review see ref. (Althobaiti and Sari, 2016a)].
On the other hand, we found that METH initially caused reduction followed by gradual restoration of ethanol intake. The cause of this effect is unclear. However, it might be due to possible interactions between METH and ethanol on dopaminergic pathways in the NAc. Indeed, in vivo microdialysis studies have reported an increase in dopamine release in the NAc following oral ethanol as well as METH self-administration (Jang et al., 2017; Lominac et al., 2012; Weiss et al., 1993). Dopamine in the NAc is known to regulate motivated behavior and reward to drugs of abuse [For review see ref. (Ikemoto and Panksepp, 1999)]. It is possible that METH initially increases dopamine release in the NAc with the associated motivational state, which in turn may decrease ethanol-seeking behavior. This initial effect might be followed by gradual decreases in dopamine release following METH exposure, which consequently may lead to an increase in ethanol drinking to reach the hedonic effects. Further studies are needed to investigate the neurochemical basis of this effect using no-net flex microdialysis technique.
Importantly, we showed for the first time that CA blocked the reinstatement of METH. The attenuating effect of CA on METH-induced reinstatement is unlikely caused by CA-induced changes in CPP preference or locomotor activity. Indeed, CA did not affect the CPP and locomotor activity when the drug is administered alone. Expression of GLT-1 and xCT was restored following CA treatment in the NAc shell which might be, in part, the underlying mechanism in blocking the reinstatement of METH seeking and reducing ethanol drinking. It has been reported that CA is capable of increasing the expression of GLT-1 in the NAc (Kim et al., 2016). In this study, we showed that CA increased the expression of xCT and restored the GLT-1 expression in the shell subregion of the NAc but not the core. Moreover, CA treatment increased the expression of mGluR2/3 in both the NAc shell and dmPFC. It is important to note that the elevation of mGluR2/3 expression in the PFC was associated with reduction in ethanol seeking (Meinhardt et al., 2013). Activation of mGluR2/3 in the NAc shell but not the NAc core blocked context-induced reinstatement of heroin seeking (Bossert et al., 2006). Interestingly, activation of mGluR2/3 in the NAc was shown to block amphetamine-induced release of dopamine (Pehrson and Moghaddam, 2010). Together, restoring the expression of GLT-1 and xCT in the NAc shell as well as increasing the expression of mGluR2/3 in the NAc shell and dmPFC can improve the glutamatergic homeostasis, which in part attenuate ethanol drinking as well as METH reinstatement. It is important to note that the effects of METH alone on these glutamate transporters and receptors were not examined in this study. Further studies are needed to examine the neurochemical changes related to METH exposure alone and any neuroprotective effects of CA treatment.
In conclusion, our findings showed for the first time that CA attenuated reinstatement to METH seeking and decreased ethanol drinking behavior. These behavioral effects of CA are most likely due to its effect on GLT-1, xCT andmGluR2/3 expressions in NAc shell and dmPFC. These findings suggest that CA could be a potential safe therapeutic agent for management of drug dependence and relapse.
ACKNOWLEDGEMENTS
The work was supported in part by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism and fund provided by The University of Toledo. The authors would like to thank Dr. F. Scott Hall for generously giving the access to use ANY-maze video tracking system and thank Molly Hill for contribution in the experiments.
Footnotes
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y (2015). Effects of (R)-(–)-5- methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. Journal of neuroscience research. 93, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi D-S, Holschneider DP (2012). Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain research. 1456, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji M, Bowers M, Knackstedt L, Damaj M (2013). Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 228, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y (2014). Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 231, 4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri FS, Hakami AY, Althobaiti YS, Sari Y (2018). Effects of ceftriaxone on hydrocodone seeking behavior and glial glutamate transporters in P rats. Behavioural brain research. 347, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Alshehri FS, Almalki AH, Sari Y (2016a). Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Frontiers in Neuroscience. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Alshehri FS, Almalki AH, Sari Y (2016b). Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Frontiers in neuroscience.10, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Sari Y (2016a). Alcohol interactions with psychostimulants: an overview of animal and human studies. Journal of addiction research & therapy. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Sari Y (2016b). Alcohol Interactions with Psychostimulants: An Overview of Animal and Human Studies. J Addict Res Ther. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezoomandan R, Moradi M, Attarzadeh-Yazdi G, Tomaz C, Haghparast A (2016). Administration of activated glial condition medium in the nucleus accumbens extended extinction and intensified reinstatement of methamphetamine-induced conditioned place preference. Brain Research Bulletin. 125, 106–116. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ (2003). Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 48,154–156. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience. 22, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, Kalivas PW (2003). Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 6, 743–749. [DOI] [PubMed] [Google Scholar]
- Bardo MT (1998a). Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol.12, 37–67. [DOI] [PubMed] [Google Scholar]
- Bardo MT (1998b). Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Critical Reviews™ in Neurobiology.12. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, LaLumiere RT, Kalivas PW, McGinty JF (2009). A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. The Journal of Neuroscience. 29, 3715–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y (2006). Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 31,2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, Allen V, Hartwell E, Leventhal A, Rohrbaugh T, Ray LA (2014). The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug and alcohol dependence.142, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M (2008). Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. European journal of pharmacology. 591, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG (1989). Conditioned place preference as a measure of drug reward. [DOI] [PubMed]
- Cornish JL, Kalivas PW (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. JNeurosci. 20, RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nature protocols.1,1662–1670. [DOI] [PubMed] [Google Scholar]
- Danbolt NC (2001). Glutamate uptake. Progress in neurobiology. 65,1–105. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y (2015a). Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y (2015b). Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 97, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Kral T, Clusmann H, Friedl M, Schramm J (2002). Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 42, 297–305. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ (2015). The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addiction biology. 20, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY (2007). mGlu1 receptor blockade attenuates cue-and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 52, 263–269. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV (2013a). Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. The Journal of Neuroscience. 33, 9319–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV (2013b). Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Nemosci. 33, 9319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S (2005). Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine-and morphine-induced conditioned place preference in rats. European Journal of Neuroscience. 22, 2744–2754. [DOI] [PubMed] [Google Scholar]
- Furr CD, Delva J, Anthony JC (2000). The suspected association between methamphetamine (‘ice5) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug and Alcohol Dependence. 59, 89–93. [DOI] [PubMed] [Google Scholar]
- Goodwani S, Rao P, Bell RL, Sari Y (2015). Amoxicillin and amoxicillinたlavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain research. 1622, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Hammad AM, Sari Y (2016). Effects of Amoxicillin and Augmentin on Cystine-Glutamate Exchanger and Glutamate Transporter 1 Isoforms as well as Ethanol Intake in Alcohol-Preferring Rats. Front Neurosci.10,171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Sari Y (2017). β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neuroscience Wters. 657, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Alasmari F, Althobaiti YS, Sari Y (2017a). Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behavioural brain research. 332, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Althobaiti YS, Das SC, Sari Y (2017b). Effects of repeated cocaine exposure and withdrawal on voluntary ethanol drinking, and the expression of glial glutamate transporters in mesocorticolimbic system of P rats. Mol Cell Neurosci. 82, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan G, Dhaher R, Knight CP, Wilden JA, McBride WJ, Rodd ZA (2015). D 1 receptors in the nucleus accumbens-shell, but not the core, are involved in mediating ethanol-seeking behavior of alcohol-preferring (P) rats. Neuroscience. 295, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Chen Y, Dong H, Su R, Gong Z, Yan L (2014). Inhibition of vesicular glutamate transporters contributes to attenuate methamphetamine-induced conditioned place preference in rats. Behavioural brain research. 267, 1–5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J (1999). The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 31,6–41. [DOI] [PubMed] [Google Scholar]
- Jang EY, Yang CH, Hedges DM, Kim SP, Lee JY, Ekins TG, Garcia BT, Kim HY, Nelson AC, Kim NJ (2017). The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addiction biology. 22,1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Oster SM, Ding Z-M, Deehan GA, Toalston JE, Hauser SR, McBride WJ, Rodd ZA (2011). Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administered directly into the nucleus accumbens shell. Pharmacology Biochemistry and Behavior. 99, 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley A, Domesick V, Nauta W, 1981. The amygdalostriatal projection in the rat In: ANATOMICAL RECORD. Vol. 199, ed.^eds. WILEY-LISS DIV JOHN WILEY & SONS INC, 605 THIRD AVE, NEW YORK, NY 10158–0012, pp. A134–A135. [Google Scholar]
- Kim J, John J, Langford D, Walker E, Ward S, Rawls SM (2016). Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino acids. 48, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL (2012). Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 219, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW (2009). The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 65, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010). Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 67, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE (1988). Cellular and molecular mechanisms of drug dependence. Science. 242, 715–723. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW (2008). Glutamate release in the nucleus accumbens core is necessary for heroin seeking. Journal of Neuroscience. 28, 3170–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD (2005). Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2- methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. Journal of Pharmacology and Experimental Therapeutics. 312, 1232–1240. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Davanger S, Danbolt NC (1997). Localization of the glutamate transporter protein GLAST in rat retina. Brain research. 744, 129–137. [DOI] [PubMed] [Google Scholar]
- Li J, Olinger A, Dassow M, Abel M (2003). Up-regulation of GABA B receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience. 118, 451–462. [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L, Doolittle DP (1993). Selective breeding for alcohol preference and associated responses. Behavior genetics. 23,163–170. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE (2012). Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 37, 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau K, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA (2007). Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. Journal of Neuroscience. 27,13968–13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li T-K (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Critical Reviews™ in Neurobiology.12. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003). Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal of neuroscience. 23, 3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004). Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. Journal of Neuroscience. 24,1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE (2003). Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology.168, 57–65. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH (2013). Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. Journal of Neuroscience. 33, 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW (2005). Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 29, 326–333. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV (2008). Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 153, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005). Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. Journal of Neuroscience. 25, 6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J (2000). Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behavioural brain research. 115, 39–47. [DOI] [PubMed] [Google Scholar]
- Münch R, Lüthy R, Blaser J, Siegenthaler W (1981). Human pharmacokinetics and CSF penetration of clavulanic acid. Journal of Antimicrobial Chemotherapy. 8, 29–37. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Yamada M, Tokiyoshi K, Miyawaki Y, Kanayama T(1994a). [Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients]. The Japanese journal of antibiotics. 47, 93–101. [PubMed] [Google Scholar]
- Nakagawa H, Yamada M, Tokiyoshi K, Miyawaki Y, Kanayama T(1994b). Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients. The Japanese journal of antibiotics. 47, 93–101. [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates, Vol., Academic Press/Elsevier, Amsterdam ; Boston: ;. [Google Scholar]
- Pehrson AL, Moghaddam B (2010). Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology. 211, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson O, Griffiths A (1985). The topographic order of inputs to nucleus accumbens in the rat. Neuroscience.16, 275–296. [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y (2013). Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of psychopharmacology. 27, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA (2003). ePKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. Journal of Neuroscience. 23, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Hunter GA, Beaman CM, Hubbell CL (1985). Toward understanding ethanol’s capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacology Biochemistry and Behavior. 22, 483–487. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994). Localization of neuronal and glial glutamate transporters. Neuron.13, 713–725. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995). Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurohgy. 38, 73–84. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Hoberg MD, Vidensky S, Chung DS (2005). β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 433, 73–77. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV (2009). Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. Journal of Neuroscience. 29, 9239–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL (2011). Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and alcoholism. 46, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula S (2012). Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 227, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi D-S (2013). Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of Molecular Neuroscience. 51,779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, Tolman NG, McKenna FF, Watkins KL, Passeri SM, Hsu AH, Shinn BR, Rawls SM (2014). Clavulanic acid reduces rewarding, hyperthermic and locomotor-sensitizing effects of morphine in rats: a new indication for an old drug? Drug Alcohol Depend. 142, 41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. w., Scofield MD, Boger H, Hensley M, Kalivas PW (2014). Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. Journal of Neuroscience. 34, 5649–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinpreis RE, Rutell AL, Parrett FA (1996). Methadone produces conditioned place preference in the rat. Pharmacology Biochemistry and Behavior. 54, 339–341. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T (2005). Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol dependence. 80,105–116. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA (2004). Antagonism at metabotropic glutamate 5 receptors inhibits nicotine-and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. European journal of pharmacology. 499, 121–133. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ (1995). N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neuroscience letters. 193, 37–40. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (1998). Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in neurobiology. 56, 613–672. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF (1993). Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics. 267, 250–258. [PubMed] [Google Scholar]
- Winkler MC, Greager EM, Stafford J, Bachtell RK (2016). Methamphetamine self-administration reduces alcohol consumption and preference in alcohol-preferring P rats. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompré P-P (1989). Brain dopamine and reward. Annual review of psychology. 40, 191–225. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S (2009). Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behavioural brain research. 198, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tan Y (2011). Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience. 193, 72–79. [DOI] [PubMed] [Google Scholar]