Abstract
Background
Theoretically, the estrogen deprivation induced by aromatase inhibitors (AIs) might cause ischemic heart disease, but empiric studies have shown mixed results. We aimed to compare AIs and tamoxifen with regard to cardiovascular events among older breast cancer patients outside of clinical trials. We hypothesized that AIs increase the risk of myocardial infarction.
Methods
We identified women age ≥67 years diagnosed with breast cancer from June 30, 2006 to June 1, 2008 in the surveillance, epidemiology, and end results (SEER)‐Medicare database, treated with either tamoxifen or an AI, and followed through December 31, 2012. To compare myocardial infarction (MI) risk for the treatment groups of AIs vs tamoxifen, we developed and assigned stabilized probability of treatment weights and used the Fine and Gray model for time to MI with death not related to MI as a competing risk.
Results
Of the cohort of 5648 women, 4690 were treated with AIs and 958 with tamoxifen; a total of 251 patients developed MI, and 22 patients died of MI during the study period while 476 died of other causes. The hazard for MI was not significantly different between AI vs tamoxifen groups (HR = 1.01, 95% CI 0.72‐1.42), after adjusting for the following known MI risk factors at the start of adjuvant therapy: diabetes, ischemic heart disease, congestive heart failure, MI, and peripheral vascular disease.
Conclusions
In this SEER‐Medicare‐based population study, there were no significant differences in the risk of MI between AI and tamoxifen users after adjustment for known risk factors.
Keywords: breast cancer, adjuvant hormonal therapy, aromatase inhibitors, tamoxifen, and cardiotoxicity
1. INTRODUCTION
Aromatase inhibitors (AIs) are the standard of care for adjuvant therapy in postmenopausal, estrogen, and progesterone receptor positive (ER/PR) breast cancer.1, 2, 3, 4 Currently, AIs are either prescribed as upfront adjuvant therapy for 5 years or as a switch after 2 to 3 years of tamoxifen use.5, 6, 7 While longer‐term therapies like AIs have substantially improved oncologic outcomes, there are concerns that AIs' effects upon lipids and estrogen levels may increase cardiovascular risk, particularly for ischemic cardiac disease, among breast cancer survivors.8, 9, 10
Concern about the potential for cardiovascular risk with aromatase inhibitors was increased when the two large upfront AI trials both reported more cardiovascular events with aromatase inhibitors. The Arimidex, Tamoxifen Alone or in Combination (ATAC) trial reported that 2.5% of subjects had ischemic cardiovascular events with anastrozole compared to 1.9% of those with tamoxifen, and the Big International Group (BIG 1‐98) reported a 1.4% risk with letrozole vs a 1.2% risk with tamoxifen.11, 12 The Tamoxifen Exemestane Adjuvant Multinational (TEAM) study evaluating adjuvant tamoxifen followed by exemestane also reported 1% of subjects had myocardial infarctions compared with 2% receiving exemestane alone (MI).13 Although none of these AI trials' cardiovascular event findings were statistically significant either in these reports or with longer follow‐up,2, 14, 15 a meta‐analysis by Amir et al.16 of the upfront AI trials reported higher odds of cardiovascular disease with AIs. But, this analysis combined ischemic events with other cardiac outcomes, including heart failure (odds ratio [OR] = 1.26, 95% confidence interval [CI] = 1.10‐1.43), as did a meta‐analyses of all of the large AI trials by Cuppone.17
Some reassurance about the potential importance of those findings was provided by the recent Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta‐analyses of individual subjects' data, which showed no significant difference in cardiovascular mortality by hormonal therapy type.1 However, important concerns about cardiovascular risk persisted. In particular, as aromatase inhibitors became more widely used, it is possible that patients at higher baseline cardiovascular risk began to receive them, potentially increasing the effects of even small medication risks. The FDA's warning on anastrozole, which suggested higher cardiotoxicity risk for subjects with pre‐existing comorbidities, heightened these concerns.18 A recent Canadian study which focused on ischemic cardiac disease specifically provided support for these concerns with their findings in a population‐based cohort of users of hormonal therapy of a doubling of the risk of myocardial infarction with AIs.19
To further examine the conflicting findings of previous studies, and particularly the risk of ischemic events among less selected patients, we sought to evaluate the risk of ischemic events in a population‐based cohort of older US adults at substantially higher baseline cardiovascular risk. This study examined myocardial infarction in a surveillance epidemiology and end results (SEER)‐Medicare‐based breast cancer cohort who received AIs or tamoxifen as their adjuvant endocrine therapy for breast cancer.
2. METHODS
2.1. Data sources and study sample
We used the linked Medicare and SEER national databases for this observational study. Our cohort included subjects 67 years of age and older, with a diagnosis of breast cancer between June 30, 2006 and June 1, 2008. Subjects were required to have modified American Joint Committee on Cancer (AJCC) clinical stages I to III breast cancer, be continuously enrolled in Medicare parts A and B for at least 24 months prior to the diagnosis, and enrolled in part D for 1 month after diagnosis. Patients who received adjuvant hormonal therapy with tamoxifen or one of the AIs were eligible regardless of their ER/PR receptor status. All the patients were required to be HER2 negative and have no evidence of any therapy with trastuzumab. The final study cohort consisted of 5648 patients. The Institutional Review Board of the Medical College of Wisconsin approved this study.
2.2. Hormonal therapy
Medicare D prescription drug event (PDE) files were used for measures of drug fills and number of pills. Hormonal therapy drug use was ascertained from this event file; all subjects were required to fill at least one prescription for tamoxifen or one of the AIs (anastrozole, letrozole, or exemestane) within 12 months after the date of breast cancer diagnosis. Subjects with no hormonal therapy claims for 90 consecutive days were considered to have discontinued therapy.
2.3. Study covariates
Patient demographic characteristics, such as age, race, and marital status were obtained from the Medicare files, and geographic region and urbanicity were obtained from the SEER database. The per capita income and education level of the subjects' zip code were obtained from Census tract information linked by the SEER to the subject's records. We measured patients' stage and estrogen receptor and progesterone (ER and PR) positive, negative or unknown hormone status from the SEER variables. Breast cancer treatments (cytotoxic chemotherapy and radiation) were obtained from Medicare and SEER claims, respectively.20
2.4. Comorbidities and cardiovascular risk factors
Comorbidities from Medicare inpatient, outpatient and physician claims from 24 months prior through the date of breast cancer diagnosis were categorized using the combined National Cancer Institute's Comorbidity Index (NCI).21 In addition, we identified the following risk factors for myocardial infarction based on validated algorithms (when possible) or prior publications.22, 23, 24 We categorized subjects with diabetes (DM) into three separate groups; (a) mild diabetes, (b) diabetes without complications, and (c) diabetes with chronic complications.25 Other risk factors included a history of hypertension (HTN), transient ischemic attack (TIA) and ischemic stroke, congestive heart failure (CHF), and peripheral vascular disease (PVD). Patients with prior coronary artery disease (CAD) were identified by codes for coronary atherosclerosis, ischemic heart disease, angina pectoris, and Prinzmetal angina. Subjects with a history of MI up to 24 months prior to breast cancer diagnosis were defined as having prior MI.
2.5. Study outcome
The study outcome was the first diagnosis of MI after initiation of tamoxifen or AIs. We chose MI as our primary outcome because it is a precisely defined diagnosis that can be ascertained through the claims. ICD 9 codes (410, 410.01, 410.11, 410.21, 410.31, 410.51, 410.61, 410.71, 410.81, and 410.91) were used for acute MI diagnosis based on a validated algorithm.22, 23, 24 ICD 10 codes were used for deaths related to MI (I21.0‐I21.4, I12.9, I22.0, I22.1, I22.8, I22.9, I248, and 249). Ventricular fibrillation and sudden cardiac death were excluded because of the uncertainty of exact etiology.
2.6. Statistical analyses
Patient demographic and pathological characteristics of the tamoxifen and AI groups were compared. Imbalances in characteristics between treatment groups were then addressed by assigning the inverse probability of weighting (IPTW) to observations.26, 27 Standardized differences to compare between‐group balances after weighting were also computed.
Patients were followed through the end of December 2012 as SEER‐Medicare data was available through this period. Subjects were censored for the end of the observation period, treatment discontinuation, switch to a different adjuvant endocrine therapy, or end of Medicare parts A, B, and D. A multivariate fine and gray model with MI/death from MI as the event of main interest and non‐MI death as a competing risk was implemented; demographic characteristics, staging, ER/PR status, cancer treatments (chemotherapy and radiation), comorbidity score and prior cardiac conditions were included as covariates.28, 29 We also examined patients in three different sub‐groups, which included: (a) patients with prior history of cardiac risk factors (DM, HTN, MI, CHF, and CAD), (b) patients with only prior cardiac disease (MI, CHF, and CAD), (c) patients without prior cardiac disease. To examine whether our results were sensitive to the specifications for our variables and/or models,19 we repeated our analyses using a cause‐specific hazard model and also with an alternate outcome of MI identified using only inpatient claims (Appendix, Tables 4 and 5).
To assess whether AI nonadherence might reduce any association between AI use and MI, we performed additional sensitivity analysis among users of AIs.30, 31 Adherence was estimated using a cumulative medication possession ratio (MPR) during the study period. The MPR is defined as the ratio of the number of days of AI dispensed to the patients divided by the total number of days of the observation period. An analysis was then conducted to assess the association between MPR and the outcome of MI.
3. RESULTS
A total of 5648 patients with stages I to III breast cancer were included. A total of 4690 women received AIs (anastrozole (n = 3151), letrozole (n = 1358), and exemestane n = 181) and 958 women received tamoxifen. Of these, 251 subjects had MI, and 22 subjects died of MI, while 476 died of other causes during a mean follow‐up of 873 days.
Patient characteristics by AI and tamoxifen groups, before and after inverse propensity score weighting, are shown in Table 1. AI users were from more urban geographical locations and higher‐income zip codes than tamoxifen users. They were also younger, more likely to have stages 2 and 3 breast cancer and more likely to receive chemo and radiation treatments, but were less likely to have diabetes. After the propensity weights, the baseline variables were well balanced with standardized differences of less than 0.04 (Appendix, Table 3).
Table 1.
Cohort characteristics among aromatase inhibitor and tamoxifen users
| AI (n = 4690) | Tamoxifen (n = 958) | All (n = 5648) | Unweighted P‐value | Weighted P‐valuea | |
|---|---|---|---|---|---|
| Age at diagnosis | — | — | — | <0.001 | 0.962 |
| 66‐70 | 1191 (25.4%) | 187 (19.5%) | 1378 (24.4%) | — | — |
| 71‐75 | 1280 (27.3%) | 249 (26.0%) | 1529 (27.1%) | — | — |
| 76‐80 | 1153 (24.6%) | 227 (23.7%) | 1380 (24.4%) | — | — |
| 81‐85 | 755 (16.1%) | 205 (21.4%) | 960 (17.0%) | — | — |
| 86‐90 | 311 (6.6%) | 90 (9.4%) | 40 (7.1%) | — | — |
| Race | — | — | — | 0.535 | 0.999 |
| Black | 299 (6.4%) | 64 (6.7%) | 363 (6.4%) | — | — |
| Hispanic | 72 (1.5%) | 18 (1.9%) | 90 (1.6%) | — | — |
| Non‐Hispanic white | 4089 (87.2%) | 838 (87.5%) | 4927 (87.2%) | — | — |
| Other | 230 (4.9%) | 38 (4.0%) | 268 (4.7%) | — | — |
| Marital status | — | — | — | 0.009 | 0.839 |
| Married | 1925 (41.0%) | 356 (37.2%) | 2281 (40.4%) | — | — |
| Not married | 2573 (54.9%) | 574 (59.9%) | 3147 (55.7%) | — | — |
| Unknown | 192 (4.1%) | 28 (2.9%) | 220 (3.9%) | — | — |
| Urban‐rural | — | — | — | <0.001 | 0.928 |
| Big metro | 2506 (53.4%) | 395 (41.2%) | 2901 (51.4%) | — | — |
| Metro | 1388 (29.6%) | 282 (29.4%) | 1670 (29.6%) | — | — |
| Non metro | 530 (11.3%) | 191 (19.9%) | 721 (12.8%) | — | — |
| Urban | 266 (5.7%) | 90 (9.4%) | 356 (6.3%) | — | — |
| Tract per capita income | — | — | — | <0.001 | 0.919 |
| 1 $4 k‐20 k | 1110 (23.9%) | 279 (29.7%) | 1389 (24.9%) | — | — |
| 2 $20 k‐27 k | 1125 (24.3%) | 265 (28.2%) | 1390 (24.9%) | — | — |
| 3 $27 k‐37 k | 1179 (25.4%) | 213 (22.7%) | 1392 (25.0%) | — | — |
| 4 $37 k‐$143 K | 1222 (26.4%) | 182 (19.4%) | 1404 (25.2%) | — | — |
| Missing | 54 | 19 | 73 | — | — |
| Have low income subsidy | 1313 (28.0%) | 260 (27.1%) | 1573 (27.9%) | 0.590 | 0.748 |
| AJCC breast cancer stage | — | — | — | <0.001 | 0.768 |
| Stage 1 | 2598 (55.4%) | 595 (62.1%) | 3193 (56.5%) | — | — |
| Stage 2 | 1651 (35.2%) | 296 (30.9%) | 1947 (34.5%) | — | — |
| Stage3 | 441 (9.4%) | 67 (7.0%) | 508 (9.0%) | — | — |
| Estrogen and progesterone receptor status | — | — | — | 0.064 | 0.867 |
| Negative | 53 (1.1%) | 15 (1.6%) | 68 (1.2%) | — | — |
| Positive | 4408 (94.0%) | 881 (92.0%) | 5289 (93.6%) | — | — |
| Unknown | 229 (4.9%) | 62 (6.5%) | 291 (5.2%) | — | — |
| Any chemotherapy | 728 (15.5%) | 95 (9.9%) | 823 (14.6%) | <0.001 | 0.748 |
| Radiation treatment | 2711 (57.8%) | 474 (49.5%) | 3185 (56.4%) | <0.001 | 0.784 |
| Comorbidity score | — | — | — | 0.089 | 0.172 |
| Missing | 41 (0.8%) | 88 (0.84%) | 49 (0.9%) | — | — |
| No comorbidities | 2410 (51.4%) | 463 (8.2%) | 2873 (50.9%) | — | — |
| Low | 1081 (23.1%) | 237 (24.7%) | 1318 (23.3%) | — | — |
| Medium | 242 (5.2%) | 37 (3.9%) | 279 (4.9%) | — | — |
| High | 916 (18.9%) | 213 (22.2%) | 1129 (20.0% | — | — |
| Prior diabetes | — | — | — | 0.027 | 0.983 |
| No diabetes | 3052 (65.1%) | 672 (70.1%) | 3724 (65.9%) | — | — |
| Mild diabetes | 1116 (23.8%) | 193 (20.1%) | 1309 (23.2%) | — | — |
| No complications | 96 (2.0%) | 17 (1.8%) | 113 (2.0%) | — | — |
| With complications | 426 (9.1%) | 76 (7.9%) | 502 (8.9%) | — | — |
| Prior hypertension | 3992 (85.1%) | 810 (84.6%) | 4802 (85.0%) | 0.655 | 0.793 |
| Prior peripheral vascular disease | 926 (19.7%) | 182 (19.0%) | 1108 (19.6%) | 0.596 | 0.845 |
| Prior stroke, transient ischemic attack | 362 (7.7%) | 60 (6.3%) | 422 (7.5%) | 0.118 | 0.932 |
| Prior coronary artery disease | 1533 (32.7%) | 303 (31.6%) | 1836 (32.5%) | 0.524 | 0.963 |
| Prior congestive heart failure | 904 (19.3%) | 176 (18.4%) | 1080 (19.1%) | 0.517 | 0.783 |
| Prior myocardial infarction | 152 (3.2%) | 27 (2.8%) | 179 (3.2%) | 0.496 | 0.725 |
These were after weighting using the inverse probability of treatment by propensity score method.
Table 2 and Figure 1 display the results of the competing risks analysis. There was no difference in the hazard for MI among users of AIs compared with users of tamoxifen (HR = 1.01, CI, 0.72‐1.42, P = 0.96) in our primary, competing risks model. As expected, the hazard ratios for a number of pre‐existing conditions showed significant associations with MI: prior diabetes (P < 0.003) with complications (HR, 1.94; 95% CI, 1.36‐2.77), prior heart failure (HR, 1.45; 95% CI, 1.06‐1.99, P = 0.02), prior MI (HR, 2.95; 95% CI, 1.97‐4.43, P = <0.001), prior coronary artery disease (HR, 1.83; 95% CI, 1.34‐2.48, P = <0.001), and history of peripheral vascular disease (HR, 1.65; 95% CI, 1.22‐2.22, P = 0.001). In analyses of subgroups defined by cardiovascular risk, there were also no statistically significant differences between AI and tamoxifen users (Figure 2).
Table 2.
Myocardial infarction Hazard ratios for aromatase inhibitors vs tamoxifen in surveillance, epidemiology, and end results (SEER)‐Medicare breast cancer patientsa
| Hazard ratio | 95% confidence interval | P‐value | |
|---|---|---|---|
| Drug assignment | |||
| Tamoxifen | Ref | — | — |
| Aromatase inhibitors | 1.01 | 0.72‐1.42 | 0.960 |
| Age group | — | — | 0.097 |
| 67‐70 | Ref | — | — |
| 71‐75 | 1.23 | 0.79‐1.93 | — |
| 76‐80 | 1.51 | 0.96‐2.37 | — |
| 81‐85 | 1.54 | 0.95‐2.51 | — |
| 86‐90 | 2.05 | 1.19‐3.51 | — |
| AJCC cancer stage | — | — | 0.363 |
| Stage 1 | Ref | — | — |
| Stage 2 | 1.03 | 0.77‐1.37 | — |
| Stage 3 | 1.38 | 0.88‐2.16 | — |
| Any chemo | 0.75 | 0.47‐1.18 | 0.209 |
| ER/PR status | — | — | 0.249 |
| Positive | Ref | — | — |
| Negative | 1.06 | 0.33‐3.45 | — |
| Unknown | 0.55 | 0.27‐1.11 | — |
| Previous diabetes | — | — | <0.003 |
| None | Ref | — | — |
| Mild | 0.90 | 0.36‐2.22 | — |
| No complications | 1.31 | 0.95‐1.78 | — |
| With complications | 1.94 | 1.36‐2.77 | — |
| Prior cardiac heart failure | 1.45 | 1.06‐1.99 | 0.020 |
| Prior hypertension | 0.69 | 0.39‐1.23 | 0.211 |
| Prior myocardial infarction | 2.96 | 1.97‐4.43 | <0.001 |
| Prior coronary artery disease | 1.83 | 1.34‐2.48 | <0.001 |
| Prior peripheral vascular disease | 1.65 | 1.23‐2.22 | 0.001 |
| Prior stroke | 1.03 | 0.69‐1.53 | 0.894 |
Fine and gray competing risks model results. Other variables in the analysis were race, marital status, census tract per capita income quartile, SEER region, and urban size category.
Figure 1.
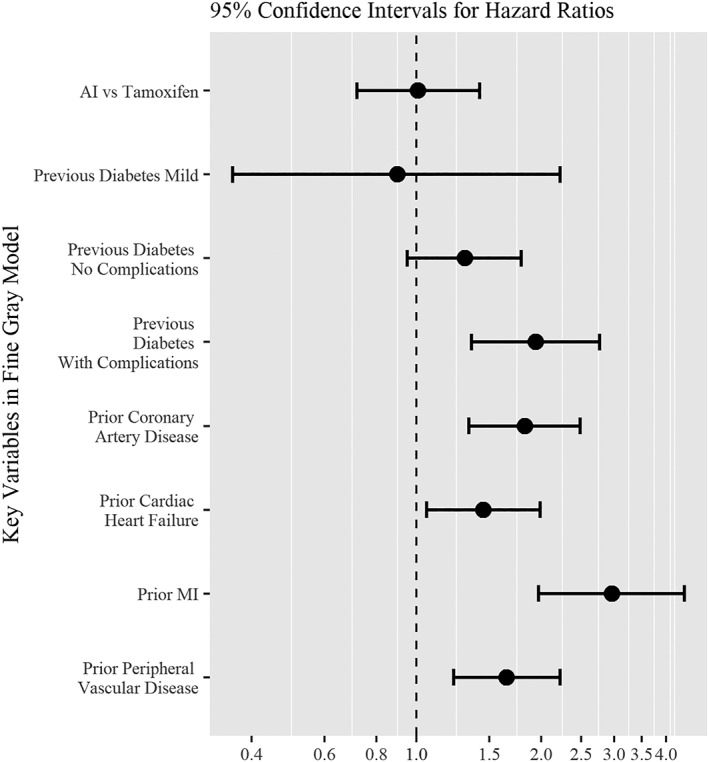
Aromatase inhibitor vs tamoxifen therapy confidence intervals for Hazard of myocardial infarction ratios for selected variables
Figure 2.
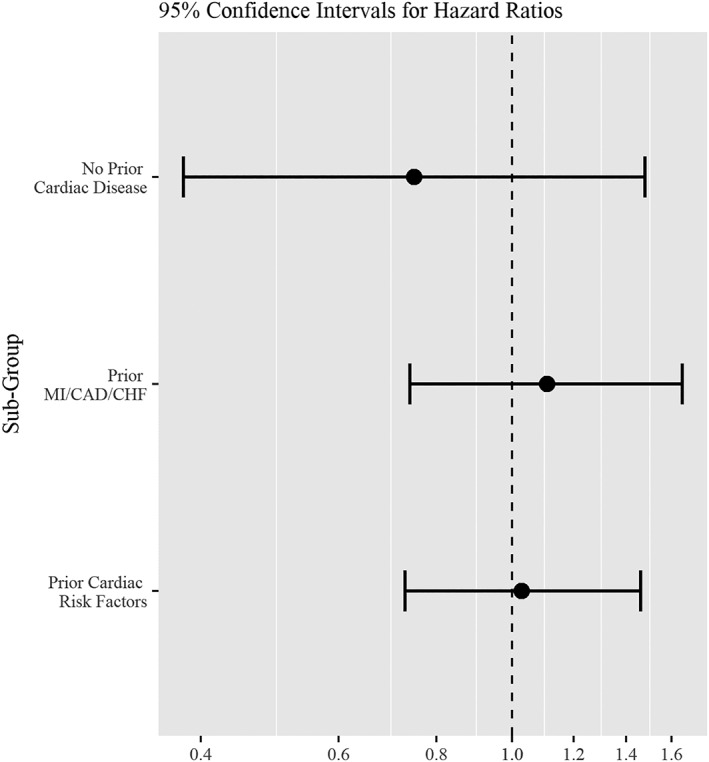
Hazard ratios for MI by hormone therapy, AI vs tamoxifen, in selected sub‐groups
The results were not changed when analyses were repeated using the cause‐specific hazards model, or when the analysis was limited to the outcome of MI as defined only by inpatient claims. (Appendix, Tables 4 and 5). Furthermore, adherence with aromatase inhibitors as measured by MPR was not associated with MI (P = 0.38) (Appendix, Figure 3).
4. DISCUSSION
Our evaluation of a SEER‐Medicare‐based breast cancer cohort of 5648 patients followed for up to 5 years revealed no association between MI and adjuvant hormonal therapy type. Although the risk of MI appeared substantially higher than in the AI randomized trials, and the hazard for MI was substantially increased in subjects with pre‐existing cardiovascular risk, there was no difference in MI between the AI vs tamoxifen groups, HR 1.01 (95% CI 0.72‐1.42), P = 0.96 (Table 2, Figure 1). Additional analyses within differing subgroups based on cardiovascular risk also demonstrated no overall differences in the risk of MI between AI and tamoxifen (Figure 2, Appendix, Tables 4 and 5).
Our paper is consistent with the findings of the large trials as well as the EBCTCG individual‐level meta‐analysis of those trials, in which a similar number of women in AI and tamoxifen groups died of cardiac causes.1 This large study was able to examine individual patient level data and examined outcomes through 9 years. However, our findings appear to conflict with two meta‐analyses16, 17 and a Canadian observational study by Abdel‐Qadir et al. that examined nonfatal outcomes.19 Our study cannot directly address the reasons for these differences. However, unlike our study which focused on only ischemic events to examine a consistent potential mechanism, the two meta‐analyses, neither of which used individual patient data, combined all cardiovascular events.16, 17 While both reported risk increases, the increased risk was small in the study by Amir et al., (OR 1.26, 95% CI 1.10‐1.43), and the CIs could be consistent with our findings.16
It is somewhat surprising that our study differs from the population‐based Canadian cohort study.19 It is possible that the differences in the results of the studies are due to baseline differences between study subjects. In particular, the average age of patients in our study was slightly higher, and our cohort appeared to include a larger number of patients with cardiovascular risk factors. However, the Canadian study was notable for the small number of events, including only 17 MIs among tamoxifen users, raising the possibility of a chance finding.19 Furthermore, the differences between our study and the Canadian one do not seem likely to be due to differences in the analytic approach. Similar to Abdel‐Qadir et al.,19 we utilized state‐of‐the‐art propensity score inverse probability weighting, to reduce measurable covariate imbalances by hormonal therapy type.
Furthermore, while we used the Fine and Gray competing risks model in the primary analysis, our results did not change in sensitivity analyses with models as used by the Canadian group,. Finally, we considered the role of adherence to hormonal therapy.30, 31 Although more Canadian subjects appeared to be adherent,19 our analyses finding no association between adherence and MI in our cohort suggest that adherence differences are unlikely to explain the differences between our study and that from Canada (Appendix, Figure 3).
Despite the reassuring negative findings of our study, the relatively large CI around our results does not exclude the possibility of a small to moderate increased risk of MI with aromatase inhibitors compared with tamoxifen in the United States. The risk of MI was higher in our study than in the Canadian study or any of the trials. Our study cohort was substantially older than, and consequently had a higher risk of MI, than women in an earlier US examination of MI and breast cancer.32 We used the large SEER‐Medicare cohort, and the likelihood of a larger study in the U.S. appears remote. Nonetheless, the conflicting findings of studies of AI cardiotoxicity and the inherent limitations of retrospective studies suggest that future clinical trials should attempt to collect adverse event information in a way that maximizes their precision in key areas like cardiovascular outcomes.
The design of our study has several strengths: (a) our cohort size and population‐based setting, (b) subjects were older and had pre‐existing cardiac risk factors, making them suitable for a study of MI as a cardiac outcome, (c) our 5‐year study follow‐up which not only allowed many patients to complete the adjuvant endocrine therapy, but also is optimal to capture any cardiac events,9 (d) our precise definition of MI, which avoids the inconsistencies in previously published reports of cardiac outcomes. Like other observational studies, our paper was limited because assignment to treatment was nonrandom. However, we utilized rigorous epidemiologic approaches to provide findings with high validity, including a new user design (ie, capturing patients at the time they were first prescribed hormonal therapy), censoring subjects who switched to different AIs, and use of inverse probability of treatment weights computed from a propensity score model with achieved covariate balance confirmed through standardized differences, to counteract targeted prescription of aromatase inhibitors by informed physicians. In addition, performing an analysis stratified by the type of AI use was not possible. The majority of our study cohort received anastrozole, perhaps in part because it was the first to have a generic approved. Unfortunately, we were unable to include other risk factors such as smoking and body mass index which play a significant role in cardiovascular disease and which may be causing US oncologists to steer patients away from AIs in a way that could not be captured in our or the Canadian observational19 studies. Because use of administrative data to detect several other new cardiovascular conditions that might predispose to MI, such as congestive heart failure, have poor positive predictive value,22 we were also not able to adjust for these conditions. Finally, we, lacked information regarding cardio‐protective medications such as statins, although the validated cardiovascular risk factors that we did include in our propensity score are likely to have helped us avoid potential major imbalances in risk between groups.
In summary, we found no significant differences in MI between AI vs tamoxifen users in this cohort of older women with early breast cancer. Given the growing evidence for the extended use of adjuvant hormonal therapy for more than 5 years, cardiotoxicity remains an important question for breast cancer survivors. It is well known that the mechanisms of cardiotoxicity vary among different antineoplastic regimens and utilizing precise definitions of cardiac outcomes is paramount to clinical practice. Future trials should focus on precise definitions of cardiotoxicity and clear guidelines for subjects with preexisting comorbidities to guide therapy and surveillance.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Author contributions
Sailaja Kamaraju is contributed in conceptualization, coordination of methodology, data collection, writing the original draft, and editing/review and writing of the final manuscript. Yushu Shi is contributed in data collection, formal analysis, writing the original manuscript, reviewing the final manuscript. Elizabeth Smith is contributed in methodology, and statistical analysis, reviewing the final draft. Ann B. Nattinger is contributed in conceptualization, and review and editing of the final manuscript. Purushottam Laud is contributed in design of methodology, statistical analysis, data analysis, review and editing of the final manuscript. Joan Neuner is contributed in conceptualization, data collection, methodology, an overview of the statistical analysis, overall supervision, and writing ‐review, and editing of the manuscript.
ACKNOWLEDGMENTS
Supported by NIH‐Minority Health and Health Disparities grant (R01MD010728) and American Cancer Society's grant‐RSG‐11‐098‐01‐CPHPS).
Kamaraju S, Shi Y, Smith E, Nattinger AB, Laud P, Neuner J. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population‐based study. Clin Cardiol. 2019;42:93–100. 10.1002/clc.23114
Funding information NIH Blueprint for Neuroscience Research, Grant/Award Number: Supported by NIHMinority Health and Health Dispar
Precis: In this large population‐based study, there were no significant differences in the risk of MI between AI and tamoxifen users after adjustment for known risk factors.
REFERENCES
- 1. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient‐level meta‐analysis of the randomised trials. Lancet (London, England). 2015;386(10001):1341‐1352. 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 2. Cuzick J, Sestak I, Baum M, et al. ATAC/LATTE investigators: effect of anastrozole and tamoxifen as adjuvant treatment for early‐stage breast cancer: 10‐year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135‐1141. 10.1016/S1470-045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 3. Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine‐responsive early breast cancer: Update of study BIG 1–98. J Clin Oncol. 2007;25(5):486‐492. JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 4. Van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomized phase 3 trial. Lancet (London, England). 2011;377(9762):321‐331. 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 5. Boccardo F, Guglielmini P, Rubagotti A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long‐term results of the Italian tamoxifen anastrozole (ITA) trial. European J Cancer. 2013;49(7):1546‐1554. [DOI] [PubMed] [Google Scholar]
- 6. Kesisis G, Markis A, Miles D, et al. Update on the use of aromatase inhibitors in early‐stage breast cancer. Breast Cancer Res. 2009;11:211 10.1186/bcr2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliss JM, Kilburn LS, Coombes RC, et al. Disease‐related outcomes with long‐term follow‐up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012;30(7):709‐717. 10.1200/JCO.2010.33.7699. [DOI] [PubMed] [Google Scholar]
- 8. Bell LN, Nguyen AT, Li L, et al. Comparison of changes in the lipid profile of postmenopausal women with early‐stage breast cancer treated with exemestane or letrozole. J Clin Pharmacol. 2012;52(12):1852‐1860. 10.1177/0091270011424153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandhi S, Verma S. Aromatase inhibitors and cardiac toxicity: getting to the heart of the matter. Breast Cancer Res and Treatment. 2007;106(1):1‐9. 10.1007/s10549-006-9470-y. [DOI] [PubMed] [Google Scholar]
- 10. Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for two years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23(22):5126‐5137. doi: 10.1200/JCO.2005.07.097 [DOI] [PubMed] [Google Scholar]
- 11. Baum M, Buzdar AU. ATAC Trialists' Group, et al.: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomized trial. Lancet. 2002;359(9324):2131‐2139. S0140673602090888. [DOI] [PubMed] [Google Scholar]
- 12. Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine‐responsive early breast cancer: Update of study BIG. J Clin Oncol. 2007;25(5):486‐492. JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 13. Van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomized phase 3 trial. Lancet. 2011;377(9762):321‐331. 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 14. Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1‐98 trial. J Clin Oncol. 2007;25(36):5715‐5722. 10.1200/JCO.2007.12.1665. [DOI] [PubMed] [Google Scholar]
- 15. Bliss JM, Kilburn LS, Coleman RE, et al. Disease‐related outcomes with long‐term follow up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2011;30(7):709‐717. 10.1200/JCO.2010.33.7899. [DOI] [PubMed] [Google Scholar]
- 16. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta‐analysis. J Natl Cancer Inst. 2011;103(17):1299‐1309. 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 17. Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta‐analysis of randomized trials. Cancer. 2008;112(2):260‐267. 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 18.Anastrozole (package insert). AstraZeneca Pharmaceuticals LP, Wilmington, DE; 2010. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
- 19. Abdel‐Qadir H, Amir E, Fischer HD, et al. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post‐menopausal women with early‐stage breast cancer. Eur J Cancer. 2016;68:11‐21. S0959‐8049(16)32395‐4. [DOI] [PubMed] [Google Scholar]
- 20. Giordano SH, Duan Z, Kuo YF, et al. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750‐2756. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 22. Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self‐report, hospital discharge codes, and adjudication of cardiovascular events in the women's health initiative. Am J Epidemiol. 2004;160(12):1152‐1158. [DOI] [PubMed] [Google Scholar]
- 23. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290‐296. S0002870302000819. [DOI] [PubMed] [Google Scholar]
- 24. Birman‐Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Medical Care. 2005;43(5):480‐485. 00005650–200505000‐00009. [DOI] [PubMed] [Google Scholar]
- 25. Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims‐based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584‐590. [DOI] [PubMed] [Google Scholar]
- 26. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2007;168(6):656‐664. 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC, Stuart EA. Moving towards best practice when using the inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661‐3679. 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statis Asso. 1999;94(446):496‐509. [Google Scholar]
- 29. Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301‐2308. 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst. 2015;107(8):pii djv130 10.1093/jnci/djv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis‐Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early‐stage breast cancer. J Clin Onc. 2008;26(4):556‐562. 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 32. Ligibel JA, O'Malley JA, Fisher M, et al. Risk of myocardial infarction, stroke, and fracture in a cohort of community‐based breast cancer patients. Breast Cancer Res Treat. 2012;131(2):589‐597. 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]


