Abstract
Objectives:
There is a growing need to understand the underlying mechanisms of age-related olfactory dysfunction with the increasing proportion of older adults over the next 20 years. Despite the importance of olfactory cleft (OC) volumes on odorant deposition and olfactory function, little is known about age-related changes OC volume. The goal of this study was to use automated techniques in a cross-sectional design to investigate the extent to which OC volumes vary with age and determine the spatial specificity of any age-related effects.
Methods:
Deformation-based morphometry was utilized to measure OC and sinus volumes in two independent samples of 101 (MUSC sample) and 95 (Hammersmith sample) healthy adults who underwent T1-weighted magnetic resonance imaging, with the Hammersmith sample serving as a replication sample.
Results:
The mean age of the MUSC and Hammersmith samples were 54.9 ± 17.0 years and 52.1 years ± 15.7 years, respectively. In both samples, there was a significant positive association between age and OC volume that occurred at a constant rate across the lifespan (Cohen’s f2 of 0.065 in the MUSC sample and 0.110 in the Hammersmith sample). Age-associated OC volume increases occurred in conjunction with decreases in sinus volumes as well as increases in non-OC nasal cavity volumes.
Conclusions:
In this cross-sectional study, there is an increase in OC volume with increasing age that occurs in the context of broad age-associated differences in sinonasal anatomy. Future studies should investigate the impact of age-associated differences in intranasal anatomy on nasal airflow, odorant deposition, and olfactory function.
Keywords: olfactory cleft, intranasal volume, aging, nasal cavity, deformation-based morphometry
Introduction
The word presbynasalis was termed to describe the cumulative histologic and structural changes that occur in the aging sinonasal tract.1 Symptoms associated with presbynasalis include dryness, nasal obstruction, and olfactory dysfunction, which is observed in 50% of adults >60 years of age.1–3 In recent years, both computed tomography (CT) and acoustic rhinometry studies have identified global increases in intranasal volumes among older adults which may contribute to this constellation of symptoms.4–6 Complaints of dryness and nasal obstruction may be related to alteration of intranasal heat and humidity exchange due to changing airflow patterns.6–9 Volumes of specific intranasal regions [olfactory cleft (OC), nasal valve] can also modulate olfactory function, likely through alterations in odorant deposition.10–13
Although age has been correlated with global intranasal volume differences, the underlying mechanisms driving this finding have not been rigorously defined. Atrophy of nasal and turbinate mucosa has been suggested as one mechanism for the age-related increase in intranasal volume.6, 14 Global intranasal volumes may also be influenced by age-related changes to the midfacial skeleton. Studies in the plastic surgery literature have documented both maxillary retrusion and pyriform aperture widening with increasing age.15, 16 There is also evidence of contraction of bony structures surrounding the intranasal space as sinus volumes have been found to decrease with age, potentially resulting in increased intranasal volumes through outward bowing of the nasal cavity’s bony walls.17–19 Depending on the underlying cause of the global increase in intranasal volume, age-related changes to specific regions may not be uniform throughout the intranasal space (i.e., differ based on proximity to turbinate tissue or the bony borders of the nasal cavity) and vary in their impact at different points across the lifespan.
Given the importance of OC anatomy in overall olfactory function, it is important to determine the extent to which there are locally specific aging effects on OC structure, and how these aging effects relate to broader sinonasal structural differences. Previous studies investigating sinonasal anatomy have largely utilized manual measurements on computed tomography (CT) or magnetic resonance imaging (MRI) images.11, 17, 19 Disadvantages of manual image segmentation include labor-intensive measurements that can have limited intra- and inter-rater reliability. Deformation-based morphometry (DBM) is a computational imaging analysis technique that can provide reliable analysis of volumetric changes within the OC and across voxels representing the intranasal space for a large number of images.20 Although it is most commonly employed in the study of neuroanatomic structures, DBM can be implemented extracranially, and has previously been used in the study of craniofacial morphology.21 The goal of the present study was to use a cross-sectional design to investigate age-related differences in OC volumes and determine their local specificity using DBM.
Methods
Subjects
The study of retrospective data included 105 subjects (56.2% female, mean age = 54.9 years, standard deviation (SD) = 17.0 years) who were recruited for an Institutional Review Board approved study of presbyacusis at the Medical University of South Carolina (MUSC). Exclusion criteria included history of head trauma, central nervous system (CNS) acting medications, self-reported CNS disorders, and gross CNS anatomical abnormalities on MRI. A second sample of subjects from the Hammersmith open access database was included to replicate findings from the MUSC sample. This dataset consisted of 182 healthy subjects who underwent imaging at Hammersmith Hospital (http://brain-development.org/ixi-dataset/). Images from the two samples were reviewed and excluded for evidence of prior sinonasal surgery, or significant dental artifact (n = 0 MUSC sample, n = 8 Hammersmith sample).
A subset of Hammersmith subjects were age- (± 5 years) and sex-matched to the 105 MUSC subjects using SPSS 24 (IBM SPSS Inc, Armonk, NY). 98 subjects in the Hammersmith dataset were matched to the MUSC sample (57.1% female, mean age = 52.1 years, SD = 15.7 years), with 7 MUSC sample subjects not having a suitable match. There were no significant differences in age or sex ratio between the matched MUSC and Hammersmith samples (ps > 0.20).
Imaging Protocols
A Siemens Tim Trio 3T scanner was used at MUSC to obtain T1-weighted images with the following parameters: 160 slices, 256 × 256 matrix, repetition time (TR) = 8.13 msec, echo time (TE) = 3.7 msec, 8-degree flip angle, 1.0 mm slice thickness, and zero slice gap. A Philips 3T scanner as used at Hammersmith Hospital to collect T1-weighted images with the following parameters: 208 × 208 matrix, TR = 9.60 msec, TE = 4.60 msec, and 8-degree flip angle. A subset of images exhibited motion artifact and were identified and excluded based on a structural covariance method which quantified how dissimilar an image was from every other image in the sample (> 2 SD; n = 4 MUSC sample; n = 3 Hammersmith sample).22, 23
Imaging Pre-Processing
The T1-weighted images were rigidly aligned and segmentation was performed in SPM12 (Wellcome Department of Imaging Neuroscience Group, London, UK) to estimate the gray matter, white matter, CSF, skull, and dura/soft tissue probability at each voxel of the individual images.24 The volumes of the segmented gray matter, white matter, CSF, and skull images were summed to obtain an estimate for the total intracranial volume (TICV). The dura/soft tissue images were then added to the TICV for a volume estimate of the overall head size of each subject. The SPM12 diffeomorphic (DARTEL) normalization procedure was then used to compute the DARTEL deformation fields to spatially transform the native space segmented images into the sample-specific anatomical templates.25 Jacobian determinant (JD) images were then calculated using the deformation fields to represent the volumetric displacement required to deform an individual subject’s image to the template.
Regions of Interest & DBM Analyses
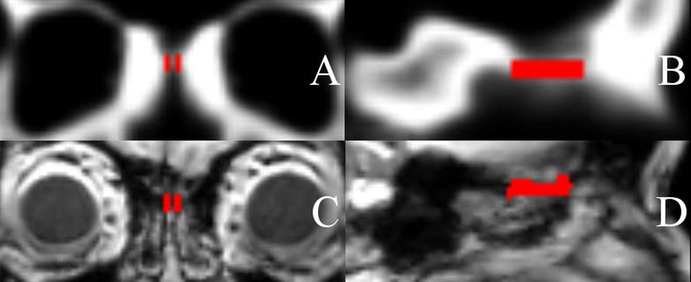
An OC region of interest (ROI) was defined on the sample-specific templates using MRIcron software as a 3-dimensional (3D) space directly inferior to the cribiform plate.26 The ROI was bounded posteriorly by the thickening of the skull base at the junction of the cribiform plate and planum, and anteriorly by a plane through the junction of the anterior edge of the crista galli and posterior wall of the frontal sinus. The cleft space was defined to extend inferiorly such that it would encompass approximately the upper 25% of the middle turbinate height at its anterior aspect. Medially the space was designed to be bound by the septum and laterally by the middle turbinate. The ROI was outlined on the template images from each site to generate a roughly rectangular space (Figure 1A-B). In Figure 1C-D an OC ROI has been inverse-warped from a template image into native space T1-weighted images to show the alignment of the ROI with the OC region.
Figure 1.
(A) Coronal and (B) sagittal images of an olfactory cleft region of interest drawn in the sample-specific template space. (C) Coronal and (D) sagittal images of an olfactory cleft region of interest inverse-warped into subject T1-weighted images demonstrating the alignment between the template space olfactory cleft and the olfactory clefts of the individual subjects.
A sinus region of interest (ROI) was defined on the sample-specific templates as a control region to verify that DBM was sensitive to volumetric changes around the nasal cavity. Sinus volumes are highly correlated with one another, and unlike the intranasal space, they have been shown to be negatively correlated with age.17, 18 Age-related differences in sinus volumes could serve as a marker of bony contraction around the intranasal space, and were examined as an explanation for age-related OC volume differences. The sinus ROI encompassed the bilateral sphenoid, ethmoid, and frontal sinuses (the maxillary sinus was incompletely imaged on some scans and was excluded).
Mean values of the sinus and OC ROI’s were extracted from the individual subject JD images as measures of OC and sinus volumes. The mean JD for an individual ROI represents the volumetric displacement required to deform the individual ROI to the template ROI (e.g., an individual OC with a mean JD of 1.25 corresponds to an OC volume 25% larger than the average OC volume in the sample). Hierarchical regression was then performed with SPSS 24 (IBM Corp.) to determine the association between age and sinus volume with sex and the head size estimate serving as covariates. A second regression was then performed to evaluate the association between age and OC volume with sex, head size, and paranasal sinus volume serving as covariates. Analyses were repeated with TICV as a covariate in place of the head size estimate and yielded similar age effects. Cohen’s f2 was computed to assess the local effect size of age within the multivariate models.27
Voxel-based DBM regression analyses were performed to examine age-related volumetric differences at each voxel across the paranasal sinuses and nasal cavity with sex and head size as covariates. The entire nasal cavity including all air and soft tissue from the nasal vestibule to the posterior edge of the inferior turbinate at the choanae was included in the analysis as the variation in intranasal anatomy and resolution of the images prohibited a clear delineation of intranasal airspace and soft tissue. A Bonferroni corrected threshold (p < 0.05) with a cluster size of 25 voxels was used to define significant effects outside of the a priori ROI with results reported as peak T-scores and cluster sizes (voxels).
Results
Age Effects on the Olfactory Cleft
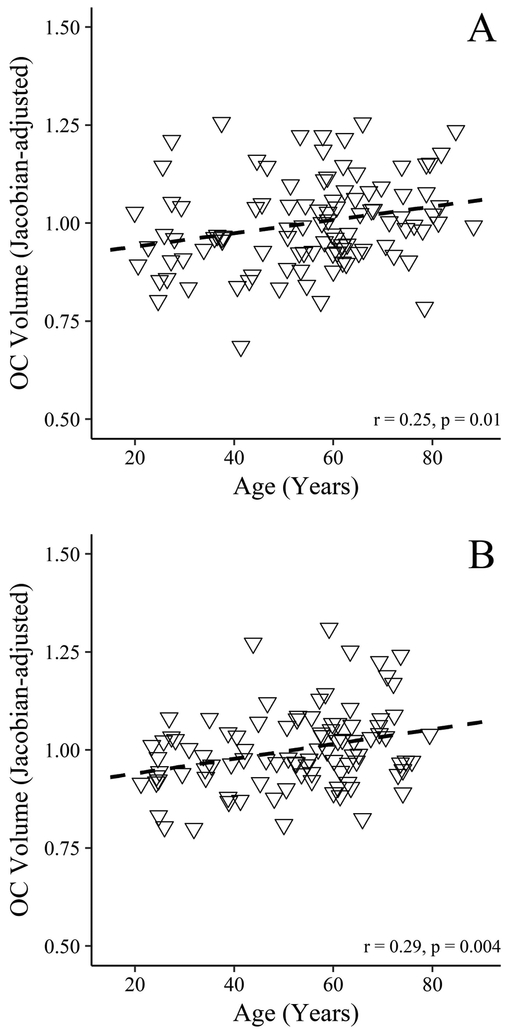
Hierarchical regression demonstrated that age explained a significant amount of unique variance in OC volume in both samples (p = 0.008 and 0.001 for the MUSC and Hammersmith samples, respectively) with a modest overall effect (Cohen’s f2 = 0.065 and 0.110 for the MUSC and Hammersmith samples, respectively) (Table 1). In both samples, OC volume increased each year by 0.2% (95% Confidence Interval [CI], 0–0.3% in the MUSC sample and 0.1–0.4% in the Hammersmith sample). Figure 2 displays plots of the OC volume versus age for the two samples after controlling for sex, head size, and sinus volume. Both samples demonstrated a progressive linear increase in OC volume that appears constant across the adult lifespan.
Table 1.
Hierarchical Linear Regression of Olfactory Cleft Volume (Expressed as the Mean Jacobian Determinant)
| Level 1 (R2 = 0.279, p < 0.001) |
Level 2 (R2 = 0.323, p < 0.001)* |
|||||
|---|---|---|---|---|---|---|
| B | 95% CI | P | B | 95% CI | P | |
| MUSC Sample* | ||||||
| Female Sex | −0.030 | [−0.088, 0.029] | 0.313 | −0.033 | [−0.090, 0.023] | 0.244 |
| Head Size Estimate | 0.221 | [0.133, 0.309] | <0.001 | 0.197 | [0.110, 0.284] | <0.001 |
| Sinus Volume | −0.012 | [−0.085, 0.061] | 0.745 | 0.009 | [−0.063, 0.081] | 0.802 |
| Age (years) | 0.002 | [0.000, 0.003] | 0.008 | |||
| Level 1 (R2 = 0.424, p < 0.001) |
Level 2 (R2 = 0.481, p < 0.001)* |
|||||
|---|---|---|---|---|---|---|
| B | 95% CI | P | B | 95% CI | P | |
| Hammersmith Sample* | ||||||
| Female Sex | −0.036 | [−0.095, 0.023] | 0.225 | −0.020 | [−0.076, 0.037] | 0.488 |
| Head Size Estimate | 0.278 | [0.147, 0.409] | <0.001 | 0.299 | [0.173, 0.424] | <0.001 |
| Sinus Volume | 0.067 | [−0.018, 0.151] | 0.120 | 0.112 | [0.035, 0.208] | 0.007 |
| Age (years) | 0.002 | [0.001, 0.004] | 0.001 | |||
Abbreviations: B, Regression Coefficients; CI, Confidence Interval;
P for ΔR2 < 0.01
Figure 2.
Scatterplots for the (A) MUSC and (B) Hammersmith samples with associations between olfactory cleft volumes and age (expressed as the mean Jacobian determinants after adjusting for the effects of sex, sinus volume, and the head size estimate). The individual olfactory cleft volumes were scaled to demonstrate their relationship to the overall sample mean (centered at 1).
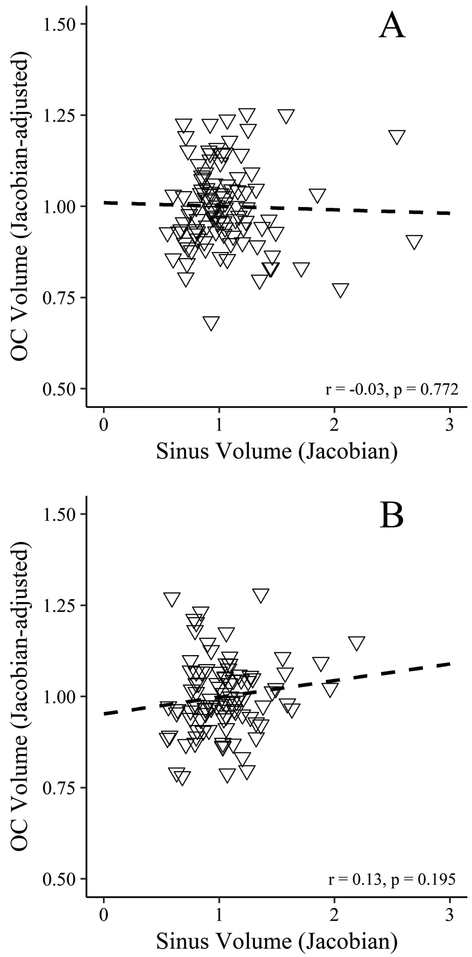
The effects described above were significant when head size was included in the regression models, despite significant effects of head size on OC volume. Overall head size was positive correlated with OC volume in both samples, with male head sizes being 9.9% larger than females in the MUSC sample and 10.4% larger than females in the Hammersmith sample. After adjusting for overall head size, there were no significant differences in female versus male OC volumes in the two samples. Sinus volume was also not correlated with OC volume after adjusting for gender and head size (p = 0.772 and 0.195 for the MUSC and Hammersmith samples, respectively) (Figure 3).
Figure 3.
Scatterplots for the (A) MUSC and (B) Hammersmith samples with associations between olfactory cleft volumes (expressed as the mean Jacobian determinants after adjusting for the effects of sex and the head size estimate) and sinus volumes. The individual olfactory cleft volumes were scaled to demonstrate their relationship to the overall sample mean (centered at 1).
Age Effects on the Sinuses
Hierarchical regression revealed that age explained a significant amount of unique variance in the sinus volumes of both samples (p = 0.033 and < 0.001 for the MUSC and Hammersmith samples, respectively) after accounting for sex and head size. In the MUSC sample, sinus volume decreased each year by 0.4%, (95% CI, 0–0.9%) and in the Hammersmith sample, sinus volume decreased by 0.6% (95% CI, 0.3–0.9%) each year (Cohen’s f2 = 0.038 and 0.147 for the MUSC and Hammersmith samples, respectively). In the final model, female sex was correlated with decreased sinus volumes in both samples with sinuses from female subjects being 29.2% (95% CI, 14.5–43.8%) smaller than males in the MUSC sample and 15.6% (95% CI, 2.4–28.8%) smaller than males in the Hammersmith sample.
Voxel-based DBM Analyses
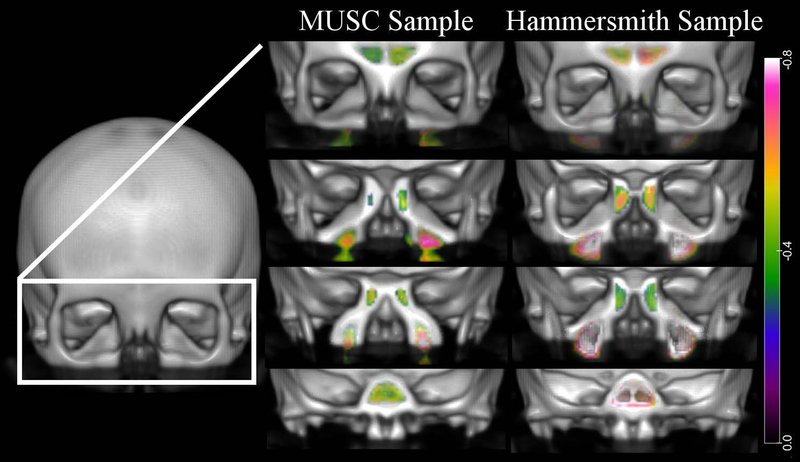
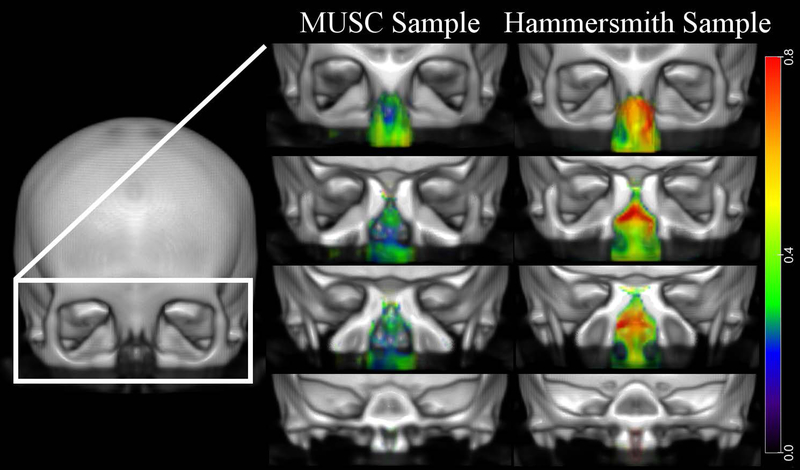
The MUSC sample exhibited no sinus or nasal cavity regions with significant age-related differences in volume in the voxel-based DBM analysis after multiple comparison correction. In the Hammersmith sample, the sphenoid sinus (Peak T-score 6.22, Cluster size 341) and bilateral maxillary sinuses (Left- Peak T-score 6.52, Cluster size 809; Right- Peak T-score 6.99, Cluster size 1069) had regions with significant age-associated decreases in volume. The posterior midline nasal cavity at the level of the choanae (Peak T-score 5.96, Cluster size 179) and the left lateral nasal cavity (Peak T-score 4.88, Cluster Size 96) had age-associated increases in volume. Figure 4 and 5 visually demonstrate the regions of the nasal cavity with age-related volumetric differences at an uncorrected threshold (absolute Cohen’s d = 0.2). The maxillary, sphenoid, ethmoid, and frontal sinuses of both samples display age-related decreases in volume with increases in volume seen in the region of the OC as well as throughout the nasal cavity. These volumetric differences were modest and had a larger effect size in the Hammersmith sample consistent with no effects surviving Bonferonni correction in the MUSC sample.
Figure 4.
Voxel-based DBM results: Age-related contraction. Regions across the intranasal cavity and paranasal sinuses with age-associated volumetric contraction are colored according to the magnitude of the effects (Cohen’s d) as shown in the color bar on the far right. The left column displays results for the MUSC sample and the right column displays results for the Hammersmith sample. Results were thresholded at an absolute Cohen’s d = 0.2 (small effect size).
Figure 5.
Voxel-based DBM results: Age-related expansion. Regions across the intranasal cavity and paranasal sinuses with age-associated volumetric expansion are colored according to the magnitude of the effects (Cohen’s d) as shown in the color bar on the far right. The left column displays results for the MUSC sample and the right column displays results for the Hammersmith sample. Results were thresholded at an absolute Cohen’s d = 0.2 (small effect size).
Discussion
The results demonstrate significant age-related differences in OC volumes in two independent samples across a broad age range. We observed a progressive linear increase in OC volumes which appeared to occur at a constant rate across the lifespan for this cross-sectional study. Our voxel-based analyses demonstrated that aging effects were not specific to the OC and occurred with modest age-related differences in nasal cavity volumes, with statistically unique effects in the paranasal sinuses. Together, these results highlight the presence of broad age-associated variations in sinonasal anatomy, which may have important implications for nasal airflow, humidification, and olfactory function.
There was previously no data regarding the extent to which there are local aging effects on the OC. We found that increasing age was associated with a 0.2% increase in OC volume for each year of life in two independent samples, corresponding to an overall small effect size. This result is similar to age-associated effects reported in previous studies of the entire intranasal space.4–6 In a CT study of 66 patients, Loftus et al. found that for two cohorts with a mean age difference of 59.7 years, the older cohort’s global intranasal volume was 11% larger in females and 20% larger in males.5 Lindemann et al. also reported age-associated differences in intranasal volumes measured with acoustic rhinometry in 40 older and 40 younger adults. Using a measurement of the intranasal volume between 20–50 mm from the nasal entrance, the older adult cohort was found to have a 20% larger intranasal space.6 Evaluating the volumetric differences reported by Loftus et al. and Lindemann et al. on a per year basis, the magnitude of volume change observed in their studies is similar to our OC-specific results. The voxel-based analyses demonstrated that these OC volume differences occurred in conjunction with modest volumetric expansion throughout much of the nasal cavity. The fact that only the Hammersmith sample had regions achieving statistical significance was not surprising given the modest effects we observed were similar to those reported in studies of global intranasal volumes.4–6
One purported mechanism for the age-associated increase in intranasal volumes is mucosal atrophy of the nasal and turbinate mucosa.5, 6, 14 To examine the association between age and nasal mucosa histology, Schrӧdter et al. obtained biopsies of middle turbinate tissue from subjects age 5–75. They observed that ciliated epithelium decreases with age, and that atrophic epithelium initially begins to be observed in patients at 40 years of age.14 Interestingly, we observed a constant linear increase in OC volume beginning in subjects as young as age 18. Our findings add to a previous report which showed non-significant increases in intranasal volumes in adults <40 years of age.5 At this time, it is unclear the extent to which more minor epithelial changes could be occurring in younger adults, or if increases in intranasal volumes in this cohort reflect a different etiology. Changes in the sympathetic/parasympathetic tone to the nasal vasculature could influence nasal soft tissue and intranasal volume without a histologic change to the mucosa. Previous work has suggested that nasal cycle patterns decrease with age, which may reflect changes in autonomic innervation to the nasal cavity.28, 29 Unfortunately, we were unable to define a measure of soft tissue volume around the OC to investigate its correlation with the age-related volumetric changes. Future studies using high-resolution sinus CT scans could quantify the volume of soft tissue surrounding the OC and assess its association with OC volumes.
The nasal cavity volume may also be impacted by age-related changes to the midfacial skeleton. Cross-sectional and cohort studies have documented age-related decreases in midfacial mineral bone density as well as pyriform aperture widening and volumetric contraction of the paranasal sinuses.15–18 For this reason, we investigated the extent to which paranasal sinus volume contraction was associated with the age-related expansion of the OC (i.e., was the OC expanding near the ethmoid and sphenoid as these structures contracted). Though our ROI and voxel-based analyses demonstrated contraction of the paranasal sinuses, we did not observe a negative correlation between sinus and OC volumes. Therefore, age-related OC volumetric change appears more likely due to mucosal atrophy or another mechanism and not due to bony changes in the surrounding sinuses. Further research is needed on the potential relationship between the aging midfacial skeleton and changes within the nasal cavity. Maxillary and ethmoid sinus contraction may influence the volumes of other intranasal regions (middle meatus, etc.), or be correlated with age-related orbital expansion.30 There is precedent from studies following endoscopic sinus surgery that expansion of the orbit (via medial bowing of the lamina) can decrease ethmoid volumes.31, 32
Although we observed relatively small increases in OC volume across the lifespan (0.2% per year), even modest differences may significantly influence odorant deposition. Zhao et al. found that an approximately 4-fold change in OC width could increase OC airflow 50-fold.13 Despite the intuitive link between OC volume and OC airflow, there is a growing body of computational fluid dynamics literature demonstrating the complexity of intranasal airflow patterns and their interdependence on the anatomy throughout the nasal cavity.13, 33–35 Anatomic changes in regions distant from the OC can modulate odorant deposition, potentially outweighing the impact of local anatomic changes, and highlighting the importance of understanding the cumulative impact of age-related effects throughout the nasal cavity.13, 34 In addition, changes in intranasal airflow patterns due to airspace volume increases can impair intranasal heat and humidity exchange which may lead to symptoms of nasal obstruction and rhinorrhea.6–9 The implications of age-related changes in intranasal humidification and temperature to mucus viscosity and odorant transport at the olfactory cleft is unknown. Therefore, further study is needed regarding the cumulative impact of age-related intranasal airspace changes on nasal airflow, local mucosal conditions, and odorant deposition and transport at the OC.
This study has several important limitations. Although both study cohorts consisted of healthy adults, pertinent rhinologic comorbidities including allergies and chronic rhinosinusitis were unknown. The use of nasal steroids or other medications, which can influence measures of intranasal volumes in patients with rhinitis, was also unknown.36 The variability of intranasal anatomy and the resolution of the MR images did not allow for clear definition of the soft tissue and air boundaries on the template images. Therefore, we included the entire nasal cavity in our voxel-based analyses. Implementation of similar computational analysis techniques with high-resolution sinus CT scans could facilitate a more focused study of regional differences in intranasal airspace and soft tissue volumes. The cross-sectional design limited us to making observations of age-related anatomic differences across subjects, and did not provide data on how sinonasal anatomy changes with age in individual subjects. Future well-controlled prospective studies could confirm our cross-sectional observations and provide subject-level data on the role of age in sinonasal anatomic change.
Conclusions:
There is an increase in OC volume with increasing age that occurs in the context of broad age-associated differences in sinonasal anatomy. Future studies should investigate the impact of age-related differences in intranasal anatomy on nasal airflow, odorant deposition, and olfactory function.
Acknowledgments
Funding and Conflicts of Interest: This work was supported by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (P50 DC00422).
Footnotes
Meeting Information: N/A
References:
- 1.DelGaudio JM, Panella NJ. Presbynasalis. Int Forum Allergy Rhinol. 2016;6(10):1083–1087. [DOI] [PubMed] [Google Scholar]
- 2.Duffy VB, Backstrand JR, Ferris AM. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J Am Diet Assoc. 1995;95(8):879–884; quiz 885–876. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 4.Kalmovich LM, Elad D, Zaretsky U, et al. Endonasal geometry changes in elderly people: acoustic rhinometry measurements. J Gerontol A Biol Sci Med Sci. 2005;60(3):396–398. [DOI] [PubMed] [Google Scholar]
- 5.Loftus PA, Wise SK, Nieto D, Panella N, Aiken A, DelGaudio JM. Intranasal volume increases with age: Computed tomography volumetric analysis in adults. Laryngoscope. 2016;126(10):2212–2215. [DOI] [PubMed] [Google Scholar]
- 6.Lindemann J, Sannwald D, Wiesmiller K. Age-related changes in intranasal air conditioning in the elderly. Laryngoscope. 2008;118(8):1472–1475. [DOI] [PubMed] [Google Scholar]
- 7.Lindemann J, Tsakiropoulou E, Keck T, Leiacker R, Wiesmiller KM. Nasal air conditioning in relation to acoustic rhinometry values. Am J Rhinol Allergy. 2009;23(6):575–577. [DOI] [PubMed] [Google Scholar]
- 8.Lindemann J, Brambs HJ, Keck T, Wiesmiller KM, Rettinger G, Pless D. Numerical simulation of intranasal airflow after radical sinus surgery. Am J Otolaryngol. 2005;26(3):175–180. [DOI] [PubMed] [Google Scholar]
- 9.Lindemann J, Keck T, Wiesmiller KM, Rettinger G, Brambs HJ, Pless D. Numerical simulation of intranasal air flow and temperature after resection of the turbinates. Rhinology. 2005;43(1):24–28. [PubMed] [Google Scholar]
- 10.Jun BC, Song SW, Kim BG, et al. A comparative analysis of intranasal volume and olfactory function using a three-dimensional reconstruction of paranasal sinus computed tomography, with a focus on the airway around the turbinates. Eur Arch Otorhinolaryngol. 2010;267(9):1389–1395. [DOI] [PubMed] [Google Scholar]
- 11.Damm M, Vent J, Schmidt M, et al. Intranasal volume and olfactory function. Chem Senses. 2002;27(9):831–839. [DOI] [PubMed] [Google Scholar]
- 12.Hornung DE, Leopold DA. Relationship between uninasal anatomy and uninasal olfactory ability. Arch Otolaryngol Head Neck Surg. 1999;125(1):53–58. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chem Senses. 2004;29(5):365–379. [DOI] [PubMed] [Google Scholar]
- 14.Schrodter S, Biermann E, Halata Z. Histological evaluation of age-related changes in human respiratory mucosa of the middle turbinate. Anat Embryol (Berl). 2003;207(1):19–27. [DOI] [PubMed] [Google Scholar]
- 15.Paskhover B, Durand D, Kamen E, Gordon NA. Patterns of Change in Facial Skeletal Aging. JAMA Facial Plast Surg. 2017;19(5):413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw RB Jr., Kahn DM Aging of the midface bony elements: a three-dimensional computed tomographic study. Plast Reconstr Surg. 2007;119(2):675–681; discussion 682–673. [DOI] [PubMed] [Google Scholar]
- 17.Yonetsu K, Watanabe M, Nakamura T. Age-related expansion and reduction in aeration of the sphenoid sinus: volume assessment by helical CT scanning. AJNR Am J Neuroradiol. 2000;21(1):179–182. [PMC free article] [PubMed] [Google Scholar]
- 18.Emirzeoglu M, Sahin B, Bilgic S, Celebi M, Uzun A. Volumetric evaluation of the paranasal sinuses in normal subjects using computer tomography images: a stereological study. Auris Nasus Larynx. 2007;34(2):191–195. [DOI] [PubMed] [Google Scholar]
- 19.Ariji Y, Kuroki T, Moriguchi S, Ariji E, Kanda S. Age changes in the volume of the human maxillary sinus: a study using computed tomography. Dentomaxillofac Radiol. 1994;23(3):163–168. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner J, Hutton C, Frackowiak R, Johnsrude I, Price C, Friston K. Identifying global anatomical differences: deformation-based morphometry. Hum Brain Mapp. 1998;6(5–6):348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarty MM, Aleong R, Leonard G, et al. Automated analysis of craniofacial morphology using magnetic resonance images. PLoS One. 2011;6(5):e20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert MA, Berninger VW, Hoeft F, Vaden KI Jr. A case of Bilateral Perisylvian Syndrome with reading disability. Cortex. 2016;76:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VBM8 Toolbox Manual [computer program]. Version. Jena: University of Jena; 2010. [Google Scholar]
- 24.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 26.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. [DOI] [PubMed] [Google Scholar]
- 27.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A Practical Guide to Calculating Cohen’s f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol. 2012;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirza N, Kroger H, Doty RL. Influence of age on the ‘nasal cycle’. Laryngoscope. 1997;107(1):62–66. [DOI] [PubMed] [Google Scholar]
- 29.Williams MR, Eccles R. The nasal cycle and age. Acta Otolaryngol. August 2015;135(8):831–834. [DOI] [PubMed] [Google Scholar]
- 30.Masters M, Bruner E, Queer S, Traynor S, Senjem J. Analysis of the volumetric relationship among human ocular, orbital and fronto-occipital cortical morphology. J Anat. 2015;227(4):460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt MP, Cunnane ME, Curtin HD, Metson R. Anatomical changes of the ethmoid cavity after endoscopic sinus surgery. Laryngoscope. 2008;118(12):2240–2244. [DOI] [PubMed] [Google Scholar]
- 32.Cunnane ME, Platt M, Caruso PA, Metson R, Curtin HD. Medialization of the lamina papyracea after endoscopic ethmoidectomy: comparison of preprocedure and postprocedure computed tomographic scans. J Comput Assist Tomogr. 2009;33(1):79–81. [DOI] [PubMed] [Google Scholar]
- 33.Zhao K, Jiang J. What is normal nasal airflow? A computational study of 22 healthy adults. Int Forum Allergy Rhinol. 2014;4(6):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Jiang J, Kim K, et al. Nasal Structural and Aerodynamic Features that may Benefit Normal Olfactory Sensitivity. Chem Senses. 2018;43(4):229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey KP, Borojeni AA, Koenig LJ, Rhee JS, Garcia GJ. Correlation between Subjective Nasal Patency and Intranasal Airflow Distribution. Otolaryngol Head Neck Surg. 2017;156(4):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan KO, Huang ZL, Wang DY. Acoustic rhinometric assessment of nasal obstruction after treatment with fluticasone propionate in patients with perennial rhinitis. Auris Nasus Larynx. 2003;30(4):379–383. [DOI] [PubMed] [Google Scholar]