Abstract
Background:
Children with brain tumors can experience symptom burden throughout their disease continuum. The aim of the study was to evaluate symptom burden reported by children with brain tumors and factors that potentially were associated with their symptoms.
Methods:
Data from 199 children with brain tumors aged 7–22 (mean age=14 years; 52% males; 76% white) were analyzed. Symptom burden was assessed using Patient Reported Outcomes Measurement Information System (PROMIS) via computerized adaptive testing (CAT) – Anxiety, Depression, Fatigue, Mobility, Upper Extremity Function, Peer Relationship, and Cognition. Patients and parents completed Symptom Distress Scales (SDS). Test-statistics and ANOVA were used to evaluate relationships between PROMIS measures and potentially influential variables.
Results:
Significant results (p<0.01) showing impact of symptom burden included: PROMIS measures correlated with Symptom Distress Scales reported by patients and parents on all comparisons. Fatigue, Mobility and Upper Extremity Function were associated with Karnofsky functional performance status, number of treatment modalities (0–3), and time since last treatment (<=1 yr, >1 yr). Fatigue and Cognition were associated with educational program (regular classroom without an individualized education plan versus those that had an individualized education plan); Mobility and Upper Extremity Function were associated with time since last radiation. Mobility, Upper Extremity Function and Anxiety were associated with time since last chemotherapy.
Conclusions:
Significant associations were found between PROMIS and SDS as well as clinical and demographic characteristics. Brief-yet-precise PROMIS CATs can be used to systematically assess symptom burden experienced by children with brain tumors.
Keywords: Children, Brain Tumor, Patient-Centered Outcomes, CAT, PROMIS
INTRODUCTION
Cancer is one of the leading causes of death and disability in children under 15 years of age. With advanced medical technology, the cure rate in the United States for children and adolescents is approaching 85%, and the number of long-term survivors is steadily rising.1 Successful pediatric cancer therapy is often associated with detrimental effects across the lifespan.2–5 Children with a brain tumor are particularly vulnerable to poor health-related quality of life (HRQOL) because of adverse cognitive outcomes following treatment.6–10 Oeffinger et al2 demonstrated that brain tumor survivors, in comparison to other pediatric cancer survivors, are most likely to be functionally impaired. Greenen et al,7 in a retrospective cohort study of 1,362 childhood cancer survivors, found more than 80% of survivors of brain tumors experienced a moderate or severe adverse event according to the Common Terminology Criteria for Adverse Events (v3.0).11 A recent study on 5,522 adult childhood cancer survivors found survivors of central nervous system tumors had the highest cumulative burden of chronic health conditions at age 50 years among survivors of all cancers,12 which further highlighted the importance of systematically monitoring symptom burden in children with brain tumors and its impact to patients’ quality of life.
Because their experiences are considered unique compared to the majority of pediatric cancer survivors, brain tumor patients have typically been excluded from pediatric cancer HRQOL studies. Furthermore, pediatric brain tumor patients are a particularly challenging group to study, as brain tumors are both uncommon (though the second most common type of cancer in children) and diverse. The functional impact of brain tumors and the range of surgical and treatment effects can vary based on characteristics of tumors such as location, size, and type,9,13,14 making it challenging to evaluate comparative effectiveness with this population. Our project is the first to focus on children with brain tumors evaluating common HRQOL domains, using the established measurement system PROMIS® (Patient-Reported Outcomes Measurement Information System) and offers an opportunity to address this deficit. PROMIS is a National Institutes of Health Common Fund Initiative to measure patient-reported symptoms and HRQOL across various conditions and disease populations for both adults and children.15,16 The PROMIS measurement system consists of item banks measuring outcomes of chronic diseases in the domains of physical functioning, pain, fatigue, emotional distress, and peer relationships,17 representing potentially important outcomes in children who are treated for brain tumors. Most PROMIS item banks were developed using item response theory models, and scores are reported using T-score metric centering on the norming sample. This strategy allows us to compare the HRQOL of pediatric brain tumor patients to that of the norming sample. The aim of this study was to assess HRQOL of children with brain tumors by using the PROMIS measures of Anxiety, Depression, Fatigue, Mobility, Upper Extremity Function, Peer Relationships and Cognition, as well as other factors that could potentially be associated with their symptoms and HRQOL such as time since last treatment.
METHODS
Participants
Participants were recruited from the Ann and Robert H. Lurie Children’s Hospital of Chicago (including Northwestern Medicine Chicago Proton Center and Marianjoy Rehabilitation Hospital), Boston Children’s Hospital/Dana Farber Cancer Institute, and Maryland Proton Treatment Center from February 2013 to June 2017. Patients were eligible for this study if they: 1) were between the ages of 5 and 22; 2) had a diagnosis of a brain tumor; 3) were at any stage of treatment, from on- to off-therapy; and 4) received any type of treatment. Both patients and/or their parents were required to have sufficient English literacy to read/sign informed consent/assent forms as determined by patients’ treating clinicians.
A research assistant (RA) approached eligible participants after receiving permission from their treating clinicians. Once informed consents/assents were obtained, patients aged 7–22 years and parents of patients aged 5–17 were each given unique login information to complete study surveys using an iPad in the clinic or at home, if they were unable to complete surveys in clinic. Participants completed surveys by themselves. Parents and study team members were not allowed to influence patients’ responses. Patients aged 7–22 completed Symptom Distress Scale (SDS), and PROMIS pediatric measures via computerized adaptive testing (CAT; described in the next section) of Anxiety, Depression, Fatigue, Mobility, Upper Extremity Function, Peer Relationships, and Cognition short-form. Parents of patients completed SDS, a single 5-point global HRQOL item about their child, and proxy versions of the PROMIS measures. Although pediatric PROMIS measures were validated with children ages 8–17, we included children with an age of 7 (n=5) based on previous literature demonstrating 7-year-olds are able to complete self-reported symptom and HRQOL measures.18 We also included patients ages 18–22 (n=15) to capture patients who were transitioning into adult clinics. The PROMIS items included in this study have been used to capture data from patients’ ages 18–25 years,19 supporting the inclusion of this age group. This manuscript focuses on patients’ perception of symptom burden, and thus data from the proxy version of the PROMIS measures are not reported here.
PROMIS Measurement System
Participants completed PROMIS Anxiety,20 Depression,20 Fatigue,21 Mobility,22 Upper Extremity Function,22 Peer Relationships,23 and Cognition24–26 (a.k.a., pediatric perceived cognitive function item bank, pedsPCF). Items included in these item banks were developed using PROMIS methodology, including literature review, focus groups with children and parents, drafting of items, cognitive interviews with children, and large scale data collection. Unidimensionality and reliability of items were supported, and item response theory (IRT) was used to calibrate items to allow for computerized adaptive testing (CAT) and the development of short-forms. In CAT, all participants first complete a screening item. An initial score is then estimated based on the response using the pre-programmed algorithm, and the next most informative item around the estimated score will be selected by the algorithm; the score is re-estimated based on the participant’s response to that item. This iterative estimation process continues until the stopping rule is met. Thus, precise estimation can be achieved by using just a few items.27–29 To measure cognition, a 10-item short-form was created by the study team based on the content and psychometric properties of items included in the cognition item bank. This short-form was associated with the presence of leukoencephalopathy in children with brain tumors.25 As with other PROMIS CATs, cognition short-form scores were reported as T-scores relative to a general population mean of 50, with a standard deviation of 10. For Anxiety, Depression, and Fatigue, higher scores represent greater symptom burden. For Mobility, Upper Extremity Function, Peer Relationships and Cognition, higher scores indicate better functioning.
Analysis
Descriptive statistics were used to describe patients’ HRQOL relative to the norming sample. We evaluated the association between PROMIS measures and clinical and outcomes variables (educational program, parent-reported HRQOL and symptom burden). We used analysis of variance (ANOVA) to evaluate the distribution of PROMIS measures’ scores between groups defined by symptom burden, as measured by items of the SDS, reported by patients themselves and parents. To further understand the factors associated with patient-rated HRQOL, as measured by PROMIS, we also looked at associations with parent-rated global QOL (single item), types of treatments received, time since last treatment, clinician-rated Karnofsky performance rating, and educational program (with versus without individualized educational programs). We then divided PROMIS scores into above (inclusive) and below (exclusive) norms and evaluated the association of PROMIS score groups with the variables mentioned above using chi-square statistics.
Institutional Research Boards (IRB) at each recruitment site approved this study. All participants provided informed consent (parents and patients with ages 18 years or older) or assent (ages varied depending on each IRB’s requirements) prior to participation in this study.
RESULTS
Participants
A total of 382 dyads were approached. Of those approached, 330 patient-parent dyads (participants=567) signed the informed consent (participation rate=86.4%). Of these who agreed to participate, 250 patients aged 8–22 years, 254 parents of patients aged 8–22 years and 63 parents of patients aged 5–7 years were registered to the study online in clinic by the research coordinator. As this manuscript focused on patients’ perception of symptom burden, the following was based on results from patients aged 7–22, who completed at least one PROMIS measure (n=199; 158 from the Ann and Robert H. Lurie Children’s Hospital, 35 from Boston Children’s Hospital, and 6 from Maryland Proton Treatment Center ). Mean age was 14.1 years (SD=3.4). About half of the participants (51.6%) were male, most (75.9%) were white and had new onset brain tumor (84.4%). The most common histology was low grade glioma (grades I & II) (26.3%), followed by medulloblastoma & other embryonal tumors (18.7%) and glioneuronal tumors (12.4%); 21.7% had one or more lesions in the posterior fossa, 10.9% in the thalamus and 10.4% in the brain stem. In terms of treatment, 73.8% received surgery, 74.1% chemotherapy, 56.8% radiation (41.9% received proton therapy), and 34.1% received all three modes of therapy. Average years since diagnosis was 4.1 years (SD=4.5), and average years since last treatment was 2.6 (SD=3.4), with 86.5% receiving the most recent treatment within one year of study participation. Clinical and demographic details are shown in Table 1.
Table 1.
Patient Demographic and Clinical Information
| Variable | Mean | SD (Range) | |
|---|---|---|---|
| Age (in years) | 13.7 | 3.9 (7–22) | |
| Years since diagnosis | 4.8 | 4.4 (0–15.8) | |
| Years since most recent treatment | 0.4 | 1.4 (0–12.6) | |
| Variable | Categories | n | % |
| Gender | Male | 107 | 54.0 |
| Female | 91 | 46.0 | |
| Ethnicity | White | 129 | 79.1 |
| Black or African-American | 10 | 6.1 | |
| Does your child go to school? | Yes | 156 | 94.6 |
| Educational programs attending | Mainstream classroom, no IEP | 84 | 54.2 |
| Mainstream classroom, with IEP | 53 | 34.2 | |
| Special education classroom within a regular school | 7 | 4.5 | |
| Special education school | 3 | 1.9 | |
| Other | 8 | 5.2 | |
| How do you rate your child’s quality of life in general? | |||
| Poor | 2 | 1.2 | |
| Fair | 18 | 11.0 | |
| Good | 41 | 25.2 | |
| Very good | 62 | 38.0 | |
| Excellent | 40 | 24.5 | |
| Histology* | Medulloblastoma & other embryonal tumors | 37 | 18.7 |
| Low grade glioma (grades I & II) | 52 | 26.3 | |
| High grade glioma (grades III & IV) | 9 | 4.6 | |
| Glioneuronal tumors | 25 | 12.4 | |
| Germinoma | 15 | 7.6 | |
| Ependymoma | 13 | 6.6 | |
| Craniopharyngioma | 8 | 4.0 | |
| Lesion location* | Posterior fossa | 37 | 18.7 |
| Thalamus | 24 | 12.1 | |
| Brain Stem | 23 | 11.6 | |
| Ventricle (not otherwise specified) | 21 | 10.6 | |
| Optic pathway tumor (nerve, chiasm, tract) | 20 | 10.1 | |
| Current Status of Tumor | First onset | 167 | 84.3 |
| Recurrent | 31 | 15.7 | |
| Treatment modalities received (surgery, chemotherapy, radiation) |
None | 7 | 3.6 |
| 1 of 3 possible treatments | 49 | 25.1 | |
| 2 of 3 possible treatments | 72 | 36.9 | |
| Chemotherapy + radiation + surgery | 67 | 34.4 | |
| Treatments | Radiation | ||
| No radiation | 84 | 42.4 | |
| <=1 year | 57 | 28.8 | |
| > 1 year | 57 | 28.8 | |
| Chemotherapy | |||
| No chemotherapy | 49 | 25.0 | |
| <=1 year | 68 | 34.7 | |
| > 1 year | 79 | 40.3 | |
| Surgery | |||
| No surgery | 53 | 26.9 | |
| <=1 year | 43 | 21.8 | |
| > 1 year | 101 | 51.3 | |
| Type of radiation received | Proton | 55 | 48.2 |
| Photon | 55 | 48.2 | |
| Both proton and photon | 4 | 3.6 | |
| Years since last treatment | <= 1 year | 167 | 83.9 |
| > 1 year | 32 | 16.1 | |
| Performance Status Rating | 50–70 | 6 | 3.2 |
| 80 | 17 | 9.1 | |
| 90 | 55 | 29.6 | |
| 100 | 108 | 58.1 | |
The most prevalent histology and lesion locations were listed
Patient- and parent-rated symptom distress are shown in Figure 1 (1a for parent-rated and 1b for patient-rated). The top three most distressful (scores 3 and worse) symptoms rated by patients were fatigue (39%), sleep (27.2%), and emotional distress (25.6%). In comparison, parents rated fatigue (38.3%), emotional distress (28.8%), and appetite (27%) as being most stressful. Despite this, most parents rated their child’s quality of life in general as either excellent (24.7%), very good (36.3%), or good (25.3%).
Figure 1.
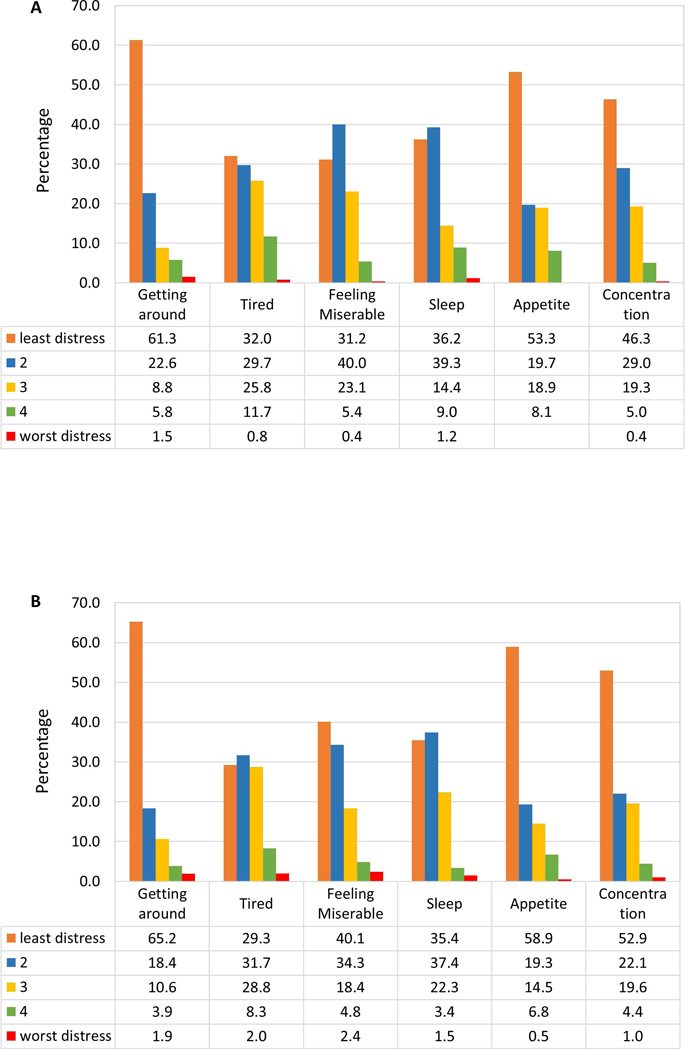
Symptom Burden
1a. Parent-rated Symptom Distress
1b. Patient-rated Symptom Distress
Analysis Results
Average number of items used by CAT administrations ranged from 8 (SD=3.2, Peer Relationships CAT) to 10.3 (SD=2.8, Upper Extremity Function CAT). As patients were allowed to stop the assessments at any time and complete the assessments at their convenience, we did not report time to complete each CAT here. Compared to the norming sample, patients reported similar levels of Upper Extremity Function (mean=48.4; SD=9.5), Peer Relationships (mean=49.5, SD=10.7), and Cognition (mean=49.5, SD=7.7). Although patients reported worse Mobility (mean=47.7, SD=9.6), they reported less Anxiety (mean=43.1, SD=10.9), Depression (mean=45.6, SD=11.1), and Fatigue (mean=44.6, SD=13.0) than the norming sample. However, it was noted that many patients reported their symptoms worse than the norm, ranging from 30.8% (Anxiety) to 62.1% (Mobility), as shown in Figure 2.
Figure 2.
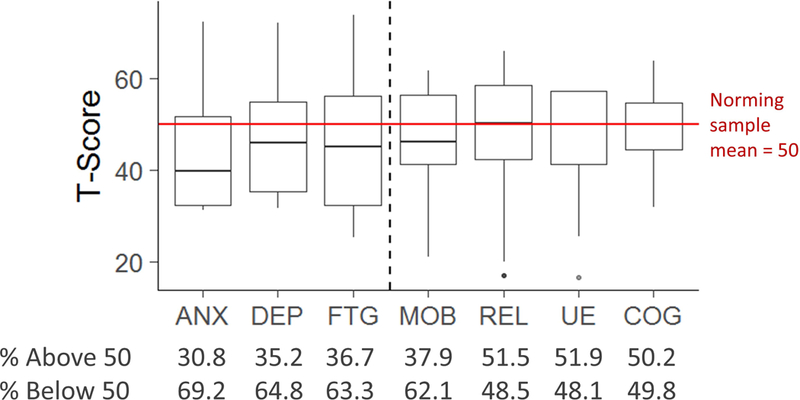
Patient-reported PROMIS T-Score distributions across domains
PROMIS measures were significantly associated with symptom distress severity as measured by SDS rated by patients as well as parents on all planned comparisons (patient-rated results in Figure 3). Of all possible comparisons, 37 and 33 out of 42 comparisons were significant on child-rated and parent-rated SDS, respectively. Fatigue, Mobility, and Upper Extremity Function significantly differentiated patients with different levels of Karnofsky performance status with ANOVA F=5.43 (p=0.0051), F=18.99 (p<0.0001), F=14.4 (p<0.0001), respectively. Fatigue and Cognition were significantly associated with the educational programs that patients attended (regular classroom without any IEP versus any type of IEP regardless of classroom type), t=4.6 (p=0.0336) and t=25.29 (p<0.0001) respectively. As shown in Table 2, Anxiety, Fatigue, Mobility, Upper Extremity Function and Cognition significantly differentiated clinical variables and educational programs that patients attended. Specifically, Mobility, Upper Extremity Function, and Fatigue significantly differentiated time since the last radiation that patients received (“no radiation” vs. “radiation within one year” vs. “radiation > 1 year”), ANOVA F=4.44 (p=0.0131) and F=4.89 (p=0.0086) for Mobility and Upper Extremity Function, respectively. Physical functioning and Anxiety significantly differentiated time since the last chemotherapy, ANOVA F=4.18 (p=0.017), 3.38 (p=0.0363) and 6.45 (p=0.002) for Anxiety, Mobility and Upper Extremity Function, respectively. Fatigue (F=3.03, p=0.0188), Mobility (F=3.26, p=0.0132), and Upper Extremity Function significantly discriminated patients by number of treatment modalities (none, chemotherapy + radiation + surgery, 2 of 3 modalities, and 1 of 3 modalities) and time since the last treatment. All PROMIS measures significantly (p<0.01) differentiated children’s global quality of life rated by their parents.
Figure 3.
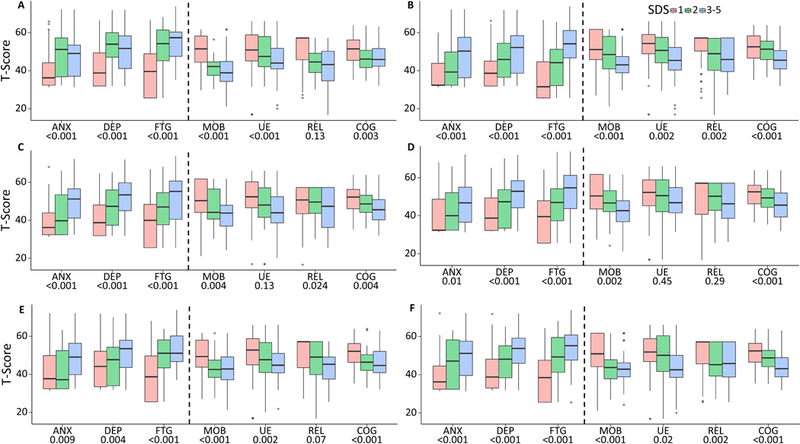
Comparisons of PROMIS measures (patient-rated) versus the Symptom Distress Scales rated by patients
Table 2.
Comparisons between PROMIS scores versus clinical variables, educational programs and parent-rated patient’s global quality of life
| Parent-rated global quality of life |
Karnofsky Performance Rating |
Educational Program |
Time since last radiation |
Time since last chemotherapy |
Treatment modalities & time since last treatment |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Higher scores represents worse symptomatic | ||||||||||||
| Anxiety | 4.14 | 0.003** | 2 | 0.140 | 0.24 | 0.624 | 0.2 | 0.817 | 3.85 | 0.023* | 0.93 | 0.450 |
| Depression | 7.28 | <.001*** | 1.37 | 0.258 | 0.31 | 0.580 | 1.36 | 0.260 | 1.89 | 0.155 | 1.51 | 0.202 |
| Fatigue | 13.18 | <.001*** | 5.54 | 0.005** | 4.12 | 0.044* | 4.69 | 0.010* | 1.04 | 0.354 | 3.06 | 0.018* |
| Higher scores represents better functioning | ||||||||||||
| Mobility | 7.5 | <.001*** | 18.67 | <.001*** | 2.5 | 0.116 | 4.29 | 0.015* | 3.44 | 0.034* | 3.19 | 0.015* |
| Upper Extremity Function | 3.32 | 0.012* | 14.22 | <.001** | 2.43 | 0.121 | 4.53 | 0.012* | 6.4 | 0.002** | 3.38 | 0.011* |
| Peer Relationships | 4.22 | 0.003** | 0.9 | 0.407 | 1.53 | 0.219 | 1.11 | 0.331 | 2.55 | 0.081 | 1.89 | 0.115 |
| Cognition | 5.63 | <0.001*** | 2.61 | 0.077 | 22.44 | <.001** | 0.03 | 0.966 | 0.66 | 0.518 | 0.33 | 0.859 |
F: F-statistic from analysis of variance (ANOVA) models with PROMIS score as the dependent variable and grouping variable as indicated by the top row
p < 0.05
p <0.01
p<0.001
No significantly different PROMIS scores were found on “time since last surgery” (“no surgery” vs. “received radiation within one year, inclusive” vs. “received radiation > 1 year”) and types of radiation (“proton”, “photon”, and “both proton and photon”)
Parent-rated global quality of life “How do you rate your child’s quality of life in general?”: Poor, Fair, Good, Very good, Excellent
Performance Rating: 40–80 vs. 90 vs. 100
Educational program: “Regular classroom without individual educational program (IEP)” vs. “any other classroom types and special education”
Time since last radiation: “never received radiation” vs. “received radiation within one year, inclusive” vs. “received radiation > 1 year”
Time since last chemotherapy: “never received chemotherapy” vs. “received chemotherapy within one year, inclusive” vs. “received chemotherapy > 1 year”
Treatment modalities & time since last treatment: number of treatment modalities received (0, 1, 2 or 3) and received treatment within 1 year (inclusive), and received the last treatment more than one year.
DISCUSSION
This study is the first trial for children with brain tumors using PROMIS, an established national resource for precise and efficient measurement of patient-reported symptoms, functioning, and HRQOL that is appropriate for patients with a wide variety of chronic diseases and conditions. Children with brain tumors are at risk of experiencing significant challenges later in life, as shown by lower achievement, education, full-time employment, and income, as well as decreased likelihood of marriage in comparison to children with other types of cancers.10 Extending the use of the pediatric PROMIS measures to children with brain tumors will enhance the understanding of children’s HRQOL/symptom burden by referencing them to a standard metric. As many children with brain tumors share similar symptoms to children with other conditions (e.g., fatigue and pain), this standard metric allows for evaluation of the impact from symptoms beyond those directly associated with brain tumors. As recommended by standards of care, children with brain tumors and their families should routinely receive systematic psychosocial assessments,30 and be monitored and assessed for neuropsychological deficits during and after treatment.31 PROMIS makes it feasible to comply with these standards. Advantages of PROMIS such as availabilities of a large normative sample and the individualized, tailored CAT assessments make it possible for clinical investigators to monitor the long-term effects of treatment, ultimately resulting in improved care for this beleaguered yet under-studied population.
Significant planned comparisons between PROMIS measures and SDS reported by patients as well as by parents support the clinical utility of the PROMIS measures in assessing patient symptom burden. Compared to using one single item to measure each domain as used by the SDS, PROMIS CATs can provide more precise estimations using robust psychometrically sound methods. Although there were no planned comparisons with Peer Relationship, as SDS did not include an item measuring social functioning, Peer Relationship significantly differentiated SDS-fatigue, SDS-emotion and SDS-cognition reported by patients and SDS-emotion and SDS-sleep reported by parents. Using the established PROMIS T-score metric, we found patients reported their HRQOL, at the group level, as similar to or better than the norming sample. However, at the individual level, we noticed that many patients experienced worse HRQOL than their peers, from 31% on Anxiety to 62% on Mobility, with some patients being 3 SDs worse than the norm. Contributing factors to inferior HRQOL were domain dependent. Physical functioning (Mobility and Upper Extremity Function) was significantly associated with all treatment-related variables listed in Table 2, except the educational programs that patients attended. The educational programs, as expected, were associated with Cognition and Fatigue. Depression and Peer Relationships were not significantly associated with treatment-related variables. However, it was interesting to note that among patients who received chemotherapy, patients who completed therapy within 1 year reported better peer relationships than those who completed chemotherapy longer than 1 year (p=0.036); however, the overall model became non-significant when patients not receiving chemotherapy were included in the model. Inconsistent results regarding impacts of cancer treatment on peer relations have been found and very few studied on-therapy patients.32–34 Most of these studies investigated survivors with mixed types of cancer; however, brain tumor patients are a unique population and have demonstrated lower functioning than children with other types of cancer. Multiple factors can affect children’s perceived peer relationship such as attitudes from concerned adults, including parents and teachers. Patients’ poor health status might be related to parental over-protectiveness, which further can influence their peer relationships. Salley et al35 suggested that changes in temperament did not explain some of the problematic changes in social interaction patterns observed for brain tumor survivors and suggested that other aspects of social information processing should be further investigated. Bonner et al found that pediatric brain tumor survivors demonstrated difficulties in interpreting adult facial expressions which impaired their social functioning.36 Further studies are needed to monitor patients’ social function from on-therapy to off-therapy, and evaluate factors contributing to changes in survivors’ social functioning.
The main limitation of this study, as in other brain tumor studies, was the heterogeneity of samples. To increase the generalizability of the study results, efforts were made to recruit a relatively large size of pediatric patients with brain tumors. Yet given the diverse nature of brain tumors (sizes, location, histology etc.) and their associated treatments, we were unable to further compare patients by specific treatment, location, or types with a robust sample size within each sub-group. Consequently, replicability of the study results should be evaluated in patients from other institutions. Most patients received more than one treatment modality. Treatment intensities were also not consistent within those who received the same treatment modality. Some patients who received proton therapy at the time of this study had received either photon and/or proton therapy prior to current proton therapy. These complexities made it difficult to compare HRQOL by each specific treatment regimen, which can only be accomplished through collaborations across multiple institutions such as via the Pediatric Brain Tumor Consortium. However, results from this study have established a foundation for comparison from future studies. This study was limited to English-speaking patients only because the pediatric PROMIS was not available in other languages when this study started. Pediatric PROMIS is now available in other languages (e.g., Spanish, German, Dutch and Chinese), replicability of the study results to other languages should be confirmed.
In conclusion, this study investigated HRQOL of children with brain tumors in reference to the PROMIS norming sample. All PROMIS measures were significantly associated with symptom distress reported by patients and parents, and most measures were associated with clinical variables and educational programs attended. Though many patients reported inferior HRQOL to the norming sample, these inferior scores were averaged out at the group level, suggesting the need of individualized, tailored assessment such as PROMIS CATs when monitoring patients’ HRQOL across the cancer continuum. As indicated by literature,37 administering CATs in pediatric neuro-oncology clinics was feasible and future studies should consider PROMIS CATs when evaluating patients’ HRQOL or symptom burden.
Acknowledgements:
This study was supported by the National Cancer Institute (1R01CA174452; PI: Jin-Shei Lai)
Funding Source:
This study was funded by National Institutes of Health/National Cancer Institute (R01CA174452; PI: Jin-Shei Lai)
Abbreviations
- HRQOL
Health-related quality of life
- PROMIS
Patient-Reported Outcomes Measurement Information System
- IRT
Item response theory
- CAT
Computerized adaptive testing
- SDS
Symptom Distress Scale
- ANOVA
Analysis of variance
- IEP
Individual educational program
Footnotes
Conflict of Interest Statement:
All authors have no conflicts of interest to declare
Contributor Information
Jin-Shei Lai, Medical Social Sciences and Pediatrics, Northwestern University Feinberg School of Medicine.
Mary Jo Kupst, Pediatrics, Medical College of Wisconsin.
Jennifer L. Beaumont, Medical Social Sciences, Northwestern University Feinberg School of Medicine, Terasaki Research Institute, Los Angeles, California, USA.
Peter E. Manley, Children’s Hospital Boston and Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA.
John Han-Chih Chang, Radiation Oncology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
William F. Hartsell, Northwestern Medicine Chicago Proton Center, Warrenville, Illinois, USA, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois, USA.
Young Kwok, Radiation Oncology, University of Maryland School of Medicine.
Allison Piazza Fisher, Medical Social Sciences, Northwestern University Feinberg School of Medicine.
Stewart Goldman, Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois, USA.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- 3.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol 2005; 30(1): 65–78. [DOI] [PubMed] [Google Scholar]
- 4.Wright MJ, Twose DM, Gorter JW. Gait characteristics of children and youth with chemotherapy induced peripheral neuropathy following treatment for acute lymphoblastic leukemia. Gait Posture 2017; 58: 139–45. [DOI] [PubMed] [Google Scholar]
- 5.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. J Clin Oncol 2013; 31(33): 4242–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. The Journal of the American Medical Association 2007; 297(24): 2762–4. [DOI] [PubMed] [Google Scholar]
- 7.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. The Journal of the American Medical Association 2007; 297(24): 2705–15. [DOI] [PubMed] [Google Scholar]
- 8.Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology 2004; 62(4): 544–7. [DOI] [PubMed] [Google Scholar]
- 9.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol 2005; 30(1): 9–27. [DOI] [PubMed] [Google Scholar]
- 10.Ellenberg L, Liu Q, Yasui Y, et al. Neurocognitive Status in Long-Term Survivors of Childhood CNS Malignancies: A Report From the Childhood Cancer Survivor Study. Neuropsychology 2009; 23(6): 705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute CTEP. Common Terminology Criteria v3.0 Bethesda, USA: National Cancer Institute, CTEP, 2003. [Google Scholar]
- 12.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). The Lancet 2017; 390(10112): 2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micklewright JL, King TZ, Morris RD, Krawiecki N. Quantifying Pediatric Neuro-oncology Risk Factors: Development of the Neurological Predictor Scale. J Child Neurol 2008; 23(4): 455–8. [DOI] [PubMed] [Google Scholar]
- 14.Chapman CA, Waber DP, Bernstein JH, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: Role of age and perioperative factors. J Child Neurol 1995; 10(3): 209–12. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010; 63(11): 1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella D, Stone AA. Health-related quality of life measurement in oncology: advances and opportunities. Am Psychol 2015; 70(2): 175–85. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group During its First Two Years. Med Care 2007; 45(5 Suppl 1): S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai JS, Cella D, Peterman A, Barocas J, Goldman S. Anorexia/cachexia related quality of life for children with cancer: Testing the psychometric properties of the Pediatric Functional Assessment of Anorexia/Cachexia Therapy (peds-FAACT). Cancer 2005; 104(7): 1531–9. [DOI] [PubMed] [Google Scholar]
- 19.Reeve B, Thissen D, DeWalt D, et al. Linkage between the PROMIS® pediatric and adult emotional distress measures. Qual Life Res 2016; 25(4): 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin DE, Stucky B, Langer MM, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res 2010; 19(4): 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai J-S, Stucky B, Thissen D, et al. Development and psychometric properties of the PROMIS® pediatric fatigue item banks. Qual Life Res 2013; 22(9): 2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeWitt EM, Stucky BD, Thissen D, et al. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol 2011; 64(7): 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewalt DA, Thissen D, Stucky BD, et al. PROMIS Pediatric Peer Relationships Scale: development of a peer relationships item bank as part of social health measurement. Health Psychol 2013; 32(10): 1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai JS, Zelko F, Butt Z, et al. Parent-perceived child cognitive function: results from a sample drawn from the US general population. Childs Nerv Syst 2011; 27(2): 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai J-S, Bregman C, Zelko F, et al. Parent-reported cognitive function is associated with leukoencephalopathy in children with brain tumors. Qual Life Res 2017; 26(9): 2541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai J-S, Zelko F, Krull K, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Qual Life Res 2014; 23(4): 1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai J-S, Butt Z, Zelko F, et al. Development of a Parent-Report Cognitive Function Item Bank Using Item Response Theory and Exploration of its Clinical Utility in Computerized Adaptive Testing. J Pediatr Psychol 2011; 36(7): 766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JS, Cella D, Choi SW, et al. How Item Banks and Their Application Can Influence Measurement Practice in Rehabilitation Medicine: A PROMIS Fatigue Item Bank Example. Arch Phys Med Rehabil 2011; 92(10 Supplement): S20–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS):depression, anxiety, and anger. Assessment 2011; 18(3): 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazak AE, Abrams AN, Banks J, et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr Blood Cancer 2015; 62(S5): S426–S59. [DOI] [PubMed] [Google Scholar]
- 31.Annett RD, Patel SK, Phipps S. Monitoring and Assessment of Neuropsychological Outcomes as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer 2015; 62(S5): S460–S513. [DOI] [PubMed] [Google Scholar]
- 32.Schultz KAP, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2007; 25(24): 3649–56. [DOI] [PubMed] [Google Scholar]
- 33.Katz LF, Leary A, Breiger D, Friedman D. Pediatric cancer and the quality of children’s dyadic peer interactions. J Pediatr Psychol 2010; 36(2): 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung L, Yanofsky R, Klaassen RJ, et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer 2011; 128(5): 1213–20. [DOI] [PubMed] [Google Scholar]
- 35.Salley CG, Hewitt LL, Patenaude AF, et al. Temperament and social behavior in pediatric brain tumor survivors and comparison peers. J Pediatr Psychol 2015; 40(3): 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonner MJ, Hardy KK, Willard VW, Anthony KK, Hood M, Gururangan S. Social functioning and facial expression recognition in survivors of pediatric brain tumors. J Pediatr Psychol 2008; 33(10): 1142–52. [DOI] [PubMed] [Google Scholar]
- 37.Lai J-S, Beaumont JL, Nowinski CJ, et al. Computerized Adaptive Testing In Pediatric Brain Tumor Clinics. J Pain Symptom Manage 2017; 54(3): 289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


