Abstract
Background.
Eosinophilic esophagitis (EoE) is a chronic Th2-assocated inflammatory condition accompanied by substantial impairments in epithelial barrier function and increased numbers of IL-9 expressing inflammatory cells. While IL-9 is known to affect barrier function in the intestine, the functional effects of IL-9 on the esophagus are unclear. Herein we aimed to understand the expression of the IL-9 receptor and effects of IL-9 on the epithelium in EoE.
Methods.
We used esophageal biopsies from pediatric EoE patients with active and inactive disease to analyze the expression of the IL-9 receptor, the adherens junction protein E-cadherin and the tight junction protein claudin-1. We treated primary human esophageal epithelial cells with IL-9 to understand its effects on E-cadherin expression and function.
Results.
Active EoE subjects had increased epithelial expression of IL-9 receptor mRNA and protein (p<0.05) and decreased membrane bound E-cadherin (p<0.01) and claudin-1 (p<0.05) expression. IL-9 receptor expression and mislocalized claudin-1 positively correlated and while membrane bound E-cadherin expression negatively correlated with the degree of histologic epithelial remodeling (p<0.05). IL-9 decreased epithelial resistance in stratified primary human esophageal epithelial cells (p<0.01) and membrane bound E-cadherin in epithelial cell monolayers (p<0.01).
Conclusion.
These data suggest that IL-9, its receptor, and its effects on E-cadherin may be important mechanisms for epithelial barrier disruption in EoE.
Introduction
Eosinophilic esophagitis (EoE) is an antigen-induced Th2 inflammatory disease of increasing prevalence and incidence in children and adults 1,2. Inflammation-dependent and independent esophageal remodeling mediate disease complications 3–6. Histologic epithelial remodeling includes basal cell hyperplasia with loss of differentiated squamous cells, dilated intercellular spaces, and desquamation and functionally associates with loss of appropriate barrier integrity 7–9. Diminished epithelial barrier function the EoE esophagus is not always fully reversed with reduced inflammation and may be an important instigating and/or propagating mechanism for the disease 8.
The mechanisms for barrier dysfunction in EoE include down-regulation of epithelial cell expression of claudin-7 and desmoglein-1 by TGFβ1 and IL-13 7,10,11. Reports of E-cadherin expression have been mixed with some studies showing decreased expression and others reporting no changes 10,12,13. Decreased levels of claudin-1 and occludin have been reported 12. Esophageal resistance to both electrical current and the flux of extracellular tracers is decreased in active EoE biopsies and in esophageal epithelial cell culture models treated with TGFβ1 or IL-13 7,8,10,11.
We have previously demonstrated that there are increased numbers of IL-9 expressing eosinophils in the active EoE esophagus 14. IL-9 expressing mast cells are functionally important in intestinal food allergy 15 and there is a significant mastocytosis in EoE subjects, especially those who have food sensitization 16–18. IL-9 induces epithelial barrier disruption in inflammatory bowel diseases such as ulcerative colitis by promoting the expression of the pore forming protein, claudin-2, and decreasing the expression of tight junction proteins, ultimately resulting in the loss of barrier integrity and increased bacterial translocation 15,19–21. We hypothesized that IL-9 might have detrimental effects on esophageal epithelial barrier function by altering the expression of proteins, such as E-cadherin, that are essential for epithelial integrity and stratification. Herein, we demonstrate that Il-9 can alter the localization and expression of E-cadherin in primary esophageal epithelial cells and that children with active EoE have altered expression of both E-cadherin and claudin-1.
Methods
EoE subjects/biopsies and immunohistochemistry
Using our University of California, San Diego (UCSD)/Rady Children’s Hospital, San Diego (RCHSD) EoE database, we selected a cohort of EoE subjects who had active and inactive EoE, defined as ≥15 eosinophils and <15 eosinophils per hpf, respectively. Biopsies from subjects with inactive and active disease (unpaired samples) were utilized in qPCR studies. Twenty individual subject biopsies were stained as previously described.22,23 Paired biopsy specimens were used for all of the E-cadherin and claudin-1 stains. IL-9 receptor (IL-9R) staining was done in 9 subjects, 4 had paired biopsy specimens while the other 5 were unpaired specimens. Paired biopsies came from the same subject prior to and following topical steroid treatment as part of standard of care therapy. Stains for IL-9R, E-cadherin, claudin-1 were performed in 9, 9, and 10 individuals, respectively, with 7 subjects whose biopsies were stained for both E-cadherin and claudin-1 and one subject biopsy that was stained for both IL-9R and claudin-1 (Table 1). Written informed consent/assent was obtained under UCSD/RCHSD IRB approved protocol 091485. Procurement and use of esophageal epithelial cells from deceased organ donors was not considered human subjects research and approved under UCSD IRB protocol 130835.
Table 1.
Subject Characteristics
| Patient | Gender | Age | Eosinophils per hpf | Stain | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Claudin-1 | E-cadherin | IL-9R | |||
| 1 | M | 1 | 21 | 7 | X | ||
| 2 | M | 11 | 85 | 0 | X | X | |
| 3 | F | 5 | 59 | 0 | X | ||
| 4 | M | 2 | 51 | 11 | X | X | |
| 5 | F | 7 | 4 | 0 | X | X | |
| 6 | M | 14 | 104 | 0 | X | X | |
| 7 | M | 4 | 88 | 2 | X | X | |
| 8 | M | 7 | 90 | 1 | X | X | |
| 9 | M | 16 | 81 | 12 | X | X | |
| 10 | F | 4 | 44 | 0 | X | ||
| 11 | F | 2 | 168 | 0 | X | ||
| 12 | M | 2 | Unpaired | 0 | X | ||
| 13 | M | 9 | 79 | 0 | X | X | |
| 14 | M | 5 | Unpaired | 1 | X | ||
| 15 | M | 9 | 155 | Unpaired | X | ||
| 16 | M | 8 | 200 | 8 | X | ||
| 17 | M | 5 | 60 | 9 | X | ||
| 18 | M | 5 | 80 | Unpaired | X | ||
| 19 | F | 8 | 30 | Unpaired | X | ||
| 20 | M | 1 | 90 | 4 | X | ||
hpf: high power field; M: male; F: female; IL-9R: IL-9 receptor
Hematoxylin and eosin stained, formalin fixed, paraffin embedded specimens were scored by a single pathologist (R.N.) using our previously published standardized histology scoring tool 24. The numbers of epithelial eosinophils, the severity of basal zone hyperplasia (scored 0–3), the presence/absence of dilated intercellular spaces (scored 0/1), and the presence/absence of epithelial desquamation (scored 0/1) were quantified. The epithelial remodeling score is a composite index of the latter three epithelial cell features (maximum score of 5).
Epithelial staining was quantified using color detection and image analysis (Image J) to assess the height of positively staining cells or cell layers with membrane bound or cytoplasmic E-cadherin and claudin1 relative to the total epithelial height (the same index utilized for calculating basal zone hyperplasia). For IL-9 receptor quantification, positively staining epithelial layers were selected using the plug-in Image J IHC profiler to objectively quantify the percent of high positive, low positive, and negative pixels. Only those layers with “high positive” on optical vector density determination were quantitatedFor any stain, 3–5 image fields were captured and the mean value for a given subject was utilized. All images were analyzed under identical light microscopic conditions, including magnification, gain, camera position, and background illumination.
Quantitative PCR
Biopsies were collected in RNA later (Life Technologies, Carlsbad CA) for RT qPCR experiments as previously described 26. Primer sequences were IL-9R CCA ATG ACC ACA CTT and reverse- GCC TCA TGG ATA AAG CCCA GGG 3 and RPL13A forward- CAT AGG AAG CTG GGA GCA AG and reverse- GCC CTC CAA TCA GTC TTC TG 3’
Cell culture and treatment
Human organ transplant donor esophagi provided by the National Disease Research Interchange and the Arkansas Regional Organ Recovery Agency were processed to isolate epithelial cells. Cells were treated with IL-9, for 48 hours. Staining studies were repeated three times in duplicate using esophageal epithelial cells derived from 2 donors.
Transepithelial Resistance
Epithelial cells were plated on transwell permeable supports (6.5 mm insert, 1.9cm2, 0.4um polyester membrane, Costar, Cambridge, MA) coated with collagen (0.033 mg/ml), human fibronectin (0.01 mg/ml), and BSA (0.02 mg/ml) 27. Resistance was measured daily using a voltohmmeter (World Precision Instruments, Sarasota FL) and measurements corrected by subtracting the resistance of a blank insert without cells and multiplying by the area of insert. Epithelial resistance studies were repeated 3 times in duplicate to triplicate wells using epithelial cells derived from 3 organ donors.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (San Diego, CA). A Student’s t-test for unpaired variables was used to compare between two groups with Gaussian distribution; non-Gaussian data/non-continuous variables were compared using a Mann-Whitney test. A t-test for paired variables was used for pre-post comparisons of Gaussian data/continuous variables; a Wilcoxon’s test was used for non-parametric paired data. A Spearman’s coefficient was calculated for correlations. A two tailed p value <0.05 was considered statistically significant. To ensure this overall error rate, we used the false discovery rate (FDR) to control for multiple comparisons. The FDR is a popular alternative to the classic Bonferroni correction as the latter is too conservative, especially with large number of tests as in the current study. We also computed estimated power for the all of the tests performed in our study. In each case, we set power = 80% and type I error alpha = 0.05, and estimated sample size required to detect an effect size as small as the one observed. Given the large effect sizes in both the paired t-test and correlation, our estimated sample sizes were very small (e.g. 3 and 7) and all were below the actual sample size used in the test.
Results
Clinical characteristics
We studied biopsy specimens from a total of 20 children. Of these, 75% were male, 70% failed PPI monotherapy, and 75% had allergies to aeroallergens and/or foods. The inactive biopsies were due to topical steroid treatment with budesonide in all subjects (Table 1).
IL-9 receptor expression in the EoE esophagus
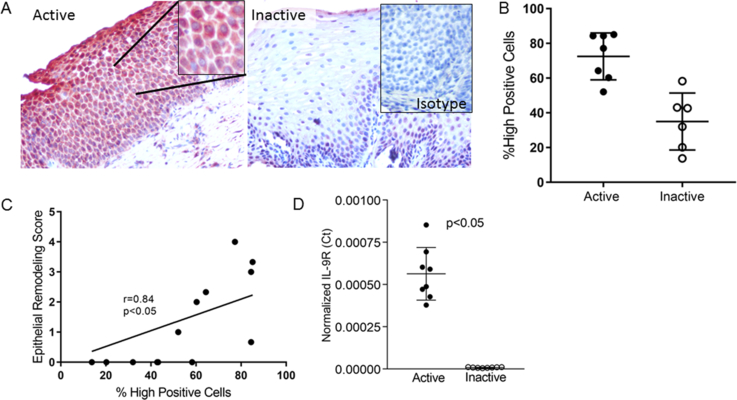
In order to assess whether IL-9 might play a role in regulating the function of the esophageal epithelium in EoE, we analyzed IL-9 receptor (IL-9R) expression in paired active and inactive EoE biopsies (n=7). Immunohistochemistry and image analysis demonstrated that active EoE biopsies expressed significantly more IL-9R in epithelial cells than inactive biopsies (active mean=74, 95% CI 63–85, inactive mean=35, 95% CI 21, 49) p=0.0005) (Figure 1a, b). The degree of epithelial IL-9R expression significantly correlated with the epithelial remodeling score (r=0.82, p=0.0007) (Figure 1c). A sub-analysis demonstrated that the parameter that best correlated with the severity of IL-9R expression was the degree of basal zone hyperplasia (not shown). Consistent with protein data, IL-9R transcript was significantly higher in active EoE biopsies (p=0.0002) (Figure 1d).
Figure 1. IL-9 receptor (IL-9R) expression in EoE.
Representative images (A) and quantitation (B) of IL-9R positive cells in the esophageal epithelium of active and inactive EoE biopsies. Inset in inactive biopsy shows isotype control on an active EoE biopsy. Lines represent mean and standard deviation. The correlation between IL-9R expression and epithelial remodeling (C). Normalized IL-9R mRNA expression in active and inactive biopsies. Bars represent the mean and standard deviation (D).
Barrier protein expression in the EoE esophagus
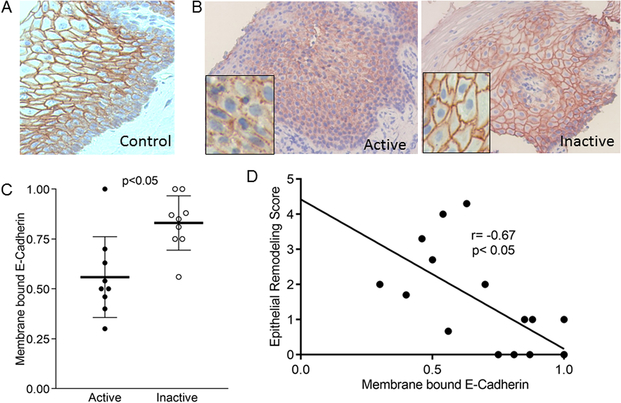
To understand whether IL-9 could have detrimental effects on the esophageal epithelium, we systematically analyzed the distribution pattern of the adherens and tight junction proteins E-cadherin and claudin-1 in normal specimens of esophagus from human donors and children with active and inactive EoE. In both normal specimens and biopsies from subjects with inactive EoE, the majority of E-cadherin was membrane-bound and localized predominantly to stratum basale and spinosum with less expression in the most luminal stratified layers (Figure 2a, b). Conversely, E-cadherin staining in active EoE biopsies was decreased, more punctate, and less membrane-localized (Figure 2b). Quantitation using paired biopsy specimens demonstrated that there were significantly fewer epithelial cell layers expressing membrane bound E-cadherin in active as opposed to inactive EoE (active mean=0.56, 95% CI 0.40, 0.71; inactive mean=0.83, 95% CI 0.73, 0.93 p=0.004) (Figure 2c). The amount of membrane-bound E-Cadherin staining inversely correlated with the epithelial remodeling score (95% CI −0.89, 0.28; p=0.0025), demonstrating that the severity of epithelial cell abnormalities is associated with less properly localized E-cadherin (Figure 2d).
Figure 2. E-cadherin expression in EoE.
Representative images of E-cadherin in control (A), inactive, and active EoE (B). Quantitation of membrane bound E-cadherin in active and inactive EoE. Lines represent means, bars are standard deviation (C). Correlation between the severity of esophageal epithelial remodeling and membrane bound E-cadherin (D)
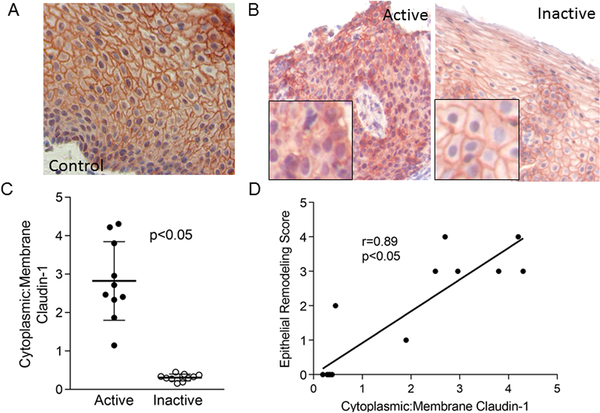
Membrane-bound claudin-1 was expressed through all layers of normal and inactive EoE biopsies (Figure 3a, b). In active EoE there was significantly less membrane bound and more cytosolic claudin-1 (ratio of cytoplasmic to membrane bound claudin-1 active 95% CI 2.1, 3.6; inactive 95% CI 0.25, 0.38; p=0.002) (Figure 3b, c). The ratio of cytoplasmic to membrane bound claudin-1 positively correlated with the severity of epithelial remodeling (r=0.89, 95% CI 0.67 0.97; p<0.001), demonstrating that the degree of epithelial cell abnormalities associated with mislocalized claudin-1. In addition, the degree of mislocalized claudin-1 correlated with the severity of esophageal eosinophilia (r= 0.72, p<0.05, data not shown). Together, these data demonstrate that there is a significant dysregulation of the barrier proteins E-cadherin and claudin-1 in children with active EoE and that the expression and localization of these proteins can improve following therapy that diminishes eosinophilic inflammation.
Figure 3. Claudin-1 expression in the EoE.
Representative images of claudin-1 in control (A), active, and inactive EoE (B) esophageal biopsies. Quantitation of the ratio of cytoplasmic : membrane- bound claudin-1 in active and inactive EoE Lines represent means, bars are standard deviation (C) and its correlation with the severity of epithelial remodeling (D).
IL-9 regulates barrier protein expression and function
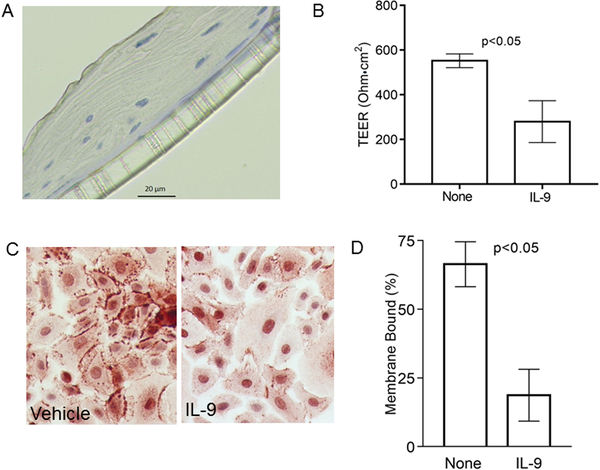
Based on its role in other diseases such as inflammatory bowel disease and asthma, we hypothesized that IL-9 may have deleterious effects on the esophagus by altering epithelial barrier function. We seeded primary human esophageal epithelial cells isolated from human donor mucosae onto transwells until both stratification (Figure 4a) and transepithelial electrical resistance (TEER) were present. Once stratification and TEER were maximal, the cells were placed at an air liquid interface (ALI) and treated with IL-9 or its vehicle control. IL-9 significantly decreased TEER by day 4 as compared with vehicle treated cells (no treatment TEER mean 552, 95% CI 476, 628; IL-9 TEER mean=279, 95% CI 47, 513; p=0.009) (Figure 4b). In order to assess if IL-9 affected esophageal epithelial barrier protein expression and/or localization of E-cadherin, we treated primary esophageal epithelial cell monolayers with IL-9 or vehicle for 48 hours. As compared with control wells, IL-9 treatment significantly decreased the amount of membrane bound E-cadherin (mean percent membrane bound E-cadherin with vehicle=66 95% CI 58, 75; mean IL-9=19, 95% CI 9, 29; p=0.002) (Figure 4c, d).
Figure 4. IL-9 effects on esophageal epithelial barrier and E-cadherin.
Representative image of stratified primary esophageal epithelial cells epithelium on transwells (A) and transepithelial resistance following treatment with IL-9 or vehicle. Bars represent mean and standard deviation (B). Representative image (C) and quantitation of membrane bound E-cadherin expression on cultured epithelial cells treated with vehicle or IL-9. Bars represent means and standard deviations (D).
Discussion
In this manuscript, we demonstrate a number of findings in EoE related to barrier proteins, function, and IL-9. We demonstrate decreased and mislocalized expression of the adherens junction protein E-cadherin and the tight junction protein claudin-1 during active EoE which significantly improves following therapy. We demonstrate that the epithelium expresses the IL-9 receptor during active EoE, consistent with the ability of increased IL-9 to damage the epithelial barrier during active disease. Lastly, we demonstrate that non-immortalized human esophageal epithelial cells respond to IL-9 treatment in vitro with decreased transepithelial resistance and diminished and mislocalized E-cadherin.
Prior studies have implicated both IL-13 and TGFβ1 in barrier disruption in EoE via alterations in claudin-7 and desmoglein-1 7,10,11. In addition, decreased expression of E-cadherin and claudin-1 have been reported in pediatric and adult EoE 12,13. However, the findings reported for E-cadherin have been inconsistent since one study demonstrated no change in expression while others showed decreased expression 12,13,28. These studies utilized a limited group of patients. In addition, one recent study documented no changes in E-cadherin expression during topical steroid therapy in adults but it was not clear if paired biopsy specimens were utilized in the analysis or if the subjects were histologic responders to therapy 13. Herein we systematically assessed barrier protein expression in paired specimens from children who had an adequate histologic response to topical corticosteroids and demonstrate a significant decreases in membrane-bound E-cadherin during active EoE that is reversed by successful therapy. While non-diseased esophagi and those from inactive EoE showed E-cadherin expression in the stratum basale and the stratum spinosum, the active EoE esophagus had decreased and mislocalized E-cadherin seen throughout the epithelium. This expression pattern in active disease was closely related to the severity of epithelial remodeling defined as a composite index of basal zone hyperplasia, dilate intercellular spaces, and epithelial desquamation. Even in the normal esophagus, the basal layers closest to the lamina propria express less membrane-bound E-cadherin and the loss of E-cadherin could be reflective of an increased proportion of these less differentiated epithelial cells in active EoE.
Interestingly, E-cadherin is essential for barrier function as well as proper epithelial stratification and flattening of cell morphology in the skin 29,30. Mice that are deficient in E-cadherin have transepithelial water loss, perinatal demise, and decreased numbers of epithelial cells with the flattened morphology normally present in differentiated squamous cells 30. It is possible that the loss of E-cadherin in the EoE epithelium is a primary insult that leads to subsequent dysregulation of morphologic changes in the normally stratified squamous epithelium. Indeed, there striking histologic similarities between the skin biopsies from E-cadherin/p-cadherin deficient mice and the esophagi of active EoE patients.
In addition, proper E-cadherin expression is essential for claudin-1 localization 30. Loss of E-cadherin causes improper tight junction protein localization, specifically that of claudin-1, but does not affect the expression of desmosomal proteins. Claudin-1 is essential for survival and skin barrier function and claudin-1 deficient mice have a rapid postnatal demise due to transepithelial water loss 31. As such, it may be that the loss of E-cadherin has subsequent downstream impacts on tight junction proteins important for epithelial barrier function. Doubtlessly, barrier integrity is a complex process and it is more than likely that multiple pathways for epithelial cell dysregulation exist in the EoE esophagus. In addition, distinguishing barrier endotypes in EoE may elucidate why there is heterogeneity in treatment response and disease severity/progression in EoE. For example, the ability of IL-9 to induced E-cadherin loss versus that of IL-13 to induce calpain-14 and desmoglein-1 loss likely represent two unique mechanisms for epithelial barrier dysfunction in EoE. In this regard, it is possible that the convergence of multiple pathways could predispose to a more severe and/or therapy resistant phenotype. Additional complexities such as phosphorylation-dependent changes in claudin-1 remain to be unraveled. Thus, it will be of interest to explore whether interleukin signals can change the membrane bound state by specifically altering the phosphorylation status of junctional proteins.
IL-9 can have deleterious effects on the intestinal epithelium both directly, by altering the expression of pore forming proteins, and indirectly, through the recruitment and retention of mast cells 20. In ulcerative colitis, IL-9 up-regulates the pore-forming molecule, claudin-4, allowing bacterial translocation from the colon 20. Further, IL-9R expression in ulcerative colitis can serve as a marker of disease severity. IL-9 is an important regulator of mastocytosis and the recent description of a novel group of IL-9 producing mast cells further supports a role for IL-9 in food allergic intestinal disorders 14,15,32. Indeed, the presence of IL-9 induced barrier disruption could represent a sub-phenotype of food allergy driven EoE.
In conclusion, our data suggest that IL-9 may be an important mediator of esophageal epithelial dysfunction in EoE and could represent a new therapeutic target. Future larger studies to better understand the complexity of EoE endotypes should include an assessment of the severity and mechanisms driving epithelial barrier dysfunction.
What is known:
Barrier impairment is involved in the pathogenesis of EoE
IL-9 is increased in EoE
Tissue remodeling accompanies EoE
What is new:
IL-9 receptor is expressed in pediatric active EoE biopsies
E-cadherin and claudin-1 are mislocalized in active EoE
IL-9 impairs esophageal epithelial cell barrier function and protein expression
Acknowledgements:
We thank Tom Yang, B.S. and Arjun Anilkumar, B.S. for technical assistance. The UCSD–RCHSD database is maintained by the Altman Clinical Research Institute at UCSD. The project described was partially supported by NIH grant UL1TR001442. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding Sources: NIH/NIAID AI 092135 (S.S.A), NIH/NIAID AI 135034 (S.S.A), Rady Children’s Hospital Academic Enrichment Grant (S.S.A.), NIH/NCRR/NCATS UL1TR000039 (R.C.K.)
Footnotes
The authors have no relevant conflicts of interest
References
- 1.Dellon ES. Epidemiology of Eosinophilic Esophagitis. Gastroenterology clinics of North America 2014; 43(2): 201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. The Journal of allergy and clinical immunology 2011; 128(1): 3–20 e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 3.Nhu QM, Aceves SS. Tissue Remodeling in Chronic Eosinophilic Esophageal Inflammation: Parallels in Asthma and Therapeutic Perspectives. Front Med (Lausanne) 2017; 4: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointestinal endoscopy 2014; 79(4): 577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145(6): 1230–6 e1–2. [DOI] [PubMed] [Google Scholar]
- 6.Tkachenko E, Rawson R, La E, et al. Rigid substrate induces esophageal smooth muscle hypertrophy and eosinophilic esophagitis fibrotic gene expression. The Journal of allergy and clinical immunology 2016; 137(4): 1270–2 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis BP, Stucke EM, Khorki ME, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight 2016; 1(4): e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rhijn BD, Verheij J, van den Bergh Weerman MA, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. The American journal of gastroenterology 2015; 110(9): 1289–97. [DOI] [PubMed] [Google Scholar]
- 9.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE 2017; 30(3): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal immunology 2014; 7(3): 718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen N, Fernando SD, Biette KA, et al. TGF-beta1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, et al. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clinical immunology 2013; 148(2): 265–78. [DOI] [PubMed] [Google Scholar]
- 13.Simon D, Page B, Vogel M, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid-treated eosinophilic esophagitis. Allergy 2017. [DOI] [PubMed] [Google Scholar]
- 14.Otani IM, Anilkumar AA, Newbury RO, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. The Journal of allergy and clinical immunology 2013; 131(6): 1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, Lee JB, Liu B, et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abonia JP, Blanchard C, Butz BB, et al. Involvement of mast cells in eosinophilic esophagitis. The Journal of allergy and clinical immunology 2010; 126(1): 140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. The Journal of allergy and clinical immunology 2010; 126(6): 1198–204 e4. [DOI] [PubMed] [Google Scholar]
- 18.Dellon ES, Chen X, Miller CR, et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. The American journal of gastroenterology 2011; 106(2): 264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird L T cells: IL-9 breaks down barriers. Nature reviews Immunology 2014; 14(7): 432. [DOI] [PubMed] [Google Scholar]
- 20.Gerlach K, Hwang Y, Nikolaev A, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nature immunology 2014; 15(7): 676–86. [DOI] [PubMed] [Google Scholar]
- 21.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. International archives of allergy and immunology 2004; 134(1): 79–87. [DOI] [PubMed] [Google Scholar]
- 22.Rawson R, Yang T, Newbury RO, et al. TGF-beta1-induced PAI-1 contributes to a profibrotic network in patients with eosinophilic esophagitis. The Journal of allergy and clinical immunology 2016; 138(3): 791–800 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beppu L, Yang T, Luk M, et al. MMPs-2 and −14 are Elevated in Eosinophilic Esophagitis and Reduced Following Topical Corticosteroid Therapy. Journal of pediatric gastroenterology and nutrition 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2009; 103(5): 401–6. [DOI] [PubMed] [Google Scholar]
- 25.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology 2007; 119(1): 206–12. [DOI] [PubMed] [Google Scholar]
- 26.Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-beta1-induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. The Journal of allergy and clinical immunology 2014; 134(5): 1100–7 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Oshima T, Shan J, Fukui H, Watari J, Miwa H. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. American journal of physiology Gastrointestinal and liver physiology 2012; 303(2): G199–208. [DOI] [PubMed] [Google Scholar]
- 28.Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal immunology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proceedings of the National Academy of Sciences of the United States of America 2008; 105(40): 15405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tunggal JA, Helfrich I, Schmitz A, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 2005; 24(6): 1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. The Journal of cell biology 2002; 156(6): 1099–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearley J, Erjefalt JS, Andersson C, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. American journal of respiratory and critical care medicine 2011; 183(7): 865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]