Abstract
Juvenile-onset fibromyalgia (JFM) is typically diagnosed in adolescence and characterized by widespread pain and marked functional impairment. The long-term impact of JFM into adulthood is poorly understood. The objectives of this study were to describe physical and psychosocial outcomes of youth diagnosed with JFM in early adulthood (~ 8-year follow-up), examine longitudinal trajectories of pain and depressive symptoms from adolescence to young adulthood, and examine the impact of pain and depressive symptoms on physical functioning over time. Participants were 97 youth with JFM enrolled in a prospective longitudinal study in which pain symptoms, physical and psychosocial functioning were assessed at four time points over approximately eight years. At the Time 4 follow-up (Mage = 24.2 years), the majority continued to suffer from pain and impairment in physical, social, and psychological domains. However, trajectories of pain and emotional symptoms showed varying patterns. Longitudinal analysis using growth mixture modeling revealed two pain trajectories (Steady Improvement and Rapid Rebounding Improvement); whereas depressive symptoms followed three distinct trajectories (Low-Stable, Improving, and Worsening). Membership in the Worsening Depressive symptoms group was associated with poorer physical functioning over time (p < .001) compared to the Low-Stable and Improving groups. This study offers evidence that while JFM symptoms persist for most individuals, pain severity tends to decrease over time. However, depressive symptoms follow distinct trajectories that indicate subgroups of JFM. In particular, JFM patients with worsening depressive symptoms showed decreasing physical functioning and may require more intensive and consistent intervention to prevent long-term disability.
Keywords: juvenile fibromyalgia, pediatric pain, longitudinal study, trajectory analysis, subgroups, physical function, depressive symptoms
Summary:
Longitudinal trajectory analyses showed decreasing pain over time in juvenile-onset fibromyalgia. A worsening depressive symptom trajectory was associated with the poorest outcomes in adulthood.
Juvenile-onset Fibromyalgia (JFM) is a chronic pain condition characterized by widespread musculoskeletal pain, pain sensitivity (hyperalgesia), mood difficulties [16; 17; 29; 42; 52] and impaired physical functioning. [30; 46; 49] Studies have examined outcomes of youth with JFM over time, with follow-up ranging from 1 to 6 years, [7; 29; 41; 42; 48] but no research to date has examined long-term outcomes of these adolescents as they transition into adulthood. Examining how pain and psychological symptoms unfold through the crucial developmental years of adolescence into early adulthood could provide important information about the long-term impact of JFM, help identify those at greatest risk for continued impairment, and provide new insights into the origins of subgroups of fibromyalgia (FM) that have been described in the adult literature.[18; 38; 54; 59]
Prior studies of adult outcomes of youth with functional abdominal pain have linked higher pain intensity and poorer emotional functioning in adolescence to poor outcomes in adulthood.[43; 55] Three patterns of pain, disability and psychological functioning (high pain dysfunctional, high pain adaptive, and low pain adaptive[55]) were identified, with long-term risk for pain and disability being associated with poorer emotional functioning.[43] The subgroups described for adults with childhood-onset abdominal pain show similarities to subgroups described in adult FM studies, suggesting subtypes characterized as having a greater nociceptive element versus having both a nociceptive and affective component, with the latter showing the greatest impairment.[6; 18] Little is known about FM subtypes development, particularly during the critical transition period from adolescence to adulthood. A recent prospective study examined the impact of early depressive symptoms on trajectories of musculoskeletal pain in a cohort of Swedish youth from adolescence to adulthood.[35] Baseline depressive symptoms strongly predicted trajectory membership (i.e., high-stable pain or increasing pain) into adulthood. Examination of the trajectories of both pain and depressive symptoms from adolescence to adulthood in JFM may elucidate how these trajectories contribute to physical impairment in adulthood.
Cross-sectional studies have shown that youth with JFM had persisting pain and significantly greater physical and psychological impairment than healthy controls (HC) in adolescence, late adolescence and emerging adulthood [25; 27–29] and approximately half (51.1%) eventually met criteria for adult FM.[58] Comorbid mood disorders were associated with greater physical impairment at all time points,[11] however these relationships were not examined longitudinally.
This study aimed to: 1) describe physical and psychosocial outcomes of JFM participants after the transition to young adulthood, 2) examine trajectories of pain intensity and depressive symptoms, and 3) examine the longitudinal impact of pain and depressive symptom trajectories on physical functioning. Consistent with findings for this cohort in late adolescence and emerging adulthood [25; 29], it was expected youth with JFM would exhibit continued pain and impairment in young adulthood. Based on previous studies on pain and mood in FM [11; 14; 19], we predicted at least two distinct trajectories of pain and depressive symptoms reflecting worsening or improving symptoms. It was hypothesized that worsening pain and depressive symptom trajectories would be significantly associated with increasing physical impairment.
Methods
Participants
The original cohort for this study (Time 1: Mage = 15.9 years; SD = 1.6; N=159) consisted of 116 adolescents diagnosed with JFM recruited from a pediatric rheumatology clinic at a Midwestern children’s hospital. Characteristics of the sample have been previously published [25] but briefly, inclusion criteria at initial enrollment were: 1) ages 12–18 years, 2) diagnosis of primary JFM according to Yunus and Masi criteria, [60] and 3) no underlying rheumatologic conditions (e.g. systemic lupus erythematosus, juvenile idiopathic arthritis). Of the initial cohort, 93.1% were retained and assessed at Time 2 (N = 108; Mage = 19.3 years; SD = 2.4, 81.9% at Time 3 (N = 95; Mage = 22.0 years; SD = 2.0) and 74.1% at Time 4 (N = 86; Mage = 24.2 years; SD = 2.2). The initial (Time 1) assessments were conducted between 2001–2006, Time 2 assessments were conducted between 2007–2009, Time 3 between 2010–2013, and Time 4 between 2012–2015. Cross sectional findings on physical and psychosocial functioning for this cohort at Time 2 and Time 3 have been published previously. [25; 29]
Criteria for inclusion in the final (Time 4) assessment were 1) at least 2 years elapsed since their prior assessment and 2) at least 21 years of age. Retention in this long-term study was maximized by maintaining multiple contacts (e.g., family members, close friends) and sending an annual newsletter to enhance engagement. Additionally, assessment burden was minimized (use of web-based and in-home surveys) and modest compensation was provided (gift cards). These procedures were approved by the Children’s Hospital Institutional Review Board and conducted in accordance with current ethical standards for human subject research.
Procedure
At each follow-up time point (Time 2, 3 and 4), participants were contacted by phone to obtain verbal consent and if they agreed, signed informed consent was obtained by mail. Participants received a unique login and password to access a secure website to complete self-report study questionnaires assessing recent symptoms of pain, FM, anxiety and depression, physical and psychosocial functioning, medications and health care visits. Once they completed the questionnaires, a home visit was scheduled at a time convenient for participants. The in-person component of the assessment was conducted by a trained assessor (post-doctoral fellow or senior research coordinator) and included a semi-structured psychiatric interview and confirmation of FM symptoms using the American College of Rheumatology criteria [58] (for adults) and performing a tender point exam. Assessors were trained by a board-certified psychiatrist [LMA] and licensed psychologist [SKZ] in conducting psychiatric interviews and a board-certified pediatric rheumatologist [TVT] in administering the tender point exam.
Objective 1. Outcomes at 8-year follow-up (Time 4)
Background and Demographics.
Information about marital status, education and occupation was obtained from all participants, as well as current living situation and primary sources of financial support.
Fibromyalgia classification based on American College of Rheumatology (ACR) 2010 Criteria.
Participants were asked to complete the Widespread Pain Index (WPI) and Symptom Severity Scale (SS), which has been validated in adults with FM [58] and was adapted for self-report use (with confirmation by trained examiners) in this study. On the WPI, participants were asked to identify up to 19 body areas in which they felt pain over the past week (WPI range 0–19). The SS scale assesses 1) cardinal symptoms (fatigue, not feeling rested upon waking, and cognitive disturbances) for which the severity of each symptom is rated on a 4-point Likert scale (range 0–9) and 2) other somatic symptoms (e.g., dizziness, nausea, irritable bowel, numbness) associated with FM based on which a rating of 0 = no symptoms, 1 = few symptoms, 2 = moderate symptoms, or 3 = great deal of symptoms was assigned. The SS score comprises the sum of the 3 primary symptoms with the rating of physical symptom severity (final range 0–12). Based on the published ACR guidelines, a participant was considered to meet classification for adult FM if they had: 1) WPI score ≥ 7 and SS score ≥ 5, or WPI = 3–6 and SS ≥ 9; 2) symptom duration of at least 3 months; 3) no underlying medical condition that would otherwise explain the pain. For the purposes of this study, participants were classified as having “sub-clinical FM” symptoms if they continued to experience pain and one or more of the cardinal symptoms (widespread pain, sleep difficulty and/or cognitive symptoms) but did not meet full ACR criteria based on the WPI and SS score cut points for FM as described above.
Standardized tender point examinations were conducted to provide additional clinical confirmation, but tender point exams were not used for determination of FM status per the ACR 2010 criteria.
Pain Intensity:
Based on the Brief Pain Inventory (BPI),[9; 31] an 11-point numeric rating scale was used to gather ratings of average pain intensity (0-“No Pain” to 10-”Pain as bad as you can imagine”), with the modification that participants were asked to rate their average pain “in the past week” instead of the original BPI pain rating for the past 24 hours.
Physical Function and Perceived Health Status.
The Short Form – 36 Health Survey – Version 2 (SF-36) [56] was used to evaluate impairment in physical function (Physical Function subscale) and all other domains of perceived health status (including General Health, Social, Emotional Functioning and Role Functioning). The SF-36 is an established and well-validated self-report instrument of perceived health status for individuals ≥ 14 years of age and has been used in research assessing physical function and health status of youth and adult patients with FM.[25; 57] Subscale scores were transformed to standardized scores according to standard SF-36 norm-based scoring (T-score mean = 50, SD = 10) with lower scores reflecting poorer functioning.
Anxiety and Depressive Symptoms.
The Beck Anxiety Inventory (BAI) and Depression Inventory (BDI-2) are well-validated, brief, self-report instruments used to assess anxiety (BAI) and mood (BDI-2) in adults and adolescents over 17 years of age.[4; 5] Participants rated the severity of each symptom on a 4-point Likert scale. Higher scores on each instrument reflect greater symptom severity (range 0–63). For the BAI, established clinical cut-off scores are as follows: Minimal, 0–9; Mild, 10–16; Moderate, 17–29; and Severe 30–63. For the BDI-2, established clinical cut-off scores are as follows: Minimal, 0–13; Mild, 14–19; Moderate, 20–28; and Severe Depression; 29–63.
Healthcare Utilization.
Participants were asked to report on their utilization of healthcare services over the past one year. Number of outpatient visits (i.e., primary care, family physician, specialty care [e.g., pain clinic, orthopedist], mental health [e.g., psychologist/psychiatrist visits) and emergency department visits were gathered using a previously developed survey.[22] Information on current medications was also obtained.
Objectives 2 and 3. Longitudinal assessments
Pain Intensity.
Assessment of pain intensity in the past week at all time points was conducted using Numeric Rating Scale (NRS) derived from the Brief Pain Inventory described above.[9; 31] The NRS for pain intensity is commonly used in both pediatric and adult pain populations;[13; 40; 47] therefore, the identical measure was used to assess pain across all assessment time points (Time 1–4).
Depressive Symptoms.
The Children’s Depression Inventory (CDI), a brief 27-item self-report questionnaire validated for youth up to age 17, was used to assess depressive symptoms [34] in adolescents at Time 1. Mood symptoms are rated on a 3-point Likert scale, with higher scores indicating greater severity of depressive symptoms (range 0–54). The CDI has been widely used in pediatric pain research. [26; 28; 36] The Beck Depression Inventory (BDI-2),[5] described in the previous section, was used for assessment of depressive symptoms in the youth when they reached adulthood (≥18 years) and was administered at Times 2–4. CDI and BDI-2 scores were subjected to a psychometric linkage procedure to enable longitudinal analyses (described in analytic plan).[23]
Physical Function:
The Physical Functioning Scale of the Short Form – 36 Health Survey – Version 2 (SF-36), described previously, was used to assess physical function at Times 2–4. This measure is validated for older adolescents (14+) and adults and was not administered at Time 1 when many of the participants (23%) were younger than 14 years of age. There is no pediatric version of the SF-36 or a comparable measure that would allow for linkage analysis as was possible for the CDI-BDI measures described above. Hence, this measure was used for Time 4 group comparisons, and Times 2, 3 and 4 SF-36 scores were included in the longitudinal SEM analysis evaluating the contributions of pain and depression to functioning.
Analytic Plan
Descriptive statistics were computed using SPSS v. 23 (IBM Corp., N.Y.) for cross sectional data on all JFM participants (n=86) who completed the Time 4 assessment. Trajectory analyses were conducted on a subset of the 116 participants in the original cohort (N=97, i.e., 83.6%). This subset was used to increase confidence in the results of the longitudinal growth models – which require that age heterogeneity is minimized and that the length of time between assessments is consistent. Preliminary analyses of the full cohort showed that age had a bi-modal distribution, with one younger cohort comprising the majority of participants (N=97) and an older cohort (N=19). It was determined that the older cohort corresponded to a group of participants that were recruited and enrolled some years earlier than the rest of the cohort (the initial cohort was enrolled from 2001–2006, a longer time frame than the 2–3 year-time frames for the follow-up assessments). Although their age range at baseline (Time 1) was comparable (between 12–18 years), the group of 19 JFM participants were somewhat older than other participants through the longitudinal data collection phase. Because the older cohort was assessed on a very different and unbalanced longitudinal schedule, they were excluded and only data from the younger cohort was used for longitudinal mixture and subsequent prediction analyses.
Of note, this older subgroup did not differ from the younger group on either depressive symptoms (p = .303) or pain severity (p = .286).
Outcomes at 8-year follow-up (Objective 1)
Descriptive data for all participants who completed the Time 4 assessment (n = 86) were computed for the domains of FM symptom severity, pain, health status, and mood (see Table 2). Descriptive data were also computed on additional variables of interest – such as number and type of medications and healthcare utilization.
Table 2:
Symptom severity, pain, health status and mood at Time 4 (n = 86)
| Variable | |||||
|---|---|---|---|---|---|
| Mean | SD | Min | Max | Moderate to Severe (%) | |
| Fibromyalgia Symptom Severity | 14.42 | 6.46 | 2 | 30 | |
| Pain intensity, NRS (0–10) | 3.34 | 2.50 | 0 | 8 | 36.1%* |
| Number of pain locations (0–19) | 7.29 | 4.11 | 1 | 18 | |
| SF-36 Norm-based T-scores (Normative Mean = 50; SD =10) | |||||
| Physical Functioning | 44.08 | 10.82 | 17 | 57 | 23.8%ϯ |
| Physical Role Functioning | 41.46 | 10.66 | 18 | 57 | 26.2%ϯ |
| Bodily Pain | 40.22 | 9.96 | 20 | 62 | 33.3%ϯ |
| General Health | 36.55 | 11.78 | 19 | 64 | 51.2%ϯ |
| Vitality | 38.90 | 12.16 | 21 | 68 | 40.5%ϯ |
| Social Functioning | 40.68 | 11.75 | 13 | 57 | 22.6%ϯ |
| Emotional Role Functioning | 41.49 | 14.50 | 9 | 56 | 28.6%ϯ |
| Mental Health Functioning | 41.96 | 11.91 | 13 | 64 | 29.8%ϯ |
| Mood | |||||
| Anxiety, BAI (0–60) | 20.80 | 13.31 | 0 | 59 | 59.5%Ɵ |
| Depressive Symptoms, BDI (0–63) | 13.80 | 10.63 | 0 | 44 | 26.2%Ɵ |
Percent of pain scores above 4
Percent of scores below T-score of 35 (or 1.5 SD below normative mean)
Percent meeting clinical cut-offs for moderate to severe symptoms
Longitudinal trajectory analyses of pain and depressive symptoms (Objective 2)
Prior to conducting trajectory analysis it was necessary to first psychometrically link [23] the CDI and BDI raw scores to allow comparison of scores on depressive symptoms longitudinally from adolescence to adulthood. Equipercentile equating, a form of linking, uses a nonlinear transformation so that scores from two measures of the same construct can be transformed to a common metric and have the same distributional characteristics. [33] We used equipercentile equating with loglinear smoothing,[33] which reduces irregularities in the frequency distributions, to transform CDI scores to the BDI metric for trajectory analysis.
Statistical analyses were conducted using two separate growth mixture modeling (GMM) analyses in MPlus (version 8; Muthén & Muthén, 1998–2017)[44] to identify and independently describe unobserved subgroup trajectories for both depression and pain. GMM assumes that a set of longitudinal data is comprised of a mixture of several distinct, separate, and latent subpopulations (i.e., unobserved groups) that each show a different longitudinal change trajectory for the response variables of interest (depression & pain). Prior studies identifying subgroups of individuals with FM have used methods such as cluster analysis.[18; 54] However, GMM was chosen over cluster analysis because it is most appropriate for the longitudinal design and because it outperforms cluster analysis in detecting sample heterogeneity even with smaller sample sizes.[39; 50; 53] Several criteria were used in determining the best fitting GMM to the data, including: 1) minimized Bayesian Information Criteria (BIC) and sample size-adjusted BIC (NBIC) model fit statistics, 2) maximized entropy[3; 8; 37] (which quantifies both model fit and how accurately participants are classified into mixture solutions) statistic values, 3) statistical significance of all trajectory (i.e., intercept, linear slope, quadratic deviation, etc.) components within a mixture solution, and 4) mixture trajectory solutions that contained at least 8–10% of sample (N=8–10) participants. Extraction of latent mixture trajectories continued until none of the four criteria could be improved upon.
Study attrition was minimized due to the strong longitudinal participant retention rates (81.9% at Time 3, 74.1% at Time 4); as such, missing data was handled via maximum likelihood parameter estimation algorithm for all analyses.
Impact of depressive symptom trajectory on physical functioning (Objective 3)
Latent depression trajectory group memberships (from the GMM analyses above) were used as predictors of longitudinal physical functioning trajectory (i.e., intercept and slope) variance during the adult years (Times 2–4) using longitudinal structural equation modeling. Pain trajectories were not analyzed as predictors of physical functioning given that a) over the span of the study both pain trajectories improved, and b) at Time 4, participants in the two pain trajectory groups did not significantly differ from one another in physical functioning (p = .90). Thus, there was little utility in examining functional outcomes by pain trajectory in this sample.
Results
Sample Characteristics
The majority of participants were female (94.2%) and white (87.2%). The mean age of the cohort at initial assessment was 15.9 ± 1.6 years, with average age of symptom onset at 12.7 ± 2.6 years. The average age of this cohort at Time 4 follow-up was 24.24 ± 2.2 years. There were no significant differences between retained participants and those who dropped out during the course of the study based on age or baseline socioeconomic status, pain, or depressive symptoms. Over 80% had at least some college/vocational education. With regard to marital status, 67.4% were single, 25.6% were married and 5.8% were divorced or separated; and 26.7% had one or more children.
Objective 1 – Outcomes in young adulthood.
Fibromyalgia Status and Pain Symptoms.
At Time 4, 100% participants reported having one or more fibromyalgia symptoms such as pain, fatigue and/or sleep difficulties, with 50 (58.1%) meeting full ACR 2010 criteria for adult FM at follow-up. The remaining (n = 36; 41.9%) reported subclinical symptoms based on the suggested WPI and SS score cut-points. Participants reporting having pain in an average of 7 body areas with average pain intensity in the mild to moderate range overall (3.34 on a 0–10 Numeric Rating Scale).
Physical and Emotional Functioning.
The JFM cohort reported impaired physical functioning as compared to healthy norms on the SF-36, and lower perceived health status and role functioning across all domains of physical, emotional and social functioning (Table 2). Nearly 60% reported moderate to severe anxiety based on cut-off scores on the BAI and 26% reported having moderate to severe depressive symptoms based on BDI cut-off scores. Over 40% of the cohort was taking at least one medication for pain and nearly 30% reported being on antidepressant medication (although whether they were prescribed the antidepressant medication to address pain or mood symptoms was not specifically assessed). With regard to health care utilization within the past year (see Table 3), participants reported 7–8 outpatient physician visits on average and about 3 visits to mental health providers (psychologist/psychiatrist). While most participants reported less than one ED visit per year (M = .55), 39% (n=30) reported at least one visit in the last 12 months. About half of those (46.7%) were for pain-related issues (e.g., headache/migraine, abdominal, chest, limb pain) not associated with an acute injury. The most commonly used medications (for pain and related symptoms) were analgesic and non-steroidal anti-inflammatory medications (40.7%) and antidepressants (29.1%). Very few (< 5%) reported opioid use.
Table 3.
Health Care Utilization and Medications at Time 4 (n = 86)
| Variable | |||||
|---|---|---|---|---|---|
| Mean | Median | SD | Min | Max | |
| Healthcare Utilization | |||||
| Number of Emergency Department Visits | 0.55 | 0.00 | 1.20 | 0 | 6 |
| Number of Visits to Primary Care Physician | 1.14 | 1.00 | 0.35 | 1 | 2 |
| Number of Visits to Pain Clinic/Physician | 0.87 | 0.00 | 2.31 | 0 | 12 |
| Number of Visits to Family Doctor | 2.61 | 2.00 | 2.60 | 0 | 12 |
| Number of Visits to Psychologist/Psychiatrist | 2.77 | 0.00 | 8.17 | 0 | 45 |
| Number of Visits to Orthopedists | 0.30 | 0.00 | 1.08 | 0 | 7 |
| Number of Visits to Other Doctors/Specialists | 2.90 | 1.00 | 4.11 | 0 | 16 |
| Medication usage | |||||
| No. of current medications | 1.31 | 1.00 | 1.53 | 0 | 6 |
| Antidepressants (%) | 25 (29.1%) | ||||
| Analgesic/NSAIDS (%) | 35 (40.7%) | ||||
| Anticonvulsants (%) | 13 (15.1%) | ||||
| Opioid analgesics (%) | 4 (4.7%) |
Objective 2 - Longitudinal Trajectory Analysis
Pain Intensity Trajectories.
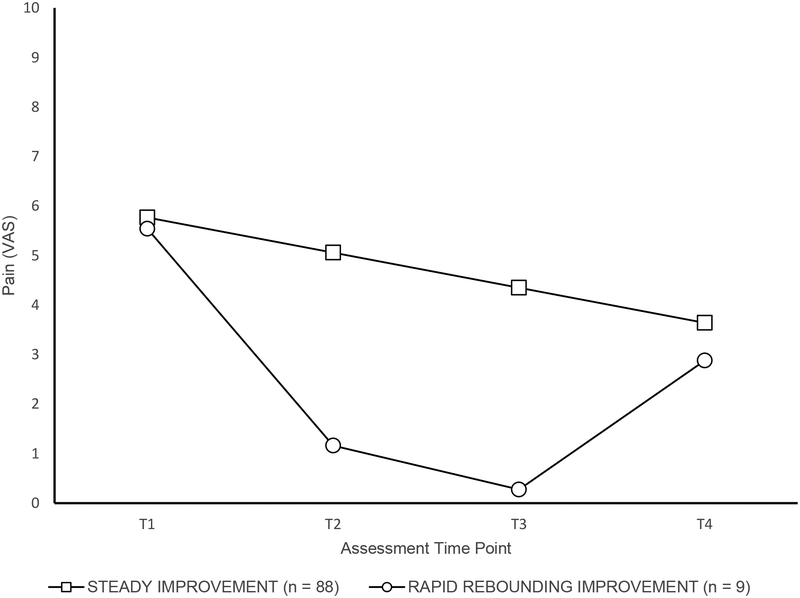
GMM analysis results showed that a two trajectory solution best described longitudinal pain intensity changes across participants (entropy = 0.88, showing excellent model fit,[8] see Figure 1). Although both subgroups showed comparable levels of moderately high pain at baseline (Time 1: M = 5.77 and 5.54, respectively), two distinct patterns of pain levels emerged over time. The first group, (i.e., “Steady Improvement” pain class, 91% of N) demonstrated a steady linear decrease in pain levels over time, with a significant intercept (5.77; p < .001) and negative slope (−0.71; p < .001). The second group (i.e., “Rapid Rebounding Improvement”, 9% of N), exhibited marked decreases in pain from Time 1 to Time 3, with a significant intercept (5.54, p < .001) and negative linear slope (−6.13, p < .001); however, this group also exhibited a significant and positive quadratic “rebound” in pain levels between Time 3 and Time 4 (1.75, p < .001; see Figure 1). Pain levels were in the low-moderate range at the final time point for both groups (Time 4: M = 3.64 and 2.88, respectively). Given that trajectories consisting of less than 10% of the sample should be interpreted with caution, [45] a single trajectory solution was also considered, however, a two-class solution for longitudinal pain intensity demonstrated superior fit and was therefore the two trajectory solution was retained.
Figure 1.
Longitudinal trajectories of pain intensity in juvenile fibromyalgia.
Depressive Symptom Trajectories.
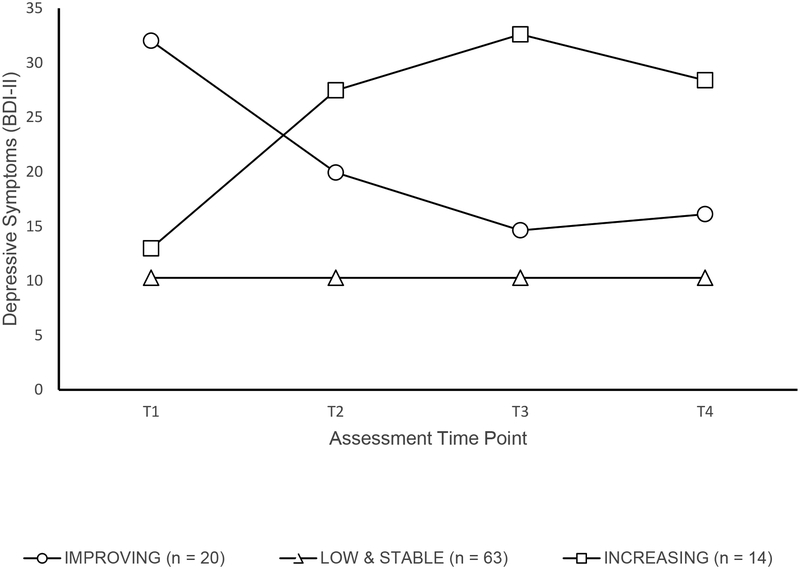
GMM results for depressive symptoms showed a three trajectory solution (see Figure 2) that best fit the data (entropy = 0.85, showing excellent model fit). The first trajectory class (i.e., “Low and Stable” depression, 65% of N) was characterized by low levels of depressive symptoms that remained low over time, and defined by a significant intercept (10.28, p < .001) only. The second trajectory class (i.e., “Improving” depressive symptoms, 21% of N) was marked by higher levels of depressive symptoms initially (with a significant intercept, 32.02, p < .001), a large improvement in symptoms (significant and negative linear slope, −15.48, p < .001), and a smaller but significant worsening of symptoms between Time 3 and Time 4 (significant quadratic change, 3.39, p < .001). The third trajectory class (i.e., “Worsening” depressive symptoms, 14% of N) was defined by initial depressive symptoms similar to the “Low and Stable depressive symptoms” class (intercept of 12.98, p < .001), but with significant increase in depressive symptoms after Time 1 (a large, positive, and significant linear slope, 19.17, p < .001), and a small improvement after Time 3 (significant negative quadratic change (−4.68, p < .001; See Figure 2).
Figure 2.
Longitudinal trajectories of depressive symptoms in juvenile fibromyalgia.
Objective 3 - Differences in Physical Functioning by Depression Trajectories
The “Improving” and “Worsening” depression trajectory classes were dummy-coded (the Low and Stable trajectory was the reference class) and entered as time-invariant predictors into a longitudinal SEM model that quantified changes in physical functioning at assessment points Time 2, Time 3 and Time 4. Both the “Improving” (−5.08, p < .001) and “Worsening” depression (−6.10, p < .001) trajectory classes showed significantly lower physical functioning at Time 2 compared to the “Low and Stable depression” trajectory. However, only the “Worsening” depressive symptoms class showed significantly decreasing physical functioning over time (−3.82, p < .001). In comparing the outcomes of JFM participants in adulthood based on their membership in the three trajectory classes, those in the “Worsening” trajectory showed markedly lower functioning across physical and psychosocial domains on the SF-36 (Table 4).
Table 4 –
Post-hoc comparisons of health and functioning of JFM group in adulthood (Time 4) based on depression trajectories
| 1. Improving (n = 11–13) |
2. Low & Stable (n = 39–42) |
3. Worsening (n = 10–11) |
Low vs. Worsening | Improving vs. Worsening | Improving vs. Low | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | Z | P | d | Z | P | d | Z | P | d | |
| FM Symptom Severity | 16.18 (5.88) | 13.18 (6.74) | 19.30 (5.25) | −2.50 | .012 | 0.77 | −0.91 | .363 | 0.36 | 1.71 | 0.088 | 0.47 |
| Physical Functioning | 69.23 (23.53) | 74.52 (22.97) | 41.36 (19.76) | 5.57 | <.001 | 1.53 | 3.51 | <.001 | 1.36 | −0.78 | 0.433 | 0.24 |
| Physical Role Limitations | 60.10 (23.04) | 66.52 (26.90) | 30.11 (17.42) | 5.94 | <.001 | 1.46 | 3.84 | <.001 | 1.51 | −0.93 | 0.354 | 0.26 |
| Emotional Well-being | 55.38 (17.97) | 68.81 (18.00) | 32.73 (14.73) | 7.34 | <.001 | 2.11 | 3.95 | <.001 | 1.43 | −2.45 | 0.014 | 0.76 |
| Emotional Role Limitations | 66.03 (24.41) | 77.78 (25.08) | 31.06 (32.30) | 5.01 | <.001 | 1.80 | 3.49 | <.001 | 1.30 | −1.56 | 0.119 | 0.48 |
| Social Functioning | 57.69 (19.46) | 69.35 (24.72) | 32.95 (25.17) | 4.69 | <.001 | 1.50 | 3.08 | .002 | 1.15 | −1.93 | 0.053 | 0.52 |
Observed group means and standard deviations presented. Wald Z, Cohen’s d, and significance values (p) correspond to differences between estimated group means
To test whether a sample size of 97 with 4 time points (as in the current study) had acceptable power to select the best mixture solution that best fit the data, Monte Carlo simulation power analyses were performed in Mplus (Version 8.1). [44] Simulations were performed assuming analysis model results for both the GMM for depressive symptoms (Objective 2) and conditional longitudinal physical functioning growth models (Objective 3) were population models, from which r = 1000 replications of N = 97 data were generated from both. The GMM simulation results showed acceptable power for three of four enumeration indices (AIC: power = .704; BIC: power = .994; Entropy: power = .62) used to determine the mixture solution that best fit the data. Conditional growth model simulation results showed acceptable power for physical functioning intercept variance (“Improving” = −5.08; power = .73; “Worsening” = −6.10; power = 0.79) and physical functioning slope variance (“Worsening” = −3.82: power = .64) prediction.
Discussion
This study was the first of its kind to longitudinally examine pain and depressive symptoms in a clinical sample of individuals with JFM, capturing the progression of symptoms over the critical developmental transition years from adolescence to early adulthood. Prospective longitudinal studies such as the current investigation make it possible to disentangle the evolving nature of chronic pain and mood symptoms starting early in life and offer a new perspective for pain research using a developmentally-informed paradigm.
In line with our prior findings for this cohort,[25] we confirmed that symptoms of JFM persist into adulthood, which supports the notion that fibromyalgia is a chronic condition; adolescent JFM patients do not simply “grow out of it.” As a group, young adults previously diagnosed with JFM remained significantly affected by widespread pain and other FM symptoms. More than half (58%) met criteria for adult FM with the remaining experiencing “sub-clinical” levels of FM symptoms including pain, fatigue and sleep difficulties. Anxiety and depressive symptoms were generally elevated, and both physical and psychosocial functioning were significantly impaired. Over a third of individuals were taking pain and/or antidepressant medication.
Despite continued FM symptoms, trajectory analyses showed that overall, mean pain intensity levels decreased from moderate in adolescence (>5 on a 0–10 VAS) to low/moderate levels (< 4) in early adulthood, with two subgroups of pain trajectories. One trajectory group (“Steady Improvement”) consisting of the majority of the sample (91%) showed small but gradual improvement in pain over time, whereas the remainder of the sample (“Rapid Rebounding Improvement”) demonstrated marked early improvement, with a gradual rebound in pain by the time they were adults. However, both groups reported comparable levels of (reduced) pain in adulthood compared to the pain levels reported at their initial assessment. The reasons for why pain ratings decline over time are not currently known but may be due to a natural progression towards improvement in pain over time, the effects of treatment (participants were initially enrolled from a clinic setting so they had sought treatment early in life), or simply adjusting to their diagnosis, learning better ways to manage their symptoms or recalibrating their pain reports as they became older.
In comparison to the trend for improvements in pain, trajectory analyses revealed strikingly different progression of depressive symptoms characterized by three trajectory groups. The majority (~66% of the sample) experienced low to mild depressive symptoms (the “Low and Stable” symptom trajectory) that remained stable from mid adolescence to early adulthood. Another subset of participants (“Improving symptoms”; 21%) initially reported severe levels of depression at Time 1, with gradual improvement over time reducing to mild levels by Time 4. Finally, a relatively small subgroup of the cohort (14%) constituted a third trajectory group (“Worsening symptoms”) and initially reported minimal depressive symptoms, with increases over time, and severe levels of depression by adulthood. Most notably, membership in this “Worsening” depressive symptoms group predicted worsening physical impairment over time and marked impairment in adulthood compared to the other two trajectory groups. This longitudinal study shows that there may be a substantial number of youth with JFM who may still experience FM symptoms as adults but their pain levels generally tend to reduce over time. On the other hand, there is a subgroup that may have poorer outcomes in adulthood such as worsening depressive symptoms and increasing physical impairment over time.
Defining key developmental periods where risk for long-term impairment can be identified is one of the important goals of a prospective study of this nature. A closer examination of the three depression trajectories in Figure 2 shows that Time 3 (late adolescence or emerging adulthood, approximately ages 19–22) was the period when differences in the trajectories first became evident. Hence, higher or lower levels of depressive symptoms in earlier adolescence were not necessarily predictive of later progression of mood symptoms. It was only in late adolescence/emerging adulthood when the three subgroups of stable, decreasing or worsening depressive symptoms became more established. From a developmental point of view, emerging adulthood is considered a crucial phase [25] when expectations of increasing independence from family, greater influence of peers, career decisions and formation of romantic attachments can test the coping abilities and resilience of youth. The ability of young individuals who are also dealing with chronic pain to take on these additional challenges may well be an important indicator of their long-term outcomes. Monitoring the progression of mood symptoms in the transition years between adolescence and emerging adulthood among youth with JFM and intervening as early as possible for those showing worsening symptoms, may be the best strategy to prevent a spiral of increasing comorbidity and disability into the adult years. As such, continuity of care during this transitional period is an area that likely requires greater attention in JFM. Understanding of practices associated with transitional care has only begun to be explored in pediatric chronic pain. [21] However, much can be learned from transition models developed in other pediatric chronic health conditions, such as juvenile arthritis, [24] diabetes, [10] epilepsy, [1; 12] and IBD. [20] Ideally, a transition plan involving both the patient and provider should be put into place well in advance and may involve the services of a care coordinator or a medical transition team. In addition, direct communication between child-adult pain management providers may facilitate the transition process [10] and optimize patient outcomes.
An interesting observation in this longitudinal study was that youth with JFM were likely to marry relatively early and have children, as we have previously observed and discussed in the prior (Time 3) assessment of this cohort,[25] and further demonstrates the importance of how developmental transitions may be affected in youth with JFM. Differential attainment of certain developmental milestones have been documented in other populations experiencing early life adversity or illness, and show that while educational and vocational goals may be delayed, other milestones (such as marrying or having a child) are achieved earlier than is normative. [15; 32] The impact of chronic pain on intimate relationships in early adulthood and developmental outcomes have not been well-studied in young adults with chronic pain but clearly deserves further investigation.
Subgroup profiles of pain and emotional symptoms have been described in prior studies of adults with FM.[18; 19; 51] Specifically, FM appears to manifest as one of two general subtypes, one categorized by heightened pain in the relative absence of psychological comorbidities (“nociceptive” subtype), and the other profile categorized by both pain and comorbid depressive symptoms. The latter profile has been hypothesized to be associated with poorer long term outcomes. In a recent study, Vincent et al. [54] described several clusters of FM in adults based on progressively greater symptoms and disability. Interestingly, two clusters were relatively similar in their physical symptom profile, but diverged based on their psychological profile, with the profile that includes psychological symptoms such as depressive symptoms also experiencing greater impairment, similar to findings reported by Giesecke et al.[18] Results from the current longitudinal study offer the additional perspective that for individuals diagnosed with JFM early in life, pain intensity tends to improve gradually over time. A minority of patients, i.e., those with worsening mood symptoms, appear to be at high risk for long term impairment. Early intervention in this subgroup (particularly in the emerging adulthood years, between the ages of 19–22 when symptom trajectories tend to solidify) may well prevent progression of functional impairment and comorbid depressive symptoms in some patients by the time they are adults.
Strengths of this study were the longitudinal design, high retention (nearly 75% over the approximately 8-year follow-up period) and robust statistical methods used to examine pain and depressive symptom trajectories resulting in excellent model fit. A limitation of the study was the relatively small sample size of patients from one geographic area (the US Midwest). It should also be noted that this was a clinical sample of patients recruited from a tertiary care pediatric hospital and results may not generalize to children with chronic widespread pain in the community who do not seek specialized medical care. Consistent with the predominance of fibromyalgia sufferers being female and Caucasian, we had very few males or minorities in the sample. As such, gender and race/ethnicity effects were not possible to investigate, so results are primarily applicable for adolescent and young adult Caucasian females. Expanding to additional sites with a more diverse group of individuals would improve generalizability and replicability of the trajectory model solutions from this investigation.
In conclusion, results of this prospective longitudinal study offer a developmental perspective on the progression of JFM from adolescence into young adulthood. Results identified depressive symptom trajectories as being particularly important in predicting long-term outcomes in physical impairment. The key developmental period of transition into emerging adulthood appeared to be when mood trajectories became more entrenched, and impacted later physical functioning. Early identification of mood symptoms and monitoring of emotional functioning throughout this time with targeted and timely interventions, would greatly enhance our ability to prevent and treat the disabling impact of JFM over the years.
Table 1:
Demographic information at Time 4 (n=86)
| Variable | ||
|---|---|---|
| Age (Mean, SD) |
Mean 24.24 |
SD 2.20 |
| Female |
N 81 |
% 94.2 |
| Race | ||
| White | 75 | 87.2 |
| African American | 5 | 5.8 |
| Asian | 1 | 1.2 |
| American Indian | 5 | 5.8 |
| Marital Status | ||
| Single | 58 | 67.4 |
| Married/Divorced/Separated | 28 | 32.6 |
| Living situation | ||
| With parents | 28 | 32.6 |
| With roommate/in dormitory | 16 | 18.6 |
| With spouse/significant other | 34 | 39.5 |
| Alone | 8 | 9.3 |
| Years of Education | ||
| 9th to 11th grade | 1 | 1.2 |
| High School/GED | 9 | 10.5 |
| Vocational, trade, associate’s degree | 18 | 20.9 |
| Some college/bachelor’s/graduate degree | 58 | 67.4 |
| Primary source of income | ||
| Job/scholarship/student loans | 49 | 57.0 |
| Parents/spouse/partner | 33 | 38.4 |
| Public assistance | 2 | 2.3 |
| Other/unknown | 2 | 2.3 |
| Have one or more children | 23 | 26.7 |
Acknowledgements:
We would like to acknowledge Soumitri Sil PhD, Emily Verkamp MSc. and Daniel Strotman BS, for their assistance with data collection, recruitment and data management for this project. We are also grateful to all the participants who graciously took time to complete repeated assessments in this long-term study. The study was funded by NIH/NIAMS Grants R01AR054842 and K24AR056687 to the first author (SKZ). The authors have no other financial disclosures or conflicts of interest to report in relation to this project.
Funding Source: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health Grants R01AR054842 and K24AR056687 to the first author (SKZ).
Footnotes
Financial Disclosure: The authors have no other financial disclosures in relation to this study.
Conflict of Interest: The authors have no conflict of interest to disclose in relation to this study.
References
- [1].Andrade DM, Bassett AS, Bercovici E, Borlot F, Bui E, Camfield P, Clozza GQ, Cohen E, Gofine T, Graves L, Greenaway J, Guttman B, Guttman-Slater M, Hassan A, Henze M, Kaufman M, Lawless B, Lee H, Lindzon L, Lomax LB, McAndrews MP, Menna-Dack D, Minassian BA, Mulligan J, Nabbout R, Nejm T, Secco M, Sellers L, Shapiro M, Slegr M, Smith R, Szatmari P, Tao L, Vogt A, Whiting S, Carter Snead O 3rd., Epilepsy: Transition from pediatric to adult care. Recommendations of the Ontario epilepsy implementation task force. Epilepsia 2017;58(9):1502–1517. [DOI] [PubMed] [Google Scholar]
- [2].Arnett JJ. Emerging Adulthood: Understanding the New Way of Coming of Age In: Arnett JJ, Tanner JL, editors. Emerging Adults in America: Coming of Age in the 21st Century. Washington, DC: American Psychological Association, 2006. pp. 3–19. [Google Scholar]
- [3].Bakk Z, Oberski DL, Vermunt JK. Relating Latent Class Assignments to External Variables: Standard Errors for Correct Inference. Political Analysis 2014; 22(04):520–540. [Google Scholar]
- [4].Beck A, Steer R. Manual for the Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation, 1990. [Google Scholar]
- [5].Beck A, Steer R, Brown G. BDI-II Manual. San Antonio, TX: The Psychological Corporation, 1996. [Google Scholar]
- [6].Buskila D Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res Ther 2009;11(5):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buskila D, Neumann L, Hershman E, Gedalia A, Press J, Sukenik S. Fibromyalgia syndrome in children: An outcome study. Journal of Rheumatology 1995;22(3):525–528. [PubMed] [Google Scholar]
- [8].Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification 1996;13(2):195–212. [Google Scholar]
- [9].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore 1994;23(2):129–138. [PubMed] [Google Scholar]
- [10].Crowley R, Wolfe I, Lock K, McKee M. Improving the transition between paediatric and adult healthcare: a systematic review. Arch Dis Child 2011;96(6):548–553. [DOI] [PubMed] [Google Scholar]
- [11].Cunningham NR, Tran ST, Lynch-Jordan AM, Ting TV, Sil S, Strotman D, Noll JG, Powers SW, Arnold LM, Kashikar-Zuck S. Psychiatric Disorders in Young Adults Diagnosed with Juvenile Fibromyalgia in Adolescence. J Rheumatol 2015;42(12):2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Disabato JA, Cook PF, Hutton L, Dinkel T, Levisohn PM. Transition from Pediatric to Adult Specialty Care for Adolescents and Young Adults with Refractory Epilepsy: A Quality Improvement Approach. J Pediatr Nurs 2015;30(5):e37–45. [DOI] [PubMed] [Google Scholar]
- [13].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- [14].Epstein SA, Kay G, Clauw D, Heaton R, Klein D, Krupp L, Kuck J, Leslie V, Masur D, Wagner M, Waid R, Zisook S. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 1999;40(1):57–63. [DOI] [PubMed] [Google Scholar]
- [15].Forthofer MS, Kessler RC, Story AL, Gotlib IH. The effects of psychiatric disorders on the probability and timing of first marriage. Journal of health and social behavior 1996;37(2):121–132. [PubMed] [Google Scholar]
- [16].Gedalia A, Press J, Klein M, Buskila D. Joint hypermobility and fibromyalgia in schoolchildren. Ann Rheum Dis 1993;52(7):494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerloni V, Ghirardini M, Fantini F. Assessment of nonarticular tenderness and prevalence of primary fibromyalgia syndrome in healthy Italian schoolchildren. Arthritis and Rheumatism 1998;41(9):1405. [Google Scholar]
- [18].Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis & Rheumatism 2003;48(10):2916–2922. [DOI] [PubMed] [Google Scholar]
- [19].Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain Res Treat 2012;2012:486590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hait E, Arnold JH, Fishman LN. Educate, communicate, anticipate-practical recommendations for transitioning adolescents with IBD to adult health care. Inflammatory bowel diseases 2006;12(1):70–73. [DOI] [PubMed] [Google Scholar]
- [21].Higginson A, Forgeron P, Dick B, Harrison D. Moving on: A survey of Canadian nurses’ self-reported transition practices for young people with chronic pain. Canadian Journal of Pain 2018;2(1):169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ho IK GK, Kashikar-Zuck S, Kotagal U, Tessman C, & Jones BA Healthcare utilization and indirect burden among families of pediatric patients with chronic pain. Journal of Musculoskeletal Pain 2008;16(3):155–164. [Google Scholar]
- [23].Holland PW, Dorans NJ. Linking and equating In: Brennan RL, editor. Educational measurement. West Port, CT: Praeger, 2006. pp. 187–220. [Google Scholar]
- [24].Jensen PT, Karnes J, Jones K, Lehman A, Rennebohm R, Higgins GC, Spencer CH, Ardoin SP. Quantitative evaluation of a pediatric rheumatology transition program. Pediatric rheumatology online journal 2015;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, Lovell DJ, Arnold LM. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics 2014;133(3):e592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain 2001;17(4):341–349. [DOI] [PubMed] [Google Scholar]
- [27].Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis and Rheumatism 2007;57(3):474–480. [DOI] [PubMed] [Google Scholar]
- [28].Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis and Rheumatism 2008;59(10):1392–1398. [DOI] [PubMed] [Google Scholar]
- [29].Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, Graham TB. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 2010;49(11):2204–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: Findings from a clinical trial of cognitive–behavioral therapy. Arthritis care & research 2013;65(3):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain 2004;20(5):309–318. [DOI] [PubMed] [Google Scholar]
- [32].Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, Wang PS, Zaslavsky AM. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. Am J Psychiatry 2008;165(6):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kolen MJ, Brennan RL. Test Equating, Scaling, and Linking. New York: Springer, 2014. [Google Scholar]
- [34].Kovacs M Children’s Depression Inventory Multi-Health systems, Inc., 908 Niagara Falls Blvd, North Tonawanda, N.Y., 1992. [Google Scholar]
- [35].Leino-Arjas P, Rajaleid K, Mekuria G, Nummi T, Virtanen P, Hammarstrom A. Trajectories of musculoskeletal pain from adolescence to middle age: the role of early depressive symptoms, a 27-year follow-up of the Northern Swedish Cohort. Pain 2017. [DOI] [PubMed] [Google Scholar]
- [36].Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, Wilson AC, Zhou C. Factor structure of the children’s depression inventory in a multisite sample of children and adolescents with chronic pain. J Pain 2013;14(7):689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lubke G, Muthén BO. Performance of Factor Mixture Models as a Function of Model Size, Covariate Effects, and Class-Specific Parameters. Structural Equation Modeling: A Multidisciplinary Journal 2007;14(1):26–47. [Google Scholar]
- [38].Luciano JV, Forero CG, Cerda-Lafont M, Penarrubia-Maria MT, Fernandez-Vergel R, Cuesta-Vargas AI, Ruiz JM, Rozadilla-Sacanell A, Sirvent-Alierta E, Santo-Panero P, Garcia-Campayo J, Serrano-Blanco A, Perez-Aranda A, Rubio-Valera M. Functional Status, Quality of Life, and Costs Associated With Fibromyalgia Subgroups: A Latent Profile Analysis. Clin J Pain 2016;32(10):829–840. [DOI] [PubMed] [Google Scholar]
- [39].Martin DP, von Oertzen T. Growth mixture models outperform simpler clustering algorithms when detecting longitudinal heterogeneity, even with small sample sizes. Structural Equation Modeling: A Multidisciplinary Journal 2015;22(2):264–275. [Google Scholar]
- [40].McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain 2008;9(9):771–783. [DOI] [PubMed] [Google Scholar]
- [41].Mikkelsson M One year outcome of preadolescents with fibromyalgia. Journal of Rheumatology 1999;26(3):674–682. [PubMed] [Google Scholar]
- [42].Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents. Prevalence and 1-year persistence. Pain 1997;73(1):29–35. [DOI] [PubMed] [Google Scholar]
- [43].Mulvaney S, Lambert EW, Garber J, Walker LS. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 2006;45(6):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muthén LK, Muthén BO. Mplus user’s guide (6th ed.). Los Angeles: Muthén & Muthén, 1998–2010. [Google Scholar]
- [45].Nagin DS, NAGIN D. Group-based modeling of development: Harvard University Press, 2005. [Google Scholar]
- [46].Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis and Rheumatism 1997;40(4):752–760. [DOI] [PubMed] [Google Scholar]
- [47].Ruskin D, Lalloo C, Amaria K, Stinson JN, Kewley E, Campbell F, Brown SC, Jeavons M, McGrath PA. Assessing pain intensity in children with chronic pain: convergent and discriminant validity of the 0 to 10 numerical rating scale in clinical practice. Pain Res Manag 2014;19(3):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Siegel DM, Janeway D, Baum J. Fibromyalgia syndrome in children and adolescents: Clinical features at presentation and status at follow-up. Pediatrics 1998;101(3 Pt 1):377–382. [DOI] [PubMed] [Google Scholar]
- [49].Sil S, Thomas S, DiCesare C, Strotman D, Ting TV, Myer G, Kashikar-Zuck S. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis Care Res (Hoboken) 2015;67(1):102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Steinley D, Brusco MJ. Evaluating mixture modeling for clustering: recommendations and cautions. Psychol Methods 2011;16(1):63–79. [DOI] [PubMed] [Google Scholar]
- [51].Thieme K, Turk DC, Gracely RH, Maixner W, Flor H. The relationship among psychological and psychophysiological characteristics of fibromyalgia patients. J Pain 2015;16(2):186–196. [DOI] [PubMed] [Google Scholar]
- [52].Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology adult fibromyalgia criteria for use in an adolescent female population with juvenile fibromyalgia. The Journal of pediatrics 2016;169:181–187. e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vermunt JK. K-means may perform as well as mixture model clustering but may also be much worse: comment on Steinley and Brusco (2011). Psychol Methods 2011;16(1):82–88; discussion 89–92. [DOI] [PubMed] [Google Scholar]
- [54].Vincent A, Hoskin TL, Whipple MO, Clauw DJ, Barton DL, Benzo RP, Williams DA. OMERACT-based fibromyalgia symptom subgroups: an exploratory cluster analysis. Arthritis Res Ther 2014;16(5):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012;153(9):1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ware JE, Snow KK, Kosinski M, Gandek B, Institute NEMCHH. SF-36 health survey: manual and interpretation guide: The Health Institute, New England Medical Center, 1993. [Google Scholar]
- [57].Wittink H, Turk DC, Carr DB, Sukiennik A, Rogers W. Comparison of the redundancy, reliability, and responsiveness to change among SF-36, Oswestry Disability Index, and Multidimensional Pain Inventory. Clinical Journal of Pain 2004;20(3):133–142. [DOI] [PubMed] [Google Scholar]
- [58].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. [DOI] [PubMed] [Google Scholar]
- [59].Yim YR, Lee KE, Park DJ, Kim SH, Nah SS, Lee JH, Kim SK, Lee YA, Hong SJ, Kim HS, Lee HS, Kim HA, Joung CI, Kim SH, Lee SS. Identifying fibromyalgia subgroups using cluster analysis: Relationships with clinical variables. Eur J Pain 2016. [DOI] [PubMed] [Google Scholar]
- [60].Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis & Rheumatism 1985;28(2):138–145. [DOI] [PubMed] [Google Scholar]