Abstract
We previously reported that delayed treatment with Mito-tempo (MT), a mitochondrial targeted superoxide dismutase mimetic, protects against the early phase of acetaminophen (APAP) hepatotoxicity by inhibiting peroxynitrite formation. However, whether this protection is sustained to the late phase of toxicity is unknown. To investigate the late protection, C57Bl/6J mice were treated with 300mg/kg APAP followed by 20mg/kg MT 1.5h or 3h later. We found that both MT treatments protected against the late phase of APAP hepatotoxicity at 12 and 24h. Surprisingly, MT-treated mice demonstrated a significant increase in apoptotic hepatocytes, while the necrotic phenotype was observed almost exclusively in mice treated with APAP alone. In addition, there was a significant increase in caspase-3 activity and cleavage in the livers of MT-treated mice. Immunostaining for active caspase-3 revealed that the positively stained hepatocytes were exclusively in centrilobular areas. Treatment with the pan-caspase inhibitor ZVD-fmk (10mg/kg) 2h post-APAP neutralized this caspase activation and provided additional protection against APAP hepatotoxicity. Treatment with N-acetylcysteine (NAC), the current standard of care for APAP poisoning, protected but did not induce this apoptotic phenotype. Mechanistically, MT treatment inhibited APAP-induced RIP3 kinase expression, and RIP3-deficient mice showed caspase activation and apoptotic morphology in hepatocytes analogous to MT treatment. These data suggest that while necrosis is the primary cause of cell death after APAP hepatotoxicity, treatment with the antioxidant MT may switch the mode of cell death to secondary apoptosis in some cells. Modulation of mitochondrial oxidative stress and RIP3 kinase expression play critical roles in this switch.
Keywords: acetaminophen hepatotoxicity, mitochondria, oxidative stress, Mito-Tempo, necrosis, secondary apoptosis
INTRODUCTION
Acetaminophen (APAP) overdose causes severe liver injury in both murine models and humans. APAP hepatotoxicity is responsible for more than 70,000 hospitalizations each year and around 50% of cases of acute liver failure in the US and many Western countries (Budnitz et al. 2011; Manthripragada et al. 2011). APAP toxicity is initiated by the cytochrome P450-mediated formation of a reactive electrophile, N-acetyl-p-benzoquinone imine (NAPQI) (Nelson 1990), which depletes hepatic glutathione (GSH) levels and binds to cellular proteins (McGill and Jaeschke 2013). However, most critical for the injury process is the formation of protein adducts in mitochondria (Tirmenstein and Nelson 1979; Xie et al. 2015), which causes impairment of the mitochondrial respiration (Meyer et al. 1988) and induces mitochondrial oxidant stress and peroxynitrite formation (Jaeschke 1990; Cover et al. 2005). The mitochondrial oxidative and nitrosative stress subsequently trigger the opening of the mitochondrial permeability transition pore (MPTP) (Kon et al. 2004; Masubuchi et al. 2005; Ramachandran et al. 2011; LoGuidice and Boelsterli 2011), nuclear DNA fragmentation (Ray et al. 1990; Lawson et al. 1999) and finally necrotic cell death (Gujral et al. 2002).
It has been conclusively demonstrated in numerous studies that cell death following APAP hepatotoxicity occurs almost exclusively via oncotic necrosis rather than apoptosis (Gujral et al. 2002; Jaeschke et al. 2011). Evidence supporting this includes the typical morphology of cell necrosis in the hepatic centrilobular areas, most notably karyorrhexis, cell swelling and loss of membrane integrity (Jaeschke and Lemasters 2003). This is further supported by the release of cell contents into the extracellular space and a robust inflammatory response during the toxicity (Jaeschke et al. 2012; Woolbright and Jaeschke 2017). In addition, caspase-3, the main executioner of apoptosis, is not activated after APAP overdose, and pan-caspase inhibitors do not protect against APAP hepatotoxicity (Lawson et al. 1999; Adams et al. 2001; Gujral et al. 2002; Jaeschke et al. 2006). Intriguingly, some features that were previously considered specific for apoptosis are still noticed during APAP toxicity, such as mitochondrial bax translocation and release of cytochrome C and Smac/DIABLO from mitochondria through bax pores (Adams et al. 2001; Knight and Jaeschke 2002; Bajt et al. 2008; Du et al. 2016b). Although the lower ATP levels in the fasted mice were thought to be responsible for the absence of caspase activation and the subsequent caspase-mediated apoptotic cell death (Antione et al. 2009; 2010), no morphological evidence for apoptosis and no protection with caspase inhibitors were found in fed mice despite their higher ATP levels in the liver (Williams et al. 2011). Therefore, the reason why there is no caspase activation and apoptosis despite release of pro-apoptotic mediators from mitochondria is still unknown. Importantly, studies of the mode of APAP-induced cell death in humans also showed evidence for predominantly necrosis in human hepatocytes (Xie et al. 2014) and in humans (Antoine et al. 2012; McGill et al. 2012). However, based on plasma biomarkers of caspase-cleaved cytokeratin-18, there appears to be evidence of moderate caspase activation in some patients suggesting that there might be a low degree of apoptosis (Antoine et al., 2012, 2013). Interestingly, all patients are treated with the standard of care, N-acetylcysteine (NAC). NAC supports the synthesis of GSH, which at these later stages of the toxicity, mainly acts as a scavenger of reactive oxygen and peroxynitrite (Saito et al. 2010).
We recently reported that Mito-Tempo (MT), a mitochondria-targeted superoxide dismutase mimetic, dramatically reduced APAP-induced mitochondrial oxidant stress and the opening of MPTP, and thus effectively protected the mice against early APAP toxicity (Du et al. 2017). However, whether the protection by MT is sustained until the late phase of APAP hepatotoxicity is still unknown. It has been well recognized that oxidative stress can dose-dependently affect caspase activation and the mode of cell death. Short and long term MPTP opening can induce apoptosis and necrosis, respectively (Skulachev 2006; Golbidi et al. 2014). Therefore, we hypothesized that MT may affect the model of cell death following APAP overdose by modulating mitochondrial oxidative stress and dysfunction. Because one of the primary protective mechanisms of NAC is through the scavenging mitochondrial oxidative stress (Knight et al. 2002; Bajt et al. 2004; James et al. 2003; Saito et al. 2010), we further hypothesized that NAC treatment manipulates mitochondrial oxidative stress and may lead to changes in mode of cell death in those patients. Therefore, to explore our hypothesis, we evaluated the effect of MT or NAC treatment following APAP overdose on the late liver injury and mode of cell death together with the underlying mechanisms.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) 8-12 weeks of age were kept in an environmentally controlled room with a 12h light/dark cycle. RIP3-deficient mice (C57BL/6N background) were kindly provided by Dr. Vishva Dixit (Genentech, Inc). C57BL/6N wild type animals were from Charles River, Frederick, Maryland, USA). The mice were acclimated before experiments with free access to diet and water. All experimental protocols followed the criteria of the National Research Council for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Experimental design
Overnight fasted mice (16-18h) were treated i.p. with 300 mg/kg APAP (Sigma-Aldrich, St. Louis, MO) dissolved in warm saline. Some mice were treated with 200mg/kg APAP in experiments evaluating effect of RIP3 deficiency. A dose of 20 mg/kg Mito-Tempo (Sigma-Aldrich) dissolved in saline was administered i.p. 1.5 or 3 h after APAP. Some mice were subsequently treated (i.p.) with 10 mg/kg Z-VD fmk (EP1013; a generous gift from Dr. S.X. Cai, Epicept Corp., San Diego, CA) dissolved in Tris-buffered saline or vehicle 2 h after APAP. To mimic the clinical care of APAP-overdose patients, some mice received the antidote NAC (i.p., 500 mg/kg) at 1.5 or 3 h after APAP overdose. Groups of mice were euthanized at 0-24 h post-APAP by exsanguination under isoflurane anesthesia. Additional mice were treated i.p. with 100 μg/kg Salmonella abortus equi endotoxin (ET) and 700 mg/kg galactosamine (Gal) for 6 h. Blood was drawn into a heparinized syringe and centrifuged to obtain plasma. Plasma ALT activities were measured using the ALT assay kit from Pointe Scientific, MI. The liver tissue was cut into pieces and fixed in 10% phosphate-buffered formalin for histology or flash frozen in liquid nitrogen and subsequently stored at −80°C.
In vivo morpholino treatment
All vivo-morpholinos were from Gene Tools, LLC (Philomath, OR, USA). The antisense sequence used for RIP3 was 5’-TAGGCCATAACTTGACAGAAGACAT-3’. The standard control in vivo oligo sequence from Gene Tools was used for all control morpholino treatments. Morpholinos were used as provided by the manufacturer and administered ip to mice (12.5 mg/kg body weight) every 24h for 2 days. Treatment with APAP was then done on day 3.
Glutathione measurement
Hepatic GSH and GSSG were measured using the Tietze assay with modifications as described (McGill and Jaeschke 2015). Briefly, frozen liver tissue was homogenized in 3% sulfosalicylic acid and centrifuged to remove precipitated proteins. The sample was then assayed for total GSH using dithionitrobenzoic acid and measured spectrophotometrically at 412 nm. GSSG was measured using the same method after removal of reduced GSH with N-ethylmaleimide.
Caspase activity measurements and western blotting
Liver caspase activity was measured as described (Lawson et al. 1999). In brief, frozen liver tissue was homogenized in 25 mM HEPES buffer containing 5 mM EDTA, 2 mM DTT and 0.1% CHAPS, and then centrifuged to get the homogenate. A fluorogenic substrate (Ac-DEVD-AFC, Enzo Life Sciences, Plymouth Meeting, PA) was added to the homogenate and fluorescence was measured with or without the presence of pan-caspase inhibitor (z-VAD-fmk, Enzo). Results are expressed as RFU per unit time per mg protein concentration. Western blotting was performed as described (Bajt et al. 2000) using a rabbit anti-caspase 3 antibody and a rabbit anti-beta-actin antibody (Cell Signaling Technology, Danvers, MA), and an anti-RIP3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The proteins were visualized using a goat anti-rabbit HRP conjugated antibody (Santa Cruz Biotechnology Santa Cruz, CA).
Histology
Liver tissue samples embedded in paraffin were cut in 5 μm sections and stained with hematoxylin and eosin (H&E) for assessment of apoptosis versus necrosis (Gujral et al. 2002). Nitrotyrosine staining was performed as previously described (Knight et al. 2002), using a rabbit polyclonal anti-nitrotyrosine antibody (Life Technologies, Grand Island, NY) and the Dako LSAB peroxidase kit (Dako, Carpinteria, CA). Active caspase-3 staining was performed using a cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, Danvers, MA). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed for cell death using the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) following manufacturer’s instructions.
Statistics
All results were presented as mean ± SEM. For normally distributed data, statistical significance was evaluated using the Student's t-test for comparisons between two groups, or one-way analysis of variance (ANOVA) for multiple groups, followed by Student-Newman-Keul’s test. For non-normally distributed data, ANOVA was performed using Kruskal–Wallis Test, followed by Dunn’s multiple comparisons. P < 0.05 was considered significant.
RESULTS
Mito-Tempo protects against APAP-induced liver injury
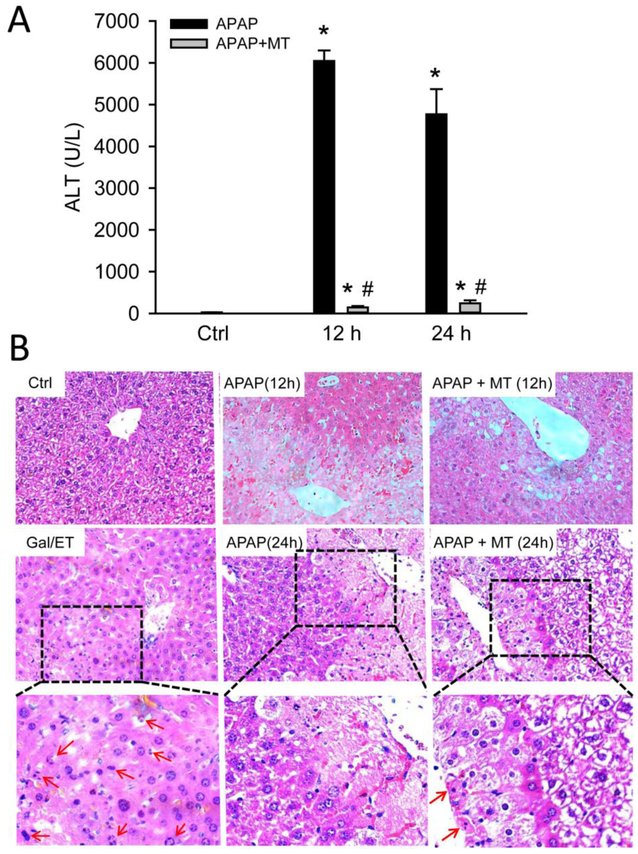
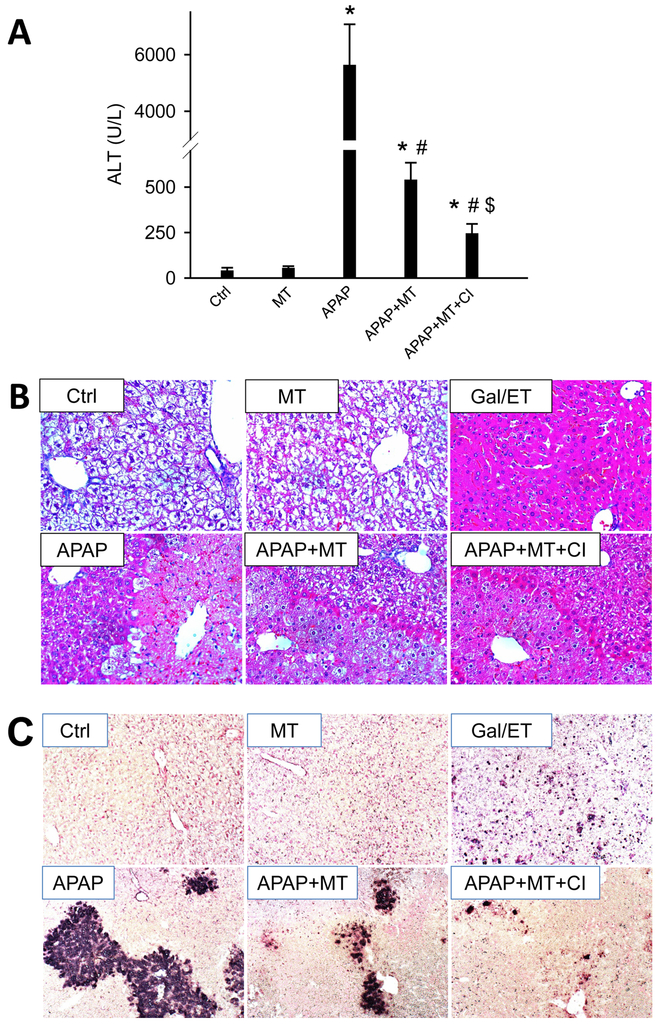
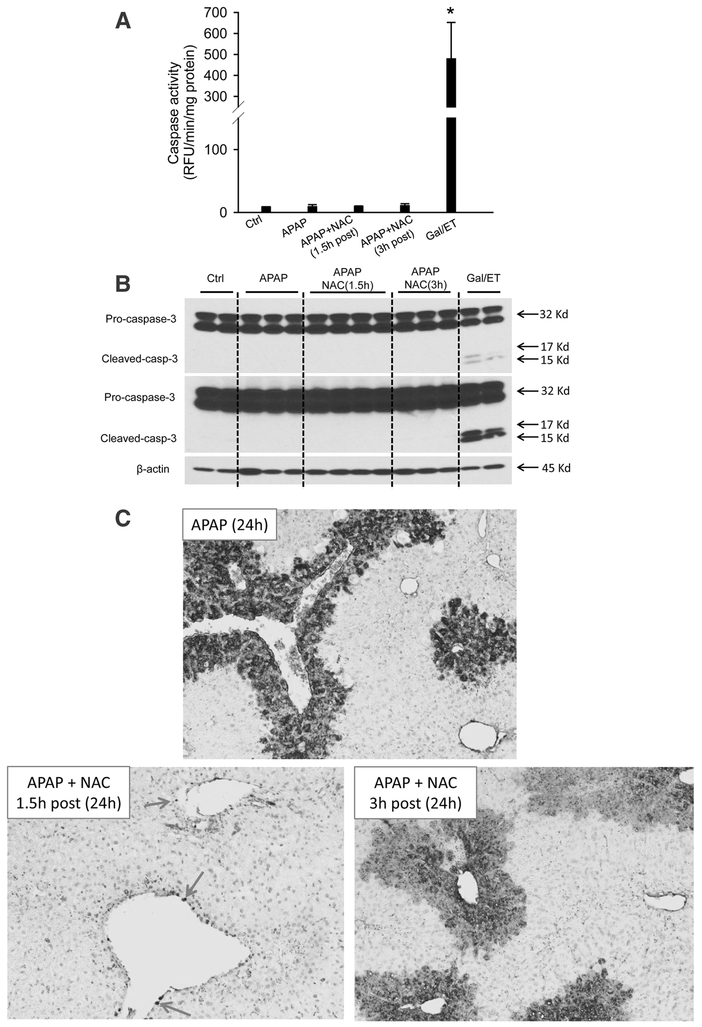
We previously reported that MT as a 1.5 h post treatment protects against APAP-induced liver injury at 3 and 6 h (Du et al. 2017). Here, we extended the time course to the later phase of liver injury at 12 and 24 h. APAP overdose (300 mg/kg) induced acute liver injury, as indicated by significantly increased plasma ALT activities (Fig. 1A) and extensive centrilobular necrosis in H&E-stained liver sections (Fig. 1B). MT greatly attenuated the increase in ALT activities (Fig. 1A) and reduced the areas of necrosis at both time points (Fig. 1B), indicating that the protection by MT is sustained until at least 24 h post-APAP. Interestingly, histopathologic analysis of the liver tissue at high resolution demonstrated a significant increase in cells exhibiting apoptotic morphology in MT-treated mice (most notably cell shrinkage and chromatin condensation), while the necrotic phenotype was observed in animals treated only with APAP (most notably cell vacuolization, cell swelling and karyorrhexis). As a positive control for apoptosis, animals were treated for 6 h with Gal/ET. At this time, a large number of hepatocytes show characteristic features of apoptotic cell death including cell shrinkage, chromatin condensation and margination, and formation of apoptotic bodies (Figure 1B, arrows).
Figure 1: MT protected against late phase of APAP hepatotoxicity.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline was given 1.5 h later. (A) Plasma levels of ALT. (B) Representative H&E stained liver sections (200x). Bottom panel is an enlargement of the boxed area. A liver section from a Gal/ET-treated mouse was used as a positive control of apoptosis, and arrows denote the hepatocytes showing apoptotic morphology. Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
Mito-Tempo attenuates APAP-induced mitochondrial peroxynitrite formation
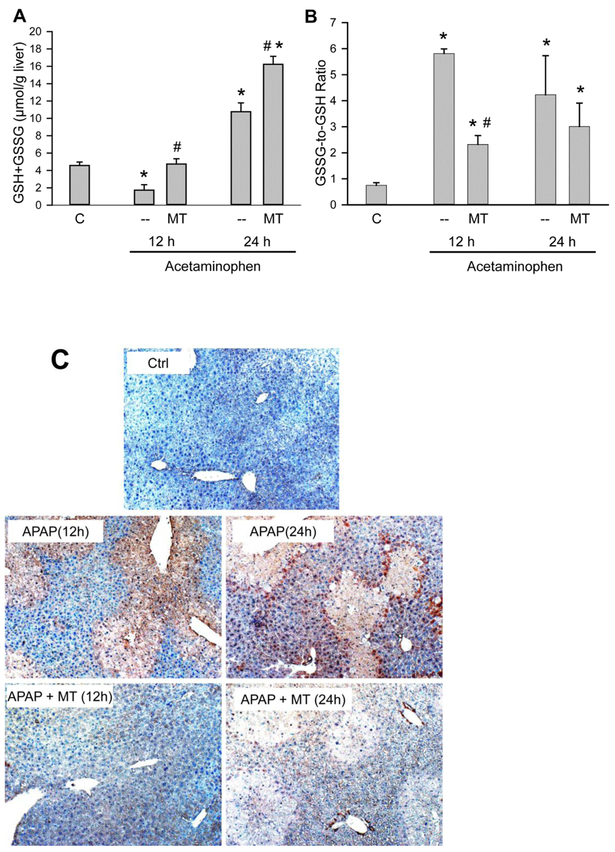
We previously demonstrated that MT alleviates the mitochondrial oxidant stress, especially peroxynitrite formation, during the early liver injury (Du et al. 2017). During the late phase, the recovery of hepatic GSH levels was improved in MT-treated animals compared with the animals treated with APAP alone (Figure 2A) but there was no significant difference in the GSSG levels between both groups (data not shown). However, the GSSG-to-GSH ratio was significantly lower in the MT-treated animals at 12 h but not at 24 h (Figure 2B). In contrast, the extensive nitrotyrosine protein adduct staining in the centrilobular areas observed in APAP-treated mice as indicator for peroxynitrite formation was almost eliminated in MT-treated mice (Fig. 2C). This supports the hypothesis that the SOD mimetic MT predominantly prevents peroxynitrite formation and only partially reduces the overall oxidant stress during the later phase of APAP-induced liver injury.
Figure 2: MT attenuated APAP-induced mitochondrial oxidant stress.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline was given 1.5 h later. (A) Total liver GSH levels. (B) GSSG-to-GSH ratios. (C) Representative nitrotyrosine staining of liver sections (50x). Bars represent mean ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
Mito-Tempo induced secondary apoptosis in the late phase of APAP hepatotoxicity
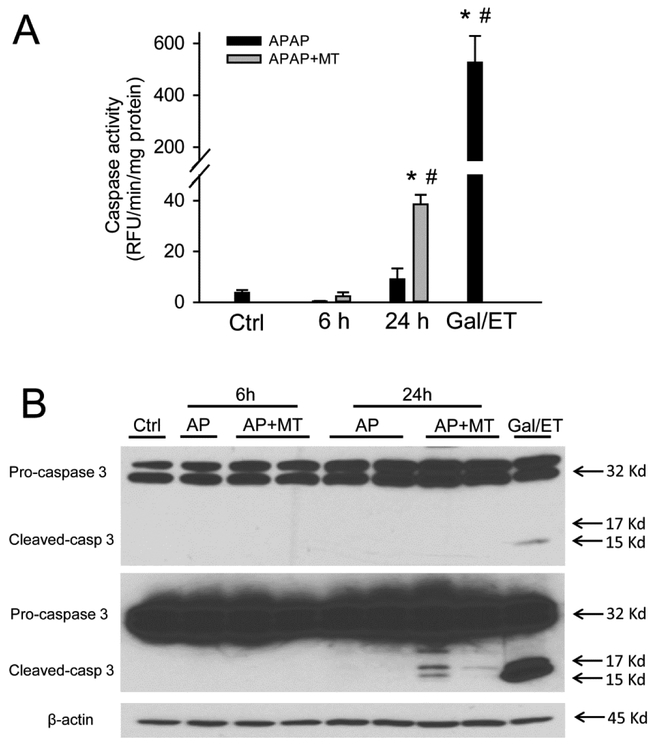
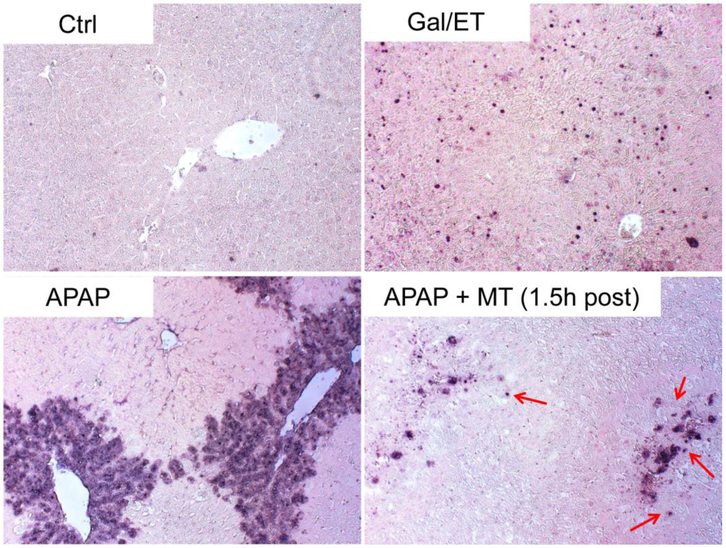
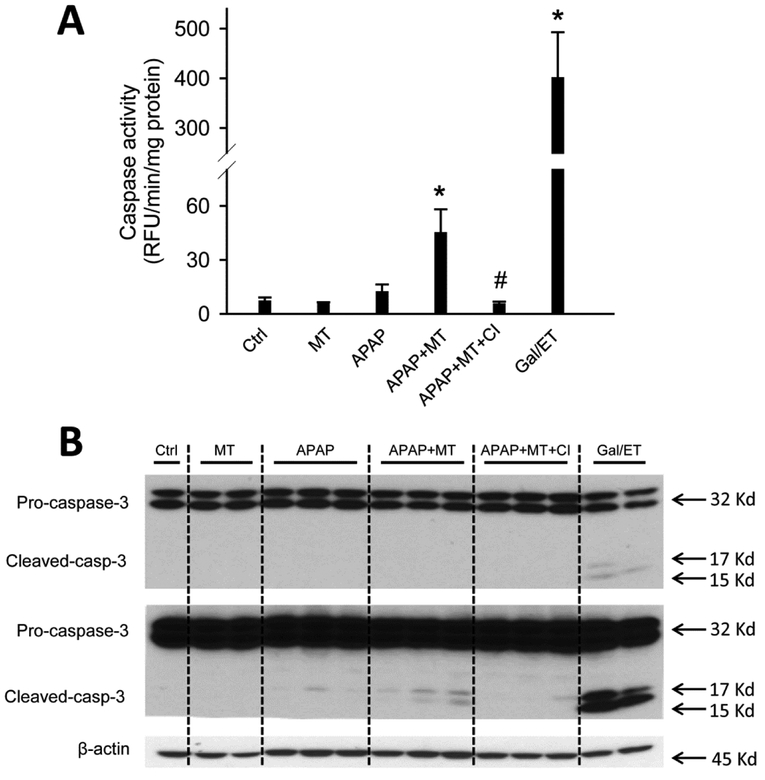
To test whether MT affects the mode of cell death during APAP hepatotoxicity, we assayed for increased caspase-3 enzyme activity and pro-caspase-3 cleavage, which are prerequisites for inducing apoptosis. Gal/ET treatment is well known to induce caspase-mediated apoptosis and liver injury in mice (Jaeschke et al. 1998), and thus was used as a positive control in our study. As expected, Gal/ET-treated mice displayed a more than 100-fold increase of hepatic caspase-3 activity compared to untreated controls (Figure 3A). In addition, cleavage of pro-caspase-3 to the active fragments was also observed (Figure 3B, top panel, right lane). In contrast, neither a significant increase in caspase-3 activity nor procaspase-3 processing was detected in APAP-treated mice at either 6 or 24 h post-APAP (Figure 3A, B). Interestingly, however, MT treatment significantly increased the caspase activity (Figure 3A) and the cleavage of pro-caspase-3, clearly visible after longer exposure of the blots, at 24 h post-APAP (Figure 3B, middle panel). Immunostaining for active caspase-3 revealed that individual cells positive for cleaved caspase-3 were exclusively located in the centrilobular areas of animals treated with MT+APAP but not with APAP alone (Supplemental Figure 1). In the Gal/ET-treated animals, cleaved caspase-3-positive cells were detectable with no zonal preference (Supplemental Figure 1). In support of these results, only few TUNEL-positive cells as indicator of DNA strand breaks, were seen in control tissue (Figure 4). However, an exclusive necrotic pattern of TUNEL staining (positive staining of both cytosol and nuclei) was observed in centrilobular hepatocytes in only APAP-treated mice, whereas an apoptotic pattern of staining (distinct nuclear staining) was seen in some hepatocytes in mice with MT treatment (Figure 4). A similar apoptotic TUNEL staining pattern was also observed in Gal/ET-treated animals (Figure 4). Together, these results indicate that MT dramatically reduced the area of necrosis at both early and late stages of APAP-induced liver injury. However, MT treatment did not completely rescue all hepatocytes but switched APAP-induced cell death from necrosis to secondary apoptosis in some hepatocytes in the centrilobular areas.
Figure 3: MT treatment resulted in caspase-3 activation and pro-caspase cleavage in the liver.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline was given 1.5 h later. A group of mice was treated with Gal/ET for 6h as the positive control of apoptosis. (A) Caspase-3 activity in liver homogenate. (B) Pro-caspase-3 cleavage analyzed with western blotting. Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
Figure 4: MT treatment resulted in apoptotic morphology in centrilobular hepatocytes at late phase of APAP hepatotoxicity.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline was given 1.5 h later. Mice treated with Gal/ET for 6h were used as the positive control for apoptosis. Representative TUNEL staining of liver sections, with arrows denoting hepatocytes showing apoptotic morphology (100x).
Caspase inhibition further protected against APAP hepatotoxicity in Mito-tempo treated mice
To further test the pathophysiological role of secondary apoptosis in MT-treated mice after APAP overdose, liver injury was assessed in mice with or without the additional treatment of a pan-caspase inhibitor. As we expected, neither APAP nor MT caused any caspase activation or cleavage, while both of them were induced in mice treated with a combination of APAP and MT (Figure 5A,B). The caspase inhibitor completely blocked APAP+MT-induced caspase activation and cleavage (Figure 5A, B). More importantly, the caspase inhibitor further alleviated the remaining liver injury in APAP+MT treated mice, as indicated by the further decrease in plasma ALT activity (Figure 6A) and the reduction in cell death in both H&E- (Figure 6B) and TUNEL-stained liver sections (Figure 6C).
Figure 5: Pan-caspase inhibitor prevented caspase activation in MT treated mice.
Mice were treated with 300 mg/kg APAP, followed by 20 mg/kg MT or saline 1.5 h later, with or without 10 mg/kg ZVD-fmk or its vehicle 2h later. (A) Caspase-3 activity in liver homogenate. (B) Caspase-3 cleavage analyzed with western blotting. Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. APAP. #p<0.05 vs. APAP+MT.
Figure 6: Pan-caspase inhibitor further protected against late phase of APAP hepatotoxicity.
Mice were treated with 300 mg/kg APAP, followed by 20 mg/kg MT or saline 1.5 h later, with or without 10 mg/kg ZVD-fmk or its vehicle 2h later. (A) Plasma levels of ALT. (B) H&E stained liver sections (200x). (C) TUNEL stained liver sections (100x). Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP. $p<0.05 vs. APAP+MT.
Late post-treatment of Mito-Tempo did not induce secondary apoptosis during APAP hepatotoxicity
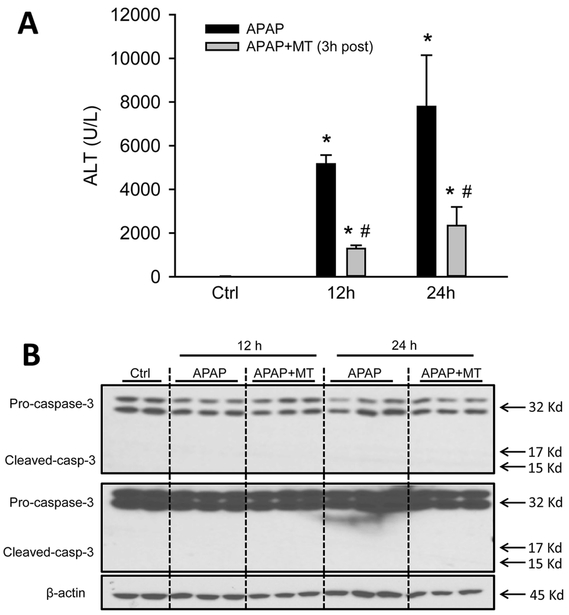
To test whether MT as a late post-treatment can still induce secondary apoptosis in APAP hepatotoxicity, MT was given at 3 h post-APAP, and mice were euthanized at 12 or 24 h post-APAP. Surprisingly, while MT as a 3 h post-treatment still significantly reduced APAP-induced liver injury at both time points (Figure 7A), it did not induce apoptotic morphology in the H&E stained sections (not shown) or caused any caspase cleavage at either time point (Figure 7B). Together with the data in Figures 5 and 6, this suggests that MT-induced secondary apoptosis during APAP hepatotoxicity occurs only within a certain time window.
Figure 7: Late post-treatment of MT, while protective, did not induce apoptosis.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline was given 3 h later. (A) Plasma levels of ALT. (B) Pro-caspase-3 cleavage in liver homogenate. Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
N-acetylcysteine did not induce secondary apoptosis during APAP hepatotoxicity
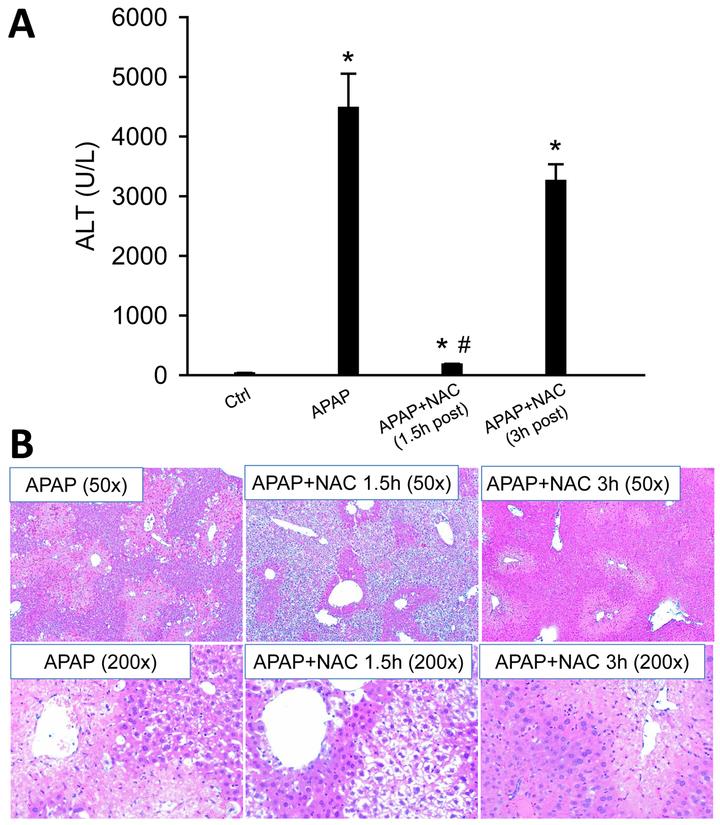
Although it has been shown that APAP causes necrosis in pathophysiologically relevant human cell culture models (e.g. HepaRG cells and primary mouse/human hepatocytes) (McGill et al. 2011; Xie et al. 2014), some human studies of APAP overdose patients reported evidence for the release of caspase-cleaved cytokeratin-18 into the plasma (Antione et al. 2012, 2013; Thulin et al. 2014). The reason for the discrepancy between humans and human cell culture models remains unknown. We hypothesized that N-acetylcysteine (NAC) treatment, which is the current standard of care for APAP poisoning, may play a role in this. To mimic the clinical care of APAP-overdose patients, the antidote NAC was given at 1.5 or 3 h after APAP overdose, and mice were euthanized at 24 h post-APAP. Consistent with previous studies (James et al. 2003; Saito et al. 2010), NAC as a 1.5 h post-treatment reduced over 95% of the injury, while a 3 h post-treatment provided only marginal protection against APAP toxicity (Figure 8A, B). However, treatment with NAC did not induce any caspase enzyme activation or cleavage (Figure 9A, B). Histological analysis of the liver sections by TUNEL staining pinpointed very few cells with apoptotic staining pattern in early 1.5 h NAC-treated mice, while it was completely absent in APAP group and the APAP+NAC (3 h) group (Figure 9C). In the latter 2 groups, all TUNEL staining was mainly cytosolic suggesting necrotic cell death consistent with the H&E-stained sections and the high ALT values (Figure 9C).
Figure 8: Posttreatment with NAC did not induce apoptotic morphology.
Mice were treated with 300 mg/kg APAP, and 500 mg/kg NAC was given 1.5 h or 3 h later. (A) Plasma levels of ALT. (B) Representative H&E stained liver sections (50x). Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
Figure 9: Posttreatment with NAC did not induce caspase activation or apoptosis.
Mice were treated with 300 mg/kg APAP, and 500 mg/kg NAC was given 1.5 h or 3 h later. (A) Caspase-3 activity in liver homogenate. (B) Caspase-3 cleavage analyzed with western blotting. (C) TUNEL stained liver sections (100x). Bars represent means ± SEM for n = 4 mice per group. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
Mito-Tempo induces secondary apoptosis after APAP overdose by inhibition of RIP3
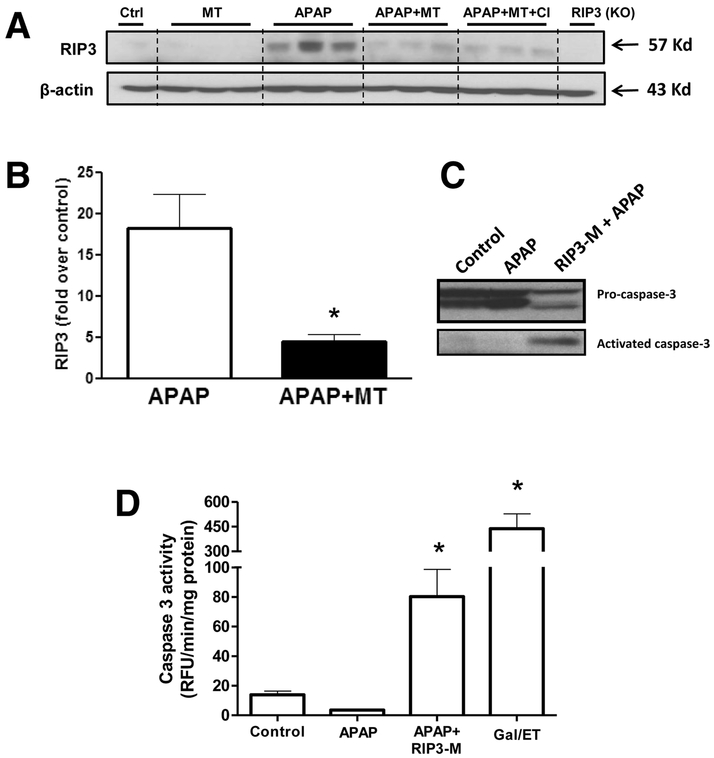
The data so far indicated that early post treatment (within 90 minutes) with MT prevented necrotic cell death but induced apoptosis in some hepatocytes. The RIP3 kinase has been identified as an important mediator of necrotic cell death (Li et al. 2012), and we have earlier shown significant upregulation of RIP3 after APAP overdose (Ramachandran et al. 2013). Since RIP3 has also been shown to switch cell death from apoptosis to necrosis (Zhang et al. 2009), we investigated whether RIP3 could be a molecular regulator mediating effects seen with MT. Significant elevations in RIP3 protein levels are sustained at 24 h after an APAP overdose, and treatment with MT significantly blunted this increase (Figure 10A,B). Interestingly, caspase inhibitor treatment had no significant effect on the blunting of RIP3 induction by MT. This suggests that RIP3 induction is upstream of caspase activation. To directly examine the role of RIP3 in switching necrosis to apoptosis after APAP, experiments were repeated after treatment of mice with a RIP3-morpholino, which significantly decreased RIP3 protein expression (Ramachandran et al. 2013). While animals treated only with 200mg/kg APAP showed no evidence of caspase cleavage or activation (Figure 10 C,D), those with RIP3 morpholino treatment prior to APAP showed evidence of caspase processing (Fig. 10 C) as well as increased caspase 3 activity (Fig. 10 D), though this was much lower than that seen with the positive control, galactosamine/endotoxin.
Figure 10: MT treatment inhibits APAP-induced RIP3 kinase activation and RIP3 kinase deficiency promotes caspase 3 activity.
Mice were treated with 300 mg/kg APAP, followed by 20 mg/kg MT or saline 1.5 h later. (A) Western blot for RIP3 kinase protein levels 24h after APAP and (B) densitometric quantitation of western blot. (C) Western blot showing caspase cleavage and (D) caspase 3 activity in liver homogenates. Bars represent means ± SEM for n = 3 mice per group. *p<0.05 vs. APAP (B) and *p<0.05 vs. Ctrl. (D).
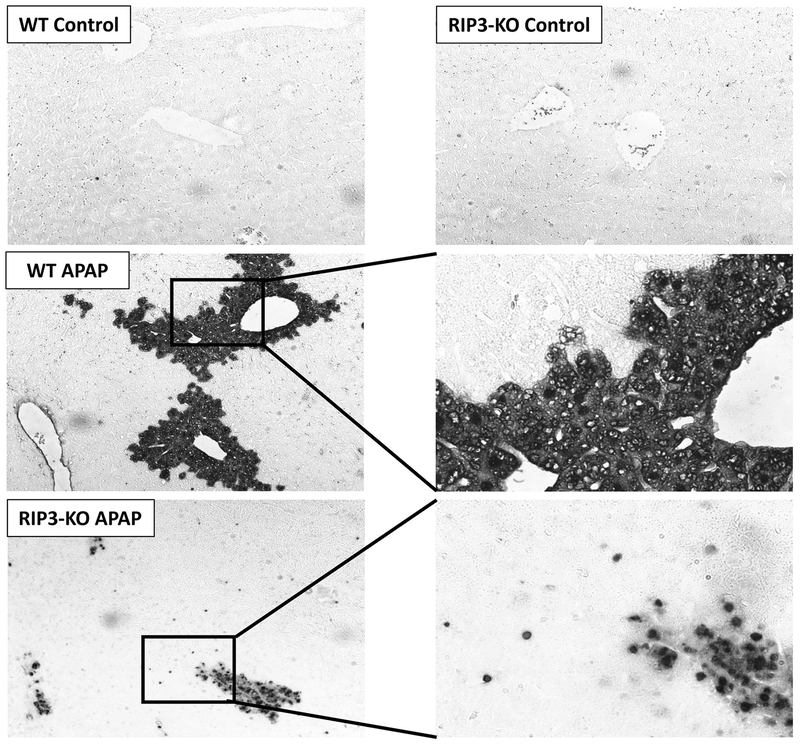
To further examine induction of apoptosis in RIP3 deficient animals, experiments were repeated with RIP3 knockout mice treated with 300mg/kg APAP, followed by TUNEL staining of liver sections at 6 hours. While WT animals treated with APAP showed characteristic TUNEL staining throughout the cell, a number of hepatocytes in RIP3 knockout animals treated with APAP showed distinct staining exclusively in the nucleus (Figure 11) suggestive of apoptotic cell death. These features were corroborated in animals treated with RIP3-morpholino, where a similar distinct nuclear TUNEL staining was evident (Supplemental Figure 2).
Figure 11: RIP3 kinase knockout mice show hepatocytes with apoptotic staining after APAP overdose.
TUNEL stained liver sections from control as well as APAP treated (300mg/kg) wild type and RIP3 kinase knockout mice sacrificed at 6h post APAP. The lower panels on the right are magnified views of the area in boxes on the left.
DISCUSSION
Role of apoptosis in APAP-induced liver injury in mice
It has been well established that APAP overdose exclusively causes oncotic necrosis rather than apoptosis in both in vivo and in vitro models (Bajt et al. 2004; Gujal et al. 2002; Jaeschke et al. 2011; Kon et al. 2004, McGill et al. 2011). Critical evidence comes from a typical necrotic morphology in the centrilobular areas of the liver during APAP hepatotoxicity, which is characterized by cell vacuolization, cell and organelle swelling, and karyorrhexis (Gujral et al. 2002; Jacob et al. 2007; Jaeschke et al. 2004). This is also accompanied by an extensive membrane leakage with release of cell contents followed by a robust inflammatory response (Lawson et al. 2000; Jaeschke et al. 2012). In contrast, the typical histological features of apoptosis, including cell shrinkage, chromatic condensation and formation of apoptotic bodies, are rarely noticeable in APAP toxicity (Gujral et al. 2002). While extensive caspase activation and dramatic protection by caspase inhibitors are routinely observed in Gal/ET- or Fas-induced hepatic apoptosis (Bajt et al. 2000; Jaeschke et al. 1998; Mignon et al. 1999; Schüngel et al. 2009), neither of these biochemical characteristics can be reproduced during APAP hepatotoxicity (Gujral et al. 2002; Jaeschke et al. 2006; Lawson et al. 1999; Williams et al. 2010). Together these findings strongly support necrosis as the dominant mode of cell death during APAP-induced liver injury in mice and argue against the relevance of apoptosis in the mechanism of toxicity. Interestingly, however, some previously assumed specific parameters of apoptosis, such as mitochondrial bax translocation, mitochondrial cytochrome c release, BH3 interacting domain death agonist (bid) cleavage and DNA strand breaks (TUNEL and DNA ladder), are all detected during APAP hepatotoxicity (Adams et al. 2001; Bajt et al. 2008; Cover et al., 2005; Jaeschke and Bajt 2006; Lawson et al. 1999; Ray et al. 1990). Use of these parameters has led to the assumption in some earlier studies and also more recently that apoptosis is a relevant cell death mechanism in the toxicity (El-Hassan et al. 2003; Hu et al. 2017; Wang et al. 2018, Zhang et al. 2017), while in reality none of these parameters is specific for apoptosis, and all of them can also be observed in cell necrosis (Jaeschke et al. 2018; Jaeschke and Lemasters 2003; Krassl-Graup et al. 1995). On the other hand, this also raises the intriguing question “why does APAP cause exclusive necrosis rather than apoptosis despite the appearance of these classical apoptotic parameters?”
The role of apoptosis in APAP overdose patients
Recent translational studies have used circulating plasma biomarkers to study the mode and mechanisms of cell death following APAP overdose in patients (McGill and Jaeschke 2014). One commonly used cell death biomarker is cytokeratin-18, an intermediate filament protein, which comprises part of the cytoskeleton. During necrosis, cytokeratin is released in its full-length form and can be measured in the plasma. In contrast, during apoptosis, active caspases cleave cytokeratin into two smaller fragments. Thus, the relative amount of full-length to cleaved cytokeratin-18 can provide information as to which mode of cell death predominates in a pathophysiology at any given time (Antoine et al. 2012; Eguchi et al. 2014; Weemhoff et al. 2017; Woolbright et al. 2017). In mice, very little (4%) cleaved cytokeratin-18 compared to the full-length form can be measured in plasma following APAP overdose (Antoine et al. 2009). Importantly, a pan-caspase inhibitor reduced the levels of caspase-cleaved cytokeratin-18 but did not reduce the overall liver injury (Antoine et al. 2009), supporting the conclusion that necrosis is the primary mode of cell death in mice. In addition, experiments with a metabolically competent human hepatoma cell line (HepaRG) and in primary human hepatocytes showed exclusively necrotic cell death in response to APAP (McGill et al. 2011; Xie et al. 2014), which was consistent with the lack of caspase-3 activity detectable in serum of APAP overdose patients (McGill et al. 2012). Interestingly, however, cleaved cytokeratin-18 was detected in some APAP overdose patients, and could even account for up to 20% of the total cytokeratin levels (Antione et al. 2012, 2013). Even more, the levels of cleaved cytokeratin on admission correlated with poor outcome of the patients (Possamai et al., 2013). The reason for some of the discrepancies between humans and mice, especially when using cytokeratin-18, triggered a heated debate in recent years. Antoine et al. (2010) proposed that this might be due to the fact that fasted mice are typically used in APAP toxicity studies, in whom ATP level are decreased and apoptosis signaling is inhibited. This contrasts to clinical practice where patients are normally in a non-fasted status at the time of overdose and they receive nutritional support upon hospitalization, which may sustain hepatocellular ATP and permit the onset of apoptosis (Possamai et al. 2013). This is supported by the findings that minor and transient caspase-3 activation was observed in fed mice after APAP overdose (Antione et al. 2010). However, a follow-up study demonstrated that the transient caspase activation in fed mice is more related to the mouse strain rather than the ATP levels (Williams et al. 2011). More importantly, this minor caspase activation never resulted in noticeable apoptotic cell death and a caspase inhibitor did not protect against the overall cell death (Williams et al. 2011). Thus, there is very limited evidence for apoptosis as a primary mode of cell death during APAP-induced liver injury in mice or humans (Jaeschke et al. 2018).
Apoptosis as a secondary mode of cell death during APAP toxicity
Consistent with all previous reports, we did not observe any morphological evidence for apoptosis, did not detect evidence for caspase activation (enzyme activity and cleavage of the pro-enzyme) and did again not find a protection with a caspase inhibitor. Interestingly, however, in animals with drastically reduced cell necrosis due to treatment with MT, there was clear evidence for individual cells with apoptotic morphology, selective nuclear TUNEL staining typical for apoptosis and a moderate increase of caspase-3 enzyme activity with cleavage to the active fragment. Importantly, a caspase inhibitor prevented these effects and caused an overall additive protection with MT. These data suggest that although most hepatocytes are effectively protected against APAP toxicity by MT, a small fraction of these cells are now undergoing apoptosis.
It is well known that APAP overdose triggers a mitochondrial oxidant stress through enhanced electron leakage from the electron transport chain (Du et al. 2016a). Superoxide anion radicals, the first product being formed can react in 2 ways: a) it can rapidly combine with the radical nitric oxide to form peroxynitrite, a potent oxidant and nitrating species, or b) it can dismutate, facilitated by SOD2, to form oxygen and hydrogen peroxide, which can be reduced to water by glutathione peroxidase. Previous studies have provided evidence that peroxynitrite is the most relevant oxidant for APAP toxicity (Cover et al. 2005; Knight et al. 2002). This is the reason why the mitochondria-targeted SOD mimetic MT is highly protective (Du et al. 2017). MT promotes superoxide dismutation and prevents peroxynitrite formation as shown by the dramatic reduction of nitro-tyrosine adducts in the liver of MT- treated animals. As a result, MT effectively prevents mitochondrial dysfunction as indicated by the reduced release of intermembrane proteins such as endonuclease G, AIF and Smac, some of which translocate to the nucleus and cause DNA fragmentation (Du et al., 2017). However, MT treatment does not eliminate the oxidant stress as can be seen by the very high levels of GSSG. This means, that although the formation of the most aggressive oxidant is prevented by MT, there is still some oxidant stress in these cells, which may promote apoptosis. In support of this hypothesis, it has been long recognized that oxidative stress causes dose-dependent cell death (Sen and Roy 2001). This is consistent with previous findings where a low dose of oxidant stress (e.g. H2O2) induced caspase activation and caused apoptosis in Jurkat T cells while a high dose of it inactivated caspases and switched the mode of cell death from apoptosis to necrosis (Hampton and Orrenius 1997; Borutaite and Brown 2001). In line with these findings, it was previously shown that when APAP-induced necrosis of cultured hepatocytes is inhibited by glycine and fructose, apoptotic cell death develops several hours later (Kon et al. 2004). This again shows that if a cell death mechanism is inhibited, in this case the MPTP formation is prevented, the upstream stress (protein adducts, initial oxidant stress) is still present and can eventually lead to cell death. This suggests that although an intervention can reduce the overall cell injury, the residual stress may trigger limited apoptosis. This concept is also supported by a recent report that GSH treatment reduces mitochondrial ROS and damage and thus shifts cell death from necrosis to apoptosis in hearts of ageing diabetic mice during exercise (Golbidi et al. 2014). However, despite the mounting evidence for the occurrence of secondary apoptosis when mechanisms of necrotic cell death are inhibited, we observed that MT can only trigger this effect within a limited time frame. Late post-treatment still attenuated the overall injury but the remaining cell death was still necrosis. This suggests that the extent of the residual stress in each cell will determine whether the cell dies by apoptosis or necrosis.
Although protection with MT triggered limited apoptotic cell death after APAP overdose, NAC protected effectively when treated at 1.5 h but not anymore when given at 3 h after APAP. These data are consistent with previous reports (James et al. 2003; Knight et al. 2002, Saito et al. 2010). However, no evidence for apoptotic cell death was found with NAC treatment. The reason for this discrepancy might be that NAC has multiple modes of action. The GSH synthesized from NAC can assist in scavenging any residual NAPQI being formed (Corcoran et al. 1985), can directly scavenge peroxynitrite and help in detoxifying H2O2 through glutathione peroxidase in the mitochondria, and degradation of the NAC surplus supports the mitochondrial bioenergetics (Saito et al. 2010). This makes NAC more versatile; however, the fact that it requires GSH synthesis and transport into mitochondria may also limit the therapeutic window. Nevertheless, although MT effectively prevents peroxynitrite formation, MT shifts some of the superoxide more towards hydrogen peroxide, which could be responsible for the secondary apoptosis described here. This effect needs to be considered as potential side-effect for SOD mimetics that are currently under clinical evaluation (Dear et al., 2017).
Mechanisms of apoptosis induction during APAP hepatotoxicity
So how did post treatment with MT shift the mode of cell death from necrosis to apoptosis in some hepatocytes? Recent studies have illuminated the role of RIP3 kinase in mediating programmed necrosis, where RIP3 kinase forms part of the necrosome complex, necessary for cellular signaling during this mode of cell death (Li et al. 2012). RIP3K has been suggested to switch the mode of cell death from apoptosis to necrosis (Zhang et al. 2009) and we have earlier demonstrated that RIP3 protein levels and mRNA were significantly elevated after APAP overdose, and inhibition of RIP3 provides early protection against APAP induced hepatotoxicity (Ramachandran et al. 2013). These studies were corroborated by a number of investigators showing elevations in RIP3 subsequent to APAP overdose (Deutsch et al. 2015; Horng et al. 2017; Lu et al. 2017) as well as protection against APAP induced liver injury by blockade of RIP3 (Deutsch et al. 2015; Li et al. 2014). In spite of these studies, and the fact that RIP3 has also been shown to be upregulated in the liver in chronic ethanol feeding, and mice lacking RIP3 protected against liver injury (Roychowdhury et al. 2013), one group was unable to detect RIP3 expression in the liver and have questioned the relevance of the protein (Dara et al. 2015). In addition to protection against liver injury, we also observed that inhibition of RIP3 prevented mitochondrial dysfunction and release of mitochondrial proteins such as apoptosis inducing factor and endonuclease G into the cytosol (Ramachandran et al. 2013). RIP3 was also shown to regulate TNF-induced reactive oxygen species production, which facilitated RIP3's ability to promote necrosis (Zhang et al. 2009). The data with MT now seem to suggest that the relationship between mitochondrial oxidative stress/dysfunction and RIP3 activation is reciprocal, with scavenging of mitochondrial radicals with MT blocking APAP-induced RIP3 induction. The role of RIP3 inhibition in switching hepatocytes to an apoptotic mode of cell death was confirmed by the experiments with direct knockdown of RIP3, which showed effects similar to that observed with MT, namely procaspase cleavage, increases in caspase activity and cells with apoptotic morphology. It has to be noted, however, that these changes were relatively minor when compared to the positive control galactosamine/endotoxin, again emphasizing that APAP induced cell death under normal conditions is predominantly necrosis. All these data suggest that RIP3 kinase is the molecular mediator switching APAP-induced necrotic cell to apoptosis in cells where mitochondrial oxidant stress was blocked.
In conclusion, while the predominant mode of cell death after APAP overdose is necrosis, this seems to be dictated by activation of the RIP3 kinase, and interference with this signaling seems to be capable of switching the mode of cell death, at least in some hepatocytes, to apoptosis. These effects are intricately dictated by mitochondrial events, and their modulation can thus influence mode of cell death.
Supplementary Material
ACKNOWLEDGEMENTS
This investigation was supported in part by the National Institutes of Health grant R01 DK102142 and by grants from the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) from the National Institutes of Health. Additional support came an award from the Biomedical Research Training Program (BRTP) from the University of Kansas Medical Center (to K.D.).
List of Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- CYP
cytochrome P450
- Gal
D-galactosamine
- GSH
glutathione
- GSSG
glutathione disulfide
- ET
endotoxin (lipopolysaccharide)
- MPT
mitochondrial permeability transition
- MT
Mito-Tempo
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- ROS,
reactive oxygen species
- RFU
relative fluorescence units
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The manuscript does not contain clinical studies or patient data.
CONFLICT OF INTEREST DISCLOSURE
The authors declare that they have no conflict of interest.
REFERENCES
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA (2001) Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol 60:907–15. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK (2013) Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology 58:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK (2012) Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol 56:1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK (2009) High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci 112:521–31. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK (2010) Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med 16:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H (2008) Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther 324:8–14. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetylcysteine. Toxicol Sci 80:343–9. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H (2000) Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci 58:109–17. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE (2011) Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med 40:585–92. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Brown GC (2001) Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett 500:114–8. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR (1985) Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther 232:864–72. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H (2005) Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–87. [DOI] [PubMed] [Google Scholar]
- Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N (2015) Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 62:1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear JW, Morrison E, Henriksen D, Nasstrom J (2017) Calmangafodipir is a new treatment for late stage liver toxicity after acetaminophen overdose. Hepatology 66 (Supplement 1): 4A–5A.28370190 [Google Scholar]
- Deutsch M, Graffeo CS, Rokosh R, Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R, Tomkotter L (2015) Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis 6:e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Farhood A, Jaeschke H (2017) Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol 91:761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Jaeschke H (2016a) Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 10:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Weemhoff JL, Chavan H, Xie Y, Krishnamurthy P, Jaeschke H (2016b) Editor's Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction. Toxicol Sci 154:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi A, Wree A, Feldstein AE (2014) Biomarkers of liver cell death. J Hepatol 60:1063–74. [DOI] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE (2003) Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol 191:118–29. [DOI] [PubMed] [Google Scholar]
- Golbidi S, Botta A, Gottfred S, Nusrat A, Laher I, Ghosh S (2014) Glutathione administration reduces mitochondrial damage and shifts cell death from necrosis to apoptosis in ageing diabetic mice hearts during exercise. Br J Pharmacol 171:5345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21:1465–8. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–8. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414:552–6. [DOI] [PubMed] [Google Scholar]
- Horng CT, Liu ZH, Huang YT, Lee HJ, Wang CJ (2017) Extract from Mulberry (Morus australis) leaf decelerate acetaminophen induced hepatic inflammation involving downregulation of myeloid differentiation factor 88 (MyD88) signals. J Food Drug Anal 25:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JN, Liu Z, Wang Z, Li XD, Zhang LX, Li W, Wang YP (2017) Ameliorative effects and possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules 22(4): pii: E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M, Mannherz HG, Napirei M (2007) Chromatin breakdown by deoxyribonuclease1 promotes acetaminophen-induced liver necrosis: an ultrastructural and histochemical study on male CD-1 mice. Histochem Cell Biol 128:19–33. [DOI] [PubMed] [Google Scholar]
- Jaeschke H (1990) Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255:935–41. [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML (2006) Role of caspases in acetaminophen-induced liver injury. Life Sci 78:1670–6. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Duan L, Akakpo JY, Farhood A, Ramachandran A (2018) The role of apoptosis in acetaminophen hepatotoxicity. Food Chem Toxicol 118:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA (1998) Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol 160:3480–6. [PubMed] [Google Scholar]
- Jaeschke H, Gujral JS, Bajt ML (2004) Apoptosis and necrosis in liver disease. Liver Int 24:85–9. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125:1246–57. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Farhood A (2011) No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 53:718–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML (2012) Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int 32:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA (2003) Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75:458–67. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H (2002) Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther 303:468–75. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H (2002) Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol 181:133–41. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–9. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H (2000) The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci 54:509–16. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H (1999) Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol 156:179–86. [DOI] [PubMed] [Google Scholar]
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, Shen YY, Chen Y, Xiong B, Yang CH, Ding J (2014) The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis 5:e1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA (2011) Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology 54:969–78. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang C, Chen YH, Wang H, Zhang ZH, Chen X, Xu DX (2017) Immature mice are more susceptible than adult mice to acetaminophen-induced acute liver injury. Sci Rep 7:42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthripragada AD, Zhou EH, Budnitz DS, Lovegrove MC, Willy ME (2011) Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf 20:819–26. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–6. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H (2014) Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol 10:1005–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H (2015) A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods 25:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122:1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H (2011) HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53:974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD (1988) Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol 93:378–87. [DOI] [PubMed] [Google Scholar]
- Mignon A, Rouquet N, Fabre M, Martin S, Pagès JC, Dhainaut JF, Kahn A, Briand P, Joulin V (1999) LPS challenge in D-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am J Respir Crit Care Med 159(4 Pt 1):1308–15. [DOI] [PubMed] [Google Scholar]
- Nelson SD (1990) Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10:267–78. [DOI] [PubMed] [Google Scholar]
- Possamai LA, McPhail MJ, Quaglia A, Zingarelli V, Abeles RD, Tidswell R, Puthucheary Z, Rawal J, Karvellas CJ, Leslie EM, Hughes RD, Ma Y, Jassem W, Shawcross DL, Bernal W, Dharwan A, Heaton ND, Thursz M, Wendon JA, Mitry RR, Antoniades CG (2013) Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure. Crit Care Med 41:2543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H (2011a) Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res 45:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H (2011b) The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 251:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H (2013) Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB (1990) Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol 106:346–51. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE (2013) Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57:1773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H (2010) Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüngel S, Buitrago-Molina LE, Nalapareddy Pd, Lebofsky M, Manns MP, Jaeschke H, Gross A, Vogel A (2009) The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology 50:1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Roy S (2001) Redox control of caspases. Environ Toxicol Pharmacol 10:215–20. [DOI] [PubMed] [Google Scholar]
- Skulachev VP (2006) Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 11:473–85. [DOI] [PubMed] [Google Scholar]
- Thulin P, Nordahl G, Gry M, Yimer G, Aklillu E, Makonnen E, Aderaye G, Lindquist L, Mattsson CM, Ekblom B, Antoine DJ, Park BK, Linder S, Harrill AH, Watkins PB, Glinghammar B, Schuppe-Koistinen I (2014) Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int 34(3):367–78. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD (1989) Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem 264:9814–9. [PubMed] [Google Scholar]
- Wang X, Liu J, Zhang X, Zhao S, Zou K, Xie J, Wang X, Liu C, Wang J, Wang Y (2018) Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine 38:90–7. [DOI] [PubMed] [Google Scholar]
- Weemhoff JL, Woolbright BL, Jenkins RE, McGill MR, Sharpe MR, Olson JC, Antoine DJ, Curry SC, Jaeschke H (2017) Plasma biomarkers to study mechanisms of liver injury in patients with hypoxic hepatitis. Liver Int 37(3):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H (2010) Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol 247(3):169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Koerner MR, Lampe JN, Farhood A, Jaeschke H (2011) Mouse strain-dependent caspase activation during acetaminophen hepatotoxicity does not result in apoptosis or modulation of inflammation. Toxicol Appl Pharmacol 257:449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Bridges BW, Dunn W, Olson JC, Weinman SA, Jaeschke H (2017) Cell Death and Prognosis of Mortality in Alcoholic Hepatitis Patients Using Plasma Keratin-18. Gene Expr 17(4):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H (2017) Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol 66:836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H (2014) Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol 279:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H (2015) Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol 289:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Song Q, Han X, Zhang Y, Zhang Y, Zhang X, Chu X, Zhang F, Chu L (2017) Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int Immunopharmacol 47:95–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.