Abstract
Objective:
Comparative survival between neoadjuvant chemotherapy (NC) and adjuvant chemotherapy (AC) for patients with cT2-4N0-1M0 non-small cell lung cancer (NSCLC) has not been extensively studied.
Methods:
Patients with cT2-4N0-1M0 NSCLC who received platinum-based chemotherapy were retrospectively identified. Exclusion criteria included stage IV disease, induction radiotherapy, and targeted therapy. The primary endpoint was disease-free survival (DFS). Secondary endpoints were overall survival (OS), chemotherapy tolerance, and ability of Response Evaluation Criteria In Solid Tumors (RECIST) response to predict survival. Survival was estimated using the Kaplan-Meier method, compared using the log-rank test and Cox proportional hazards models, and stratified using matched pairs following propensity score–matching.
Results:
In total, 330 patients met the inclusion criteria (n=92/group after propensity-score matching; median follow-up, 42 months). Five-year DFS was 49% (95% confidence interval [CI]: 39%-61%) for NC versus 48% (95% CI: 38%-61%) for AC (p=0.70). On multivariable analysis, DFS was not associated with NC or AC (hazard ratio: 1.1 [95% CI: 0.64-1.90]; p=0.737), nor was OS (hazard ratio: 1.21 [95% CI: 0.63-2.30]; p=0.572). The NC group was more likely to receive full doses and cycles of chemotherapy (p=0.014/0.005) and had fewer grade ≥3 toxicities (p=0.001). RECIST response to NC was associated with DFS (p=0.035); 15% of NC patients (14/92) had a major pathologic response.
Conclusions:
Timing of chemotherapy—before or after surgery—is not associated with an improvement in overall or disease-free survival among patients with cT2-4N0-1M0 NSCLC who undergo complete surgical resection.
Introduction
We have recently shown that even with a complete resection (R0) in patients with pathologic node-negative lung adenocarcinoma there was a very high incidence (20-50%) of distant recurrence for increasing T stage tumors, suggesting that surgery alone in this patient population is inadequate therapy.1 The National Comprehensive Cancer Network (NCCN) guidelines recommended surgery followed by adjuvant chemotherapy (AC) for patients with T2-4N0-1 non-small cell lung cancer (NSCLC), with a footnote that neoadjuvant chemotherapy (NC) should also be considered for these patients.2 The recommendation for AC is based on multiple phase III randomized controlled trials and a meta-analysis that established an approximately 5% better 5-year overall survival (OS) for surgery plus AC versus surgery alone.3-5 Although associated with improved survival, up to 51% of patients who receive AC experience adverse events, 23% of which are grade 4.5 In addition, compliance with AC regimens is poor: in one study, 9% of patients did not receive any of the prescribed AC, only 59% received the full dose, and 25% received ≤2 cycles of AC.4
NC offers potential benefits over AC for patients with cT2-4N0-1 NSCLC, including additional time for preoperative cessation of smoking, reduction in tumor size, treatment of micro-metastatic disease, and ability to assess treatment response which can impact decisions on appropriate selection of additional adjuvant therapies. Radiographic tumor response to NC may also provide additional prognostic information and allows the ability to consider cessation or switch of therapy in the absence of a response.6 In addition, NC has been associated with better tolerance and compliance, as demonstrated in a phase III randomized trial in which 97% of patients in the NC group started planned chemotherapy, compared with 66% in the AC group.7
The preponderance of available data support surgery followed by AC over surgery alone. In contrast, the evidence base supporting the use of NC for patients with cT2-4N0-1 NSCLC is limited, and comparisons with AC are not robust and have limitations.7 With the increasing interest in the use of induction immunotherapy for resectable NSCLC, it is becoming ever more important to understand the contemporary effects of NC versus AC on outcomes in these patients8. Therefore, the primary objective of this study was to evaluate disease-free survival (DFS) among patients with cT2-4N0-1 NSCLC treated with NC or AC. Secondary objectives included assessment of OS and compliance with chemotherapy regimen(s). Additionally, among patients receiving NC, the relationship between survival and major pathologic response (MPR) rates and radiographic response to chemotherapy were investigated.
Materials and Methods
Patient Population
Following Institutional Review Board approval (IRB Protocol # 16-1395), we performed a retrospective review of a prospectively maintained, single-institution surgical database for patients with cT2-4N0-1 NSCLC who were treated with platinum-based chemotherapy and surgical resection from January 2000 to December 2015. The decision to administer NC versus proceed directly to surgical resection was based on physician assessment as well as multidisciplinary tumor board discussion. At our institution, patients who receive neoadjuvant chemotherapy are typically re-evaluated radiographically after two cycles of therapy; if there is no response, the decision is to proceed directly to surgery or to switch chemotherapy agents as previously described by our group.6 Patients were excluded from analysis if they had undergone induction radiotherapy, any targeted therapy (with or without chemotherapy), both NC and AC, or a nonconventional chemotherapy regimen (including additional treatment for a separate metastatic cancer); if they didn’t undergo a pretreatment positron emission tomography [PET] scan; if they had stage IV disease (American Joint Committee on Cancer 8th edition) or microscopic or macroscopic residual disease (R1/R2 resection); or if >90 days had elapsed between surgery and either NC or AC. Patient demographic characteristics, primary tumor maximum standardized uptake value (SUVmax), tumor response to NC, chemotherapy details, pathologic tumor subtype (American Joint Committee on Cancer 8th edition), postoperative complications, and follow-up data were documented. Patients who received NC underwent restaging with posttreatment computed tomography (CT) and PET/CT. Mediastinal surgical restaging was performed on a selective basis.9
Tumor response to NC was assessed by change in size on CT scan in accordance with Response Evaluation Criteria In Solid Tumors (RECIST).10 Pathologic responses to NC were verified by re-review of pathologic slides and was confirmed by a pathologist (J.M., W.T.). MPR was defined as ≥90% necrosis of the tumor.11 Chemotherapy tolerance was defined as the receipt of full doses and full cycles without alteration secondary to intolerance or adverse reactions. Adverse reactions to chemotherapy and postoperative complications were graded according to the Common Terminology Criteria for Adverse Events (versions 5.0 and 4.0, respectively). Prolonged air leak was defined as air leak lasting >5 days.12, 13
Patient follow-up was performed by history and physical examination and CT scan every six months for the first two years and then annually thereafter, in accordance with NCCN guidelines. The timing and location of recurrences were noted. Metachronous primary lung cancers were differentiated from recurrent disease using the criteria established by Martini and Melamed,14 as well as using molecular genomic data, when available.
Statistical Analysis
To reduce potential selection bias related to a nonrandomized cohort, we included results from propensity score–matched analyses and generated two groups (NC and AC) with comparable characteristics (i.e., balanced). Propensity scores were computed as the conditional probability of receiving AC (vs. NC) using a logistic regression model that included 10 variables: year of surgery (2000-2005, 2006-2010, 2011-2015), age at surgery, gender, body mass index (kg/m2), Charlson comorbidity score, procedure (pneumonectomy vs bilobectomy, lobectomy, or segmentectomy), tumor laterality, histologic subtype (adenocarcinoma, squamous, vs. large cell, mixed, or other), clinical stage (IB, IIA, IIB, IIIA), and pretreatment tumor SUVmax.
Propensity score–matching resulted in matched pairs of patients (one from each chemotherapy group) with a similar propensity for receiving AC versus NC. Propensity score–matched pairs were identified without replacement using a 1:1 nearest-neighbor matching algorithm with estimated caliper width. The caliper width was based on the recommendation by Austin (equal to 0.2 of the standard deviation of the logit of the propensity score).15, 16 Balance in variables between groups was assessed by the absolute standardized mean difference (ASMD) before and after the matching procedure. An ASMD <0.1 indicates balance in the covariates between NC and AC. Following matching, 92 pairs that were comparable across patient characteristics were available for analysis. Univariable and multivariable analyses were performed on the matched set. As a sensitivity analysis and to model an intention-to-treat analysis, the propensity score–matching procedures were repeated to include a subset of previously excluded patients (R1/R2 resection or stage IV disease). There were too few R1/R2 and stage IV cases to achieve adequate balance between the two chemotherapy groups, as none of these additional cases had an adequate match in the other group. The resulting propensity score–matched cohort, obtained using an optimal caliper width that ensured balanced between the two chemotherapy groups (not shown), did not include any R1/R2 or stage IV cases. The study analysis was limited to patients who had R0 resection.
To assess the effect of NC and AC on long-term outcomes, we chose DFS and OS as the endpoints of interest. Survival was measured from the time of the first treatment to the time of death (OS) or recurrence or death (DFS). Patients who do not die or experience recurrence were censored on the date of the last follow-up. DFS and OS were estimated using the Kaplan-Meier approach and compared between NC and AC groups using the log-rank test. Associations between variables and DFS and OS were quantified using Cox proportional hazard models. All analyses were stratified by matched pairs. Chemotherapy tolerance, adverse reactions to chemotherapy, and postoperative complications were compared between the propensity score–matched NC and AC groups using Fisher’s exact test.
Last, in the NC group we evaluated whether RECIST, tumor pathologic response, and ypStage were associated with DFS and OS. For this analysis, we used the entire cohort of patients who underwent NC (n=142). Survival was estimated using the Kaplan-Meier approach and compared between RECIST classifications and between MPR strata (<90% vs ≥90% tumor necrosis) using the log-rank test.
Statistical analyses were conducted using R 3.1.1 (R Development Core Team, Vienna, Austria). The propensity score–matching procedures used the MatchIt and tableone packages. All statistical tests were 2-sided, and p<0.05 was considered to indicate statistical significance.
Results
Patient Population
In total, 330 patients met the inclusion criteria (NC=142, AC=188) (see CONSORT diagram, Fig. 1). Eighty-eight percent of patients who received NC had an R0 resection (142/162). Patients with incomplete resection had microscopic tumor (R1) present at the margin (N=9), positive pericardial fluid (N=1), bulky tumor/nodes inseparable from the superior vena cava (N=4) or aorta (N=3), or the surgeon did not believe a R0 resection was possible even with a pneumonectomy (N=3). Of the 20 patients in who had an incomplete resection and NC, 13 died (65%). As stated in the methods section, when the propensity-score matching was performed including these patients, all were eliminated as part of that analysis. Before propensity-score matching, NC patients were more likely to have earlier year of surgery, pneumonectomy, higher clinical stage, higher pretreatment tumor SUVmax, and adenocarcinoma (Table 1). After matching, the ASMD between AC and NC for year of surgery, age, gender, body mass index, Charlson comorbidity score, resection type, tumor histologic subtype, pretreatment tumor SUVmax, and clinical stage were all <0.1, indicating proper matching (Table 1). Additional clinicopathologic characteristics of the matched cohort are listed in Supplementary Table 1.
Figure 1.
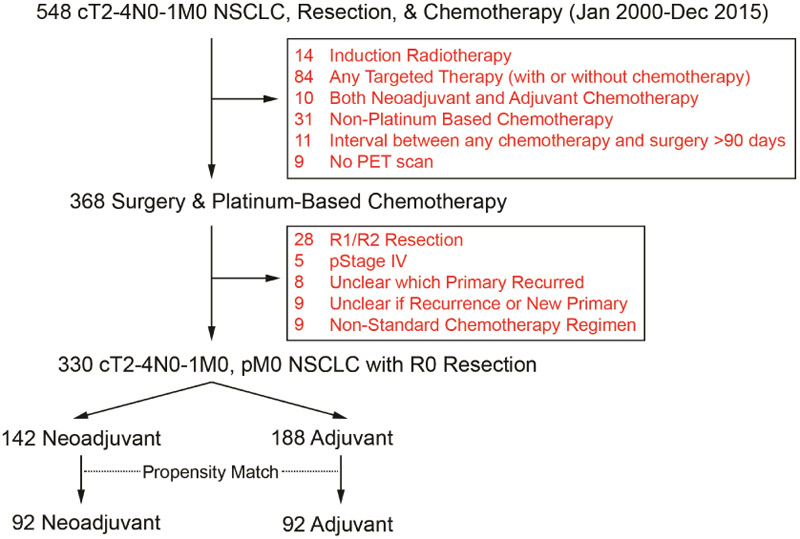
CONSORT diagram. NSCLC, non-small cell lung cancer; PET, positron emission tomography.
Table 1.
Distribution of Variables Between Adjuvant and Neoadjuvant Groups Before and After Propensity-Score Matching
| Variable | Before Propensity-Score Matching | After Propensity-Score Matching | ||||
|---|---|---|---|---|---|---|
| Neoadjuvant (N=142) |
Adjuvant (N=188) |
ASMD | Neoadjuvant (N=92) |
Adjuvant (N=92) |
ASMD | |
| Year of surgery | ||||||
| 2000-2005 | 55 (39) | 44 (23) | 0.346 | 25 (27) | 22 (24) | 0.081 |
| 2006-2010 | 38 (27) | 70 (37) | 28 (30) | 28 (30) | ||
| 2011-2015 | 49 (35) | 74 (39) | 39 (42) | 42 (46) | ||
| Age at surgery, years | 65 (58-73) | 65 (59-72) | 0.021 | 64 (58-74) | 65 (59-71) | 0.017 |
| Gender | ||||||
| Female | 75 (53) | 86 (46) | 0.142 | 40 (43) | 39 (42) | 0.022 |
| Male | 67 (47) | 102 (54) | 52 (57) | 53 (58) | ||
| BMI, kg/m2 | 26.4 (23.6-29.7) | 27.2 (24.0-30.4) | 0.152 | 26.6 (23.8-30.0) | 27.3 (23.7-29.8) | 0.006 |
| Charlson comorbidity score | 1 (0-1) | 1 (0-2) | 0.024 | 1 (0-2) | 1 (0-2) | 0.022 |
| Procedure | ||||||
| Pneumonectomy | 22 (15) | 16 (9) | 0.302 | 10 (11) | 11 (12) | 0.087 |
| Bilobectomy | 10 (7) | 9 (5) | 5 (5) | 6 (7) | ||
| Lobectomy | 106 (75) | 160 (85) | 74 (80) | 73 (79) | ||
| Segmentectomy | 4 (3) | 2 (1) | 3 (3) | 2 (2) | ||
| Wedge | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | ||
| Laterality | 0.062 | 0.044 | ||||
| Right | 78 (55) | 109 (58) | 52 (57) | 54 (59) | ||
| Left | 64 (45) | 79 (42) | 40 (43) | 38 (41) | ||
| Histologic subtype | ||||||
| Adenocarcinoma | 69 (49) | 109 (58) | 0.189 | 45 (49) | 44 (48) | 0.077 |
| Squamous | 43 (30) | 47 (25) | 27 (29) | 30 (33) | ||
| Large cell, mixed, other | 30 (21) | 32 (17) | 20 (22) | 18 (20) | ||
| Clinical stage | ||||||
| IB | 11 (8) | 59 (31) | 0.8 | 9 (10) | 11 (12) | 0.078 |
| IIA | 20 (14) | 44 (23) | 14 (15) | 14 (15) | ||
| IIB | 60 (42) | 58 (31) | 42 (46) | 42 (46) | ||
| IIIA | 51 (36) | 27 (14) | 27 (29) | 25 (27) | ||
| Clinical nodal stage | ||||||
| 0 | 86 (61) | 152 (81) | 0.457 | 55 (60) | 59 (64) | 0.09 |
| 1 | 56 (39) | 36 (19) | 37 (40) | 33 (36) | ||
| Clinical T stage | ||||||
| 2a | 25 (18) | 71 (38) | 0.583 | 22 (24) | 21 (23) | 0.072 |
| 2b | 32 (23) | 53 (28) | 21 (23) | 22 (24) | ||
| 3 | 57 (40) | 48 (26) | 33 (36) | 35 (38) | ||
| 4 | 28 (20) | 16 (9) | 16 (17) | 14 (15) | ||
| Preinduction tumor SUVmax | 13.8 (10.9-18.0) | 11 (7.4-15.0) | 0.526 | 12.7 (9.2-17.3) | 12.8 (9.0-16.4) | 0.081 |
Data are no. (%) or median (interquartile range), unless otherwise noted. An ASMD <0.1 indicates balance in the covariate between the two groups. ASMD, absolute standardized mean difference; BMI, body mass index; SUVmax, maximum standardized uptake value.
Survival and Recurrence
Following propensity-score matching, both groups (NC and AC) comprised 92 patients. The median duration of follow-up was 69.6 months (interquartile range [IQR]: 38.8-121.2 months). Median follow-up for the propensity-score matched NC group was 74.4 months (IQR: 42-138 months) versus 64.4 months (IQR: 42.7-108 months). Postoperative radiation therapy was administered to 11% of patients in the NC group and 4% of patients in the AC group (Supplementary Table 1; Fisher’s exact test p=0.16). In this propensity-score matched cohort, 33 patients in the NC group recurred, 4 of which were locoregional, 20 were distant, and 9 were both distant and locoregional. In the AC group, of 41 patients who recurred, 1 was locoregional only, 34 were distant recurrences, and 6 were both locoregional and distant.
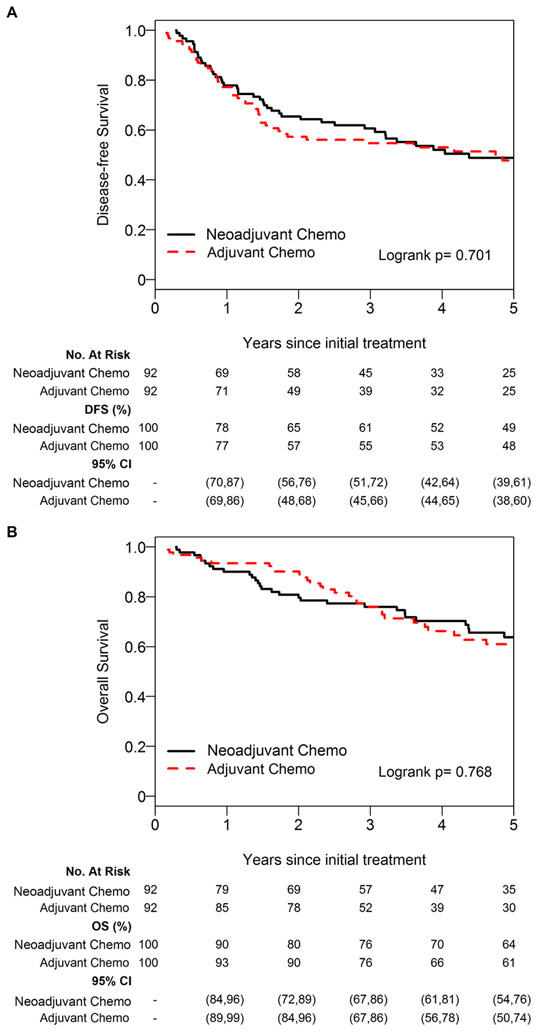
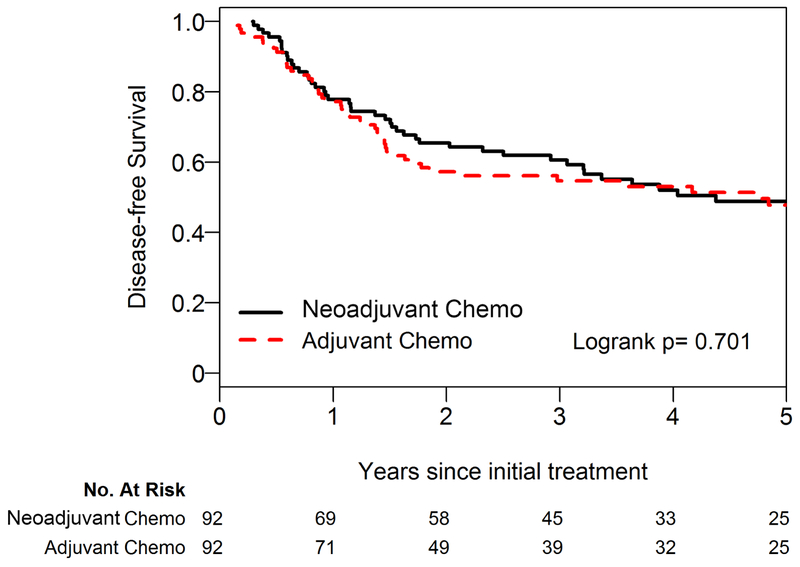
We first evaluated whether there was an association between DFS and NC or AC. Median DFS was 4.38 years (95% confidence interval [CI]: 2.92-6.90 years) in the NC group versus 4.74 years (95% CI: 1.63-NA years) in the AC group. The 5-year DFS was 48% (95% CI: 39%-61%) for the NC group versus 48% (95% CI 38%-60%) for the NC group (Fig. 2A; log-rank p=0.701). On univariable analysis, there was no significant association between DFS and NC or AC (AC vs NC hazard ratio [HR]: 1.09 [95% CI: 0.61-1.95]; p=0.768). We next evaluated DFS using a multivariable model, and similarly there was no significant association between DFS and NC or AC (AC vs. AC HR: 1.10 [95% CI: 0.64-1.90]; p=0.737) (Table 2).
Figure 2.
Five-year disease-free survival (A) and overall survival (B) for the neoadjuvant versus adjuvant group using the propensity-matched cohort. Chemo, chemotherapy.
Table 2.
Multivariable Cox Proportional Hazards Model for Disease-Free and Overall Survival
| Variable | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Chemotherapy (vs neoadjuvant) | 1.00 | — | 1.00 | — |
| Adjuvant | 1.10 (0.64-1.90) | 0.737 | 1.21 (0.63-2.30) | 0.572 |
| Age | 1.00 (0.96-1.04) | 0.805 | 1.01 (0.96-1.06) | 0.765 |
| Gender | — | — | 1.00 | — |
| Male | — | — | 2.03 (0.58-7.11) | 0.270 |
| Procedure (vs pneumonectomy) | 1.00 | 1.00 | — | |
| Lobectomy, bilobectomy, segmentectomy | 0.58 (0.14-2.45) | 0.461 | 0.54 (0.10-2.89) | 0.467 |
| Histologic subtype (vs adenocarcinoma) | 1.00 | — | 1.00 | — |
| Squamous | 0.48 (0.17-1.39) | 0.175 | 0.68 (0.20-2.34) | 0.54 |
| Large cell, mixed, other | 0.62 (0.22-1.75) | 0.367 | 1.13 (0.33-3.87) | 0.84 |
| Clinical stage (vs IB) | 1.00 | — | 1.00 | — |
| IIA | 0.77 (0.15-4.06) | 0.759 | 1.34 (0.16-10.96) | 0.787 |
| IIB | 3.30 (0.59-18.5) | 0.175 | 2.74 (0.35-21.71) | 0.339 |
| IIIA | 2.86 (0.45-18.1) | 0.264 | 1.72 (0.18-16.23) | 0.635 |
CI, confidence interval; HR, hazard ratio.
Median OS was 9.22 years (95% CI: 5.14-12.92 years) in the NC group versus 8.98 years (95% CI: 4.62-NA years) in the AC group. The 5-year OS was 64% (95% CI 54%-75%) for the NC group versus 61% (95% CI 50%-74%) for the AC group (Fig. 2B; log-rank p=0.768). On univariable analysis, there was no significant association between OS and NC or AC (HR: 1.09 [95% CI: 0.61-1.95]; p=0.77). On multivariable analysis, there remained no significant association between OS and NC or AC (AC vs. NC HR: 1.21 [95% CI: 0.63-2.30]; p=0.572) (Table 2).
We also evaluated whether postoperative complications and operative approach differed between NC and AC. There were 41 (45%) total postoperative complications in the NC group versus 32 (35%) in the AC group (Fisher’s exact test p=0.23). Most complications were low grade with only 13 patients in the NC group (14%) having grade ≥3 complications, compared with 6 patients in the AC group (7%) (Fisher’s exact test p=0.14). In the unmatched cohort, 30-day mortality was 0.7% for the NC group versus 0% for the AC group (p=0.4); 90-day mortality was 2.8% for the NC group versus 1.1% for the AC group (p=0.4). After propensity-score matching, 30-day mortality was 1.1% for the NC group versus 0% for the AC group (p=0.99), and 90-day mortality was 3.3% for the NC group versus 2.2% for the AC group (p=0.99). Unsurprisingly, after propensity-score matching, fewer patients in the NC group underwent minimally invasive approach (7 patients, 6.9%) versus the AC group (19 patients, 19%) (p=0.02).
Chemotherapy Tolerance
Tolerance and completion of chemotherapy were examined using the propensity score–matched cohort. There was no significant association between NC or AC and whether patients received cisplatin-based chemotherapy or carboplatin-based chemotherapy (p=0.8, Appendix). In the propensity-score matched cohort, 6 patients in the NC group stopped therapy completely versus 20 in the AC group. In addition, 9 had their regimens altered in the NC group versus 7 in the AC group. We found that patients in the NC group were more likely to receive the full cycle and full dose and had fewer high-grade toxicities related to chemotherapy than patients in the AC group (Table 3).
Table 3.
Chemotherapy Tolerance Between the Neoadjuvant and Adjuvant Groups
| Category | Neoadjuvant (N=92; 50%) |
Adjuvant (N=92; 50%) |
P |
|---|---|---|---|
| Full dose of chemotherapy | 72 (78) | 58 (63) | 0.014 |
| Full cycles of chemotherapy | 84 (91) | 72 (78) | 0.005 |
| Grade ≥3 adverse reaction to chemotherapy | 14 (15) | 35 (38) | 0.001 |
Response to NC
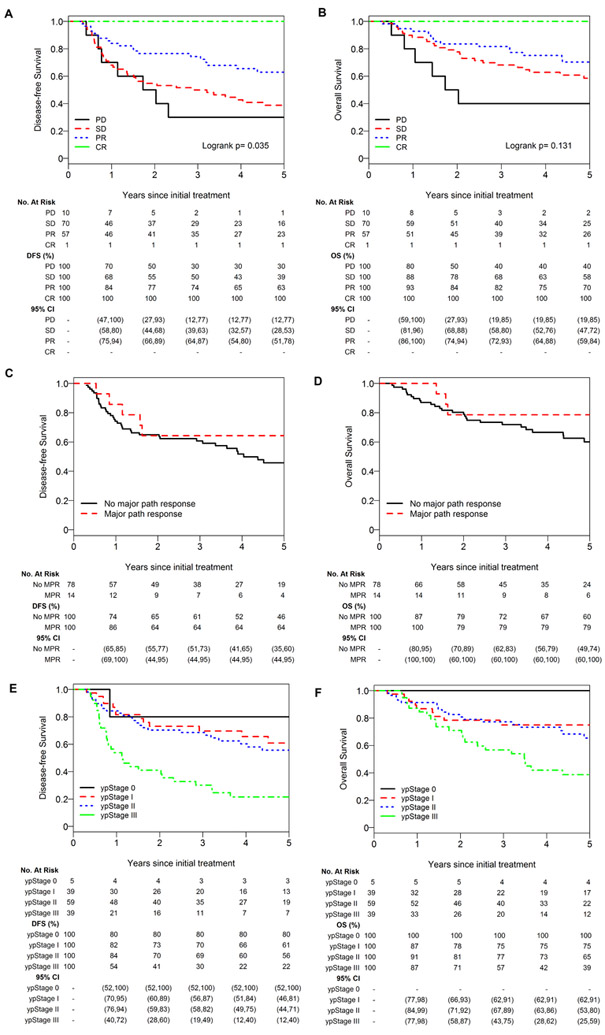
Finally, we examined whether response to NC provides additional prognostic information about survival. Characteristics of the entire NC cohort (n=142), including RECIST response and pathologic response, are listed in Supplementary Tables 2, 3, and 4. We found that RECIST response was significantly associated with DFS (p=0.035) but not OS (p=0.13) (Fig. 3A and 3B). Kaplan-Meier curves for MPR versus no MPR indicated better OS and DFS among patients with an MPR to NC (Fig. 3C and 3D). As MPR status was available for a subset of patients in the NC group (92/142), we did not perform a log-rank test in this subanalysis (Fig. 3C and 3D). Fourteen patients in our study (15%) had an MPR to NC: 5 (36%) classified as stable disease and 9 (64%) classified as partial response by RECIST. We also found that ypStage was associated with DFS (p<0.001) but not OS (p=0.1) (Figs. 3E and 3F).
Figure 3.
Five-year disease-free survival and overall survival by RECIST response (A, B), major pathologic response (C, D), and ypStage (E, F). CR, complete response; path, pathologic; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
Our primary observation is that timing of chemotherapy (whether before or after surgery) was not associated with DFS or OS in patients with clinically higher T-stage (T2-4), N0-1 NSCLC. At present, there are more robust data on AC than NC for the treatment of patients with cT2-4N0-1M0 NSCLC. In 2008, Pignon et al. reported that AC is associated with an 11% decrease in risk of death, a 5.8% benefit in DFS, and a 5.4% absolute improvement in OS, compared with surgery alone.4 Similarly, the superiority of NC plus surgery over surgery alone for patients with cT2-4N0-1M0 NSCLC has been established by other trials. SWOG S9900 evaluated neoadjuvant carboplatin-paclitaxel in patients with stage IB-IIIA NSCLC (excluding patients with Pancoast tumors and cN2 disease).17 This trial, which was powered to detect a 33% increase in median survival, was closed early after trials of AC establishing its efficacy were published. However, analysis of the abbreviated SWOG 9900 trial revealed a 9% improvement in both progression-free survival and OS for NC plus surgery versus surgery alone.17 Another trial, which similarly closed early and evaluated neoadjuvant cisplatin and gemcitabine, found an 8% improvement in 3-year OS for NC versus surgery alone.18 Similar to our study, Lim and colleagues reported a meta-analysis of 32 clinical trials and found no difference in OS or DFS between neoadjuvant or adjuvant chemotherapy in resected NSCLC patients.19 In contrast, a 2014 meta-analysis comprising 15 randomized trials (n=2385 patients) compared NC followed by surgery and surgery alone and found that NC was associated with better recurrence-free survival and distant recurrence rates and a 5% absolute improvement in OS, with an HR identical to that of the pooled adjuvant studies.20 Collectively, the available evidence suggests that NC may offer a survival advantage over surgery alone for patients with locoregionally advanced NSCLC.
In contrast to the above studies, our study directly compares NC with AC—the current standard of care—in patients with locoregionally advanced (non-cN2) NSCLC. As discussed, multiple studies have demonstrated that NC and AC are associated with a survival benefit over surgery alone; however, very few studies have directly compared the two chemotherapy approaches.4, 17, 18 Only the NATCH trial evaluated both NC versus surgery alone and AC versus surgery alone in patients with T1N0-T3N1 NSCLC. In that study, the addition of chemotherapy (either NC or AC) to surgery had no effect on DFS, however the study included an overly optimistic power analysis of a 15% difference in DFS at 5 years and a preponderance of patients with clinical stage I disease, for whom chemotherapy has been shown to have limited benefit. 7, 20-23. While our findings are similar to those from the NATCH trial and the meta-analysis comparing NC followed by surgery and surgery alone, there are important differences.20, 24 In the NATCH study and the meta-analysis, 75% and 50% of the NC cohorts, respectively, had clinical stage I disease.7, 17 In contrast, our propensity-score matched cohort consisted of patients with higher-stage tumors (53% cT3-4, 38% cN1), and these patients are more likely to benefit from the addition of chemotherapy to their treatment plan. In addition, in the NATCH trial, all patients received carboplatin-based chemotherapy, whereas in the present study 63% of patients received cisplatin-based chemotherapy; however, it should be noted that the meta-analysis found no difference in survival between these two regimens.17 More patients in the present study had adenocarcinoma histologic subtype (48% vs. 29% in NATCH). Moreover, in both NATCH and the meta-analysis, women made up a small portion of the study population (13% in NATCH and 22% in the meta-analysis vs. 43% in the present study).7, 20 Therefore, our study population perhaps more closely reflects the population of NSCLC patients who would receive either NC or AC, and is likely better suited to evaluate the putative benefits of NC.
In our study, significantly more patients in the NC group received the full dose and full cycle of chemotherapy than in the AC group. In addition, there were fewer high-grade adverse events in the NC group. Our findings support those of the NATCH study7 in that NC is associated with better chemotherapy tolerance than AC. Unfortunately, in both studies, the improved tolerance among patients receiving NC was not associated with improved DFS or OS, although a similar improvement in compliance has been shown to be associated with improved survival in breast cancer.25 Despite the lack of a survival benefit, the other benefits of NC, especially the improved tolerance and the ability to monitor response and stop or alter therapy if no response is observed, can make it appealing for patients and clinicians.
Our findings that RECIST response to NC was associated with improved DFS but not OS are similar to those from a study from MD Anderson that evaluated OS in 160 patients with clinical stage I-IV NSCLC who underwent NC followed by resection. Although the authors of that study initially found that RECIST response was associated with OS, when pathologic response was included in multivariable analysis, the association was no longer present.26 In contrast, a group from Moffitt Cancer Center evaluated RECIST in 89 patients and found that CT RECIST response, but not PET RECIST response was associated with OS and that the association was more pronounced among patients with higher-stage disease.27 It is possible that the lack of association between OS and RECIST response in our study can be explained by our exclusion of patients with cN2 or higher stage disease. The aforementioned studies had more patients with stage III/IV disease (56% in the MD Anderson study and 40% in the Moffitt Cancer Center study). In addition, both studies divided the RECIST response into two groups (partial response/complete response vs. progressive disease/stable disease); we chose to examine the criteria as four distinct groups.
It has been suggested that MPR is an assessable and reliable surrogate measurement of survival following NC for NSCLC.28 MPR rates following NC were not reported in the NATCH or CHEST trials or in the meta-analysis.7, 18, 20 In our study, the MPR rate following NC was 15%, which is similar to that in other studies26, 29 but significantly less than the 43% recently reported in a small series of patients with NSCLC receiving two cycles of neoadjuvant nivolumab followed by surgical resection.8 Unfortunately, there is a significant discordance between RECIST response (CT and PET) and MPR in patients with NSCLC treated with NC. William et al. found a 41% discordance rate between CT RECIST response and histopathologic response26; we observed a discordance rate of at least 36% in our study.
The limitations of our study include its retrospective, single-institution nature, which may limit the generalizability of the results. Additionally, we used clinical stage to match cohorts, which has known inaccuracies, but has been used by other groups when evaluating outcomes following NC.17 Some patients may develop progressive disease during NC and are unable to undergo surgery; although this number is likely to be small (3%-4% in published trials of NC), we cannot account for these patients in our data set.17, 18 Although in our unmatched cohort, more patients had an incomplete resection in the NC group, the Lancet meta-analysis found that pre-operative chemotherapy was not associated with ability to undergo complete resection (Odd’s ratio 0.88), and our rate of R2 resection in the NC group (6.2%) is comparable to that published in the previous trials.7 We also do not know the number of patients who underwent surgery and were eligible for AC but did not receive it. These are common limitations of a retrospective design which would be addressed in a prospective intention-to-treat study. Finally, although this was a large study, it still may be underpowered to fully address the effect of the timing of chemotherapy on DFS or OS in patients with advanced, non-N2 NSCLC.
Conclusions
Despite the higher levels of tolerance and full doses and full cycles of chemotherapy in the NC group, neoadjuvant administration did not result in better DFS or OS, compared with adjuvant administration, among patients with cT2-4N0-1M0 NSCLC who had R0 resection. However, RECIST response to chemotherapy was associated with better DFS. Our study provides a contemporary benchmark against which to compare outcomes of future neoadjuvant regimens, including immunotherapy, for locally advanced NSCLC. It also amplifies the need to more fully understand and develop genomic, molecular, or pathologic biomarkers (alone or in combination) to more accurately predict which tumors will respond to neoadjuvant or adjuvant regimens as part of the treatment paradigm for patients with surgically resectable NSCLC.
Supplementary Material
Central Picture:
Five-year disease-free survival for the neoadjuvant versus adjuvant group.
Central Message:
Timing of chemotherapy (before or after surgical resection) is not associated with survival for patients with cT1-3N0-1M0 NSCLC; however, tolerance is improved with neoadjuvant therapy.
Perspective Statement:
For patients with cT1-3N0-1M0 NSCLC, timing of chemotherapy is not associated with survival. The benefits of neoadjuvant therapy over adjuvant therapy, including improved tolerance, ability to stop/alter therapy if no response, and potential to determine prognosis based on tumor response, make neoadjuvant chemotherapy a favorable option for patients and clinicians.
Acknowledgments
Funding:
This work was supported by NIH grants R01 CA217169 (to D.R.J.) and T32 CA009501 (to W.S.B.). This work was also supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748. The sponsor played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- AC
adjuvant chemotherapy
- ASMD
absolute standardized mean difference
- CI
confidence interval
- CT
computed tomography
- DFS
disease-free survival
- HR
hazard ratio
- MPR
major pathologic response
- NC
neoadjuvant chemotherapy
- NCCN
National Comprehensive Cancer Network
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PET
positron emission tomography
- RECIST
Response Evaluation Criteria In Solid Tumors
- SUVmax
standardized uptake value
Chemotherapy Regimens for Propensity-Matched Cohort
| Chemotherapy Regimen | Neoadjuvant Chemotherapy N (%) |
Adjuvant Chemotherapy N (%) |
|---|---|---|
| Carboplatin, Docetaxel | 10 (11%) | 1 (1.1%) |
| Cisplatin, Gemcitabine | 14 (15%) | 1 (1.1%) |
| Cisplatin, Vinorelbine | 2 (2.2%) | 34 (37%) |
| Carboplatin, Paclitaxel | 9 (9.8%) | 12 (13%) |
| Cisplatin, Docetaxel, Gemcitabine | 1 (1.1%) | 0 (0%) |
| Carboplatin, Gemcitabine | 7 (7.6%) | 7 (7.6%) |
| Cisplatin, Docetaxel | 22 (24%) | 6 (6.5%) |
| Cisplatin, Etoposide | 3 (3.3%) | 3 (3.3%) |
| Cisplatin, Pemetrexed | 17 (18%) | 9 (9.8%) |
| Carboplatin, Pemetrexed | 7 (7.6%) | 14 (15%) |
| Cisplatin, Vinorelbine then carboplatin, paclitaxel | 0 (0%) | 2 (2.2%) |
| Cisplatin, Docetaxel, Vinorelbine | 0 (0%) | 1 (1.1%) |
| Carboplatin, Paclitaxel, Vinorelbine | 0 (0%) | 1 (1.1%) |
| Carboplatin, Paclitaxel, Pemetrexed | 0 (0%) | 1 (1.1%) |
Footnotes
Conflicts of interest:
J.E.C. has received consulting fees from AstraZeneca, Merck, Genentech, and BMS and research funding from AstraZeneca, Genentech, and BMS. M.G.K. has received consulting fees from AstraZeneca, Pfizer, and Regeneron. W.D.T. has served in a nonpaid consulting role for Genentech. All other authors have no conflicts of interest to disclose.
IRB Approval: IRB# 16-1395 – Approved 9/26/2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandt WS, Bouabdallah I, Tan KS, et al. Factors associated with distant recurrence following R0 lobectomy for pN0 lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1212–1224 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN guidelines): Non-small cell lung cancer. Version 6. August 17, 2018. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed August 18, 2018.
- 3.Berry MF, Coleman BK, Curtis LH, Worni M, D'Amico TA, Akushevich I. Benefit of adjuvant chemotherapy after resection of stage II (T1-2N1M0) non-small cell lung cancer in elderly patients. Ann Surg Oncol 2015;22:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–3559. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–360. [DOI] [PubMed] [Google Scholar]
- 6.Chaft JE, Dunphy M, Naidoo J, et al. Adaptive neoadjuvant chemotherapy guided by (18)F-FDG PET in resectable non-small cell lung cancers: The NEOSCAN trial. J Thorac Oncol 2016;11:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138–3145. [DOI] [PubMed] [Google Scholar]
- 8.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripley RT, Suzuki K, Tan KS, et al. Postinduction positron emission tomography assessment of N2 nodes is not associated with ypN2 disease or overall survival in stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2016;151:969–977, 979 e961-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 11.Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690–699 e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of persistent air leaks. Chest. 2017;152:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606–612. [PubMed] [Google Scholar]
- 15.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisters KM, Vallieres E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172–178. [DOI] [PubMed] [Google Scholar]
- 19.Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380–1388. [DOI] [PubMed] [Google Scholar]
- 20.Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589–2597. [DOI] [PubMed] [Google Scholar]
- 22.Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719–727. [DOI] [PubMed] [Google Scholar]
- 24.McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis 2014;6 Suppl 2:S224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 1995;332:901–906. [DOI] [PubMed] [Google Scholar]
- 26.William WN Jr., Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanvetyanon T, Eikman EA, Sommers E, Robinson L, Boulware D, Bepler G. Computed Tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol 2008;26:4610–4616. [DOI] [PubMed] [Google Scholar]
- 28.Hellmann MD, Chaft JE, William WN Jr., et al. Pathological response after neoadjuvant chemotherapy in resectable non-small cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaft JE, Rusch V, Ginsberg MS, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol 2013;8:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.