Abstract
Objective:
Macrophages express three Akt isoforms, Akt1, Akt2 and Akt3, which display isoform-specific functions but may be redundant in terms of Akt survival signaling. We hypothesize that loss of two Akt isoforms in macrophages will suppress their ability to survive and modulate the development of atherosclerosis.
Approach and Results:
To test this hypothesis, we reconstituted male Ldl-receptor null (Ldlr−/−) mice with double Akt2/Akt3 knockout hematopoietic cells expressing only the Akt1 isoform (Akt1only). There were no differences in body weight and plasma lipid levels between the groups after 8 weeks of the Western diet, however Akt1only→Ldlr−/− mice developed smaller (57.6% reduction) atherosclerotic lesions with more apoptotic macrophages than control mice transplanted with wild-type (WT) cells. Next, male and female Ldlr−/− mice were reconstituted with double Akt1/Akt2 knockout hematopoietic cells expressing the Akt3 isoform (Akt3only). Female and male Akt3only→Ldlr−/− recipients had significantly smaller (61 and 41%, respectively) lesions than the control WT→Ldlr−/− mice. Loss of two Akt isoforms in hematopoietic cells resulted in markedly diminished levels of white blood cells, B-cells and monocytes, and compromised viability of monocytes and peritoneal macrophages compared to WT cells. In response to LPS, macrophages with a single Akt isoform expressed low levels of inflammatory cytokines, however Akt1only macrophages were distinct in expressing high levels of anti-apoptotic Il10 compared to WT and Akt3only cells.
Conclusions:
Loss of two Akt isoforms in hematopoietic cells, preserving only a single Akt1 or Akt3 isoform, markedly compromises monocyte and macrophage viability and diminishes early atherosclerosis in Ldlr−/−mice.
Keywords: Atherosclerosis, Macrophages, Akt signaling, Apoptosis, Monocytopenia
Graphical Abstract
Introduction
Atherosclerosis is a chronic inflammatory disease that arises from an imbalance in lipid metabolism and a maladaptive immune response driven by the accumulation of cholesterol-laden macrophages in the artery wall 1. Macrophages and macrophage-derived foam cells are the major cell type of early atherosclerotic lesions, and they play crucial roles in every stage of atherosclerosis 2. Therefore, changes in the ability of blood monocytes or macrophages to respond to inflammatory or pro-apoptotic stimuli may significantly modulate atherogenesis.
Akt is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and migration 3, 4. Akt has three isoforms, Akt1, Akt2 and Akt3, that share similar domain structure with 80% sequence homology 5, which are largely redundant with respect to Akt signaling but exhibit distinct isoform-specific functions 6. Studies with Akt isoform-specific knockout mice demonstrated that each isoform gives a distinct phenotype. For example, mice lacking Akt1 have increased perinatal mortality and reduced body weight 7, 8. In contrast, Akt2 deficient mice display normal growth and a diabetes-like syndrome 9, whereas Akt3 knockout mice exhibit impaired brain development and reduced brain volume 10. In regard to atherogenesis, loss of Akt1 in apolipoprotein E deficient (Apoe−/−) mice leads to severe atherosclerosis 11. Akt3 deficiency in macrophages of Apoe−/− mice promoted foam cell formation and atherosclerosis 12. Knockout of Akt2 in LDL-receptor null mice has been reported to impair glucose tolerance and induce more complex atherosclerotic plaques compared to controls 13. Finally, we and others have shown that loss of macrophage Akt2 suppresses their ability to undergo M1 polarization resulting in impaired recruitment and migration, which reduces both early and advanced atherosclerosis 14, 15. Together these results demonstrate isoform-specific effects of Akt in macrophage biology and atherogenesis.
In contrast to inactivation of a single Akt isoform, which is generally well tolerated and does not impact mouse survival, loss of two Akt isoforms produces detrimental effects in mice. For example, double Akt1/Akt3 deficient mice were embryonic lethal (at 11-12 day) producing severe developmental defects in the cardiovascular and nervous systems 16. There was also a clear dose-dependent effect with combined Akt1 and Akt3 deficiency indicating that both isoforms are required for embryo development and mouse survival 16. Similarly, Akt1/Akt2 knockout mice displayed a rather severe phenotype with dwarfism, impaired skin development, delayed bone development, reduced adipogenesis, and early lethality after birth 17. In contrast, Akt2/Akt3 knockout mice survive both the embryonic and postnatal periods, but they later develop insulin intolerance and a reduction in body weight compared to wild-type mice 18. Moreover, the presence of a single functional allele of Akt1 (Akt1+/−Akt2−/−Akt3−/−) in mice appears to be sufficient for embryonic development and postnatal survival 18. This data is consistent with the notion that Akt1 is the principal pro-survival isoform 11, 19, 20. Further studies are necessary to clarify the impact of the different Akt isoforms on cell viability and atherosclerosis.
Beyond the impressive impact of total loss of two Akt isoforms on mouse survival, the PI3K/Akt pathway plays critical roles in the viability of hematopoietic cells. For example, Akt signaling promotes both the maturation and the survival of thymocytes 21, 22 and peripheral B-cells 23. Therefore, mice reconstituted with Akt1/Akt2 knockout hemotopoietic cells expressing the Akt3 isoform (Akt3only) had developmental defects of thymocytes 24, 25, whereas follicular B cells exhibited a profound survival defect in a competition assay against wild-type B cells in vivo 23. Similarly, the Akt1/Akt3 knockout, leaving only a single Akt2 isoform, interferes with the differentiation of thymocytes 25. In addition, hematopoietic stem cells with a single Akt3 isoform exhibited decreased ROS production and survival 26. There is tremendous interest in developing inhibitors of PI3K/Akt pathway for the treatment of cancer and to modulate antitumor responses 27; therefore, it is crucial to understand the impact of reduced Akt expression by hematopoitic cells, including macrophages, on the development of atherosclerosis. Importantly, it remains unknown whether mouse blood monocytes and macrophages have a threshold effect for Akt protein levels in survival. For comparison, the Akt3 isoform accounts for only ~25% of total macrophage Akt content and has little impact on macrophage viability 12; whereas Akt2 is the predominant isoform in mouse macrophages 14 and the Akt1 isoform is crucial for their survival 11. Since monocyte/macrophages are key cells in atherosclerosis1, it would be important to elucidate the impact of Akt protein reduction to a single isoform in monocytes/macrophages on atherogenesis.
Here we generated chimeric Ldlr−/− mice reconstituted with hematopoietic cells expressing a single Akt1 or Akt3 isoform. These mice had drastically lower levels of white blood cell counts and blood monocytes compared to control mice transplanted with WT hematopoietic cells. Loss of two Akt isoforms in hematopoietic cells was detrimental for the viability of monocytes/macrophages and resulted in dramatic reductions in the extent of early atherosclerosis.
Material and Methods
The authors declare that all additional data that support the findings of this study are available in the online-only version as Supplemental Data.
Animal Procedures:
Mice homozygous for Akt1 (stock# 004912) 8 and Akt2 (stock# 006966) 9 knockout mutations (both on C57BL/6 background), recipient Ldlr−/− (on C57BL/6 background) and C57BL/6 mice were purchased from the Jackson Laboratories. Akt3 knockout mice 10 (on C57BL/6 background) were provided by Dr. Morris J. Birnbaum, from Perelman School of Medicine, University of Pennsylvania. Mice were maintained in microisolator cages on a rodent chow diet containing 4.5% fat (PMI 5010, St. Louis, MO) or a Western type diet containing 21% milk fat and 0.15% cholesterol (Teklad, Madison, WI). Animal care and experimental procedures were performed according to the regulations of Vanderbilt University’s Institutional Animal Care and Usage Committee. The mouse experiments were designed, performed and reported using approaches consistent with the recommendations of the ATVB Council Statement28.
Fetal liver cell (FLC) transplantation:
FLC were isolated on day 14-16 of gestation and the gender of the embryos were determined by PCR as described 29. Recipient Ldlr−/− or C57BL6 mice were lethally irradiated (9Gy) and transplanted (4×106) with FLCs as described30.
Analysis of Serum Lipids and Aortic Lesions:
Serum total cholesterol and triglyceride levels were determined after overnight fasting by the enzyme method. All measurements were performed according to the guidelines for experimental atherosclerosis studies described in the AHA Statement 31. Aortas were flushed through the left ventricle and the entire aorta was dissected for en face analysis as described 32. Cryosections of the proximal aorta were analyzed using an Imaging system KS 300 (Kontron Electronik GmbH).
Peritoneal macrophage isolation and reagents.
To analyze peritoneal macrophages, we reconstituted male C57BL6 mice with male WT, Akt1only or Akt3only FLC. Eight weeks post-transplantation, thioglycollate-elicited peritoneal macrophages were isolated and cultured. Two days later, macrophages were used in vitro experiments
Western blotting.
Cells were lysed in a lysis buffer (Cell Signaling Technology, Danvers, MA) containing protease and phosphatase inhibitors. Proteins were measured with the DC Protein assay kit (Bio-Rad Laboratories) and resolved by NuPAGE Bis-Tris electrophoresis and transferred onto nitrocellulose membranes (Amersham Bioscience). Blots were probed with primary rabbit antibodies and secondary goat anti-rabbit horseradish peroxidase-conjugated antibodies (Sigma). Proteins were visualized with ECL western blotting detection reagents (GE Healthcare) and quantified by densitometry using ImageJ software (NIH).
Immunocytochemistry:
Serial 5-micron cryosections of the proximal aorta were fixed with 2% paraformaldehyde-PBS and stained with the appropriate antibodies or non-immune rabbit or rat IgG were used as a negative control. Double staining of macrophages with MOMA-2 and cell nuclei with DAPI with analysis of nucleus numbers in macrophage area as previously described 33.
RNA isolation and real-time PCR.
Total RNA was isolated and relative quantitation of the target mRNA was performed as described 33 using the gene expression assays that were normalized with 18S ribosomal RNA as an endogenous control.
Flow Cytometry:
Blood cells were stained with a cocktail of antibodies against lineage markers and Ly-6C . Then the cells were treated with Lysing buffer (BD Pharm, cat#555899), washed and analyzed using FACS DiVa v6.1 software (BD Biosciences) in the Research Flow Cytometry Core Laboratory, Veterans Administration Medical Center.
Apoptosis assessment.
Cultured cells in Laboratory-Tek chambers (Nalge Nunc International) or 5-micron cryosections from the proximal aorta were fixed in 4% paraformaldehyde in PBS, treated with 3% citric acid and apoptotic cells were detected by the in situ cell death detection kit (Roche Applied Science). TUNEL-positive (TUNEL+) cells were counted in 4 different sections of each aorta as described32. For in vitro analysis, peritoneal macrophages were seeded in Laboratory-Tek chambers (Nalge Nunc International), then treated with BSA or 0.5M PA-BSA with or without a mouse recombinant Il-10 (100ng/ml; R&D System) for 24 hours or human Ox-LDL (100mg/ml) or Ac-LDL (100mg/ml) with ACAT inhibitor Sandoz 58035 (2mg/ml) for 48 hours. Apoptotic cells were detected by an Alexa Fluor488 Annexin V/Dead Cell Apoptosis kit (Life Technologies, catalog number V13241). The number of TUNEL+ cultured cells were counted as a percentage of the total number of cells in at least four separate fields (containing ≈1,000 cells) from duplicate chambers.
Statistical Analysis:
Data are provided as Means ± SEM. For the comparison of two groups, data were initially tested for normal distribution and equal variance. If the data were normally distributed, a Student t test was performed. For nonparametric data the Mann–Whitney rank-sum test was used. To compare multiple groups, one way ANOVA and Kruskal-Wallis one way ANOVA on Ranks were used. For one way ANOVA, when the data met the assumption of homogeneity of variances, we used Tukey’s post hoc test. In the case of Kruskal-Wallis analysis, the Games Howell post hoc test was run. If the data were not normally distributed, the nonparametric Kruskal–Wallis one-way ANOVA on ranks was performed. P<0.05 was considered significant. All statistical analyses were performed using SigmaPlot 14.0 a SPSS Statistics Premium 22 (IBM, Armonk, NY) or GraphPad Prism 7.
Results
Mice with a single Akt1 isoform in hematopoietic cells have reduced atherosclerosis.
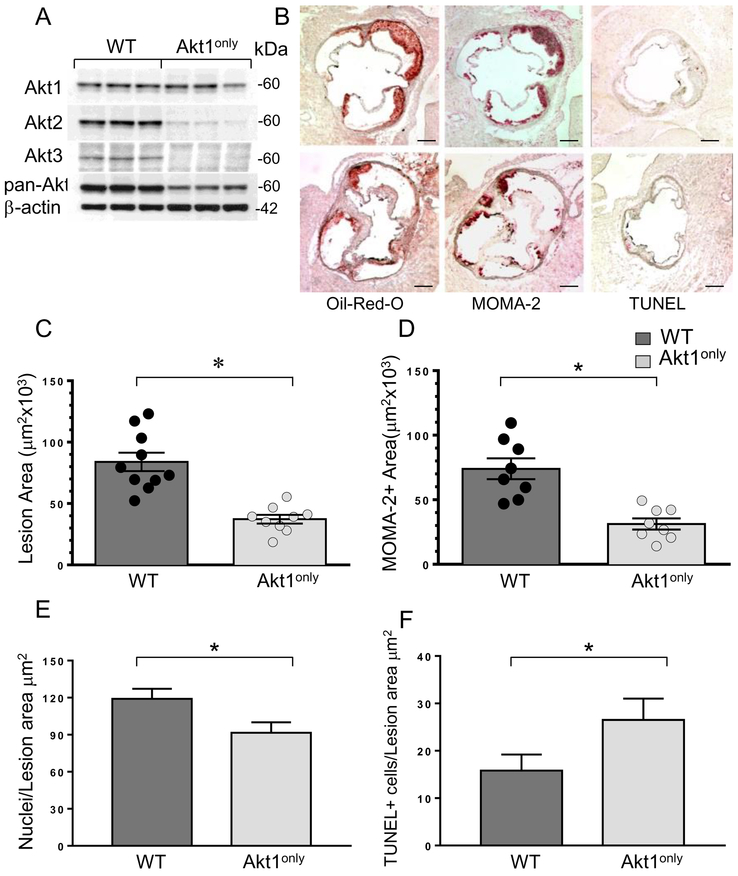
Because mice with double knockouts of Akt isoforms have significant neonatal mortality, we used a fetal liver cell (FLC) transplantation approach 29 to generate mice chimeric for loss of two Akt isoforms in hematopoietic cells. First, Akt2−/− and Akt3−/− mice were crossed, then their littermates were intercrossed and FLCs were collected on day 16-18. Next, ten-week-old male Ldlr−/− mice were lethally irradiated and transplanted with male WT (control group; n=10) or Akt2/3 knockout FLC expressing only Akt1 (Akt1only; n=10) FLC. Four weeks after transplantation, the recipients were challenged with the Western diet for 8 weeks. At sacrifice, thioglycollate-elicited peritoneal macrophages were isolated from the recipient mice. Western bot analysis demonstrated that macrophages did not express Akt2 or Akt3, preserving only the Akt1 isoform (Figure 1A). Importantly, there were no statistically significant differences between the groups of recipient mice in body weight, serum total cholesterol or triglyceride levels (Table 1A). In contrast, analysis of atherosclerotic lesions in the aortic sinus (Figure 1B) demonstrated that Akt1only→Ldlr−/− mice developed smaller (57.6% reduction) atherosclerotic lesions than control WT→Ldlr−/− mice (Figure 1C). Compared to WT→Ldlr−/− mice, Akt1only→Ldlr−/− mice also had a smaller area of the lesions occupied by macrophages as determined by staining with the anti-macrophage antibody, MOMA-2 (Fig 1D) and fewer nuclei in the MOMA-2-positive area (Figure 1E) analyzed as previously described 34. In addition, Akt1only→Ldlr−/− mice had increased numbers of apoptotic TUNEL-positive cells in the atherosclerotic lesions (Figure 1F). Double staining for TUNEL and macrophage marker CD68 verified the presence of apoptotic cells in the atherosclerotic lesions that are macrophage in origin (Figure I of Supplemental Data). Since this marker may be expressed by smooth muscle cells after lipid loading 35, we used another specific marker of macrophages, MOMA2 (Figure SII). Similar double staining of WT macrophages with CD68 and DAPI combined with antibodies to Akt1 or Akt3 revealed that macrophages expressed both Akt1 and Akt3 isoforms but did not stain with the isotype control (Figure SIII). Staining of serial aortic sections of mice reconstituted with Akt1only hematopoietic cells demonstrated that macrophages in the atherosclerotic lesions contained only Akt1 but not Akt2 and Akt3 isoforms (Figure SIV). Thus, reduction of Akt to a single Akt1 isoform in hematopoietic cells significantly suppresses early atherosclerotic lesion formation.
Figure 1. Reconstitution of Akt1only hematopoietic cells into male Ldlr−/− mice significantly decreases Akt protein levels in macrophages and reduces early atherosclerosis.
(A) Double Akt2/Akt3 knockout macrophages preserve only the Akt1 isoform. Peritoneal macrophages were isolated from WT→Ldlr−/− and Akt1only→Ldlr−/− mice (n=3/group), proteins were extracted from cells and analyzed by Western blot;
(B) Detection of atherosclerotic lesions in the aortic sinus of WT→Ldlr−/− (top panel) and Akt1only→Ldlr−/− mice (bottom panel); Serial aortic cryosections were stained to detect lipids by Oil-Red-O stain, reveal macrophages using antibody to MOMA-2 or identify apoptotic cells by TUNEL assay; scale bars, 200μm;
(C-F) The extent of atherosclerotic lesions (C), MOMA-2-positive area (D), numbers of nuclei in MOMA-2+ area (E) and TUNEL+ cells (F) in atherosclerotic lesions of WT→Ldlr−/− and Akt1only→Ldlr−/− mice FLC; Graphs represent data (mean ± SEM, ∗p<0.05 by Mann-Whitney Rank Sum and *p<0.05 by t-tests).
Table 1.
Body weight (BW), total serum cholesterol (TC) and triglyceride (TG) levels in Ldlr−/− mice reconstituted with WT and male Akt1only (A), female and male Akt3only (B,C) hematopoietic cells after 8 weeks of the Western diet.
| Recipient Mice |
BW (g) |
TC (mg/dl) |
TG (mg/dl) |
|---|---|---|---|
|
A. Male FLC→male Ldlr−/− mice WT (n=10) |
25.7+1.1 | 880±39 | 197±5 |
| Akt1only (n=10) p = |
23.2±0.6 0.17 |
872±48 0.87 |
201±24 0.90 |
|
B. Female FLC →female Ldlr−/− mice WT (n=10) |
21.5±0.4 | 673±42 | 229±7 |
| Akt3only (n=10) p = |
21.2±0.5 0.58 |
660±33 0.82 |
200±23 0.62 |
|
C. Male FLC→male Ldlr−/− mice WT (n=9) |
29.3±0.9 | 906±76 | 237±16 |
| Akt3 only (n=11) p = |
27.4±0.7 0.46 |
835±48 0.46 |
216±15 0.37 |
Data are (Mean ± SEM). The number of recipient mice in each group is indicated by n;
p, there are no statistically significant differences between the groups.
Female and male mice with a single Akt3 isoform in hematopoietic cells have reduced atherosclerosis.
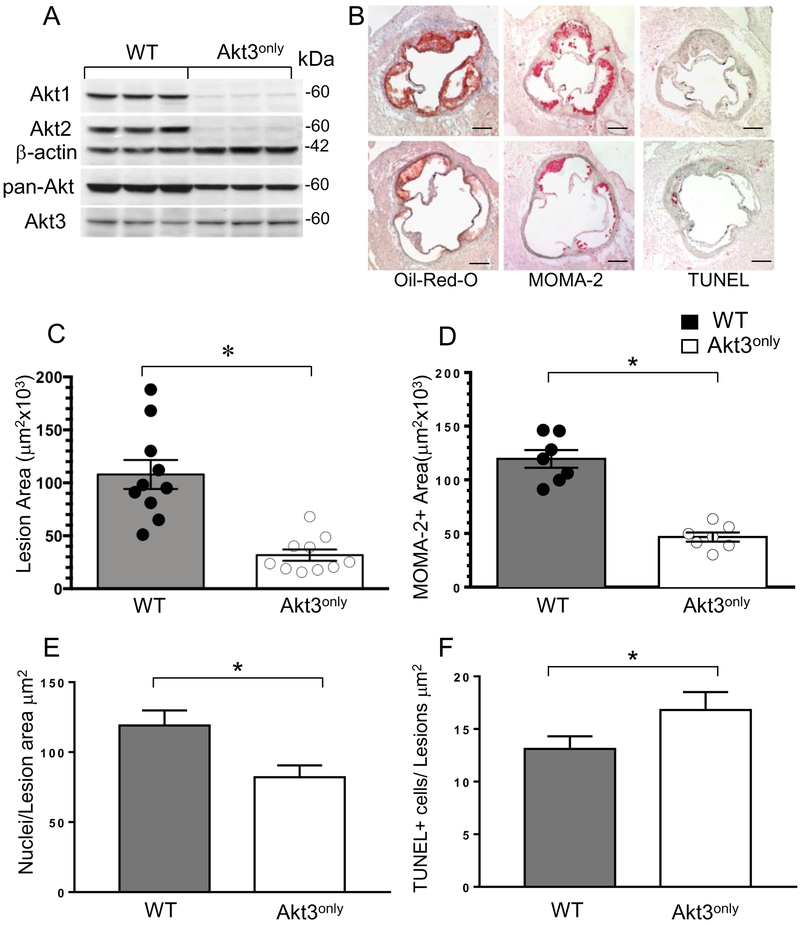
In the next set of experiments, we examined whether the presence of a single Akt3 isoform in hematopoietic cells has an impact on atherogenesis in vivo. Ten-week-old female Ldlr−/− mice were lethally irradiated and transplanted with female WT(n=10) or Akt3only(n=10) FLC, and, four weeks later, the recipient mice were challenged with the Western diet for 8 weeks. At sacrifice, the analysis of proteins extracted from peritoneal macrophages demonstrated that macrophages of Akt3only→Ldlr−/− mice contained only the Akt3 isoform (Figure 2A). No differences in the ratio of Akt3/β-actin were noted between WT and Akt3only macrophages (0.63±0.08 vs. 0.53±0.02, respectively, p=0.29). Moreover, we compared Akt1 and Akt3 gene expression levels in WT, Akt1only and Akt3only macrophages and found no compensatory increase in the levels of the preserved gene (Figure SV). There were no statistically significant differences between recipient mice of different groups in body weight, serum total cholesterol or triglyceride levels (Table 1B). However, mice reconstituted with Akt3only FLC had markedly smaller (61% reduction) atherosclerotic lesions in the aortic sinus than control WT→Ldlr−/− mice (Figure 2B,C). Akt3only→Ldlr−/− mice had a significantly smaller area of the lesions occupied by macrophages (Figure 2D), with fewer nuclei in the MOMA-2+ area (Figure 2E) and an increased number of apoptotic TUNEL-positive cells in the atherosclerotic lesions (Figure 2F) than WT→Ldlr−/− mice. Thus, female Ldlr−/− mice with Akt3only hematopoietic cells had dramatically suppressed early atherosclerosis.
Figure 2. Reconstitution of Akt3only hematopoietic cells into female Ldlr−/− mice significantly decreases Akt protein levels in macrophages and diminishes atherosclerotic lesions.
(A) Double Akt2/Akt3 knockout macrophages expressed the Akt3 isoform. Peritoneal macrophages were isolated from WT→Ldlr−/− and Akt3only→Ldlr−/− mice (n=3/group), proteins were isolated from cells and analyzed by Western blot.
(B) Detection of atherosclerotic lesions in the aortic sinus of WT→Ldlr−/− (top panel) and Akt3only→Ldlr−/− mice (bottom panel); Serial sections were stained with Oil-Red-O, MOMA-2 or TUNEL assay; scale bars, 200μm.
(C-F) The percent of atherosclerotic lesions (C), macrophage area (D), numbers of nuclei (E) and apoptotic cell numbers (F) in atherosclerotic lesions of female Ldlr−/− mice reconstituted with WT or Akt3only FLC; Graphs represent data (mean ± SEM, ∗p<0.05 by Mann-Whitney Rank Sum and *p<0.05 by t-tests).
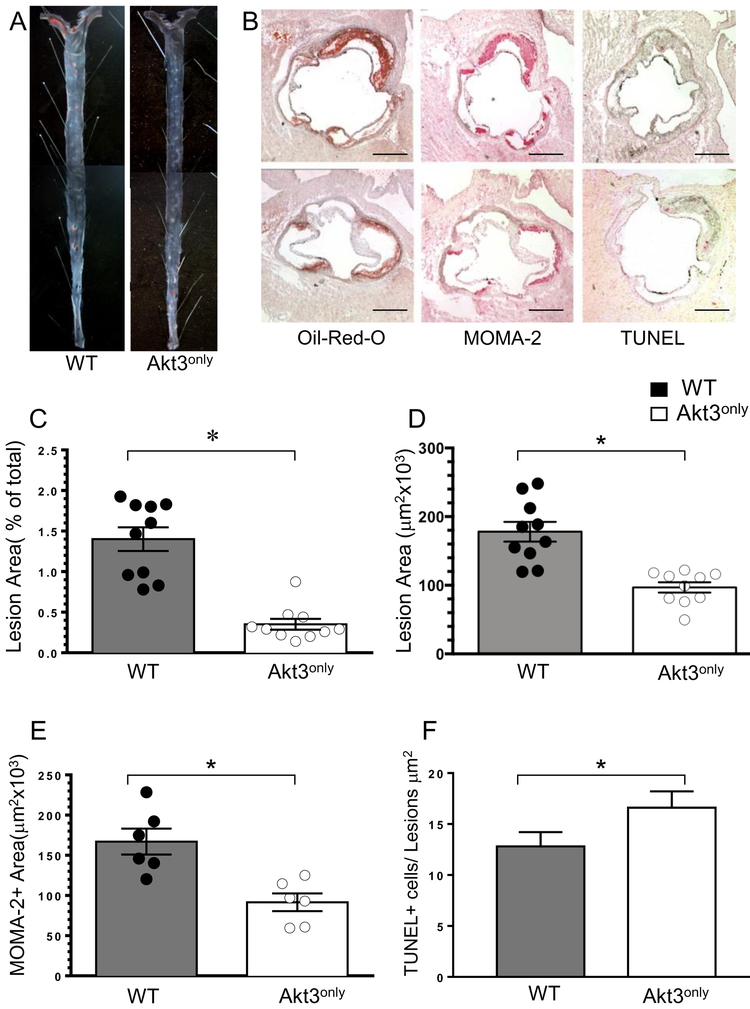
Next, to elucidate whether these findings were gender specific, twenty-four-week-old male Ldlr−/− mice were lethally irradiated and transplanted with male FLCs expressing WT (n=9) or Akt3only(n=11). Four weeks post-transplantation, the recipient mice were challenged with the Western diet for 8 weeks. Again, there were no statistically significant differences in body weight and serum lipid levels between the two groups of recipients (Table 1C). Interestingly, the 24-week old male WT→Ldlr−/− mice developed larger atherosclerotic lesions than the 10-week old male or female recipients used in the previous experiments, despite spending a similar amount of time on the Western diet. This allowed us to analyze the extent of atherosclerosis in the aortas en face (Figure 3A), which showed that male Ldlr−/− mice reconstituted with Akt3only FLC had a 75% reduction in atherosclerotic lesion area versus control WT→Ldlr−/− mice (Figure 3C). In addition, Akt3only→Ldlr−/− mice had smaller (41%) atherosclerotic lesions in the aortic sinus (Figure 3D), less MOMA2-positive area (Figure 3E), fewer nuclei in the MOMA-2+ area and more TUNEL-positive cells in the atherosclerotic lesions (Figure 3F) than WT→Ldlr−/− mice. Again, double staining of atherosclerotic lesions of mice transplanted with Akt3only bone marrow cells revealed that macrophages expressed only the Akt3 isoform (Figure SVI). Together these results demonstrate that both female and male Ldlr−/− mice reconstituted with Akt3only hematopoietic cells have significantly suppressed early atherosclerotic lesions compared to the same gender of control WT→Ldlr−/− mice.
Figure 3. Reconstitution of Akt3only hematopoietic cells into male Ldlr−/− mice reduces atherosclerosis.
(A) Detection of atherosclerotic lesions in pinned out aorta en face of WT→Ldlr−/− and Akt3only→Ldlr−/− mice; Aortas were stained with Sudan IV, pin size 10μm.
(B) Detection of atherosclerotic lesions, MOMA-2+ area and apoptotic cells in aortic sinus of WT→Ldlr−/− (top) and Akt3only→Ldlr−/− mice (bottom); Aortic sections were stained with Oil-Red-0, MOMA-2, or TUNEL assay; Scale bars, 200μm.
(C-F). The percent of atherosclerotic lesions in pinned out aortas (C), the extent of lesion area (D), MOMA-2+ area (E) and number of TUNEL+ cells (F) in aortic sinus of male Ldlr−/− mice reconstituted with WT or Akt3 FLC; Graphs represent data (mean ± SEM, ∗p<0.05 by Mann-Whitney Rank Sum and *p<0.05 by t-tests).
Mice with a single Akt isoform in hematopoietic cells have low levels of white blood cells, B-cells and monocytes, and increased monocyte apoptosis.
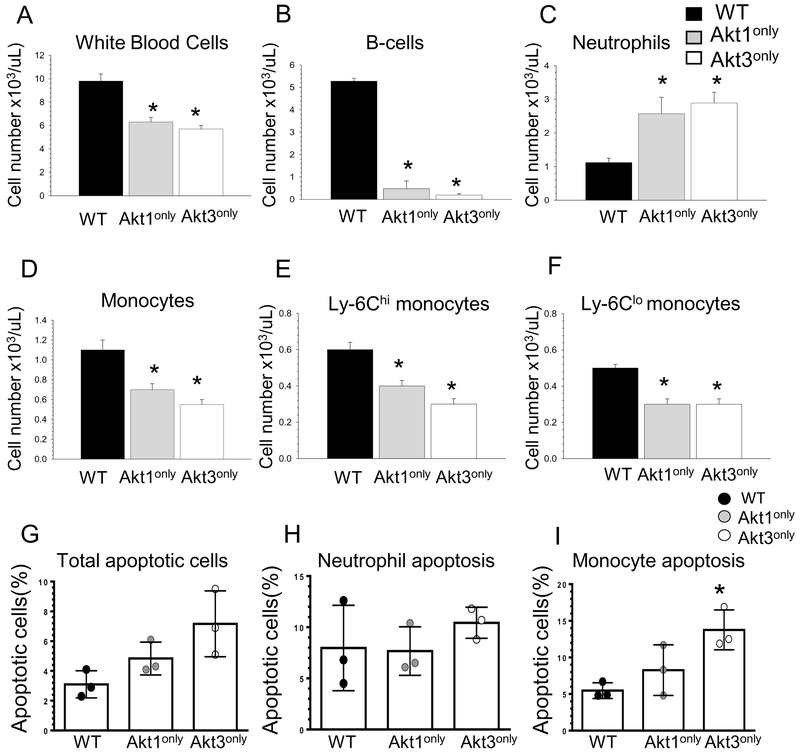
To evaluate the impact of Akt1only or Akt3only isoforms in hematopoietic cells of recipient mice on peripheral blood leukocytes, blood cells were analyzed by multicolor flow cytometry. Remarkably, both groups of Akt1only→Ldlr−/− and Akt3only →Ldlr−/− mice had similar changes in blood cells: dramatic reductions of white blood cell counts (Figure 4A) and B-cells (Figure 4B), and significant increases (>2-fold) in neutrophils (Figure 4C) compared to control WT→Ldlr−/− mice. In addition, mice reconstituted with Akt1only and Akt3only hematopoietic cells exhibited substantial reductions (38 and 52%, respectively) in blood monocyte numbers (Figure 4C) with equally diminished levels of inflammatory Ly-6Chi/CCR2-positive and patrolling Ly-6Clo/CX3CR1-positive subsets (Figure 4E,F). Thus, expression of only a single Akt1 or Akt3 isoform in hematopoietic cells induced leukopenia with dramatically reduced numbers of white blood cells, B-cells and monocytes, whereas neutrophil numbers were increased.
Figure 4. Mice with a single Akt isoform in hematopoietic cells have low levels of white blood cells, B-cells and monocytes but increased numbers of neutrophils and augmented monocyte apoptosis.
(A-F); Blood cells were collected from retro-orbital sinus of mice reconstituted with WT, Akt1only or Akt3only FLC (n=4/group). Cells were incubated with antibodies to CD19, CD11b, Ly-6G, Ly-6C and CCR2, and then analyzed by multicolor flow cytometry. Graphs represent data (mean ± SEM, *p<0.05 compared to WT cells by One Way Analysis of Variance (Tukey test). The experiment was repeated three times.
(G-I) Total number of apoptotic cells (G), apoptotic neutrophils (H) and monocytes (I) in blood of mice transplanted with WT, Akt1only and Akt3only FLC. Cells were isolated from mice (n=3/group) and incubated with antibodies to CD11b, Ly-6G and then treated with Annexin V Alexa Flour 647 to detect apoptotic cells. The experiment was repeated two times. Graphs represent data (mean ± SEM), *p<0.05 compared to WT cells by One Way ANOVA.
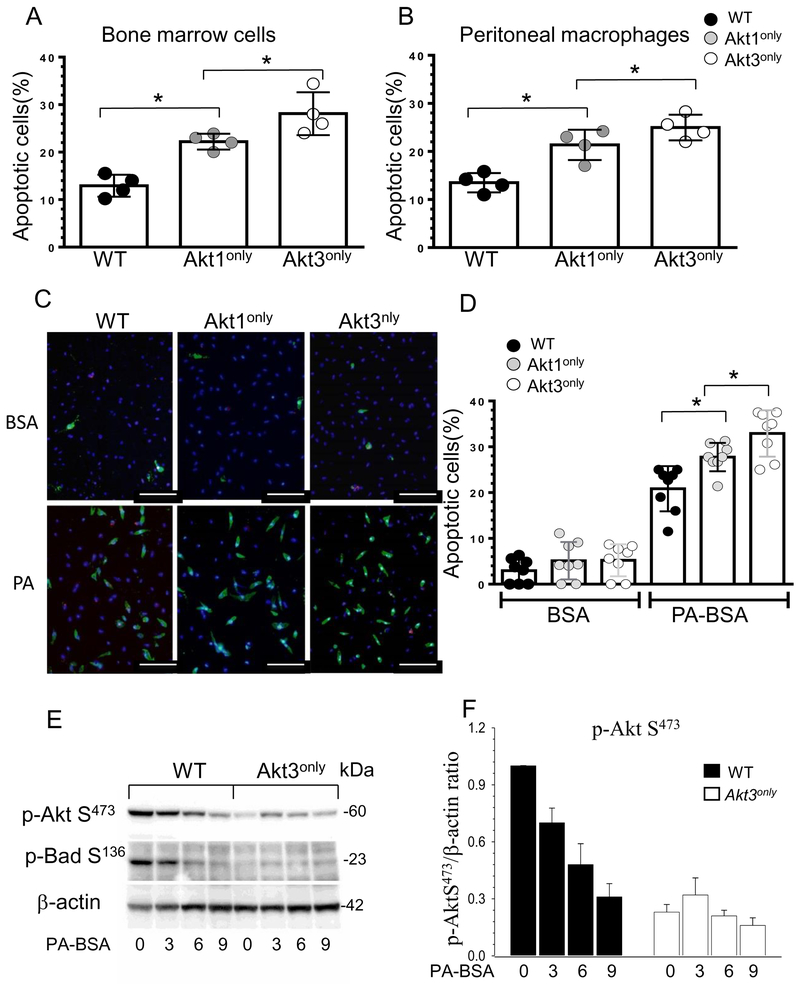
Our flow cytometry analysis also detected an increase in total blood apoptotic cells in Akt1only→Ldlr−/− and Akt3only→Ldlr−/− recipients compared to control WT→Ldlr−/− mice (Figure 4G). There were no differences in neutrophil apoptosis (Figure 4H), but the levels of apoptotic monocytes were significantly increased in mice expressing only the Akt1 or Akt3 isoform in hematopoietic cells (Figure 4I). To further elucidate this phenomenon, freshly isolated WT, Akt1only and Akt3only bone marrow cells and peritoneal macrophages were analyzed by flow cytometry. Again, there was a statistically significant increase of apoptosis in Akt3only cells versus Akt1only bone marrow and peritoneal macrophages compared to control WT cells (Figure 5A, B).
Figure 5. Blood Akt1only or Akt3only monocytes are more sensitive to ER stress than WT cells(A,B).
Apoptotic cells in bone marrow and peritoneal macrophages freshly isolated from Ldlr−/− mice reconstituted with WT, Akt1only and Akt3only FLC; cells were incubated with Alexa Fluor 647 Annexin V antibody and analyzed by flow cytometry; *p<0.05 compared to WT cells by One Way Analysis of Variance (Tukey test).
(C,D) Detection and the percent of apoptotic monocytes after treatment with BSA or PA-BSA overnight. Monocytes were isolated from blood of Ldlr−/− mice reconstituted with Akt1only, Akt3only or WT FLC, seeded in culture chambers and, two days later, treated with BSA or a pro-apoptotic stress factor, PA-BSA in the presence of 3% fetal bovine serum and 10nM of mouse macrophage colony-stimulating factor overnight, then apoptotic cell numbers were analyzed using Alexa Flour 488 Annexin V/Dead cell apoptosis kit. Graphs represent data (mean ± SEM) obtained from the same (n=8/group) mice; *p<0.05 compared to WT cells treated with PA by One Way Analysis of Variance, Dunnett’s method); Scale bar is 50mm.
(E,F) ER stress inhibits Akt signaling faster in Akt3only macrophages than in WT cells. WT, Akt3only peritoneal macrophages were treated with BSA or 0.5M PA-BSA for 0, 1, 3 and 6 hours. Extracted proteins were used for analysis of Akt and BadS136 signaling. Graphs represent data (mean ± SEM) of three experiments.
Next, blood monocytes were isolated from the recipient mice, seeded in culture chambers and treated with control BSA or a lipotoxic pro-apoptotic factor palmitic acid (PA) complexed to BSA to induce ER stress 36. Analysis of apoptosis by Annexin V staining demonstrated that all of these cells had similar levels of apoptosis after incubation with BSA, but PA treatment significantly increased apoptosis in a step-shaped manner in WT, Akt1only and Akt3only monocytes, respectively (Figure 5C,D). Thus, Akt3only monocytes have significantly higher susceptibility to apoptosis than Akt1only cells.
Macrophages with a single Akt1 or Akt3 isoform are less resistant to ER stress.
Since Akt signaling plays a pro-survival role preventing cells from undergoing apoptosis3 and the threshold of Akt protein levels is important for cell survival under stressed conditions37, we examined Akt phosphorylation in a short-term response to insulin treatment and under conditions of long-lasting ER stress. Compared to WT, Akt3only cells had comparable levels of Akt phosphorylation in response to insulin treatment (Figure SVII) indicating that short-term Akt signaling is essentially preserved in these cells. In contrast, when WT and Akt3only peritoneal macrophages were incubated in the presence of lipotoxic PA-BSA for several hours, the PA-induced stress gradually weakened Akt signaling in WT cells; whereas, cells expressing the Akt3only isoform exhibited less p-Akt than WT cells at baseline and in response to PA-BSA (Figure 6A,B). Correspondingly, the Akt down-stream target and anti-apoptotic member of the BCL-2 family, p-Bad S136, was also drastically reduced in Akt3only macrophages (Figure 6A). Similar results were seen in macrophages expressing the Akt1only isoform, which exhibited slightly lower levels of Akt signaling at every time point of ER stress than WT cells (Figure SVIII). Thus, ER stress exhausts anti-apoptotic Akt signaling more rapidly in Akt3only macrophages than in WT and Akt1only cells.
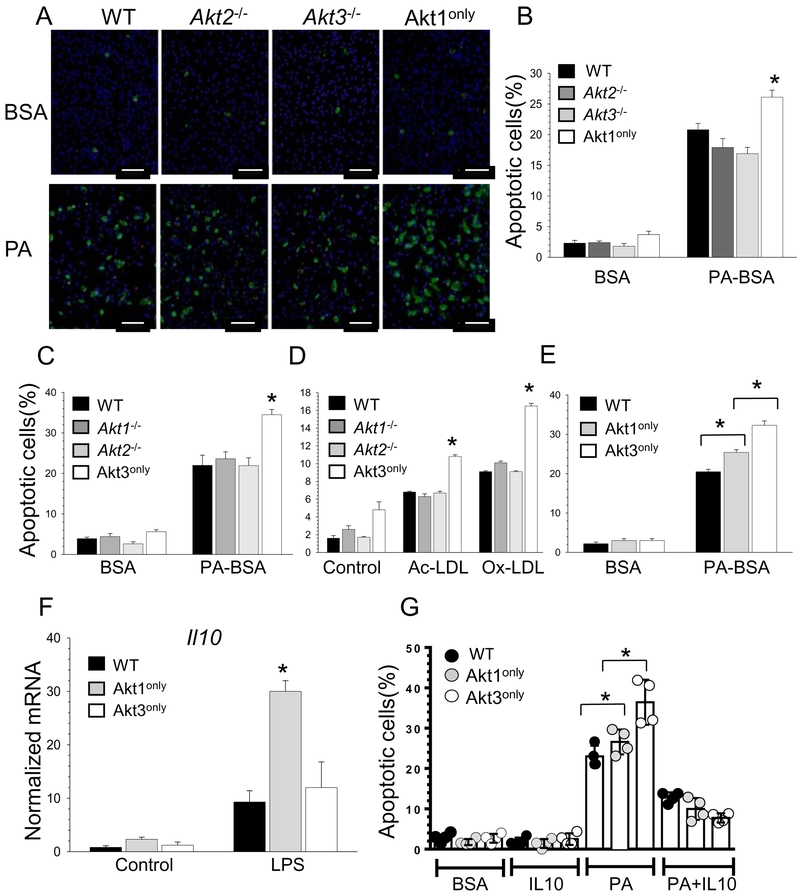
Figure 6. Akt1only and Akt3only macrophages are more sensitive to pro-apoptotic cells stimuli than WT and a single Akt isoform knockout cells, and Il-10 suppresses apoptosis in macrophages.
(A,B) WT, Akt2−/−, Akt3−/− or Akt1only peritoneal macrophages were isolated from recipient mice (n=4/group) and two days later treated with BSA or 0.5mM PA-BSA overnight. Apoptotic cell numbers were detected by Alexa Flour 488 Annexin V/Dead cell apoptosis kit. Graphs represent data (mean ± SEM; *p<0.05 compared to WT cells treated with PA-BSA by One Way Analysis of Variance); Scale bar is 50mm.
(C-D) The percent of apoptosis in WT, Akt1−/−, Akt2−/− or Akt3only macrophages after treatment with BSA or PA-BSA overnight, or incubated with human Ac-LDL (100mg/ml) plus an ACAT inhibitor Sandoz 58035 (2mg/ml) or with Ox-LDL (100mg/ml) for 48 hours. Graphs represent data (mean ± SEM) obtained from the same number (n=3/group) of mice, *p<0.05 compared to WT cells treated with PA-BSA, Ac-LDL or Ox-LDL by One Way Analysis of Variance.
(E) Akt1only macrophages are more resistant to apoptosis than Akt3only cells
(F) WT, Akt1only and Akt3only macrophages were treated with LPS (20ng/ml) for 6 hours and gene expression levels were measured by real-time PCR. Graphs represent data (mean ± SEM) obtained from the same number (n=3/group) of mice (*p<0.05 compared to WT cells treated with LPS by One Way Analysis of Variance).
(G) Macrophages were isolated from mice reconstituted with WT, Akt1only and Akt3only FLC and, after two days in culture, were treated with BSA or a mouse recombinant Il-10 (100ng/ml) or 0.5M PA-BSA with or without of IL-10 for 24 hours. Apoptotic cells were detected by Alexa Flour 488 Annexin V. Graphs represent data (mean ± SD) obtained from the same number (n=4/group) of mice (*p<0.05 compared to WT cells treated with PA-BSA by One Way Analysis of Variance).
Next, we compared the ability of WT, Akt1only and Akt3only macrophages to resist pro-apoptotic stimuli. Treatment with BSA had no impact on apoptosis and there were no differences between the groups (Figure 6A). In contrast, PA-BSA significantly increased apoptosis in all four groups but to a significantly greater extent in Akt1only macrophages than WT cells and cells with a single isoform deletion (Figure 6A,B). Similarly, BSA alone did not induce apoptosis in any of the different groups (Figure SIX), whereas PA-BSA treatment induced less apoptosis in WT, Akt1−/− and Akt2−/− macrophages than Akt3only macrophages, indicating that loss of two Akt isoforms markedly decreases cell survival (Figure 6C). These Akt3only macrophages also showed an enhanced sensitivity to different pro-apoptotic stimuli such as oxidized LDL or free cholesterol loading with acetylated LDL in combination with an ACAT inhibitor (Figure 6D). A direct comparison of cells with only a single Akt isoform demonstrated that Akt3only macrophages have the lowest levels of survival compared to Akt1only and especially WT cells (Figure 6E). Together these data demonstrate that under conditions of ER stress, loss a single Akt isoform alone is dispensable for macrophage survival, and the loss of two Akt isoforms markedly compromises cell survival, with higher levels of resistance to apoptosis in Akt1only cells compared to Akt3only macrophages.
In response to LPS, Akt1only macrophages express high levels of Il-10 that likely increases their survival.
Akt1 and Akt3 isoforms exert different functions in macrophages 11,12. Previously we have shown that loss of Akt1 but not Akt2 significantly suppressed Il-10 gene expression in macrophages 14 indicating that the Akt1 isoform may play a role in regulation of the gene. Moreover, a recent report specifies that IL-10 is an important anti-inflammatory cytokine that mediates metabolic reprogramming of macrophages 38. To examine whether loss of two Akt isoforms impacts cytokine expression, WT, Akt1only and Akt3only macrophages were treated with LPS and analyzed by real-time PCR. Both Akt1 and Akt3 macrophages showed markedly reduced expression of pro-inflammatory genes including Tnfα, Il12a, Il6, Nfkb1 (p50) and Rela (p60) (Figure SIX). However, Akt1only macrophages expressed significantly higher (3-fold) levels of Il10 gene than WT and Akt3only cells (Figure 6F). To test whether IL-10 is facilitating a pro-survival effect in mouse macrophages, we treated WT, Akt1only and Akt3only peritoneal macrophages with BSA, a mouse recombinant IL-10 and PA-BSA alone or together with IL-10 for 24 hours. Interestingly, IL-10 alone had no impact on apoptosis but when combined with PA it dramatically reduced levels of apoptosis in every type of macrophage (Figure 6G). Importantly, Akt1only macrophages were significantly more protected from apoptosis via incorporation of exogenous IL-10 than WT and Akt3only cells (73.6% vs. 31.8 and 43.5% reduction, respectively). Thus, IL-10 protects macrophages from apoptosis and high levels of Il-10 gene expression in Akt1only macrophages likely contributes to their survival advantage over Akt3only cells.
Discussion
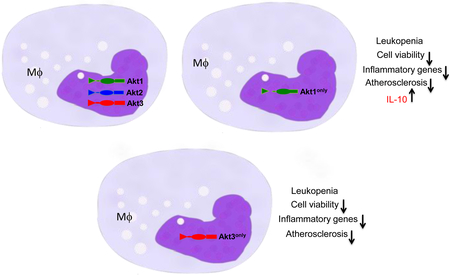
The viability of monocyte/macrophages and their survival in the artery wall plays a crucial role in the pathogenesis of atherosclerotic lesions 39. Liu and co-workers19 were the first to demonstrate that constitutive activation of Akt1 is vital for the survival of human macrophages and that inhibition of Akt signaling induced apoptosis in human and mouse macrophages32. Macrophages express three Akt isoforms, Akt1, Akt2 and Akt3, which are likely redundant with regard to Akt signaling. Recent studies have reported that mice with hematopoietic cells expressing only the Akt3 isoform exhibited low viability of B-, T-cells and stem cells 23-26. Based on these data, we hypothesized that marked reduction of Akt to a single isoform may compromise the viability of monocytes and macrophages, which would be expected to alter atherosclerosis. To test this hypothesis, we generated mice expressing Akt1only or Akt3only in hematopoietic cells. This novel approach allowed us to compare the impact of expression of the individual Akt1 and Akt3 isoforms on macrophage viability and atherosclerosis. Remarkably, both groups of mice reconstituted with Akt1only or Akt3only hematopoietic cells exhibited dramatic reductions in white blood cell counts and monocytopenia. In addition, monocytes and macrophages of these mice had increased sensitivity to apoptotic stimuli. As a result, male Ldlr−/− mice reconstituted with Akt1only FLC had significantly smaller atherosclerotic lesions compared to control mice with WT cells. Similarly, male and female Ldlr−/− mice transplanted with Akt3only FLC had reduced atherosclerotic lesions compared to control WT→Ldlr−/− mice. Thus, the reduction of Akt protein to a single isoform compromised monocyte and macrophage viability and this significantly diminished early atherosclerosis.
A striking finding of our studies is the fact that mice expressing only the Akt1 or Akt3 isoform in hematopoietic cells had lower levels of white blood cells but increased numbers of neutrophils. Recently Chen and co-authors 40 have shown that Akt2 knockout mice exhibited a 3-fold increase in blood neutrophils due to their defects in migration, and release of O2. In our experiments, Akt2 was deleted in both groups of Akt1only or Akt3only hematopoietic cells, and this may explain the increased number of neutrophils. The decreased number of white blood cells in these mice has been reported to be due in part to a profound survival defect in B-cells 23 as well as developmental defects of thymocytes 24, 25 and stem cells 26. In addition, we report here for the first time that the reduction of Akt protein to a single isoform in hematopoietic cells significantly lowered levels of blood monocytes, both Ly-6Chi and Ly-6Clow subsets. These monocytes were less viable than WT cells in response to increased ER stress. Importantly, Akt1only monocytes were more resistant to apoptosis than Akt3only cells (Figure 5C-F), and these data support the concept that Akt1 is the principal pro-survival isoform in macrophages 11, 19, 20.
Monocyte/macrophages are central cells of the innate immune system, and they play a key role in the initiation and progression of atherosclerosis 2. There is a strong correlation between blood monocyte count and atherosclerosis 41 suggesting that monocytopenia may significantly suppress atherogenesis. Indeed, Smith et al. 42 first demonstrated that Apoe null mice deficient in macrophage colony-stimulating factor (M-CSF), which have very low numbers of blood monocytes, developed significantly smaller atherosclerotic lesions than control mice. Later, Rajavashisth and co-workers 43 revealed that Ldlr−/− mice homozygous for M-CSF gene deficiency (op/op) have dramatically reduced atherosclerotic lesions (~0.3% of control lesions), whereas heterozygous mice had lesions < 1% in size of controls. They highlighted the fact that a 2-fold decrease in M-CSF expression reduced lesion size approximately 100-fold. Similarly, Ldlr−/− mice deficient for monocyte chemoattractant protein-1 developed smaller atherosclerotic lesions compared to control Ldlr−/− mice 44. Consistently, genetic deficiency 45 and pharmacological inhibition 46 of the chemokine receptor CX3CR1 in Apoe−/− mice significantly decreased atherosclerosis. Similarly, combined loss of CCL2, CX3CR1, and CCR5 in Apoe−/− mice significantly reduced circulating monocytes and markedly suppressed (90%) atherosclerosis 47. Moreover, depletion of monocytes from the circulation using clodronate (dichloromethylene bisphosphonate) considerably reduced plaque formation in rabbits 48. Finally, Stoneman et al 49 utilized transgenic mice expressing diphtheria toxin in CD11b-positive cells to demonstrate that depletion of monocytes profoundly reduced early atherosclerotic lesions. Together these data indicate that genetic reduction as well as chemical depletion of blood monocytes markedly suppresses atherosclerosis. Thus, the dramatic reduction of blood monocytes in Akt1only→Ldlr−/− and Akt3only→Ldlr−/− mice likely contributes to the suppression of atherosclerosis seen in these mice.
Since the loss of two Akt isoforms in hematopoietic cells impairs lymphocyte survival and increases neutrophils in the blood, we cannot conclude that compromised viability of monocyte/macrophages is the only mechanism responsible for the reduction atherosclerosis in Ldlr−/− mice reconstituted with Akt1only and Akt3only FLC. Future studies will be required to evaluate the impact of expression of a single Akt isoform by neutrophils, T- and B-cells on atherosclerosis. Here we demonstrated that both monocytes and macrophages expressing a single Akt isoform had increased apoptosis under conditions of ER stress, whereas loss of either Akt1 or Akt2 alone in macrophages did not compromise cell survival. A potential limitation of the in vitro macrophage apoptosis studies is the small number of mice (n=3/group) used; however, hundreds of peritoneal macrophages were studied per mouse and the responses to different pro-apoptotic factors (Figure 6B-E) were very similar and consistent for the different genotypes of macrophages (WT, Akt1−/−, Akt2−/−, Akt1only and Akt3only). Furthermore, these results are consistent with the finding that further depletion of Akt3 protein in Akt1/2 knockout mouse embryonic fibroblast spontaneously induced apoptosis 37, supporting the notion that Akt is critical for cell survival. Together, our results provide strong in vitro and in vivo evidence that a reduction of total Akt content in monocytes and macrophages markedly reduces their resistance to apoptosis. Dramatic reductions in atherosclerotic lesion area of mice reconstituted with Akt1only or Akt3only hematopoietic cells were coupled with finding fewer macrophages and more apoptotic cells that support the current concept 50 that an inverse relationship exists between macrophage apoptosis and lesion size in early atherosclerotic lesions. By showing that reduced macrophage Akt protein levels promote increased sensitivity to apoptosis and that this suppresses early atherosclerosis, our data demonstrate that Akt protein content is crucial for macrophage survival in the highly stressed environment of atherosclerotic lesions.
We have previously reported that mouse peritoneal macrophages have significantly higher levels of Akt1 and Akt2 isoforms than Akt3 14. However, it remains unknown whether macrophage-derived foam cells of atherosclerotic lesions may have differential expression of the individual Akt isoforms compared to other macrophages, such as bone marrow-derived or peritoneal macrophages. To examine this possibility, we analyzed publicly available microarray gene (mRNA) expression datasets that utilized laser capture micro-dissection and examined gene expression in CD68-positive macrophages of atherosclerotic lesions of Reversa mice 51. These mice are LDL receptor deficient and express an apolipoprotein B100-only allele, but they also harbor a floxed microsomal triglyceride transfer protein gene (Ldlr−/− Apob100/100Mttpfl/flMx1Cre+/+) that allows reversal of the hyperlipidemia and atherosclerosis by conditional deletion of MTP 33, 52. Surprisingly, expression of the Akt3 isoform was significantly higher in macrophage-derived foam cells of both control mice (Supplemental Figure XIA) and in mice with deletion of the floxed Mttp gene in the liver (Figure XIB) than expression of Akt1 or Akt2 isoforms. In contrast, the expression of Akt1 and Akt2 isoforms were significantly higher than Akt3 expression in bone marrow-derived macrophages 53 (Figure XIC). A potential limitation of these results with bone marrow-derrived macrophages is the small sample size, but there was a large difference between the groups with little variation within each group and this pattern of Akt isoform expression is consistent with our previous results for peritoneal macrophages 14. Most importantly, our results show differential expression of Akt isoforms in macrophage-derived foam cells of atherosclerotic lesions with increased levels of Akt3 compared to the expression pattern seen in bone marrow-derived and peritoneal macrophages.
Previously, Arranz and co-workers 54 demonstrated that Akt isoforms play opposing roles in macrophage polarization with the Akt1 isoform promoting the M2 phenotype and Akt2 initiating the M1 phenotype. More recently, we have shown that Akt2 null macrophages are skewed to the M2 phenotype with decreased expression of pro-inflammatory genes and reduced migration, and these changes suppress atherogenesis 14. In the current study, we verified that the Akt3 isoform expression alone in macrophages has no impact on cell polarization. In response to LPS, both Akt1only and Akt3only macrophages exhibited significantly lower levels of inflammatory gene expression. However, Akt1only macrophages expressed markedly increased levels of Il-10 gene compared to WT and Akt3only macrophages. Moreover, IL-10 protects cells from apoptosis, including astrocytes 55, myeloid progenitor cells 56 and cardiomyocytes 57. Here we demonstrated that recombinant IL-10 significantly reduces levels of apoptosis in monocytes and macrophages. These findings may explain, at least in part, pro-survival advantages of Akt1only cells over Akt3only macrophages.
In conclusion, we generated chimeric Ldlr−/− mice expressing a single Akt1 or Akt3 isoform in hematopoietic cells. These mice had low levels of white blood cell counts and monocyte numbers. In addition, they also exhibited increased apoptosis in blood monocytes, in freshly isolated peritoneal macrophages and bone marrow cells. In conditions of ER stress, loss of two Akt isoforms in hematopoietic cells was detrimental for the viability of monocytes and macrophages. These changes in viability of monocyte/macrophages resulted in dramatic reductions in the extent of early atherosclerosis. Furthermore, our findings may have important implications for the development of new therapeutic approaches. In recent years, the PI3K/Akt pathway has emerged as one of the most promising targets of anticancer drug therapy 58, and a number of clinical trials with inhibitors of Akt are currently underway in cancer patients 59. Therefore, our current data predict a potential unanticipated impact of therapeutic inhibition of Akt signaling on monocyte/macrophage survival that is likely to reduce atherogenesis, which may have important clinical relevance.
Supplementary Material
Highlights:
Akt protein reduction to a single Akt1 or Akt3 isoform in blood monocyte and macrophage markedly compromises their survival under conditions of ER stress.
Ldlr−/−mice reconstituted with Akt1only or Akt3only hematopoietic cells develop low white blood cell counts and monocytopenia, which dramatically reduces early atherosclerosis.
Akt1only macrophages expressed significantly higher levels of Il10 gene, which may contribute to their survival advantages over Akt3only cells.
Acknowledgements
The authors are grateful to Dr. Morris J. Birnbaum from Perelman School of Medicine, University of Pennsylvania for providing Akt3 knockout mice. This work was supported by National Institutes of Health grants HL116263, HL105375, DK50435 and DK59637 (Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers).
Abbreviations:
- Akt
protein kinase B
- FLC
fetal liver cells
- LDLR
LDL-receptor
Footnotes
Conflict Interests: The authors declare no conflicts of interest.
References:
- 1.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arter Thromb Vasc Biol. 2011;31:1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duronio V The life of a cell: Apoptosis regulation by the pi3k/pkb pathway. Biochemical Journal. 2008;415:333–344 [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. Akt/pkb signaling: Navigating downstream. Cell. 2007;129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heron-Milhavet L, Khouya N, Fernandez A, Lamb NJ. Akt1 and akt2: Differentiating the aktion. Histol Histopathol. 2011;26:651–662 [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Littlewood T, Bennett M. Akt isoforms in vascular disease. Vascular Pharmacology. 2015;71:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen WS, Xu P-Z, Gottlob K, Chen M-L, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes & Development. 2001;15:2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/pkbalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352 [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase akt2 (pkb beta). Science. 2001;292:1728–1731 [DOI] [PubMed] [Google Scholar]
- 10.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VMY, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for akt3/protein kinase bγ in attainment of normal brain size. Molecular and Cellular Biology. 2005;25:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metabolism. 2007;6:446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Biswas S, Morton Richard E, Smith Jonathan D, Hay N, Byzova TV, Febbraio M, Podrez Eugene A Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab. 2012;15:861–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rensing KL, de Jager SCA, Stroes ES, Vos M, Twickler MTB, Dallinga-Thie GM, de Vries CJM, Kuiper J, Bot I, von der Thüsen JH. Akt2/ldlr double knockout mice display impaired glucose tolerance and develop more complex atherosclerotic plaques than ldlr knockout mice. Cardiovasc Res. 2014;101:277–287 [DOI] [PubMed] [Google Scholar]
- 14.Babaev VR, Hebron KE, Wiese CB, Toth CL, Ding L, Zhang Y, May JM, Fazio S, Vickers KC, Linton MF. Macrophage deficiency of akt2 reduces atherosclerosis in ldlr null mice. J Lipid Res. 2014;55:2296–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotllan N, Chamorro-Jorganes A, Araldi E, Wanschel AC, Aryal B, Aranda JF, Goedeke L, Salerno AG, Ramírez CM, Sessa WC, Suárez Y, Fernández-Hernando C. Hematopoietic akt2 deficiency attenuates the progression of atherosclerosis. The FASEB Journal. 2015;29:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z-Z, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of akt1/protein kinase ba(pkba) and akt3/pkbg on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng XD, Xu PZ, Chen ML, et al. Dwarfism a., impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking akt1 and akt2. Genes & Development. 2003;17:1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dummler B, Tschopp O, Hynx D, Yang Z-Z, Dirnhofer S, Hemmings BA. Life with a single isoform of akt: Mice lacking akt2 and akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated akt-1 is vital for the survival of human monocyte-differentiated macrophages: Role of mcl-1, independent of nuclear factor (nf)-{kappa}b, bad, or caspase activation. J. Exp. Med. 2001;194:113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BD, Jabbour AM, Sandow JJ, Riffkin CD, Masouras D, Daunt CP, Salmanidis M, Brumatti G, Hemmings BA, Guthridge MA, Pearson RB, Ekert PG. Akt1 is the principal akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013;20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Borlado L, Barber DF, Hernández C, Rodríguez-Marcos MA, Sánchez A, Hirsch E, Wymann M, Martínez-A. C Carrera AC Phosphatidylinositol 3-kinase regulates the cd4/cd8 t cell differentiation ratio. J Immunol. 2003;170:4475–4482 [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of pi3kγ in thymocyte development, t cell activation, and neutrophil migration. Science. 2000;287:1040–1046 [DOI] [PubMed] [Google Scholar]
- 23.Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and akt2 promote peripheral b-cell maturation and survival. Blood. 2010;115:4043–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and akt2 are required for ab thymocyte survival and differentiation. Proc Nat Acad Sci USA. 2007;104:12105–12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, Horie K, Gaitanaris GA, Fidanza V, Ludwig T, Wiest DL, Gounari F, Tsichlis PN. Unequal contribution of akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178:5443–5453 [DOI] [PubMed] [Google Scholar]
- 26.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. Akt1 and akt2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: Focus on kinase inhibitors. J Amer College Card. 2015;66:1160–1178 [DOI] [PubMed] [Google Scholar]
- 28.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies—statement from atvb council. Arterioscl Thromb Vasc Biol. 2018;38:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein e-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037 [DOI] [PubMed] [Google Scholar]
- 31.Daugherty A, Tall AR, Daemen MJAP, Falk E, Fisher EA, García-Cardeña G, Lusis AJ, Owens AP, Rosenfeld ME, Virmani R Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the american heart association. Arterioscl Thromb Vasc Biol. 2017;37:e131–e157 [DOI] [PubMed] [Google Scholar]
- 32.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage ep4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metabol. 2008;8:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage ppar{gamma} increases atherosclerosis in c57bl/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–1653 [DOI] [PubMed] [Google Scholar]
- 35.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Nati Acad Sci. 2003;100:13531–13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006;47:2726–2737 [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Shi Y, Birnbaum MJ, Ye K, De Jong R, Oltersdorf T, Giranda VL, Luo Y. Quantitative analysis of anti-apoptotic function of akt in akt1 and akt2 double knock-out mouse embryonic fibroblast cells under normal and stressed conditions. J Biol Chem. 2006;281:31380–31388 [DOI] [PubMed] [Google Scholar]
- 38.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of il-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabas I Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Ather Thromb Vasc Biol. 2005;25:2255–2264 [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Tang H, Hay N, Xu J, Ye RD. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115:4237–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony- stimulating factor (op) and apolipoprotein e. Proc Nat Acad Sci. 1995;92:8264–8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in ldl receptor- deficient mice. J Clin Invest. 1998;101:2702–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2:275–281 [DOI] [PubMed] [Google Scholar]
- 45.Combadière C, Potteaux S, Gao J-L, Esposito B, Casanova S, Lee EJ, Debré P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in cx3cr1/apolipoprotein e double knockout mice. Circulation. 2003;107:1009–1016 [DOI] [PubMed] [Google Scholar]
- 46.Poupel L, Boissonnas A, Hermand P, Dorgham K, Guyon E, Auvynet C, Charles FS, Lesnik P, Deterre P, Combadiere C. Pharmacological inhibition of the chemokine receptor, cx3cr1, reduces atherosclerosis in mice. Arter Thromb Vasc Biol 2013;33:2297–2305 [DOI] [PubMed] [Google Scholar]
- 47.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6chi and ly6clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657 [DOI] [PubMed] [Google Scholar]
- 48.Ylitalo R, Oksala O, Yla-Herttuala S, Ylitalo P. Effects of clodronate (dichloromethylene bisphosphonate) on the development of experimental atherosclerosis in rabbits. J Lab Clin Med. 1994;123:769–776 [PubMed] [Google Scholar]
- 49.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in cd11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circulation Res. 2007;100:884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50:S382–S387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramsey SA, Vengrenyuk Y, Menon P, Podolsky I, Feig JE, Aderem A, Fisher EA, Gold ES. Epigenome-guided analysis of the transcriptome of plaque macrophages during atherosclerosis regression reveals activation of the wnt signaling pathway. PLOS Genetics. 2014;10:e1004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, Wong JS, Hamilton RL, Fisher EA, Young SG. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321 [DOI] [PubMed] [Google Scholar]
- 53.Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining gm-csf– and macrophage-csf–dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752–5765 [DOI] [PubMed] [Google Scholar]
- 54.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C Akt1 and akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA. 2012;109:9517–9522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pahan K, Khan M, Singh I. Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J Neurochem. 2000;75:576–582 [DOI] [PubMed] [Google Scholar]
- 56.Zhou J-H, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW. Il-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3′-kinase. J Immunol. 2001;167:4436–4442 [DOI] [PubMed] [Google Scholar]
- 57.Dhingra S, Bagchi AK, Ludke AL, Sharma AK, Singal PK. Akt regulates il-10 mediated suppression of tnfα-induced cardiomyocyte apoptosis by upregulating stat3 phosphorylation. PLOS ONE. 2011;6:e25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fruman DA, Rommel C. Pi3k and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13:140–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu N, Lao Y, Zhang Y, Gillespie DA. Akt: A double-edged sword in cell proliferation and genome stability. J Oncology. 2012;ID 951724:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.