Abstract
Broadly neutralizing, anti-HIV-1 gp120 monoclonal antibodies have been isolated from infected individuals, and there is considerable interest in developing these reagents for antibody-based immunoprophylaxis and treatment. As a means to identify potentially new anti-HIV antibodies, we exploited humanized NOD-scid IL2rgnull (NSG) mice systemically infected with HIV-1, to generate a wide variety of antigen-specific human monoclonal antibodies. The antibodies were encoded by a diverse range of variable gene families and immunoglobulin classes, including IgA, and several showed significant levels of somatic mutation. Moreover, the isolated antibodies not only bound target antigens with similar affinity as broadly neutralizing antibodies, they also demonstrated neutralizing ability against multiple HIV-1 clades. The use of humanized mice will allow us to utilize our knowledge of HIV-1 gp120 structure and function, and the immune response targeting this protein, to generate native human prophylactic antibodies to reduce the infection and spread of HIV-1.
Introduction
HIV-1 continues to be a worldwide health issue with an estimated 35 million lives lost to date (1). In 2016, 1 million people died from HIV-1-related causes. Despite the length of time HIV-1 has been wreaking havoc on its victims, further improvements in the prevention and treatment of HIV-1 are still critically needed.
Humanized mice offer invaluable animal models to study the treatment and prevention of HIV-1 infection since human tissues engrafted in these mice can be infected with HIV-1. Generally, there are three different humanized models for engraftment of human immune systems in immunodeficient mice: engraftment with human peripheral blood mononuclear cells (Hu-PBL-SCID), engraftment with human CD34+ hematopoietic stem cells (HSC, Hu-SRC-SCID), and engraftment with human fetal tissues (bone marrow, liver, thymus, BLT and SCID-Hu). Hu-PBL-SCID mice are generated by injection of human peripheral blood leukocytes and support examination of human T cell function (2). However, due to the rapid onset of T-cell mediated xenogeneic graft-versus-host disease (GVHD), there is a limited window of opportunity for experiments with Hu-PBL-SCID mice. In the second model, Hu-SRC-SCID mice, HSC derived from fetal liver, cord blood, bone marrow, or granulocyte colony-stimulating factor mobilized peripheral blood are injected (2, 3). Hu-SRC-SCID mice support engraftment of a functional human immune system, including B cells, T cells, myeloid cells and antigen-presenting cells (APCs). However human innate immune cell populations developing in Hu-SRC-SCID mice are present at very low numbers in the blood, and human T cells develop primarily within the murine thymus, which lacks HLA expression needed for development of HLA-restricted T cells (2). Finally, the BLT model involves the transplantation of human fetal liver and thymus, and intravenous injections of autologous fetal liver HSC. This model enables robust development of a functional immune system, provides much higher percentages of human T cells and, supports efficient development of HLA-restricted conventional and regulatory T cells, and is the only model that leads to the generation of a robust mucosal human immune system (3). This combination of features is ideal for studying HIV-1infection, as it predominantly occurs at the mucosal surfaces. Of course, there are caveats to BLT mice as well, including a limited supply of fetal tissue for engraftment, the requirement for skilled technicians to perform engraftment protocols, development of a wasting syndrome that limits the life span of the mice and difficulty in generating class switched, affinity matured B cell responses following antigenic challenge.
For our studies on preventing and treating HIV-1 infection with monoclonal antibodies (mAbs), we selected the BLT model. Studies using SCID-hu and hu-HSC mice revealed the characteristics of latency during the early stages of infection. (4). However, as improvements to the engraftment of BLT’s have been made, they have become a powerful model for studying HIV-1 for their unique characteristics allowing for the mimicry of a full human immune system. We describe here the generation of human mAbs to HIV-1 from infected NSG-BLT mice. Despite the BLT mouse model having previously been shown difficult to illicit a robust antibody response (5, 6), there are unique characteristics of HIV-1 infection such as the chronic production of viral antigens with inflammation helping to drive the response (7). The mAbs isolated here were incredibly diverse in variable repertoire, isotype and subclass, and displayed neutralization activity. Thus, the engraftment of immunodeficient mice with human immune cells in combination with infection of HIV-1 enables the generation and isolation of fully human mAbs to specific targets and antigens for which immunized individuals are either not available or fail to generate a humoral immune response
Materials and Methods
Infection of NSG-BLT Mice
Stock NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD-scid IL2rγnull, NSG) mice were obtained from colonies maintained at The Jackson Laboratory (Bar Harbor, ME) by LDS. All procedures with animals were done in accordance with the guidelines of the Animal Care and Use Committee of the University of Massachusetts Medical School and The Jackson Laboratory and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, Eighth Edition 2011). NSG-BLT mice were generated using standard protocols previously described (8, 9). Mice were infected with virus isolate BAL, or with virus generated by cloned pNL43 bearing the JR-FL env, via intraperitoneal injection. Plasma samples were collected over time via submandibular or tail nick bleeds and used in ELISAs to monitor antibody development, as well as to measure HIV-1 genomic RNA equivalents by RT-PCR. A total of four animals were chosen to perform fusions.
Hybridoma Isolation
Splenocytes were fused with human myeloma cells HMMA 2.5 using polyethylene glycol (PEG solution, Sigma P7181) and selection with 1% hypoxanthine-aminopterin-thymidine (Mediatech, HAT), and 0.2% ouabain as previously described (10, 11). Fusions were screened for HIV-1 antibody by capture ELISA using gp41 (Meridian Life Science), gp120, or gp120-CD4 FLSC (full length single chain, gp120 plus CD4 D1 and D2 domains (12)). gp120 and gp120-CD4 FLSC were made by transient transfection of 293T with expression plasmids and purified using Lectin from Galanthus nivalis (snowdrop) agarose (L8275 SIGMA) as previously described (13). Positive producing hybrids were scaled up and antibody purified from supernatants using a Protein G Sepharose (IgG, GE healthcare), or Capture Select Affinity Matrix (IgA, Thermo Scientific).
Antibody Sequencing
RNA extraction from hybridomas was performed using QIAGEN RNEasy Plus Mini Kit. cDNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen) and then amplified using either heavy, light, or alternate lambda chain primer sets (Table 1). PCR reactions were run on an agarose gel; bands extracted and purified using QIAGEN Gel Extraction Mini Kit. 10μL of gel-extracted product was sent to Genewiz with separate reactions for each sequencing primer (Table 2). Sequences were then analyzed using IMGT.
Table I:
Primers for Amplification of Isolated Antibodies
| Heavy Chain Primers | Sequence |
|---|---|
| NB-VH1/5/7 | CAGGTGCAGCTGGTGCAGTCTGGGGCTGAGGTG |
| NB-VH2 | CAGATCACCTTGAAGGAGTCTGGTCCTACGCTGGTGAAACCCAC |
| NB-VH3 | GAGGTGCAGCTGGTGGAGTCTGGGGGAG |
| mutNB-VH4/6 | CAGGTGCAGCTGCAGGAGTCAGGCCCAGGACTG |
| CLONE-HeavyREV | CCAAGCTGCTGGAGGGCACGGTCACCACGC |
| CLONE-IgAREV | GCACTGTGTGGCCGGCAGGGTCAGCTGG |
| Light Chain Primers | |
| modNB-VK1 | GACATCCAGATGACCCAGTCTCCATCCTCCCTGTCTGCATC |
| modNB-VK2 | GATATTGTGATGACCCAGACTCCACTCTCCCTGCCCGTCACCC |
| modNB-VK3 | GAAATTGTGTTGACGCAGTCTCCAGCCACCCTGTCTTTGTCTCCAGG |
| modNB-VK4 | GACATCGTGATGACCCAGTCTCCAGACTCCCTGGCTGTG |
| modNB-VK5 | GAAATTGTGCTGACTCAGTCTCCAGACTTTCAGTCTGTGACTCC |
| mod-VL1/2 | CTCATCACTCACTGTGCAGGGTCCTGGGCC |
| mod-VL3r | CTCGGCGTCCTTGCTTACTGCACAGGATC |
| mod-VL3p | CTCCCCCTCCTCACTCTCTGCACAGTCTC |
| NB-LightREV | GCGTTATCCACCTTCCACTGTACTTTGGCCTCTCTG |
| LambdaRev | CCTTCCAGGCCACTGTCACGGCTCC |
| Lambda Primers | |
| HuIgLVL5-A* | GGG AAT TCA TGR CCT GSW CYC CTC TCY TYC TSW YC |
| HuIgLVL3–1* | CCC AAG CTT GAA GCT CCT CAG AGG AGG G |
Table II.
Sequencing Primer Sets
| HvSeq2 primer | CTGAGTTCCACGACACCGTCA |
| IgASeq primer | GGGAAGTTTCTGGCGGTCAC |
| NB-PfxLtSeqR primer | GCGTTATCCACCTTCCACTG |
| LambdaSeqR primer | CCTTCCAGGCCACTGTCAC |
| HuIgLSeqR | GTCACTCTGTTCCCACCCTC |
Viral Neutralization
The neutralization activity of the antibodies against four isolates of HIV-1 was determined by a luciferase assay using TZM-bl cells. Antibodies were titered in serial dilutions and incubated with virus stock diluted to 100 TCID50 for 1 hour at 37⁰ C. TZM-bl cells were then added at a concentration of 1 × 10⁴ cells/well with DEAE-Dextran (Sigma). After 48 hours of incubation at 37⁰ C, 100ul of supernatant was removed from the wells and 100ul of Britelite Plus substrate was added and incubated for 5 minutes. Plates were then read on a luminometer to determine relative light units (RLUs). Percent neutralization was determined based on control wells of virus and media, and IC50 and IC90 values were calculated by regression analysis.
Results
Isolation of Human Monoclonal Antibodies
Three months post- tissue engraftment, NSG-BLT mice were screened for the level of human cell chimerism in the blood by flow cytometry. Peripheral blood samples were evaluated for human CD45+ cells, CD3+ T cells and CD20+ B cells. Successfully engrafted NSG-BLT mice with >20% human CD45+ cells and >20% human CD3+ T cells (as a percentage of CD45) in blood were used in experiments. Within each cohort, >75% of animals were engrafted with at least 20% human CD45+ cells. For those animals engrafted with >20% of human CD45+ cells, among the CD45+ cells the range of either CD3+ T cells, or CD20+ B cells, was 20–70%. Successfully engrafted mice were infected with HIV-1 BaL or NL43-JRFL via IP injection. Blood samples were tested by ELISA for anti-HIV-1 antibody using gp41, gp120, or gp120-CD4 FLSC antigen ELISA and viral load. The inclusion of gp120-CD4 FLSC, which is a single chain construct of CD4 and gp120, allows reactivity with epitopes only exposed upon CD4 binding (gp120-CD4 FLSC) in contrast to gp120 without CD4 (12). Figure 1 shows a representative animal, in which there was an increase in serum antibody reactive against gp41 or gp120-CD4 FLSC, as a function of time post-HIV-1-infection. Figure 2 demonstrates two other animals of the same cohort and their antibody response to gp120-CD4 FLSC over time comparing IgG vs. IgA. It should be noted that there was no correlation between the number of B cells (as indicated by CD20+ cells) and antibody titers, albeit this is a small dataset.
Figure 1:
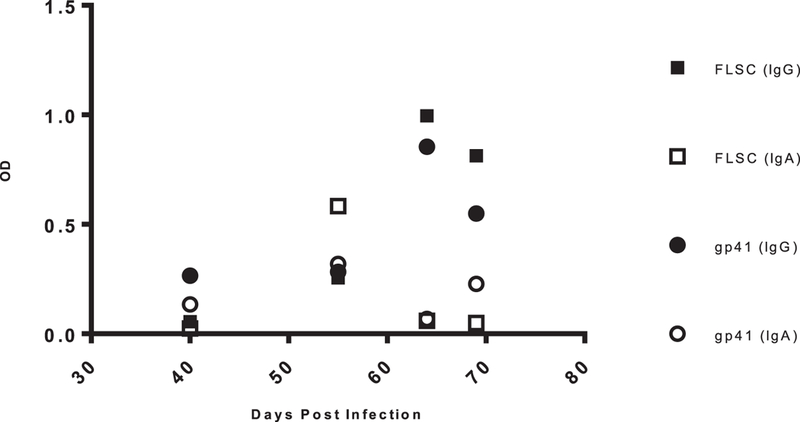
Antigen Specific Antibody Titers. Serum samples for all mice were collected weekly over time and measured for antigen specific antibody by ELISA. Above is data representing animal C4m1 being tested against either gp120-CD4 FLSC or gp41. All sera samples were run at a 1:10 dilution and detected using goat anti-human IgG or IgA HRP conjugated antibodies. Plates were developed and read at 450nm.
Figure 2:
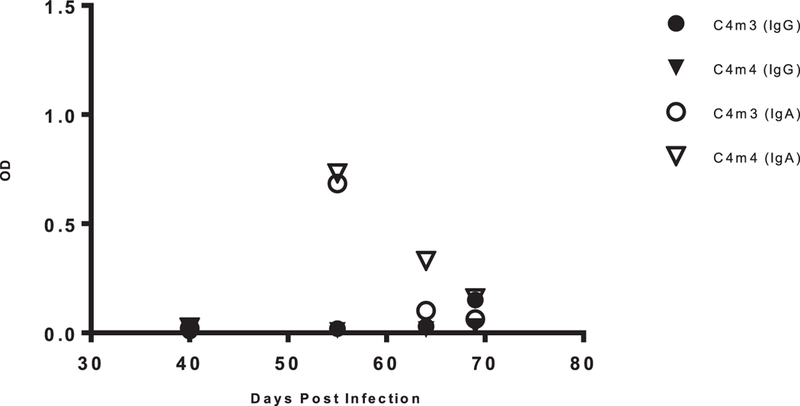
gp120-CD4 FLSC Specific Antibody Titers. Serum samples for all mice were collected weekly over time and measured for antigen specific antibody by ELISA. Above is data representing animals C4m3 and C4m4 being tested against gp120-CD4 FLSC. All sera samples were run at a 1:10 dilution and detected using goat anti-human IgG or IgA HRP conjugated antibodies. Plates were developed and read at 450nm.
All but one animal were euthanized within three months of infection (one animal was euthanized 8 months post infection), spleens were harvested, and single cell suspensions were collected for fusion. Out of 20 total mice, between three different cohorts, four mice were selected for fusion based on antibody titer (and survival for at least three months post infection). As shown in Table 3, recovery of splenocytes varied, which in turn led to variation in the number of hybrids to be screened. Altogether, between three different fusions, more than 70 different antibodies to either gp41 or gp120-CD4 FLSC were isolated during the initial screening process, with a random selection undergoing further characterization (Table 4). Perhaps due to splenocytes being frozen previously, the yield of hybridomas to be screened from fusion F878 was less than for fusions in which fresh splenocytes were used. It should be noted that >80% of the screened wells with hybridomas secreted either IgG or IgA immunoglobulin, demonstrating the ability to capture antibody producing B cells using these methods.
Table III.
Fusion Summarya
| Sera Reactivity (IgG/IgA) |
|||||||
|---|---|---|---|---|---|---|---|
| Mouse | Virus | gp120 or gp120- CD4 FLSCb |
gp41 | Fusion | Weeks Infectedc |
Splenocyte number |
Percent Positived |
| C5M3 | BaL | +/+ | −/− | F860 | 8 | 12X106 | 5% |
| C5M2 | BaL | +/− | +/− | F861 | 8 | 30X106 | 7% |
| Ms#1 | BaL | +/+ | +/+ | F862 | 4 | 54X106 | 22% |
| 13.41 M1 | JrFl | +/NTe | +/NT | F878 | 34 | 3.3X106 | 18% |
A total of four separate fusions were performed under same conditions
Sera was collected from animals prior to sacrifice and tested for IgG or IgA immunoreactivity with gp120, gp120-CD4 FLSC or gp41
The length of time mice were infected prior to euthanasia/fusion is indicated in weeks
Percent positive is the number of HIV-1 specific antibodies isolated
NT= not tested
Table IV.
Genetic Diversity of Human Monoclonal Antibodies
| Antibody | Isotype | Reactivity | Gene Family | Germline Homology (Vh/Vl) |
|---|---|---|---|---|
| F860 A2e10 | IgG2, k | gp120-CD4 FLSC | IgHV1–69(*01F or *12F) IgkV1–13*02 |
95.1%/96.1% |
| F860 B1d3 | IgG1, λ | gp41 | --a | -- |
| F860 B2c9 | IgG2, λ | gp41 | -- | -- |
| F860 B2e12 | IgG1, λ | gp41 | -- | -- |
| F861 A3h3 | IgG1,k | gp120-CD4 FLSC | -- | -- |
| F861 A7f10 | IgG3,λ | gp120-CD4 FLSC | IgHV1–69(*01F or *12F) IgLV5–45*02F |
99.6/99.0 |
| F862 A1f6 | IgG1, λ | gp120-CD4 FLSC | IgHV1–69*05F IgLV3–9*01F | 95.5/100 |
| F862 A3b11 | IgG2,λ λ | gp41 | IgHV4–34*01F IgLV2–23*02F |
96.8/99.7 |
| F862 A5a7 | IgG1, λ | gp120-CD4 FLSC | IgHV3–30(*04F or *03F) IgLV2–23*02F |
100/100 |
| F862 A5d2 | IgG2, λ | gp120-CD4 FLSC | -- | -- |
| F862 B4h11 | IgG1, λ | gp120-CD4 FLSC | -- | -- |
| F862 B12h6 | IgA, λ | gp120-CD4 FLSC | IgHV4–30-2*04F IgLV1–47*01F |
91.1/98.3 |
| F878 A2a9 | IgG1, λ | gp120 | IgHV1–69*01F IgLV3–19*01F |
94.1/97.1 |
| F878 B2e12 | IgG1, k | gp120, gp120-CD4 FLSC | IgHV3–33(*01F or *06F) IgKV1–17*03F |
91.5/96.7 |
-- = Antibodies not characterized
Immunoreactivity of HIV-1 Specific Antibodies
The immunoreactivity of purified antibody with HIV-1 env-encoded antigens was determined using gp41 and gp120 or gp120-CD4 FLSC (Figure 3). Human mAbs F240, reactive with gp41, F425-A1g8, reactive with CD4i epitope (expressed by gp120-CD4 FLSC), and b12, reactive with gp120/CD4 binding site were included for comparison. Endpoint concentrations were determined by linear regression based on the best-fit curve for the control. For each assay, it was determined that the immunoreactivity for each mAb isolated from the humanized mice was very similar, if not more immunoreactive than that observed for a known positive control ab. It is interesting to note that the antibody isolated as an IgA showed higher affinity for gp120-CD4 FLSC when compared to its control IgG.
Figure 3:
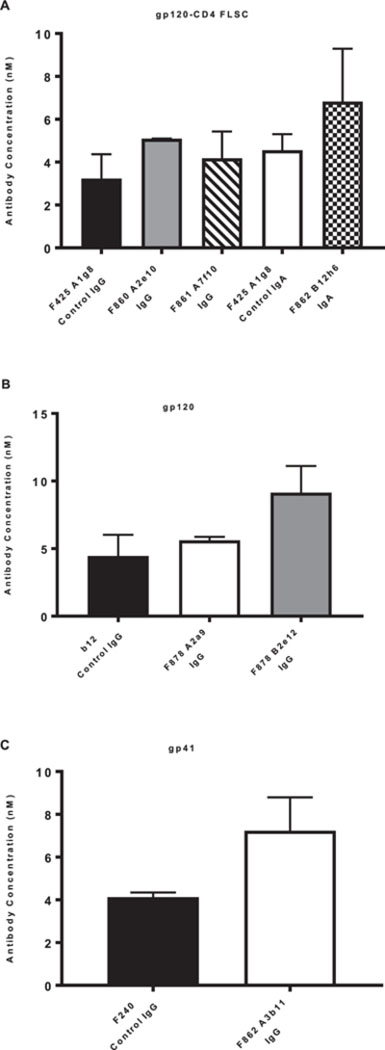
Immunoreactivity Endpoint Concentrations: ELISA plates were coated with gp120-CD4 FLSC (A), gp120 (B), or gp41 (C) protein. HIV-specific antibodies were set up in triplicate and titered 1:2 alongside positive control antibodies F425A1g8 (IgG and IgA in A), b12 IgG in B, and F240 IgG in C. Bound antibody was detected using anti-human IgG (A, B and C) or IgA (A) HRP labeled antibodies were used for detection. Plates were developed and read at 450nm. Endpoint concentrations were determined by linear regression based off the best-fit curve for the control in three separate experiments with the error bars representing the standard deviation of the mean.
Neutralization activity against HIV-1 by isolated human monoclonal antibodies
Neutralization of HIV-1 was tested in vitro using TZM-bl cells as targets and a panel of four HIV-1 isolates (67970, BaL, JR-CSF, and SF-162) grown in PBMCs. Serial dilutions of all mAb were tested and IC50 and IC90 values were calculated (Table 5). The antibody F861 A7f10, had the most neutralizing activity across all HIV-1 isolates, with IC50’s ranging from <0.02 to 25ug/ml, while IC90’s ranged from 0.02–39ug/ml, depending on the virus. Tested antibodies from fusions F860, F862 and F878 displayed neutralizing activity against isolate SF-162, with IC50 values 2.64, 12.14, 4.65, and 1.3ug/ml, respectively (Table 5). Thus, antibodies can be isolated from HIV-1 infected humanized mice that vary in immunoreactivity and neutralization across more than one clade of virus and target antigen.
Table V.
Neutralization of HIV-1 by Human Monoclonal Antibodiesa
| 67960 Clade B, X4 IC50/IC90 b |
BAL Clade B, R5 IC50/IC90 |
JR-CSF Clade B, IC50/IC90 |
SF-162 Clade B, R5 IC50/IC90 |
|
|---|---|---|---|---|
| F860 A2e10 | >40 | >40 | >40 | 2.64/18.49 |
| F861 A7f10 | 1.28/39.36 | 25.01/ >40 | <0.01/ 9.39 | <0.02/0.02 |
| F862 A3b11 | >40 | >40 | >40 | 12.14/ >40 |
| F862 B12h6 | >40 | >40 | >40 | 4.65/ >40 |
| F878 B2e12 | >40 | >40 | >40 | 1.3/16.98 |
The results are the mean of triplicate wells and representative of at least three independent experiments
IC50: or IC90: concentration (μg/ml) of antibody required for 50% or 90% inhibition of HIV, respectively
Diversity of isolated human monoclonal antibodies
To explore the variety of antibodies generated from fusions, a random sampling of isolated human mAb were chosen for sequencing and gene family usage was explored (Table 4). The heavy and light chains of several human mAb were sequenced and analyzed using ImMunoGeneTics (IMGT) V-Quest software to determine variable gene family usage and somatic hypermutation and affinity maturation. Based on these analyses, it is clear that antibody isotype and subclass was not restricted, but there was a much higher representation of lambda light chains (78%). In looking at gene family usage for the selected human mAb, our data displays comparable results to previous studies that explored Ig repertoires in NSG mice in comparison to humans (14). Though no single gene family was dominant, VH1 and VH3 families were most common, which is comparable to gene family usage in humans (15, 16). It is of interest that mAbs F860 A2e10 and F861 A7f10, despite being different isotypes, share the same gene family IgHV1–69*01, and yield more neutralizing ability and wider breath than members of other families respectively. In preceding studies comparing gene family usage in healthy versus HIV-1 infected individuals, an increase of VH1 gene families could be seen (15). Most of the light chain genes were relatively unchanged from germline sequences (>95% homology), there was more divergence from germline for heavy chain genes. Two antibodies were 85–90% homologous to germline, one was 94% homologous with the remaining five >95% homologous to germline. Broadly neutralizing antibodies to HIV tend to have high somatic mutation rates (17). However, here, the most effective antibody, F861A7f10 was not highly mutated. Classically, somatic mutation occurs predominantly in the CDR regions whereas for HIV, framework regions can also be found significantly mutated (18). Such extensive somatic mutation, especially of the framework regions, may not be necessarily critical for neutralizing activity (19, 20) but perhaps it is specific mutations that may only occur in the presence of high mutation activity that are critical (21). It can also be possible that further maturation of this antibody response would increase potency and breadth of neutralization with this particular (or other) antibody. While the sample size here was limited, these data would suggest that though it has been observed that most B-cells in BLT mice do not undergo selection processes in the germinal center (22), some B cells can be stimulated to undergo affinity maturation and somatic hypermutation.
Discussion
Although much has been learned in the field of HIV-1 research in regards to disease pathogenesis, there is still a need for a more effective prophylactic that will not only impede the spread of HIV-1, but will prevent infection from occurring. With infected individuals being capable of producing broadly neutralizing antibodies, treating HIV-1 using a prophylactic antibody is under active development (23–26). As we have shown here, antigen-specific, affinity-matured, and somatically mutated antibodies can be generated in the BLT mouse model, making it ideal for producing broadly neutralizing antibodies. Furthermore, given that the animals have human immune systems, the potential for countless immunization strategies that will ensure the production of millions of splenocytes against the exact target being examined is possible.
HIV-1 infection of the mice was monitored by viral load and human IgG and IgA antibody response using antigen-specific ELISA with gp120, gp41 or gp120/CD4 complex as antigens. Approximately 8–12 weeks post infection, spleens were harvested and splenocytes were fused with human fusion partner HMMA 2.5 to isolate antibody-expressing hybridomas. Interestingly, mouse 13.41 that was infected with JRFL, had survived up to 8 months at time of sacrifice. This is rare in that given the high level of augmentation the mice undergo, in combination with potential onset of GVHD, they do not normally survive for such extended periods of time (2, 3, 27). Lead clones were scaled and purified for testing in TZM-bl neutralization functional assays to determine neutralization and cytotoxic ability of the antibodies.
Previous studies using humanized mice for immunization with virus or antigen have generally demonstrated lower serum antibody titers characterized by mostly IgM antigen specific responses with lower IgG (14). In addition to serum reactivity, IgM mAb have been isolated from humanized mice (28). When the human IL-6 gene was knocked into the murine locus, there was an increase in serum antigen specific IgG with improved diversity (29). As shown in Figure 1, even without insertion of the human IL-6 gene, we observed a dramatic rise in antigen-specific antibody following infection. In addition, we were not limited to detecting an IgG response, but detected an IgA response as well. This finding is intriguing as IgA antibodies may be the first encountered at the mucosal surface upon infection. However, intriguingly, there is a limited IgA response to HIV-1 especially as compared to other mucosal infections (30–32). It should be noted that neutralizing activity of the mAbs was not necessarily restricted to the infected isolate. As shown in Table 5, antibody F861 A7f10 neutralized Tier 1 and Tier 2 strains, with an IC50 of 25.01 when being tested against BAL. The same antibody had an IC50 of <0.01 and an IC90 of <0.02 against JR-CSF and SF-162 respectively. Out of the sampling of antibodies tested that were reactive with gp120-CD4 FLSC, F861 A7f10 was the only IgG3 that had neutralizing ability across multiple clades. It has been shown that IgG3 antibodies typically appear before other IgG subclasses throughout the course of viral infection (33).
Antibody sequences were also determined. A robust, specific antibody response, of both IgG and IgA isotypes, occurred in response to HIV-1 infection. Over 70 hybridomas were created that were not only immunoreactive with env antigens, but some of which also had neutralization activity. Moreover, variable family usage was not limited and somatic mutation was evident indicating ongoing stimulation and proliferation of B cells in response to infection. Ultimately, by using this mouse model, not only were we able to generate large amounts of antigen specific antibodies, but it offered astounding variation of isotypes and gene family usage. Thus, in contrast to some other mouse models, such as the HuMab mouse, there are no limitations to the gene families that are available for use (14, 34).
In summary, we have demonstrated that human mAbs can be isolated from HIV-infected humanized mice. The antibodies were highly immunoreactive, functional, somatically mutated and represented diverse gene family usage as well as isotype selection. Further use of these animals, in HIV-1 as well as other disease areas, may lead to the isolation of additional diverse, functional human mAbs for active or passive immunotherapy.
1.
This work was supported in part by National Institutes of Health grants R01AI106478 to L.C; R01AI111809 and DP1DA034990 to J.L.; R24 OD018259 and NIDDK-supported Human Islet Research Network (HIRN, https://hirnetwork.org; UC4 DK104218 to MAB, DLG), R01 AI132963 (MAB), R01 DK1035486 (MAB) and CA34196 (LDS).
Abbreviations:
- BLT
immunodeficient mice engrafted with human fetal tissues (bone marrow, liver, thymus)
- FLSC
full-length single chain construct
- SCID-hu
immunodeficient mice engrafted with human fetal tissues (bone marrow, liver, thymus)
- hu-HSC
immunodeficient mice engrafted with human CD34+ hematopoietic stem cells
- Hu-SRC-SCID
immunodeficient mice engrafted with human CD34+ hematopoietic stem cells
- GVHD
graft-versus-host disease
- mAb
monoclonal antibody
References
- 1.WHO. 2016. Global health sector strategy on HIV, 2016–2021. WHO Document Production Services, Geneva, Switzerland. [Google Scholar]
- 2.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, and Shultz LD. 2017. Humanized Mouse Models of Clinical Disease. Annu Rev Pathol 12: 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shultz LD, Brehm MA, Garcia-Martinez JV, and Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12: 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Policicchio BB, Pandrea I, and Apetrei C. 2016. Animal Models for HIV Cure Research. Front Immunol 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, and Tager AM. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol 83: 7305–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akkina R 2013. New generation humanized mice for virus research: comparative aspects and future prospects. Virology 435: 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt K, Charlins P, Veselinovic M, Kinner-Bibeau L, Hu S, Curlin J, Remling-Mulder L, Olson KE, Aboellail T, and Akkina R. 2018. Zika viral infection and neutralizing human antibody response in a BLT humanized mouse model. Virology 515: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aryee KE, Shultz LD, and Brehm MA. 2014. Immunodeficient mouse model for human hematopoietic stem cell engraftment and immune system development. Methods Mol Biol 1185: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, and Brehm MA. 2013. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clinical and experimental immunology 174: 372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavacini LA, Emes CL, Wisnewski AV, Power J, Lewis G, Montefiori D, and Posner MR. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses 14: 1271–1280. [DOI] [PubMed] [Google Scholar]
- 11.Cavacini L, Duval M, Song L, Sangster R, Xiang SH, Sodroski J, and Posner M. 2003. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. Aids 17: 685–689. [DOI] [PubMed] [Google Scholar]
- 12.Fouts T, Godfrey K, Bobb K, Montefiori DC, Hanson CV, Kalyanaramen VS, DeVico A, and Pal R. 2002. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc Natl Acad Sci 99: 11842–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts TR, Tuskan R, Godfrey K, Reitz M, Hone D, Lewis GK, and DeVico AL. 2000. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol 74: 11427–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ippolito GC, Hoi KH, Reddy ST, Carroll SM, Ge X, Rogosch T, Zemlin M, Shultz LD, Ellington AD, Vandenberg CL, and Georgiou G. 2012. Antibody repertoires in humanized NOD-scid-IL2Rgamma(null) mice and human B cells reveals human-like diversification and tolerance checkpoints in the mouse. PLoS One 7: e35497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Wang XH, Banerjee S, Volsky B, Williams C, Virland D, Nadas A, Seaman MS, Chen X, Spearman P, Zolla-Pazner S, and Gorny MK. 2012. Different pattern of immunoglobulin gene usage by HIV-1 compared to non-HIV-1 antibodies derived from the same infected subject. PLoS One 7: e39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stollar BD 1995. The expressed heavy chain V gene repertoire of circulating B cells in normal adults. Ann N Y Acad Sci 764: 265–274. [DOI] [PubMed] [Google Scholar]
- 17.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, and Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458: 636–640. [DOI] [PubMed] [Google Scholar]
- 18.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, and Nussenzweig MC. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiev IS, Rudicell RS, Saunders KO, Shi W, Kirys T, McKee K, O’Dell S, Chuang GY, Yang ZY, Ofek G, Connors M, Mascola JR, Nabel GJ, and Kwong PD. 2014. Antibodies VRC01 and 10E8 neutralize HIV-1 with high breadth and potency even with Ig-framework regions substantially reverted to germline. J Immunol 192: 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sok D, Laserson U, Laserson J, Liu Y, Vigneault F, Julien JP, Briney B, Ramos A, Saye KF, Le K, Mahan A, Wang S, Kardar M, Yaari G, Walker LM, Simen BB, St John EP, Chan-Hui PY, Swiderek K, Kleinstein SH, Alter G, Seaman MS, Chakraborty AK, Koller D, Wilson IA, Church GM, Burton DR, and Poignard P. 2013. The effects of somatic hypermutation on neutralization and binding in the PGT121 family of broadly neutralizing HIV antibodies. PLoS Pathog 9: e1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiehe K, Bradley T, Meyerhoff RR, Hart C, Williams WB, Easterhoff D, Faison WJ, Kepler TB, Saunders KO, Alam SM, Bonsignori M, and Haynes BF. 2018. Functional Relevance of Improbable Antibody Mutations for HIV Broadly Neutralizing Antibody Development. Cell Host Microbe 23: 759–765 e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpel ME, Boutwell CL, and Allen TM. 2015. BLT humanized mice as a small animal model of HIV infection. Curr Opin Virol 13: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O’Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, and Chun TW. 2016. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 375: 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, and Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O’Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, and Team VRCS. 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Science translational medicine 7: 319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JC, Parrish EH, Learn GH, West AP, Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, and Nussenzweig MC. 2016. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science (New York, N.Y.) 352: 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavender KJ, Pace C, Sutter K, Messer RJ, Pouncey DL, Cummins NW, Natesampillai S, Zheng J, Goldsmith J, Widera M, Van Dis ES, Phillips K, Race B, Dittmer U, Kukolj G, and Hasenkrug KJ. 2018. An advanced BLT-humanized mouse model for extended HIV-1 cure studies. AIDS 32: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker PD, Legrand N, van Geelen CM, Noerder M, Huntington ND, Lim A, Yasuda E, Diehl SA, Scheeren FA, Ott M, Weijer K, Wedemeyer H, Di Santo JP, Beaumont T, Guzman CA, and Spits H. 2010. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Borsotti C, Schickel JN, Zhu S, Strowig T, Eynon EE, Frleta D, Gurer C, Murphy AJ, Yancopoulos GD, Meffre E, Manz MG, and Flavell RA. 2017. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood 129: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni V, and Ruprecht RM. 2017. Mucosal IgA Responses: Damaged in Established HIV Infection-Yet, Effective Weapon against HIV Transmission. Front Immunol 8: 1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nabi R, Moldoveanu Z, Wei Q, Golub ET, Durkin HG, Greenblatt RM, Herold BC, Nowicki MJ, Kassaye S, Cho MW, Pinter A, Landay AL, Mestecky J, and Kozlowski PA. 2017. Differences in serum IgA responses to HIV-1 gp41 in elite controllers compared to viral suppressors on highly active antiretroviral therapy. PLoS One 12: e0180245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TA, Murphy BR, Radl J, Haaijman JJ, and Mestecky J. 1985. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol 22: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidarsson G, Dekkers G, and Rispens T. 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz LD, Ishikawa F, and Greiner DL. 2007. Humanized mice in translational biomedical research. Nat Rev Immunol 7: 118–130. [DOI] [PubMed] [Google Scholar]


