Abstract
Background:
This study aimed to evaluate the preliminary validation and application of a pain screening tool to identify biopsychosocial risk factors for chronic pain in pediatric sickle cell disease (SCD) and classify youth with SCD into prognostic risk groups.
Method:
Youth presenting to a pediatric SCD clinic completed the Pediatric Pain Screening Tool (PPST), a brief 9-item self-report questionnaire developed for rapid identification of risk in youth with pain complaints. Youth also completed a battery of standardized patient-reported outcomes, including pain characteristics, pain burden, functional disability, pain interference, depressive symptoms, pain catastrophizing, and fear of pain. Healthcare utilization was extracted from medical chart review.
Results:
73 8–18-year-olds (94% Black, 57% female) with SCD participated. The PPST demonstrated discriminant validity that ranged from fair to excellent (AUCs=0.74–0.93, ps < .001) for identifying significant pain frequency, disability, pain interference, and psychosocial distress. Receiver operating characteristic curve analyses indicated that previously established cutoff scores were appropriate for the SCD sample. Participants were classified into Low Risk (28.8%), Medium Risk (38.4%), and High Risk (32.9%) groups with significant group differences across measures, F(18, 116) = 6.67, p < .001. The High Risk group reported significantly higher pain intensity, pain frequency, pain burden, functional disability, pain interference, and depressive symptoms relative to both Low and Medium Risk groups (ps<.005).
Conclusions:
The High Risk group demonstrated a pain and psychosocial profile consistent with chronic SCD pain. The PPST may be useful for efficiently identifying youth with chronic SCD pain or those at risk of poor outcomes.
Keywords: pediatric, sickle cell disease, chronic pain, screening, biopsychosocial, risk factors
As children with sickle cell disease (SCD) age into adolescence, pain frequency and severity increase along with the use of opioids1–3. There is increasing awareness that chronic pain is part of the pain experience for many adolescents with SCD4. The Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks – American Pain Society Pain Taxonomy (AAPT) identified core diagnostic criteria for chronic SCD pain, including ongoing pain present on most days over the past 6 months5. Although further validation is needed, the chronic SCD pain definition suggests the pain frequency characteristic of “most days” corresponds to ≥15 pain days per month and pain duration of at least 6 months. The AAPT also recommends classifying chronic pain along dimensions of psychological consequences and risk factors. Approximately 20% of children and adolescents report chronic pain consistent with AAPT criteria4. Common psychosocial risk factors and consequences of chronic SCD pain in youth include functional impairment, frequent school absences, elevated depressive symptoms, high levels of catastrophic thinking, and twice the number of inpatient admissions for pain relative to patients with SCD without chronic pain4,6, which is consistent with other pediatric chronic pain conditions7–9. Thus, there is a pressing need to identify youth with SCD who are at risk for adverse outcomes to guide targeted treatments.
The treatment of chronic SCD pain remains challenging given the likely complex integration of vaso-occlusion and peripheral and central sensitization that is further complicated by biopsychosocial determinants10. The mechanisms that contribute to the transition from acute to chronic pain in SCD are not well-understood. Nonetheless, early identification of youth who may be at risk of developing chronic SCD pain is needed to help disrupt the onset and prevent the maintenance and exacerbation of chronic pain and its consequences. To date, there is no well-developed pain-related screening tool available to help identify youth with chronic pain in SCD. The Pediatric Pain Screening Tool (PPST) was recently adapted and validated in large samples of youth with mixed pain complaints (primarily musculoskeletal pain) as well as headaches11,12. Cutoff scores for the PPST were derived for grouping patients into categories to determine risk severity and allocation of treatment needs: low-risk (few prognostic risk factors), medium-risk (moderately unfavorable prognosis, high number of physical and low number of psychosocial risk factors), and high-risk (unfavorable prognosis, high levels of physical and psychosocial risk factors). Given the overlap in biopsychosocial factors (pain intensity, pain interference, depressive and anxiety symptoms) that can predict risk of poor outcomes in youth with different pain conditions including SCD13, it is important to examine the utility of a brief screening tool in a sample of youth with SCD pain.
The primary aim evaluated the preliminary validation of the PPST compared to a standardized battery of well-validated patient-reported outcomes in a sample of youth with SCD. We expected the PPST would adequately discriminate patients with clinically significant levels (i.e., reference standard cases) on standardized pain and psychosocial measures from patients whose scores fell within the range that is not clinically significant (i.e., non-cases). Standardized measures included pain frequency, pain duration, functional disability, pain interference, healthcare utilization, depression, pain catastrophizing, and fear of pain. Next, we examined whether receiver operating characteristic (ROC) curve-derived cutoff score for the PPST in SCD was similar to the cutoff score derived from mixed chronic pain and headache samples used in previous validation studies. It was hypothesized that youth with SCD identified as “high risk” on the PPST would have the most impaired scores on pain characteristics (intensity, frequency, duration) and biopsychosocial risk factors (functional disability, pain interference, healthcare utilization, depressive symptoms, pain catastrophizing, fear of pain) consistent with a chronic pain profile.
Method
Recruitment
Participants were children and adolescents presenting to outpatient comprehensive SCD clinics at three campus locations of a tertiary care children’s hospital over 14 months. Youth were eligible if they had SCD (any genotype), aged 8 to 18 years, and English-speaking. Patients receiving active treatment with hydroxyurea or transfusion for pain also were eligible for inclusion. Exclusion criteria included significant documented cognitive or developmental disabilities, chronic transfusions indicated for central nervous system complications (e.g., stroke), or comorbid medical condition in which pain is common (e.g., rheumatological or gastrointestinal conditions).
Study Procedures
Institutional Review Board approval was obtained prior to study initiation. Potentially eligible patients and parents were introduced to the study, and a trained research coordinator assessed eligibility and provided greater detail during clinic visits. Parents and youth provided written informed consent and assent. Patients completed web-based or pencil-and-paper measures while waiting for their provider. Families who previously expressed interest in research were contacted in clinic or by phone. Patients recruited by phone provided verbal consent and assent and either completed web-based or paper-and-pencil measures at home. Patients received a monetary incentive for participation time (about 30 minutes for survey completion).
Measures
Demographics
Detailed demographic and background information, including patient age, race, sex, annual family income, and common SCD treatment were collected from parents.
Pediatric Pain Screening Tool
The PPST is a 9-item self-report measure developed and validated among youth aged 8–18 with mixed pain complaints11 and headaches12. Items 1 through 8 are answered dichotomously (no=0, yes=1), and item 9 is rated from “not at all” to “a whole lot” referencing the past 2 weeks. The ratings “a lot” and “a whole lot” are scored as 1, whereas ratings “not at all”, “a little”, and “some” are scored as 0. Total scores (range 0–9) include two subscales: physical (range 0–4) and psychosocial (range 0–5). In samples of youth with mixed pain complaints and headaches, cutoff scores for risk severity were: Low Risk (Total Score ≤ 2); Medium Risk (Total Score ≥ 3 and Psychosocial subscale ≤ 2); and High Risk (Total Score ≥ 3 and Psychosocial subscale ≥ 3).
Pain characteristics
Patients reported their average pain intensity over the last two weeks using a numeric rating scale (0 = no pain to 10 = worst possible pain). Patients reported the number of days they had pain in the past month (0–31 days), and how long (i.e., duration) they experienced the current level of pain frequency on a 5-point Likert rating (1=only this month to 5 = over 1 year)4. Per AAPT criteria, chronic SCD pain was defined as pain frequency ≥ 15 days per month with duration ≥ 6 months5.
Sickle Cell Pain Burden
The Sickle Cell Pain Burden Interview (SCPBI) is a 7-item self-report of pain burden in the past month (0 = none, 1 = a few, 2 = some, 3 = many, 4 = every)3. Higher total scores (0–28) indicate severe pain burden. Clinical cutoff scores are not yet established. Therefore, item 1 “How many days have you had any pain [in the last month]?” was used to further evaluate pain frequency. Total scores were used in analysis to compare differences among PPST risk groups. Internal reliability for this study sample was 0.88.
Functional Disability
The Functional Disability Inventory (FDI) is a well-validated 15-item self-report of perceived difficulty performing daily activities across settings14. Items are rated from 0 (no trouble) to 4 (impossible) and totaled (range 0–60). Established clinical reference points are 0–12 (no/minimal disability), 13–29 (moderate disability), and 30–60 (severe disability)15. The FDI has high internal consistency, moderate to high test-retest reliability, and good predictive validity14,16. Internal reliability for this study sample was 0.96.
Pain Interference
The PROMIS Pediatric Pain Interference Short Form is a well-validated 8-item self-report of pain-related difficulties in the past 7 days17. Total scores (0–32) are transformed into T-scores. Higher T-scores indicate greater pain interference. Internal consistency for the sample was 0.96.
Depressive Symptoms
The Children’s Depression Inventory-2 (CDI-2) is a well-validated 24-item self-report of depressive symptoms in the past two weeks18 frequently used in pediatric pain research19–22. Total scores (0–54) are transformed into T-scores based on patient age and sex. Higher T-scores indicate greater symptom severity. Study coordinators immediately reviewed CDI results and informed a licensed psychologist of reports of suicidality. A risk assessment and safety plan were devised and mental health service referrals were provided, as appropriate. Internal reliability for the current sample was 0.90.
Pain Catastrophizing
The Pain Catastrophizing Scale – Child (PCS-C) is a well-validated 13-item questionnaire on thoughts and feelings about pain23,24 commonly used in pediatric pain24–26. Items are rated from 0 (mildly) to 4 (extremely). Higher total scores (0–52) reflect greater catastrophic thinking about pain. Established clinical reference points include: low (0–14), moderate (15–25), and high (≥ 26)25. Internal reliability for the current sample was 0.95.
Fear of Pain
The Fear of Pain Questionnaire – Child (FOPQ-C) is a validated 24-item self-report of pain-related fear and avoidance27. Items are rated from 0 (Strongly Disagree) to 4 (Strongly Agree). Higher total scores (0–96) indicate higher fear. Internal consistency was 0.96.
Healthcare Utilization
Electronic medical record data were reviewed to gather the number of inpatient hospitalizations (admissions) and emergency department (ED) visits related to SCD pain within the last 12 months. Three or more admissions or ≥ 3 ED visits within the past 1 year were considered clinically significant28–30.
Statistical Analyses
Data were analyzed using SPSS version 23. ROC curve analysis was used to evaluate the potential utility of the PPST to detect diagnostically or clinically significant levels of risk or disorder and develop cutoff scores to allocate patients into a risk group31. Utility was determined by the PPST’s ability to be both sensitive and specific to detecting risk, as determined by the area under the curve (AUC) metric.
Discriminant Validity
ROC curves calculated the AUC for the PPST total score and psychosocial subscale score by comparing scores against reference standard cases, defined as patients met criteria for clinically significant symptoms based on pre-existing, established cutoffs for each measure. Each standardized measure was dichotomized based on established cutoffs to determine “cases” and “non-cases.” Case or non-case was determined for each measure independently. Established reference standard cases were defined as: Pain frequency ≥ 15 days/month5 or SCPBI item 1 ≥ 3 (i.e., “many” or “every” days/month); Pain duration ≥ 6 months5; FDI ≥ 1315; Pain Interference T-Score ≥6532; CDI-2 T-score ≥ 6533; PCS-C ≥ 2625; FOPQ-C ≥ 5127; Admissions ≥ 330; or ED visits ≥328. Responses that did not meet the established cutoff for a measure were categorized as non-cases for that measure. PPST total and psychosocial subscale scores were compared to all measures. Consistent with previous work11,12, cases reflective of “psychosocial distress” also were classified based on patients who were cases on two or more of the psychosocial measures (i.e., depression, catastrophizing, and fear of pain). Strength of discrimination criteria of the AUC was classified based on the following standards: poor (<0.70), fair (0.70–0.79), good (0.80–0.89), and excellent discrimination (≥0.90)31.
Validating High-Risk Cutoff Scores
PPST cutoff scores were previously recommended and validated using pediatric samples of musculoskeletal pain and headaches11,12. First, ROC curves for PPST total scores were examined against reference standard cases of disability. Classification into the high-risk group is primarily driven by psychosocial factors. Therefore, ROC curve analysis examined the PPST psychosocial subscale score against cases for psychosocial distress to compare the data-driven cutoff for the SCD sample with the established cutoff score. Sensitivity was considered more important than specificity to support the goal of the PPST as a screener to avoid missing cases that truly have chronic SCD pain31.
Concurrent Validity
Chi-square and multivariate analysis of variances (MANOVA) examined differences across the PPST risk groups based on frequency of cases and continuous scores on pain and psychosocial measures.
Results
Recruitment
Of the 124 eligible participants approached for the study, 93 provided consent (75% enrollment rate). Most patients who did not consent (n=26, 21%) expressed interest in the study but were unable to enroll due to time constraints (e.g., arrived late to clinic). Only 5 families (4%) refused to participate. Of the 93 who consented, 73 patients completed study procedures (78.5% participation rate). Primary barriers to participation were completing and returning surveys after completing verbal consent. There was no significant difference in child age or sex between those who completed participation and those who consented but did not complete study procedures.
Participants
Patients were on average 14.3 years old (SD = 2.61, range 8–18). Detailed sample characteristics are presented in Table I. Patients reported mild average pain intensity of 3.38 (SD = 2.93, range 0–10) with an average of 8.61 pain days (SD = 9.41, range 0–31) in the past month. There were no significant differences in patient or parent demographics by campus or recruitment method (clinic vs. phone).
TABLE 1.
Sample characteristics (n=73)
| Child | |
| Mean Age (SD) | 14.3 (2.6), range 8–18 |
| Sex (% Female) | 57.5 |
| Race (% Black or African American) | 94.3 |
| Hemoglobin Type (%) | |
| HbSS | 75.0 |
| HbSC | 13.9 |
| HbSβ+ | 8.3 |
| HbSβ0 | 1.4 |
| HbSO-Arab | 1.4 |
| SCD Treatments (%) | |
| Hydroxyurea | 81.7 |
| Chronic Transfusions | 15.7 |
| Parent | |
| Reporter (mother/stepmother) | 93.1 |
| Race (% Black or African American) | 88.9 |
| Marital Status (%) | |
| Married/Partnered | 40.8 |
| Single | 32.4 |
| Divorced/Separated | 21.1 |
| Widowed | 2.8 |
| Prefer not to answer | 2.8 |
| Highest grade completed (%) | |
| High School or GED | 24.7 |
| Some college or trade | 27.4 |
| College or trade degree | 35.6 |
| Graduate or Professional degree | 12.3 |
| Annual family income (%) | |
| ≤$10,000 | 3.1 |
| $10,001–20,000 | 26.2 |
| $20,001–30,000 | 15.4 |
| $30,001–50,000 | 23.1 |
| $50,001–75,000 | 13.8 |
| ≥$75,001 | 15.4 |
| Prefer not to answer | 3.1 |
PPST Item Endorsement
Descriptive statistics of measures revealed skewness and kurtosis within the limits of normal distribution. PPST total scores averaged 4.15 (SD = 2.65) with full possible range (0–9). Frequency of PPST item endorsement is presented along with the previous validation pain samples11,12 to support comparison across conditions in Table II. In the SCD sample, “My pain is in more than one body part” was the most frequently endorsed item whereas “It is not really safe for me to be physically active” was the least frequently endorsed item. For the validation samples, “How much has pain been a problem in the last 2 weeks” and “It is difficult for me to be at school all day” were most frequently endorsed for mixed pain and headache samples, respectively. In contrast, “I feel that my pain is terrible, and it is never going to get any better” and “It is not really safe for me to be physically active” were least frequently endorsed by mixed pain and headache samples, respectively.
TABLE 2.
Frequency of PPST Item Endorsement
| Current sample (sickle cell) |
Validation samplea (mixed pain) |
Validation sampleb (headache) |
|
|---|---|---|---|
| PPST items | Agree (%) | Agree (%) | Agree (%) |
| Physical subscale | |||
| My pain is in more than one body part | 83.6 | 69.4 | 40.9 |
| I can only walk a short distance because of my pain | 50.7 | 56.8 | 25.6 |
| It is difficult for me to be at school all day | 52.1 | 73.1 | 76.9 |
| It is difficult for me to fall asleep and stay asleep at night | 49.3 | 63.6 | 54.5 |
| Psychosocial subscale | |||
| It is not really safe for me to be physically active | 30.1 | 45.9 | 19.4 |
| I worry about my pain a lot | 52.1 | 48.3 | 54.5 |
| I feel that my pain is terrible and it is never going to get any better | 31.5 | 36.6 | 45.9 |
| In general, I do not have as much fun as I used to | 32.9 | 61.7 | 54.5 |
| Overall, how much has pain been a problem in the past 2 weeks?c | 32.8 | 79.2 | 73.1 |
Note. PPST=Pediatric Pain Screening Tool.
Data taken from Simons et al (2015)
Data taken from Heathcote et al (2017)
Item responses include “not at all,” “a little,” “some,” “a lot,” and “a whole lot.” Responses of “a lot” and “a whole lot” are coded as endorsement of bothersomeness for the total score and are reflected in the Agree column.
Discriminant Validity
The AUC for the PPST total score significantly discriminated cases from non-cases for most measures (AUCs = 0.74–0.93, ps < .001), indicative of fair to excellent discrimination. Primary exceptions included poor discrimination for ED visits (AUC=.69, p<.05) and non-significant discrimination for pain duration (AUC=0.63, p=.06, see Table III). The AUC for the PPST psychosocial subscale revealed fair to good discrimination of cases from non-cases for most measures (AUCs = 0.72–0.86, ps < .001). The PPST psychosocial subscale demonstrated poor discrimination for pain duration and ED visits (AUCs = 0.65–0.67, ps < .05).
TABLE 3.
Discriminant Validity: area under the curve (AUC) for screening tool total scores and psychosocial subscale scores against reference standard cases
| Reference standards | Case definition | Total scores, AUC (95% CI) |
Psychosocial subscale scores, AUC (95% CI) |
|---|---|---|---|
| High pain frequency | Pain frequency ≥ 15 days/month | .82 (.71 - .93)*** | .81 (.68 - .93)*** |
| SCD Pain Burden Days ≥ many | .83 (.74 - .92)*** | .82 (.71 - .93)*** | |
| Persistent pain duration | Duration ≥ 6 months | .63 (.50 - .77) | .65 (.52 - .78)* |
| Moderate to severe disability | FDI ≥ 13 | .93 (.87 - .98)*** | .86 (.78 - .95)*** |
| High pain interference | Pain Interference T-score ≥ 65 | .80 (.68 - .93)*** | .80 (.66 - .94)*** |
| High inpatient admissions | Admissions for pain ≥ 3 | .74 (.62 - .86)** | .77 (.66 - .89)*** |
| High ED visits | ED visits for pain ≥ 3 | .69 (.55 - .82)* | .67 (.53 - .82)* |
| Elevated depressive symptoms | CDI T-score ≥ 65 | .76 (.63 - .88)*** | .69 (.53 - .85)* |
| High pain catastrophizing | PCS-C ≥ 26 | .75 (.64 - .86)*** | .72 (.60 - .84)*** |
| High pain-related fear | FOPQ ≥ 51 | .83 (.73 - .92)*** | .80 (.70 - .90)*** |
| Psychosocial distress index (CDI, PCS, FOPQ) | Case on two or more psychosocial variables┼ | .78 (.67 - .89)*** | .76 (.64 - .88)*** |
Note: FDI = Functional Disability Inventory; ED = emergency department; CDI = Children’s Depression Inventory-2; PCS-C = Pain Catastrophizing Scale for Children; FOPQ = Fear of Pain Questionnaire.
Psychosocial variables include depressive symptoms, catastrophizing, and fear of pain
p < .05,
p < .01,
p < .001
Validating High-Risk Cutoff Score
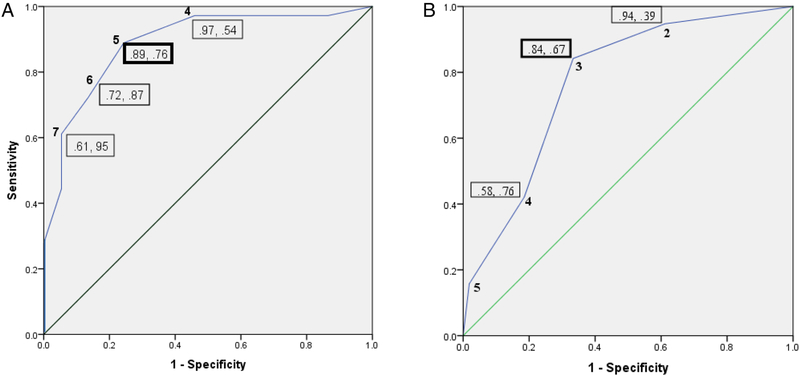
ROC curve analyses for the PPST total score and psychosocial subscale against cases for disability and psychosocial distress are illustrated in Figure 1. Comparable to previous validation studies, a PPST total score ≥ 5 was the best predictor of cases of moderate to severe functional disability. A psychosocial subscale score ≥ 3 was the best concurrent predictor of cases of psychosocial distress. These two cutoff scores are similar to previously established PPST cutoff scores in mixed pain and headache samples. Therefore, the following cutoff criteria were used based on previously established values: Low Risk (Total Score ≤ 2); Medium Risk (Total Score ≥ 3 and Psychosocial subscale ≤ 2); and High Risk (Total Score ≥ 3 and Psychosocial subscale ≥ 3). For the current sample, 28.8% were classified as Low Risk, 38.4% were Medium Risk, and 32.9% were classified as High Risk.
Figure 1.
Receiver operating characteristic (ROC) curves for the (A) PPST total score against reference standard cases for disability, and (B) PPST psychosocial subscale score against reference standard case for psychosocial distress defined using depression, pain catastrophizing, and fear of pain. Boxed numbers indicate sensitivity and specificity values. The straight line signifies the null.
Concurrent Validity
The percentages of patients meeting clinically significant or elevated levels of pain and psychosocial measures across the 3 PPST risk groups were examined (see Table IV). As expected, a low percentage of cases meeting clinically significant levels on standard measures (0–7%) fell into the Low Risk group, a moderate percentage of cases (24–45%) across measures were classified as Medium Risk, and the largest percentage of cases (48–71%) across measures were classified into High Risk. Consistent with discriminant validity analyses, PPST risk groups did not significantly differ in the distribution of cases meeting clinically significant levels for pain duration or ED visits and thereby excluded from further analysis.
TABLE 4.
Chi-Square Results for Risk Group across Clinically Significant or Elevated Pain Characteristics and Pain-Related Outcomes
| PPST Risk Group | ||||
|---|---|---|---|---|
| Low | Medium | High | χ2 value | |
| High pain frequency | 0% (n=0) | 29% (n=4) | 71% (n=10) | 13.52*** |
| Burden days frequency | 0% (n=0) | 30% (n=7) | 70% (n=16) | 23.96*** |
| Persistent pain duration | 22% (n=7) | 31% (n=10) | 47% (n=15) | 3.97 |
| Moderate to severe disability | 0% (n=0) | 39% (n=13) | 61% (n=20) | 31.43*** |
| High pain interference | 6% (n=1) | 24% (n=4) | 71% (n=12) | 14.89*** |
| High admissions for pain | 5% (n=1) | 37% (n=7) | 58% (n=11) | 9.84** |
| High ED visits for pain | 7% (n=1) | 43% (n=6) | 50% (n=7) | 4.45 |
| Elevated depressive symptoms | 0% (n=0) | 40% (n=6) | 60% (n=9) | 9.67** |
| High pain catastrophizing | 7% (n=2) | 45% (n=14) | 48% (n=15) | 13.17*** |
| High pain-related fear | 3% (n=1) | 44% (n=14) | 53% (n=17) | 20.56*** |
p<.01,
p<.001
MANOVA analysis examining pain intensity, pain frequency, pain burden, disability, pain interference, pain admissions, depressive symptoms, pain catastrophizing, and pain-related fear as continuous variables revealed significant PPST risk group differences across measures (Wilks’ λ = 0.24, F(18, 116) = 6.67, p < .001, see Table V). Post-hoc analysis with Bonferroni correction (p < .005) revealed the High Risk group reported significantly higher pain intensity, pain frequency, pain burden, functional disability, pain interference, and depressive symptoms relative to both Low and Medium Risk groups. The High Risk group had significantly more admissions, higher pain catastrophizing and fear of pain compared to the Low Risk group. The Medium Risk group reported significantly higher levels of pain catastrophizing and fear of pain relative to Low Risk, but no significant differences emerged between the Medium and High Risk group on these two psychosocial measures. One-way ANOVA and chi-square analyses revealed no significant differences between PPST Risk Groups by demographic characteristics (i.e., child age, sex, annual family income, hemoglobin type, or treatment with hydroxyurea or chronic transfusions; all p’s > .05).
TABLE 5.
Univariate Analysis of Variance Results for Risk Group across Pain Characteristics and Pain-Related Outcomes
| PPST Risk Group | ||||
|---|---|---|---|---|
| Variable (range of scores) | Low M (SD) N=21 |
Medium M (SD) N=28 |
High M (SD) N=24 |
F-value |
| Pain intensity (0–10) | 1.20 (1.47)a | 2.77 (2.42) | 5.78 (2.58)c | 23.46** |
| Pain frequency (0–31) | 2.60 (2.89)a | 7.46 (8.17)b | 15.52 (10.35)c | 14.62** |
| SCD Pain Burden (0–28) | 2.20 (2.04)a | 9.65 (4.48)b | 14.82 (4.87)c | 51.29** |
| Functional disability (0–60) | 1.85 (2.35)a | 15.19 (12.21)b | 27.30 (12.21)c | 32.16** |
| High pain interference | 43.23 (11.27)a | 55.47 (11.50)b | 66.36 (9.67)c | 22.25** |
| Admissions for pain (0–18) | 0.30 (0.73)a | 2.04 (3.98) | 3.22 (3.41) | 4.57* |
| Depressive symptoms (40–100) | 48.05 (6.84)a | 54.85 (9.88)b | 61.04 (11.07)c | 9.89** |
| Pain catastrophizing (0–54) | 12.80 (10.63)a | 25.31 (11.99)b | 31.26 (11.63) | 14.23** |
| Pain-related fear (0–96) | 18.70 (16.53)a | 50.31 (15.33)b | 55.96 (22.64) | 25.20** |
Note: Superscripts represent significant group differences using Bonferroni-corrected post-hoc comparisons between
Low-High,
Low-Medium,
Medium-High
p<.01,
p<.001
Discussion
There is preliminary evidence that the PPST may be a valid and useful screening tool to identify youth at risk for chronic SCD pain who also demonstrate a psychosocial profile consistent with chronic pain in SCD4 and other pain conditions7–9. The PPST performed well in identifying prognostic factors commonly associated with adverse outcomes among a sample of youth with SCD and classified pediatric patients with SCD into risk groups that may efficiently inform treatment decision-making.
The PPST adequately identified patients who met clinically significant levels of pain frequency, functional disability, pain interference, inpatient admissions, depressive symptoms, pain catastrophizing, and pain-related fear. The data-derived cutoff score for the PPST “high risk” classification was both sensitive and specific for identifying youth with elevated psychosocial distress and was comparable to the cutoff score resulting from two prior validation samples of pediatric musculoskeletal pain11 and headaches17. Therefore, previous recommendations for the PPST cutoff scores for classifying patients into risk groups may be meaningfully applied to youth with SCD. Use of a single, universal pain screening tool (rather than multiple disease-specific measures) offers several potential benefits, including ease of comparison and interpretation across chronic pain conditions, generalization of findings that advance the field of pediatric SCD and chronic pain, and implementation into busy clinical practice.
As expected, the PPST performed well on discriminating patients with high pain frequency. The High Risk group reported an average of 15.5 pain days per month, consistent with the AAPT chronic SCD pain diagnostic criteria (i.e., pain on most days [≥ 15] per month)5. However, the PPST did not adequately discriminate cases of pain duration (i.e., pain frequency lasting ≥ 6 months). Study findings indicate that pain duration is relatively stable across varying pain frequency, with up to 60% of youth in each risk group reporting pain duration of over 1 year. The PPST was developed and originally administered in pain clinics where typical referrals are patients with chronic pain; thus, the screening tool may not have been designed to capture pain duration as a diagnostic criterion or risk factor for poor prognostic outcomes. Although pain duration is a key component used to distinguish chronic from episodic SCD pain, the larger chronic pain literature suggests that how long a patient has experienced chronic pain has no significant or reliable relation with pain or functional outcomes34. Therefore, pain duration may not be critical for identifying youth at risk for poor functional outcomes but remains important for diagnosing chronic SCD pain.
The clinical utility and applicability of the PPST as a brief screening tool in pediatric SCD is promising. The screener, which takes 1–2 minutes to complete, may offer a brief overview that is consistent with a more detailed battery of well-established, standardized patient-reported outcome measures that can take up to 30 minutes to complete to identify psychosocial risk factors indicative of poor prognostic outcomes. Use of a single screening tool may offer improved time efficiency and reduce patient burden in completing patient-reported outcomes while offering clinically useful data to guide further assessment and treatment decision-making. Furthermore, brief, routine screening offers a key opportunity for risk stratification that can help inform a stepped care model for allocating treatment and resources based on patient need. For example, youth in the Low Risk group may be responsive to medication and standard care that integrates education about SCD self-management whereas the Medium Risk group may require targeted education about the biopsychosocial impact on pain and introduction to coping skills to facilitate improved functioning. Youth in the High Risk group may require more intensive, interdisciplinary treatment that combines medical care; psychological services such as cognitive-behavioral therapy; physical therapy; and integrative treatments such as acupuncture, yoga, or tai-chi35. Therefore, brief and routine screening can allow for early identification of youth experiencing SCD pain and co-morbid psychosocial risks and may facilitate early, targeted intervention to help improve pain and functioning.
Focused attention on the Medium Risk group may help identify youth transitioning from acute to chronic pain. The biopsychosocial model of pain describes pain and disability as a multidimensional and dynamic bi-directional interaction among physiological, psychological, and social factors resulting in chronic pain13. Youth in the Medium Risk group report subclinical levels of many functional and psychosocial factors commonly associated with chronic pain, including mild functional disability, depressive symptoms, pain catastrophizing, and pain-related fear. These elevated psychosocial factors combined with physiological changes can play essential roles in the transition to chronic SCD pain. Thus, the PPST may facilitate identification of youth in need of closer monitoring and early intervention to prevent the development of chronic SCD pain.
The interpretation of study findings should be considered within the context of a few limitations. The cross-sectional study design, convenience sample, and modest sample size limit the ability to examine the predictive validity of the screening tool on long-term pain and biopsychosocial outcomes. Additional validation of the PPST with a larger representative sample is needed to further support the validation of the screening tool among youth with SCD, which may support future studies in the development of a diagnostic measure for chronic SCD pain. Healthcare utilization data may be under-represented if families sought emergent care from medical settings outside of our healthcare system and may account for PPST scores being invariant by ED visits. Future validation studies may consider collecting additional reports of healthcare use, opioid consumption, amending or adding an item to capture pain duration for diagnostic benefit, and exploring the development of parent proxy report.
Despite these limitations, study findings provide preliminary evidence that the PPST can efficiently and effectively identify modifiable biopsychosocial factors that classify youth based on risk for poor pain and functional outcomes. The PPST may be a useful tool to identify youth who are at risk of transitioning from acute to chronic SCD pain as well as identify youth who have chronic pain and present with a biopsychosocial profile consistent with chronic SCD pain. Future research is warranted to evaluate the utility of the PPST to inform targeted patient needs and guide treatment decision-making.
Acknowledgments
The authors thank the children and families for their time and participation in this research. Special thanks are also extended to the clinical research coordinators for their time and effort in supporting the study: Farida Abudulai, Morgan Barnett, Shelley Mays, Natasha Morris, Bailey Sturdivant, and Amanda Watt.
Abbreviation: Full term or phrase:
- AAPT
Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks – American Pain Society Pain Taxonomy
- AUC
Area under the curve
- CDI-2
Children’s Depression Inventory – 2
- FDI
Functional Disability Inventory
- FOPQ-C
Fear of Pain Questionnaire – Child
- M
Mean
- PCS-C
Pain Catastrophizing Scale – Child
- PPST
Pediatric Pain Screening Tool
- ROC
Receiver operating characteristic
- SCD
Sickle cell disease
- SCPBI
Sickle Cell Pain Burden Interview
- SD
Standard deviation
Footnotes
Note: Aspects of this work were presented previously at American Society of Hematology Annual Meeting, Atlanta, GA, December 2017 and Society of Pediatric Psychology Annual Conference, Orlando, FL, April 2018.
Disclosures
Funding for this study was supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award UL1TR000454. Preparation of this paper was supported by the National Heart, Lung, and Blood Institute (NHLBI) Award 1K23Hl133457–01A1 to Soumitri Sil, Ph.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to report.
References Cited
- 1.Dampier C, Ely B, Brodecki D, O’Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002;3(6):461–70. Epub 2003/11/19. PubMed PMID: 14622732. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro BS, Dinges DF, Orne EC, Bauer N, Reilly LB, Whitehouse WG, Ohene-Frempong K, Orne MT. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain. 1995;61(1):139–44. 10.1016/0304-3959(94)00164-A. PubMed PMID: 7644237. [DOI] [PubMed] [Google Scholar]
- 3.Zempsky WT, O’Hara EA, Santanelli JP, Palermo TM, New T, Smith-Whitley K, Casella JF. Validation of the Sickle Cell Disease Pain Burden Interview–Youth. The Journal of Pain. 2013;14(9):975–82. doi: 10.1016/j.jpain.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sil S, Cohen LL, Dampier C. Psychosocial and Functional Outcomes in Youth With Chronic Sickle Cell Pain. Clin J Pain. 2016;32(6):527–33. 10.1097/ajp.0000000000000289. PubMed PMID: 26379074. [DOI] [PubMed] [Google Scholar]
- 5.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT Diagnostic Criteria for Chronic Sickle Cell Disease Pain. J Pain. 2017. 10.1016/j.jpain.2016.12.016. PubMed PMID: 28065813. [DOI] [PubMed] [Google Scholar]
- 6.Sil S, Dampier C, Cohen LL. Pediatric Sickle Cell Disease and Parent and Child Catastrophizing. J Pain. 2016;17(9):963–71. 10.1016/j.jpain.2016.05.008. PubMed PMID: 27263990. [DOI] [PubMed] [Google Scholar]
- 7.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain. 2007;131(1–2):132–41. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and Functional Disability in Chronic Pediatric Pain. The Clinical Journal of Pain. 2001;17(4):341–9. [DOI] [PubMed] [Google Scholar]
- 9.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. Journal of Developmental & Behavioral Pediatrics. 2000;21(1):58–69. [DOI] [PubMed] [Google Scholar]
- 10.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. European journal of haematology. 2014;93(2):89–95. 10.1111/ejh.12340. PubMed PMID: 24735098. [DOI] [PubMed] [Google Scholar]
- 11.Simons LE, Smith A, Ibagon C, Coakley R, Logan DE, Schechter N, Borsook D, Hill JC. Pediatric Pain Screening Tool: rapid identification of risk in youth with pain complaints. Pain. 2015;156(8):1511–8. 10.1097/j.pain.0000000000000199. PubMed PMID: 25906349; PubMed Central PMCID: PMCPmc4504741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heathcote LC, Rabner J, Lebel A, Hernandez JM, Simons LE. Rapid Screening of Risk in Pediatric Headache: Application of the Pediatric Pain Screening Tool. J Pediatr Psychol. 2018;43(3):243–51. 10.1093/jpepsy/jsx123. PubMed PMID: 29048551. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain. 2016;17(9 Suppl):T70–92. 10.1016/j.jpain.2016.01.001. PubMed PMID: 27586832; PubMed Central PMCID: PMCPmc5012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16(1):39–58. PubMed PMID: 1826329. [DOI] [PubMed] [Google Scholar]
- 15.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1–2):77–84. 10.1016/j.pain.2005.12.002. PubMed PMID: 16480823; PubMed Central PMCID: PMCPmc3144698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dampier C, Jaeger B, Gross HE, Barry V, Edwards L, Lui Y, DeWalt DA, Reeve BB. Responsiveness of PROMIS(R) Pediatric Measures to Hospitalizations for Sickle Pain and Subsequent Recovery. Pediatric blood & cancer. 2016;63(6):1038–45. 10.1002/pbc.25931. PubMed PMID: 26853841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs M Children’s Depression Inventory Available from Multi-Health systems, Inc., 908 Niagara Falls Blvd., North Tonawanda, N.Y. 14120–2060; 1992. [Google Scholar]
- 19.Conte P, Walco G, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis and Rheumatism. 2003;48(10):2923–30. [DOI] [PubMed] [Google Scholar]
- 20.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108(3):221–9. 10.1016/j.pain.2003.11.008S0304395903004585 [pii]. PubMed PMID: 15030941. [DOI] [PubMed] [Google Scholar]
- 21.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain. 2001;17(4):341–9. PubMed PMID: 11783815. [DOI] [PubMed] [Google Scholar]
- 22.Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, Wilson AC, Zhou C. Factor Structure of the Children’s Depression Inventory in a Multisite Sample of Children and Adolescents With Chronic Pain. The Journal of Pain. 2013;14(7):689–98. doi: 10.1016/j.jpain.2013.01.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): A preliminary validation. PAIN. 2006;123(3):254–63. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 25.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: Proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. PAIN®. 2014;155(11):2360–7. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;7(4):524. doi: Doi 10.1037//1040-3590.7.4.524. PubMed PMID: WOS:A1995TL18000014. [DOI] [Google Scholar]
- 27.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): Assessment of Pain-Related Fear Among Children and Adolescents With Chronic Pain. The Journal of Pain. 2011;12(6):677–86. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Aisiku IP, Smith WR, McClish DK, Levenson JL, Penberthy LT, Roseff SD, Bovbjerg VE, Roberts JD. Comparisons of high versus low emergency department utilizers in sickle cell disease. Annals of emergency medicine. 2009;53(5):587–93. 10.1016/j.annemergmed.2008.07.050. PubMed PMID: 18926599. [DOI] [PubMed] [Google Scholar]
- 29.Carroll CP, Haywood C, Lanzkron S. Prediction of onset and course of high hospital utilization in sickle cell disease. Journal of Hospital Medicine. 2011;6(5):248–55. doi: 10.1002/jhm.850. [DOI] [PubMed] [Google Scholar]
- 30.Epstein K, Yuen E, Riggio JM, Ballas SK, Moleski SM. Utilization of the office, hospital and emergency department for adult sickle cell patients: a five-year study. Journal of the National Medical Association. 2006;98(7):1109–13. Epub 2006/08/10. PubMed PMID: 16895280; PubMed Central PMCID: PMCPMC2569470. [PMC free article] [PubMed] [Google Scholar]
- 31.Youngstrom EA. A primer on receiver operating characteristic analysis and diagnostic efficiency statistics for pediatric psychology: we are ready to ROC. J Pediatr Psychol. 2014;39(2):204–21. 10.1093/jpepsy/jst062. PubMed PMID: 23965298; PubMed Central PMCID: PMCPmc3936258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan EM, Mara CA, Huang B, Barnett K, Carle AC, Farrell JE, Cook KF. Establishing clinical meaning and defining important differences for Patient-Reported Outcomes Measurement Information System (PROMIS((R))) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Qual Life Res. 2017;26(3):565–86. 10.1007/s11136-016-1468-2. PubMed PMID: 27913986; PubMed Central PMCID: PMCPmc5311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs M Chidlren’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems Inc.; 1992. [Google Scholar]
- 34.McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976). 2002;27(22):2564–73. 10.1097/01.Brs.0000032130.45175.66. PubMed PMID: 12435995. [DOI] [PubMed] [Google Scholar]
- 35.Tick H, Nielsen A, Pelletier KR, Bonakdar R, Simmons S, Glick R, Ratner E, Lemmon RL, Wayne P, Zador V. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore (New York, NY). 2018;14(3):177–211. 10.1016/j.explore.2018.02.001. PubMed PMID: 29735382. [DOI] [PubMed] [Google Scholar]