Abstract
Objectives
The patterns of care for salivary gland adenoid cystic carcinomas (ACC) is unknown. We sought to assess predictors of receiving postoperative radiation and/or chemotherapy for patients with non-metastatic, definitively resected ACC, as well as report unexpected nodal disease.
Methods
The National Cancer Data Base was queried for definitively resected non-metastatic ACC from 2004 to 2014. Logistic regression, Kaplan-Meier, and Cox proportional-hazard models were utilized. Propensity-score matched (PSM) analysis was employed to reduce confounding variables.
Results
3,136 patients met entry criteria: 2,252 (71.8%) received postoperative radiation with 223 (7.4%) also receiving concurrent chemotherapy. Median follow up was 4.87 years. In cN0 patients, 7.4% had pN+ after elective neck dissection. Patients who lived closer to their treatment facility and had positive margins were more likely to receive postoperative radiation. Black patients and uninsured patients were less likely to receive radiation. Older age, male sex, advancing stage, and positive surgical margins were associated with worse OS. With limited follow-up, receipt of radiation or chemotherapy was not associated with overall survival (OS).
Conclusion
Postoperative radiation was frequently given for resected ACC with a minority receiving chemotherapy. Black patients and uninsured patients were less likely to receive radiation. Postoperative radiation and/or chemotherapy had no association with OS but was given more frequently in more advanced disease, and our series is limited by short follow-up. The disparity findings for this rare disease need to be addressed in future studies.
Keywords: Adenoid cystic carcinoma, postoperative radiation, postoperative chemotherapy, healthcare disparities, National Cancer Data Base, salivary gland tumors
INTRODUCTION
Adenoid cystic carcinomas (ACC) represent approximately 10% of salivary gland tumors and less than 1% of all head and neck tumors1–3. Given its rarity, the optimal management of non-metastatic ACC has been influenced by retrospective reviews from large centers rather than randomized trials4–7. ACC grow relatively slowly compared to other head and neck cancers, have lower risk of lymph node metastases, and have high-propensity for perineural invasion3,7,8. ACC have a tendency for hematogenous spread at early stages, mostly to the lungs, liver, and bones3. Chemotherapy has been investigated but low response rates have been disappointing9. The standard therapy for localized disease is surgical resection followed by adjuvant radiotherapy5–7,10,11.
Current National Comprehensive Cancer Network (NCCN) guidelines state that postoperative radiotherapy should be “considered” for completely resected ACC and is recommended for patients with positive margins12. Given the lack of clarity in national guidelines for postoperative radiation, the low prevalence of the disease, and to further understand practice patterns of adjuvant therapy in the United States (US), we used the National Cancer Data Base (NCDB) to identify a large cohort of patients with non-metastatic salivary gland ACC who underwent definitive primary surgical resection. Our primary goal was to identify demographic, tumor, and treatment related factors associated with the receipt of postoperative radiation or chemotherapy. Secondarily we sought to determine the rate of unexpected positive nodal disease in patients who were clinically node negative before an elective neck dissection.
METHODS
Patient Selection
The NCDB is a database capturing cases at Commission on Cancer accredited facilities within the United States. The database catalogs 70% of newly diagnosed malignancies and includes detailed demographic, socioeconomic, disease, surgical and radiation treatment details in addition to OS outcomes.
The salivary gland NCDB file was queried for patients diagnosed between 2004 and 2014. Our inclusion criteria included only patients with non-metastatic ACC. We excluded patients who did not receive definitive upfront surgery, patients with incomplete treatment records, and patients who had a previously diagnosed malignancy (Figure 1). Staging was done per the American Joint Committee on Cancer 7th Edition guidelines13. The following patient characteristics were examined: age, sex, race (white, black, and other), insurance status (not insured, Medicaid/Medicare, and private), co-morbidities as quantified by the Charlson-Deyo Score14,15, county of residence (urban, rural, or metro as defined by the US Census Bureau), percentage of residents without a high school degree in patient’s census tract (<14, 14–19.9, 20–28.9, and ≥29% quartiles), median income of patient’s census tract (<30,000, 30,000–35,999, 36,000–45,999, and ≥46,000 dollars as determined by the American Community Survey), and the distance from patient’s census tract to treatment facility (≤10 miles, 10–50 miles, and >50 miles). The following tumor characteristics were examined: primary site (parotid, palate, submandibular, sublingual, and not specified), clinical lymph node positivity (cN0, cN+, cNX, and missing), pathological T stage, pathological N stage, and pathological overall stage. The following treatment characteristics were examined: time from diagnosis to definitive resection (0–3 months, 3–6 months, >6 months), treatment center case volume (high vs. low as defined using the 80th percentile for the number of cases treated at each facility), surgical margins (positive or negative), receipt of adjuvant radiation (yes or no), receipt of adjuvant chemotherapy (yes or no), treatment facility type (community cancer center, academic cancer center, comprehensive community cancer center), and facility location (northeast, south, Midwest, and west). Age was evaluated as a continuous variable after it was determined it had a linear effect on OS.
Figure 1.
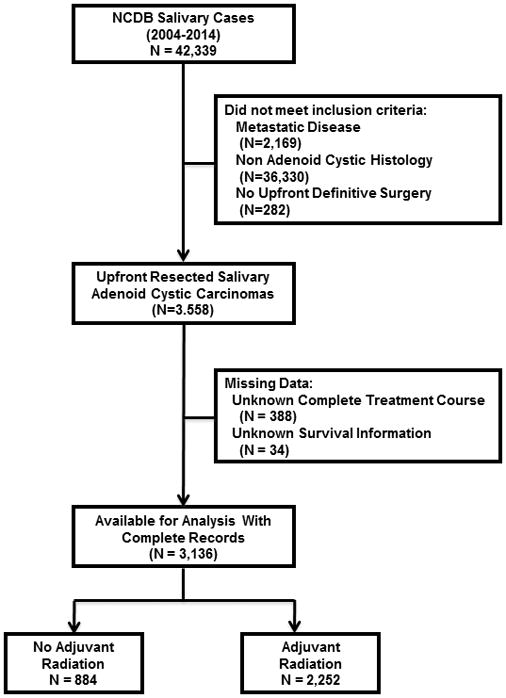
The Consolidated Standards of Reporting Trials (CONSORT) diagram of patients with non-metastatic resected salivary gland adenoid cystic carcinomas in the National Cancer Data Base.
Statistical Methods
All statistics were computed using SAS software version 9.4 (Cary, NC) and SAS macros16. Univariable and multivariable logistic regression models were fit to each patient, tumor, and treatment variable to determine predictors of receiving adjuvant radiation and adjuvant chemotherapy. The collinearity of among all variables was checked by removing any variance inflation factors greater than ten. Given the high collinearity of clinical node positivity, pathological T, N, and overall stage, only pathological stage was incorporated in multivariable models, otherwise any significant variable from univariable analysis was included in the multivariable analysis. ANOVA testing was done to compare radiation dosing amongst age cohorts, race cohorts, and treatment facility cohorts. Separate univariable and multivariable analyses were performed to determine factors associated with positive margins at surgery. OS was defined as months from diagnosis to death or last follow up. Univariable and multivariable Cox proportional hazard models for OS were generated. Kaplan-Meier curves were generated for OS for the entire cohort stratified by adjuvant therapy, with comparisons using log-rank tests. Propensity score-matching was utilized to reduce treatment selection bias; a logistic regression model for predicting receipt of adjuvant radiation was carried out to estimate the propensity score of all covariates. All variables that were associated with OS were included in the propensity-matched analysis. Patients were then matched 1:1 based on propensity score using a greedy 5-1-digit match algorithm17, where a patient receiving radiation were matched to a patient not receiving radiation over the set of variables detailed above. Once a match was made, no additional matching was considered. After matching, the balance of the two groups was evaluated by standardized differences with values <0.1 considered negligible18. The OS effect in the matched sample was estimated using a Cox model with a robust variance estimator19,20. For all analyses a p<0.05 was considered statistically significant. For each survival model, the proportional hazard assumption was assessed.
RESULTS
Patient Characteristics
A total of 3,136 patients met entry criteria (Figure 1), with 607 (19.7%) pathological stage I, 583 (18.9%) pathological stage II, 515 (16.7%) pathological stage III, and 1,431 (44.7%) pathological stage IVA/IVB. The median follow-up time was 4.87 years (range 0.34 to 11.88 years). Table 1 summarizes the remaining characteristics of our population.
Table 1.
Summary of patient, tumor, and treatment characteristics of all 3,136 patients with definitively resected non-metastatic adenoid cystic carcinomas of salivary gland origin.
| Variable | Level | N (%) |
|---|---|---|
| Patient Characteristics | ||
| Age (years) | Median (range) | 55.8 (18.1 – 90.0) |
| Sex | Male | 1,286 (41.0) |
| Female | 1,850 (59.0) | |
| Race | White | 2,507 (79.9) |
| Black | 348 (11.1) | |
| Other | 281 (9.0) | |
| Insurance Status | Not Insured | 229 (7.3) |
| Medicaid/Medicare | 1,125 (35.9) | |
| Private | 1,782 (56.8) | |
| Charlson-Deyo Comorbidity Score | 0 | 2,764 (88.1) |
| 1+ | 372 (11.9) | |
| County of Residence | Metro | 2,494 (82.5) |
| Urban | 459 (15.2) | |
| Rural | 69 (2.3) | |
| Missing | 114 | |
| Percentage of Patient’s Census Tract without a High-School Degree (quartiles) | <14% | 1,176 (39.0) |
| 14–19.9% | 723 (24.0) | |
| 20–28.9% | 643 (21.3) | |
| ≥29% | 473 (15.7) | |
| Missing | 121 | |
| Median Income of Patient’s Census Tract | < $30,000 | 357 (11.8) |
| $30,000 – $35,999 | 509 (16.9) | |
| $36,000 – %45,999 | 825 (27.4) | |
| ≥ $46,000 | 1,324 (43.9) | |
| Missing | 121 | |
| Distance from patient’s census tract to treatment facility | ≤10 miles | 1,298 (42.0) |
| 10–50 miles | 1,256 (40.6) | |
| >50 miles | 540 (17.5) | |
| Missing | 42 | |
| Tumor Characteristics | ||
| Primary Site | Parotid Gland | 1,249 (39.8) |
| Palate | 571 (18.2) | |
| Submandibular Gland | 1,057 (33.7) | |
| Sublingual Gland | 110 (3.5) | |
| Not Specified | 149 (4.8) | |
| Clinical Nodal Status | cN+ | 194 (6.4) |
| cN0 | 2,059 (67.4) | |
| cNX | 801 (26.2) | |
| Missing | 82 | |
| Pathological T Stage | T1 | 779 (25.4) |
| T2 | 760 (24.8) | |
| T3 | 575 (18.7) | |
| T4a/T4b | 956 (31.1) | |
| Missing | 66 | |
| Pathological N Stage | No Neck Dissection Done | 809 (26.8) |
| N0 | 1,855 (61.5) | |
| N+ | 354 (11.7) | |
| Missing | 118 | |
| Overall Pathological Stage | I | 607 (19.7) |
| II | 583 (18.9) | |
| III | 515 (16.7) | |
| IVA/IVB | 1,374 (44.7) | |
| Missing | 57 | |
| Treatment Characteristics | ||
| Time from diagnosis to definitive resection | 0–3 months | 1,857 (60.2) |
| 3–6 months | 184 (6.0) | |
| >6 months | 1,044 (33.8) | |
| Missing | 51 | |
| Surgical Margins | Positive | 1,365 (46.2) |
| Negative | 1,591 (53.8) | |
| Missing | 180 | |
| Receipt of Adjuvant Radiotherapy | Yes | 2,252 (71.8) |
| No | 884 (28.2) | |
| Receipt of Adjuvant Chemotherapy | Yes | 223 (7.4) |
| No | 2,779 (92.6) | |
| Missing | 134 | |
| Treatment Center Case Volume | High | 2,076 (66.2) |
| Low | 1,060 (33.8) | |
| Treatment Facility Type | Community Cancer Center | 495 (18.7_ |
| Academic Cancer Center | 1,317 (49.7) | |
| Comprehensive Community Cancer Center | 837 (31.6) | |
| Missing | 487 | |
| Treatment Facility Location | Northeast | 544 (20.5) |
| South | 927 (35.0) | |
| Midwest | 697 (26.3) | |
| West | 481 (18.2) | |
| Missing | 487 | |
Predictors of Receiving Adjuvant Radiation
A total of 2,252 (71.8%) patients received adjuvant radiation. The median total dose was 64.0 Gy (range 45.0 Gy to 66.6 Gy), 1,982 (88.0%) patients were treated with intensity-modulated radiation techniques, and 64 patients (2.8%) received neutron radiotherapy. Median radiation doses did not differ amongst age, race, and treatment facility (all p>0.52). On univariable analysis, younger patients, privately insured patients, patient’s living in a zip code <50 miles from their treatment facility, parotid gland tumors, clinically node positive tumors, positive surgical margins, patients receiving chemotherapy, and advancing pathological T, N, and overall stage tumors were more likely to receive adjuvant radiation while black patients, patients living in a lower-educated census tract, and patients treated in the south were less likely to receive radiation (Table 2). On multivariable analysis patient’s living in a zip code ≤10 miles from their treatment facility (OR=1.76, 95%CI: 1.27–2.45), pathological stage III (OR=1.77, 95%CI: 1.26–2.50) or stage IVA/B (OR=2.06, 95%CI: 1.48–2.88) patients, patients with positive margins (OR=1.71, 95%CI: 1.34–2.17), and patients receiving chemotherapy (OR=20.31, 95%CI: 4.94–83.49) were more likely to receive radiation while black patients (OR=0.66, 95%CI: 0.46–0.95), uninsured patients (OR=0.52, 95%CI: 0.30–0.89), younger patients (OR=0.97, 95%CI: 0.96–0.98) and palate primary tumors (OR=0.51, 95%CI: 0.37–0.72) compared to parotid primary tumors were less likely to receive radiation (Table 2).
Table 2.
Univariable and multivariable analysis of all patient, tumor, and treatment factors and their association with receiving adjuvant radiation after definitive resection.
| Univariable Analysis | Multivariable Analysis* | ||||
|---|---|---|---|---|---|
| Variable | Level | Odds Ratio (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value |
| Patient Characteristics | |||||
| Age (years) | Median (range) | 0.98 (0.97–0.99) | <0.01 | 0.97 (0.96–0.98) | <0.01 |
| Sex | Male | 1.11 (0.94–1.30) | 0.22 | -- | -- |
| Female | -- | -- | -- | -- | |
| Race | White | -- | -- | -- | -- |
| Black | 0.76 (0.59–0.96) | 0.02 | 0.66 (0.46–0.95) | 0.02 | |
| Other | 1.19 (0.89–1.60) | 0.23 | 0.96 (0.62–1.49) | 0.85 | |
| Insurance Status | Not Insured | 1.18 (0.86–1.61) | 0.30 | 0.52 (0.30–0.89) | 0.02 |
| Medicaid/Medicare | -- | -- | -- | ||
| Private | 1.69 (1.43–1.99) | <0.01 | 1.06 (0.78–1.43) | 0.71 | |
| Charlson-Deyo Comorbidity Score | 0 | 1.25 (0.99–1.59) | 0.06 | -- | -- |
| 1+ | -- | -- | -- | -- | |
| County of Residence | Metro | 1.25 (0.74–2.10) | 0.41 | -- | -- |
| Urban | 1.24 (0.72–2.14) | 0.44 | -- | -- | |
| Rural | -- | -- | -- | -- | |
| Percentage of Patient’s Census Tract without a High-School Degree (quartiles) | <14% | -- | -- | -- | |
| 14–19.9% | 0.93 (0.75–1.15) | 0.51 | -- | -- | |
| 20–28.9% | 0.91 (0.73–1.14) | 0.43 | -- | -- | |
| ≥29% | 0.78 (0.61–0.98) | 0.04 | -- | -- | |
| Median Income of Patient’s Census Tract | < $30,000 | 0.82 (0.63–1.06) | 0.13 | -- | -- |
| $30,000 – $35,999 | 0.88 (0.70–1.10) | 0.26 | -- | -- | |
| $36,000 – $45,999 | 1.02 (0.84–1.25) | 0.84 | -- | -- | |
| ≥ $46,000 | -- | -- | -- | ||
| Distance from patient’s census tract to treatment facility | ≤10 miles | 1.34 (1.07–1.67) | 0.01 | 1.76 (1.27–2.45) | <0.01 |
| 10–50 miles | 1.26 (1.01–1.57) | 0.04 | 1.30 (0.94–1.78) | 0.11 | |
| >50 miles | -- | -- | -- | -- | |
| Tumor Characteristics | |||||
| Primary Site | Parotid Gland | -- | -- | -- | -- |
| Palate | 0.62 (0.50–0.77) | <0.01 | 0.51 (0.37–0.72) | <0.01 | |
| Submandibular Gland | 1.13 (0.93–1.37) | 0.22 | 1.07 (0.82–1.39) | 0.61 | |
| Sublingual Gland | 0.64 (0.42–0.97) | 0.04 | 0.59 (0.34–1.04) | 0.07 | |
| Not Specified | 0.60 (0.42–0.86) | <0.01 | 0.62 (0.37–1.05) | 0.08 | |
| Clinically Lymph Node Positive (Prior To Surgery) | Yes | 1.85 (1.26–2.73) | <0.01 | -- | -- |
| No | -- | -- | -- | -- | |
| Pathological T Stage | T1 | -- | -- | -- | -- |
| T2 | 1.41 (1.13–1.77) | <0.01 | -- | -- | |
| T3 | 1.86 (1.44–2.40) | <0.01 | -- | -- | |
| T4a/T4b | 1.69 (1.33–2.16) | <0.01 | -- | -- | |
| Pathological N Stage | N0 | -- | -- | -- | -- |
| N+ | 1.83 (1.36–2.47) | <0.01 | -- | -- | |
| Overall Pathological Stage | I | -- | -- | -- | -- |
| II | 1.24 (0.96–1.59) | 0.10 | 1.22 (0.90–1.65) | 0.19 | |
| III | 2.00 (1.51–2.64) | <0.01 | 1.77 (1.26–2.50) | <0.01 | |
| IVA/IVB | 1.95 (1.51–2.53) | <0.01 | 2.06 (1.48–2.88) | <0.01 | |
| Treatment Characteristics | |||||
| Time from diagnosis to definitive resection | 0–3 months | 0.55 (0.26–1.20) | 0.14 | -- | -- |
| 3–6 months | 0.40 (0.12–1.31) | 0.13 | -- | -- | |
| >6 months | -- | -- | -- | -- | |
| Surgical Margins | Positive | 2.03 (1.71–2.40) | <0.01 | 1.71 (1.34–2.17) | <0.01 |
| Negative | -- | -- | -- | -- | |
| Receipt of Adjuvant Chemotherapy | Yes | 7.85 (4.26–14.47) | <0.01 | 20.31 (4.94–83.49) | <0.01 |
| No | -- | -- | -- | -- | |
| Treatment Center Case Volume | High | 1.12 (0.95–1.31) | 0.17 | -- | -- |
| Low | -- | -- | -- | -- | |
| Treatment Facility Type | Community Cancer Center | 0.99 (0.78–1.25) | 0.94 | -- | -- |
| Academic Cancer Center | -- | -- | -- | -- | |
| Comprehensive Community Cancer Center | 1.01 (0.83–1.22) | 0.96 | -- | -- | |
| Treatment Facility Location | Northeast | 1.01 (0.75–1.35) | 0.96 | 0.97 (0.65–1.44) | 0.87 |
| South | 0.70 (0.54–0.90) | <0.01 | 0.82 (0.58–1.16) | 0.26 | |
| Midwest | 0.78 (0.60–1.02) | 0.07 | 0.90 (0.63–1.30) | 0.59 | |
| West | -- | -- | -- | -- | |
CI = Confidence Interval
Bold indicates statistical significance
Of note, clinical node positivity, pathological T and N stage were not included in the multivariable model due to their high collinearity with pathological overall stage
Predictors of Receiving Adjuvant Chemotherapy
A total of 223 (7.4%) patients received adjuvant chemotherapy. On univariable analysis, male patients, younger patients, patients with clinically positive lymph nodes, patients treated in the northeast, patients treated at a high-volume center, positive surgical margins, patients receiving adjuvant radiation, patients treated at an academic center, and advancing T, N, and overall pathological stage were associated with receipt of chemotherapy (Supplemental Table 1). On multivariable analysis, patients with pathological stage III (OR=3.19, 95%CI: 1.42–7.21) or stage IVA/IVB (OR=7.66, 95%CI: 3.59–16.37) tumors, patients with positive surgical margins (OR=1.70, 95%CI: 1.14–2.54), and patients receiving adjuvant radiation (OR=17.81, 95%CI: 4.34–73.04) were more likely to receive adjuvant chemotherapy while patient’s who lived in an urban census tract were less likely to receive adjuvant chemotherapy (OR=0.18, 95%CI: 0.06–0.54) (Supplemental Table 1).
Clinical Nodal Status Relationship to Pathological Nodal Status
A total of 194 (6.4%) patients were reported clinically node positive prior to resection. Of these, 170 patients underwent a neck dissection with 139 (81.8%) being pathologically node positive. No information is reported as to why 24 clinically node positive patients did not receive a neck dissection. A total of 2,059 (67.4%) patients were reported clinically node negative prior to resection: 747 (36.3%) patients did not undergo a neck dissection and 1,312 (63.7%) underwent a neck dissection. Of the patients who had a neck dissection, 98 (7.5%) had unexpected pathologically positive nodes, with the rest having a negative neck.
Predictors of Positive Margin at the Time of Surgery
A total of 1,365 (46.2%) patients had positive margins after surgery, 625 (50.0%) patients with parotid tumors, 433 (40.9%) patients with submandibular tumors, 246 (43.1%) patients with palate tumors, 52 (47.2%) patients with sublingual tumors, and 9 patients with unknown site tumors. On univariable analysis, non-black or Caucasian patients, having clinically positively nodes pre-operatively, advancing T, N, and overall pathological stage, and resection within 3 months of diagnosis were associated with positive margins (Table 3). On multivariable analysis, advancing stage was associated with positive margins, with stage II (OR=1.76, 95%CI: 1.33–2.31), stage III (OR=2.96, 95%CI: 2.22–3.93), and stage IVA/IVB (OR=3.57, 95%CI: 2.72–4.70) patients more likely to have positive margins than stage I patients. Additionally, delay from diagnosis to surgery more than 3 months (OR=1.36, 95%CI: 1.09–1.68) was associated with positive margins at surgery (Table 3).
Table 3.
Univariable and multivariable analysis of all patient, tumor, and treatment factors and their association with positive margin status at the time of definitive resection.
| Univariable Analysis | Multivariable Analysis* | ||||
|---|---|---|---|---|---|
| Variable | Level | Odds Ratio (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value |
| Patient Characteristics | |||||
| Age (years) | Median (range) | 1.00 (1.00–1.01) | 0.41 | - | - |
| Sex | Male | 1.00 (0.87–1.16) | 0.97 | - | - |
| Female | - | - | - | - | |
| Race | White | - | - | - | - |
| Black | 0.87 (0.69–1.10) | 0.26 | 1.03 (0.76–1.40) | 0.85 | |
| Other | 1.31 (1.01–1.68) | 0.04 | 1.44 (0.99–2.07) | 0.05 | |
| Insurance Status | Not Insured | 0.73 (0.53–1.02) | 0.07 | - | - |
| Medicaid/Medicare | 0.97 (0.83–1.13) | 0.67 | - | - | |
| Private | - | - | - | - | |
| Charlson-Deyo Comorbidity Score | 0 | 0.90 (0.80–1.10) | 0.88 | - | - |
| 1+ | - | - | - | - | |
| County of Residence | Metro | 0.99 (0.90–1.08) | 0.72 | - | - |
| Urban | 1.14 (0.82–1.44) | 0.65 | - | - | |
| Rural | - | - | - | - | |
| Percentage of Patient’s Census Tract without a High-School Degree (quartiles) | <14% | - | - | - | - |
| 14–19.9% | 0.98 (0.81–1.19) | 0.85 | - | - | |
| 20–28.9% | 1.14 (0.93–1.38) | 0.20 | - | - | |
| ≥29% | 1.01 (0.81–1.26) | 0.92 | - | - | |
| Median Income of Patient’s Census Tract | < $30,000 | 1.21 (0.95–1.54) | 0.13 | - | - |
| $30,000 – $35,999 | 0.99 (0.80–1.22) | 0.89 | - | - | |
| $36,000 – $45,999 | 1.19 (1.01–1.43) | 0.06 | - | - | |
| ≥ $46,000 | - | - | - | - | |
| Distance from patient’s census tract to treatment facility | ≤10 miles | 1.00 (0.81–1.23) | 0.99 | - | - |
| 10–50 miles | 0.93 (0.76–1.15) | 0.53 | - | - | |
| >50 miles | - | - | - | - | |
| Tumor Characteristics | |||||
| Primary Site | Parotid Gland | - | - | - | - |
| Palate | 0.74 (0.60–0.91) | <0.01 | 0.87 (0.64–1.17) | 0.36 | |
| Submandibular Gland | 1.04 (0.88–1.24) | 0.62 | 1.03 (0.83–1.29) | 0.77 | |
| Sublingual Gland | 0.95 (0.64–1.42) | 0.81 | 0.90 (0.54–1.51) | 0.69 | |
| Not Specified | 0.84 (0.58–1.20) | 0.33 | 0.93 (0.58–1.49) | 0.76 | |
| Clinically Lymph Node Positive (Prior To Surgery) | Yes | 2.01 (1.47–2.73) | <0.01 | 0.97 (0.88–1.08) | 0.15 |
| No | - | - | - | - | |
| Pathological T Stage | T1 | - | - | - | - |
| T2 | 1.54 (1.25–1.90) | <0.01 | - | - | |
| T3 | 2.77 (2.21–3.48) | <0.01 | - | - | |
| T4a/T4b | 2.88 (2.30–3.60) | <0.01 | - | - | |
| Pathological N Stage | N0 | - | - | - | - |
| N+ | 1.94 (1.53–2.46) | <0.01 | - | - | |
| Overall Pathological Stage | I | - | - | - | - |
| II | 1.51 (1.18–1.92) | <0.01 | 1.76 (1.33–2.31) | <0.01 | |
| III | 2.72 (2.12–3.49) | <0.01 | 2.96 (2.22–3.93) | <0.01 | |
| IVA/IVB | 2.94 (2.32–3.72) | <0.01 | 3.57 (2.72–4.70) | <0.01 | |
| Treatment Characteristics | |||||
| Time from diagnosis to definitive resection | 0–3 months | 1.28 (1.10–1.50) | <0.01 | 1.36 (1.09–1.68) | <0.01 |
| 3–6 months | 1.29 (0.93–1.79) | 0.12 | 1.33 (0.88–2.01) | 0.17 | |
| >6 months | - | - | - | - | |
| Treatment Center Case Volume | High | 0.94 (0.81–1.09) | 0.42 | - | - |
| Low | - | - | - | - | |
| Treatment Facility Type | Community Cancer Center | 0.97 (0.79–1.20) | 0.80 | - | - |
| Academic Cancer Center | 1.17 (0.98–1.40) | 0.08 | - | - | |
| Comprehensive Community Cancer Center | - | - | - | - | |
| Treatment Facility Location | Northeast | 0.88 (0.68–1.13) | 0.33 | 1.03 (0.75–1.41) | 0.86 |
| South | 0.77 (0.61–0.96) | 0.02 | 0.75 (0.56–1.01) | 0.06 | |
| Midwest | 0.71 (0.56–0.90) | <0.01 | 0.76 (0.56–1.02) | 0.07 | |
| West | - | - | - | - | |
CI = Confidence Interval
Bold indicates statistical significance
Of note, clinical node positivity, pathological T and N stage were not included in the multivariable model due to their high collinearity with pathological overall stage
Factors Influencing Overall Survival
On unadjusted Kaplan-Meier analysis, the 5-year OS rate for patients receiving adjuvant radiation was 82.7% (95%CI: 80.7%–84.4%) compared to 78.3% (74.7%–81.4%) for those that did not receive adjuvant radiation (p=0.08).
On univariable analysis, older age, male sex, patients with clinically positive nodes before surgery, patients with non-Medicaid/Medicare insurance, patients with advancing pathological T, N, and overall stage, patients with positive surgical margins, patients receiving adjuvant chemotherapy, patients treated at a comprehensive community cancer center (versus academic center), and patients treated in the south were associated with worse OS while patients with improved comorbidity status with a Charlson-Deyo score of 0 and patients living between 10–50 miles from their treatment facility were associated with improved OS (Supplemental Table 2). Receipt of adjuvant radiation had a non-significant trend for improved OS in this model (p=0.08). On multivariable analysis, older age (HR=1.03, 95%CI: 1.02–1.04), male sex (HR=1.27, 95%CI: 1.03–1.56), advancing pathological tumor stage, positive surgical margins (HR=1.24, 95%CI: 1.01–1.53), and treatment facility located in the south (HR=1.48, 95%CI: 2.02) were associated with worse OS while having private insurance (HR=0.67, 95%CI: 0.51–0.89) and lower comorbidities with a Charlson-Deyo score of 0 (HR=0.72, 95%CI: 0.56–0.94) were associated with improved OS (Supplemental Table 2). Receipt of adjuvant radiation had no statistical association with OS (p=0.57).
As patients who received adjuvant radiation were more likely to have positive surgical margins and more advanced tumors, propensity-score matched analysis was conducted. After balancing for patient, tumor, and treatment characteristics that were associated with OS, 376 patients who received adjuvant radiation were matched to 376 who did not. Receipt of adjuvant radiation was not associated with OS on PSM (HR=0.90, 95%CI: 0.65–1.23).
DISCUSSION
Our series is the largest in the literature examining patterns of postoperative therapy and survival outcomes for non-metastatic salivary gland ACC. Among 3,136 patients, the majority (71.8%) received adjuvant radiation with a minority (7.4%) receiving adjuvant chemotherapy. Patients who lived closer to their treatment facility, had positive surgical margins, and had more advanced tumors were more likely to receive postoperative radiation while younger patients, black patients, and uninsured patients were less likely to receive adjuvant radiation. We report a 7.5% neck node positive rate in patients who were clinically node negative undergoing an elective neck dissection. Our series also confirmed known factors that influence overall survival in head and neck cancers including age, sex, comorbidity status, extent of resection, and tumor stage 7,10,13,21–23. Interestingly in this analysis we found that patients with private insurance and patients living closer to their treatment facility had improved OS, perhaps owing to improved access to care. In our series, with limited follow-up, the receipt of adjuvant radiation or chemotherapy was not associated with improved OS.
Surgery is the mainstay of therapy for resectable ACC, with a neck dissection only to be performed in patients with clinically positive nodes3,10,24. Historically, the incidence of lymph node involvement for ACC was thought to be low25, but more recent international collaboration efforts have seen positive neck lymph node rates of 15–30%, dependent on stage7,22,26,27. Our series, which represents the largest collection of patients with definitively treated ACC, found that 2,209 patients (70.4%) underwent neck dissection. We found that 194 patients (6.4%) had clinically positive nodes prior to resection, and 139 (81.8%) of those undergoing a neck dissection had confirmed pathological disease in the nodes. Our series lower rates of clinical node positivity is congruent with historical reports25 but contrary to recent international studies26, perhaps owing to our patient population representing a much larger sample of the cancer community in the United States. Our series also reports that 7.5% of patients had unexpected pathologically positive nodes at the time of elective neck dissection. This information may help guide the use of elective neck dissections for this rare disease.
Locoregional control rates with surgery alone are reported between 30–70%, with a wide range related to the rarity of the disease and mostly single-institution publications1,3–5. Given these results, postoperative radiation is frequently administered, mostly based on retrospective single institution evidence, with most series reporting a 20–30% locoregional control benefit at 10 years1,23,28. Our series confirms that postoperative radiation is frequently given in the US as 71.8% of patients in our series received adjuvant radiation. Despite a benefit of conventional adjuvant radiation for locoregional disease control, the impact of radiation on OS is less clear. ACC can recur many years after initial treatment, and thus long-term survival estimates can be challenging3,7. Many of the single-institution series that found a locoregional control benefit to radiation subsequently found no differences in survival1,22,23,29. There was no OS benefit to adjuvant radiation in our series, even when balancing for patient, tumor, and treatment factors. However, the NCDB is limited in endpoint reporting and the median follow up in our series was 4.87 years which may not be long enough to witness a benefit to postoperative therapy for ACC, which have a long natural history. We cannot comment on a local or regional disease control benefit to postoperative radiation.
The role of chemotherapy in resected ACC is unclear, with most literature estimates indicating response rates between 0 to 29%9, with one recent review found that in eight separate studies involving a total of 151 patients, there were no complete responses and one partial response to chemotherapy3. The low response rates to cytotoxic therapy have been attributed to the slow growth kinetics of ACC3. Given the lack of published prospective trials with systemic therapy, generally chemotherapy is reserved for palliation of symptomatic metastases or rapidly progressing disease if not a candidate for other therapies3,12. Our series representing approximately 70% of all nationwide malignancy diagnoses, found that 7.4% of patients received postoperative chemotherapy, with more advanced disease and positive margins associated with its delivery. There was no OS benefit to adjuvant chemotherapy, and in fact its receipt was associated with an OS detriment in our series (HR=1.48, 95%CI: 1.05–2.09), likely related to unfavorable patient selection. Taken together, there does not currently appear to be a role of adjuvant chemotherapy in resected ACC. The enrolling prospective randomized trial RTOG 1008, comparing adjuvant concurrent chemoradiation versus adjuvant radiation alone, in resected high-risk salivary gland tumors (including ACC), will hopefully answer this question in the future30.
Our series confirmed known prognostic factors with ACC. Male sex (HR=1.27, 95%CI: 1.03–1.56) was associated with worse OS, in line with Surveillance, Epidemiology, and End Results (SEER) reports for ACC21. Patients with less medical comorbidities, delineated with a Charlson-Deyo score of 0 (HR=0.72, 95%CI: 0.56–0.94) had improved OS, further validating these indices14,15. Positive surgical margins (HR=1.24 95%CI: 1.01–1.53) was associated with worse OS, congruent with previously published ACC reports as well as in other head and neck subsites5,21–23,29,31, and this series provides the largest assessment of surgical margins by ACC subsite. More advanced pathological stage was also associated with worse OS in our series, confirming the accuracy of the modern staging system for this rare disease13. On multivariable analysis, advancing stage was found to be associated with positive resection margins. Our series also found socioeconomic and demographic factors related to OS. Patients with private insurance had improved OS. This benefit is possibly related to access to healthcare, known to be important in clinical outcomes for many cancer subsites32–36.
This study has several strengths and limitations. The strengths include the largest number of resected non-metastatic head and neck ACC of any study to date, all treated in the modern era. Our series provides the most comprehensive examination of postoperative practice patterns in the US, with almost two-thirds of patients receiving adjuvant radiation and a small number receiving chemotherapy. We also report unexpected lymph node positivity in 7.5% patients with clinically negative necks, which may help guide surgical management. Half of the patients in our series had their therapy at an academic center. However, like other studies using registries, the NCDB does not capture all variables. We do not have information on disease-specific control, including locoregional outcomes. We did not find an OS benefit to adjuvant radiation, but there may be a disease-control benefit that our data did not allow us to investigate. Additionally, as ACC can recur many years after initial resection, our median follow-up time of 4.87 years is likely not long enough to assess survival outcomes. Perineural invasion and solid tumor histology, both known to be associated with worse outcomes in ACC1,4,37, are not captured in the NCDB and there may be potential imbalance of these factors in our series. The NCDB does not include patients treated at non-Commission on Cancer accredited sites, which may have different practice patterns. This series cannot comment on the impact of neutron therapy. The NCDB records detailed surgical and radiation information, but the information on chemotherapy types, number of cycles, and compliance is not available. Treatment toxicity information is not available, so short or long-term morbidity from therapy cannot be assessed.
CONCLUSION
In this analysis, 71.8% of resected non-metastatic salivary gland ACC receive adjuvant radiation with 7.4% of cases receiving adjuvant chemotherapy. Patients receiving adjuvant radiation or adjuvant chemoradiation were more likely to have more advanced disease and positive surgical margins. Black patients and patients living far away from their treatment facility were less likely to receive adjuvant radiation. Receipt of adjuvant radiation, with or without chemotherapy, had no statistical association with OS, which is limited by a median follow up of 4.87 years. This series cannot comment on locoregional control outcomes. The rate of unexpected nodal disease after elective neck dissection was 7.5%. Among other variables, several socioeconomic factors influenced survival as patients with private insurance and patients who lived closer to their treatment facility had improved OS while male patients and patients treated in the southern US had worse OS. This information further needs to be investigated and addressed by the oncologic community for this rare disease.
Supplementary Material
Supplemental Table 1: Univariable and multivariable analysis of all patient, tumor, and treatment factors and their association with receiving adjuvant chemotherapy after definitive resection.
Supplemental Table 2: Univariable and multivariable Cox-proportional hazard analysis of all patient, tumor, and treatment factors and their association with overall survival.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Conflicts of Interest/Disclosures: None
CONFLICTS OF INTEREST: None
LEVEL OF EVIDENCE: 2c
References
- 1.Chen AM, Bucci MK, Weinberg V, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66:152–159. doi: 10.1016/j.ijrobp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Papaspyrou G, Hoch S, Rinaldo A, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33:905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 3.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: A review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38:620–627. doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–162. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang DQ, Liang LZ, Zheng GS, et al. Risk factors and prognosis for salivary gland adenoid cystic carcinoma in southern china: A 25-year retrospective study. Medicine (Baltimore) 2017;96:e5964. doi: 10.1097/MD.0000000000005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512–520. doi: 10.1016/0002-9610(74)90265-7. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Ge M, Chen X, Xu J, Chen C. Clinical outcomes and prognostic factors of salivary gland adenoid cystic carcinomas: a case control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 doi: 10.1016/j.oooo.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Azumi N, Battifora H. The cellular composition of adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1987;60:1589–1598. doi: 10.1002/1097-0142(19871001)60:7<1589::aid-cncr2820600729>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12:815–824. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Xu L, Zhao H, El-Naggar AK, Sturgis EM. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012;118:3945–3953. doi: 10.1002/cncr.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskaluk CA. Adenoid cystic carcinoma: clinical and molecular features. Head Neck Pathol. 2013;7:17–22. doi: 10.1007/s12105-013-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfister DG, Spencer S, et al. [Accessed 3/15/2017];NCCN Guidelines Version 1.2017 Head and Neck Cancers. 2017 Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 13.Edge SB. AJCC cancer staging manual. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Nickleach DLY, Shrewsberry A, et al. SAS® Macros to Conduct Common Biostatistical Analyses and Generate Reports. SESUG 2013: The Proceeding of the SouthEast SAS User Group. 2013 [Google Scholar]
- 17.LSP Reducing bias in a propensity score matched pair sample using greedy matching techniques. SAS SUGI. 2001;26:214–226. [Google Scholar]
- 18.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson P. On robustness and model flexibility in survival analysis: transformed hazard models and average effects. Biometrics. 2007;63:69–77. doi: 10.1111/j.1541-0420.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin DYLW. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 21.Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 22.Ko YH, Lee MA, Hong YS, et al. Prognostic factors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn J Clin Oncol. 2007;37:805–811. doi: 10.1093/jjco/hym119. [DOI] [PubMed] [Google Scholar]
- 23.Balamucki CJ, Amdur RJ, Werning JW, et al. Adenoid cystic carcinoma of the head and neck. Am J Otolaryngol. 2012;33:510–518. doi: 10.1016/j.amjoto.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615–619. doi: 10.1002/1097-0142(19920201)69:3<615::aid-cncr2820690303>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Amit M, Binenbaum Y, Sharma K, et al. Incidence of cervical lymph node metastasis and its association with outcomes in patients with adenoid cystic carcinoma. An international collaborative study. Head Neck. 2015;37:1032–1037. doi: 10.1002/hed.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International H Neck Scientific G. Cervical lymph node metastasis in adenoid cystic carcinoma of the major salivary glands. J Laryngol Otol. 2017;131:96–105. doi: 10.1017/S0022215116009749. [DOI] [PubMed] [Google Scholar]
- 28.Miglianico L, Eschwege F, Marandas P, Wibault P. Cervico-facial adenoid cystic carcinoma: study of 102 cases. Influence of radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13:673–678. doi: 10.1016/0360-3016(87)90284-7. [DOI] [PubMed] [Google Scholar]
- 29.Oplatek A, Ozer E, Agrawal A, Bapna S, Schuller DE. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope. 2010;120:65–70. doi: 10.1002/lary.20684. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez CAD. RTOG 1008: A Randomized Phase II/Phase III Study of Adjuvant Concurrent Radiation and Chemotherapy versus Radiation Alone in Resected High-Risk Malignant Salivary Gland Tumors. [Accessed 3/26/2017];2017 Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1008.
- 31.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 32.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 33.Lee W, Nelson R, Mailey B, Duldulao MP, Garcia-Aguilar J, Kim J. Socioeconomic factors impact colon cancer outcomes in diverse patient populations. J Gastrointest Surg. 2012;16:692–704. doi: 10.1007/s11605-011-1809-y. [DOI] [PubMed] [Google Scholar]
- 34.Tawk R, Abner A, Ashford A, Brown CP. Differences in Colorectal Cancer Outcomes by Race and Insurance. Int J Environ Res Public Health. 2015;13 doi: 10.3390/ijerph13010048. ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassidy RJ, Switchenko JM, Cheng E, et al. Health care disparities among octogenarians and nonagenarians with stage II and III rectal cancer. Cancer. 2017 doi: 10.1002/cncr.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassidy RJ, Zhang X, Switchenko JM, et al. Health care disparities among octogenarians and nonagenarians with stage III lung cancer. Cancer. 2018 doi: 10.1002/cncr.31077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Weert S, van der Waal I, Witte BI, Leemans CR, Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015;51:71–76. doi: 10.1016/j.oraloncology.2014.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Univariable and multivariable analysis of all patient, tumor, and treatment factors and their association with receiving adjuvant chemotherapy after definitive resection.
Supplemental Table 2: Univariable and multivariable Cox-proportional hazard analysis of all patient, tumor, and treatment factors and their association with overall survival.


