Abstract
In this study, we evaluate the role of the thalamus in the neural circuitry of arousal. Level of consciousness within the first 12 hours of a thalamic stroke is assessed with lesion symptom mapping. Impaired arousal correlates with lesions in the paramedian posterior thalamus near the centromedian and parafascicular nuclei, posterior hypothalamus, and midbrain tegmentum. All patients with severely impaired arousal (coma, stupor) had lesion extension into the midbrain and/or pontine tegmentum, whereas purely thalamic lesions did not severely impair arousal. These results are consistent with growing evidence that pathways most critical for human arousal lie outside the thalamus.
Arousal is a critical feature of consciousness, yet the neural circuitry that maintains it is poorly understood. Early models of an ascending reticular activating system (ARAS) originating from diffuse neurons in the brainstem reticular formation have been revised to include discrete brainstem regions and individual nuclei that are preferentially involved in maintaining a state of wakefulness.1–5
Classically, ARAS projections were thought to synapse in the “nonspecific” nuclei of the midline and intralaminar thalamus, which project diffusely to the cerebral cortex.5 It soon became dogmatic in the field that the thalamus was the critical relay of the human arousal system.6,7 Yet the assumption that critical, arousal-promoting projections from the brainstem must synapse in the thalamus has been challenged by a growing body of research in experimental animals. For example, extensive ablation or inhibition of thalamic neurons fails to alter the normal pattern and amount of waking and sleeping behavior, and the only apparent electroencephalographic abnormality that results is a loss of spindle activity during sleep.8–10
Outside the thalamus, neurons in the posterior hypothalamus and basal forebrain appear more important than the thalamus for linking brainstem arousal- promoting projections to the cerebral cortex. Ablating the posterior hypothalamus or inhibiting corticopetal neurons in the basal forebrain nuclei produces coma, whereas exciting these regions is potently wake-promoting.3,9,11–13
Unlike these advances in understanding the ascending arousal system in experimental animals, we lack detailed information about the importance of the thalamus for human arousal. A study of atrophy patterns in a large cohort of patients with disorders of consciousness observed no association between thalamic atrophy and wakefulness, but this study could not determine whether the thalamus is necessary for maintaining a basic level of arousal.14 As such, the question of whether the human thalamus is necessary for maintaining a basic level of arousal remains unanswered. Answering this question is more than just an academic concern; it has direct, therapeutic implications for patient care. Based on the idea that the ascending arousal system relays in the thalamus, patients with disorders of consciousness have had electrodes implanted into their thalami by several groups of investigators, with inconsistent results.15 It is imperative that we learn more about the importance of the human thalamus and adjoining brain regions for maintaining arousal, because this information holds the potential to improve therapeutic targeting for neuromodulation in coma and other disorders of consciousness.
To help inform this debate, we identify patients with ischemic lesions involving the thalamus to address whether the thalamus itself is responsible for the reduced level of consciousness in patients with artery of Percheron and related stroke syndromes involving the thalamus. We hypothesize that severe impairments in arousal (coma, stupor) do not occur with focal lesions limited to the thalamus, but may be observed when thalamic lesions extend into the brainstem.
Patients and Methods
For this retrospective study, we identified patients by searching the electronic medical records of the University of Iowa Hospitals and Clinics using the key terms “thalamic infarct,” “cerebral infarct,” “coma,” “artery of Percheron,” “basilar tip infarct,” and “paramedian infarct” between 2000 and 2017. We reviewed clinical records to determine whether patients had corresponding magnetic resonance (MR) imaging with abnormal diffusion signal in the thalamus. Exclusion criteria included the presence of sepsis, alcohol or drug overdose, seizure, or trauma. This search yielded 23 patients (17 men, ages 63.30 ± 14.25). An additional 10 patients (5 men, ages 50.30 ± 16.23) were added from a previously published study on artery of Percheron infarcts conducted at the University of Utah Hospital.16 Cases were analyzed using the same protocols as those from Iowa. In total, 33 patients were included in the analysis. The previously published study did not perform lesion-symptom mapping, and their results do not overlap with the current analysis. This study was approved by the Institutional Review Board of the University of Iowa (Iowa City, IA).
The level of arousal displayed by each patient during the first 12 hours after the onset of symptoms was extrapolated from review of the electronic medical record based primarily on neurological exam findings. Level of arousal was rated on a 6-point scale (coma, 1; stupor, 2; obtunded, 3; somnolent, 4; lethargic, 5; awake, 6, terms defined previously17) by 2 neurologists blinded to patient imaging (J.C.G. and A.D.B.).
Location of stroke was identified using the clinical MR images. Lesion location was reproduced onto a 3D template brain (MNI152 atlas, 1 × 1 × 1 mm) using FSL (http://fsl.fmrib.ox.ac.uk/fsldownloads/), as performed previously,1 and accuracy of each lesion was reviewed independently by 2 neurologists blinded to clinical outcome (J.C.G. and A.D.B.).
Lesion-symptom mapping (LSM) was used to conduct a statistical test to identify regions that, when lesioned, were associated with greater impairment in arousal. LSM was performed using LESYMAP, a lesion-symptom mapping package in R (R Foundation for Statistical Computing) that uses sparse canonical correlation analysis to find a pattern of normalized voxel weights that is most strongly correlated with impairment in arousal. Statistical significance of the map of voxel weights was determined through a 4-fold cross-validation correlation between predicted and observed arousal scores. This approach has several advantages compared to mass univariate (ie, “voxelwise”) lesion-symptom mapping.18 Statistical significance of the overall model was determined, and each voxel received a weight between 0 and 1, with higher values associated with greater impairment in arousal. Resulting voxel weights were color-coded and overlaid onto the template brain for display. Statistical analysis was limited to voxels in which 3 or more patients had damage, excluding areas with sparse coverage with less than 10% of the cohort, as performed previously.18
Results
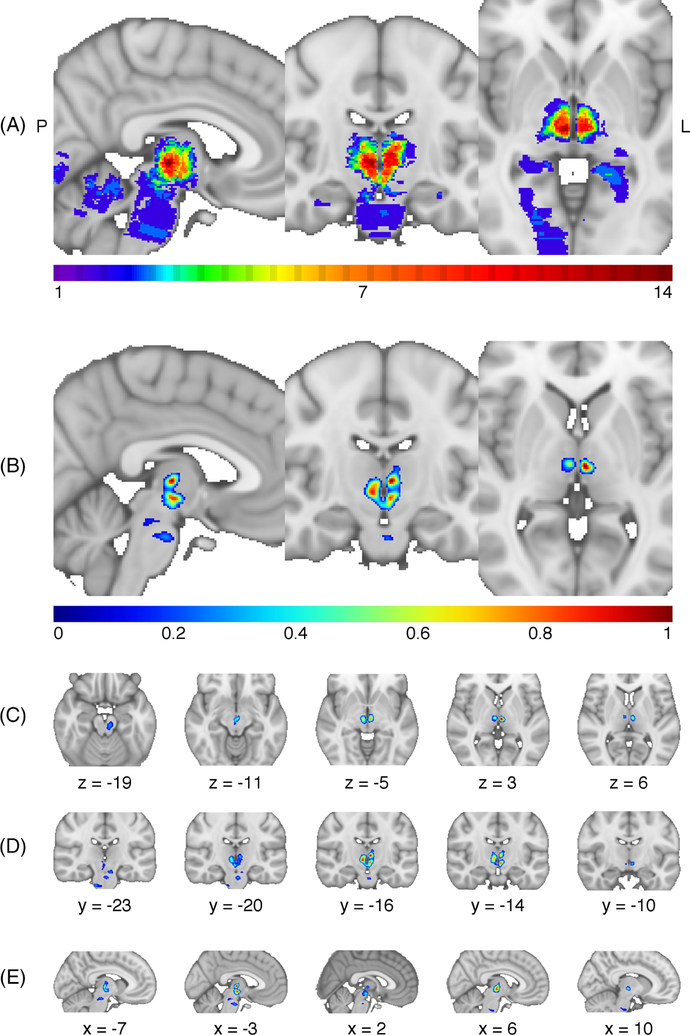
The lesion overlap (Fig 1A) demonstrates coverage of the thalamus and limited coverage of the brainstem and other structures supplied by the posterior circulation. The overall LSM is significant (cross-validated correlation = 0.59; p < 0.001). Regions associated with impaired arousal extend from the bilateral ventromedial thalamus in the region of the centromedian and parafascicular nuclei (Fig 1B–E) with a peak at Montreal Neurological Institute (MNI) coordinate: −3, −16, 3. The 4 patients with severe impairments in arousal (2 coma, 2 stupor) all had infarcts extensively involving the brainstem in addition to the thalamus (Fig 2). In contrast, 12 patients with thalamic lesions with minimal to no extension into the brainstem were awake on presentation with no impairment of consciousness. The remaining 17 patients with intermediate extension into the midbrain had partial impairments in level of arousal (obtunded, somnolent, or lethargic).
FIGURE 1:
(A) Overlap of all 33 lesions in this study. All involve the thalamus, and some extend into the brainstem, cerebellum, or cortical regions supplied by the posterior cerebral artery. The maximum lesion overlap was 14/33 at MNI coordinate 5, −17, −2. (B) Lesion-symptom mapping (LSM) result. Voxel weights derived from the LSM analysis are shown centered on the voxel with the greatest weight (−3, −16, 3). The color-coded scale represents the normalized voxel weights derived from the LSM analysis. Greater weights are associated with a more-severe impairment in arousal. Representative slices of the LSM result are depicted in (C) axial, (D) coronal, and (E) sagittal views. MNI, Montreal Neurological Institute.
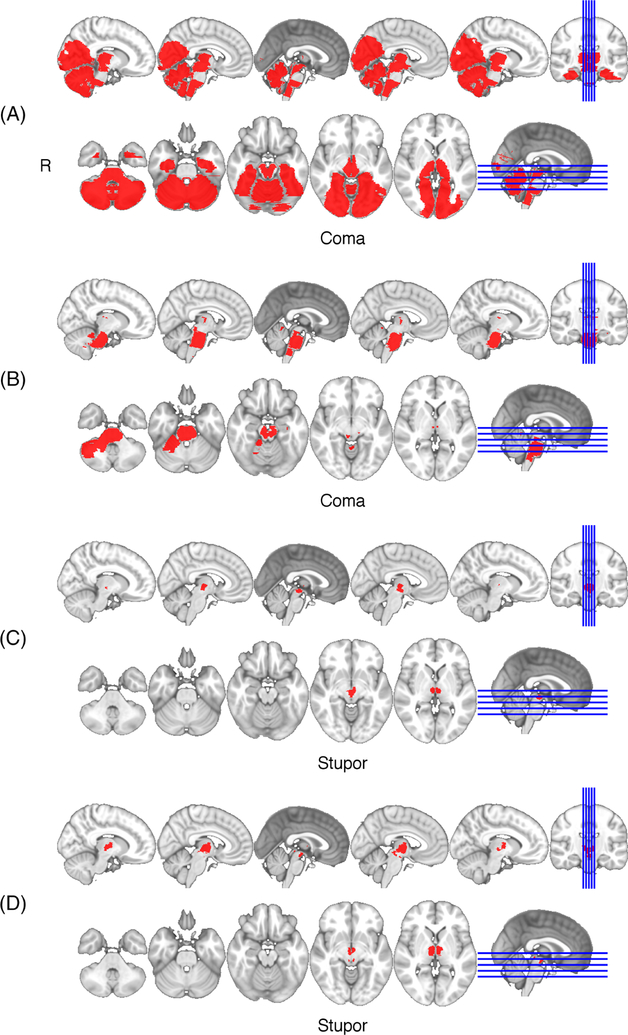
FIGURE 2:
Lesions in patients presenting with coma (A,B) or stupor (C,D). In each, bilateral thalamic infarcts extended into the posterior hypothalamus and midbrain (A–D) and in 2, the pons (A,B). No patients with a lesion restricted to the thalamus had a severe impairment in arousal (coma or stupor).
Conclusions
This study provides a formal analysis of human thalamic lesions attempting to localize subregions that may impair arousal. Our analysis identifies an association between impaired arousal and a bilateral region of the ventromedial thalamus near the centromedian and parafascicular nuclei, extending into posterior hypothalamus and midbrain. The association of this thalamic region with impaired arousal must be interpreted cautiously because every patient with a thalamic lesion causing a severe impairment in arousal had extension into the brainstem, whereas no patients with lesions entirely limited to the thalamus demonstrated severe impairment in arousal. These results are consistent with evidence from experimental animals that the thalamus is not critical for arousal,8–10 and suggest that historically the thalamus was implicated because of its shared vascular supply with brainstem structures that are critical for arousal.19 These findings are consistent with previous research highlighting the role of the brainstem and posterior hypothalamus as critical regions in the arousal pathway,1–4 dating back to Moruzzi and Magoun’s demonstration that the brainstem and caudal diencephalon show the lowest stimulation threshold for cortical arousal.5 Similarly, our findings are consistent with previous work linking dorsolateral pontine tegmentum lesions to coma in humans1,4 and coma-like outcomes in animals.9 An alternative explanation for our findings would be that lesions must damage both the thalamus and brainstem to severely impair arousal, but this is not consistent with past evidence that ischemic strokes and other lesions limited to the brainstem cause coma.1,4
This analysis is limited to 33 patients, and we cannot exclude the possibility that a purely thalamic lesion can severely impair consciousness, though we did not observe such a case. We also note that most, if not all, cases from the literature describing “thalamic coma” have radiographic evidence of lesions extending into the hypothalamus and brainstem, have exam findings not consistent with the diagnosis of coma, or have clinical signs like ophthalmoplegia that signify damage to the pons or midbrain.20–22 Our findings are also limited in that we focus on level of arousal in the first 12 hours and do not evaluate hypersomnia or circadian rhythm disruptions that have been reported with diencephalic lesions.
In conclusion, the current results are consistent with studies in experimental animals that challenge the long-held view that the thalamus is a critical node in the arousal pathway.8–10,13 Future work with larger sample sizes will be needed to confirm and extend these findings, both to better understand the neural circuitry of arousal and inform targeted neuromodulation approaches for disorders of consciousness.
Acknowledgment
This study was supported by a Carver College of Medicine Research Fellowship (J.H.). A.D.B. was supported by NIH/NINDS grant K12HD027748–24, K12NS098482–01 and the Roy J. Carver Trust. J.C.G. was supported by K08-NS099425.
We thank A. Andres Capizzano, MD, for assistance acquiring imaging data.
Footnotes
Potential Conflicts of Interest
A.D.B. participated in a data safety and monitoring board for Ekso Bionics.
References
- 1.Fischer DB, Boes AD, Demertzi A, et al. A human brain network derived from coma-causing brainstem lesions. Neurology 2016;87: 2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 2016;19:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen NP, Ferrari L, Venner A, et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun 2017;8: 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain 2003;126:1524–1536. [DOI] [PubMed] [Google Scholar]
- 5.Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1949;1:455–473. [PubMed] [Google Scholar]
- 6.Steriade M Ascending control of thalamic and cortical responsiveness. Int Rev Neurobiol 1970;12:87–144. [DOI] [PubMed] [Google Scholar]
- 7.Papez JW. Central reticular path to intralaminar and reticular nuclei of thalamus for activating EEG related to consciousness. Electroencephalogr Clin Neurophysiol 1956;8:117–128. [DOI] [PubMed] [Google Scholar]
- 8.Villablanca J, Salinas-Zeballos ME. Sleep-wakefulness, EEG and behavioral studies of chronic cats without the thalamus: the “athalamic” cat. Arch Ital Biol 1972;110:383–411. [PubMed] [Google Scholar]
- 9.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 2011;519:933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzsaki G, Bickford RG, Ponomareff G, et al. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 1988;8:4007–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol 2017;44:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Chung S, Zhang S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci 2015;18:1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anaclet C, Pedersen NP, Ferrari LL, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun 2015;6:8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutkenhoff ES, Chiang J, Tshibanda L, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol 2015;78:68–76. [DOI] [PubMed] [Google Scholar]
- 15.Vanhoecke J, Hariz M. Deep brain stimulation for disorders of consciousness: systematic review of cases and ethics. Brain Stimul 2017; 10:1013–1023. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaro NA, Wright B, Castillo M, et al. Artery of percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol 2010;31:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner JB, Saper CB, Schiff N, Plum F. Plum and Posner’s Diagnosis of Stupor and Coma, 4th ed. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 18.Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 2018;115:154–166. [DOI] [PubMed] [Google Scholar]
- 19.Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003; 34:2264–2278. [DOI] [PubMed] [Google Scholar]
- 20.Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 1988;38:837–848. [DOI] [PubMed] [Google Scholar]
- 21.Zappella N, Merceron S, Nifle C, et al. Artery of Percheron infarction as an unusual cause of coma: three cases and literature review. Neurocrit Care 2014;20:494–501. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Lara L, Henninger N. Delayed sudden coma due to artery of percheron infarction. Arch Neurol 2011;68:386–387. [DOI] [PubMed] [Google Scholar]