Abstract
Tanycytes are highly specialized bipolar ependymal cells that line the ventrolateral wall and the floor of the third ventricle in the brain and form a blood-cerebrospinal fluid barrier at the level of the median eminence. They play a pivotal role in regulating metabolic networks that control body weight and energy homeostasis. Due to the glucosensing function of tanycytes they could be considered as a critical player in the pathogenesis of type 2 diabetes. Genetic fate mapping studies have established the role of tanycytes for the newly detected adult hypothalamic neurogenesis with important implications for metabolism as well as pathophysiology of various neurodegenerative diseases. We believe that a comprehensive understanding of the physiological mechanisms underlying their neuroplasticity, glucosensensing and crosstalk with endothelial cells will enable us to achieve metabolic homeostasis in type 2 diabetes patients and possibly delay the progression of Alzheimer’s disease and hopefully improve cognitive function.
Keywords: Alzheimer’s disease, glucose homeostasis, tanycytes, type 2 diabetes
Introduction:
The worldwide incidence of type 2 diabetes is increasing at an alarming pace and is indeed a significant health problem especially due to an increase in life expectancy and an aging population [1]. As per American Diabetes Association, in the year 2015, 84.1 million Americans were prediabetic and 30.3 million had diabetes leading to a total healthcare economic burden of $322 billion. type 2 diabetes is associated with various types of complications including brain insulin resistance and cognitive impairment. Perusal of current literature suggests a strong linkage between type 2 diabetes and Alzheimer's disease (AD) [2-8]. AD is a progressive neurodegenerative disorder that causes an irreversible cognitive decline in an estimated 5.5 million Americans with $259 billion in healthcare costs in 2017 (Alzheimer's Association). If the present trend continues, by 2050 the number of AD patients will surpass 16 million with ~$1.1 trillion in healthcare costs thereby necessitating the development of novel therapeutic strategies to effectively treat AD. Towards fulfilment of the unmet clinical need for an effective therapy to combat AD, our ultimate goal is to develop a robust AD patient-specific personalized precision-guided targeted gene editing and stem cell therapy. Although, the current literature suggests that there is a strong linkage between TYPE 2 DIABETES and AD [4-6], there is a significant knowledge gap regarding the precise molecular mechanism/s underlying the linkage and interaction between type 2 diabetes and AD. A complex neuronal network in the brain regulates cell metabolism and energy homeostasis.
Recent studies have shown that tanycytes play a crucial role in regulating peripheral metabolic signals and controlling energy balance. Prevot et al. have very recently published an in-depth review article on the role of tanycytes in reproduction and energy metabolism [9]. The current review is focused upon deciphering the role of tanycytes during health and disease states and establishing a nexus between type 2 diabetes and Alzheimer’s Disease (AD). The objective of this review is to understand the role of tanycytes in blood glucose regulation in type 2 diabetes patients and their concurrent risk of developing AD. Here we discuss the latest developments in the field and provide a new paradigm that will hopefully enable the generation of novel therapeutic targets to treat type 2 diabetes and AD.
Tanycytes and their anatomical placement
The historic origins and the discovery of tanycytes have been elegantly described by Prevot et al. recently [9]. Adult hypothalamus harbors neural stem cells (NSCs) in a niche near the third ventricle. Tanycytes, ependymocytes, subventricular astrocytes and parenchymal glial cells all reside near the third ventricle and each is a potential adult stem and progenitor cell candidate. Tanycytes represent a highly specialized glial cell type found lining the wall of the third ventricle in the median eminence of the hypothalamus [10,11]. Hypothalamic tanycytes resemble embryonic radial glial cells and possess a cell body with an unusually long basally extending process. Using combined immunohistochemical and permeability studies, Langlet et al. have demonstrated that tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain [12]. Morphological studies have mapped and defined various subpopulations of the tanycytes according to their position and process projection types. Ventral-most β-tanycytes line the infundibulum and median eminence, while adjacent α-tanycytes line regions of the ventricular zone (VZ) that are adjacent to hypothalamic nuclei and project laterally, contacting capillaries and neurons of the arcuate and ventromedial nuclei en passant. The α-tanycytes are classified as dorsal α1d, ventral α1v and α2 tanycytes. The β-tanycytes are further sub classified as dorsal β1d, ventral β1v, lateral β2la and medial β2me tanycytes. Furthermore, identifying tanycyte and tanycyte-subtype-specific marker genes will allow the development of genetic tools for achieving cell-subtype-specific manipulation for dissecting the function of tanycyte and tanycyte subtypes. Such an invention can help achieve long-term remission in diabetics and in turn reduce the risk of development of AD by combating the excessive activation of inflammatory pathways.
Tanycyte Genetic Markers
It is important to identify molecular markers that would enable tanycytes to be distinguished from the other cell types. The radial glia markers nestin and vimentin were highly transcribed in tanycytes suggesting that these cells originate from embryonic radial glia and function as neural stem cells in adult hypothalamus. However, since nestin and vimentin are highly expressed in ependymal cells both these markers cannot be considered as true tanycyte specific markers. Besides nestin and vimentin, tanycytes also express Sox2, brain lipid-binding protein (BLBP), glutamate/aspartate transporter (GLAST), Mushashi-1 and Glial Fibrillary Acidic Protein (GFAP), Notch1, Notch2, Lhx2, Rax and Hes5 [13-16]. The tanycyte-enriched genes include Col23a1, Slc16a2, Lhx2, and Ptn some of which have been linked to tanycyte development and function, such as Lhx2 and Slc16a2. Lhx2 activates Rax transcription in hypothalamic tanycytes and controls tanycyte differentiation [16]. However, there are different types of tanycytes and whether these different tanycyte subtypes can be characterized using a molecular signature is necessary. Using the scRNA-seq data, Chen et al. have identified Slc17a8 and Col25a1 as potential markers for α1 and β tanycyte subtypes, respectively [17]. Clustering analysis has identified Rax as tanycyte cell cluster marker, indicating that tanycytes are transcriptionally distinct from ependymocytes and other cell types [17]. Notably, although specific marker genes (or combinations of marker genes) are used to roughly separate tanycyte subtypes, many genes exhibit a gradient, rather than a clear-cut distribution across tanycyte subpopulations which are consistent with the notion that tanycytes may be composed of continuous cell trajectory with transition zones between different subtypes. Recently two new markers UGS148 and Prss56 have been identified and found to be enriched in tanycytes [18,19].
Campbell et al. have profiled gene expression in 20,921 cells in and around the adult mouse hypothalamic arcuate-median eminence complex (ARC-ME) using Drop-seq [20]. They identified 50 transcriptionally distinct Arc-ME cell populations including a rare tanycyte population. Further, their studies revealed certain genes with very restricted gene expression patterns. They identified Sprr1a as a specific marker of tanycytes and demonstrated that it was found only at the border between Arc and ME where tanycytes are thought to form a diffusion barrier. Based upon the current information and especially those described by Campbell et al., we have compiled the list of old and new tanycyte markers as shown in Table 1 and 2. It would be interesting to investigate the functional significance of these markers during normal homeostasis, type 2 diabetes as well as AD. We believe that these studies will be very crucial to establish the role of tanycytes and their nexus between type 2 diabetes and AD.
Table 1:
Established Tanycyte Molecular Markers
| Marker | EpyI | EpyII | α1I | α1II | α2 | β1 | β2I | β2II |
|---|---|---|---|---|---|---|---|---|
| Sox2 | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Vim | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Slc2a1 | +++++ | +++++ | +++ | + | + | ++ | ++ | + |
| Fgfr1 | +++ | +++++ | ++ | + | + | ++ | ++++ | +++++ |
| Fgfr2 | + | ++ | + | + | + | + | + | + |
| Fabp7 | + | +++ | ++ | +++ | ++ | + | + | + |
| Cntfr | + | − | + | + | + | + | + | + |
| Fgf10 | + | +++ | ++ | ++ | ++ | + | + | + |
| GLUT1 | + | + | + | + | + | + | − | − |
| GLT-1 | − | − | + | + | + | − | − | − |
| GLAST | + | + | − | − | − | + | + | + |
| GKRP | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| Nes | ++++ | +++++ | +++ | +++ | +++ | ++ | ++ | +++ |
| Gpr50 | + | ++++ | +++++ | +++ | +++ | ++++ | +++ | ++ |
| Ppp1r1b | + | ++++ | + | + | + | ++ | ++++ | +++ |
| 6330403K07Rik | +++++ | +++++ | +++++ | +++++ | +++ | +++ | +++ | +++ |
| Trhde | + | + | + | − | + | + | + | + |
| Rbp1 | ++ | +++ | + | + | + | + | ++ | + |
| Rax | ++ | ++ | ++ | +++ | +++ | ++++ | ++++ | ++++ |
| Rab4 | + | + | − | − | − | +++ | +++ | |
| Stra6 | + | − | + | − | + | + | + | + |
| Dio2 | + | + | + | + | ++ | ++ | ++++ | ++++ |
| Foxj1 | +++++ | ++++ | +++ | +++ | +++ | +++ | + | + |
| Gfap | + | +++++ | +++ | + | + | + | + | + |
| Slc1a3 | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Rarres2 | +++++ | +++++ | +++++ | ++ | ++ | ++ | + | + |
| S100b | +++++ | +++++ | +++++ | ++ | + | + | + | + |
| Gja1 | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Cntf | − | + | − | − | + | + | + | + |
Table 2:
Newly Discovered Tanycyte Molecular Markers
| Marker | EpyI | EpyII | α1I | α1II | α2 | β1 | β2I | β2II |
|---|---|---|---|---|---|---|---|---|
| Prdx6 | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Mt1 | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Mt2 | +++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Dlk1 | ++++ | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ |
| Cdhr4 | +++++ | ++++ | + | − | − | + | + | − |
| Calb1 | +++++ | +++++ | + | + | + | + | + | + |
| Ccdc153 | +++++ | ++++ | + | − | + | + | + | + |
| Tmem212 | +++++ | ++++ | ++ | − | + | + | − | + |
| Itih5 | +++++ | ++++ | ++ | + | + | + | + | + |
| Flt1 | + | ++ | ++ | + | + | + | + | + |
| Rspo3 | + | +++ | +++ | ++ | + | + | + | − |
| Slc17a8 | + | + | + | +++++ | + | + | − | − |
| Lyz2 | + | + | + | ++ | + | + | + | − |
| Pdzph1 | + | − | + | + | ++ | + | + | + |
| P3h2 | + | + | + | + | ++ | + | + | + |
| Ribp1 | + | +++ | + | + | + | ++ | + | + |
| Lrm1 | + | ++ | ++ | + | + | ++ | + | + |
| Frzb | + | + | ++ | + | +++ | ++ | ++ | + |
| Cldn10 | + | + | + | + | + | ++ | ++++ | ++++ |
| Col25a1 | + | + | + | − | + | ++ | ++++ | ++++ |
| Scn7a | ++ | ++ | + | − | + | + | ++++ | ++++ |
| Rgs7bp | + | + | + | + | + | + | ++ | +++ |
| Cysltr1 | − | − | − | − | + | + | + | ++ |
| Lrrtm3 | + | + | + | − | + | + | + | ++ |
Glucose Homeostasis by Tanycytes
Growing evidence points to the brain as a potential target for the treatment of type 2 diabetes. In vitro calcium imaging studies using brain slices have revealed that the tanycytes respond to glucose treatment by displaying P2Y1 receptor-dependent spreading of calcium in a wave-like pattern between the neighboring cells due to the release and extracellular diffusion of ATP [21]. These studies were the first to demonstrate that tanycytes are glucosensors. Tanycytes possess the glucosensing machinery including GLUT1, GLUT2, glucokinase as well as ATP-sensitive potassium channels that are found in the insulin producing pancreatic β cells within the pancreatic islets [22,23]. These findings suggest that actually tanycytes and pancreatic β cells use very similar glucosensing mechanism. However, recent studies indicate that there is another glucosensing machinery in tanycytes based on sweet taste receptor which is a heterodimer consisting of Tas1r2 and Tas1r3 receptor subunits [24]. Their findings using Tas1r2 null mice suggest that the proportion of glucose-insensitive tanycytes is increased. As compared to wild type mice wherein 53% of tanycytes respond to glucose puffs, only 22% of tanycytes do so in the Tas1r2 null mice thereby suggesting the existence of 2 different tanycyte populations that utilize either the sweet taste receptor or another glucose sensing mechanism. A recent study has shown that VEGF-A expression in tanycytes orchestrates the modulation of the blood brain barrier and that the neutralization of VEGF signaling blocks fasting-induced barrier remodeling thereby significantly impairing the physiological response to refeeding [25]. These findings highlight VEGF-dependent mechanism operational in the tanycytes that is responsible for glucosensing. There is a distinct possibility that there are additional glucose sensing mechanisms employed by the tanycytes. How these glucose-sensing mechanisms are perturbed in diabetes and diabetic AD patients remain unexplored. Interestingly, it would be possible to explore the role of tancytes as well as tanycyte-mediated novel glucose sensing mechanisms in the well-established diabetes as well as AD mouse models.
Salgado et al. demonstrated the expression of both glucokinase (GK) and glucokinase regulatory protein (GKRP) in the primary tanycyte cultures [26]. Their in vitro kinetic studies demonstrated GK activity and its inhibition by GKRP. Utilizing highly enriched primary tanycyte cultures, their studies suggest that the intranuclear localization of GK and GKRP increases in the presence of high glucose concentrations. Based on these data they have postulated that the nuclear compartmentalization of GK and GKRP might play a role in glucosensing in tanycytes. They had reported earlier that increased glucose (2-10 mM) generates a high glycolytic flux that leads to the release of ATP by Cx43 hemichannels, activating P2Y receptors and thereby increasing [Ca2+]i at the expense of intracellular stores [27]. However, an increase in [Ca2+]i in acute in situ application of glucose and nonmetabolizable analogs in α-tanycytes was observed. These research findings need to be studied more carefully to determine whether different tanycyte subpopulations could be involved in metabolic and non-metabolic glucosensing mechanisms.
Uranga et al. developed an adenovirus expressing GK shRNA to inhibit GK expression in tanycytes in vivo [28]. Their in vivo GK knockdown studies have revealed an increased food intake and altered feeding behavior. GK knockdown did not alter the expression of GLUT2 or GKRP in the tanycytes. In response to an intracerebroventricular glucose injection, they found that the mRNA levels of anorexigenic POMC and CART and orexigenic AgRP and NPY neuropeptides were altered in GK knockdown animals. It is well known that POMC and CART levels increase while NPY and AgRP decrease during hyperglycemia. Based on their in vivo data, they have proposed that there is an existence of the metabolic coupling between tanycytes and neurons. Since there are different types of tanycytes, it is possible that different tanycyte populations could detect glucose differently.
Recently there is an enhanced interest in exploring the role of tau in pancreatic β cell function as well as insulin signalling. Interestingly, utilizing immunohistochemical analysis of AD and non-AD brain sections as well as by examining the tau hyperphosphorylation cellular models, Rodriguez et al. have demonstrated that the hyperphosphorylated form of tau promotes the oligomerization and accumulation of insulin in the neurons [29]. Most recently, Marciniak et al. have identified a putative novel function of tau protein as a regulator of insulin signalling in the brain through IRS-1 and PTEN dysregulation [30]. Using the tau knockout mice, they were able to show that intracerebroventricular (icv) insulin injection leads to reduction of anorexigenic effects and development of glucose intolerance as well as peripheral hyperinsulenimeia. These studies are exciting and provide compelling evidence of a strong linkage between brain insulin signalling, insulin resistance, energy metabolism and the nexus with AD pathophysiology. However, it remains to be determined whether or not tanycytes are the nexus between AD and type 2 diabetes. Utilizing a tau knockout mice, Wijesekara et al. have demonstrated that the tau knockout mice were hyperglycemic, glucose intolerant, had reduced islet insulin content, increased hepatic glucose production but significantly elevated proinsulin levels resulting in impaired glucose stimulated insulin secretion [31]. Further, loss of tau led to increased epididymal fat mass and leptin levels, insulin resistance at later stages leading to complete onset of diabetes. They further demonstrated that tau is expressed in the brain as well as pancreatic β cells in the wild type mice. Previously, Miklossy et al. have successfully demonstrated beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes patients [32]. These significant findings highlight the potential nexus between type 2 diabetes and AD.
Schwartz et al. have previously shown that a brain-centred glucoregulatory system (BCGS) can lower blood glucose levels via both insulin-dependent and -independent mechanisms [33]. These authors suggested that normal glucose homeostasis is a result of a complex and highly co-ordinated interaction between the BCGS and the pancreatic islets. Furthermore, the activation of either regulatory system compensates for the failure of the other regulatory system and as a result the development of diabetes is a consequence of the defects in both the systems. Taken together these findings indicate that therapies targeting both BCGS as well as pancreatic islets simultaneously are likely to be more successful than targeting them individually.
Fibroblast growth Factor (FGF), Tanycytes and Diabetes Treatment
It has been previously demonstrated that the FGF receptors 1 and 2 and the ligands FGF1, FGF2, FGF4, FGF5, FGF7 and FGF10 are expressed in adult mouse pancreatic β cells and FGFR1c perturbation leads to development of diabetes [34]. Furthermore, these studies also suggested that Pdx1 which acts upstream of FGFR1 signaling in pancreatic β cells is essential for the maintenance of proper glucose homeostasis. In murine type 2 diabetes models, hyperglycemia can be ameliorated transiently by either systemic or intracerebroventricular (icv) administration of FGF19 [35-38] or FGF21 [39]. Morton et al. have shown that systemic FGF19 administration improved glucose tolerance through its action via a central mechanism [35]. Utilizing ob/ob mice, they showed that a single icv injection of FGF19 significantly improved glucose tolerance. They further showed that the antidiabetic effect of icv injected FGF19 was solely due to increased glucose effectiveness and not because of the changes either in insulin secretion or insulin sensitivity and was mediated at least in part via a melanocortin-independent mechanism. Unfortunately, these studies did not investigate the role of FGF19 in tanycyte-mediated glucosensing. Scarlett et al. have explored the antidiabetic efficacy of centrally administered FGF1 [40]. Their findings indicate that the glucose lowering induced by icv injection of FGF1 into the lateral or third ventricle of the brain occurs only in hyperglycemic but not in nondiabetic mice. Another interesting finding was that the FGF1-mediated glucose lowering effect requires FGFR1 signalling in adipose tissue. Further, these studies also revealed that icv FGF1 and not icv FGF19 administration led to robust activation of tanycytes and sustained lowering of glucose for 18 weeks. These glucose-lowering effects were also demonstrated post ivc rFGF1 injection in a ZDF rat TYPE 2 DIABETES model. The translational potential of this discovery is heightened by the feasibility of therapeutic FGF1 delivery to the CNS via the intranasal route, since intra-nasal insulin administration has been well established in human patients [41-43]. The translational significance of these exciting findings is that if FGF1 therapy is successful in human type 2 diabetes patients such an approach could also be very useful for AD patients suffering from type 2 diabetes.
Yet another possibility to achieve long-term sustained correction of hyperglycemia could be to transplant glucose responsive insulin producing cells derived from the patient specific iPS cells. We have performed feasibility studies in diabetic mice by transplanting human iPS as well as mouse ES cell-derived insulin producing cells [44-49] as well as pancreatic endoderm-like cells [50]. Our data suggest that transplantation of both mouse ES cell as well as human iPS cell-derived insulin producing cells are able to achieve sustained long-term correction of hyperglycemia.
Do Tanycytes Mimic Stem Cells?
Whether or not tanycytes are neural stem cell capable of generating other cell types, subset of β-tanycytes, the β2-tanycytes, can proliferate and are neurogenic, contributing new neurons to hypothalamic nuclei in the postnatal/juvenile period. This reflects the neurogenic stem cell property of tanycytes. Initial studies utilizing an adenoviral vector expressing GFP injected into the third ventricle, Xu et al. demonstrated labelling of tanycytes and ependymal cells [51]. Migration of the GFP labelled tanycytes into the hypothalamic parenchyma and their subsequent differentiation led to development of neurons, which integrated into neural networks. However, it is important to note that in these studies Xu et al. have used an adenoviral vector in which the CMV promoter drives the expression of EGFP. Since CMV is a strong constitutively active promoter, which is highly active in a wide variety of cell types, it is very likely that besides transducing the tanycytes, their adenoviral vector also cotransduced the neighboring neurons thereby making the data interpretation rather difficult. Instead, it would be worthwhile to employ a capsid engineered recombinant adeno-associated viral vector with a tanycyte specific promoter driving the expression of the reporter. Furthermore, a recent complementary study suggests that ventrally located tanycytes, including β2-tanycytes, can proliferate in early adulthood, giving rise to new neurons [15]. Lee et al. have utilized genetic fate mapping to identify median eminence tanycytes as being responsible for the generation of newborn neurons [15]. Bromodeoxyuridine (BrdU) labelling of mice fed with either normal chow or high fat diet revealed that high fat diet in adult mice led to quadruplicating the neurogenesis rate. To further prove whether or not β2- tanycytes could differentiate into neurons; transgenic Nestin:CreERR26stopYFP driver mice were bred with ROSA26stopYFP mice to inducibly and selectively fate map tanycytes and their progeny. Administration of 4-hydroxytamoxifen (4-OHT) led to the induction and labelling of Nestin+ as well as Sox2+ β2 tanycytes with YFP but no co-labelling of YFP and GFAP or Hu was observed immediately post transduction. However, 1 month post 4-OHT induction a substantial fraction of Hu+ MEm neurons were labelled with YFP thereby suggesting that β2-tanycytes directly give rise to the neurons. Lineage tracing studies revealed that very few of these cells were derived from Nestin+ tanycytes thereby suggesting the existence of an as-yet-unidentified non-tanycyte progenitor cell population in the postnatal hypothalamus.
Based on lineage cell tracing experiments, the existence of the stem cell potential of hypothalamic tanycytes has now been very well established [15,52,14,53]. Prior studies by Rodriguez et al. indicated that α2 tanycytes act as the potential neural stem cells [10]. In an unrelated study utilizing long-term in vivo lineage tracing using a GLAST::CreERT2 conditional mouse model, Robins et al. have demonstrated that α-tanycytes are self-renewing cells that constitutively give rise to new β1 tanycytes, astrocytes and sparse number of neurons [14]. These studies further suggest that GLAST::CreERT2 cells are gliogenic as well as neurogenic. GLAST+ α-tanycytes include neural stem/progenitor populations that are capable of proliferating and differentiating into astrocytic and neuronal lineages at a lower frequency. The neural stem cell population present in the SVZ and SGZ actively responds to FGF2 and undergoes increased proliferation and survival and is neurospherogenic with an expression profile similar to NSC including Sox2, Nestin, Vimentin, GFAP and pErk1/2. The neurosphere formation and propagation requires exogenous EGF and FGF supplementation.
In a separate study, Haan et al. have shown that FGF10+ tanycytes express molecular markers of neural stem/progenitor cells, are able to proliferate and differentiate into neurons as well as astrocytes in vivo [13]. These FGF10+ tanycytes divide at least once every 9-15 days between P28-P75 and based on lineage tracing studies contribute mostly to the neurons of the arcuate and to a lesser extent to the ventromedial, dorsomedial and lateral hypothalamic nuclei. In an interesting study, the loss of hypothalamic neural stem cells have been shown to be an important cause of ageing in the whole body [54]. Using various mouse models in which hypothalamic stem/progenitor cells that co-express Sox2 and Bmi1 were ablated, these authors revealed an accelerated aging phenotype and a shortened life span due to substantial loss of hypothalamic cells. Implantation of healthy hypothalamic stem/progenitor cells engineered to survive in the ageing-related hypothalamic inflammatory microenvironment led to reversal of the ageing process and extension of the life span in mid-aged mice. Mechanistically, anti-ageing effect of hypothalamic neural stem cells which were achieved in a relatively short period were partially due to age dependent differential expression of exosomal microRNAs in a neurogenesis-independent manner. However, it is not clear from these studies whether hypothalamic neural stem cells were tanycytes.
Most recently, Pellegrino et al. have reported on a comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse rat and gray mouse lemur using a panel of 5 neural stem/progenitor cell (NPCs)-specific markers including Sox2, Nestin, Vimentin, GLAST and GFAP [55]. Their data suggest that unlike the mouse, rat and the gray mouse lemur, adult human hypothalamus contains 4 different NPC cell types express including a ribbon of small stellate cells lining the third ventricular wall, ependymal cells, tanycytes and a population of small stellate cells in the suprachiasmatic nucleus. Thus, it would be very interesting to investigate the effect of hyperglycemia as well as neuroinflammation and neurodegeneration on the neurogenic potential of tanycytes. We believe that these studies will enable us to decipher the nexus between tanycytes, type 2 diabetes as well as AD.
Future Directions
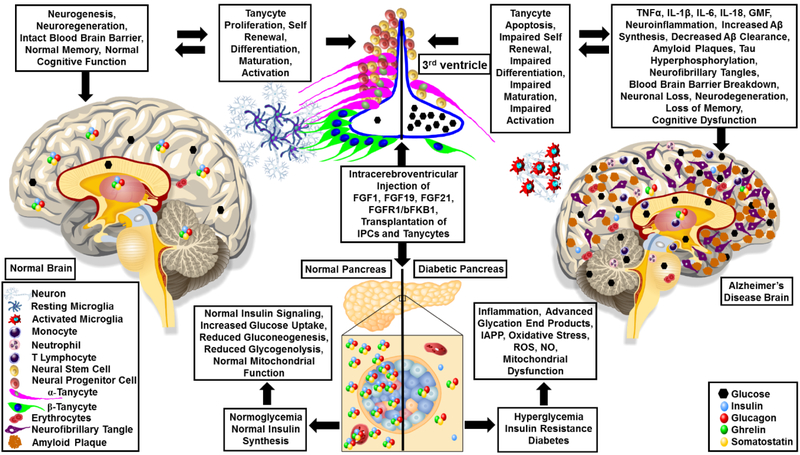
Currently, there is no report to suggest any role of tanycytes in AD pathophysiology. However, we believe that tanycytes play a crucial role in neurogenesis as well as regulation of glucose metabolism during normal homeostasis, which might become impaired during AD progression (Figure 1). To establish tanycytes as a nexus between type 2 diabetes and AD, it would be necessary to investigate the alterations in the tanycyte population in the brains of normal, type 2 diabetes as well as AD patients. For example, it would be interesting to investigate how chronic neuroinflammation and hyperglycemia induce functional alterations in the circumventricular organ composed of the β tanycytes that form blood-cerebrospinal fluid barrier at the level of median eminence located in the tuberal region of the hypothalamus. Further, it will be equally important to study the α-tanycytes that line the ventricular wall of the arcuate nucleus in the mediobasal hypothalamus and their association with the endothelial cells. Especially, it would be interesting to look at the differential expression of VEGF-A, VEGFR1, VEGFR2, hypoxia inducible factor 1α, P2Y1, Rax, Lhx2, insulin, leptin, GLUT1, GLUT2, FGFR1, ZO1, Occludin, Vimentin, along with the markers of neuroinflammation in the human AD and age-matched non AD brain. Similar studies in wild type, TYPE 2 DIABETES and AD mouse models will uncover novel molecular mechanisms and improve our understanding of the role of tanycytes in both of these diseases. It is expected that with aging, molecular mechanisms governing homeostasis also enter a phase of dormancy. However, significant advancements in the field of gene therapy, gene editing, molecular imaging and stem cells make it possible to rejuvenate, reactivate, reduce or reverse the aging related defects in the molecular metabolism.
Figure 1: Alzheimer’s Disease, Diabetes and Tanycyte Axis:
Normal healthy functioning pancreas maintains normal homeostasis by regulating insulin synthesis, insulin signalling, facilitating glucose uptake and metabolism. As a result, in the normal brain there is maintenance of the normal blood brain barrier, normal neurogenesis, neuroregeneration, normal Aβ synthesis and clearance, normal memory and normal cognitive function. However, in type 2 diabetes, the normal insulin synthesis and insulin signaling becomes impaired thereby leading to hyperglycemia, inflammation, increased oxidative stress, islet amyloid polypeptide (IAPP), reactive oxygen species (ROS), nitric oxide (NO) production and generation of advanced glycation products and mitochondrial dysfunction. As a result, tanycytes as well as pancreatic β cells become dysfunctional thereby initiating a cascade of events in the brain involving neuroinflammation, secretion of inflammatory cytokines, blood brain barrier breakdown, increased amyloid beta (Aβ) synthesis, decreased Aβ clearance, formation of amyloid plaques and neurofibrillary tangles. Cumulative effects lead to neuronal loss, neurodegeneration, impaired microglial function, loss of memory and cognitive dysfunction as observed in AD patients. Perusal of literature suggest that intracerebroventricular injection of either fibroblast growth factor 1 (FGF1), FGF19, FGF21 or FGFR1/β-klotho bispecific antibody (bFKB1) has the potential to improve the diabetic phenotype by modulating tanycyte as well as pancreatic beta cell functions. We propose that transplantation of embryonic stem (ES) and induced pluripotent stem (iPS) cell-derived insulin producing cells (IPCs) and tanycytes can reverse hyperglycemia and delay or possibly halt the progression of AD thereby improving cognitive function.
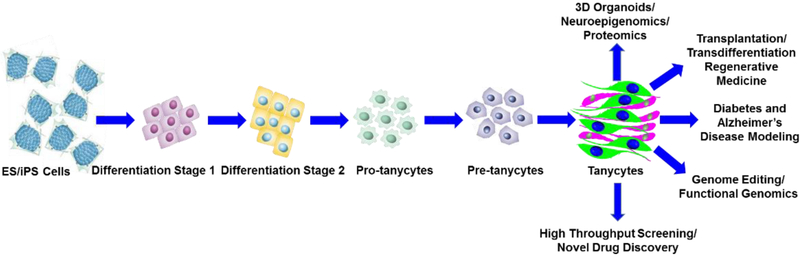
Current perusal of literature indicate the lack of studies and efforts to directly differentiate either embryonic stem (ES) cells or induced pluripotent stem (iPS) cells into tanycytes. Thus, it would be indeed interesting to develop novel approaches for the directed differentiation of ES cells or patient-specific iPS cells into tanycytes (Figure 2). It would be very interesting to derive tanycytes using AD patient specific iPS cells. Once successful, iPS-derived tanycytes could then be treated with Aβ oligomers to explore the molecular mechanism/s underlying their proliferation, survival, apoptosis as well as their differentiation. In addition, it will be very interesting to study these mechanisms using mixed tanycyte and neuronal and glial cell cultures. Besides in vivo lineage tracing studies in the normal and AD murine models will enable to conceptualize the role of tanycytes in neurogenesis. These studies will enable us to decipher the precise role of tanycytes in diabetes and AD pathophysiology which in turn will benefit the development of the next generation of precision targeted diabetes and AD-patient specific molecular therapies.
Figure 2: Derivation of functional tanycytes from ES and iPS cells:
The derivation of tanycytes from the ES and iPS cells has not yet been attempted. We propose that the lineage commitment and the directed differentiation of ES and iPS cells into tanycytes can be achieved in vitro. A simplified version of the directed differentiation as depicted in our scheme has the potential to generate an unlimited source and supply of tanycytes. The ES and iPS cell-derived tanycytes can be successfully used to study their normal physiological function using 3D organoids, as well as to develop disease in a dish model, perform genome editing to decipher functional genomics, neuroepigenomics, proteomics, high throughput screening of chemical libraries for novel drug discovery as well as transplantation and in vivo transdifferentiation studies to develop tanycyte-based regenerative therapies to treat type 2 diabetes and AD.
Latest developments in the field of CRISPR-Cas9 have enabled precisely targeted gene editing of disease causing genes [56-63]. Recent advancements in the emerging field of CRISPR-Cas9 based gene editing could be exploited to enhance the directed differentiation of ES and iPS cells into functional tanycytes as well as tanycytes harbouring specific gene knockouts. Such an approach is likely very important to gain an in-depth insight into the developmental regulation of tanycytes during normal development, neuroinflammation as well as in various neurodegenerative diseases. ES and iPS cell-derived tanycytes could be very useful to study the transcriptomic, metabolomic as well as epigenetic signature profiles. Further, ES and iPS cell-derived tanycytes could be used for transplantation in AD mouse models to investigate whether such an approach is beneficial to delay the progression of AD pathophysiology and hopefully restore memory and improve cognitive function.
MicroRNAs as well as long noncoding RNAs play a critical role during normal development as well as during pathogenesis of various neurodegenerative diseases. Thus, it will be interesting to investigate the microRNA and long noncoding RNA profiles of tanycytes at various stages of development as well as during pathogenesis of diabetes and various neurodegenerative diseases. Identification of microRNAs that are differentially expressed during healthy and diseased states could be exploited for inducing differentiation of tanycytes into neurons as well as for targeted gene therapy of neurodegenerative diseases. Once the robust protocols to differentiate ES and iPS cells into tanycytes are successful, it would enable high throughput screening of small molecules with therapeutic potential to successfully treat various neurodegenerative disorders associated with tanycyte dysfunction. These approaches will not only provide novel mechanistic insights but will also enable novel drug discovery efforts to develop patient-specific precision medicine against a wide variety of neurodegenerative diseases.
Table 3:
Expression of Selective Molecular Markers in Glial Cells and their comparison with Tanycytes
| Marker | Astrocytes | Microglia | Oligodendrocytes | Tanycytes |
|---|---|---|---|---|
| GFAP | + | − | − | − |
| EAAT1/GLAST | + | − | − | − |
| Glutamine Synthase | + | − | − | − |
| S100 beta | + | − | − | − |
| ALDH1L1 | + | − | − | − |
| Aquaporin 4 | + | − | − | − |
| CD11b | − | + | − | − |
| CD45 | − | + | − | − |
| Iba1 | − | + | − | − |
| F4/80 | − | + | − | − |
| CD68 | − | + | − | − |
| CD40 | − | + | − | − |
| IGTAM | − | + | − | − |
| TREM2 | − | + | − | − |
| ITGB2 | − | + | − | − |
| ADORA3 | + | + | − | − |
| TMEM119 | − | + | − | − |
| LGMN | − | + | − | − |
| PROS1 | − | + | − | − |
| C1QA | − | + | + | − |
| SELPLG | − | + | − | − |
| HEXB | − | + | − | − |
| LTC4S | − | + | − | − |
| CCL2 | − | + | − | − |
| Olig1 | − | − | + | − |
| Olig2 | − | − | + | − |
| Olig3 | − | − | + | − |
| OSP | − | − | + | − |
| MBP | − | − | + | − |
| SOX10 | − | − | + | − |
| MOG | − | − | + | − |
| NG2 | +/− | + | + | − |
Acknowledgements
The authors would like to acknowledge the Veteran Affairs Merit Award I01BX002477 and the National Institutes of Health Grant AG048205 to AZ.
Footnotes
Conflict of Interest
The authors confirm that they have no conflict of interest.
Bibliography
- 1.Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S (2017) Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol doi: 10.1016/S2213-8587(17)30186-9 [DOI] [PubMed] [Google Scholar]
- 2.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB (2011) Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br J Clin Pharmacol 71 (3):365–376. doi: 10.1111/j.1365-2125.2010.03830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3 (3):169–178. doi: 10.1016/S1474-4422(04)00681-7 [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Gonzalez I, Edwards Iii G, Salvadores N, Shahnawaz M, Diaz-Espinoza R, Soto C (2017) Molecular interaction between type 2 diabetes and Alzheimer's disease through cross-seeding of protein misfolding. Mol Psychiatry 22 (9):1327–1334. doi: 10.1038/mp.2016.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta 1863 (5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara M, Sato N (2017) Bidirectional interactions between diabetes and Alzheimer's disease. Neurochem Int 108:296–302. doi: 10.1016/j.neuint.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 7.Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer's disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta 1863 (5):1078–1089. doi: 10.1016/j.bbadis.2016.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley M, Macauley SL, Holtzman DM (2016) Changes in insulin and insulin signaling in Alzheimer's disease: cause or consequence? J Exp Med 213 (8):1375–1385. doi: 10.1084/jem.20160493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J (2018) The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev doi: 10.1210/er.2017-00235 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P (2005) Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164. doi: 10.1016/S0074-7696(05)47003-5 [DOI] [PubMed] [Google Scholar]
- 11.Chauvet N, Prieto M, Alonso G (1998) Tanycytes present in the adult rat mediobasal hypothalamus support the regeneration of monoaminergic axons. Exp Neurol 151 (1): 1–13. doi: 10.1006/exnr.1998.6784 [DOI] [PubMed] [Google Scholar]
- 12.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B (2013) Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521 (15):3389–3405. doi: 10.1002/cne.23355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK (2013) Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci 33 (14):6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M (2013) alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun 4:2049. doi: 10.1038/ncomms3049 [DOI] [PubMed] [Google Scholar]
- 15.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S (2012) Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 15 (5):700–702. doi: 10.1038/nn.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, de Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S (2014) The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J Neurosci 34 (50):16809–16820. doi: 10.1523/JNEUROSCI.1711-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R, Wu X, Jiang L, Zhang Y (2017) Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep 18 (13):3227–3241. doi: 10.1016/j.celrep.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma MS, Brouwer N, Wesseling E, Raj D, van der Want J, Boddeke E, Balasubramaniyan V, Copray S (2015) Multipotent stem cell factor UGS148 is a marker for tanycytes in the adult hypothalamus. Mol Cell Neurosci 65:21–30. doi: 10.1016/j.mcn.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Jourdon A, Gresset A, Spassky N, Charnay P, Topilko P, Santos R (2016) Prss56, a novel marker of adult neurogenesis in the mouse brain. Brain Struct Funct 221 (9):4411–4427. doi: 10.1007/s00429-015-1171-z [DOI] [PubMed] [Google Scholar]
- 20.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT (2017) A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20 (3):484–496. doi: 10.1038/nn.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayling C, Britton R, Dale N (2011) ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol 589 (Pt 9):2275–2286. doi: 10.1113/jphysiol.2010.202051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia M, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zuniga F, Vera JC, Onate SA, Nualart F (2003) Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem 86 (3):709–724 [DOI] [PubMed] [Google Scholar]
- 23.Garcia MA, Carrasco M, Godoy A, Reinicke K, Montecinos VP, Aguayo LG, Tapia JC, Vera JC, Nualart F (2001) Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J Cell Biochem 80 (4):491–503 [PubMed] [Google Scholar]
- 24.Benford H, Bolborea M, Pollatzek E, Lossow K, Hermans-Borgmeyer I, Liu B, Meyerhof W, Kasparov S, Dale N (2017) A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia 65 (5):773–789. doi: 10.1002/glia.23125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B (2013) Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17 (4):607–617. doi: 10.1016/j.cmet.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salgado M, Tarifeno-Saldivia E, Ordenes P, Millan C, Yanez MJ, Llanos P, Villagra M, Elizondo-Vega R, Martinez F, Nualart F, Uribe E, de Los Angeles Garcia-Robles M (2014) Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes. PLoS One 9 (4):e94035. doi: 10.1371/journal.pone.0094035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Saez JC, Garcia MA (2012) Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia 60 (1):53–68. doi: 10.1002/glia.21246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uranga RM, Millan C, Barahona MJ, Recabal A, Salgado M, Martinez F, Ordenes P, Elizondo-Vega R, Sepulveda F, Uribe E, Garcia-Robles MLA (2017) Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior. Sci Rep 7 (1):3697. doi: 10.1038/s41598-017-03928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Rodriguez P, Sandebring-Matton A, Merino-Serrais P, Parrado-Fernandez C, Rabano A, Winblad B, Avila J, Ferrer I, Cedazo-Minguez A (2017) Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain 140 (12):3269–3285. doi: 10.1093/brain/awx256 [DOI] [PubMed] [Google Scholar]
- 30.Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, Issad T, Gerhardt E, Pagesy P, Vileno M, Bournonville C, Hamdane M, Bantubungi K, Lancel S, Demeyer D, Eddarkaoui S, Vallez E, Vieau D, Humez S, Faivre E, Grenier-Boley B, Outeiro TF, Staels B, Amouyel P, Balschun D, Buee L, Blum D (2017) Tau deletion promotes brain insulin resistance. J Exp Med 214 (8):2257–2269. doi: 10.1084/jem.20161731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijesekara N, Goncalves RA, Ahrens R, De Felice FG, Fraser PE (2018) Tau ablation in mice leads to pancreatic beta cell dysfunction and glucose intolerance. FASEB J:fj201701352. doi: 10.1096/fj.201701352 [DOI] [PubMed] [Google Scholar]
- 32.Miklossy J, Qing H, Radenovic A, Kis A, Vileno B, Laszlo F, Miller L, Martins RN, Waeber G, Mooser V, Bosman F, Khalili K, Darbinian N, McGeer PL (2010) Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol Aging 31 (9):1503–1515. doi: 10.1016/j.neurobiolaging.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, D'Alessio D (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503 (7474):59–66. doi: 10.1038/nature12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart AW, Baeza N, Apelqvist A, Edlund H (2000) Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 408 (6814):864–868. doi: 10.1038/35048589 [DOI] [PubMed] [Google Scholar]
- 35.Morton GJ, Matsen ME, Bracy DP, Meek TH, Nguyen HT, Stefanovski D, Bergman RN, Wasserman DH, Schwartz MW (2013) FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 123 (11):4799–4808. doi: 10.1172/JCI70710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA (2004) Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145 (6):2594–2603. doi: 10.1210/en.2003-1671 [DOI] [PubMed] [Google Scholar]
- 37.Marcelin G, Jo YH, Li X, Schwartz GJ, Zhang Y, Dun NJ, Lyu RM, Blouet C, Chang JK, Chua S Jr. (2014) Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab 3 (1):19–28. doi: 10.1016/j.molmet.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ (2013) Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154 (1):9–15. doi: 10.1210/en.2012-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg R, Veniant MM (2009) Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 297 (5):E1105–1114.doi: 10.1152/ajpendo.00348.2009 [DOI] [PubMed] [Google Scholar]
- 40.Scarlett JM, Rojas JM, Matsen ME, Kaiyala KJ, Stefanovski D, Bergman RN, Nguyen HT, Dorfman MD, Lantier L, Wasserman DH, Mirzadeh Z, Unterman TG, Morton GJ, Schwartz MW (2016) Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 22 (7):800–806. doi: 10.1038/nm.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avgerinos KI, Kalaitzidis G, Malli A, Kalaitzoglou D, Myserlis PG, Lioutas VA (2018) Intranasal insulin in Alzheimer's dementia or mild cognitive impairment: a systematic review. J Neurol. doi: 10.1007/s00415-018-8768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29 (10):1326–1334. doi: 10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 43.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70 (6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36 [DOI] [PubMed] [Google Scholar]
- 44.Raikwar SP, Zavazava N (2013) Differentiation and lineage commitment of murine embryonic stem cells into insulin producing cells. Methods Mol Biol 1029:93–108. doi: 10.1007/978-1-62703-478-4_7 [DOI] [PubMed] [Google Scholar]
- 45.Raikwar SP, Zavazava N (2012) PDX1-engineered embryonic stem cell-derived insulin producing cells regulate hyperglycemia in diabetic mice. Transplant Res 1 (1):19. doi: 10.1186/2047-1440-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raikwar SP, Zavazava N (2009) Insulin producing cells derived from embryonic stem cells: are we there yet? J Cell Physiol 218 (2):256–263. doi: 10.1002/jcp.21615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raikwar SP, Zavazava N (2009) Real-time non-invasive imaging of ES cell-derived insulin producing cells. Methods Mol Biol 590:317–334. doi: 10.1007/978-1-60327-378-7_21 [DOI] [PubMed] [Google Scholar]
- 48.Chan KM, Raikwar SP, Zavazava N (2007) Strategies for differentiating embryonic stem cells (ESC) into insulin-producing cells and development of non-invasive imaging techniques using bioluminescence. Immunol Res 39 (1–3):261–270 [DOI] [PubMed] [Google Scholar]
- 49.Raikwar SP, Kim EM, Sivitz WI, Allamargot C, Thedens DR, Zavazava N (2015) Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PLoS One 10 (1):e0116582. doi: 10.1371/journal.pone.0116582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raikwar SP, Zavazava N (2011) Spontaneous in vivo differentiation of embryonic stem cell-derived pancreatic endoderm-like cells corrects hyperglycemia in diabetic mice. Transplantation 91 (1):11–20 [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C (2005) Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192 (2):251–264. doi: 10.1016/j.expneurol.2004.12.021 [DOI] [PubMed] [Google Scholar]
- 52.Li J, Tang Y, Cai D (2012) IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 14 (10):999–1012. doi: 10.1038/ncb2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaker Z, George C, Petrovska M, Caron JB, Lacube P, Caille I, Holzenberger M (2016) Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol Aging 41:64–72. doi: 10.1016/j.neurobiolaging.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D (2017) Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548 (7665):52–57.doi: 10.1038/nature23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellegrino G, Trubert C, Terrien J, Pifferi F, Leroy D, Loyens A, Migaud M, Baroncini M, Maurage CA, Fontaine C, Prevot V, Sharif A (2018) A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J Comp Neurol 526 (9):1419–1443. doi: 10.1002/cne.24376 [DOI] [PubMed] [Google Scholar]
- 56.Jarrett KE, Lee CM, Yeh YH, Hsu RH, Gupta R, Zhang M, Rodriguez PJ, Lee CS, Gillard BK, Bissig KD, Pownall HJ, Martin JF, Bao G, Lagor WR (2017) Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Sci Rep 7:44624. doi: 10.1038/srep44624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murlidharan G, Sakamoto K, Rao L, Corriher T, Wang D, Gao G, Sullivan P, Asokan A (2016) CNS-restricted Transduction and CRISPR/Cas9-mediated Gene Deletion with an Engineered AAV Vector. Mol Ther Nucleic Acids 5 (7):e338. doi: 10.1038/mtna.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohmori T, Nagao Y, Mizukami H, Sakata A, Muramatsu SI, Ozawa K, Tominaga SI, Hanazono Y, Nishimura S, Nureki O, Sakata Y (2017) CRISPR/Cas9-mediated genome editing via postnatal administration of AAV vector cures haemophilia B mice. Sci Rep 7 (1):4159. doi: 10.1038/s41598-017-04625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stover JD, Farhang N, Berrett KC, Gertz J, Lawrence B, Bowles RD (2017) CRISPR Epigenome Editing of AKAP150 in DRG Neurons Abolishes Degenerative IVD-Induced Neuronal Activation. Mol Ther doi: 10.1016/j.ymthe.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F (2015) In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 33 (1):102–106. doi: 10.1038/nbt.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang JZ, Wu P, Shi ZM, Xu YL, Liu ZJ (2017) The AAV-mediated and RNA-guided CRISPR/Cas9 system for gene therapy of DMD and BMD. Brain Dev 39 (7):547–556. doi: 10.1016/j.braindev.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 62.Yu W, Mookherjee S, Chaitankar V, Hiriyanna S, Kim JW, Brooks M, Ataeijannati Y, Sun X, Dong L, Li T, Swaroop A, Wu Z (2017) Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun 8:14716. doi: 10.1038/ncomms14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M (2016) Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533 (7601):125–129. doi: 10.1038/nature17664 [DOI] [PubMed] [Google Scholar]