Abstract
Objective:
Angiotensinogen (AGT) is the unique precursor of the renin-angiotensin system that is sequentially cleaved by renin and angiotensin-converting enzyme (ACE) to produce angiotensin II (AngII). In this study, we determined how these renin-angiotensin components interact with megalin in kidney to promote atherosclerosis.
Approach and Results:
AGT, renin, ACE, and megalin were present in the renal proximal convoluted tubules of wild type mice. Hepatocyte-specific AGT deficiency abolished AGT protein accumulation in proximal tubules and diminished AngII concentrations in kidney, while renin was increased. Megalin was most abundant in kidney and exclusively present on the apical side of proximal tubules. Inhibition of megalin by antisense oligonucleotides (ASO) led to ablation of AGT and renin accumulation in proximal tubules, while leading to striking increases of urine AGT and renin concentrations, and 70% reduction of renal AngII concentrations. However, plasma AngII concentrations were unaffected. To determine whether AGT and megalin interaction contributes to atherosclerosis, we used both male and female low-density lipoprotein receptor −/− mice fed a saturated fat-enriched diet and administered vehicles (PBS or control ASO) or megalin ASO. Inhibition of megalin did not affect plasma cholesterol concentrations, but profoundly reduced atherosclerotic lesion size in both male and female mice.
Conclusion:
These results reveal a regulatory role of megalin in the intrarenal renin-angiotensin homeostasis and atherogenesis, positing renal AngII to be an important contributor to atherosclerosis that is mediated through AGT and megalin interactions.
Keywords: atherosclerosis, angiotensin, angiotensinogen, megalin, renin
Introduction
The renin-angiotensin system plays a pivotal role in the development of atherosclerosis.1–3 Angiotensinogen (AGT) is the sole substrate of this hormonal system, which is cleaved by renin to release angiotensin I (Ang)I. AngI is subsequently cleaved by angiotensin-converting enzyme (ACE) to produce AngII.4,5 There is compelling evidence that genetical or pharmacological inhibition of the renin-angiotensin components reduces atherosclerosis through inhibition of AngII production or action.1,6–10 However, it remains unclear where AngII is produced to exert its proatherogenic effects.
AGT originating from liver, renin from kidney, and ACE from lung constitute the systemic renin-angiotensin system. This classic notion has been expanded by discovery of these components in many tissues and organs.11 Kidney has all of the renin-angiotensin components required to produce AngII, and it has the potential to be an important locus for AngII production based on its concentrations in proximal convoluted tubule far exceeding those in plasma.12–14 It is also well-documented that activation of renal AngII contributes to the pathogenesis of hypertension and kidney disease,13,15–18 but the role of AngII produced in kidney to atherosclerosis has not been determined.
In kidney, hepatocyte-derived AGT is endocytosed into proximal convoluted tubule cells by megalin.19 Megalin, also known as low-density lipoprotein-related protein 2 or gp330, is a multiligand endocytic receptor in the low density-lipoprotein (LDL) receptor family.20–22 Present in polarized epithelial cells,20,22–24 megalin is most abundant along the apical aspect of proximal tubules,23 where it is primarily responsible for reabsorption of some glomerular filtered substances.24 Megalin also interacts with AGT and determines its homeostasis in kidney.19,25 The regulatory role of megalin in AGT homeostasis and renal AngII production that contributes to atherosclerosis has not been explored. In the present study, we identified potent actions of megalin in intrarenal renin-angiotensin regulation and atherogenesis, which provide evidence for a novel concept that kidney is an important source to promote atherosclerosis through a megalin-mediated mechanism.
Materials and Methods
Detailed Materials and Methods are available in the online-only Data Supplement.
Animals
Generation of hepatocyte-specific AGT deficient mice has been reported in our previous studies.26,27 Male angiotensinogen floxed (Agt f/f) × albumin-Cre +/− mice and female Agt f/f × albumin-Cre −/− mice were bred to generate Agt f/f × albumin-Cre −/− (hepAGT+/+) and Agt f/f × albumin-Cre +/− (hepAGT−/−) littermates in an LDL receptor −/− background for experiments described in this manuscript.
C57BL/6J (Stock # 000664) mice and LDL receptor −/− mice (B6.129S7 - LdlrtmlHer/J, Stock# 002207) were purchased from The Jackson Laboratory. C57BL/6J mice were fed a normal laboratory diet (Diet # 2918; Envigo). To induce atherosclerosis, LDL receptor −/− mice were fed a diet supplemented with saturated fat (milk fat 21% wt/wt) and cholesterol (0.2% wt/wt; Diet # TD. 88137; Envigo; termed “Western diet”) for 12 weeks. Eight - 12 week old littermates, both male and female mice, were used for experiments reported in this manuscript following the recent ATVB Council statement.28 Quantification and characterization of atherosclerosis followed the recent AHA statement and our standard protocols.29,30
All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Kentucky (Protocol # 2006–0009 or 2015–2050).
Administration of Antisense Oligonucleotides
Control or megalin antisense oligonucleotides (ASOs) were provided by Ionis Pharmaceuticals Inc. (Carlsbad, CA, USA). Control ASOs showed no complementary binding to any known rodent mRNA including megalin. Optimal dose information (lowest dose with the maximal inhibition of megalin mRNA) of megalin ASOs was provided by Ionis Pharmaceuticals Inc for Gen 2.0 ASOs at 120 mg/kg body weight and Gen 2.5 ASOs at 6 mg/kg body weight, respectively. ASOs were administered via subcutaneous injections once a week prepared in sterile PBS.
Statistical Analysis
SigmaPlot Version 13 (Systat Software Inc.) was used for statistical analyses. To compare continuous response variables between two groups, an unpaired two-tailed Student’s t-test was used for normally distributed variables that passed the equal variance test, and Mann-Whitney U test was performed for variables not passing either normality or equal variance test. To compare more than two groups, one-way ANOVA followed by Holm-Sidak method was used for normally distributed variables that passed equal variance test and Kruskal-Wallis one way ANOVA on Ranks with Dunn’s method for variables not passing normality or equal variance test, respectively. P<0.05 was considered as statistically significant.
Results
Hepatocyte-derived AGT Contributed to AGT Protein and AngII Production in Kidney
AGT is present in many tissues and organs.4,31 In agreement with previous findings,19 we found that hepatocyte-specific AGT deficiency (hepAGT−/−) diminished renal AGT protein accumulation without affecting mRNA abundance of AGT in kidney (Figure 1A and Figures I and II in online-only Data Supplement). Renin is an aspartyl protease, which cleaves AGT to release the decapeptide AngI. Plasma renin concentrations were much higher in hepAGT−/− mice than their wild type littermates.26 Immunofluorescent staining showed that both AGT and renin were present in proximal convoluted tubule cells (Figure 1A). We also observed profound increases of renin protein abundance in kidneys of hepAGT−/− mice, as demonstrated by Western blotting and a 5-fold increase of renin mRNA (Figures I and II in online-only Data Supplement). ACE and AT1a receptor abundance in kidney were not significantly different between hepAGT+/+ and hepAGT−/− mice (Figures I and II in online-only Data Supplement).
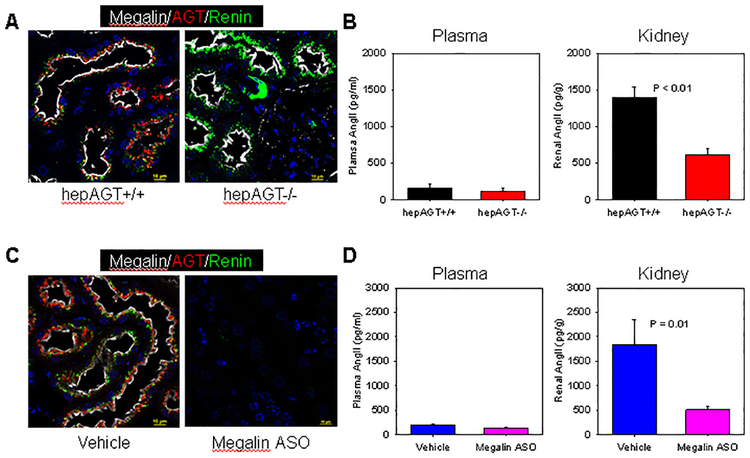
Figure 1. Hepatocyte-derived AGT was the source of renal AGT, and megalin regulated AGT homeostasis and contributed to renal AngII production.
(A) Immunofluorescent staining demonstrates the presence of megalin (white), AGT (red), and renin (green) in kidney tissue sections from hepAGT+/+ and hepAGT−/− mice.(B) AngII concentrations in plasma and kidney from hepAGT+/+ and hepAGT−/− mice. P < 0.01 by Student’s t-test. (C) Immunofluorescent staining of megalin (white), AGT (red), and renin (green) in kidney sections from mice injected with either vehicle or megalin ASO. (D) Plasma and renal AngII concentrations in mice injected with either vehicle or megalin ASO. N = 9 – 10/group. P = 0.01 by Mann-Whitney Rank Sum test.
Since the predominant function of AGT is to produce AngII, we determined whether depletion of this precursor from hepatocytes would affect plasma and renal AngII concentrations. Although hepatocyte-derived AGT was responsible for the majority of plasma AGT concentrations,19,26,27 plasma AngII concentrations were not different between hepAGT +/+ and −/− mice. In contrast, a profound reduction of AngII in kidney was evident in hepAGT−/− mice, compared with wild type littermates (Figure 1B).
Inhibition of Megalin Disturbed AGT and Renin Homeostasis and Reduced Renal AngII Production
Consistent with the report by Pohl and colleagues,25 we found AGT and renin were present in the subapical compartment of megalin-positive renal proximal tubules, and ACE was observed on brush borders (Figure I in online-only Data Supplement). Therefore, the essential renin-angiotensin components to produce AngII were localized with megalin in renal proximal tubules. To investigate whether megalin regulates these renin-angiotensin components, megalin ASO were applied to inhibit megalin.
Megalin was expressed most abundantly in kidney while being barely detectable in other organs in both male and female adult C57BL/6 mice fed normal diet or LDL receptor −/− mice fed Western diet (Figure III in online-only Data Supplement). Administration of megalin ASO efficiently suppressed megalin mRNA and protein abundance in kidney (Figure IVA and B in online-only Data Supplement). Inhibition of megalin by ASO had no effects on mRNA abundance of both AGT and renin in kidney (Figures VA and B in online-only Data Supplement), but ablated their proteins from proximal tubules, as demonstrated by immunofluorescent staining (Figure 1C), We observed striking increases of urine AGT and renin concentrations in mice receiving megalin ASO, whereas no major effects on plasma AGT and renin concentrations were detected (Figure V C–F in online-only Data Supplement). In agreement with our observation in mice with hepatocyte-specific deficiency of AGT, inhibition of megalin led to pronounced reduction of renal AngII concentrations without influence on plasma AngII concentrations (Figure 1D).
Inhibition of Megalin Attenuated Hypercholesterolemia-induced Atherosclerosis
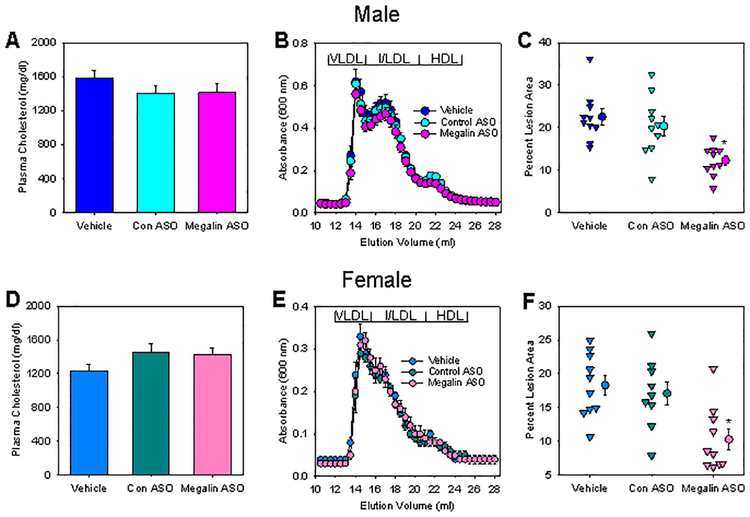
Our previous studies demonstrated that hepatocyte-specific deficiency of AGT led to pronounced reductions of atherosclerotic lesion area in mice.26,27 Since AngII production in kidney is regulated by megalin, we examined the involvement of megalin in atherosclerosis. Both male and female LDL receptor −/− mice were injected subcutaneously with vehicle (PBS only), control ASO, or Gen 2.5 megalin ASO once every week for 13 weeks. Western diet feeding started 1 week after the first injection and continued for 12 weeks. Inhibition of megalin did not affect plasma total cholesterol concentrations or lipoprotein-cholesterol distributions (Figure 2A and B). En face analysis revealed reductions of atherosclerotic lesions in megalin ASO injected mice in both male and female mice (Figure 2C). To validate this result, a second Gen 2.5 megalin ASO was developed, which targeted a different sequence of mouse megalin mRNA. A pronounced amelioration of atherosclerosis was also observed with administration of this second megalin ASO (Figure VI in online-only Data Supplement), providing consistent evidence that inhibition of megalin reduced development of atherosclerosis.
Figure 2. Inhibition of megalin reduced hypercholesterolemia-induced atherosclerosis.
(A and D) Plasma total cholesterol concentrations in mice were measured using an enzymatic colorimetric method in mice fed Western diet for 12 weeks. N = 9 – 10/group. (B and E) Lipoprotein distributions of cholesterol in plasma were determined using FPLC size exclusion chromatography. N = 4/group. (C and F) Percent atherosclerotic lesion area was measured using an en face technique. N = 9 – 10/group. * P < 0.001 (C) or P = 0.002 (F) by one way ANOVA followed by Holm-Sidak method.
We also measured atherosclerotic lesions in the aortic root. Consistent with our findings by en face analysis, atherosclerotic lesions in the aortic root were smaller in mice injected with megalin ASO, compared to mice injected with vehicle (Figure VII in online-only Data Supplement). Despite the difference of lesion size between mice injected with vehicle and megalin ASO, the major cell type in atherosclerotic lesions of both groups was the lipid-laden macrophages as demonstrated by Oil Red O staining and immunostaining of CD68 (Figure VIII and XI in online-only Data Supplement).
AGT has been implicated as an acute phase protein.32 A previous study reported that hepatocyte-specific gp130 deficiency in apolipoprotein E deficient mice reduced atherosclerotic lesions, accompanied by reduced plasma serum amyloid A concentrations.33 We measured plasma concentrations of serum amyloid A, but did not detect differences of this acute phase protein marker between hepAGT+/+ and −/− mice, and between vehicle and megalin ASO injected mice fed Western diet (Figure X in online-only Data Supplement).
Discussion
The present study reports two novel findings. First, inhibition of megalin reduces hypercholesterolemia-induced atherosclerosis. Second, renal, rather than plasma, AngII contributes to the development of atherosclerosis.
Since leukocyte infiltration is a prominent feature of atherosclerosis,34–36 it was hypothesized that the renin-angiotensin activation in leukocytes would contribute to atherosclerosis. This hypothesis was negated by previous studies that AGT deficiency in leukocytes had no effects on atherosclerosis,27 deficiency of renin or ACE in leukocytes only led to modest reduction of atherosclerosis,37,38 and AT1a receptor deficiency on leukocytes had no effects37,39,40 or modest effect on atherosclerosis.41 As AT1a receptor is abundant on endothelial and smooth muscle cells of the vasculature,42 another widely circulated view persists that AngII contributes to atherosclerosis by direct provocation of AT1a receptor on resident cells in vasculature. This hypothesis was not substantiated by our studies demonstrating that AT1a receptor deletion on either endothelial cells or smooth muscle cells had no effects on atherosclerosis.43 Therefore, the major source for AngII to promote atherosclerosis is not in the vascular wall or the circulation.
It is well recognized that AGT synthesized by liver, renin by kidney and ACE by lung contribute to their presence in circulation.5,26,27,44–46 However, it is unclear whether these organs provide each component to plasma to produce AngII that contributes to physiological and pathophysiological functions, or they are predominantly transported through blood flow to target organs to facilitate local production of AngII. Remarkably, kidney expresses AGT mRNA in S3 segment,25,47,48 but AGT protein only accumulates in S1 and S2 segments of proximal convoluted tubules.19 The function of AGT in S3 segment, if any, remains unclear. The molecular weight of AGT is approximately 50 kDa. The glomerular permeability of AGT in vivo was observed using multiphoton microscopy.49 After filtration, AGT is retained in proximal convoluted tubules and becomes the primary source of intrarenal AGT protein.19 Our findings also confirm that liver is not only the major provider of AGT in plasma, but also the source of AGT protein in kidney. Hepatocyte-derived AGT did not affect plasma AngII concentrations, but had a profound influence on AngII concentrations in kidney. Given that AngII concentrations in kidney are far higher than concentrations in plasma,12–14,50 and depletion of AGT in plasma reduced renal rather than plasma AngII concentrations, we hypothesize that kidney is an important organ for AGT derived from liver to produce AngII.
We confirmed that all the components needed for AngII production were present in proximal tubules. Since these renin-angiotensin components to produce AngII are localized with megalin in proximal tubules, we presumed that megalin can regulate AngII production in kidney. Our studies clearly showed that reduction of megalin by ASOs ablated both AGT and renin accumulation from proximal tubules, which support the notion that megalin plays an important role in retaining intratubular AGT and renin. Diminished accumulation of both AGT and renin in proximal convoluted tubule cells by megalin ASOs did not lead to profound reductions of plasma AGT or renin concentrations despite their substantial losses in urine. Similar losses occur in patients with Dent disease or Lowe syndrome in whom reabsorption by megalin is impaired, implicating human relevance of our findings in mice.51 These results are consistent with endocytosed AGT and renin in proximal convoluted tubules being processed locally. Reduction of megalin led to approximately 70% reduction of renal AngII concentrations, without influence on plasma concentrations. These findings demonstrate that megalin plays a crucial role in maintaining AGT and renin homeostasis in kidney, which orchestrates the crosstalk of intrarenal renin-angiotensin components to determine AngII production locally. Pronounced reductions of atherosclerosis were observed in hepAGT−/− mice26,27 and mice administered megalin ASOs, although their plasma AngII concentrations were not different compared with their relative controls. These data support that renal, rather than the systemic renin-angiotensin activation, is the contributor to the development of atherosclerosis. Because serum amyloid A, a well-established biomarker of systemic inflammatory stress, was unchanged in hepAGT−/− mice or mice administered megalin ASO, the mechanisms whereby renal AngII contributes to atherosclerosis may not be via increases in systemic inflammation.
In summary, either deletion of AGT or inhibition of megalin profoundly reduced AngII production in kidney and was associated with pronounced reduction of atherosclerosis. These data support an important role of megalin in intrarenal renin-angiotensin homeostasis and atherosclerosis, and indicate kidney as a primary source of AngII to promote atherosclerosis. This effect is accomplished via megalin integrating with the renin-angiotensin components in proximal tubules, providing rationale for future studies to determine the mechanisms by which renal AngII contributes to atherosclerosis.
Supplementary Material
Highlights.
Megalin regulates AGT and renin homeostasis in kidney and regulates renal AngII production.
Inhibition of megalin ameliorates hypercholesterolemia-induced atherosclerosis.
AngII in kidney, rather than in plasma, contributes to atherosclerosis.
Acknowledgment
We thank Dr. Craig Vander Kooi (Molecular and Cellular Biochemistry, University of Kentucky) for his constructive advice on experimental design and data interpretation.
Sources of Funding
The authors’ research work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01HL139748. Dr. Feiming Ye and Ya Wang were supported by grants (81320108003 and 31371498) to Jian’an Wang from National Natural Science Foundation of China. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation
- ACE
Angiotensin-converting enzyme
- AGT
Angiotensinogen
- Ang
Angiotensin
- ASO
Antisense Oligonucleotides
- LDL
Low-density lipoprotein
Footnotes
Disclosures
Adam E. Mullick is an employee and Mark J. Graham was an employee of Ionis Pharmaceuticals, Inc.
References
- 1.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. [DOI] [PubMed] [Google Scholar]
- 2.Mazzolai L, Hayoz D. The renin-angiotensin system and atherosclerosis. Curr Hypertens Rep. 2006;8:47–53. [DOI] [PubMed] [Google Scholar]
- 3.van Thiel BS, van der Pluijm I, te Riet L, Essers J, Danser AH. The renin-angiotensin system and its involvement in vascular disease. Eur J Pharmacol. 2015;763:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston). 2011;4:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Howatt DA, Balakrishnan A, Moorleghen JJ, Wu C, Cassis LA, Daugherty A, Lu H. Angiotensin-converting enzyme in smooth muscle cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 7.ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. [DOI] [PubMed] [Google Scholar]
- 10.Wu CH, Mohammadmoradi S, Chen JZ, Sawada H, Daugherty A, Lu HS. Renin-angiotensin system and cardiovascular functions. Arterioscler Thromb Vasc Biol. 2018;38:e108–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danser AH. Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol. 2003; 35:759–778. [DOI] [PubMed] [Google Scholar]
- 12.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–506. [DOI] [PubMed] [Google Scholar]
- 15.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT(1a) receptors induces blood pressure responses to intracellular angiotensin II in AT(1a) receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2013;304:R588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Niimi M, Yang D et al. Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits. Arterioscler Thromb Vasc Biol. 2017;37:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satou R, Shao W, Navar LG. Role of stimulated intrarenal angiotensinogen in hypertension. Ther Adv Cardiovasc Dis. 2015;9:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raychowdhury R, Niles JL, McCluskey RT, Smith JA. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Sci. 1989;244:1163–1166. [DOI] [PubMed] [Google Scholar]
- 21.Saito A, Pietromonaco S, Loo AKC, Farquhar MG. Complete cloning and sequencing of rat gp330/”megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc. Natl. Acad. Sci. USA 1994;91:9725–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. [DOI] [PubMed] [Google Scholar]
- 23.Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 2016;89:58–67. [DOI] [PubMed] [Google Scholar]
- 25.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Xu Y, Lu H, Howatt DA, Balakrishnan A, Moorleghen JJ, Kooi CW, Cassis LA, Wang JA, Daugherty A. Cys18-Cys137 disulfide bond in mouse angiotensinogen does not affect AngII-dependent functions in vivo. Hypertension. 2015; 65:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, Feldman DL, Cassis LA, Vander Kooi CW, Daugherty A. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler Thromb Vasc Biol. 2016;36:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-Statement From ATVB Council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daugherty A, Tall AR, Daemen MJAP, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Rateri DL, Daugherty A. Immunostaining of mouse atherosclerosis lesions. Methods Mol Med. 2007; 139:77–94. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Cassis LA, Vander Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bing J Relation between renin substrate and acute phase proteins. Studies of normal and adrenalectomized rats. Acta Pathol Microbiol Scand A. 1972;80:646–650. [DOI] [PubMed] [Google Scholar]
- 33.Luchtefeld M, Schunkert H, Stoll M et al. Signal transducer of inflammation gp130 modulates atherosclerosis in mice and man. J Exp Med. 2007;204:1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross R Atherosclerosis--an inflammatory disease. N. Engl. J. Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 35.Brasier AR, Recinos A, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 36.Lusis AJ. Atherosclerosis. Nature. 2000;407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu H, Rateri DL, Feldman DL, Charnigo RJ Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen XC, Lu H, Zhao M, Tashiro K, Cassis LA, Daugherty A. Angiotensin-converting enzyme promotes atherosclerosis through an angiotensin I to angiotensin II pathway involving leukocytes. Arterioscler Thromb Vasc Biol. 2013;33: 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007; 27:380–386. [DOI] [PubMed] [Google Scholar]
- 40.Koga J, Egashira K, Matoba T, Kubo M, Ihara Y, Iwai M, Horiuchi M, Sunagawa K. Essential role of angiotensin II type 1a receptors in the host vascular wall, but not the bone marrow, in the pathogenesis of angiotensin II-induced atherosclerosis. Hypertens Res. 2008;31:1791–1800. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda D, Sata M, Ishizaka N, Nagai R. Critical role of bone marrow angiotensin II type 1 receptor in the pathogenesis of atherosclerosis in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2007; 28:72–77. [DOI] [PubMed] [Google Scholar]
- 42.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. [DOI] [PubMed] [Google Scholar]
- 43.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One. 2012;7:e51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000; 275:5–8. [DOI] [PubMed] [Google Scholar]
- 46.Ng KK, Vane JR. The conversion of angiotensin I to angiotensin II in vivo. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259:188–189. [DOI] [PubMed] [Google Scholar]
- 47.Taugner R, Hackenthal E, Helmchen U, Ganten D, Kugler P, Marin-Grez M, Nobiling R, Unger T, Lockwald I, Keilbach R. The intrarenal renin-angiotensin-system. An immunocytochemical study on the localization of renin, angiotensinogen, converting enzyme and the angiotensins in the kidney of mouse and rat. Klin Wochenschr. 1982;60:1218–1222. [DOI] [PubMed] [Google Scholar]
- 48.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. [DOI] [PubMed] [Google Scholar]
- 49.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison-Bernard LM, El-Dahr SS, O’Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension. 1999;33:340–346. [DOI] [PubMed] [Google Scholar]
- 51.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ, Danser AH. On the Origin of Urinary Renin: A Translational Approach. Hypertension. 2016;67:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.