Abstract
Apoptosis of CD8 T cells is an essential mechanism that maintains immune system homeostasis, prevents autoimmunity, and reduces immunopathology. CD8 T cell death also occurs early during the response to both inflammation and co-stimulation blockade. Here we studied the effects of a combined deficiency of Fas (extrinsic pathway) and Bim (intrinsic pathway) on early T cell attrition in response to lymphocytic choriomeningitis virus (LCMV) infection and during co-stimulation blockade during transplantation. Loss of Fas and Bim function in Bcl2l11−/−Faslpr/lpr mice inhibited apoptosis of T cells and prevented the early T cell attrition resulting from LCMV infection. Bcl2l11−/−Faslpr/lpr mice were also resistant to prolonged allograft survival induced by co-stimulation blockade targeting the CD40-CD154 pathway. These results demonstrate that both extrinsic and intrinsic apoptosis pathways function concurrently to regulate T cell homeostasis during the early stages of immune responses and allograft survival during co-stimulation blockade.
INTRODUCTION
Apoptosis is a critical mechanism regulating T cell homeostasis and is essential for T cell development, for suppression of autoreactive T cells, and for the contraction phase of an antigen-specific T cell response (1, 2). The well-described attrition of T cells occurring early after both viral infection (3) and co-stimulation blockade (CoB) therapy to extend allograft survival (4) also involves apoptosis of T cells. However specific cell death pathways regulating the early apoptosis of T cells after viral infection or CoB are not well defined. Two distinct pathways regulate T cell apoptosis: the intrinsic and extrinsic pathways (5). The intrinsic pathway is regulated by the members of the B-cell lymphoma 2 (Bcl-2) family and includes pro-survival proteins and pro-apoptotic BH3 proteins and pro-apoptotic pore-formers (6). The extrinsic pathway is activated by the binding of death ligands such as Fas ligand (FasL) to cognate death receptors (Fas) and results in the formation of the death inducing signaling complex (DISC) and activation of the initiator caspase 8 (1).
The immune response to viral infections involves two distinct stages of T cell apoptosis, which have been studied during acute infection with LCMV in mice (3). The first wave of apoptosis occurs early after LCMV infection (2 to 4 days post-infection), and the second wave occurs after antigen is cleared during the contraction phase (3, 7). During the early T cell attrition phase, memory phenotype (CD44hi) CD8+ T cells are more susceptible to deletion than naïve (CD44lo) CD8+ T cells (3). The viral dsRNA mimetic poly(I:C) simulates the early apoptosis observed during LCMV infection, and cell death is dependent on type I interferons IFN-α/β (3, 8). FasL-deficient gld mice are not resistant to early CD8+ T cell deletion induced by poly(I:C) suggesting that the extrinsic death receptor pathway regulated by Fas-FasL interactions is not necessary for early T cell apoptosis (3). Mice lacking the pro-apoptotic protein Bim show a partial resistance to early deletion of CD44hi CD8+ T cells after infection with LCMV suggesting a partial role for the intrinsic apoptosis pathway during this early T cell death (9).
Strategies to prolong allograft survival that target the CD28-B7 and CD40-CD154 pathways have been tested extensively in animal models (10). Death of alloreactive T cells is an important component for prolonged allograft survival during blockade of co-stimulation pathways (11). CoB utilizing the reagents CTLA4-Ig and anti-CD154 mAb induce tolerance to skin, islets, heart and kidney allografts in mice (12). CoB (CTLA4-Ig + anti-CD154) prolongs survival of allografts in FasL deficient gld mice and in Fas deficient lpr mice, suggesting that the Fas-FasL pathway is not necessary for tolerance induction (13–15). Moreover, Bim deficient mice are sensitive to tolerance induction by CoB (CTLA4-Fc + anti-CD154), indicating that the intrinsic cell death pathway regulated by Bim is dispensable for peripheral tolerance induction (16).
In the present study, we investigated the effects of a combined deficiency of Fas and Bim on T cell apoptosis during early stages of viral infection and CoB-induced prolonged survival of skin allografts by generating mice lacking Bim and harboring the lpr mutation in Fas (Bcl2l11−/−Faslpr/lpr). Mice lacking Bim and bearing the lpr mutation have a block in the contraction of antigen-specific T cells in chronic and certain acute viral infections, and they have dysregulated homeostatic proliferation and develop lymphadenopathy and autoimmunity (2, 7, 17, 18). Our studies show that Bcl2l11−/−Faslpr/lpr mice are resistant to the early T cell attrition resulting from LCMV infection and this resistance was due to inhibition of T cell apoptosis. Moreover, Bcl2l11−/−Faslpr/lpr mice were resistant to prolonged survival of skin allografts induced by a CoB therapy consisting of DST/anti-CD154 mAb. These results indicate that Fas and Bim both function to regulate T cell apoptosis in early stages of viral infections and during CoB.
MATERIALS AND METHODS
Mice
C57BL/6J (H2b), Bcl2l11−/− (H2b), Faslpr/lpr (H2b), and BALB/c (H2d) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Bcl2l11−/− and Faslpr/lpr mice were backcrossed (ten generations) to the C57BL/6J strain in the animal facility of the University of Massachusetts Medical School (UMMS) and then interbred to generate the Bcl2l11−/−Faslpr/lpr double knockout mouse line. Mice were given autoclaved food and housed in microisolator cages in a specific pathogen free facility accredited by the American Association for Laboratory Animal Care (AALAC), and all studies were approved by the UMMS Institutional Animal Care and Use Committee.
Preparation of leukocytes
Spleens were recovered from mice and single cell suspensions were prepared. To remove contaminating erythrocytes, leukocyte preparations were treated with 0.84% ammonium chloride.
Virus
LCMV, strain Armstrong, was propagated in BHK cells as previously described (19). Mice were inoculated intraperitoneally (IP) with 5 × 104 PFU (plaque forming units) of LCMV.
Flow Cytometry
Leukocytes were stained for CD8β (YTS156.7.7; Biolegend), CD4 (RM4–5; BD Pharmingen), CD44 (IM7; eBiosciences), IFNγ (XMG1.2; BD Pharmingen). For intracellular cytokine staining, splenocytes from recipient mice were incubated with LPS-matured, irradiated syngeneic (H2b) or allogeneic (H2d) stimulator cells and assessed for intracellular IFNγ production as described previously (20). Samples were analyzed on an LSRII flow cytometer (Becton Dickinson).
TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay
For TUNEL staining, 1 × 106 leukocytes were incubated in 48 well plates at 37°C directly ex-vivo for 5 hours in culture media. TUNEL assay (APO-DIRECT KIT; BD Pharmigen) staining was carried out according to the manufacturer’s protocol.
Transplantation
Recipient mice of the specified strain were transplanted with complete MHC-mismatched skin and treated with a tolerizing regimen of donor-derived splenocytes (donor specific transfusion or DST) and anti-CD154 mAb, as described previously (21). A DST of 1×107 donor splenocytes was injected IV on day −7 relative to skin transplantation on day 0. Anti-CD154 mAb (0.5mg, clone MR1, BioXCell) was given IP on days −7, −4, 0 and +4 relative to skin transplantation. Skin graft rejection was defined as the first day when the entire graft was necrotic.
Statistical analysis
Statistical analyses were performed using GraphPad PRISM software. Data significance (p values) was calculated using an unpaired Students t test. To compare 3 or more means, one-way ANOVA were used. All error bars represent the Standard Error of the Mean (SEM). Allograft survival curves were plotted by the Kaplan-Meier method and the incidence of graft rejection between groups was compared by chi-square analysis (22). For all statistical analyses significance is defined as p < 0.05 and indicated as ns (not significant).
RESULTS AND DISCUSSION
Memory T cell attrition in the early phase of acute viral infection is dependent on Fas and Bim
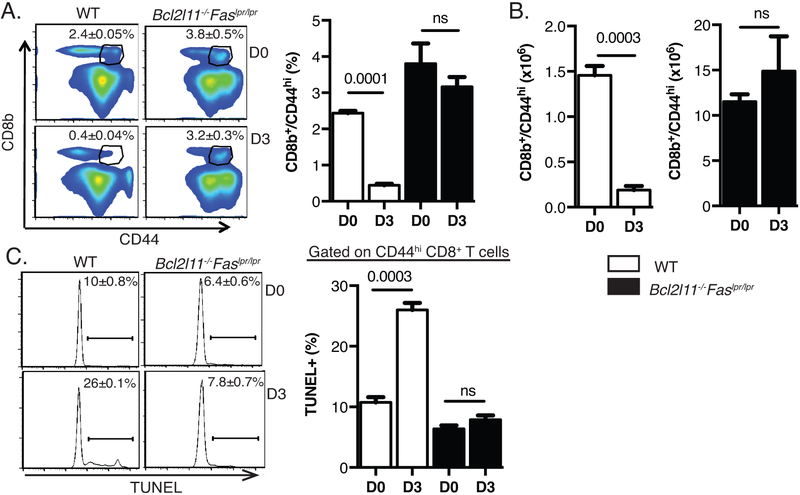
CD44hi CD8 T cells undergo apoptotic attrition in the early phase of acute LCMV infection (3, 8). However, the apoptosis pathways mediating this early attrition have not been fully defined. Previous studies have shown that the absence of Fas signaling does not protect CD8 T cells from cell death after inflammation and that deletion of Bim is only partially protective (3, 9). To elucidate the apoptotic pathways involved in this deletion, wild type (WT) C57BL/6J and Bcl2l11−/−Faslpr/lpr mice were infected with LCMV. CD44hi CD8+ T cells underwent a significant reduction in percentages and numbers in the spleens of WT mice 3 days post-infection (Fig. 1A, 1B). Consistent with previous reports, untreated Bcl2l11−/−Faslpr/lpr mice developed splenomegaly (data not shown) and had higher levels of memory phenotype CD44hi CD8+ T cells in the spleen compared to untreated WT mice (7, 17, 18). However, these cells did not undergo attrition in response to LCMV infection (Fig. 1A, 1B). To determine whether this block in attrition of memory phenotype CD8 T cells was due to inhibition of apoptosis, we performed the TUNEL assay to measure DNA fragmentation. There was a significant increase in the frequency of TUNEL+ CD44hi CD8+ T cells following LCMV infection of WT mice compared to the untreated controls but not after LCMV infection of Bcl2l11−/−Faslpr/lpr mice (Fig. 1C). These data indicated that CD44hi CD8+ T cells from Bcl2l11−/−Faslpr/lpr mice are resistant to apoptotic cell death.
Figure 1: LCMV-induced attrition of CD44hi CD8 T cells is dependent on Bim and Fas.
C57BL/6 (wild-type) and Bcl2l11−/−Faslpr/lpr mice were infected IP with LCMV- (5 × 104 PFU). Splenocytes were harvested at day 0 and day 3 post infection. A) Representative FACS plots showing percentages of CD44hi CD8β T cells in WT and Bcl2l11−/−Faslpr/lpr mice. B) Absolute number of CD44hi CD8β T cells in WT and Bcl2l11−/−Faslpr/lpr mice. C) Splenocytes were isolated and incubated ex-vivo for 5 hours followed by TUNEL staining. Representative histographs of day 0 and day 3 LCMV-infected wild-type and Bcl2l11−/−Faslpr/lpr TUNEL+ CD8β CD44hi T cells. Each plot is representative of three mice for WT and four mice for Bcl2l11−/−Faslpr/lpr. The data are representative of two independent experiments. Percentages ± SEM are depicted.
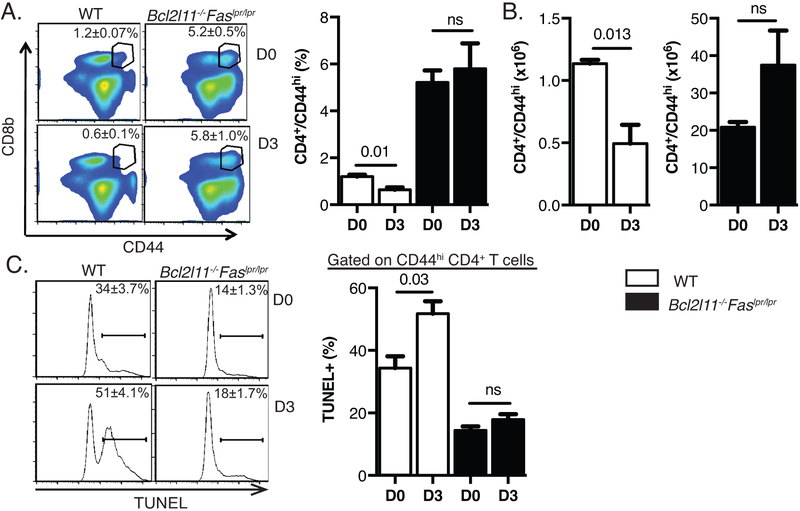
Memory phenotype CD44hi CD4+ T cells from WT mice showed a decline in percentages and numbers 3 days post-LCMV infection, although the decline was not as pronounced for CD4 T cells as for CD8 T cells (Fig. 2A, 2B). CD44hi CD4+ T cells showed a high percentage of apoptotic TUNEL+ cells (Fig. 2C). CD44hi CD4+ T cells from Bcl2l11−/−Faslpr/lpr mice were resistant to apoptotic cell death and attrition in the spleen (Fig. 2A, 2B, 2C). Together, these data indicate that memory phenotype T cells depend on both Fas and Bim to undergo apoptotic deletion in the early phase of acute viral infection.
Figure 2: LCMV-induced attrition of CD44hi CD4 T cells is dependent on Bim and Fas.
C57BL/6 (wild-type) and Bcl2l11−/−Faslpr/lpr mice were infected IP with LCMV-Armstrong (5 × 104 PFU). Splenocytes were harvested at day 0 and day 3 post infection. A) Representative FACS plots showing of percentages of CD44hi CD4 T cells in WT and Bcl2l11−/−Faslpr/lpr mice. B) Absolute number of CD44hi CD4 T cells in WT and Bcl2l11−/−Faslpr/lpr mice. C) Splenocytes were isolated and incubated ex-vivo for 5 hours followed by TUNEL staining. Representative histographs of day 0 and day 3 LCMV-infected wild-type and Bcl2l11−/−Faslpr/lpr TUNEL+ CD4 CD44hi T cells. Each plot is representative of three mice for WT and four mice for Bcl2l11−/−Faslpr/lpr. The data are representative of two independent experiments. Percentages ± SEM are depicted.
We have previously published that T cells deficient in Fas-FasL interactions or lacking Bim (Bcl2l11−/− mice) have no or only partial defects in memory T cell attrition following LCMV infection or treatment with poly (I:C) (3, 8, 9, 23). These results indicate that Fas and Bim function concurrently to regulate T cell apoptosis in early stages of viral infections. One explanation for the importance of both the intrinsic and extrinsic cell death pathways in this system is that the early T cell deletion in viral infection relies predominantly on the mitochondrial pathway activated by Bim, with a smaller contribution by the death receptor pathway activated by Fas. In Fas- or FasL- deficient mice, the functional mitochondrial pathway activated by Bim in response to viral infection would delete T cells by apoptosis (24). In Bim-deficient mice, the mitochondrial pathway that is primarily responsible for the early T cell deletion is inactivated. However, cell death would occur to a limited extent by the death receptor pathway and hence T cell attrition is partially blocked. Combined deficiency of Fas and Bim would inactivate both pathways of apoptosis, thereby completely inhibiting T cell deletion.
Prolonged allograft survival induced by CoB is dependent on Fas and Bim
CoB including the combination of DST and anti-CD154 mAb significantly prolongs survival of fully mismatched skin, heart, bone marrow and islet grafts in mice and non-human primates (25, 26). In addition, we have demonstrated that DST/anti-CD154 mAb treatment results in early deletion of alloreactive CD8 T cells (21). Previous studies have shown that CoB will significantly extend allograft survival in mice deficient in Bim or Fas (13, 16). We have also evaluated gene expression profiles of alloreactive CD8 T cells during DST/anti-CD154 mAb treatment using RNA-Seq (data not shown). No change in levels of Bim mRNA were detected between the untreated and mice treated with DST/anti-CD154 mAb. However levels of FasL mRNA increased significantly in the DST/anti-CD154 mAb (10.7 fold) treated groups as compared to untreated mice, consistent with our previous observations and suggesting a role of Fas-FasL interactions during apoptosis of T cells during CoB (27). RNA isolation, sequencing and analysis was performed as previously described (28). The raw data for this study were deposited in the Gene Expression Omnibus (Accession GSE89030, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89030).
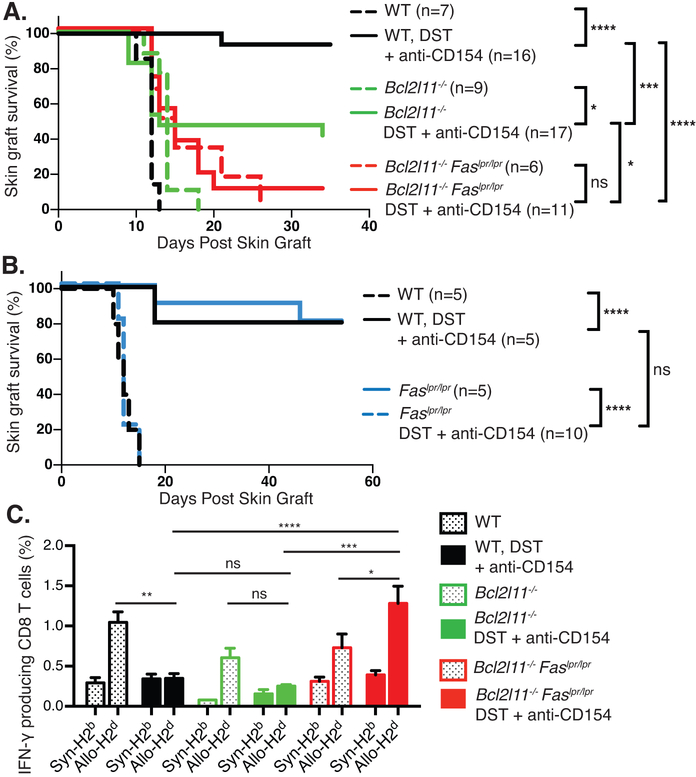
To determine the apoptotic pathways involved in allograft survival induced by CoB, WT C57BL/6J, Bcl2l11−/− and Bcl2l11−/−Faslpr/lpr mice were transplanted with MHC-mismatched BALB/c skins and treated with DST/anti-CD154 mAb. WT mice that were given no treatment rejected skin grafts rapidly, with a median survival time (MST) of 12 days (Fig. 3A) and DST/anti-CD154 mAb treatment showed prolonged allograft survival (Fig. 3A) (29). Bcl2l11−/− mice that received no treatment rejected grafts with kinetics similar to those of WT mice (MST=12 days). Interestingly, 10 out of 17 Bcl2l11−/− mice that received DST/anti-CD154 mAb rejected grafts (MST=13 days), suggesting that Bim is partially necessary for prolonged allograft survival. Skin allografts were rejected in Bcl2l11−/−Faslpr/lpr mice that received no treatment, as expected (MST=14 days) (Fig. 3A). Notably, the majority (10 out of 11 mice) of Bcl2l11−/−Faslpr/lpr mice that were treated with DST/anti-CD154 mAb rejected their grafts with a MST of 15 days (Fig. 3A). Skin allograft survival in Bcl2l11−/− mice that were treated with DST/anti-CD154 mAb was significantly increased as compared to the Bcl2l11−/−Faslpr/lpr mice (p = 0.04), indicating that mice deficient in Bim alone are partially sensitive to CoB. To confirm previous observations that mice lacking Fas signaling are still sensitive to CoB-induced survival of allografts (13, 16), WT C57BL/6J and Faslpr/lpr mice were transplanted with MHC-mismatched BALB/c skin grafts and treated with DST/anti-CD154 mAb (Fig 3B). Both untreated WT and Faslpr/lpr mice rapidly rejected the skin allografts. Treatment with DST/anti-CD154 mAb significantly prolonged allograft survival in both WT and Faslpr/lpr mice (Fig 3B), demonstrating that elimination of Fas signaling alone does not abrogate survival of skin allografts following CoB. These results indicate that loss of Fas function in addition to that of Bim completely prevents the increased survival of skin allografts in mice treated with CoB.
Figure 3: CoB induced prolonged allograft survival is dependent on Bim and Fas.
A) C57BL/6 (WT), Bcl2l11−/− and Bcl2l11−/−Faslpr/lpr mice were treated with BALB/c DST/anti-CD154 mAb and skin as described in the Materials and Methods. Skin allograft survival was then monitored for all groups. B) C57BL/6 (WT) and Faslpr/lpr mice were treated with BALB/c DST/anti-CD154 mAb and skin as described above. C) Splenocytes were harvested from the WT and Bcl2l11−/−Faslpr/lpr mice 5 weeks post-transplantation, stimulated with in vitro matured irradiated syngeneic (H2b) or allogeneic (H2d) splenocytes for 4 hours and analyzed for intracellular IFNγ by flow cytometry. The bar graphs show percentages of IFNγ producing CD8 T cells. The data are representative of two independent experiments with 2–5 mice per group. Percentages ± SEM are depicted. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We next investigated the ability of alloreactive CD8+ T cells in each group of mice to produce IFNγ in response to syngeneic (C57BL/6J) or allogeneic (BALB/c) stimulation. Consistent with our previous observation, DST/anti-CD154 mAb CoB treatment significantly reduced the frequency of donor-reactive IFNγ producing effector/memory CD8+ T cells in WT mice, correlating with increased survival of skin allografts in these mice (Fig. 3C) (20). Donor-reactive IFNγ producing CD8+ T cell proportions were lowered in Bcl2l11−/− mice treated with DST/anti-CD154 mAb despite the observation that 10 out of 17 of these mice rejected skin allografts. However, the total number of IFNγ producing CD8 T cells in the Bcl2l11−/− mice treated with DST/anti-CD154 mAb was significantly higher as compared to the numbers in WT C57BL/6J mice (5.6±0.6×104 for Bcl2l11−/− mice and 2.5±0.5×104 for WT mice, p = 0.003), which suggests that CD8 T cells in Bcl2l11−/− mice do maintain a low level of functionality. High proportions of IFNγ producing CD8+ T cells were detectable in Bcl2l11−/−Faslpr/lpr mice treated with DST/anti-CD154 mAb (Fig. 3C). Together, these data indicate that the deletion of alloreactive T cells by CoB requires both Fas and Bim.
Our data show that in a stringent BALB/c to B6 skin allograft model, Faslpr/lpr mice are sensitive to CoB induced allograft survival and Bcl2l11−/− mice are partially resistant. However Bcl2l11−/−Faslpr/lpr mice are completely resistant to prolonged allograft survival. We propose that the passive cell death pathway is the primary mechanism for the prolonged allograft survival with a small contribution by the death receptor pathway. Recent studies have shown that the anti-CD154 mAb inhibits dendritic cell expression of inflammatory cytokines that are required for productive T cell activation and expansion (30). In Fas or FasL deficient mice, the passive death pathway activated by the lack of cytokines resulting from CD154 antagonism would mediate apoptosis of alloreactive T cells. In Bim deficient mice, cell death would occur by AICD or mitochondrial apoptosis by an alternate BH3 protein such as Puma, Noxa or Bid resulting in partial susceptibility to CoB. However, in mice lacking Fas and Bim, the inhibition of both extrinsic and intrinsic apoptosis completely prevents prolonged survival of allografts.
Overall our results show that apoptosis of memory T cells in response to acute LCMV infection and death of alloantigen-specific T cells in response to alloantigen and CoB are mediated by both Fas and Bim pathways. Previous studies have shown that Fas and Bim have redundant roles in regulating cell death pathways that are important for controlling the development of autoimmunity and the contraction of virus-specific memory CD8 T cells (7, 17, 18). The results of our studies demonstrate that the Fas and Bim redundancy is also critical for cell death observed in the context of inflammation-induced attrition of memory T cells and the extension of allograft survival by CoB. Our results highlight the concurrent function of multiple apoptotic pathways in regulating immune responses during early viral infection and during CoB. Together these findings suggest that collaboration between Bim and Fas is essential to regulate T cell homeostasis and cell death at several stages during the T cell life cycle (31, 32).
Acknowledgments
This work was supported in part by National Institutes of Health grants R24 OD018259, R01 DK103546, DP3 DK111898 and R01 AI132963 (MAB), R01 DK107220 (RJD), and R01 AI17672 (RMW). RJD is an Investigator of the Howard Hughes Medical Institute. This research was supported by the NIDDK-supported Human Islet Research Network (UC4 DK104218 to MAB)
REFERENCES
- 1.Krammer PH, Arnold R, and Lavrik IN. 2007. Life and death in peripheral T cells. Nat Rev Immunol 7: 532–542. [DOI] [PubMed] [Google Scholar]
- 2.Bouillet P, and O’Reilly LA. 2009. CD95, BIM and T cell homeostasis. Nat Rev Immunol 9: 514–519. [DOI] [PubMed] [Google Scholar]
- 3.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, and Welsh RM. 2001. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 75: 5965–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, and Greiner DL. 2006. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. Journal of immunology (Baltimore, Md. : 1950) 176: 1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strasser A, Harris AW, Huang DC, Krammer PH, and Cory S. 1995. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. Embo j 14: 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kale J, Osterlund EJ, and Andrews DW. 2018. BCL-2 family proteins: changing partners in the dance towards death. Cell death and differentiation 25: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, and Grayson JM. 2008. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity 28: 218–230. [DOI] [PubMed] [Google Scholar]
- 8.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, and Welsh RM. 2006. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol 176: 4284–4295. [DOI] [PubMed] [Google Scholar]
- 9.Bahl K, Huebner A, Davis RJ, and Welsh RM. 2010. Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists. Journal of virology 84: 4866–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ML 2016. T Cell Cosignaling Molecules in Transplantation. Immunity 44: 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XC, Strom TB, Turka LA, and Wells AD. 2001. T cell death and transplantation tolerance. Immunity 14: 407–416. [DOI] [PubMed] [Google Scholar]
- 12.Maltzman JS, and Turka LA. 2013. T-cell costimulatory blockade in organ transplantation. Cold Spring Harb Perspect Med 3: a015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XC, Li Y, Dodge I, Wells AD, Zheng XX, Turka LA, and Strom TB. 1999. Induction of allograft tolerance in the absence of Fas-mediated apoptosis. Journal of immunology (Baltimore, Md. : 1950) 163: 2500–2507. [PubMed] [Google Scholar]
- 14.Wagener ME, Konieczny BT, Dai Z, Ring GH, and Lakkis FG. 2000. Alloantigen-driven T cell death mediated by Fas ligand and tumor necrosis factor-alpha is not essential for the induction of allograft acceptance. Transplantation 69: 2428–2432. [DOI] [PubMed] [Google Scholar]
- 15.Trambley J, Lin A, Elwood E, Bingaman AW, Lakkis F, Corbascio M, Pearson TC, and Larsen CP. 2001. FasL is important in costimulation blockade-resistant skin graft rejection. Transplantation 71: 537–543. [DOI] [PubMed] [Google Scholar]
- 16.Lehnert AM, Murray-Segal L, Cowan PJ, d’Apice AJ, and O’Connell PJ. 2007. Blockade of the passive cell death pathway does not prevent tolerance induction to islet grafts. Transplantation 83: 653–655. [DOI] [PubMed] [Google Scholar]
- 17.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, and Bouillet P. 2008. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK 3rd, Wu T, Li QZ, Davis LS, Mohan C, and Perlman H. 2008. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity 28: 206–217. [DOI] [PubMed] [Google Scholar]
- 19.Welsh RM Jr., Lampert PW, Burner PA, and Oldstone MB. 1976. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology 73: 59–71. [DOI] [PubMed] [Google Scholar]
- 20.Brehm MA, Mangada J, Markees TG, Pearson T, Daniels KA, Thornley TB, Welsh RM, Rossini AA, and Greiner DL. 2007. Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood 109: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, and Greiner DL. 2000. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol 164: 512–521. [DOI] [PubMed] [Google Scholar]
- 22.Hegde S, Beauregard C, Mayhew E, and Niederkorn JY. 2005. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation 79: 23–31. [DOI] [PubMed] [Google Scholar]
- 23.Zarozinski CC, McNally JM, Lohman BL, Daniels KA, and Welsh RM. 2000. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J. Virol. 74: 3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla-Sarkar M, Leaman DW, and Borden EC. 2001. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res 7: 1821–1831. [PubMed] [Google Scholar]
- 25.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, and Rossini AA. 1995. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. U S A 92: 9560–9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishimoto K, Yuan X, Auchincloss H, Sharpe AH, Mandelbrot D. a., and Sayegh MH. 2004. Mechanism of action of donor-specific transfusion in inducing tolerance: role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. Journal of the American Society of Nephrology : JASN 15: 2423–2428. [DOI] [PubMed] [Google Scholar]
- 27.Priyadharshini B, Thornley TB, Daniels KA, Cuthbert A, Welsh RM, Greiner DL, and Brehm MA. 2013. Alloreactive CD8 T cells rescued from apoptosis during co-stimulation blockade by Toll-like receptor stimulation remain susceptible to Fas-induced cell death. Immunology 138: 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Vasaikar S, Shi Z, Greer M, and Zhang B. 2017. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res 45: W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, and Rossini AA. 1997. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation 64: 329–335. [DOI] [PubMed] [Google Scholar]
- 30.Ferrer IR, Liu D, Pinelli DF, Koehn BH, Stempora LL, and Ford ML. 2012. CD40/CD154 blockade inhibits dendritic cell expression of inflammatory cytokines but not costimulatory molecules. J Immunol 189: 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upton JW, and Chan FK. 2014. Staying alive: cell death in antiviral immunity. Molecular cell 54: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weant AE, Michalek RD, Crump KE, Liu C, Konopitski AP, and Grayson JM. 2011. Defects in apoptosis increase memory CD8+ T cells following infection of Bim−/−Faslpr/lpr mice. Cell Immunol 271: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]